Abstract
Background:
Postoperative atrial fibrillation occurs in 27% to 40% of patients after cardiac surgery. One cause of postoperative atrial fibrillation is pericardial effusion, which can be a significant source of inflammation. In this study, we investigated the effect of a drain placed in a posterior site to the heart to reduce pericardial effusion in the early postoperative period to prevent postoperative atrial fibrillation.
Methods:
Participants were patients who underwent initial standby aortic valve replacement at Saga-Ken Medical Centre Koseikan from January 2010 to December 2021. Patients with a history of atrial fibrillation, complex surgery, or emergency surgery were excluded. The patients were divided into two groups: those with a posterior cardiac drain in addition to the usual intrapericardial and subpleural drains from September 2017 (group P) and those without posterior cardiac drain from January 2010 to August 2017 (group N). Multiple logistic regression analysis was performed to evaluate the usefulness of posterior cardiac drain.
Results:
Of the 79 patients included the study, 40 were male and groups P and N comprised 27 and 52 patients, respectively. Of the 79 patients, 32 developed postoperative atrial fibrillation; among whom, 7/27 (25.9%) had posterior cardiac drain and 25/52 (48.1%) had no posterior cardiac drain (p = 0.09). When adjusted for body surface area, left ventricular end-diastolic and left atrial diameter, the incidence of postoperative atrial fibrillation was significantly lower in group P than in group N (adjusted odds ratio 0.270, 95% confidence interval 0.077–0.953, p = 0.042). Furthermore, no patient in the group P underwent postoperative thoracentesis in the subanalysis.
Conclusions:
The results suggest that early postoperative reduction of pericardial effusion by posterior cardiac drain placement may reduce the incidence of postoperative atrial fibrillation.
Keywords: Aortic valve replacement, posterior cardiac drain, atrial fibrillation, pericardial effusion
Introduction
Postoperative atrial fibrillation (POAF) is the most frequent complication of cardiac surgery. 1 The occurrence of POAF is associated with decreased survival, increased incidence of stroke and heart failure, increased length of hospital stay, and increased costs. 2 Pericardial effusion is common after cardiac surgery and induces postoperative atrial arrhythmias. 3 A left posterior pericardiotomy prevents postoperative pericardial effusion by incising the pericardium on the left posterior side of the heart and draining it into the left thoracic cavity. One report showed that POAF was reduced by half by performing left posterior pericardiotomy to prevent postoperative pericardial effusion. 4 Placement of a posterior cardiac drain (PCD) has also been reported to decrease postoperative pericardial effusion. 5 In this study, we examined the hypothesis that the reduction in pericardial effusion by PCD placement would reduce POAF.
Patient and methods
Patients who underwent initial aortic valve replacement (AVR) at Saga-Ken Medical Centre Koseikan between January 2010 and December 2021 were included in the study. Patients with preoperative history of atrial fibrillation, complex and emergency procedures were excluded. Both groups underwent AVR through a median sternotomy under general anesthesia with observation during the hospitalization period. Since our hospital decided to implant PCD in addition to the usual subpleural and intrapericardial drains in September 2017, this study was divided into two groups: the group with subpleural and intrapericardial drain implantation from January 2010 to August 2017 (group N) and the group with PCD in addition to the usual drain after September 2017 (group P). Additional thoracic drains were placed, as required, in both groups. The effects of the PCD on the prevention of pericardial effusion and POAF were examined. To examine the factors involved in POAF, comparisons were made between groups with and without POAF.
Definition of POAF
POAF was detected by electrocardiographic monitoring, regardless of duration and treated by various means including oral medication. The electrocardiographic monitor was worn for 7–10 days after surgery, with wear periods extended as required.
Drain placement method
A 15-Fr soft silastic channeled drain was placed in the diaphragmatic plane posterior to the ventricle, next to the left atrium, and anterior to the pulmonary artery. The drain was connected to a portable low-pressure continuous suction system, and negative pressure was applied after the subpleural and intrapericardial drains were removed (Figure 1).
Figure 1.
