Abstract
Background:
Female athletes lag behind their male counterparts in recovery from anterior cruciate ligament (ACL) injury. Quadriceps muscle size and strength are crucial factors for regaining function after ACL injury, but little is known about how these metrics vary due to biological sex.
Hypothesis:
Female patients have reduced vastus lateralis fiber cross-sectional area (CSA) and lower quadriceps strength after ACL injury than male patients.
Study Design:
Cross-sectional study.
Level of Evidence:
Level 4.
Methods:
A total of 60 participants with recent ACL tear were evaluated for vastus lateralis muscle fiber CSA, isometric quadriceps peak torque, and quadriceps rate of torque development. Linear mixed models were fit to determine differences across sex and limb for each variable of interest.
Results:
The female group averaged almost 20% atrophy between limbs (P < 0.01), while the male group averaged just under 4% (P = 0.05). Strength deficits between limbs were comparable between female and male groups.
Conclusion:
Immediately after ACL injury, female patients have greater between-limb differences in muscle fiber CSA but between-limb strength deficits comparable with those of male patients.
Clinical Relevance:
These results indicate that the underpinnings of strength loss differ based on biological sex, and thus individual patients could benefit from a sex-specific treatment approach to ACL injury.
Keywords: ACL, fiber cross-sectional area, quadriceps strength, rate of torque development, sex-based differences
Overwhelming evidence across all sports and levels of competition indicates that female athletes are 1.7 times more likely to experience an anterior cruciate ligament (ACL) injury. 27 After reconstructive surgery, female athletes also lag in recovery and are less likely to return to previous levels of sport or competition than their male counterparts.1 -3,5,35,36 Despite greater frequency of injury and overall poorer return-to-sport outcomes after ACL reconstruction (ACLR), the physiological differences between female and male athletes after ACL injury contributing to these disparities are still relatively unknown. A critical need remains to assess these differences to make informed treatment decisions that will best serve each group.
Reductions in quadriceps muscle volume and cross-sectional area (CSA) have been associated with decreased quadriceps strength after ACLR.6,22,33 Decreased quadriceps strength and lower self-reported function after ACLR have been linked to presurgical quadriceps weakness, 12 highlighting the important need to address impairments early after injury. Presurgical rehabilitation [“prehab”] is often recommended to target quadriceps impairments before reconstruction, with varying reports of success.7,16,34 However, the effect of biological sex on quadriceps strength early after ACL injury is unknown, and thus prehab treatments lack informed sex-specific programming. Further, while quadriceps atrophy and strength post ACLR have been well characterized,15,20,25,38,39 the early effects of the initial injury on muscle fiber CSA have not been reported.
Work in other areas supports the need to evaluate sex-specific changes in muscle composition. Skeletal muscle composition varies between female and male bodies, which may lead to differential responses to injury between sexes. 31 Preclinical work in rodents has implied that, when exposed to hind limb unloading, female subjects experience greater muscle loss.31,40,41 Historical reports of quadriceps fiber atrophy in ACL-injured subjects have not reported sex-based differences, 29 limiting the development of individualized and sex-specific treatment guidelines.
The purpose of the current study was to evaluate differences in preoperative vastus lateralis muscle fiber atrophy of the ACL-deficient limb between female and male patients. We also sought to define whether there are sex-specific differences in quadriceps muscle strength. The preoperative timepoint was selected to address the gap in knowledge related to the onset of sex-specific differences after ACL injury. Defining the rapidity of sex-based differences after ACL injury may provide crucial insights needed to develop a more precise and effective rehabilitation program that considers biological sex. We hypothesized that female participants would display greater between-limb differences in muscle fiber CSA and reduced quadriceps strength of the ACL-deficient limb after the initial ACL injury when compared with male participants.
Methods
Study Participants
A total of 60 participants (demographics listed in Table 1) with a current complete ACL tear were enrolled between 2017 and 2021. This study was a cross-sectional design, level of evidence 4, including participants from 2 separate ongoing studies, 1 of which is a clinical trial (NCT03364647). The timepoint for data collection was consistent for all participants and occurred before reconstruction surgery and before randomization into a treatment program for participants enrolled in the clinical trial. Participant inclusion criteria included age between 15 and 40 years and a minimum score of 5 out of 10 on the Tegner Activity Scale. Tegner activity level was representative of participants’ preinjury activity level. Participants were excluded from the study if they had a previous ACL injury or ACLR or if they experienced a complete knee dislocation during the initial knee injury. All participants had ACL tears (and concomitant injuries) diagnosed by an orthopaedic surgeon and confirmed with magnetic resonance imaging (MRI). Time of injury was determined by self-report of injury date by the participant. Muscle biopsies were collected after muscle strength testing either on the same day or within several days of strength testing (average 3 ± 2 days). Participants were weightbearing with full range of motion of the knee at the time of testing. Participants were not currently undergoing any rehabilitation at the time of testing. The study was approved by the university Institutional Review Board and all participants completed informed consent or parental consent and participant assent before completing any study-related activities.
