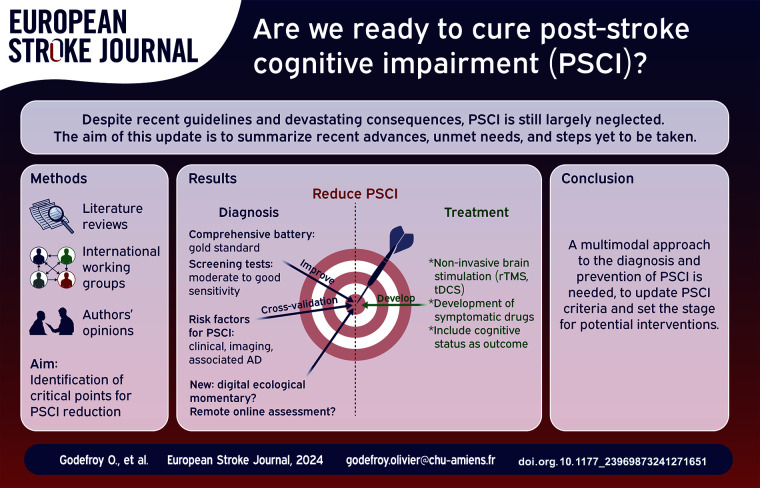
Abstract
Purpose:
Post-stroke (PS) cognitive impairment (CI) is frequent and its devastating functional and vital consequences are well known. Despite recent guidelines, they are still largely neglected. A large number of recent studies have re-examined the epidemiology, diagnosis, imaging determinants and management of PSCI. The aim of this update is to determine whether these new data answer the questions that are essential to reducing PSCI, the unmet needs, and steps still to be taken.
Methods:
Literature review of stroke unit-era studies examining key steps in the management of PSCI: epidemiology and risk factors, diagnosis (cognitive profile and assessments), imaging determinants (quantitative measures, voxelwise localization, the disconnectome and associated Alzheimer’s disease [AD]) and treatment (secondary prevention, symptomatic drugs, rehabilitation and noninvasive brain stimulation) of PSCI.
Findings:
(1) the prevalence of PSCI of approximately 50% is probably underestimated; (2) the sensitivity of screening tests should be improved to detect mild PSCI; (3) comprehensive assessment is now well-defined and should include apathy; (4) easily available factors can identify patients at high risk of PSCI; (5) key imaging determinants are the location and volume of the lesion and the resulting disconnection, associated AD and brain atrophy; WMH, ePVS, microhemorrhages, hemosiderosis, and cortical microinfarcts may contribute to cognitive impairment but are more likely to be markers of brain vulnerability or associated AD that reduce PS recovery; (6) remote and online assessment is a promising approach for selected patients; (7) secondary stroke prevention has not been proven to prevent PSCI; (8) symptomatic drugs are ineffective in treating PSCI and apathy; (9) in addition to cognitive rehabilitation, the benefits of training platforms and computerized training are yet to be documented; (10) the results and the magnitude of improvement of noninvasive brain stimulation, while very promising, need to be substantiated by large, high-quality, sham-controlled RCTs.
Discussion and conclusion:
These major advances pave the way for the reduction of PSCI. They include (1) the development of more sensitive screening tests applicable to all patients and (2) online remote assessment; crossvalidation of (3) clinical and (4) imaging factors to (5) identify patients at risk, as well as (6) factors that prompt a search for associated AD; (7) the inclusion of cognitive outcome as a secondary endpoint in acute and secondary stroke prevention trials; and (8) the validation of the benefit of noninvasive brain stimulation through high-quality, randomized, sham-controlled trials. Many of these objectives can be rapidly and easily attained.
Keywords: Stroke, Alzheimer’s disease, mild cognitive impairment, dementia, executive functions, lesion symptom mapping
Graphical abstract.
Introduction
Post-stroke (PS) cognitive impairment (CI) is frequent and its consequences well known, with an increased risk of disability, institutionalization,1–3 stroke recurrence and death.4,5 International guidelines emphasize the importance of identifying patients with PSCI, referring them for appropriate neuropsychological evaluations and initiating early treatment.6–8 However, they are still largely overlooked. 9 A large number of recent studies, pooled data analyses and meta-analyses have re-examined the epidemiology, diagnosis, imaging and management of PSCI. In this update based on literature review, our aim is to determine whether these new data answer the indispensable questions needed to define management that will reduce PSCI and its consequences in the near future. This update summarizes the recent advances, unmet needs, and steps yet to be taken before these disorders can be reduced.
Methods: Selection of critical points for PSCI reduction
Since the ultimate goal is to provide effective treatment/management for PSCI patients, recent advances in this area were reviewed. To achieve effective treatment/management, it is first necessary to diagnose patients with PSCI, so it is necessary to report on recent advances in diagnosis, that is, neuropsychological assessment (including screening tests). Since it is impossible (and probably inappropriate) to assess the cognitive and behavioral status of all stroke patients with sufficient accuracy, it is appropriate to review strategies for identifying patients at risk (i.e. patients who should be referred for neuropsychological assessment): we have focused on the two strategies that have been previously explored on the basis of the clinical and imaging characteristics of these patients. Despite their interest, new and interesting aspects such as vascular dysfunction and inflammation were not addressed because they do not currently have practical implications for patient management. The data used were derived from recent literature reviews, ongoing works by the authors, their opinions and discussions in various international working groups.
After a brief epidemiological review highlighting the limitations of current estimates of the prevalence of PSCI, the study was organized around the following questions: (1) can we improve diagnosis both through the use of screening tests and with (2) comprehensive batteries, (3) can we identify patients at risk by both clinical (4) and imaging factors, analyzing features that account for the emergence of PSCI such as (5) post-stroke cavity characteristics, (6) white matter hyperintensities (WMH), (7) markers of small vessel pathology, (8) the contribution of Alzheimer’s disease (AD) and Cerebral amyloid angiopathy (CAA), and (9) structural and functional disconnection. Finally, we reviewed (10) more recent and promising approaches, including (11) remote cognitive assessment and (12) PSCI management, including (13) non-invasive stimulation.
Epidemiology: The prevalence of PSCI
PCSI has been assessed in numerous studies, most focusing on major CI (i.e. dementia), and reported varying prevalence10–12 (Table 1). A meta-analysis reported a PSCI prevalence of 53%, two-thirds corresponding to mild CI and one-third to dementia. 11 Heterogeneity across studies is due to several factors (Table 1) the most important of which is probably the applicability of cognitive assessment, as 4%–25% of patients are non-assessable and are at high risk of CI.13–15
Table 1.
Epidemiology of poststroke cognitive impairment (PSCI): Facts and knowledge taken for granted versus yet to be clarified-unmet needs.
| Taken for granted | To be clarified-unmet needs | |
|---|---|---|
| Prevalence | • Dementia: 26.5% (varies from 7% to 67%)10,11 | • Underestimation of prevalence is likely11,14,15 |
| • Mild CI: 36.4% ( varies from 5% to 64%)11,12 | • Need for sensitive cognitive tool adapted to severe strokes21,22 | |
| • Overall PSCI: 53% with about two thirds of mild CI in initially hospitalized patients11,14,15 | • Utility of remote administration 23 | |
| • Depends on selection at baseline,10,13,16,17 attrition at follow-up, 18 applicability of cognitive assessment,13–15 sensitivity of cognitive evaluation, criterion of cognitive deficit11,19 and time of assessment11,20 | ||
| Risk factors | ||
| Stroke subtype | More frequent in hemorrhage and infarct10,11,24–26 than in ruptured aneurysm27–29 and cerebral venous thrombosis27,30 | Cognitive impairment in pure cerebellar lesions to be documented31–34 |
| Others factors | Age,10,11,35,36 cognitive reserve and educational attainment,6,10,35 APOE genotype, 37 prestroke CI, disability or frailty, 38 stroke severity,35,36 recurrent stroke, multiple or large lesions,10,35,36 left hemisphere stroke, acute complications or cognitive disorders (including delirium), low score on cognitive screening test10,35,36 and gross imaging markers6,10,35 | Predictive model based on: |
| • vascular risk factors: low predictive accuracy 39 | ||
| • clinical and gross imaging factors (age, stroke severity, multiple strokes, low score on screening test, extensive to confluent WMH): good to excellent sensitivity35,36 but large validation studies needed |
WMH: white matter hyperintensities.
This indicates that the prevalence of PSCI of approximately 50% among initially hospitalized patients is probably underestimated.
The diagnosis of PSCI
The diagnosis of PSCI relies on the accuracy of cognitive tools, which have evolved. The aim of this section is to examine whether we can improve the diagnosis of PSCI.
