Abstract
Background:
Repeat transurethral resection of bladder tumour (reTURB) is a conventional treatment for non-muscle-invasive bladder cancer (NMIBC) to enhance prognosis. However, the necessity of reTURB in NMIBC remains controversial owing to upstaging of treatments and new evidence.
Objectives:
We performed an umbrella review to determine the need for reTURB in patients with NMIBC.
Design:
We extracted data from meta-analyses that were screened out after a systematic search of PubMed, Embase, the Web of Science and the Cochrane Database of Systematic Reviews.
Methods:
Risk of Bias in Systematic Reviews and the Grading of Recommendations, Assessment, Development and Evaluation tools were used to assess the quality of each included meta-analysis and outcomes.
Results:
Our study included seven meta-analyses. Two studies assessed the efficiency of reTURB in patients who underwent en bloc resection of bladder tumours (ERBT). Patients who underwent ERBT reported low residual tumour and upstaging rates of 5.9% and 0.3%, respectively. Conversely, patients who underwent conventional transurethral resection for bladder cancer (cTURB) had high residual tumour rates. Patients who underwent cTURB and reTURB had significantly improved 1-year recurrence-free survival (RFS) compared to those who underwent initial cTURB alone. In terms of progression-free survival (PFS), a meta-analysis reported that patients who underwent cTURB and reTURB had significantly improved PFS compared with those who underwent initial cTURB alone. In the subgroup analyses of ERBT, reTURB did not affect the RFS and PFS of patients who received ERBT. Currently, only a limited number of randomised clinical trials have evaluated reTURB, and various factors have influenced its efficacy.
Conclusion:
There was significant variation in survival outcomes among patients undergoing reTURB. The necessity and efficacy of reTURB depend on numerous factors, such as surgical approach, equipment and medication usage. Patients eligible for ERBT may constitute a group that does not require reTURB. Further clinical trials are required to validate these findings.
Registration:
This umbrella review was registered with the International Prospective Register of Systematic Reviews (CRD42023439078).
Keywords: bladder cancer, en-bloc resection of bladder tumour, repeat transurethral resection of bladder tumour, umbrella review
Introduction
Bladder cancer, ranked as the ninth most common cancer worldwide and accounted for 220,349 deaths globally in 2022. 1 Nearly 70% of the initially diagnosed bladder malignancies are categorised as non-muscle-invasive bladder cancer (NMIBC). 2 Conventional transurethral resection of bladder cancer (cTURB) is the standard treatment for NMIBC, 2 which is highly heterogeneous, with a predilection for recurrence and progression even following intravesical Bacillus Calmette-Guérin administration or chemotherapy. 3 Radical cystectomy is recommended for patients with NMIBC who show disease progression to muscle-invasive bladder cancer or frequent recurrence.2,4 Therefore, many studies have focused on the diagnosis and treatment of bladder cancer.5–7 Several therapeutic strategies are adopted to prevent and control NMIBC and decrease its recurrence and progression rates.8,9
Repeat transurethral resection of bladder tumours (reTURB) is widely used in clinical practice. ReTURB can improve the yield of the detrusor muscles in a specimen, which is the diagnostic standard for NMIBC and muscle-invasive bladder cancer.10,11 Moreover, reTURB is useful for resecting residual tumours. 12 Therefore, patients who underwent reTURB may have better survival rates. 13 However, the economic burden, perioperative complications and risk of tumour cell seeding are the disadvantages of reTURB. Several studies reported no survival benefits in patients who underwent reTURB.14–16 After pooling 68 studies, Lin et al. 17 observed that reTURB provided only short-term survival benefits and had no significant beneficial effects on long-term survival outcomes. Xu et al. 16 reported that en bloc resection of bladder tumours (ERBT) may be useful for resecting tumours with an extremely low residual tumour rate, resulting in an approximately 100% yield of the detrusor muscle. Hu et al. 15 reported the same results in an investigation of 12 studies. They observed no significant survival benefit in patients who underwent ERBT alone compared to those who underwent ERBT and reTURB. Novel techniques have been adopted in the clinical practice. Pedersen et al. 18 performed photodynamic diagnosis-guided laser destruction of bladder cancer on an outpatient basis and observed favourable survival outcomes. Based on the survival data of transurethral laser resection of bladder tumours, we concluded that laser resection could decrease the recurrence rate of NMIBC. 18 These novel techniques may eliminate the need for reTURB.
To address these concerns and obtain deeper insights into this issue, we performed an umbrella review to determine the need for reTURB in patients with NMIBC. This umbrella review was registered with the International Prospective Register of Systematic Reviews (PROSPERO).
Materials and methods
We conducted an umbrella review on reTURB according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines. 19 The study was registered in PROSPERO (registration number: CRD42023439078). Additionally, data pooling was not feasible because of the limited number of randomised controlled trials (RCTs) that addressed this issue.