Table 1.
Participant demographics a
| Male (n = 30) | Female (n = 30) | Overall (n = 60) | ||
|---|---|---|---|---|
| Age, years | 20.20 (4.99) | 20.67 (6.33) | 20.43 (5.65) | P = 0.75 |
| Mass, kg | 84.61 (8.31) | 64.41 (9.94) | 74.5 (17.8) | P < 0.01 |
| Height, m | 1.79 (0.07) | 1.63 (0.08) | 1.71 (0.11) | P < 0.01 |
| Days since injury | 25 (19) | 29 (36) | 27 (28) | P = 0.56 |
| Meniscal tear | 27 | 24 | 51 |
Data presented as mean (SD).
Muscle Fiber CSA
Quadriceps Muscle Biopsies
Muscle biopsies from the ACL-deficient and noninjured limbs were collected during the same preoperative visit for each subject. Subjects were weightbearing at the time of data collection in the current study and were ambulating without an assistive device. Percutaneous muscle biopsies from the vastus lateralis were performed using a Bergström 5 mm muscle biopsy needle with suction. 32 Biopsy samples were taken from a standardized location; the lateral portion of the vastus lateralis, with the biopsy site at between 150 mm and 250 mm from the midpatella. Approximately 50 mg was mounted in tragacanth gum on cork and flash frozen in liquid-nitrogen-cooled 2-methylbutane. Samples were stored at -80°C until processing.
Muscle Biopsy Processing and Immunohistochemical Analysis
Cross-sections (7 µm) were cut in a cryostat and allowed to air dry for 1 hour. For fiber CSA, methods were adapted from our previous publications.4,13,14,28 Briefly, unfixed slides were incubated overnight at room temperature with antibodies against laminin (Sigma, catalog no. L9393). The next day, slides were incubated with a species-specific secondary antibody, goat anti-rabbit AF350 from ThermoFisher (catalog no. A11046). Slides were postfixed in methanol before mounting with fluorescent mounting media (Vectashield, Vector Labs, catalog no. H-1000).
Whole cross-sectional images of the muscle biopsies were captured at ×100 total magnification using the tiles and stitching functions on an AxioImager M2 upright microscope (Zeiss Zen 3.1). We assessed CSA (µm2) of each fiber directly and pooled individual fibers to generate a mean CSA for each biopsy using MyoVision automated software. 37 Analysis of images was performed in a manner blinded to sex and injury status.
Muscle Strength
Quadriceps strength testing was performed within 10 weeks of the initial ACL injury and before any preoperative physical therapy. Participants completed 4 trials of a 5-second maximal voluntary isometric knee extension (Biodex System 4 isokinetic dynamometer, Biodex Medical Systems, Inc). Participants were allowed a single practice trial at submaximal effort to familiarize with the task before the maximum effort trials. For all trials, the knee was placed and held at 90° of flexion and the hip at 85° of flexion. The knee joint center was aligned with the axis of rotation of the dynamometer arm and a dense foam pad at the distal end of the arm was secured approximately 3 cm above the malleoli. Chest straps, a waist strap, and a thigh strap were used to secure the participant in the dynamometer to isolate movement of just the quadriceps muscles during the isometric contraction. Participants were instructed to kick as hard and as fast as possible with each kick. Verbal encouragement from the study researchers was allowed and visual feedback of the torque-time curved was provided to promote maximum effort from participants. Peak torque and rate of torque development (RTD) were determined and averaged across the 4 maximal effort trials. Peak torque was defined as the maximum torque value for each trial. RTD was defined as the average slope of the torque-time curve calculated between 20% and 80% of the time from the start of the trial to peak torque. 19 Testing was completed on both limbs.