Screening tests
The use of cognitive-screening tests (Table 2) is part of the clinical assessment at the poststroke visit and sometimes before discharge from the acute stroke unit. The MiniMental State Examination 40 and Montreal Cognitive Assessment (MoCA) 41 are the most frequently used but their sensitivity for PSCI is only moderate.21,22 A meta-analysis 22 suggests that acute testing yields higher sensitivity (and lower specificity), although this is still too low to use it as a substitute for comprehensive assessment. Other screening tests are being developed (e.g.,42,43) but their superiority over existing tools still requires validation.
Table 2.
Diagnosis of poststroke cognitive impairment: Facts and knowledge taken for granted versus yet to be clarified-unmet needs.
| Taken for granted | To be clarified-unmet needs | |
|---|---|---|
| Screening test | MMSE 40 and MoCA 41 : moderately good sensitivity21,22 | Need for sensitive screening tool adapted to severe strokes |
| Comprehensive battery | - standard battery (e.g. Harmonization standards battery24,44) assesses: | • The usual executive tests (trail making, digit symbol coding and fluency tests) need to be supplemented for some causes of stroke such as aneurysm rupture 27 |
| • 6 major domains: processing speed, executive functions, episodic memory, language, visuoconstructive abilities and behavioral-socio-emotional changes-depression | - Crossvalidation of optimal and parsimonious combination of tests needed for hemineglect assessment | |
| • to be supplemented by optional modules if necessary | ||
| • most frequently impaired domains in stroke 45 | ||
| • closely related to disability3,46 | ||
| • executive functions and speed: the most vulnerable functions19,24,27,47 | ||
| - Hemineglect assessment: | ||
| • cancelation test associated if possible with line bisection, figure copying and baking tray tasks (EAN recommendation) 48 | ||
| • 3 tests (cancelation Bell test, bisection of long lines and a reading task) 49 : most sensitive and parsimonious test combination to detect hemineglect 50 | ||
| Predominant impairment of speed and executive functions | - slowing of processing speed: | - crossvalidation studies needed to relate patient slowing to: |
| • observed in 88% [75%–95%] of PSCI in the STROKCOG cohorts 24 | • prominent motor slowing54,55 subsequent to disruption of frontostriatal 54 and thalamofrontal 56 tracts | |
| • optimal sensitivity of digit symbol coding and Trail Making tests part B 27 | • primary attention disorders19,24,45,57: restricted to lesions of the dorsomedial prefrontal cortex,58–61 thalamus 62 and right ventrolateral prefrontal cortex54,58 | |
| -apathy: | - crossvalidation studies needed to support 2 main mechanisms: | |
| • observed in approximately one third of patients27,51,52 | • “pure apathy” due to primary loss of motivation and effort estimation 63 : lesions of ventral striatum, amygdala, mesencephalon, anterior cingulate, thalamus and inferior frontal gyrus63,64 | |
| • independently associated with disability3,27,51,53 | • may be secondary to sensory-motor, cognitive and depressive disorders51,52 (i.e. secondary apathy) 52 |
MMSE: MiniMental State Examination; MoCA: Montreal Cognitive Assessment; EAN: European Academy of Neurology; PSCI: post-stroke cognitive impairment; VCI: vascular cognitive impairment; WMH: white matter hyperintensities; PET: positron emission tomography.
This indicates that the sensitivity of screening tests should be improved to detect mild PSCI in routine practice, or that an alternative strategy should be developed to select at-risk patients.
Comprehensive assessment
The comprehensive assessment19,24,31,44 (Table 2) is the gold standard, appears to be very sensitive19,24,27,45,57,65 and is independently related to disability.3,46 Assessment should include apathy because it is observed in approximately one-third of patients27,51,52 and is independently associated with disability.3,27 The comprehensive battery needs to be supplemented in specific situations: certain executive tests need to be added for certain causes of stroke, 27 as well as language assessment in aphasics 66 and hemineglect assessment in certain right hemisphere stroke patients using recent recommendations 48 and a parsimonious combination of tests 50 (Table 1).
Given that it is impossible and unnecessary to carry out a comprehensive assessment of all stroke patients, and that the sensitivity of screening tests is moderate, we suggest developing alternative strategies based on the identification of at-risk patients.
Identification of patients at risk for PSCI on the basis of clinical factors
Although CI can be observed in all patients, clinical and gross imaging risk factors have been identified. CI is more frequent in cerebral hemorrhage and infarct11,24–26 than in ruptured aneurysm and cerebral venous thrombosis where the cerebral lesion is inconstant.27–30 Reviews have identified a long list (Table 1) of clinical factors10,24,45 and gross imaging markers.6,10,35,67 Other poststroke adverse outcomes, such as fatigue 68 or obstructive sleep apnea 69 may also be associated with PSCI.
The predictive value of these factors has been examined in a few studies using initial data to determine the six-month risk. Models mainly based on vascular risk factors showed low predictive accuracy in crossvalidation studies. 39 The combination of five to six clinical factors (Table 1) can provide good to excellent sensitivity to predict six-month PSCI defined based on a comprehensive battery 35 or MoCA, 36 although crossvalidation is required.
This indicates that easily available factors can identify patients at high risk of PSCI, although crossvalidation studies on large cohorts are warranted.
Imaging determinants of PSCI and their use to identify patients at risk
In addition to the vascular lesions themselves, associated diseases such as Alzheimer’s disease may contribute to PSCI.1,70–72 In terms of imaging, several features are associated with CI (Table 3): WMH, microhemorrhages and hemosiderosis, microinfarcts, enlarged perivascular spaces (ePVS), and cerebral and hippocampal atrophy.10,25,32,56,57,73 Quantitative measures have recently refined these imaging features (e.g. lesion volume, voxelwise analysis of lesion, brain connectivity).
Table 3.
Imaging characteristics of white matter hyperintensities and markers of small vessel disease (adapted from Strive 2 74 ).
| Definition | |
|---|---|
| White matter hyperintensities | Signal abnormality of variable size hyperintense on T2-weighted images, such as FLAIR, without cavitation (signal different from CSF) |
| Microbleeds | - Small, usually up to a few mm, round low signal intensity dots on susceptibility weighted MRI sequences, 74 |
| - Typically result from small hemosiderin deposits that are remnants of tiny past hemorrhages 75 | |
| - Strictly lobar cerebral microbleeds are often a manifestation of cerebral amyloid angiopathy, whereas deep and brainstem microbleeds are more likely to relate to arteriolosclerosis 75 | |
| Hemosiderosis | Thin areas of hypointensity on MRI sequences sensitive to susceptibility effects, in or overlying the superficial cortex |
| Cortical microinfarcts | Small lesions (size ⩽ 4 mm) strictly cortical seen on multiple MRI sequences with an appearance compatible with ischemia (hypointense on T1-weighted, hyperintense on T2-weighted or FLAIR, and isointense on T2*-weighted MRI) 76 |
| Enlarged perivascular space | Tubular, fluid-filled spaces, following the course of small penetrating vessels through the white, and—in the brainstem and basal ganglia—also the gray matter, with signal intensity on MRI similar to CSF 77 |
FLAIR: fluid-attenuated inversion recovery.
Imaging characteristics responsible for PSCI occurrence
Lesion location and volume
Voxelwise lesion symptom mapping analysis showed the prominent role of left frontotemporal, thalamus, and right parietal lesions. 31 The “strategic stroke” classically encompasses a large number of locations 45 that have been variably defined. Five strategic locations were identified using a strict definition, involving the left middle frontal gyrus, the temporoparietal junction, antero-middle thalamus, and both pyramidal tracts. 32 The contribution of cerebellar lesions, especially in the crus VIII, warrants further studies.31–33
This indicates that the location of lesions within the hemisphere should be used to identify patients at risk. A risk score based on lesion location 31 has been developed and requires crossvalidation.
White matter hyperintensity and features of small vessel disease: Simple markers or key-players?
White matter hyperintensity
Confluent WMH promote the emergence of cognitive decline when a focal ischemic or hemorrhagic lesion has occurred24,78–80 (Table 3). However, the underlying mechanisms remain unclear. WMH may reduce the brain resources needed for efficient recovery and promote persistent cognitive manifestations associated with a sudden ischemic or hemorrhagic lesion.81,82 Furthermore, they may themselves contribute to a greater cognitive deficit. When extensive, they are associated with psychomotor slowing. 83 Finally, WMH may increase the risk of recurrent stroke that will finally result in a larger number of lesions, with subsequent cognitive decline.83,84 In clinical practice, the degree of CI specifically associated with WMH in stroke patients remains difficult to estimate. Accumulating data in the literature suggest that the impact of WMH may largely vary depending on (1) their extent within the cerebral connectivity network, 85 (2) their exact location with a larger impact of lesions affecting anterior thalamic radiations or the forceps minor, 86 (3) the severity of the underlying tissue lesions, varying from simple water accumulation to severe neuronal and axonal loss,87–89 (4) the age and clinical status prior the stroke event, with protective factors, such as cognitive reserve, 90 and (5) the previous cerebral status, with the presence or absence of lacunes, neurodegenerative lesions, and cerebral atrophy. 91 Overall, these multiple factors explain why the exact impact of WMH is ultimately highly variable after stroke at the individual level, despite their generally pejorative predictive value.
