Abstract
Introduction:
Patients with acute ischemic stroke (AIS) and large-vessel occlusion are frequently transferred by emergency physicians (EPs) from primary to comprehensive stroke centers (CSC) for thrombectomy, particular when thrombolysed. Data on complications during such transfers are highly limited.
Patients and methods:
Consecutive AIS patients transferred between 01/2015 and 10/2021 to our CSC were included. Associations of major (MACO) and minor (MICO) complications with clinical and imaging data were assessed.
Results:
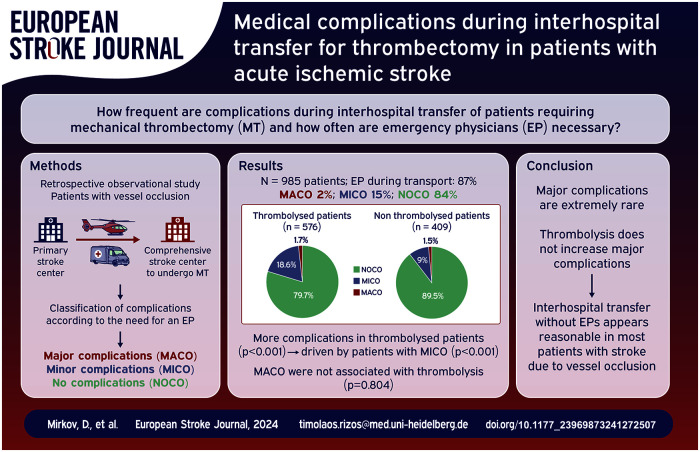
In total, 985 patients were included in the analysis (58.5% thrombolysed). MACO developed in 1.6%, MICO in 14.6%. Compared to patients without complications (NOCO), patients with MACO did not differ in terms of demographics, cerebrovascular risk factors, or site of vessel occlusion. They had more severe strokes (p = 0.026), neurological worsening was more severe (p = 0.008), and transport duration was longer (p = 0.050) but geographical distances did not differ. Thrombolysed patients had any complication more often than patients without thrombolysis (20.3% vs 10.5%; p < 0.001); however, this finding was driven by patients with MICO (p < 0.001) only (MACO: p = 0.804). No associations were observed between stroke severity and complications in either thrombolysed or nonthrombolysed patients. Neurological deterioration during transfer was observed in 21.2%, but multivariate analysis revealed no association with thrombolysis (OR 0.962; 95%CI 0.670–1.380, p = 0.832). Asymptomatic intracerebral hemorrhage was present in 1.1%, symptomatic in 0.1%.
Discussion and conclusion:
In this large cohort, no patient-specific factor increasing the risk of complications during interhospital transfer was identified. Specifically, our results do not indicate that thrombolysis increases MACO. Hence, interhospital transfer without EPs appears reasonable in most patients.
Keywords: Ischemic stroke, TIA, interhospital transfer, emergency medicine, paramedics, stroke unit
Graphical abstract.
Introduction
Endovascular stroke thrombectomy (EVT) represents a cornerstone in the management of patients who suffer acute ischemic stroke (AIS). 1 Its effectiveness, with and without additional intravenous thrombolysis, in patients with large-vessel occlusions (LVO) has been proven in numerous studies.2,3 Primary stroke centers (PSC), established to ensure access to acute stroke care and intravenous thrombolysis in large, populated areas, are, however, typically not equipped to perform EVT. 4 The predictive value of models to bypass PSCs in AIS patients in whom LVO is suspected remains limited and these models have not been established in many regions. 5 Hence, LVO patients identified at PSCs commonly must be transferred to larger comprehensive stroke centers (CSC) when EVT is indicated. 6
It is well known that shorter time intervals between onset of symptoms and both start of systemic intravenous thrombolysis (IVT) and EVT are associated with better clinical outcome.1,7 As a consequence, immediate and rapid interhospital transfer between PSCs and CSCs is crucial for LVO patients. This is underscored by the observation that prolonged transport times of stroke patients to and between hospitals represent major causes of treatment delays.8–10
Two transport modalities are available for these patients in Germany and most other European countries: transport accompanied either by paramedics only or by an additional emergency physician (EP). In particular, patients in whom IVT is administered are most commonly accompanied by an additional EP to ensure optimal safety, 11 even when respiratory and circulatory systems in these patients are deemed to be “stable.” However, in addition to the higher costs for transfers with EP, considerable time delays may result from these transfers due to the “rendezvous” between the actual transport and the physicians’ vehicles at the referring PSC and the limited availability of EPs. Unfortunately, only very few studies have explored complication rates in LVO patients during interhospital transfer.8,12–14 Thus, it remains unknown how often EPs need to perform medical interventions during these interhospital transfers. Moreover, to the best of our knowledge, clinical scores to help decide which type of transport should be chosen do not exist so far.
Here, we aimed to investigate complications during interhospital transfers between PSCs and a high-volume CSC in a large cohort of consecutive AIS patients with vessel occlusions to evaluate whether specific criteria would increase the risk of major medical complications during interhospital transports and to analyze whether interhospital transfer without an EP could be appropriate for patients with AIS.