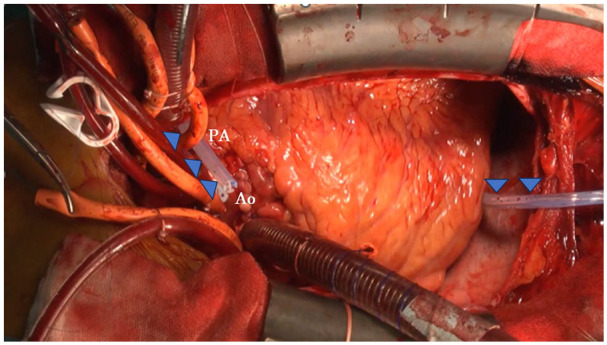
A 15-Fr drain was placed in the diaphragmatic plane, posterior to the ventricle, next to the left atrium, and anterior to the pulmonary artery (arrows).
Ao: ascending aorta, PA: main pulmonary artery.
Management of the drains
In all patients in group P, the subpleural and intrapericardial drains were removed on the first postoperative day, and negative pressure of the PCD was started. The drain was removed when the daily drainage volume of the PCD was less than 50 ml. In group N, the drain was removed when the daily drainage volume of the subpleural and intrapericardial drains reached <200 ml. Both groups underwent postoperative echocardiographic evaluation on the seventh postoperative day to assess the degree of pericardial effusion of the posterior cardiac artery and other parameters.
Statistical analysis
Continuous variables were expressed as medians and interquartile ranges. The Mann–Whitney U test was used to compare atrial fibrillation between the two groups. Categorical variables are expressed as frequencies and percentages. The two groups were compared using Fisher’s exact test. The parameters of the echocardiographic examination were evaluated using Spearman’s correlation coefficient. Multivariate logistic regression analysis was used to adjust for confounding factors. All statistical analyses were performed using R version 4.1.2, and statistical significance was set at p < 0.05.
Ethical considerations
This retrospective study was approved by the Ethics Review Committee and Conflict of Interest Review Committee at Saga-Ken Medical Centre Koseikan (Approval No. 22-09-01-01). Because this study did not involve any invasive procedures or interventions, written consent was not obtained from the participants. Instead, an opt-out approach was used, allowing patients to decline participation through the hospital’s website.
Results
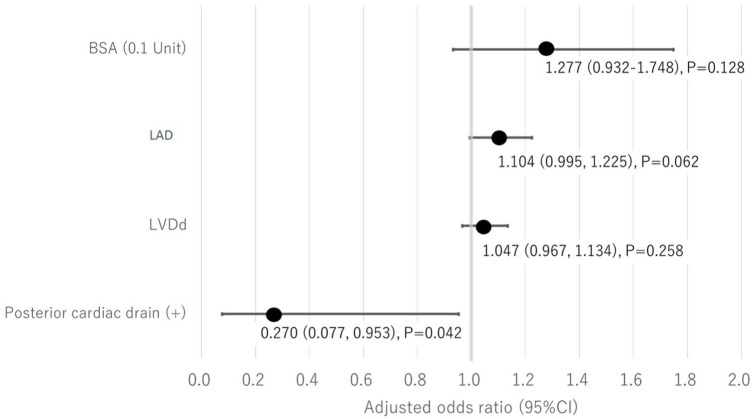
Seventy-nine patients who underwent an initial AVR between January 2010 and December 2021 at our hospital were included in this study. The mean age was 76.0 years, and the male-to-female ratio was 40:39. No cases underwent posterior pericardiotomy. The duration of monitoring did not differ between the two groups. Group P and group N consisted of 27 and 52 patients, respectively. There were no significant differences in the preoperative age, sex, medical history, or preoperative echocardiographic results (Table 1). There were no significant differences in operative, cardiopulmonary bypass, or aortic cross-clamp times. The incidence of POAF was generally half in groups P (25.9%) and N (48.1%), although the difference was not significant (p = 0.09). In group P, the subpleural and intrapericardial drains were removed on postoperative day 1, whereas in group N, the subpleural intrapericardial drain was removed on postoperative day 3 (p < 0.001). The incidence of posterior pericardial effusion greater than 5 mm on echocardiography was 3.7% and 17.3% in groups P and group N, respectively (p = 0.151). There were no cases of pleural effusion requiring thoracentesis in group P (p = 0.046) (Table 2). POAF was present in 32 patients (40.5%), with more males in the group with POAF (p = 0.039) and significantly larger body surface area (BSA) (p = 0.026). Preoperative echocardiography showed no difference in ejection fraction (EF), but the POAF group had significantly larger left ventricular end-diastolic diameters (LVDd; p = 0.004), left ventricular end-systolic diameters (LVDs; p = 0.028), and left atrial diameters (LADs; p = 0.001). There was no significant difference in the occurrence of POAF in terms of the presence or absence of PCD placement, but the POAF group tended to have less PCD placement (p = 0.09) (Table 3). After multivariate analysis adjusted for BSA, LVDd, and LAD, the incidence of POAF was significantly lower when PCD was performed (adjusted odds ratio 0.27, 95% confidence interval 0.077–0.953, p = 0.042; Figure 2). Additionally, since LVDs showed a high correlation with LVDd (Spearman’s rank correlation coefficient = 0.88), it was excluded from the model.