Markers of small-vessel disease
Cerebral microbleeds, hemosiderosis, cortical microinfarcts, and ePVS constitute MRI manifestations of small-vessel disease (Table 3). There is an expanding literature on microbleeds, ePVS, and microinfarcts as predictors of PSCI.56,92–94 Some studies found a modest association between these MRI markers and the occurrence of PSCI, whereas others did not (systematic review 92 ), likely reflecting small effect sizes. Indeed, a recent study that included a head to head comparison of these various imaging markers reported much smaller effect sizes for microbleeds and ePVS in relation to PSCI than for WMH. 56
Overall, the available data suggest that the observed associations with PSCI do not so much reflect a causative effect of these tiny lesions themselves on cognitive performance, but rather their presence indicates much more widespread microvascular disease in the brain, with a global impact on brain tissue and its resilience, thus predisposing affected individuals to a poor cognitive outcome in the event of a stroke.
The synergistic contribution of multiple imaging features
The imaging features that contribute to the assessment of cognitive performance are usually examined separately, whereas they contribute together. The few studies that have used statistical modeling of multiple imaging features suggest a dominant role for lesion location, followed by stroke volume, hippocampal and cerebral atrophy, and WMH volume, whereas the contribution of ePVS and microhemorrhages appears to be minor.32,95–98
Pending crossvalidation studies, this indicates that, in addition to lesion location, multiple imaging features (including stroke volume, hippocampal and cerebral atrophy, and WMH volume), should be used to refine the identification of at-risk patients.
The contribution of Alzheimer disease to PSCI
Although PSCI is generally attributed solely to the vascular lesions, pioneering studies suggested that a third of PS dementia is due to associated AD.1,70–72 The contribution of AD has been refined following amyloid positron emission tomography (PET) studies. First, PET studies refuted the promotion of amyloid deposition by stroke lesions.99–101 Second, they showed a prevalence of amyloid positivity of approximately 15%–20% depending on the age and frequency of CI in the sample.99,100,102–104 Third, the largest studies showed that the amyloid burden is associated with a more severe cognitive status at baseline99,102 and a high risk of developing severe CI at follow-up.102,105,106 Accordingly, PET positivity was found in 30% to 38%99,104 of PS dementia cases, thus supporting the pioneering studies.1,70,72 This converges with the additive effect of vascular and neurodegenerative lesions on cognitive outcome.107–109 Fourth, a high burden of posterior WMH, cortical microbleeds, centrum semiovale ePVS, and hippocampal atrophy has been shown to be suggestive of amyloïdopathy, 99 although this requires replication. Fifth, the use of cognitive profiles (based on the executive/memory contrast) to orient the cause of PSCI (i.e. pure vascular vs CI associated with AD) has been called into question by the finding of overlapping cognitive profiles in the two diseases47,99 due to a high rate of dysexecutive disorders in AD 110 and the predominance of the encoding-storage (also known as hippocampal) profile of vascular memory deficit. 65 Thus, only severe memory deficits (relative to executive disorders) that are not explained by stroke location should be considered an indication of associated AD. In addition to documenting the highly deleterious effect of associated AD, these data offer imaging possibilities for identifying associated AD in PSCI patients.
CAA can also be positive on amyloid PET111,112 and is a factor associated with cognitive impairment independent of associated AD. 113 Accordingly, the presence of MRI markers of CAA is associated with an increased risk of CI. 25 This suggests that CAA may represent a therapeutic target: a phase 2 trial targeting Aβ1-40, the major Aβ species deposited in the arterial wall in CAA, did not show the expected result. 114
Pending more precise predictors, this indicates that association with AD should be sought, given its frequency and major prognostic impact, in at-risk patients which include age, severe memory deficits (vs executive disorders) not explained by stroke location, high posterior WMH load, cortical microbleeds, centrum semiovale ePVS, and hippocampal atrophy. Diagnostic approach (annual cognitive and imaging follow-up, CSF biomarkers, or amyloid PET) depends on patient status, the evolving availability of amyloid PET, the development of AD-modifying drugs, and the demonstration of their benefit in patients with mild to moderate stroke.
Is disconnection the key player that determines the occurrence of PSCI?
Current predictions for PSCI based on lesion location are satisfying, although limited by the accuracy of the current model of brain function. 115 Recent evidence suggests that brain function relies more on connections within the brain than on the local contribution of regions to functions.116–119 This can be explained by the importance of the interaction between brain regions in the achievement of brain functions, as well rapid plasticity, allowing for other connected regions to take over the additional workload.120,121 In the context of brain damage, the contribution of disconnections to behavior has frequently shown higher statistical and explanatory power for the symptoms observed in patients.95,97,122–127 Hence, disconnection could be a key player in determining the occurrence of PSCI. 128 Despite the improvement provided by disconnexion, the amount of variance explained by these models is still limited.95,123
To sum up, these data suggest that the key players are the location and volume of the lesion and the resulting disconnection, associated AD and brain atrophy. WMH, ePVS, microhemorrhages, hemosiderosis, and cortical microinfarcts may contribute to cognitive impairment but are more likely to be markers of brain vulnerability or associated AD that reduce PS recovery. Further studies are warranted to accurately determine the minimal lesion load (or disconnection) that induces PSCI and refine the modeling of imaging determinants of PSCI. Thus, additional improvement is needed before using such modeling to accurately predict patients at risk of PSCI.
Recent and promising approaches
Improving the diagnosis: Remote online cognitive assessment
Pending the development of more sensitive screening tests, comprehensive cognitive assessment is needed for patients at risk of PSCI based on clinical and imaging risk factors8,83 (see above). The recent development of remote and online assessment may offer such an opportunity for selected patients. 23
Tools such as the Telephone Interview for Cognitive Status and telephone short MoCA 41 were the first available methods for remotely measuring cognitive performance in stroke patients. 129 Recent results, obtained on a small stroke sample, showed that a self-administered tablet-based neurocognitive platform is widely acceptable and has good convergent validity. 130 The organization of a remotely accessible detailed cognitive assessment on an internet platform that can be controlled under professional supervision also appears promising. The feasibility of this procedure has already been established using a flexible, integrated system. 131 Other organizational (links with professionals) and practical factors (financing of equipment, cost) must also be taken into consideration. The development of digital ecological momentary assessment presents a promising approach to monitor at-risk patients outside the hospital environment and provide access to digital therapeutics. 132 This approach needs to be validated on a large control population before standards can be established for their clinical use. Finally, remote cognitive assessment will necessarily remain limited to patients who have the necessary computer skills, are free of major deficit and who can access a high-performance internet communication network. Professional supervision will remain crucial to ensure reproducible and high-quality data. Several studies are currently underway to document these critical points.
Another advantage of online remote assessment is that it facilitates continuous assessment. PSCI is a dynamic process that can be exacerbated by mood or sleep disturbances and external factors such as social support and environment. Therefore, continuous follow-up is needed to capture potential changes in cognition and to assess the impact of these symptoms on daily life, which is not addressed in the guidelines. The current development of digital ecological momentary assessment is a promising approach to monitor patients outside the hospital setting and provide access to digital therapeutics.
Finally, such an online assessment can feed a collaborative digital platform that offers the opportunity to optimize collaboration between physicians, including neurologists, gerontologists, and primary care physicians, speech-language pathologists, occupational therapists, neuropsychologists, nurses, and allied health professionals for optimal identification and management of cognitive problems after stroke. This indicates that remote and online assessment is a promising approach for selected patients, and their value should be clarified by ongoing studies.
Management of PSCI
Interventions (Table 4) for secondary stroke prevention including vascular risk factors (hypertension, smoking, diabetes, hyperlipidemia, obesity, obstructive sleep apnea and physical activity) are mandatory but their contribution to prevent CI remains to be proven.6,7,133 The cognitive benefit of lacunar infarction treatment demonstrated in a recent small phase 2 trial requires validation in further studies. 134
Table 4.
