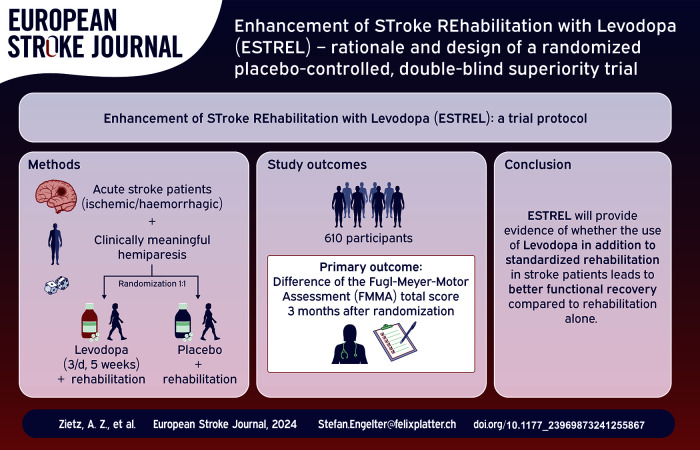
Abstract
Rationale:
Novel therapeutic approaches are needed in stroke recovery. Whether pharmacological therapies are beneficial for enhancing stroke recovery is unclear. Dopamine is a neurotransmitter involved in motor learning, reward, and brain plasticity. Its prodrug levodopa is a promising agent for stroke recovery.
Aim and hypothesis:
To investigate the hypothesis that levodopa, in addition to standardized rehabilitation therapy based on active task training, results in an enhancement of functional recovery in acute ischemic or hemorrhagic stroke patients compared to placebo.
Design:
ESTREL (Enhancement of Stroke REhabilitation with Levodopa) is a randomized (ratio 1:1), multicenter, placebo-controlled, double-blind, parallel-group superiority trial.
Participants:
610 participants (according to sample size calculation) with a clinically meaningful hemiparesis will be enrolled ⩽7 days after stroke onset. Key eligibility criteria include (i) in-hospital-rehabilitation required, (ii) capability to participate in rehabilitation, (iii) previous independence in daily living.
Intervention:
Levodopa 100 mg/carbidopa 25 mg three times daily, administered for 5 weeks in addition to standardized rehabilitation. The study intervention will be initiated within 7 days after stroke onset.
Comparison:
Matching placebo plus standardized rehabilitation.
Outcomes:
The primary outcome is the between-group difference of the Fugl-Meyer-Motor Assessment (FMMA) total score measured 3 months after randomization. Secondary outcomes include patient-reported health and wellbeing (PROMIS 10 and 29), patient-reported assessment of improvement, Rivermead Mobility Index, modified Rankin Scale, National Institutes of Health Stroke Scale (NIHSS), and as measures of harm: mortality, recurrent stroke, and serious adverse events.
Conclusion:
The ESTREL trial will provide evidence of whether the use of Levodopa in addition to standardized rehabilitation in stroke patients leads to better functional recovery compared to rehabilitation alone.
Keywords: stroke rehabilitation, neurorehabilitation, levodopa, randomized controlled trial, Fugl-Meyer-motor assessment, motor recovery, protocol
Graphical abstract.
Background and rationale
In stroke medicine, the large body of high-quality evidence proving benefits of acute revascularization therapies and secondary prevention is offset by a large gap of evidence on means to enhance stroke recovery. Levodopa as a precursor substance of dopamine is a promising candidate for the pharmacological enhancement of stroke recovery. 1 Dopamine is a key player in processes of motor learning, reward, and brain plasticity.1–4
Dopaminergic nerve terminals with dopamine receptors (D1 and D2) are present in the primary motor cortex. In rats, the inhibition or elimination of these terminals impaired motor skill acquisition and recovery. 5 Interestingly, under dopamine substitution, the motor skill learning process could be restored. 6 Dopamine may support learning and may relay reward signals to the motor cortex. 7 Next to these promising preclinical data, studies in stroke patients as well as healthy subjects support the hypothesis that neuroplasticity could be improved by levodopa. In chronic stroke patients, levodopa intake enhanced the encoding of motor memory. 8 In healthy subjects, levodopa improved motor memory levels. 9 Thus, preclinical research and studies with healthy individuals suggest that there is scope for benefit from using levodopa in addition to standardized rehabilitation therapy in stroke patients.
However, there are limited and inconsistent data from randomized controlled trials (RCTs)10–15 studying the effect of treatment with levodopa in acute and chronic stroke patients.