Literature search
A comprehensive search was performed in the PubMed, the Embase, the Web of Science and the Cochrane Database of Systematic Reviews until June 2024 (the last update) to identify relevant systematic reviews, meta-analyses and RCTs. Following the guidelines of the Scottish Intercollegiate Guidelines Network, 20 we performed an extensive literature search on reTURB using a combination of Medical Subject Heading terms, keywords and various text word variations. The search was conducted across multiple databases using the terms (repeat resection OR restaging resection OR reTURB OR second TURBT) AND (bladder tumour). Supplemental Table 1 provides the specific search formulation. Two authors (DXL and DCF) separately screened the titles and abstracts retrieved from the databases. Subsequently, the two authors independently identified meta-analyses and RCTs that met the inclusion criteria through full-text reading. A third author (RCW) was employed to resolve discrepancies in the literature screening. A fourth author (QXY) conducted a manual search to assess the references of all selected studies.
Study selection
This umbrella review aimed to assess the efficacy of reTURB and the necessity of this secondary surgical procedure. The included systematic reviews and meta-analyses met specific criteria: included studies must be RCTs, cohort studies, case-control studies or cross-sectional studies that assessed the efficacy of reTURB. The RCTs incorporated in this analysis adhered to the following conditions: (1) comparison of reTURB with no-reTURB, (2) accurate and accessible results and (3) RCT study design with full text available. Studies excluded from this analysis were those that were non-English, animal or cell culture studies.
Data extraction
The following information from included studies was independently extracted by two reviewers (DXL and DCF): (1) first author’s name, (2) publication year, number of included studies and patients, estimated summary effect (risk ratio, odds ratio, hazard ratio with 95% confidence intervals (CI)) and heterogeneity (I 2 ) in (3) perioperative (including tumour residual rate and tumour upstage rate) and survival (including recurrence-free survival (RFS); progression-free survival (PFS); and cancer-specific survival (CSS)) results. All disagreements were resolved by a third author (RCW).
Quality assessment of methods and evidence
Under the guidance of Risk of Bias in Systematic Reviews tool, 21 the included studies were assessed in three phases and assigned ratings of low, high or unclear. In the final phase, an overall assessment was performed, considering the results of phase II as low risk only when all four domains were classified as low risk. Otherwise, it was classified as high-risk. Moreover, each outcome would be evaluated and assigned a quality grade of ‘high’, ‘moderate’, ‘low’ or ‘very low’, according to Grading of Recommendations, Assessment, Development and Evaluation (GRADE) 22 (Supplemental Table 3). These evaluations were performed independently by two reviewers (DXL and DCF). Any disagreements were resolved by a third author (RCW).
Statistical analysis
The data in our study were evaluated using Review Manager 5.4.0 and R x64 4.1.3. We used the mean difference and standardised mean difference to assess continuous outcomes with 95% CI. Meanwhile, we employed OR to assess dichotomous outcomes with a 95% CI. Statistical significance was set at p < 0.05.
Results
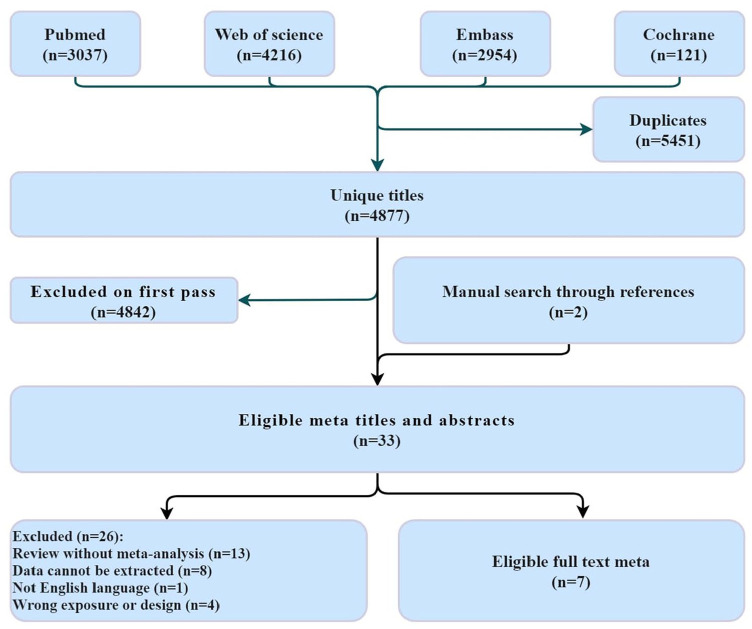
The databases provided 4877 studies after duplicates were discarded. A total of 33 eligible meta-analyses were screened after the initial screening. No eligible RCTs were identified, rendering data pooling infeasible. Finally, we included seven eligible meta-analyses (Figure 1).
Figure 1.
Workflow diagram.