Classical and promising approaches in management and brain stimulation.
| Taken for granted | To be clarified-unmet needs | |
|---|---|---|
| Secondary stroke prevention | Interventions for hypertension, smoking, diabetes, hyperlipidemia, obesity, obstructive sleep apnea and physical activity are mandatory | But effect on cognition remains unproven6,7,133 |
| Pharmacological symptomatic treatments | Cholinesterase inhibitors, memantine, dopamine agonists and selective serotonin reuptake inhibitors: no significant benefit on pure vascular PSCI7,135,136 | Refine mechanisms of certain cognitive and behavioral impairments |
| Cognitive rehabilitation | Combination of restorative and compensatory: traditional paper and pencil-based training | Platforms and computerized training: benefit to be documented6,7,137 |
| Noninvasive brain stimulation (NIBS) | Change in excitability of the underlying brain cortex potentially induces long-lasting neuroplastic changes138,139 | Overall benefit and its magnitude of all NIBS methods: needs to be substantiated by large, high-quality sham-controlled randomized trials |
| Repeated transcranial magnetic stimulation (rTMS) | Effects on cortical activity depends on frequency: | |
| - high-frequency (>1 Hz) stimulation promotes local neuronal excitability, | ||
| - low-frequency (⩽1 Hz) stimulation shows inhibitory effects 140 | ||
| Theta-burst stimulation | rTMS technique consisting of 3 pulse bursts at 50 Hz in continuous (inhibition of local cortical excitability) or intermittent forms (facilitation of local cortical excitability)140,141 | Could induce longer duration and more intense neural activity with low-intensity, short duration stimulation than conventional rTMS 142 |
| Transcranial direct current stimulation (tDCS) | Constant, low-intensity direct current (intensity of 0.5–2 mA) applied through two electrodes placed on the scalp; subdivided into anodal (enhances activity of superficial cortical neurons), cathodal (reduces activity of superficial cortical neurons) and dual (both anodal and cathodal) 143 | |
| Transcutaneous vagus nerve stimulation | May modulate brain neurotransmitters release and blood flow to brain areas such as hippocampus and thalamus 144 |
In addition, there are no standard pharmacological treatments for the treatment of PSCI as cholinesterase inhibitors, glutamate N-Methyl-D-aspartate receptor antagonist, dopamine agonists and selective serotonin reuptake inhibitors have failed to demonstrate a significant benefit on global cognitive function after stroke.7,135,136,145 The lack of efficacy of symptomatic drugs on PSCI (Table 4) may also be due to their multiple mechanisms, as recently suggested for two leading impairments in PSCI, psychomotor slowing45,54,57–62 and apathy51,52,63,64,146 (Table 1). To date, the management of PSCI mainly relies on cognitive rehabilitation, including a combination of restorative and compensatory approaches, both using traditional paper-and-pencil tasks. Recent approaches offer new opportunities to improve cognitive rehabilitation. The benefits of training platforms and computerized training are yet to be documented.6,7,137
Noninvasive brain stimulation
New hope has arisen from the promising results of noninvasive brain stimulation (Table 4), including repeated transcranial magnetic stimulation (rTMS), theta-burst stimulation (TBS), and transcranial direct current stimulation (tDCS). 140 These approaches assume that, under normal circumstances, the left and right hemispheres are in a balanced state of mutual inhibition. After a stroke, the lack of inhibitory effect of the damaged hemisphere on the undamaged hemisphere causes a relative increase in excitability of the intact hemisphere, ultimately resulting in an increase in inhibition of the damaged hemisphere. In addition noninvasive brain stimulation can induce changes in the excitability of the underlying cortex and potentially induce long-lasting neuroplastic changes by promoting neurogenesis, angiogenesis, anti-inflammatory, antioxidant, and anti-apoptosis effects. 138 Different stimulation frequencies have different effects on cortical activity, with high frequency (>1 Hz) stimulation (HF-rTMS) promoting local neuronal excitability and low frequency (⩽1 Hz) stimulation (LF-rTMS) showing inhibitory effects. 140
Given the small size of these studies, meta-analyses have been invaluable in assessing their interest. Concerning rTMS, systematic reviews and meta-analyses of randomized controlled trials (RCT) indicate a positive effect on cognitive outcome. 138 In a systematic review of 12 RCTs involving 497 patients with PSCI, Gong et al. 147 reported that rTMS had a positive effect on cognitive rehabilitation. Another study 148 pooling data from 8 studies and 336 participants found a large effect of rTMS combined with cognitive training on global cognition, executive function, and working memory, but no effect on memory. With regards to aphasia, a large number of studies converge on its benefits, 149 and it is now recommended in some countries. 150
TBS is a novel rTMS consisting of three pulse bursts at 50 Hz. Compared with conventional rTMS, TBS can induce longer and more intense neural activity with low-intensity, short-duration stimulation. Intermittent TBS (which plays a facilitating role in local cortical excitability 140 ) applied to left dorsolateral prefrontal cortex has been reported to improve executive function and semantic comprehension.151,152
Research findings on tDCS show contradictory effects153–155 with a slight improvement in general cognitive performance and attention provided by anodal tDCS. 155 In a meta-analysis 155 of 15 studies (N = 820 participants) of tDCS compared with sham tDCS or control, anodal tDCS was associated with small improvements in general cognitive and attention performance, but not in memory. However, most of these studies lacked sham tDCS and safety data.
A network meta-analysis 140 of RCTs comparing any active noninvasive brain stimulation with sham stimulation in stroke survivors showed that high-frequency rTMS improved global cognitive function while dual-tDCS improved memory performance.
Although promising, these results and the magnitude of improvement still need to be substantiated by large, high-quality, sham-controlled RCT. They should examine the influence of the timing of stimulation, treatment frequency and duration, and stimulation parameters, particularly the stimulation site. 156 Indeed, the location of the lesion and its effects on connectivity can help to select the most appropriate stimulation parameters to individualize the location of the stimulation.149,157,158 Finally, the potential benefit of combining noninvasive brain stimulation methods in the same patient should be examined, as recently suggested. 159
Conclusions
This update shows the major advances and provides guidance on the main issues to be resolved before reducing PSCI. This mainly includes (1) crossvalidation of factors that identify PS patients at risk of CI, (2) identification of the minimal vascular lesion that induces PSCI and (3) factors to prompt the search for associated AD; (4) development and validation of screening tests to improve the sensitivity and applicability to all patients, as well as (5) digital ecological momentary assessment and (6) cognitive assessment that are remotely administered online, and (7) used to monitor the course PSCI and (8) to provide access to digital therapeutics; (9) validation of the benefit of noninvasive brain stimulation by high-quality, individually-based, sham-controlled RCT; (10) systematic inclusion of cognitive outcome as a secondary endpoint in both acute and secondary prevention stroke trials, and (11) development of symptomatic drug trials targeting behavioral and cognitive disorders of pre-defined mechanisms. A significant number of these objectives can be easily and rapidly attained. Their findings may be incorporated into a revised version of the VASCOG criteria for vascular cognitive impairment. 160
Acknowledgments
Acknowledgments none related to this study.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Olivier Godefroy: has served on scientific advisory boards (Novartis and Astra Zeneca), received funding for travel and meetings from Novartis, Lilly, Genzyme, Astrazeneca, Biogen, Teva, Pfizer, CSL-Behring, GSK, Boehringer-Ingelheim, Ipsen, Covidien, Bristol-Myers Squibb. Ardalan Aarabi: none related to this study. Yannick Béjot meeting speaker: BMS, Pfizer, Boehringher-Ingelheim, Servier, Medtronic, Amgen; Consulting: Medtronic, NovoNordisk, Novartis. Geert J Biessels: Consulting : Nestle HealthScience. Bertrand Glize: Consulting: IPSEN. Vincent MT Mok: none related to this study. Michel Thiebaut de Schotten: none related to this study. Igor Sibon: meeting speaker: BMS, Pfizer, Boehringher-Ingelheim, Servier, Medtronic, Novonordisk, Novartis, Sanofi, Astra-Zeneca; Consulting: Medtronic, NovoNordisk, Novartis, Sanofi, Astra-Zeneca. Hugues Chabriat: none related to this study. M Roussel: none related to this study.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: Ethical approval was not sought for this article because it is based on literature review.
Informed consent: Informed consent was not sought for this article because it is based on literature review.