Patients and methods
Study design and modalities of patient transfer
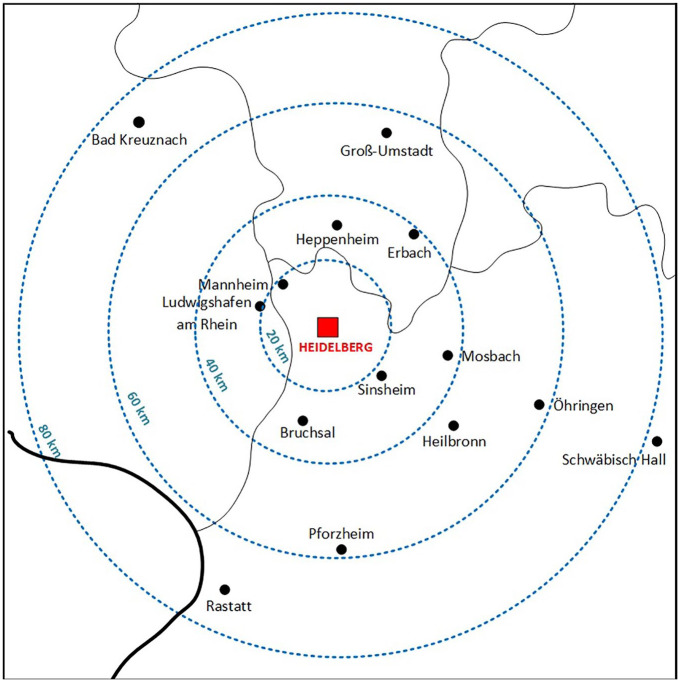
In this retrospective observational cohort study, we included consecutive AIS patients (⩾18 years) transferred between January 2015 and October 2021 from PSCs to the CSC at Heidelberg University Hospital for EVT. This high-volume EVT site is the coordinating center of a supraregional stroke network (FAST; www.fast-schlaganfall.de), covering a population of approximately 2.5 million people. Figure 1 illustrates the geographical distribution and distances between the CSC and referring PSCs.
Figure 1.
Study region in southwest Germany.
Note: Red box: comprehensive stroke center (CSC), black dots: primary stroke centers (PSC) that transferred patients for endovascular thrombectomy. Thin black line: federal state borders within Germany; thick black line: German border; blue dashed concentric circles: air distance between CSC and PSCs (kilometers, km).
Initial neurological examination, brain and vessel imaging, and, if indicated, initiation of IVT was performed at the PSCs in accordance with current guidelines. The decision for EVT was made by neurologists and neuroradiologist at the CSC following current guidelines and local standard operating procedures (SOPs). 15
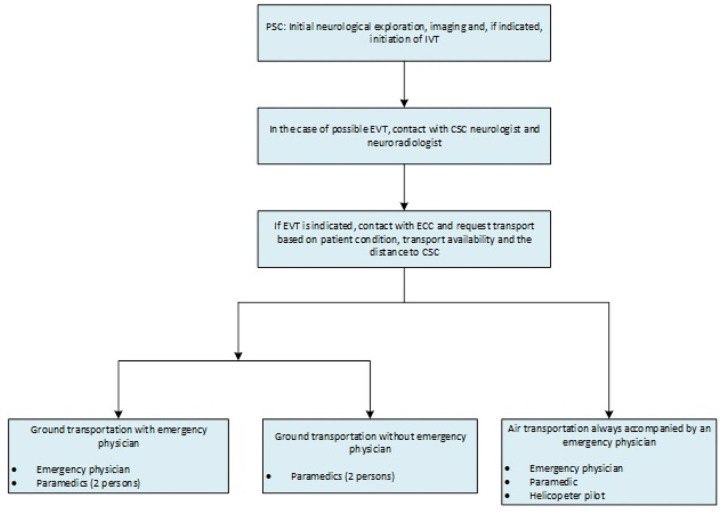
Thereafter, patients were transferred as rapidly as possible from the PSC to the CSC. Type of transportation (ground or air) depended on availability and the distance from the CSC and was set after consultation between the PSC and the rescue coordination center. Patients transported by air were always accompanied by a paramedic and an EP; patients transported by ground were accompanied either by paramedics or with an additional EP. Treating physician at the referring PSCs or/and the neurological consultant of the network determined whether an additional EP was needed for ground transport based on the patient’s condition. Figure 2 displays the decision chain regarding transport modalities.
Figure 2.
General transport modalities of patient transfers between PCS and CSC.
IVT: intravenous systemic thrombolysis; EVT: endovascular stroke thrombectomy; PSC: primary stroke center; CSC: comprehensive stroke center; ECC: emergency control center.
After arrival at our CSC, neurological follow-up examination and follow-up imaging were performed (either CCT, MRI, or DYNA CT). Type of follow-up imaging was not prespecified. Thereafter, EVT was initiated after reaching interdisciplinary consensus.
Data collection
All medical documentation from the PSCs, routine transport protocols fed to the CSC, as well as clinical and imaging data at the CSC were routinely collected. Baseline demographic parameters and stroke-specific variables, including common stroke risk factors, were extracted. Severity of stroke was measured by using the National Institutes of Health Stroke Scale (NIHSS). Transport times, vital signs, and complications during transport were extracted from transport protocols.
All transport complications were classified into major (MACO) or minor (MICO), depending on the medical severity and the necessity for physician intervention and were based on a predefined list. Resuscitation, circulatory or respiratory failure, intubation, status epilepticus, unconsciousness, tachycardia (bpm > 130/min), bradycardia (bpm < 40/min), and severe bleeding identified during transport were defined as MACO. Non-life-threatening conditions, including hypertension, hypotension without circulatory failure, mild hypoxia without respiratory failure, vomiting, nausea, and agitation were defined as MICO (Supplemental Table 1). In addition, we reviewed all imaging data upon arrival at the CSC and before EVT to identify any new intracerebral hemorrhage (ICH). These ICHs were classified as (a) symptomatic ICH and (b) asymptomatic ICH, including hemorrhagic transformations of infarcted regions. Neurological deterioration was defined as a NIHSS difference of ⩾4 between PSC and CSC.