The characteristics of eligible studies and risk of bias assessment
All eligible meta-analyses had to include retrospective studies because of the limited availability of RCTs.12,14–17,23,24 Two of the eligible meta-analyses assessed the efficiency of reTURB in patients who underwent ERBT.15,16 The studies conducted by Lin et al. 17 and Xu et al. 16 were the last to be searched in October 2021. While the former included patients who underwent curb or ERBT, the latter focused only on those who underwent ERBT.12,17 Therefore, we incorporated both meta-analyses in our study. The remaining meta-analyses had a last search >6 months ago. The specifics of the eligible meta-analyses are presented in Table 1. Over half of the meta-analyses were low risk.15–17,24 while the remaining three studies were categorised as high risk owing to language restrictions and bias assessment (Supplemental Table 2).
Table 1.
Summary of included meta-analyses and outcomes.
| Author (year) | Country | Last research | Included studies | Type | Comparison | No. of reTURB | No. of no-reTURB | Phase | CIS |
|---|---|---|---|---|---|---|---|---|---|
| Krajewski_W (2020) | Poland | March 2020 | 6 | RCT/NRCT | cTURB | 1515 | 1742 | T1 | / |
| Naselli_A (2016) | Italy | August 2016 | 29 | RCT/NRCT | cTURB | 3912 | T1 | Yes | |
| Vianello_A (2011) | Italy | June 2010 | 15 | RCT/NRCT | cTURB | / | / | Ta/T1 | No |
| Lin_LD (2023) | China | October 2021 | 68 | RCT/NRCT | ERBT/cTURB | / | / | Ta/T1 | Yes |
| Yanagisawa (2024) | Austria | February 2023 | 81 | RCT/NRCT | ERBT/cTURB | / | / | Ta/T1 | Yes |
| Xu_JN (2022) | China | October 2021 | 8 | RCT/NRCT | ERBT | 414 | Ta/T1 | / | |
| Hu_HL (2022) | China | July 2022 | 12 | RCT/NRCT | ERBT | / | / | Ta/T1 | Yes |
cTURB, conventional transurethral resection of bladder tumour; ERBT, en-Bloc transurethral resection of bladder tumour; NA, no data; NRCT, non-randomized controlled trial; RCT, randomized controlled trial; TURB, Transurethral resection of the bladder.
Perioperative outcomes in reTURB
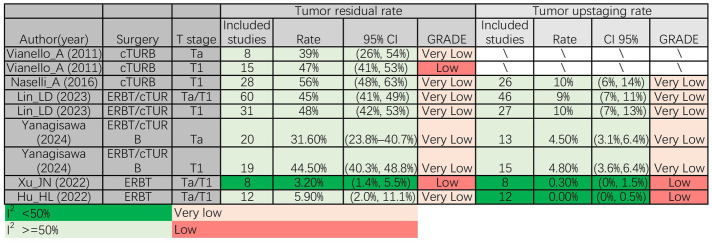
We assessed the tumour residual rate and tumour upstaging rate. Both meta-analyses15,16 that included Ta/T1 patients who received ERBT reported a low tumour residual rate, which was 3.2% and 5.9%, respectively. Conversely, in T1 patients who underwent cTURB, three meta-analyses12,17,23 revealed high residual tumour rates of 48%, 47% and 56%, respectively. Furthermore, in the subgroup analysis of Ta and Ta/T1 patients, cTURB exhibited residual tumour rates of 39% and 45%, respectively, which were higher than those observed in Ta/T1 patients who underwent ERBT. In terms of tumour upstaging rate, consistent with tumour residual rate, both meta-analyses15,16 that included Ta/T1 patients who received ERBT reported a low tumour upstaging rate, which was 0.30% and 0.0%, respectively. Similarly, two meta-analyses showed that T1 patients who accepted cTURB had a tumour residual rate of 10%, which was higher than that observed in Ta/T1 patients who underwent ERBT17,23 (Figure 2).
Figure 2.
Perioperative results. cTURB, conventional transurethral resection of bladder cancer; ERBT, en-Bloc resection of bladder tumour; reTURB, repeat transurethral resection of bladder tumours; TURB, transurethral resection of bladder tumours.