Guarantor: OG
Contributorship: OG conceived the review and wrote the first draft of the manuscript; YB researched literature on epidemiology and wrote §; AA, GJB, HC, MTdS researched literature and wrote § on imaging; OG and VTM researched literature and wrote § on Alzheimer disease; MR and OG researched literature and wrote § on cognitive assessment; HC and IS researched literature and wrote § on remote and online assessment; OG and BG researched literature and wrote § on rehabilitation and non-invasive brain stimulation. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
ORCID iDs: Olivier Godefroy  https://orcid.org/0000-0001-6789-6620
https://orcid.org/0000-0001-6789-6620
Yannick Béjot  https://orcid.org/0000-0001-7848-7072
https://orcid.org/0000-0001-7848-7072
Hugues Chabriat  https://orcid.org/0000-0001-8436-6074
https://orcid.org/0000-0001-8436-6074
References
- 1. Tatemichi TK, Foulkes MA, Mohr JP, et al. Dementia in stroke survivors in the stroke data bank cohort. Prevalence, incidence, risk factors, and computed tomographic findings. Stroke 1990; 21: 858–866. [DOI] [PubMed] [Google Scholar]
- 2. Saposnik G, Cote R, Rochon PA, et al. Care and outcomes in patients with ischemic stroke with and without preexisting dementia. Neurology 2011; 77: 1664–1673. [DOI] [PubMed] [Google Scholar]
- 3. Tasseel-Ponche S, Barbay M, Roussel M, et al. Determinants of disability at 6 months after stroke: the GRECogVASC study. Eur J Neurol 2022; 29: 1972–1982. [DOI] [PubMed] [Google Scholar]
- 4. Moroney JT, Bagiella E, Tatemichi TK, et al. Dementia after stroke increases the risk of long-term stroke recurrence. Neurology 1997; 48: 1317–1325. [DOI] [PubMed] [Google Scholar]
- 5. Lee M, Saver JL, Hong KS, et al. Cognitive impairment and risk of future stroke: a systematic review and meta-analysis. Can Med Assoc J 2014; 186: E536–E546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Quinn TJ, Richard E, Teuschl Y, et al. European stroke organisation and European academy of neurology joint guidelines on post-stroke cognitive impairment. Eur J Neurol 2021; 28: 3883–3920. [DOI] [PubMed] [Google Scholar]
- 7. El Husseini N, Katzan IL, Rost NS, et al. Cognitive impairment after ischemic and hemorrhagic stroke: a scientific statement from the American heart association/American stroke association. Stroke 2023; 54: e272–e291. [DOI] [PubMed] [Google Scholar]
- 8. Diagnostic des troubles cognitifs post-AVC: Préconisations Société Française NeuroVasculaire et Fédération des Centres Mémoires 2018. Accessed July 7, 2023. https://www.societe-francaise-neurovasculaire.fr/preconisations-sfnv
- 9. Kapoor A, Lanctôt KL, Bayley M, et al. “good outcome” isn’t good enough: cognitive impairment, depressive symptoms, and social restrictions in physically recovered stroke patients. Stroke 2017; 48: 1688–1690. [DOI] [PubMed] [Google Scholar]
- 10. Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol 2009; 8: 1006–1018. [DOI] [PubMed] [Google Scholar]
- 11. Barbay M, Diouf M, Roussel M, et al. Systematic review and meta-analysis of prevalence in post-stroke neurocognitive disorders in hospital-based studies. Dement Geriatr Cogn Disord 2018; 46: 322–334. [DOI] [PubMed] [Google Scholar]
- 12. Sexton E, McLoughlin A, Williams DJ, et al. Systematic review and meta-analysis of the prevalence of cognitive impairment no dementia in the first year post-stroke. Eur Stroke J 2019; 4: 160–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Graber M, Garnier L, Mohr S, et al. Influence of pre-existing mild cognitive impairment and dementia on post-stroke mortality. The Dijon stroke registry. Neuroepidemiology 2020; 54: 490–497. [DOI] [PubMed] [Google Scholar]
- 14. Pendlebury ST, Klaus SP, Thomson RJ, et al. Methodological factors in determining risk of dementia after transient ischemic attack and stroke: (III) applicability of cognitive tests. Stroke 2015; 46: 3067–3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Elliott E, Drozdowska BA, Taylor-Rowan M, et al. Who is classified as untestable on brief cognitive screens in an acute stroke setting? Diagnostics 2019; 9: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Béjot Y, Duloquin G, Crespy V, et al. Influence of preexisting cognitive impairment on clinical severity of ischemic stroke: the Dijon Stroke Registry. Stroke 2020; 51: 1667–1673. [DOI] [PubMed] [Google Scholar]
- 17. Pendlebury ST, Chen PJ, Bull L, et al. Methodological factors in determining rates of dementia in TIA and stroke: (I) impact of baseline selection bias. Stroke 2015; 46: 641–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pendlebury ST, Chen PJ, Welch SJ, et al. Methodological factors in determining risk of dementia after transient ischemic attack and stroke: (II) effect of attrition on follow-up. Stroke 2015; 46: 1494–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Barbay M, Taillia H, Nédélec-Ciceri C, et al. Prevalence of poststroke neurocognitive disorders using national institute of neurological disorders and stroke-Canadian stroke network, VASCOG criteria (vascular behavioral and cognitive disorders), and optimized criteria of cognitive deficit. Stroke 2018; 49: 1141–1147. [DOI] [PubMed] [Google Scholar]
- 20. Pendlebury ST, Rothwell PM. Incidence and prevalence of dementia associated with transient ischaemic attack and stroke: analysis of the population-based Oxford vascular study. Lancet Neurol 2019; 18: 248–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Godefroy O, Fickl A, Roussel M, et al. Is the Montreal cognitive assessment superior to the mini-mental state examination to detect poststroke cognitive impairment? Stroke 2011; 42: 1712–1716. [DOI] [PubMed] [Google Scholar]
- 22. Lees R, Selvarajah J, Fenton C, et al. Test accuracy of cognitive screening tests for diagnosis of dementia and multidomain cognitive impairment in stroke. Stroke 2014; 45: 3008–3018. [DOI] [PubMed] [Google Scholar]
- 23. Salvadori E, Pantoni L. Teleneuropsychology for vascular cognitive impairment: which tools do we have? Cereb Circ - Cogn Behav 2023; 5: 100173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lo JW, Crawford JD, Desmond DW, et al. Profile of and risk factors for poststroke cognitive impairment in diverse ethnoregional groups. Neurology 2019; 93: e2257–e2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moulin S, Labreuche J, Bombois S, et al. Dementia risk after spontaneous intracerebral haemorrhage: a prospective cohort study. Lancet Neurol 2016; 15: 820–829. [DOI] [PubMed] [Google Scholar]
- 26. Garcia PY, Roussel M, Bugnicourt JM, et al. Cognitive impairment and dementia after intracerebral hemorrhage: a cross-sectional study of a hospital-based series. J Stroke Cerebrovasc Dis 2013; 22: 80–86. [DOI] [PubMed] [Google Scholar]
- 27. Roussel M, Martinaud O, Hénon H, et al. The behavioral and cognitive executive disorders of stroke: the GREFEX study. PLoS One 2016; 11: e0147602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Martinaud O, Perin B, Gérardin E, et al. Anatomy of executive deficit following ruptured anterior communicating artery aneurysm. Eur J Neurol 2009; 16: 595–601. [DOI] [PubMed] [Google Scholar]
- 29. Buunk AM, Spikman JM, Wagemakers M, et al. The vanishing of the ACoA syndrome after aneurysmal subarachnoid haemorrhage: new era, different management, fewer problems? J Neuropsychol 2024; 18: 142–157. [DOI] [PubMed] [Google Scholar]
- 30. Bugnicourt JM, Guegan-Massardier E, Roussel M, et al. Cognitive impairment after cerebral venous thrombosis: a two-center study. J Neurol 2013; 260: 1324–1331. [DOI] [PubMed] [Google Scholar]
- 31. Weaver NA, Kuijf HJ, Aben HP, et al. Strategic infarct locations for post-stroke cognitive impairment: a pooled analysis of individual patient data from 12 acute ischaemic stroke cohorts. Lancet Neurol 2021; 20: 448–459. [DOI] [PubMed] [Google Scholar]
- 32. Puy L, Barbay M, Roussel M, et al. Neuroimaging determinants of poststroke cognitive performance. Stroke 2018; 49: 2666–2673. [DOI] [PubMed] [Google Scholar]
- 33. Stoodley CJ, MacMore JP, Makris N, et al. Location of lesion determines motor vs. cognitive consequences in patients with cerebellar stroke. NeuroImage Clin 2016; 12: 765–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schmahmann JD, Macmore J, Vangel M. Cerebellar stroke without motor deficit: clinical evidence for motor and non-motor domains within the human cerebellum. Neuroscience 2009; 162: 852–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Godefroy O, Yaïche H, Taillia H, et al. Who should undergo a comprehensive cognitive assessment after a stroke? A cognitive risk score. Neurology 2018; 91: e1979–e1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dong Y, Ding M, Cui M, et al. Development and validation of a clinical model (DREAM-LDL) for post-stroke cognitive impairment at 6 months. Aging 2021; 13: 21628–21641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Salah Khlif M, Egorova-Brumley N, Bird LJ, et al. Cortical thinning 3 years after ischaemic stroke is associated with cognitive impairment and APOE ε4. NeuroImage Clin 2022; 36: 103200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Munthe-Kaas R, Aam S, Saltvedt I, et al. Is frailty index a better predictor than pre-stroke modified Rankin Scale for neurocognitive outcomes 3-months post-stroke? BMC Geriatr 2022; 22: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tang EYH, Price CI, Robinson L, et al. Assessing the predictive validity of simple dementia risk models in harmonized stroke cohorts. Stroke 2020; 51: 2095–2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12: 189–198. [DOI] [PubMed] [Google Scholar]