Statistical Analysis
The primary purposes of the study were to (1) determine muscle fiber CSA changes between the ACL-deficient limb (ACL-D) and noninjured limb (NON) for female and male participants, and (2) determine whether postinjury differences in quadriceps strength between limbs exist between female and male participants. Quantitative demographic variables were compared across groups with independent 2-sample t tests. Before analysis, strength variables were normalized to body weight. All available mean peak torque and RTD data were used in the analysis, and sample sizes are included in the results. To understand fiber CSA changes of the ACL-deficient limb, the noninjured limb was used as a biological control. Data presented in figures and text are based on raw values (summarized using means ± SD), while statistical significance was determined as follows. For each outcome of interest (CSA, peak torque, and RTD), a linear mixed model was fit to examine differences across sex and between limbs, including time since injury (in days) as a covariate for adjustment and using Satterthwaite’s approximation for degrees of freedom. If the interaction for sex and injury was not significant, it was removed and the model containing only main effects was used for analysis. Post hoc tests were performed as appropriate based on the primary purposes of the study. All analyses were performed in R Version 4.1.2 (R Core Team).
Results
A total of 49 participants completed muscle strength testing and muscle biopsies. An additional 11 participants completed only the strength testing (n = 60). Muscle biopsy data for these additional 11 participants were collected but were not available at the time of analysis. There were no significant differences in average age, Tegner activity level, or time since injury between female and male participants. Based on initial imaging, 25 male and 22 female participants had either a concomitant medial, lateral, or medial and lateral meniscus tear; 2 male and 3 female participant had additional medial collateral ligament and/or lateral collateral ligament injury in combination with meniscal injury. A total of 1 male and 4 female participants had no concomitant injuries identified. Concomitant injuries based on imaging were unknown for 3 participants.
Muscle Fiber CSA
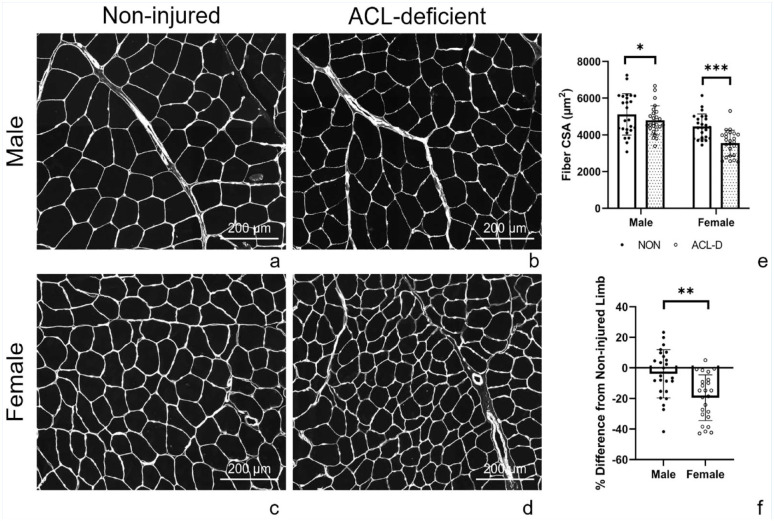
Representative images of muscle fiber CSA are shown in Figure 1a-d. After accounting for time since injury, an injury by sex interaction was identified (P = 0.02), with female participants exhibiting greater between-limb differences in fiber CSA than their male counterparts after ACL injury (Figure 1f). Full model details are presented in Appendix Tables A1 and A2, available in the online version of this article. Model-adjusted group level means are available in Appendix Table A3 (online). Muscle fiber CSA was significantly smaller for the ACL-deficient limb (3555 ± 696 µm2) than for the noninjured limb (4458 ± 678 µm2) for female participants (P < 0.01; Figure 1e). Muscle fiber CSA was significantly smaller for the ACL-deficient limb (4793 ± 791 µm2) than for the noninjured limb (5114 ± 1125 µm2) for male participants (P = 0.05; Figure 1e). Percent difference in muscle CSA between the ACL-deficient and noninjured limbs for the female group was just under 20% (19.5 ± 14.9%) compared with just under 4% (3.9 ± 15.7%) for the male group (Figure 1f). All but 1 of the female participants had lower fiber CSA of the ACL-deficient limb (Figure 2).
Figure 1.