Table 1.
Comparison of preoperative results.
| Characteristics | Group P (n = 27) | Group N (n = 52) | p-Value |
|---|---|---|---|
| Median [IQR] or N (%) | Median [IQR] or N (%) | ||
| Age (years) | 73.00 [66.00, 77.50] | 76.00 [71.00, 80.00] | 0.173 |
| Female sex | 12 (44.4) | 27 (51.9) | 0.637 |
| BSA (m2) | 1.60 [1.40, 1.75] | 1.50 [1.40, 1.60] | 0.028 |
| Hypertension | 8 (29.6) | 22 (42.3) | 0.333 |
| Diabetes mellitus | 19 (70.4) | 34 (65.4) | 0.094 |
| Dyslipidemia | 12 (44.4) | 34 (65.4) | 0.094 |
| Chronic renal failure (Cre >1.5 mg/dl) | 5 (18.5) | 9 (17.3) | 1.000 |
| Preoperative EF (%) | 66.0 [53.50, 68.00] | 65.00 [52.75, 71.00] | 0.475 |
| Preoperative LVDd (mm) | 48.00 [45.0, 50.50] | 49.50 [45.75, 54.00] | 0.328 |
| Preoperative LVDs (mm) | 31.00 [27.50, 34.00] | 32.00 [27.75, 39.25] | 0.538 |
| Preoperative LAD (mm) | 41.00 [36.00, 45.00] | 42.50 [38.00, 45.00] | 0.305 |
BSA: body surface area, EF: ejection fraction, LVDd: left ventricular end-diastolic diameters, LVDs: left ventricular end-systolic diameters, LAD: left atrial diameters; IQR: interquartile range.
Table 2.
Comparison of intraoperative and postoperative results.
| Characteristics | Group P (n = 27) | Group N (n = 52) | p-Value |
|---|---|---|---|
| Median [IQR] or N (%) | Median [IQR] or N (%) | ||
| Operation time (min) | 249.00 [222.50, 271.50] | 266.50 [233.50, 301.25] | 0.057 |
| CPB time (min) | 127.00 [112.00, 137.00] | 137.00 [115.75, 164.00] | 0.070 |
| Cross clamp time (min) | 85.00 [76.00, 91.00] | 90.50 [77.75, 103.25] | 0.171 |
| Intraoperative RCC | 10 (37.0) | 31 (59.6) | 0.063 |
| Postoperative RCC | 4 (14.8) | 25 (48.1) | 0.006 |
| Peak CK-MB (IU/l) | 29.00 [19.20, 41.15] | 28.50 [20.65, 39.48] | 0.783 |
| Peak WBC (×100/) | 161.00 [139.50, 189.00] | 162.00 [126.50, 187.50] | 0.914 |
| Peak CRP (mg/dl) | 7.52 [6.21, 9.13] | 7.61 [5.79, 9.51] | 0.788 |
| Lowest Hb (mg/dl) | 9.30 [8.80, 10.20] | 8.45 [7.88, 9.50] | 0.002 |
| Duration of subpleural and intrapericardial drain placement (days) | 1.00 [1.00, 1.00] | 3.00 [2.00, 3.00] | <0.001 |
| Duration of PCD placement (days) | 5.00 [4.00, 5.00] | NA | NA |
| Intubation time (h) | 3.00 [2.50, 4.75] | 5.50 [4.00, 9.50] | 0.001 |
| POAF(+) | 7 (25.9) | 25 (48.1) | 0.09 |
| ICU stay (days) | 3.00 [3.00, 3.00] | 4.00 [4.00, 4.00] | <0.001 |
| Hospital stay (days) | 16.00 [16.00, 20.00] | 22.50 [20.00, 27.00] | <0.001 |
| Postop cerebral infarction | 0 (0) | 2 (3.8) | 0.544 |
| Posterior pericardial effusion >5 mm | 1 (3.7) | 9 (17.3) | 0.151 |
| Pleural effusion required thoracentesis | 0 (0) | 8 (15.4) | 0.046 |
CPB: cardiopulmonary bypass, RCC: red cell concentrate, PCD: posterior cardiac drain; POAF: postoperative atrial fibrillation; IQR: interquartile range.