Patients with missing transport protocols, without imaging at PSCs, without vessel occlusions upon imaging at PSCs, and for whom medical documentation was incomplete were excluded. All patients for whom an additional EP was clinically mandatory for transport before leaving the PSC (e.g. mechanical ventilation) were also not included in the further analyses.
Statistical analysis
Descriptive data are presented as absolute and relative frequencies, ordinal and continuous data as medians and interquartile ranges (IQR). Differences between groups were examined by univariate nonparametric tests. Logistic multivariate regression was used to analyze neurological deterioration in relation to demographic and clinical variables. A two-sided p value of <0.05 was considered as exploratively significant. Data were analyzed by using the Statistical Package for the Social Sciences (SPSS 29.0).
The manuscript was developed according to STROBE guidelines for reporting secondary data. 16 This study was performed in line with the principles of the Declaration of Helsinki.
Results
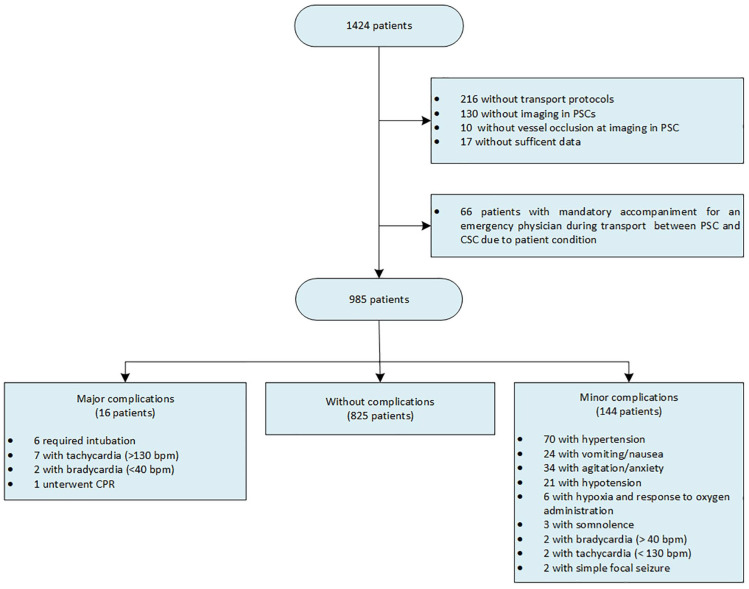
During the study period, 1424 patients with AIS were transferred from PSCs to the CSC. After excluding patients without transport protocols (N = 216), without imaging at the PSC (N = 130), without vessel occlusion at the PSC (N = 10), without sufficient data (N = 17), and with a clinical need for an additional, accompanying EP before leaving the PSC (N = 66), the final sample comprised 985 patients (Figure 3). Basic demographic data, cerebrovascular risk factors, and stroke-specific findings are outlined in Table 1. The majority of patients included were female (53.8%), median age was 77 years, median NIHSS at admission at the PSC was 14, and 576 patients (58.5%) received thrombolysis before or during transport. Most patients were transferred from PSCs to the CSC by ground transport (70.2%); EPs accompanied 87.3% of transports (Table 1). Median transport time was 38 min (IQR: 30–49.25).
Figure 3.
Flow chart representing patient inclusion and transport complications.
Note: >1 complications were recorded in single patients.
PSC: primary stroke center; CSC: comprehensive stroke center; CPR: cardiopulmonary resuscitation; bpm: beats per minute.
Table 1.
Demographic findings, cardiovascular risk factors, and stroke-specific findings and differences between patients with major, minor, and no complications during interhospital transfer.