Representative immunohistochemical images denoting laminin staining in (a) male noninjured, (b) male ACL-deficient, (c) female noninjured, and (d) female ACL-deficient limbs. (e) Quantification of mean quadriceps fiber CSA in female and male participants after ACL-injury. (f) Percent difference in fiber CSA between ACL-deficient and noninjured limbs in female and male participants. Negative percent difference indicates larger CSA of the noninjured limb. *P = 0.05, ***P < 0.01 from post hoc tests, **P = 0.02 from interaction effect of sex and injury. N = 25 male, 24 female participants. ACL, anterior cruciate ligament; CSA, cross-sectional area; NON, noninjured limb.
Figure 2.
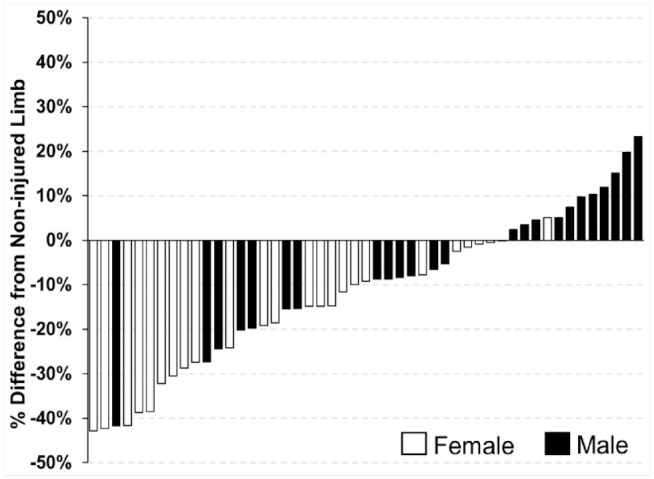
Participant-specific between-limb difference in muscle fiber CSA of the ACL-deficient limb as compared with the noninjured limb. ACL, anterior cruciate ligament; CSA, cross-sectional area.
Muscle Strength
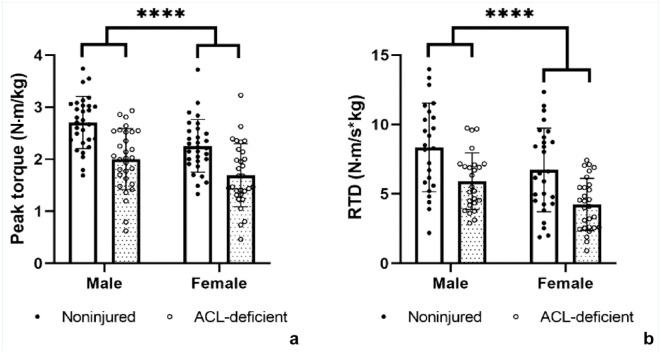
The sex by injury interaction effects were nonsignificant for both peak torque (P = 0.29) and RTD (P = 0.80), and so were removed from further analysis. Significant main effects for sex (P < 0.01, peak torque and RTD) and injury (P < 0.01, peak torque and RTD) were identified. Full model details for peak torque and RTD are presented in Appendix Tables A4, A5, A7, and A8 (online). Model-adjusted group level means are available in Appendix Tables A6 (peak torque) and A9 (RDT). Differences in peak torque between-limbs were similar for females and males, with between-limb differences of 26% and 25%, respectively (Figure 1a). Overall peak torque was lower in female (ACL-D, 1.69 ± 0.60 N.m/kg; NON, 2.26 ± 0.51 N.m/kg) than male (ACL-D, 2.00 ± 0.59 N.m/kg; NON, 2.70 ± 0.50 N.m/kg) participants. Differences in RTD between limbs were also similar for female and male participants, with between-limb differences of 23% and 26%, respectively (Figure 1b). Overall RTD was lower in female (ACL-D, 4.25 ± 1.88 N.m/kg/s; NON, 6.73 ± 3.01 N.m/kg/s) than in male (ACL-D, 5.91 ± 2.04 N.m/kg/s; NON, 8.53 ± 3.19 N.m/kg/s) participants.
Discussion
Females have poorer outcomes after an ACL injury and ACLR, and our results offer insight into the etiology of sex-specific differences in recovery trajectories. Our findings demonstrate exacerbated deficits in fiber size in female participants compared with their male counterparts after ACL injury. However, quadriceps strength asymmetries were similar between groups. Together, these results indicate that cellular and functional differences between female and male bodies develop rapidly after injury and precede differences known to occur after ACLR and rehabilitation.