Survival outcomes of patients accepted reTURB
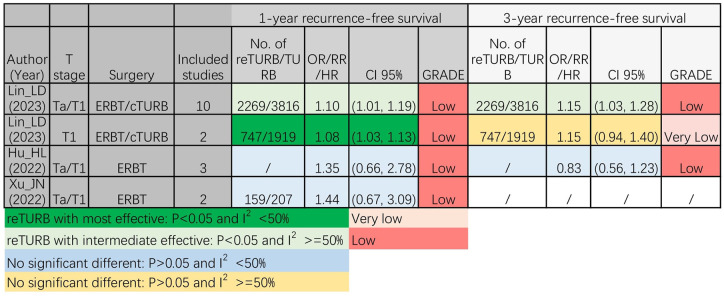
There was no significant difference in the 1-year RFS between Ta/T1 patients who received ERBT alone and those who underwent reTURB in addition to ERBT.15,16 Nevertheless, in the subgroup analyses of Ta/T1 and T1 patients who underwent cTURB, both analyses consistently demonstrated that patients who underwent reTURB were significantly associated with improved RFS compared with those who did not undergo reTURB. 17 Regarding 3-year RFS, there was no significant difference between Ta/T1 patients who received ERBT alone and those who underwent reTURB in addition to ERBT. 15 Notably, in patients who underwent cTURB, no significant difference was observed between T1 patients who underwent cTURB alone and those who underwent reTURB in the 3-year RFS. 17 In contrast, Ta/T1 patients who underwent reTURB had statistically improved 3-year RFS compared to those who underwent cTURB alone (Figure 3).
Figure 3.
The 1- and 3-year recurrence-free survival results of different meta-analyses. cTURB, conventional transurethral resection of bladder cancer; ERBT, en-Bloc resection of bladder tumour; reTURB, repeat transurethral resection of bladder tumours; TURB, transurethral resection of bladder tumours.
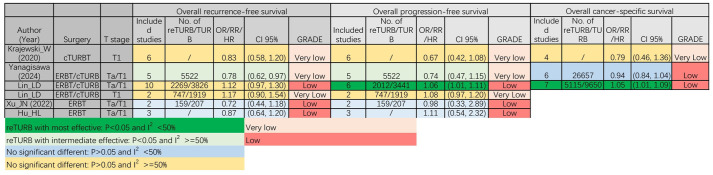
In Figure 4, five of the comparisons reported that reTURB did not have a significant impact on the overall RFS. Only one study reported the RFS benefits of reTURB, with a very low evidence level. 24 Five comparisons reported that reTURB did not have a significant impact on overall PFS. Conversely, in a subgroup analysis of Ta/T1 patients who underwent cTURB, those who underwent reTURB had significantly improved overall PFS compared with those who underwent cTURB alone. In terms of CSS, one study suggested that patients with NMIBC who underwent reTURB had a better survival outcome, while two studies did not demonstrate this result.
Figure 4.
Overall recurrence-free and progression-free survival results of different meta-analyses. cTURB, conventional transurethral resection of bladder cancer; ERBT, en-Bloc resection of bladder tumour; reTURB, repeat transurethral resection of bladder tumours; TURB, transurethral resection of bladder tumours.
Discussion
Urologists usually suggest performing reTURB in patients with high-risk NMIBC at the initial diagnosis.2,25 In this umbrella review, we observed conflicting results regarding whether reTURB can provide better perioperative and prognostic outcomes in patients with NMIBC. Of these, ERBT was associated with a reduced tumour residual rate and tumour upstaging rate compared to cTURB in patients with NMIBC. Notably, reTURB did not significantly affect the prognosis of patients with NMIBC who underwent ERBT compared to those who underwent cTURB. ERBT may be a promising therapeutic approach for NMIBC patients to avoid reTURB in patients where this technique is applicable.
Numerous factors affect the prognosis of patients with NMIBC.26,27 To improve prognosis, many treatments have been developed to improve the RFS and PFS of NMIBC. 2 Of these, patients with T1 disease have attracted the attention of urologists. T1 NMIBC is associated with a higher incidence of recurrence and progression compared with Ta NMIBC. 28 Patients need to undergo radical cystectomy if NMIBC progresses to muscle-invasive bladder cancer, which causes heavy mental and physical burdens. 29 Thus, many therapies and biomarkers have been applied in patients with T1 NMIBC to determine the recurrence and progression of NMIBC. 30 Both the European Association of Urology and the National Comprehensive Cancer Network recommend that patients with T1 undergo reTURB. Moreover, cTURB provides a high tumour residual rate and tumour upstaging rate following a low presence rate of the detrusor muscle, which could be improved by reTURB. 31 In contrast, reTURB can result in a better prognosis for patients with T1 disease. 13 Thus, Gontero et al. 25 suggested that reTURB is necessary for high-grade T1 NMIBC. However, these advantages were not observed in the patients who underwent ERBT. In survival outcomes, no significant differences in RFS and PFS were observed between patients who underwent ERBT alone and those who underwent combined ERBT and reTURB.15,16 In an early RCT, patients with T1 NMIBC who received mitomycin and initial TURB had significantly worse RFS and PFS than did those who received reTURB and mitomycin because of the high tumour residual rate and high WHO pathological grade. 32 In this RCT, the residual tumour rate in the reTURB group was 33.8%. Thus, the residual tumour rate may be a key factor in reTURB efficiency. The rate of detrusor muscle formation was approximately 70% in specimens extracted by cTURB. 13 The rate of detrusor muscle acquisition by cTURB could increase to 87.8% following reTURB, which is also lower than that of ERBT.13,33 In specimens obtained from ERBT, the presence of the detrusor muscle was observed in approximately 100% of the cases.16,34 This might be one of the reasons why ERBT decreases the residual tumour rate from approximately 30% to <6%.17,23,24 In our recent study, ERBT provided a significantly lower tumour residual rate than cTURB. 5 Furthermore, patients who intend to undergo ERBT should have a restricted number of tumours and a limited tumour size. 35 Based on the above evidence, we suggest that ERBT may be a promising approach to avoid reTURB in some patients with NMIBC.