In a systematic review and meta-analysis conducted prior to finalizing the protocol of the ESTREL trial (i.e. protocol version of July 3rd, 2019) searching Medline, the Cochrane Library, and clinicaltrials.gov using (“stroke” AND “levodopa”) and related terms combined with standard filters for randomized controlled trials, we identified six RCTs for which data on motor outcome stratified to the type of study treatment were available.10–15
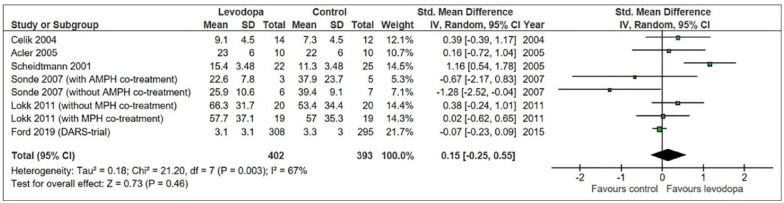
This meta-analysis suggested a – albeit statistically non-significant – more favorable outcome in levodopa-treated stroke patients than in control patients (Figure 1). However, the heterogeneity between trials was considerable. The RCTs differed regarding patient populations (chronic and acute stroke), types of strokes (ischemic and hemorrhagic), dosage and duration of levodopa treatment, length of follow-up, and outcome measures. Of note, none mentioned adaptations of concomitant rehabilitation therapies to modern forms of training and in particular the pairing of enhancing drugs with the type and amount of rehabilitation therapy, which have been shown important in pre-clinical research. 16 More importantly, there were no safety concerns related to the use of levodopa.
Figure 1.
Meta-analysis based on a systematic review dated from August 17th, 2017.
With these considerations in mind, we designed ESTREL as a randomized placebo-controlled trial to study the benefits and harms of treatment with levodopa/carbidopa in enhancing functional recovery after acute stroke.
Methods
We report this study protocol in accordance with SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) 17
Primary aim
The primary aim of the ESTREL trial is to study the benefits and harms of levodopa/carbidopa treatment in enhancing functional recovery after acute stroke.
Exploratory aims
We integrated the following nested exploratory studies in the ESTREL trial to investigate inter-individual variation in the response to levodopa/carbidopa treatment in acute stroke.
ESTREL-PRECISION aims to investigate the importance of genetic profiles on (a) outcome and (b) treatment response to levodopa.
ESTREL-BIOMARKER tests whether blood biomarkers for (a) myocardial injury and dysfunction (hs-troponinT and NT-pro-BNP) and for (b) aging processes of the vascular system (GDF-15) or of the immune system (senescence-associated secretory phenotype) as well as neurofilaments modify functional recovery in stroke patients treated with or without levodopa.
ESTREL-BENEFIT seeks to identify individual targets for personalized and novel stroke recovery treatment approaches using artificial intelligence.
ESTREL-IMAGE studies whether brain lesion characteristics modify (i) functional recovery and (ii) levodopa-treatment response for motor recovery in stroke patients.
Furthermore, we will explore the impact (i) of type and amount of rehabilitation therapies on motor recovery, providing a “dose-response-analysis” for the input of rehabilitation therapy with or without levodopa regarding motor recovery, (ii) of participation in a stroke recovery trial by comparing the outcomes of ESTREL participants with those of similarly affected patients who did not participate in ESTREL from the National (i.e. Swiss) Stroke Registry, (iii) the impact of levodopa on post-stroke fatigue or post-stroke depression, and (iv) the presence of a long-term benefit from levodopa therapy applied in addition to rehabilitation therapy.
Study design
ESTREL (Enhancement of Stroke REhabilitation with Levodopa) is an investigator-initiated clinical multicenter, placebo-controlled, double-blind, randomized (ratio 1:1), parallel-group superiority trial. The trial is conducted in certified Swiss acute care and their collaborating stroke rehabilitation centers. ESTREL uses established stroke care pathways between certified stroke units/centers (n = 13) and their partner stroke rehabilitation centers (n = 11) according to the certification criteria (https://sfcns.ch/certification/stroke).
Participants
The ESTREL trial recruits previously independent (modified Rankin Scale (mRS) ⩽3) adult patients with acute ischemic or hemorrhagic stroke (i.e. intracerebral hemorrhage excluding subarachnoid hemorrhage and cerebral venous sinus thrombosis) leading to a clinically meaningful hemiparesis that is, scoring a total of⩾3 points on the following National Institutes of Health Stroke Scale (NIHSS) items (i) motor arm, (ii) motor leg, (iii) limb ataxia (a distal arm paresis is equivalent to one of the aforementioned (i–iii)) and thus requiring in-hospital rehabilitation therapy. Patients are enrolled ⩽7 days after the onset of stroke. Table 1 lists the inclusion and exclusion criteria.
Table 1.