After injury, female participants had greater between-limb differences in muscle fiber CSA than their male counterparts. Of the female cohort, all but 1 participant had smaller fiber CSA of the ACL-deficient limb, whereas less than half of the male participants had smaller fiber CSA of the ACL-deficient limb (Figure 3). Overall, female patients showed a nearly 20% deficit in muscle fiber CSA for the ACL-deficient limb compared with the contralateral limb, while male patients had less than a 5% deficit. The 20% reduction in fiber CSA in the ACL-injured limb compared with the noninjured limb in females is the same degree of atrophy reported in adults after 2 weeks of total bed rest. 4 Although the mechanisms behind muscle atrophy after ACL injury are multifaceted, as summarized by Lepley et al, 23 our data highlight the severity of muscle fiber size loss between limbs in females.
Figure 3.
A main effect of injury was identified for (a) peak torque and (b) RTD. Similar between-limb differences were present in female and male participants. N = 30 males, 30 females (peak torque); N = 25 males, 28 females (RTD). ****P < 0.01, P value is representative of the main effect of injury. ACL, anterior cruciate ligament; RTD, rate of torque development.
Our data suggest that the etiology of preoperative quadriceps weakness may differ between sexes; female muscle weakness may be linked more tightly to quadriceps atrophy, whereas other mechanisms appear to potentiate weakness in males before surgical reconstruction. While normalized strength was lower for female participants, between-limb strength deficits were similar between both groups despite the large differences in fiber atrophy. At the cellular level, cursory reports in rodent hind limb unloading models show female rodents may be more susceptible to disuse atrophy and have fewer muscle stem cells (satellite cells) to support recovery after disuse.30,31,40,41 To the best of our knowledge, there has been only one preclinical ACL injury model to explore sex-specific differences in quadriceps fiber size. 9 Results from this study show female rats did not undergo quadriceps atrophy, while male rats exhibited sizeable deficits in quadriceps fiber size at several time points after ACL injury. 9 Given the more pronounced deficits we report in female participants after ACL injury, care should be taken in the interpretation and use of preclinical models to ensure that they recapitulate human sex-specific muscle pathophysiology.
Few studies have sought to elucidate preoperative differences in quadriceps strength and muscle size between female and male patients with ACL injury. Limited earlier work has shown that there are no preoperative differences in isokinetic strength between female and male patients. 10 However, the goal of this previous study was to evaluate pre- and postoperative strength deficits in a military population, which resulted in an overall male-dominated participant pool. With comparable group sizes, our results also showed male and female groups had comparable limb symmetry indices (LSI) for peak torque and RTD. Between-limb deficits in quadriceps strength before reconstruction surgery are predictive of poorer outcomes after surgery,12,24 and are associated with lower LSI and lower return-to-sport rates up to 9 months postsurgery.10,17 Whereas female and male participants may have had similar relative preoperative quadriceps strength losses, differences in the degree of fiber atrophy between groups suggest sex-specific rehabilitation may be required to adequately address these preoperative strength deficits.
In addition to peak torque, RTD can be a predictor of post-ACLR function, specifically gait mechanics. 19 The rate at which torque can be developed, rather than the maximum force produced, has been suggested to be related more strongly to function than quadriceps strength.8,26 Previous work has shown that deficits in RTD are present at 6 months after ACLR for both male and female participants, 19 and specifically, that female participants have reduced RTD for both the ACL-deficient and contralateral limb compared with their male counterparts up to 1-year post reconstruction. 21 Our work indicates that these differences in RTD are also present near the initial ACL injury, perhaps placing females at an early disadvantage.
Limitations were noted in the study design and must be considered with the results. Although all measures were recorded within 6 months of the initial injury, the exact timing of muscle atrophy and strength loss within that time period cannot be determined with our current study design. However, statistical analyses considered time since injury as a covariate and measurements for all but 2 participants were recorded within 10 weeks of the initial injury, further underscoring the speed with which muscle atrophy and strength loss occur. In addition, muscle biopsies are limited in scope and were taken of just the vastus lateralis, limiting assessment of fiber CSA to only 1 of the 4 quadriceps muscles. Noninvasive methods, such as MRI, provide an alternative to measuring quadriceps CSA for the more inferior muscles of the quadriceps and could be useful to expand upon the current findings.