- 41. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005; 53: 695–699. [DOI] [PubMed] [Google Scholar]
- 42. Murphy D, Cornford E, Higginson A, et al. Oxford cognitive screen: a critical review and independent psychometric evaluation. J Neuropsychol 2023; 17: 491–504. [DOI] [PubMed] [Google Scholar]
- 43. Brookes RL, Hollocks MJ, Khan U, et al. The brief memory and executive test (BMET) for detecting vascular cognitive impairment in small vessel disease: a validation study. BMC Med 2015; 13: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hachinski V, Iadecola C, Petersen RC, et al. National institute of neurological disorders and stroke-Canadian stroke network vascular cognitive impairment harmonization standards. Stroke 2006; 37: 2220–2241. [DOI] [PubMed] [Google Scholar]
- 45. Godefroy O. The behavioral and cognitive neurology of stroke. Cambridge: Cambridge University Press, 2013. [Google Scholar]
- 46. Nys GM, van Zandvoort MJ, de Kort PL, et al. The prognostic value of domain-specific cognitive abilities in acute first-ever stroke. Neurology 2005; 64: 821–827. [DOI] [PubMed] [Google Scholar]
- 47. Andriuta D, Roussel M, Barbay M, et al. Differentiating between Alzheimer’s disease and vascular cognitive impairment: is the “memory versus executive function” contrast still relevant? J Alzheimers Dis 2018; 63: 625–633. [DOI] [PubMed] [Google Scholar]
- 48. Moore M, Milosevich E, Beisteiner R, et al. Rapid screening for neglect following stroke: a systematic search and European academy of neurology recommendations. Eur J Neurol 2022; 29: 2596–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Azouvi P, Samuel C, Louis-Dreyfus A, et al. Sensitivity of clinical and behavioural tests of spatial neglect after right hemisphere stroke. J Neurol Neurosurg Psychiatry 2002; 73: 160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Azouvi P, Rousseaux M, Bartolomeo P, et al. Discriminative value of different combinations of tests to detect unilateral neglect in patients with right hemisphere damage. Eur J Neurol 2023; 30: 3332–3340. [DOI] [PubMed] [Google Scholar]
- 51. van Dalen JW, Moll van, Charante EP, Nederkoorn PJ, et al. Poststroke apathy. Stroke 2013; 44: 851–860. [DOI] [PubMed] [Google Scholar]
- 52. Aubignat M, Roussel M, Aarabi A, et al. Poststroke apathy: major role of cognitive, depressive and neurological disorders over imaging determinants. Cortex 2023; 160: 55–66. [DOI] [PubMed] [Google Scholar]
- 53. Godefroy O, Azouvi P, Robert P, et al. Dysexecutive syndrome: diagnostic criteria and validation study. Ann Neurol 2010; 68: 855–864. [DOI] [PubMed] [Google Scholar]
- 54. Ouin E, Roussel M, Aarabi A, et al. Poststroke action slowing: motor and attentional impairments and their imaging determinants. Evidence from lesion-symptom mapping, disconnection and fMRI activation studies. Neuropsychologia 2022; 177: 108401. [DOI] [PubMed] [Google Scholar]
- 55. Godefroy O, Spagnolo S, Roussel M, et al. Stroke and action slowing: mechanisms, determinants and prognosis value. Cerebrovasc Dis 2010; 29: 508–514. [DOI] [PubMed] [Google Scholar]
- 56. Georgakis MK, Duering M, Wardlaw JM, et al. WMH and long-term outcomes in ischemic stroke: a systematic review and meta-analysis. Neurology 2019; 92: e1298–e1308. [DOI] [PubMed] [Google Scholar]
- 57. Chabriat H, Joutel A, Dichgans M, et al. Cadasil. Lancet Neurol 2009; 8: 643–653. [DOI] [PubMed] [Google Scholar]
- 58. Stuss DT, Alexander MP, Shallice T, et al. Multiple frontal systems controlling response speed. Neuropsychologia 2005; 43: 396–417. [DOI] [PubMed] [Google Scholar]
- 59. Godefroy O, Lhullier C, Rousseaux M. Non-spatial attention disorders in patients with frontal or posterior brain damage. Brain J Neurol 1996; 119: 191–202. [DOI] [PubMed] [Google Scholar]
- 60. Godefroy O, Lhullier-Lamy C, Rousseaux M. SRT lengthening: role of an alertness deficit in frontal damaged patients. Neuropsychologia 2002; 40: 2234–2241. [DOI] [PubMed] [Google Scholar]
- 61. Godefroy O, Cabaret M, Rousseaux M. Vigilance and effects of fatigability, practice and motivation on simple reaction time tests in patients with lesion of the frontal lobe. Neuropsychologia 1994; 32: 983–990. [DOI] [PubMed] [Google Scholar]
- 62. Rinne P, Hassan M, Goniotakis D, et al. Triple dissociation of attention networks in stroke according to lesion location. Neurology 2013; 81: 812–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Husain M, Roiser JP. Neuroscience of apathy and anhedonia: a transdiagnostic approach. Nat Rev Neurosci 2018; 19: 470–484. [DOI] [PubMed] [Google Scholar]
- 64. Pessiglione M, Vinckier F, Bouret S, et al. Why not try harder? Computational approach to motivation deficits in neuro-psychiatric diseases. Brain 2018; 141: 629–650. [DOI] [PubMed] [Google Scholar]
- 65. Godefroy O, Roussel M, Leclerc X, et al. Deficit of episodic memory: anatomy and related patterns in stroke patients. Eur Neurol 2009; 61: 223–229. [DOI] [PubMed] [Google Scholar]
- 66. Godefroy O, Dubois C, Debachy B, et al. Vascular aphasias: main characteristics of patients hospitalized in acute stroke units. Stroke 2002; 33: 702–705. [DOI] [PubMed] [Google Scholar]
- 67. Drozdowska BA, McGill K, McKay M, et al. Prognostic rules for predicting cognitive syndromes following stroke: a systematic review. Eur Stroke J 2021; 6: 18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Graber M, Garnier L, Duloquin G, et al. Association between fatigue and cognitive impairment at 6 months in patients with ischemic stroke treated with acute revascularization therapy. Front Neurol 2019; 10: 931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zhu RL, Ouyang C, Ma RL, et al. Obstructive sleep apnea is associated with cognitive impairment in minor ischemic stroke. Sleep Breath 2022; 26: 1907–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kokmen E, Whisnant JP, O’Fallon WM, et al. Dementia after ischemic stroke: a population-based study in Rochester, Minnesota (1960-1984). Neurology 1996; 46: 154–159. [DOI] [PubMed] [Google Scholar]
- 71. Pohjasvaara T, Erkinjuntti T, Ylikoski R, et al. Clinical determinants of poststroke dementia. Stroke 1998; 29: 75–81. [DOI] [PubMed] [Google Scholar]
- 72. Hénon H, Durieu I, Guerouaou D, et al. Poststroke dementia: incidence and relationship to prestroke cognitive decline. Neurology 2001; 57: 1216–1222. [DOI] [PubMed] [Google Scholar]
- 73. Charidimou A, Boulouis G, Frosch MP, et al. The Boston criteria version 2.0 for cerebral amyloid angiopathy: a multicentre, retrospective, MRI–neuropathology diagnostic accuracy study. Lancet Neurol 2022; 21: 714–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Duering M, Biessels GJ, Brodtmann A, et al. Neuroimaging standards for research into small vessel disease—advances since 2013. Lancet Neurol 2023; 22: 602–618. [DOI] [PubMed] [Google Scholar]
- 75. Greenberg SM, Vernooij MW, Cordonnier C, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol 2009; 8: 165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. van Veluw SJ, Shih AY, Smith EE, et al. Detection, risk factors, and functional consequences of cerebral microinfarcts. Lancet Neurol 2017; 16: 730–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wardlaw JM, Benveniste H, Nedergaard M, et al. Perivascular spaces in the brain: anatomy, physiology and pathology. Nat Rev Neurol 2020; 16: 137–153. [DOI] [PubMed] [Google Scholar]
- 78. Mijajlović M, Pavlović A, Brainin M, et al. Post-stroke dementia - a comprehensive review. BMC Med 2017; 15: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Pasquier F, Leys D. Why are stroke patients prone to develop dementia? J Neurol 1997; 244: 135–142. [DOI] [PubMed] [Google Scholar]
- 80. Sachdev PS, Brodaty H, Valenzuela MJ, et al. Clinical determinants of dementia and mild cognitive impairment following ischaemic stroke: the Sydney stroke study. Dement Geriatr Cogn Disord 2006; 21: 275–283. [DOI] [PubMed] [Google Scholar]