Table 3.
Comparison with and without POAF.
| Characteristics | POAF(+) (n = 32) | POAF(−) (n = 47) | p-Value |
|---|---|---|---|
| Median [IQR] or N (%) | Median [IQR] or N (%) | ||
| Age (years) | 75.00 [66.00, 79.00] | 76.00 [69.50, 80.00] | 0.749 |
| Female sex | 11 (34.4) | 28 (59.6) | 0.039 |
| BSA (m2) | 1.55 [1.40, 1.70] | 1.50 [1.35, 1.60] | 0.026 |
| Hypertension | 22 (68.8) | 34 (72.3) | 0.803 |
| Diabetes mellitus | 5 (15.6) | 16 (34.0) | 0.077 |
| Dyslipidemia | 8 (25.0) | 25 (53.2) | 0.02 |
| Chronic renal failure (Cre >1.5 mg/dl) | 8 (25.0) | 6 (12.8) | 0.231 |
| Preoperative EF (%) | 62.50 [49.00, 71.00] | 66.00 [59.50, 69.50] | 0.481 |
| Preoperative LVDd (mm) | 52.00 [47.00, 56.00] | 47.00 [45.00, 50.00] | 0.004 |
| Preoperative LVDs (mm) | 36.00 [28.00, 42.25] | 31.00 [27.00, 33.50] | 0.028 |
| Preoperative LAD (mm) | 44.00 [41.00, 46.25] | 38.00 [36.50, 44.00] | 0.001 |
| PCD placement | 7 (21.9) | 20 (42.6) | 0.09 |
BSA: body surface area, EF: ejection fraction, LVDd: left ventricular end-diastolic diameters, LVDs: left ventricular end-systolic diameters, LAD: left atrial diameters, PCD: posterior cardiac drain; POAF: postoperative atrial fibrillation; IQR: interquartile range.
Figure 2.
Multivariate analysis with logistic regression analysis.
LVDd: left ventricular end-diastolic diameters, LAD: left atrial diameters.
Discussion
POAF after cardiac surgery is the most common complication, reported in 30% to 40% of patients, depending on the type of surgery and method of evaluation. 6 POAF is associated with decreased survival, increased incidence of stroke and heart failure, and increased hospitalization duration and costs. 2 Preventing the development of POAF can be crucial as new-onset POAF during hospitalization after AVR is associated with a 1.6- to 2-fold increase in subsequent all-cause mortality. 7 Common approaches to prevent POAF include the administration of drugs, such as beta-blockers, digoxin, and amiodarone. However, the use of these drugs may be unadvisable due to side effects, such as bradycardia and hypotension. 8
Pericardial effusion after cardiac surgery is a common complication. 3 Some reports indicate that postoperative pericardial effusion causes local inflammation of the heart, contributing to atrial fibrillation.3,9 Therefore, the prevention of pericardial effusion is widely held to prevent POAF. Decreased posterolateral pericardial effusion is a plausible mechanism for the reduction of POAF. 10 Posterior pericardiotomy prevents postoperative pericardial effusion. This method induces pericardial effusion in the chest cavity and reduce POAF. 11 Gaudino et al. reported that left posterior pericardiotomy is a very simple procedure; POAF is reduced by half and no adverse complications occur when the procedure is performed. 4 However, the authors of that study reported that more patients who underwent left posterior pericardiotomy required thoracic drainage than those who did not undergo left posterior pericardiotomy. In addition, there are drawbacks and risks to routine prophylactic pericardiotomy, such as the risk of cardiac and bypass graft hernias and adhesion formation. 3 The placement of small, soft, and silastic channeled drainage tubes is believed to promote pericardial drainage.