| Variable | All patients (N = 985) | MACO (N = 16) | MICO (N = 144) | NOCO (N = 825) | Descriptive p-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| MACO vs MICO vs NOCO | MACO vs NOCO | MICO vs NOCO | MACO vs MICO + NOCO | MACO + MICO vs NOCO | |||||
| Age: median (IQR) | 77 (66.5–83) | 74.5 (63.25–79.75) | 77.5 (66–82) | 77 (67–83) | 0.522 a | 0.289 a | 0.717 a | 0.280 a | 0.975 a |
| Female gender: N (%) | 530 (53.8%) | 9 (56.3%) | 74 (51.4%) | 447 (54.2%) | 0.809 b | 0.869 b | 0.535 b | 0.843 b | 0.592 b |
| Arterial hypertension: N (%) | 763 (77.5%) | 14 (87.5%) | 119 (82.6%) | 630 (76.4%) | 0.157 b | 0.298 b | 0.097 b | 0.333 b | 0.061 b |
| Diabetes: N (%) | 240 (24.4%) | 4 (25%) | 39 (27.1%) | 197 (23.9%) | 0.709 b | 1.000° | 0.408 b | 1.000 c | 0.419 b |
| Previous ischemic stroke: N (%) | 225 (22.8 %) | 3 (18.8%) | 32 (22.2%) | 190 (23%) | 0.905 b | 1.000 c | 0.831 b | 1.000 c | 0.750 b |
| Coronary artery disease: N (%) | 263 (26.7%) | 3 (18.8%) | 29 (20.1%) | 231 (28%) | 0.111 b | 0.577 c | 0.049 b | 0.580 c | 0.036 b |
| Atrial fibrillation: N (%) | 288 (29.2%) | 6 (37.5%) | 29 (20.1%) | 253 (30.7%) | 0.029 b | 0.558 b | 0.013 b | 0.464 b | 0.025 b |
| CHA2-DS2-VASc Score in AF patients: Median (IQR) | 5 (3–6) | 3.5 (3–4) | 5 (3–6) | 5 (4–6) | 0.142 a | 0.052 a | 0.641 a | 0.055 a | 0.238 a |
| Oral anticoagulation: N (%) | 205 (20.8%) | 5 (31.3%) | 21 (14.6%) | 179 (21.7%) | 0.089 b | 0.360 b | 0.335 b | 0.300 b | 0.120 b |
| Antihypertensive medication: N (%) | 775 (78.7%) | 15 (93.8%) | 118 (81.9%) | 642 (77.8%) | 0.179 b | 0.127 b | 0.267 b | 0.138 b | 0.134 b |
| Respiratory diseases: N (%) | 117 (11.9%) | 4 (25%) | 16 (11.1%) | 97 (11.8%) | 0.256 b | 0.115 c | 0.824 b | 0.111 c | 0.791 b |
| History of smoking: N (%) | 136 (13.8%) | 1 (6.3%) | 19 (13.2%) | 116 (14.1%) | 0.651 b | 0.712 c | 0.782 b | 0.712 c | 0.600 b |
| Wake up stroke: N (%) | 185 (18.8%) n/N = 960/985 |
3 (18.8%) | 19 (13.2%) n/N = 143/144 |
163 (19.8%) n/N = 801/825 |
0.143 b | 0.872 b | 0.049 b | 1.000 c | 0.057 b |
| IVT: N (%) | 576 (58.5%) | 10 (62.5%) | 107 (74.3%) | 459 (55.6%) | <0.001 b | 0.584 b | <0.001 b | 0.742 b | <0.001 b |
| Antiemetics treatment prior to IVT: N (%) | 18 (3.1%) n/N = 563/576 |
2 (20%) | 5 (4.7%) | 11 (2.4%) n/N = 446/459 |
0.014 c | 0.030 c | 0.221 b | 0.037 c | 0.054 b |
| Antihypertensive treatment prior to IVT: N (%) | 97 (16.8%) n/N = 563/576 |
3 (30%) | 37 (34.6%) | 57 (12.4%) n/N = 446/459 |
<0.001 b | 0.132 c | <0.001 b | 0.388 c | <0.001 b |
| NIHSS at PSC: median (IQR) | 14 (8–18) n/N = 956/985 |
18 (13–21) n/N = 15/16 |
14 (8–18) n/N = 138/144 |
14 (8–18) n/N = 803/825 |
0.081 a | 0.026 a | 0.703 a | 0.027 a | 0.316 a |
| NIHSS at CSC: median (IQR) | 15 (9–20) | 20 (18–37) | 15 (10–21) | 15 (9–20) | 0.001 a | <0.001 a | 0.163 a | <0.001 a | 0.020 a |
| Neurological deterioration during transport (NIHSS difference ⩾ 4): N (%) | 209 n/N = 956/985 |
8 (50%) n/N = 15/16 |
34 (23,6%) n/N = 138/144 |
167 (20,2%) n/N = 803/825 |
0.007 b | 0.006 c | 0.313 b | 0.007 c | 0.070 b |
| NIHSS difference between PSC and CSC: Mean (IQR) | 1.05 (−0.75–3) n/N = 956/985 |
6 (0–14) n/N = 15/16 |
1.77 (0–3) n/N = 138/144 |
0.83 (−1–3) n/N = 803/825 |
0.013 a | 0.008 a | 0.147 a | 0.010 a | 0.033 a |
| Anterior circulation occlusion: N (%) | 892 (90.6%) | 14 (87.5%) | 125 (86.8%) | 753 (91.3%) | 0.219 b | 0.644 c | 0.090 b | 0.657 c | 0.082 b |
| Posterior circulation occlusion: N (%) | 93 (9.4%) | 2 (12.5%) | 19 (13.2%) | 72 (8.7%) | |||||
| Medium-vessel occlusion: N (%) | 200 (20.3%) | 2 (12.5%) | 32 (22.2%) | 166 (20.1%) | 0.623 b | 0.751 c | 0.564 b | 0.753 c | 0.745 b |
| Large-vessel occlusion: N (%) | 785 (79,7%) | 14 (87.5%) | 111 (77.1%) | 659 (79.9%) | |||||
| Transport with emergency physician: N (%) | 860 (87.3%) | 16 (100%) | 136 (94.4%) | 708 (85.8%) | 0.005 b | 0.104 b | 0.004 b | 0.124 b | 0.001 b |
| Ground transport: N (%) | 691 (70.2%) | 12 (75%) | 96 (66.7%) | 583 (70.7%) | 0.571 b | 0.706 b | 0.333 b | 0.789 c | 0.423 b |
| Air transport: N (%) | 294 (29.8%) | 4 (25%) | 48 (33.3%) | 242 (29.3%) | |||||
| Transport duration (min): median (IQR) | 38 (30–49.25) n/N = 918/985 |
45.5 (33.75–70) | 39 (33.25–49) n/N = 136/144 |
37 (30–49) n/N = 766/825 |
0.028 a | 0.050 a | 0.054 a | 0.063 a | 0.017 a |
| Ground distance: km (median IQR) | 54.8 (35.9–83.0) | 64.7 (35.9–83.0) | 67.4 (35.9–83.0) | 54.8 (35.9–83.0) | 0.544 b | 0.892 b | 0.277 b | 0.847 b | 0.326 a |
| Air distance: km (median, IQR) | 36.0 (24.0–59.0) | 45.5 (21.0–63.5) | 48.0 (24.0–59.0) | 36.0 (24.0–59.0) | 0.519 b | 0.757 b | 0.277 b | 0.716 b | 0.351 a |
N: number; IQR: interquartile range; IVT: intravenous thrombolysis; AF: atrial fibrillation; rtPA: recombinant tissue-type plasminogen activator; NIHSS: national institutes of health stroke scale; CSC: comprehensive stroke center; PSC: primary stroke center; MACO: major complications; MICO: minor complications; NOCO: no complications. p values in bold are statistically significant.