Moreover, various factors may influence reTURB-derived benefits, such as antitumour drugs, surgical technique levels and surgical equipment.36–38 In another recent RCT, 39 the residual tumour rate in the reTURB + BCG group was 29%. No significant differences were identified between the reTURB + BCG and initial TURB + BCG groups in terms of RFS and PFS, with a median follow-up of 17 months. This result could be attributed to the limited follow-up duration and patient sample size. Notably, BCG can potentially improve RFS in patients who do not undergo reTURB. BCG is the standard treatment for high-risk NMIBC and offers significant survival benefits to patients. 40 Thus, novel treatments using biomaterials, 41 targeted therapy, 42 and radiopharmaceuticals 43 may promote reTURB in clinical practice. Meanwhile, cTURB and ERBT usually use various energies to resect tumours. 35 Of these, laser technology significantly reduces the incidence of bladder perforation and obturator nerve reflexes, providing urologists with greater precision in tumour resection. 44 This might be another reason why patients who underwent surgery using different surgical equipment had distinctive survival outcomes. Additionally, the experience of the surgeon also affects the efficiency of TURB and, therefore, determines whether to perform reTURB.24,38 Based on these results, we postulate that multiple factors, excluding ERBT, may influence the need for reTURB. It is crucial to acknowledge that numerous factors influence the surgical outcomes and efficacy of reTURBs. Therefore, future studies should strive to control for variables such as the surgeon’s experience, surgical instruments, postoperative treatment and tumour characteristics (such as tumour size, T stage and WHO grade) to minimise their impact on surgical results. Further studies are needed to determine whether ERBT patients can avoid reTURB.
This study had some limitations. All outcomes lacked moderate- or high-quality evidence, according to the GRADE assessment, primarily because of the absence of RCTs. However, this evidence was highly consistent, providing confidence in the results. However, RCTs are required to validate these results. As an umbrella review, the study’s scope for exploring additional outcomes and detailed controls was limited.
Conclusion
There is significant variation in survival outcomes among patients undergoing reTURB. The necessity and efficacy of reTURB depend on numerous factors, such as surgical approach, equipment and medication usage. Future studies should also focus on these factors. Patients eligible for ERBT may constitute a group that does not require reTURB. Further clinical trials are required to validate these findings.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359241298470 for Role of repeat transurethral resection in no-muscle-invasive bladder tumour: an umbrella review by Qing-Xin Yu, Rui-Cheng Wu, Zhou-Ting Tuo, Wei-Zhen Zhu, Jie Wang, Xing Ye, Koo Han Yoo, Wu-Ran Wei, De-Chao Feng and Deng-Xiong Li in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-2-tam-10.1177_17588359241298470 for Role of repeat transurethral resection in no-muscle-invasive bladder tumour: an umbrella review by Qing-Xin Yu, Rui-Cheng Wu, Zhou-Ting Tuo, Wei-Zhen Zhu, Jie Wang, Xing Ye, Koo Han Yoo, Wu-Ran Wei, De-Chao Feng and Deng-Xiong Li in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-3-tam-10.1177_17588359241298470 for Role of repeat transurethral resection in no-muscle-invasive bladder tumour: an umbrella review by Qing-Xin Yu, Rui-Cheng Wu, Zhou-Ting Tuo, Wei-Zhen Zhu, Jie Wang, Xing Ye, Koo Han Yoo, Wu-Ran Wei, De-Chao Feng and Deng-Xiong Li in Therapeutic Advances in Medical Oncology
Acknowledgments
We thank Figdraw (www.figdraw.com) and Chengdu Basebiotech Co., Ltd. for their assistance with drawing and data processing.
Footnotes
ORCID iDs: Qing-Xin Yu  https://orcid.org/0000-0002-4925-4568
https://orcid.org/0000-0002-4925-4568
Deng-Xiong Li  https://orcid.org/0009-0009-8071-7434
https://orcid.org/0009-0009-8071-7434
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Qing-Xin Yu, Department of Pathology, Ningbo Clinical Pathology Diagnosis Center, Ning 685 East Section of Huancheng North Road, Ningbo City, Zhejiang Province 315211, China; Department of Pathology, Ningbo Medical Centre Lihuili Hospital, Ningbo City, Zhejiang Province, 315040, China.