The drain placement pathway is important to effectively reduce posterolateral pericardial effusion. PCD is a simple method for preventing postoperative pericardial effusion by draining the effusion out of the body. In addition to the usual intrapericardial and subpleural drains, a PCD was placed intraoperatively. Negative-pressure suctioning was started the day after the removal of the intrapericardial and subpleural drains. The use of small drains for pericardial drainage has been previously reported. In a randomized controlled trial, prolonged mediastinal drainage using silastic tubes showed no advantage over standard chest drains with regard to significant effusion, tamponade, or the incidence of POAF.12,13 However, experience suggests that the use of small drains is as effective as standard drains. A drainage method similar to ours has been shown to reduce postoperative pericardial effusion. However, the occurrence of POAF in such a context has not yet been investigated. 5 One study assessing the impact of similar drainage methods on coronary artery bypass grafting demonstrated a significantly smaller volume of pericardial effusion evaluated using transthoracic echocardiography, as well as a 52% reduction in the rate of POAF associated with the use of similar drains. 14 Eryilmaz et al. reported significantly less posterior pericardial effusion and a lower incidence of POAF in patients undergoing aortic surgery with PCD than in those with anterior cardiac drainage. 15
In our study, the PCD group had less posterior pericardial effusion (> 5 mm) than the group without PCD, although the difference was not statistically significant. If sufficient drainage is achieved, PCD placement alone may be acceptable without posterior pericardiotomy. Our criterion for drain removal was a drainage volume of <50 ml/day. Consequently, the drain was removed 5 days postoperatively, on average. In the PCD group, the subpleural and intrapericardial drains were removed the day after surgery to start early rehabilitation, and PCD was performed because of concerns regarding subsequent pericardial effusions. The criteria for drain removal in the group without PCD was less than 200 ml/day, which is the standard at many centers. Notably, it cannot be overlooked that adopting removal criteria of less than 50 ml/day might have resulted in less pericardial effusion as in the PCD group. However, the long-term insertion of a large drain might have delayed the initiation of early rehabilitation owing to factors such as pain. Multivariate analysis showed that PCD significantly reduced the incidence of POAF when adjusted for BSA, LVDd, and LAD, suggesting that its effect was similar to that of left posterior pericardiotomy. There were no cases of pleural effusion requiring thoracic drainage in the PCD group, suggesting that this technique may be superior to posterior pericardiotomy. No infections or unexpected complications were observed. The amount of postoperative bleeding was evaluated based on the amount of posterior pericardial effusion observed via echocardiography because data collection was difficult owing to the retrospective nature of the study. The length of hospital stay is longer than references reported; however, the interpretation varies depending on the health insurance system of each country. In this study, we demonstrated the effectiveness of this method in AVR surgery. A single-drain tube was found to be cost-effective for suppressing POAF, thereby ameliorating various problems.
A limitation of this study is the small sample size, as it only focused on a single AVR case at a single center. Additionally, because it was a retrospective study, there is a possibility of introducing bias during the information collection process. For example, if there was no mention of POAF in the medical record, it may have been overlooked, even if it occurred. The definition of POAF also encompassed cases where drugs were used, given that the duration and other information were often not stated. Therefore, POAF incidents may have occurred without drug administration, which were not characterized as POAF. In addition, some cases did not report known factors of POAF, such as coronary artery disease, history of heart failure, or history of hyper/hypothyroidism, and data on these factors were lacking. Another limitation of the current study is the absence of power analysis for determining the sample size. Future studies should aim to overcome these limitations by conducting collaborative studies at multiple centers and including other procedures besides AVR to increase the sample size. In addition, a prospective RCT should be considered based on the present study.
Conclusion
PCD placement significantly reduced the occurrence of POAF. This procedure is simple and safe in reducing pericardial effusion, a major cause of POAF. We believe that our findings will inform standard practice in AVR surgery.
Acknowledgments
We would like to thank Dr. Kamohara for useful discussions. We are grateful to Dr. Sato for carefully proofreading the manuscript.
Footnotes
Authors’ note: Meeting/presentation:
Name: The 53rd Annual Meeting of the Japanese Society for Cardiovascular Surgery.
Location: Asahikawa Civic Culture Hall.
Date: March 23–25, 2023.
Author contributions: The conception or design of the study was provided by YK and MS, and data collection was provided by JU. Data analysis and interpretation were performed using ES. This study was written by YK. Important revisions to the article and approval of the final draft were provided by MS and KK. All the authors have read and approved the final version of the manuscript.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: This retrospective study was approved by the Ethics Committee of the Saga-Ken Medical Center, Koseikan (Approval No. 22-09-01-01).