Kruskal-Wallis test.
Chi-squared test.
Fisher’s test.
Medical complications during interhospital transfer
In total, medical complications developed in 160 patients during interhospital transfer (16.2%). Details of all medical complications are outlined in Figure 3. Most registered complications were MICO (144 patients; 14.6%); MACO were present in 16 patients (1.6%; Figure 3 and Table 1). Angioedema was not observed in any patient.
Overall, no difference was observed between ground and air transport in regard to complications (p = 0.468, Supplemental Table 3). Differences between patients with MACO and those without complications (NOCO) are given in Table 1. No differences were observed with regard to demographic data, cerebrovascular risk factors, thrombolytic treatment, and site of vessel occlusions. However, patients with MACO more often received antiemetics prior to thrombolysis (p = 0.030), they were more severely affected by their stroke (median NIHSS 18 vs 14; p = 0.026), and neurological worsening during transport was more severe than in NOCO patients (p = 0.008). Transport duration was longer in patients with MACO (p = 0.050) but geographical distances between PSC and CSC did not differ (p = 0.892 and 0.757, respectively) (Table 1).
Differences between MICO and NOCO patients are also outlined in Table 1. Coronary artery disease, atrial fibrillation, and wake-up stroke situations were less often observed in patients with MICO (p = 0.049, p = 0.013, and p = 0.049 respectively). On the other hand, these patients were more often treated with thrombolysis (p < 0.001) and antihypertensives prior to thrombolysis (p < 0.001) and they were more often transported with an EP (p = 0.004). No differences with regard to transport duration or geographical distances was observed.
Table 1 also outlines differences between patients with any complication during interhospital transfer (major or minor) and without complications. Patients with any complication were more often treated with thrombolysis (p < 0.001), they were more often treated with antihypertensives prior to thrombolysis (p < 0.001), and they were more often transported with an EP (p = 0.001). Moreover, transport duration was longer (p = 0.017) but geographical distances between PSC and CSC did not differ.
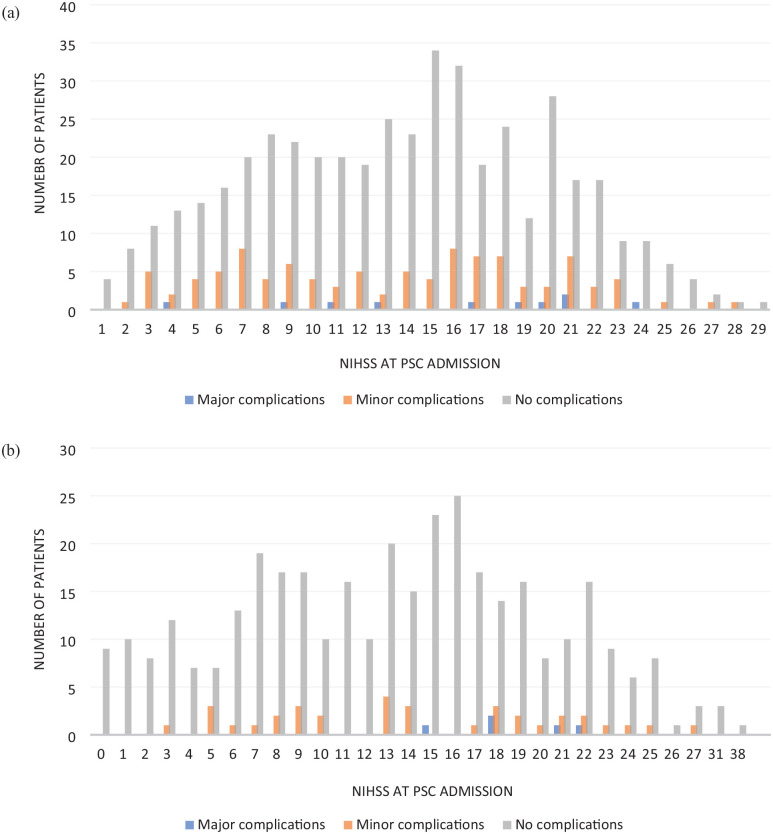
Overall, patients treated with thrombolysis were more often accompanied by an EP during transfer (535/576 vs 325/409, p < 0.001). Thrombolysed patients more often developed any medical complication during transport than patients without thrombolysis (20.3% vs 10.5%; p < 0.001). However, this result was driven by those MICO patients (p < 0.001) only; MACO were not observed more often in thrombolysed patients (p = 0.804). In addition, neither in thrombolysed nor in nonthrombolysed patients were significant associations observed between stroke severity and medical complications during transport (data not shown, Figure 4).
Figure 4.