Rui-Cheng Wu, Department of Urology, Institute of Urology, West China Hospital, Sichuan University, Chengdu, Sichuan Province, China.
Zhou-Ting Tuo, Department of Urological Surgery, Daping Hospital, Army Medical Center of PLA, Army Medical University, Chongqing, China.
Wei-Zhen Zhu, Department of Urology, Institute of Urology, West China Hospital, Sichuan University, Chengdu, Sichuan Province, China.
Jie Wang, Department of Urology, Institute of Urology, West China Hospital, Sichuan University, Chengdu, Sichuan Province, China.
Xing Ye, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
Koo Han Yoo, Department of Urology, Kyung Hee University, Seoul, Republic of Korea.
Wu-Ran Wei, Department of Urology, Institute of Urology, West China Hospital, Sichuan University, Chengdu, Sichuan Province, China.
De-Chao Feng, Department of Urology, Institute of Urology, West China Hospital, Sichuan University, Guoxue Xiang #37, Chengdu, Sichuan Province 610041, China; Division of Surgery & Interventional Science, University College London, London, UK.
Deng-Xiong Li, Department of Urology, Institute of Urology, West China Hospital, Sichuan University, Guoxue Xiang #37, Chengdu, Sichuan Province, 610041, China.
Declaration
Ethics approval and consent to participate: This was an umbrella review and meta-analysis. Therefore, ethical review and approval were not required for this study.
Consent for publication: All authors concur with publishing the study at present version. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Author contributions: Qing-Xin Yu: Conceptualization; Data curation; Formal analysis; Methodology; Software; Visualization; Writing – original draft.
Rui-Cheng Wu: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Software; Visualization; Writing – original draft.
Zhou-Ting Tuo: Data curation; Formal analysis; Investigation; Methodology; Software.
Wei-Zhen Zhu: Formal analysis; Software.
Jie Wang: Investigation; Software; Visualization.
Xing Ye: Data curation; Investigation.
Koo Han Yoo: Supervision; Validation.
Wu-Ran Wei: Project administration; Validation.
De-Chao Feng: Data curation; Funding acquisition; Methodology; Project administration; Resources; Supervision; Validation; Writing – review & editing.
Deng-Xiong Li: Conceptualization; Data curation; Investigation; Methodology; Project administration; Resources; Software; Validation; Visualization; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This research was funded by the Chinese Scholarship Council (Grant No. 202206240086) and the Project of Ningbo Leading Medicine and Health Discipline (Project Number: 2022-F30). The funders had no role in the study design, data collection or analysis, manuscript preparation or decision to publish.
The authors declare that there is no conflict of interest.
Availability of data and materials: All data were extracted from published studies. Therefore, data can be obtained. Further enquiries can be directed to the corresponding authors.
References
- 1. Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2024; 74(3): 229–263. [DOI] [PubMed] [Google Scholar]
- 2. EAU Guidelines. Edn presented at the EAU Annual Congress Paris 2024. ISBN 978-94-92671-23-3. [Google Scholar]
- 3. Deng-Xiong L, Qing-Xin Y, De-Chao F, et al. Systemic immune-inflammation index (SII) during induction has higher predictive value than preoperative SII in non-muscle-invasive bladder cancer patients receiving intravesical bacillus Calmette-Guerin. Clin Genitourin Cancer 2023; 21(3):e145–e152. [DOI] [PubMed] [Google Scholar]
- 4. Li DX, Yu QX, Wu RC, et al. Efficiency of bladder-sparing strategies for bladder cancer: an umbrella review. Ther Adv Med Oncol 2024; 16: 17588359241249068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li DX, Yu QX, Wu RC, et al. Efficiency of transurethral en-bloc resection vs. conventional transurethral resection for non-muscle-invasive bladder cancer: an umbrella review. Cancer Med 2024; 13: e7323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bicchetti M, Simone G, Giannarini G, et al. A novel pathway to detect muscle-invasive bladder cancer based on integrated clinical features and VI-RADS score on MRI: results of a prospective multicenter study. Radiol Med 2022; 127: 881–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xiang W, Peng Y, Zeng H, et al. Targeting treatment of bladder cancer using PTK7 aptamer-gemcitabine conjugate. Biomater Res 2022; 26: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Feng D, Li D, Wu R, et al. Scientific advancements in drug development and trials for urothelial carcinoma: insights from the 2023 ASCO-GU cancers symposium. Aging Dis 2023; 14(6): 1953–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lin L, Pei Y, Li Z, et al. Progress and challenges of mRNA vaccines. Interdiscip Med 2023; 15(5): e1894. [DOI] [PubMed] [Google Scholar]