Informed consent: Informed consent for this study was obtained using an opt-out method, and the patients were provided with the option to decline participation on the hospital website.
Trial registration: Not applicable
ORCID iDs: Yuichi Koga  https://orcid.org/0000-0003-0011-617X
https://orcid.org/0000-0003-0011-617X
Eiji Sadashima  https://orcid.org/0000-0001-6716-3259
https://orcid.org/0000-0001-6716-3259
Jun Ushigusa  https://orcid.org/0000-0001-8376-6201
https://orcid.org/0000-0001-8376-6201
Data availability statement: The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1. Lubitz SA, Yin X, Rienstra M, et al. Long-term outcomes of secondary atrial fibrillation in the community: the Framingham Heart Study. Circulation 2015; 131: 1648–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Eikelboom R, Sanjanwala R, Le ML, et al. Postoperative atrial fibrillation after cardiac surgery: a systematic review and meta-analysis. Ann Thorac Surg 2021; 111: 544–554. [DOI] [PubMed] [Google Scholar]
- 3. St-Onge S, Perrault LP, Demers P, et al. Pericardial blood as a trigger for postoperative atrial fibrillation after cardiac surgery. Ann Thorac Surg 2018; 105: 321–328. [DOI] [PubMed] [Google Scholar]
- 4. Gaudino M, Sanna T, Ballman KV, et al. Posterior left pericardiotomy for the prevention of atrial fibrillation after cardiac surgery: an adaptive, single-centre, single-blind, randomised, controlled trial. Lancet 2021; 398: 2075–2083. [DOI] [PubMed] [Google Scholar]
- 5. Ohuchi S, Okubo T, Mitsunaga Y, et al. Evaluation of a new method of pericardial drainage after cardiac surgery. Kyobu Geka 2010; 63: 992–994. [PubMed] [Google Scholar]
- 6. Dobrev D, Aguilar M, Heijman J, et al. Postoperative atrial fibrillation: mechanisms, manifestations and management. Nat Rev Cardiol 2019; 16: 417–436. [DOI] [PubMed] [Google Scholar]
- 7. Björn R, Nissinen M, Lehto J, et al. Late incidence and recurrence of new-onset atrial fibrillation after isolated surgical aortic valve replacement. J Thorac Cardiovasc Surg 2022; 164: 1833–1843.e4. [DOI] [PubMed] [Google Scholar]
- 8. Baker WL, White CM. Post-cardiothoracic surgery atrial fibrillation: a review of preventive strategies. Ann Pharmacother 2007; 41: 587–598. [DOI] [PubMed] [Google Scholar]
- 9. Philippou H, Adami A, Davidson SJ, et al. Tissue factor is rapidly elevated in plasma collected from the pericardial cavity during cardiopulmonary bypass. Thromb Haemost 2000; 84: 124–128. [PubMed] [Google Scholar]
- 10. Rong LQ, Di Franco AD, Rahouma M, et al. Postoperative pericardial effusion, pericardiotomy, and atrial fibrillation: an explanatory analysis of the PALACS trial. Am Heart J 2023; 260: 113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gozdek M, Pawliszak W, Hagner W, et al. Systematic review and meta-analysis of randomized controlled trials assessing safety and efficacy of posterior pericardial drainage in patients undergoing heart surgery. J Thorac Cardiovasc Surg 2017; 153: 865–875.e12. [DOI] [PubMed] [Google Scholar]
- 12. Sakopoulos AG, Hurwitz AS, Suda RW, et al. Efficacy of Blake drains for mediastinal and pleural drainage following cardiac operations. J Card Surg 2005; 20: 574–577. [DOI] [PubMed] [Google Scholar]
- 13. Moss E, Miller CS, Jensen H, et al. A randomized trial of early versus delayed mediastinal drain removal after cardiac surgery using Silastic and conventional tubes. Interact Cardiovasc Thorac Surg 2013; 17: 110–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ege T, Tatli E, Canbaz S, et al. The importance of intrapericardial drain selection in cardiac surgery. Chest 2004; 126: 1559–1562. [DOI] [PubMed] [Google Scholar]
- 15. Eryilmaz S, Emiroglu O, Eyileten Z, et al. Effect of posterior pericardial drainage on the incidence of pericardial effusion after ascending aortic surgery. J Thorac Cardiovasc Surg 2006; 132: 27–31. [DOI] [PubMed] [Google Scholar]