- 81. Kissela B, Lindsell CJ, Kleindorfer D, et al. Clinical prediction of functional outcome after ischemic stroke: the surprising importance of periventricular white matter disease and race. Stroke 2009; 40: 530–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Arsava EM, Rahman R, Rosand J, et al. Severity of leukoaraiosis correlates with clinical outcome after ischemic stroke. Neurology 2009; 72: 1403–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Verdelho A, Wardlaw J, Pavlovic A, et al. Cognitive impairment in patients with cerebrovascular disease: a white paper from the links between stroke ESO dementia committee. Eur Stroke J 2021; 6: 5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Debette S, Schilling S, Duperron MG, et al. Clinical significance of magnetic resonance imaging markers of vascular brain injury: a systematic review and meta-analysis. JAMA Neurol 2019; 76: 81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Boot EM, Mc van Leijsen E, Bergkamp MI, et al. Structural network efficiency predicts cognitive decline in cerebral small vessel disease. NeuroImage Clin 2020; 27: 102325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Duering M, Gonik M, Malik R, et al. Identification of a strategic brain network underlying processing speed deficits in vascular cognitive impairment. Neuroimage 2013; 66: 177–183. [DOI] [PubMed] [Google Scholar]
- 87. Zhang R, Ouin E, Grosset L, et al. Elderly CADASIL patients with intact neurological status. Stroke 2022; 24: 352–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. De Guio F, Vignaud A, Chabriat H, et al. Different types of white matter hyperintensities in CADASIL: insights from 7-Tesla MRI. J Cereb Blood Flow Metab 2018; 38: 1654–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Duchesnay E, Hadj Selem F, De Guio F, et al. Different types of white matter hyperintensities in CADASIL. Front Neurol 2018; 9: 526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Zieren N, Duering M, Peters N, et al. Education modifies the relation of vascular pathology to cognitive function: cognitive reserve in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Neurobiol Aging 2013; 34: 400–407. [DOI] [PubMed] [Google Scholar]
- 91. Casolla B, Caparros F, Cordonnier C, et al. Biological and imaging predictors of cognitive impairment after stroke: a systematic review. J Neurol 2019; 266: 2593–2604. [DOI] [PubMed] [Google Scholar]
- 92. Ball EL, Shah M, Ross E, et al. Predictors of post-stroke cognitive impairment using acute structural MRI neuroimaging: a systematic review and meta-analysis. Int J Stroke 2023; 18: 543–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Sagnier S, Okubo G, Catheline G, et al. Chronic cortical cerebral microinfarcts slow down cognitive recovery after acute ischemic stroke. Stroke 2019; 50: 1430–1436. [DOI] [PubMed] [Google Scholar]
- 94. Oveisgharan S, Dawe RJ, Yu L, et al. Frequency and underlying pathology of pure vascular cognitive impairment. JAMA Neurol 2022; 79: 1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Talozzi L, Forkel SJ, Pacella V, et al. Latent disconnectome prediction of long-term cognitive-behavioural symptoms in stroke. Brain 2023; 146: 1963–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kandiah N, Chander RJ, Lin X, et al. Cognitive impairment after mild stroke: development and validation of the SIGNAL2 risk score. J Alzheimers Dis 2016; 49: 1169–1177. [DOI] [PubMed] [Google Scholar]
- 97. Biesbroek JM, Weaver NA, Aben HP, et al. Network impact score is an independent predictor of post-stroke cognitive impairment: a multicenter cohort study in 2341 patients with acute ischemic stroke. NeuroImage Clin 2022; 34: 103018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Kuceyeski A, Navi BB, Kamel H, et al. Structural connectome disruption at baseline predicts 6-months post-stroke outcome. Hum Brain Mapp 2016; 37: 2587–2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Godefroy O, Barbay M, Martin J, et al. Prevalence of amyloid cerebral deposits and cognitive outcome after stroke: the IDEA3 study. Stroke 2023; 25: 315–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Wollenweber FA, Därr S, Müller C, et al. Prevalence of amyloid positron emission tomographic positivity in poststroke mild cognitive impairment. Stroke 2016; 47: 2645–2648. [DOI] [PubMed] [Google Scholar]
- 101. Sahathevan R, Linden T, Villemagne VL, et al. Positron emission tomographic imaging in stroke: cross-sectional and follow-up assessment of amyloid in ischemic stroke. Stroke 2016; 47: 113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Mok VCT, Lam BYK, Wang Z, et al. Delayed-onset dementia after stroke or transient ischemic attack. Alzheimers Dement 2016; 12: 1167–1176. [DOI] [PubMed] [Google Scholar]
- 103. Koenig LN, McCue LM, Grant E, et al. Lack of association between acute stroke, post-stroke dementia, race, and β-amyloid status. NeuroImage Clin 2021; 29: 102553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Yang J, Wong A, Wang Z, et al. Risk factors for incident dementia after stroke and transient ischemic attack. Alzheimers Dement 2015; 11: 16–23. [DOI] [PubMed] [Google Scholar]
- 105. Godefroy O, Trinchard N, et al. Do amyloid cerebral deposits influence the long-term post-stroke cognitive outcome? The IDEA3 study. medRxiv. 10.1101/2024.07.18.24310673. [DOI]
- 106. Liu W, Wong A, Au L, et al. Influence of amyloid-β on cognitive decline after stroke/transient ischemic attack: three-year longitudinal study. Stroke 2015; 46: 3074–3080. [DOI] [PubMed] [Google Scholar]
- 107. Andriuta D, Wiener E, Perron A, et al. Neuroimaging determinants of cognitive impairment in the memory clinic: how important is the vascular burden? J Neurol 2024; 271: 504–518. [DOI] [PubMed] [Google Scholar]
- 108. Snowdon DA, Greiner LH, Mortimer JA, et al. Brain infarction and the clinical expression of Alzheimer disease. The nun study. JAMA 1997; 277: 813–817. [PubMed] [Google Scholar]
- 109. Schneider JA, Wilson RS, Bienias JL, et al. Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology 2004; 62: 1148–1155. [DOI] [PubMed] [Google Scholar]
- 110. Godefroy O, Martinaud O, Verny M, et al. The dysexecutive syndrome of Alzheimer’s disease: the GREFEX study. J Alzheimers Dis 2014; 42: 1203–1208. [DOI] [PubMed] [Google Scholar]
- 111. Gurol ME, Becker JA, Fotiadis P, et al. Florbetapir-PET to diagnose cerebral amyloid angiopathy: a prospective study. Neurology 2016; 87: 2043–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Planton M, Saint-Aubert L, Raposo N, et al. Florbetapir regional distribution in cerebral amyloid angiopathy and Alzheimer’s disease: a PET study. J Alzheimers Dis 2020; 73: 1607–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Boyle PA, Yu L, Nag S, et al. Cerebral amyloid angiopathy and cognitive outcomes in community-based older persons. Neurology 2015; 85: 1930–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Leurent C, Goodman JA, Zhang Y, et al. Immunotherapy with ponezumab for probable cerebral amyloid angiopathy. Ann Clin Transl Neurol 2019; 6: 795–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Godefroy O. Brain-behaviour relationships. Some models and related statistical procedures for the study of brain-damaged patients. Brain 1998; 121: 1545–1556. [DOI] [PubMed] [Google Scholar]
- 116. Thiebaut de Schotten M, Foulon C, Nachev P. Brain disconnections link structural connectivity with function and behaviour. Nat Commun 2020; 11: 5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Fox MD. Mapping symptoms to brain networks with the human connectome. New Engl J Med 2018; 379: 2237–2245. [DOI] [PubMed] [Google Scholar]
- 118. Thiebaut de Schotten M, Urbanski M, Batrancourt B, et al. Rostro-caudal architecture of the frontal lobes in humans. Cereb Cortex 2016. Epub ahead of print 26 July 2016. DOI: 10.1093/cercor/bhw215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Thiebaut de Schotten M, Urbanski M, Valabregue R, et al. Subdivision of the occipital lobes: an anatomical and functional MRI connectivity study. Cortex 2014; 56: 121–137. [DOI] [PubMed] [Google Scholar]
- 120. Carrera E, Tononi G. Diaschisis: past, present, future. Brain 2014; 137: 2408–2422. [DOI] [PubMed] [Google Scholar]
- 121. Monakow CV. Die Lokalisation im Grosshirn Und Der Funktion durch Kortikale Herde. Wiesbaden: Verlag Von J.F. Bergmann, 1914. [Google Scholar]
- 122. Dulyan L, Talozzi L, Pacella V, et al. Longitudinal prediction of motor dysfunction after stroke: a disconnectome study. Brain Struct Funct 2022; 227: 3085–3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Salvalaggio A, De Filippo De, Grazia M, Zorzi M, et al. Post-stroke deficit prediction from lesion and indirect structural and functional disconnection. Brain J Neurol 2020; 143: 2173–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Thiebaut de Schotten M, Tomaiuolo F, Aiello M, et al. Damage to white matter pathways in subacute and chronic spatial neglect: a group study and 2 single-case studies with complete virtual “in vivo” tractography dissection. Cereb Cortex 2014; 24: 691–706. [DOI] [PubMed] [Google Scholar]