Conclusion
Our results indicate that marked sex-specific differences occur in response to an ACL injury before surgical reconstruction. Female patients have poorer outcomes (e.g., delayed return to sport, lower self-reported function, and decreased psychological readiness) after ACL tear and ACLR compared with male patients,5,11,18,35,36 but treatment decisions, including presurgical, surgical, and rehabilitation, often remain similar without consideration of biological sex. In this study, female participants displayed greater muscle fiber atrophy of the vastus lateralis between the ACL-deficient and noninjured limbs, while experiencing similar strength deficits as male participants. These results suggest that the drivers of strength loss following an initial ACL injury might differ between females and males, necessitating targeted sex-specific treatment and rehabilitation decisions.
Supplemental Material
Supplemental material, sj-docx-1-sph-10.1177_19417381241230612 for Sex Differences in Quadriceps Atrophy After Anterior Cruciate Ligament Tear by Meredith K. Owen, Kelsey R. Casadonte, Nicholas T. Thomas, Christine M. Latham, Camille R. Brightwell, Katherine L. Thompson, Gregory S. Hawk, Cale A. Jacobs, Darren L. Johnson, Christopher S. Fry and Brian Noehren in Sports Health
Acknowledgments
The project described was supported by the National Institutes of Arthritis and Musculoskeletal and Skin Disease (NIAMS) through grants R01AR072061 and R01AR071398. D.L. Johnson has received hospitality payments from Smith & Nephew.
Footnotes
The authors report no potential conflicts of interest in the development and publication of this article.
Contributor Information
Meredith K. Owen, Department of Physical Therapy, University of Kentucky, Lexington, Kentucky.
Kelsey R. Casadonte, Department of Physical Therapy, University of Kentucky, Lexington, Kentucky.
Nicholas T. Thomas, Department of Athletic Training and Clinical Nutrition, University of Kentucky, Lexington, Kentucky.
Christine M. Latham, Department of Athletic Training and Clinical Nutrition, University of Kentucky, Lexington, Kentucky.
Camille R. Brightwell, Department of Athletic Training and Clinical Nutrition, University of Kentucky, Lexington, Kentucky.
Katherine L. Thompson, Dr. Bing Zhang Department of Statistics, University of Kentucky, Lexington, Kentucky.
Gregory S. Hawk, Dr. Bing Zhang Department of Statistics, University of Kentucky, Lexington, Kentucky
Cale A. Jacobs, Department of Orthopaedic Surgery and Sports Medicine, University of Kentucky, Lexington, Kentucky.
Darren L. Johnson, Department of Orthopaedic Surgery and Sports Medicine, University of Kentucky, Lexington, Kentucky.
Christopher S. Fry, Department of Athletic Training and Clinical Nutrition, University of Kentucky, Lexington, Kentucky.
Brian Noehren, Department of Physical Therapy, University of Kentucky, Lexington, Kentucky, and Department of Orthopaedic Surgery and Sports Medicine, University of Kentucky, Lexington, Kentucky.
References
- 1. Agel J, Arendt EA, Bershadsky B. Anterior cruciate ligament injury in national collegiate athletic association basketball and soccer: a 13-year review. Am J Sports Med. 2005;33(4):524-530. [DOI] [PubMed] [Google Scholar]
- 2. Agel J, Rockwood T, Klossner D. Collegiate ACL injury rates across 15 sports: National Collegiate Athletic Association injury surveillance system data update (2004-2005 through 2012-2013). Clin J Sport Med. 2016;26(6):518-523. [DOI] [PubMed] [Google Scholar]
- 3. Ardern CL, Taylor NF, Feller JA, Webster KE. Fifty-five per cent return to competitive sport following anterior cruciate ligament reconstruction surgery: an updated systematic review and meta-analysis including aspects of physical functioning and contextual factors. Br J Sports Med. 2014;48(21):1543-1552. [DOI] [PubMed] [Google Scholar]
- 4. Arentson-Lantz EJ, English KL, Paddon-Jones D, Fry CS. Fourteen days of bed rest induces a decline in satellite cell content and robust atrophy of skeletal muscle fibers in middle-aged adults. J Appl Physiol (1985). 2016;120(8):965-975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arundale AJH, Capin JJ, Zarzycki R, Smith A, Snyder-Mackler L. Functional and patient-reported outcomes improve over the course of rehabilitation: a secondary analysis of the ACL-SPORTS trial. Sports Health. 2018;10(5):441-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Birchmeier T, Lisee C, Kane K, Brazier B, Triplett A, Kuenze C. Quadriceps muscle size following ACL injury and reconstruction: a systematic review. J Orthop Res. 2019:38(3):598-608. [DOI] [PubMed] [Google Scholar]