Distribution of patient numbers and NIHSS values with regard to complications during transfer in all thrombolysed (a) and nonthrombolysed (b) patients. Neither in thrombolysed nor in nonthrombolysed patients were associations observed between stroke severity and medical complications during transport: (a) all thrombolysed patients (N = 576); (b) all nonthrombolysed patients (N = 409).
IVT: intravenous thrombolysis; PSC: primary stroke center; CSC: comprehensive stroke center; N: number of patients; NIHSS: National Institutes of Health Stroke Scale.
In 209 patients (21.2%), neurological deterioration was observed during transport. This was associated with previous stroke (p = 0.044, OR 1.535; 95%CI 1.012–2.328), atrial fibrillation (p = 0.032 OR 1.511; 95%CI 1.036–2.204), and LVO (p = 0.029, OR 1.631; 95%CI 1.052–2.528) in multivariate analysis (Table 2). No association with thrombolysis was present (p = 0.832, OR 0.962; 95%CI 0.670–1.380).
Table 2.
Multivariate logistic regression between neurological deterioration (NIHSS difference between PSC and CSC ⩾ 4) and demographic and clinical variables (n = 209, 21.2%).
| Variable | OR | 95%CI | p-value |
|---|---|---|---|
| Age | 1.001 | 0.985–1.016 | 0.936 |
| Gender | 1.038 | 0.745–1.445 | 0.827 |
| Arterial hypertension | 0.991 | 0.606–1.621 | 0.971 |
| Diabetes | 1.064 | 0.722–1.568 | 0.755 |
| Coronary artery disease | 1.116 | 0.757–1.645 | 0.581 |
| Previous ischemic stroke | 1.535 | 1.012–2.328 | 0.044 |
| Atrial fibrillation | 1.511 | 1.036–2.204 | 0.032 |
| Peripheral artery disease | 0.612 | 0.298–1.258 | 0.182 |
| History of smoking | 1.006 | 0.602–1.682 | 0.981 |
| Antihypertensive medication | 0.767 | 0.463–1.269 | 0.302 |
| Respiratory disease | 0.961 | 0.578–1.596 | 0.876 |
| Wake-up stroke | 0.935 | 0.603–1.449 | 0.764 |
| IVT | 0.962 | 0.670–1.380 | 0.832 |
| LVO | 1.631 | 1.052–2.528 | 0.029 |
| Posterior circulation stroke | 1.039 | 0.599–1.802 | 0.892 |
| EP-led transport | 1.552 | 0.884–2.724 | 0.126 |
| Ground/air transport | 1.049 | 0.736–1.495 | 0.79 |
NIHSS: national institutes of health stroke scale; OR: odds ratio; CI: confidence interval; IVT: intravenous thrombolysis; LVO: large-vessel occlusion; EP: emergency physician. p values in bold are statistically significant.
Follow-up imaging at arrival
Follow-up imaging at CSC arrival prior to EVT was performed in 84.9% of patients (N = 836). Here, hemorrhagic transformation or asymptomatic ICH was observed in 11 (1.1%) and symptomatic ICH in one patient (0.1%). This 83-year-old male patient was treated with IVT 2 h 22 min after observed onset of stroke due to an M2 occlusion (NIHSS at PSC: 2). Medication at home consisted of 100 mg aspirin once daily. No contraindication concerning thrombolysis was present, and all laboratory values were normal. Atrial fibrillation was newly diagnosed at the PSC. During transport (accompanied by an EP), neurological symptoms worsened markedly but no hypertensive derailment and no cardiorespiratory instability were present. NIHSS at CSC arrival was 19; severe space-occupying ICH with intraventricular bleed was identified. No EVT was performed and the patient died during the further course. There was no death during transport from the PSC to the CSC.
Discussion
The main findings of our study are: (1) no patient-specific factors increasing the risk of suffering medical complications were identified during interhospital transfer between PSCs and the CSC; (2) despite a large sample size, MACO during transfer were only rarely observed (1.6%); and (3) our data do not indicate that IVT would represent a relevant factor for MACO during interhospital transfers of AIS patients with LVO. We observed that patients with MACO were more severely affected by their stroke. However, the overall number of MACO was very low and, while no significant associations were observed between stroke severity and medical complications during transport, no specific NIHSS value could be established that would help in selecting patients at risk of developing transport complications.
To the best of our knowledge, this study reports the largest consecutive sample of AIS patients transferred between PSCs to a high-volume CSC for EVT to date. Hence, comparability to other studies is limited. In a study of 377 patients 14 evaluating complications during interhospital transfer within a German stroke network, no major medical complications were observed. However, 10% of patients required medical interventions, defined as any intravenous medication being required during transfer. 14 On the other hand, an even smaller report that evaluated complications during interhospital transfer in 253 patients described a higher frequency of any complication during transport than in the present study (26.9% vs 16.2%) and life-threatening complications in 4.3%. 12 Differences in particular with regard to non-life-threatening complications between these reports and our findings may be a consequence of allocation to MACO and MICO, respectively. For example, we did not define the need of any i.v. treatment as a MACO because paramedics are usually allowed to administer antihypertensives or antiemetics themselves, at least in our region. Notably, arterial hypertension, nausea/vomiting, and agitation were the most frequent MICO during transfer both in the report of Pallesen and coworkers and the present study. These findings emphasize that additionally deploying EPs is potentially unnecessary in many cases if paramedics are adequately trained and permitted to administer basic i.v. medications during interhospital transfer of AIS patients.