- 10. Netto GJ, Amin MB, Berney DM, et al. The 2022 World Health Organization classification of tumors of the urinary system and male Genital organs-part B: prostate and urinary tract tumors. European Urology 2022; 82: 469–482. [DOI] [PubMed] [Google Scholar]
- 11. Cumberbatch MGK, Foerster B, Catto JWF, et al. Repeat transurethral resection in non-muscle-invasive bladder cancer: a systematic review. Eur Urol 2018; 73: 925–933. [DOI] [PubMed] [Google Scholar]
- 12. Vianello A, Costantini E, Del Zingaro M, et al. Repeated white light transurethral resection of the bladder in nonmuscle-invasive urothelial bladder cancers: Systematic review and meta-analysis. J Endourol 2011; 25: 1703–1712. [DOI] [PubMed] [Google Scholar]
- 13. Gordon PC, Thomas F, Noon AP, et al. Long-term outcomes from re-resection for high-risk non-muscle-invasive bladder cancer: a potential to rationalize use. Eur Urol Focus 2019; 5: 650–657. [DOI] [PubMed] [Google Scholar]
- 14. Krajewski W, Nowak Ł, Poletajew S, et al. The impact of restaging transurethral resection of bladder tumor on survival parameters in T1 nonmuscle-invasive bladder cancer: systematic review and meta-analysis. J Endourol 2020; 34: 795–804. [DOI] [PubMed] [Google Scholar]
- 15. Hu H, Zhou M, Yang B, et al. A systematic review on the role of repeat transurethral resection after initial en bloc resection for non-muscle invasive bladder cancer. J Clin Med 2022; 11: 5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xu J, Xu Z, Yin H, et al. Can a reresection be avoided after initial en bloc resection for high-risk nonmuscle invasive bladder cancer? A systematic review and meta-analysis. Front Surg 2022; 9: 849929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lin L, Guo X, Ma Y, et al. Does repeat transurethral resection of bladder tumor influence the diagnosis and prognosis of T1 bladder cancer? A systematic review and meta-analysis. Eur J Surg Oncol 2023; 49: 29–38. [DOI] [PubMed] [Google Scholar]
- 18. Pedersen GL, Erikson MS, Mogensen K, et al. Outpatient photodynamic diagnosis-guided laser destruction of bladder tumors is as good as conventional inpatient photodynamic diagnosis-guided transurethral tumor resection in patients with recurrent intermediate-risk low-grade Ta bladder tumors. A prospective randomized noninferiority clinical trial. Eur Urol 2023; 83: 125–130. [DOI] [PubMed] [Google Scholar]
- 19. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLOS Med 2009; 6: e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Scottish Intercollegiate Guidelines Network. Search Filters, https://www.sign.ac.uk/what-we-do/methodology/search-filters/
- 21. Whiting P, Savović J, Higgins JPT, et al. ROBIS: a new tool to assess risk of bias in systematic reviews was developed. J Clin Epidemiol 2016; 69: 225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011; 64: 383–394. [DOI] [PubMed] [Google Scholar]
- 23. Naselli A, Hurle R, Paparella S, et al. Role of restaging transurethral resection for T1 non-muscle invasive bladder cancer: a systematic review and meta-analysis. Eur Urol Focus 2018; 4: 558–567. [DOI] [PubMed] [Google Scholar]
- 24. Yanagisawa T, Kawada T, von Deimling M, et al. Repeat transurethral resection for non–muscle-invasive bladder cancer: an updated systematic review and meta-analysis in the contemporary era. Eur Urol Focus 2024; 10: 41–56. [DOI] [PubMed] [Google Scholar]
- 25. Gontero P, Livoti S, Soria F. A restaging transurethral resection of the bladder is always necessary for high-grade T1 non-muscle-invasive bladder cancer: con. Eur Urol Focus 2023; 9(4): 559–560. [DOI] [PubMed] [Google Scholar]
- 26. Hashemi M, Arani HZ, Orouei S, et al. Crosstalk of miRNAs with signaling networks in bladder cancer progression: therapeutic, diagnostic and prognostic functions. Pharmacol Res 2022; 185: 106475. [DOI] [PubMed] [Google Scholar]
- 27. Li DX, Wu RC, Wang J, et al. Prognostic potential of m7G-associated lncRNA signature in predicting bladder cancer response to immunotherapy and chemotherapy. Oncologie 2023; 25: 729–742. [Google Scholar]
- 28. Li DX, Wang XM, Feng DC, et al. Lymphocyte-to-monocyte ratio (LMR) during induction is a better predictor than preoperative LMR in patients receiving intravesical Bacillus Calmette -Guerin for non-muscle-invasive bladder cancer. Front Oncol 2022; 12: 937638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Feng D, Wang Z, Yang Y, et al. Incidence and risk factors of parastomal hernia after radical cystectomy and ileal conduit diversion: a systematic review and meta-analysis. Transl Cancer Res 2021; 10: 1389–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li D, Wu R, Wang J, et al. A prognostic index derived from LASSO-selected preoperative inflammation and nutritional markers for non-muscle-invasive bladder cancer. Clin Genitourin Cancer 2024; 22(3): 102061. [DOI] [PubMed] [Google Scholar]