- 7. Carter HM, Littlewood C, Webster KE, Smith BE. The effectiveness of preoperative rehabilitation programmes on postoperative outcomes following anterior cruciate ligament (ACL) reconstruction: a systematic review. BMC Musculoskelet Disord. 2020;21(1):647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cossich V, Maffiuletti NA., Early vs. late rate of torque development: relation with maximal strength and influencing factors. J Electromyogr Kinesiol. 2020;55:102486. [DOI] [PubMed] [Google Scholar]
- 9. Davi SM, Ahn A, White MS, et al. Long-lasting impairments in quadriceps mitochondrial health, muscle size, and phenotypic composition are present after non-invasive anterior cruciate ligament injury. Front Physiol. 2022;13:805213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. de Jong SN, van Caspel DR, van Haeff MJ, Saris DB. Functional assessment and muscle strength before and after reconstruction of chronic anterior cruciate ligament lesions. Arthroscopy. 2007;23(1):21-28, 28 e21-23. [DOI] [PubMed] [Google Scholar]
- 11. Devana SK, Solorzano C, Nwachukwu B, Jones KJ. Disparities in ACL reconstruction: the influence of gender and race on incidence, treatment, and outcomes. Curr Rev Musculoskelet Med. 2022;15(1):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eitzen I, Holm I, Risberg MA. Preoperative quadriceps strength is a significant predictor of knee function two years after anterior cruciate ligament reconstruction. Br J Sports Med. 2009;43(5):371-376. [DOI] [PubMed] [Google Scholar]
- 13. Fry CS, Noehren B, Mula J, et al. Fibre type-specific satellite cell response to aerobic training in sedentary adults. J Physiol. 2014;592(Pt 12):2625-2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fry CS, Porter C, Sidossis LS, et al. Satellite cell activation and apoptosis in skeletal muscle from severely burned children. J Physiol. 2016;594(18):5223-5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Garcia SA, Curran MT, Palmieri-Smith RM. Longitudinal assessment of quadriceps muscle morphology before and after anterior cruciate ligament reconstruction and its associations with patient-reported outcomes. Sports Health. 2020;12(3):271-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Giesche F, Niederer D, Banzer W, Vogt L. Evidence for the effects of prehabilitation before ACL-reconstruction on return to sport-related and self-reported knee function: a systematic review. PLoS One. 2020;15(10):e0240192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hartigan EH, Zeni J, Jr, Di Stasi S, Axe M, Synder-Mackler L. Peroperative predictors for noncopers to pass return to sports criteria after ACL reconstruction. J Appl Biomech. 2012;28(4):366-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim DK, Park WH. Sex differences in knee strength deficit 1 year after anterior cruciate ligament reconstruction. J Phys Ther Sci. 2015;27(12):3847-3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kline PW, Morgan KD, Johnson DL, Ireland ML, Noehren B. Impaired quadriceps rate of torque development and knee mechanics after anterior cruciate ligament reconstruction with patellar tendon autograft. Am J Sports Med. 2015;43(10):2553-2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Konishi Y, Ikeda K, Nishino A, et al. Relationship between quadriceps femoris muscle volume and muscle torque after anterior cruciate ligament repair. Scand J Med Sci Sports. 2007;17(6):656-661. [DOI] [PubMed] [Google Scholar]
- 21. Kuenze C, Lisee C, Birchmeier T, et al. Sex differences in quadriceps rate of torque development within 1 year of ACL reconstruction. Phys Ther Sport. 2019;38:36-43. [DOI] [PubMed] [Google Scholar]
- 22. Kuenze CM, Blemker SS, Hart JM. Quadriceps function relates to muscle size following ACL reconstruction. J Orthop Res. 2016;34(9):1656-1662. [DOI] [PubMed] [Google Scholar]
- 23. Lepley LK, Davi SM, Burland JP, Lepley AS. Muscle atrophy after ACL injury: implications for clinical practice. Sports Health. 2020;12(6):579-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Logerstedt D, Lynch A, Axe MJ, Synder-Mackler L. Pre-operative quadriceps strength predicts IKDC2000 scores 6 months after anterior cruciate ligament reconstruction. Knee. 2013;20(3):208-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lorentzon R, Elmqvist LG, Sjostrom M, Fagerlund M, Fuglmeyer AR. Thigh musculature in relation to chronic anterior cruciate ligament tear: muscle size, morphology, and mechanical output before reconstruction. Am J Sports Med. 1989;17(3):423-429. [DOI] [PubMed] [Google Scholar]