We found no association between stroke severity and risk for MICO. This is in line with the report of Pallesen and colleagues; stroke severity at PSC and at arrival at CSC was not associated with the need for medical interventions during transfer. 14 However, neurological deterioration according to usual definitions (NIHSS difference ⩾ 4) 17 was reported in 21.2% of our patients and was therefore higher than in the aforementioned study that applied NIHSS differences > 4 (N = 38, 11.1%). 14 Importantly and in line with the findings of the much smaller cohort by Leibinger and colleagues, 13 we did not observe a higher risk of clinical deterioration in case of thrombolysis in multivariate analysis (p = 0.832).
On the other hand, we observed that patients with documented MICO were treated with thrombolysis more often (p < 0.001). Explaining this finding remains speculative. Due to the observation that patients with MICO were more often transported with EPs than patients without any complications, differences in the perception of medical complications between paramedics and physicians or more precise documentation particularly in patients with ongoing thrombolysis may contribute to this result. Studying possible differences in the perception of transport complications in AIS patients could therefore be a future focus of research.
Leibinger and coworkers reported that basilar occlusions were significantly associated with transport complications 13 whereas we did not find associations between location or size of vessel occlusions or clinical deterioration during interhospital transfer. It is worthy of mention that comparability between these studies is highly limited due to different inclusion criteria. While we aimed to evaluate not only transport complications, but also the actual need for an additional EP to transfer AIS patients from PSCs to CSC in patients deemed to be “stable” with regard to their respiratory and circulatory systems, we excluded patients in whom an additional EP was clinically mandatory at the time of leaving the PSC (e.g. mechanical ventilation). Interestingly, two thirds of these excluded patients (N = 41/66; 62.1%) suffered basilar occlusions and their clinical condition definitely required the support of an accompanying EP already at the PSCs. Hence, the condition of patients with basilar occlusions frequently appears to require an additional EP already at the time of leaving the PSC.
Patients included in our analysis whose clinical condition did not definitely require EP support were nevertheless very frequently accompanied by an additional EP during transport, most likely due to concerns about feared complications particularly in thrombolysed patients. However, as demonstrated by our data, MACO were extremely rare (1.6%) and we did not find that IVT would represent a relevant factor for MACO. Considering the significantly higher costs of transfers with an additional EP, potentially significant time delays in case of “rendezvous” systems and nonavailability of the respective EPs for primary rescue missions during these transports, our data suggest that interhospital transfer without additional EPs is reasonable in most patients.
As specific factors that increase the risk of MACO in particular during interhospital transfer between PSCs and CSCs could not be identified in our cohort, we cannot provide clear recommendations that would unequivocally help select patients requiring EP-accompanied transport. On the other hand, our data support careful training of paramedics, including the administration of basic i.v. medications, so that they can manage most MICO during interhospital transfer of AIS patients with LVOs, irrespective of previous or ongoing thrombolysis, especially in German emergency medical services with a high number of preclinical emergency physicians compared to most other countries.
Recently, tenecteplase (TNK) was approved for stroke treatment by the EMA, including in patients with LVO that need to be transferred from PSCs to thrombectomy centers. We are convinced that the results of our study indicate that it is feasible to transport the majority of these patients without additional EPs in most cases, especially if IVT can then be performed with a single bolus injection, which is the case for TNK. Positive impacts on clinical outcomes or EP shortages still need to be evaluated, however.
An obvious strength of the present study is the large number of patients included and meticulous analysis of complications and their potentially associated demographic and clinical factors. Transport modalities are comparable at least in most regions of Germany, but generalizing our results to other regions might be restricted due to different geographical and organizational factors. Moreover, we had to exclude almost one third of patients transferred during the study period, predominantly due to inadequate transport documentation. The retrospective design may further limit our results, and we cannot exclude the possibility that unmeasured variables may influence medical complications during interhospital transfer of AIS patients with LVO.
Conclusion
Our study confirms that MACO are extremely rare during interhospital transfer of AIS patients and specific interventions by EPs are seldom required. Moreover, no patient-specific pattern could be detected that increases the risk of complications during transfer, including systemic thrombolysis. Our data suggest that interhospital transfer without additional EPs is reasonable in most patients with AIS and LVO, at least in our region.
Supplemental Material
Supplemental material, sj-docx-1-eso-10.1177_23969873241272507 for Medical complications during interhospital transfer for thrombectomy in patients with acute ischemic stroke by Damjan Mirkov, Ekkehart Jenetzky, Andrea S. Thieme, Adeeb Qabalan, Christoph Gumbinger, Wolfgang Wick, Peter A. Ringleb and Timolaos Rizos in European Stroke Journal
Acknowledgments
None
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Damjan Mirkov declares no conflicts of interest in relation to this work.
Ekkehart Jenetzky declares no conflicts of interest in relation to this work.
Andrea Thieme declares no conflicts of interest in relation to this work.
Adeeb Qabalan declares no conflicts of interest in relation to this work.
Christoph Gumbinger declares no conflicts of interest in relation to this work.
Wolfgang Wick declares no conflicts of interest in relation to this work.
Peter A. Ringleb received lecture honoraria from Boehringer Ingelheim, Bayer, PFIZER and Daiichi Sankyo and for advisory board activities from Boehringer Ingelheim and Pfizer not in relation to the topics of this publication.
Timolaos Rizos received consulting honoraria, speakers’ honoraria and travel support from BMS Pfizer, Boehringer Ingelheim, Bayer HealthCare and Daiichi Sankyo, outside the submitted work.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: Ethical approval for this study was obtained from the independent ethics committee of the Heidelberg University, Medical Faculty (S−762/2021).