- 31. Di Y, Li H, He C, et al. En-bloc transurethral resection vs. conventional transurethral resection for primary non-muscle invasive bladder cancer: a meta-analysis. Actas Urol Esp 2023; 47: 309–316. [DOI] [PubMed] [Google Scholar]
- 32. Divrik RT, Yildirim U, Zorlu F, et al. The effect of repeat transurethral resection on recurrence and progression rates in patients with T1 tumors of the bladder who received intravesical mitomycin: A prospective, randomized clinical trial. J Urol 2006; 175: 1641–1644. [DOI] [PubMed] [Google Scholar]
- 33. Yanagisawa T, Mori K, Motlagh RS, et al. En bloc resection for bladder tumors: an updated systematic review and meta-analysis of its differential effect on safety, recurrence and histopathology. J Urol 2022; 207: 754–768. [DOI] [PubMed] [Google Scholar]
- 34. Xu S, Cao P, Wang K, et al. Clinical outcomes of reresection in patients with high-risk nonmuscle-invasive bladder cancer treated with en bloc transurethral resection: a retrospective study with a 1-year follow-up. J Endourol 2021; 35: 1801–1807. [DOI] [PubMed] [Google Scholar]
- 35. Wang CW, Lee PJ, Wu CW, et al. Comparison of pathological outcome and recurrence rate between en bloc transurethral resection of bladder tumor and conventional transurethral resection: a meta-analysis. Cancers 2023; 15: 2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Francolini G, Garlatti P, Di Cataldo V, et al. Pattern of recurrence after stereotactic body radiotherapy for para-aortic oligo-recurrent prostate cancer, a multicentric analysis. Radiol Med 2023; 128: 1423–1428. [DOI] [PubMed] [Google Scholar]
- 37. Wang R, Huang Z, Xiao Y, et al. Photothermal therapy of copper incorporated nanomaterials for biomedicine. Biomater Res 2023; 27: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fernandes CN, Vale L, Sousa JV, et al. Surgeon experience in second-look transurethral resection of bladder cancer—a prospective study. Actas Urol Esp 2024; 48(6): 448–453. [DOI] [PubMed] [Google Scholar]
- 39. Soria F, Rosazza M, Livoti S, et al. Repeat transurethral resection (TUR) + Bacillus Calmette–Guérin (BCG) versus upfront induction BCG after TUR in high-risk non-muscle-invasive bladder cancer: feasibility phase of a randomized controlled study. Eur Urol Focus 2023: S2405-4569(23)00236-5. [DOI] [PubMed] [Google Scholar]
- 40. Li DX, Wang XM, Tang Y, et al. Prognostic value of preoperative neutrophil-to-lymphocyte ratio in histological variants of non-muscle-invasive bladder cancer. Investig Clin Urol 2021; 62: 641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Xiao M, Tang Q, Zeng S, et al. Emerging biomaterials for tumor immunotherapy. Biomater Res 2023; 27: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nardone V, Romeo C, D’Ippolito E, et al. The role of brain radiotherapy for EGFR- and ALK-positive non-small-cell lung cancer with brain metastases: a review. Radiol Med 2023; 128: 316–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liu Y, Ren YN, Cui Y, et al. Inspired by novel radiopharmaceuticals: rush hour of nuclear medicine. Chin J Cancer Res 2023; 35: 470–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gallioli A, Diana P, Fontana M, et al. En Bloc versus conventional transurethral resection of bladder tumors: a single-center prospective randomized noninferiority trial. Eur Urol Oncol 2022; 5: 440–448. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359241298470 for Role of repeat transurethral resection in no-muscle-invasive bladder tumour: an umbrella review by Qing-Xin Yu, Rui-Cheng Wu, Zhou-Ting Tuo, Wei-Zhen Zhu, Jie Wang, Xing Ye, Koo Han Yoo, Wu-Ran Wei, De-Chao Feng and Deng-Xiong Li in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-2-tam-10.1177_17588359241298470 for Role of repeat transurethral resection in no-muscle-invasive bladder tumour: an umbrella review by Qing-Xin Yu, Rui-Cheng Wu, Zhou-Ting Tuo, Wei-Zhen Zhu, Jie Wang, Xing Ye, Koo Han Yoo, Wu-Ran Wei, De-Chao Feng and Deng-Xiong Li in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-3-tam-10.1177_17588359241298470 for Role of repeat transurethral resection in no-muscle-invasive bladder tumour: an umbrella review by Qing-Xin Yu, Rui-Cheng Wu, Zhou-Ting Tuo, Wei-Zhen Zhu, Jie Wang, Xing Ye, Koo Han Yoo, Wu-Ran Wei, De-Chao Feng and Deng-Xiong Li in Therapeutic Advances in Medical Oncology