- 26. Maffiuletti NA, Aagaard P, Blazevich AJ, Folland J, Tillin N, Duchateau J. Rate of force development: physiological and methodological considerations. Eur J Appl Physiol. 2016;116(6):1091-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Montalvo AM, Schneider DK, Yut L, et al. “What’s my risk of sustaining an ACL injury while playing sports?” A systematic review with meta-analysis. Br J Sports Med. 2019;53(16):1003-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moro T, Brightwell CR, Volpi E, Rasmussen BB, Fry CS. Resistance exercise training promotes fiber type-specific myonuclear adaptations in older adults. J Appl Physiol (1985). 2020;128(4):795-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nakamura T, Kurosawa H, Kawahara H, Watarai K, Miyashita H. Muscle fiber atrophy in the quadriceps in knee-joint disorders. Histochemical studies on 112 cases. Arch Orthop Trauma Surg. 1986;105(3):163-169. [DOI] [PubMed] [Google Scholar]
- 30. Neal A, Boldrin L, Morgan JE. The satellite cell in male and female, developing and adult mouse muscle: distinct stem cells for growth and regeneration. PLoS One. 2012;7(5):e37950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rosa-Caldwell ME, Greene NP. Muscle metabolism and atrophy: let’s talk about sex. Biol Sex Differ. 2019;10(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shanely RA, Zwetsloot KA, Triplett NT, Meaney MP, Farris GE, Nieman DC. Human skeletal muscle biopsy procedures using the modified Bergstrom technique. J Vis Exp. 2014(91):51812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Thomas AC, Wojtys EM, Brandon C, Palmieri-Smith RM. Muscle atrophy contributes to quadriceps weakness after anterior cruciate ligament reconstruction. J Sci Med Sport. 2016;19(1):7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. van Melick N, van Cingel RE, Brooijmans F, et al. Evidence-based clinical practice update: practice guidelines for anterior cruciate ligament rehabilitation based on a systematic review and multidisciplinary consensus. Br J Sports Med. 2016;50(24):1506-1515. [DOI] [PubMed] [Google Scholar]
- 35. Webster KE, Feller JA. Return to Level I sports after anterior cruciate ligament reconstruction: evaluation of age, sex, and readiness to return criteria. Orthop J Sports Med. 2018;6(8):2325967118788045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Webster KE, Nagelli CV, Hewett TE, Feller JA. Factors associated with psychological readiness to return to sport after anterior cruciate ligament reconstruction surgery. Am J Sports Med. 2018;46(7):1545-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wen Y, Murach KA, Vechetti IJ, Jr, et al. MyoVision: software for automated high-content analysis of skeletal muscle immunohistochemistry. J Appl Physiol (1985). 2018;124(1):40-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Williams GN, Buchanan TS, Barrance PJ, Axe MJ, Snyder-Mackler L. Quadriceps weakness, atrophy, and activation failure in predicted noncopers after anterior cruciate ligament injury. Am J Sports Med. 2005;33(3):402-407. [DOI] [PubMed] [Google Scholar]
- 39. Williams GN, Snyder-Mackler L, Barrance PJ, Buchanan TS. Quadriceps femoris muscle morphology and function after ACL injury: a differential response in copers versus non-copers. J Biomech. 2005;38(4):685-693. [DOI] [PubMed] [Google Scholar]
- 40. Yoshihara T, Natsume T, Tsuzuki T, et al. Sex differences in forkhead box O3a signaling response to hindlimb unloading in rat soleus muscle. J Physiol Sci. 2019;69(2):235-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yoshihara T, Takaragawa M, Dobashi S, Naito H. Losartan treatment attenuates hindlimb unloading-induced atrophy in the soleus muscle of female rats via canonical TGF-β signaling. J Physiol Sci. 2022;72(6). 10.1186/s12576-022-00830-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-sph-10.1177_19417381241230612 for Sex Differences in Quadriceps Atrophy After Anterior Cruciate Ligament Tear by Meredith K. Owen, Kelsey R. Casadonte, Nicholas T. Thomas, Christine M. Latham, Camille R. Brightwell, Katherine L. Thompson, Gregory S. Hawk, Cale A. Jacobs, Darren L. Johnson, Christopher S. Fry and Brian Noehren in Sports Health