Informed consent: Informed consent was not sought for the present study because of the study design.
Guarantor: Timolaos Rizos
Contributionship: DM: Data acquisition, statistical analysis, and interpretation and drafting of the manuscript.
Ekkehart Jenetzky: Statistical analysis and interpretation, manuscript preparation, study supervision, and critical revision of the manuscript for important intellectual content.
AT: Data acquisition and critical revision of the manuscript for important intellectual content.
AQ: Data acquisition and critical revision of the manuscript for important intellectual content.
CG: Critical revision of the manuscript for important intellectual content.
WW: Critical revision of the manuscript for important intellectual content.
PAR: Interpretation, manuscript preparation, and critical revision of the manuscript for important intellectual content.
TR: Study concept and design, data acquisition, statistical analysis and interpretation, drafting of the manuscript, and study supervision
ORCID iDs: Damjan Mirkov  https://orcid.org/0009-0000-1948-2570
https://orcid.org/0009-0000-1948-2570
Timolaos Rizos  https://orcid.org/0000-0002-7250-4668
https://orcid.org/0000-0002-7250-4668
Supplemental material: Supplemental material for this article is available online.
References
- 1. Turc G, Bhogal P, Fischer U, et al. European Stroke Organisation (ESO) – European Society for Minimally Invasive Neurological Therapy (ESMINT) guidelines on mechanical thrombectomy in acute ischaemic stroke endorsed by Stroke Alliance for Europe (SAFE). Eur Stroke J 2019; 4: 6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goyal M, Demchuk AM, Menon BK, et al. ESCAPE trial investigators. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015; 372: 1019–1030. [DOI] [PubMed] [Google Scholar]
- 3. Mulder MJHL, Jansen IGH, Goldhoorn RB, et al. MR CLEAN registry investigators. Time to endovascular treatment and outcome in acute ischemic stroke: MR CLEAN registry results. Circulation 2018; 138: 232–240. [DOI] [PubMed] [Google Scholar]
- 4. Neumann-Haefelin T., Busse O., Faiss J, et al. Zertifizierungskriterien für stroke-units in Deutschland: Update 2022. DGNeurologie 2021; 4: 438–446 (German). [Google Scholar]
- 5. Romoli M, Paciaroni M, Tsivgoulis G, et al. Mothership versus drip-and-ship model for mechanical thrombectomy in acute stroke: a systematic review and meta-analysis for clinical and radiological outcomes. J Stroke 2020; 22: 317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu X, Wira CR, Matouk CC, et al. Drip-and-ship versus mothership for endovascular treatment of acute stroke: a comparative effectiveness analysis. Int J Stroke 2022; 17: 315–322. [DOI] [PubMed] [Google Scholar]
- 7. Berge E, Whiteley W, Audebert H, et al. European Stroke Organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur Stroke J 2021; 6: I-LXII. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ishihara H, Oka F, Oku T, et al. Safety and time course of drip-and-ship in treatment of acute ischemic stroke. J Stroke Cerebrovasc Dis 2017; 26: 2477–2481. [DOI] [PubMed] [Google Scholar]
- 9. Holodinsky JK, Patel AB, Thornton J, et al. Drip and ship versus direct to endovascular thrombectomy: the impact of treatment times on transport decision-making. Eur Stroke J 2018; 3: 126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boss EG, Bohmann FO, Misselwitz B, et al. Quality assurance data for regional drip-and-ship strategies- gearing up the transfer process. Neurol Res Pract 2021; 3: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. O’Carroll CB, Aguilar MI. Management of postthrombolysis hemorrhagic and orolingual angioedema complications. Neurohospitalist 2015; 5: 133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sablot D, Leibinger F, Dumitrana A, et al. Complications during inter-hospital transfer of patients with acute ischemic stroke for endovascular therapy. Prehosp Emerg Care 2020; 24: 610–616. [DOI] [PubMed] [Google Scholar]
- 13. Leibinger F, Sablot D, Van Damme L, et al. Which patients require physician-led inter-hospital transport in view of endovascular therapy? Cerebrovasc Dis 2019; 48: 171–178. [DOI] [PubMed] [Google Scholar]
- 14. Pallesen LP, Winzer S, Barlinn K, et al. Safety of inter-hospital transfer of patients with acute ischemic stroke for evaluation of endovascular thrombectomy. Sci Rep 2020; 10: 5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ringleb P, Köhrmann M, Hametner C, et al. Akuttherapie des ischämischen Hirninfarktes, S2- Leitlinie, Version 5.1. (German). https://www.awmf.org/leitlinien/detail/ll/030-046.html (Stand: November 2022).
- 16. Von Elm E, Altman DG, Egger M, et al. STROBE initiative. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007; 370: 1453–1457. [DOI] [PubMed] [Google Scholar]
- 17. Wahlgren N, Ahmed N, Dávalos A, et al. SITS-MOST investigators. Thrombolysis with alteplase for acute ischaemic stroke in the safe implementation of thrombolysis in stroke-monitoring study (SITS-MOST): an observational study. Lancet 2007; 369: 275–282. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-eso-10.1177_23969873241272507 for Medical complications during interhospital transfer for thrombectomy in patients with acute ischemic stroke by Damjan Mirkov, Ekkehart Jenetzky, Andrea S. Thieme, Adeeb Qabalan, Christoph Gumbinger, Wolfgang Wick, Peter A. Ringleb and Timolaos Rizos in European Stroke Journal