- 125. Pacella V, Foulon C, Jenkinson PM, et al. Anosognosia for hemiplegia as a tripartite disconnection syndrome. eLife 2019; 8: e46075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Thiebaut de Schotten M, Dell’Acqua F, Ratiu P, et al. From Phineas Gage and Monsieur leborgne to H.M.: revisiting disconnection syndromes. Cereb Cortex 2015; 25: 4812–4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Khalilian M, Roussel M, Godefroy O, et al. Predicting functional impairments with lesion-derived disconnectome mapping: Validation in stroke patients with motor deficits. Eur J Neurosci 2024; 59: 3074–3092. [DOI] [PubMed] [Google Scholar]
- 128. Thiebaut de Schotten M, Forkel SJ. The emergent properties of the connected brain. Science 2022; 378: 505–510. [DOI] [PubMed] [Google Scholar]
- 129. Barber M, Stott DJ. Validity of the telephone interview for cognitive status (TICS) in post-stroke subjects. Int J Geriatr Psychiatry 2004; 19: 75–79. [DOI] [PubMed] [Google Scholar]
- 130. Sloane KL, Fabian R, Wright A, et al. Supervised, self-administered tablet-based cognitive assessment in neurodegenerative disorders and stroke. Dement Geriatr Cogn Disord 2023; 52: 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Durisko C, McCue M, Doyle PJ, et al. A flexible and integrated system for the remote acquisition of neuropsychological data in stroke research. Telemed E Health 2016; 22: 1032–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Bui Q, Kaufman KJ, Pham V, et al. Ecological momentary assessment of real-world functional behaviors in individuals with stroke: a longitudinal observational study. Arch Phys Med Rehabil 2022; 103: 1327–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Weightman M, Robinson B, Fallows R, et al. Improving sleep and learning in rehabilitation after stroke, part 2 (INSPIRES2): study protocol for a home-based randomised control trial of digital cognitive behavioural therapy (dCBT) for insomnia. BMJ Open 2023; 13: e071764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Wardlaw JM, Woodhouse LJ, Mhlanga II, et al. Isosorbide mononitrate and cilostazol treatment in patients with symptomatic cerebral small vessel disease: the lacunar intervention trial-2 (LACI-2) randomized clinical trial. JAMA Neurol 2023; 80: 682–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Sami MB, Faruqui R. The effectiveness of dopamine agonists for treatment of neuropsychiatric symptoms post brain injury and stroke. Acta Neuropsychiatr 2015; 27: 317–326. [DOI] [PubMed] [Google Scholar]
- 136. Legg LA, Rudberg AS, Hua X, et al. Selective serotonin reuptake inhibitors (SSRIs) for stroke recovery. Cochrane Database Syst Rev 2021; 11: CD009286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Fava-Felix PE, Bonome-Vanzelli SRC, Ribeiro FS, et al. Systematic review on post-stroke computerized cognitive training: unveiling the impact of confounding factors. Front Psychol 2022; 13: 985438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Zhou L, Jin Y, Wu D, et al. Current evidence, clinical applications, and future directions of transcranial magnetic stimulation as a treatment for ischemic stroke. Front Neurosci 2023; 17: 1177283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Wessel MJ, Zimerman M, Hummel FC. Non-invasive brain stimulation: an interventional tool for enhancing behavioral training after stroke. Front Hum Neurosci 2015; 9: 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Wang Y, Liu W, Chen J, et al. Comparative efficacy of different noninvasive brain stimulation therapies for recovery of global cognitive function, attention, memory, and executive function after stroke: a network meta-analysis of randomized controlled trials. Ther Adv Chronic Dis 2023; 14: 20406223231168754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Huang YZ, Rothwell JC, Chen RS, et al. The theoretical model of theta burst form of repetitive transcranial magnetic stimulation. Clin Neurophysiol 2011; 122: 1011–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Kricheldorff J, Göke K, Kiebs M, et al. Evidence of neuroplastic changes after transcranial magnetic, electric, and deep brain stimulation. Brain Sci 2022; 12: 929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Nitsche MA, Cohen LG, Wassermann EM, et al. Transcranial direct current stimulation: state of the art 2008. Brain Stimul 2008; 1: 206–223. [DOI] [PubMed] [Google Scholar]
- 144. Cheng K, Wang Z, Bai J, et al. Research advances in the application of vagus nerve electrical stimulation in ischemic stroke. Front Neurosci 2022; 16: 1043446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Hankey G, Hackett M, Almeida O, et al. Twelve-month outcomes of the AFFINITY trial of fluoxetine for functional recovery after acute stroke: AFFINITY trial steering committee on behalf of the AFFINITY trial collaboration. Stroke 2021; 52: 2502–2509. [DOI] [PubMed] [Google Scholar]
- 146. Narme P, Roussel M, Mouras H, et al. Does impaired socioemotional functioning account for behavioral dysexecutive disorders? Evidence from a transnosological study. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 2017; 24: 80–93. [DOI] [PubMed] [Google Scholar]
- 147. Gong C, Hu H, Peng XM, et al. Therapeutic effects of repetitive transcranial magnetic stimulation on cognitive impairment in stroke patients: a systematic review and meta-analysis. Front Hum Neurosci 2023; 17: 1177594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Gao Y, Qiu Y, Yang Q, et al. Repetitive transcranial magnetic stimulation combined with cognitive training for cognitive function and activities of daily living in patients with post-stroke cognitive impairment: a systematic review and meta-analysis. Ageing Res Rev 2023; 87: 101919. [DOI] [PubMed] [Google Scholar]
- 149. Arheix-Parras S, Barrios C, Python G, et al. A systematic review of repetitive transcranial magnetic stimulation in aphasia rehabilitation: leads for future studies. Neurosci Biobehav Rev 2021; 127: 212–241. [DOI] [PubMed] [Google Scholar]
- 150. HAS. AVC. Premières recommandations sur la rééducation à la phase chronique. Haute Autorité de Santé. 2022. Accessed November 19, 2023. https://www.has-sante.fr/jcms/p_3344372/fr/avc-premieres-recommandations-sur-la-reeducation-a-la-phase-chronique
- 151. Chu M, Zhang Y, Chen J, et al. Efficacy of intermittent theta-burst stimulation and transcranial direct current stimulation in treatment of post-stroke cognitive impairment. J Integr Neurosci 2022; 21: 130. [DOI] [PubMed] [Google Scholar]
- 152. Li W, Wen Q, Xie YH, et al. Improvement of poststroke cognitive impairment by intermittent theta bursts: a double-blind randomized controlled trial. Brain Behav 2022; 12: e2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Begemann MJ, Brand BA, Ćurčić-Blake B, et al. Efficacy of non-invasive brain stimulation on cognitive functioning in brain disorders: a meta-analysis. Psychol Med 2020; 50: 2465–2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Khan A, Yuan K, Bao SC, et al. Can transcranial electrical stimulation facilitate post-stroke cognitive rehabilitation? A systematic review and meta-analysis. Front Rehabil Sci 2022; 3: 795737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Yan RB, Zhang XL, Li YH, et al. Effect of transcranial direct-current stimulation on cognitive function in stroke patients: a systematic review and meta-analysis. PLoS One 2020; 15: e0233903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Han K, Liu J, Tang Z, et al. Effects of excitatory transcranial magnetic stimulation over the different cerebral hemispheres dorsolateral prefrontal cortex for post-stroke cognitive impairment: a systematic review and meta-analysis. Front Neurosci 2023; 17: 1102311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Hu XY, Zhang T, Rajah GB, et al. Effects of different frequencies of repetitive transcranial magnetic stimulation in stroke patients with non-fluent aphasia: a randomized, sham-controlled study. Neurol Res 2018; 40: 459–465. [DOI] [PubMed] [Google Scholar]
- 158. Biou E, Cassoudesalle H, Cogné M, et al. Transcranial direct current stimulation in post-stroke aphasia rehabilitation: a systematic review. Ann Phys Rehabil Med 2019; 62: 104–121. [DOI] [PubMed] [Google Scholar]
- 159. Hu AM, Huang CY, He JG, et al. Effect of repetitive transcranial magnetic stimulation combined with transcranial direct current stimulation on post-stroke dysmnesia: a preliminary study. Clin Neurol Neurosurg 2023; 231: 107797. [DOI] [PubMed] [Google Scholar]
- 160. Sachdev P, Kalaria R, O’Brien J, et al. Diagnostic criteria for vascular cognitive disorders: a VASCOG statement. Alzheimer Dis Assoc Disord 2014; 28: 206–218. [DOI] [PMC free article] [PubMed] [Google Scholar]