Inclusion and exclusion criteria of the ESTREL trial.
| Inclusion criteria |
|---|
| 1. Acute ischemic or hemorrhagic (i.e. intracerebral hemorrhage excluding subarachnoid hemorrhage and cerebral venous sinus thrombosis) stroke ⩽7 days prior to randomization |
| 2. Clinically meaningful hemiparesis (i.e. scoring a total of ⩾3 points on the following NIH stroke scale score items (i) motor arm, (ii) motor leg, (iii) limb ataxia; a distal arm paresis is equivalent to one of the aforementioned (i–iii)) |
| 3. Time of randomization ⩾24 h since thrombolysis or thrombectomy |
| 4. In-hospital rehabilitation required |
| 5. Capable to participate in a standardized rehabilitation therapy |
| 6. Informed consent of patient or next to kin |
| Exclusion criteria |
| 1. Age <18 years |
| 2. Diagnosis of Parkinson’s disease |
| 3. Use of Levodopa mandatory according to judgment of treating physician |
| 4. Inability or unwillingness to comply with study procedures including adherence to study drug intake (orally, or via nasogastric tube or percutaneous endoscopic gastrostomy tube) |
| 5. Severe aphasia (i.e. unable to follow two-stage-commands) |
| 6. Previously dependent in the basal activities of daily living (defined as modified Ranking Scale prior to stroke > 3) |
| 7. Pre-existing hemiparesis |
| 8. Known hypersensitivity to Levodopa/Carbidopa and other contraindications for Levodopa/Carbidopa as outlined in the summary of product characteristics (as appended to the study protocol). |
| 9. Woman who are pregnant or breast feeding, or who intend to become pregnant during course of the study. Women of childbearing age must take a pregnancy test to be eligible for the study. |
| 10. Lack of safe contraception, defined as: Female Participants of childbearing potential, not using and not willing to continue using a medically reliable method of contraception for the entire study duration, such as oral, injectable, or implantable contraceptives, or intrauterine contraceptive devices, or who are not using any other method considered sufficiently reliable by the Investigator in individual cases. Female Participants who are surgically sterilized/hysterectomized or postmenopausal for longer than 2 years are not considered as being of child bearing potential. |
Outcomes
Primary outcome
The primary outcome is the between-group difference in the Fugl-Meyer-Motor Assessment (FMMA) total score measured at 3 months (±14 days) after randomization. The assessment of the FMMA is performed by trained study personnel only. All trainings are provided in person by two specialized FMMA experts with an academic background and teaching experience (JH, KW). Participation is confirmed and documented. Central refresher training is offered at least annually as hands-on training or via prerecorded video instructions. In the ESTREL setting, the FMMA has shown excellent interrater reliability. 18
Secondary outcomes
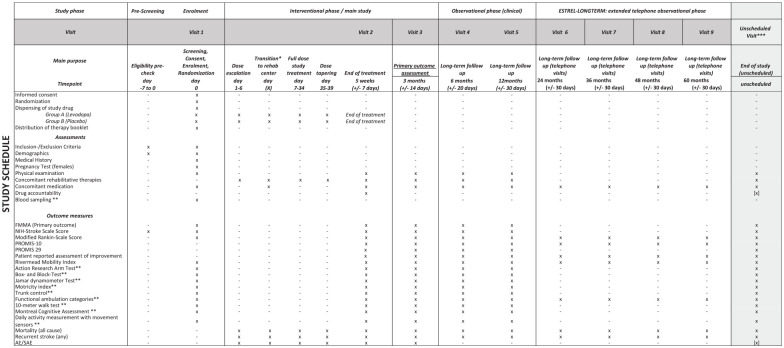
The secondary outcomes are patient-reported-outcomes-measurement-information-system (PROMIS) 10 19 and 29, 20 the patient reported assessment of improvement, FMMA (also separately for upper and lower extremity), NIHSS, modified Ranking Scale, Rivermead Mobility Index, measure at various time points as outlined in the Study Schedule (Figure 2). Further secondary outcomes are assessed in selected centers only, as outlined in the Study Schedule (Figure 2).
Figure 2.
Detailed study schedule of the ESTREL trial.
Harmful events are assessed by the following measures: In the interventional phase of the trial (i.e. ⩽3 months post-randomization) mortality (all cause), recurrent stroke (any), serious adverse events and pre-specified non-serious adverse events possibly related to the active treatment in line with the protocol of the DARS-trial 21 (also see Supplemental Table 2) are assessed. In the extended follow-up (observational phase) of the trial (i.e. 3–12 months post-randomization), mortality (all cause) and recurrent stroke (any) are assessed (Figure 2).
Randomization
Participants are randomly assigned in a 1:1 ratio either to the active treatment or the control group. Stratification is performed by center. The randomization procedure is implemented by the Clinical Trial Unit of the Department of Clinical Research of the University Basel via the Clinical Data Management Application (CDMA) secuTrial®. It includes a standard minimization algorithm which will ensure that the treatment groups are balanced within each stratum. To avoid predictable alternation of treatment allocation, and thus potential loss of allocation concealment, participants will be allocated with a probability of 80% to the treatment group that would minimize the difference between the groups within the patient’s stratum.
Blinding procedures
All investigators, study participants, care providers (i.e. therapists, physicians, nurses), outcome assessors and the study statistician will remain blinded with respect to the treatment allocation throughout the trial. The study treatment (i.e. either active treatment with levodopa/carbidopa or matching placebo) is labeled, packed, and dispensed by an independent distributor (Bichsel pharmacy). Placebo is indistinguishable (i.e. identical in aspect, texture, and taste) from the active treatment. Unblinding can be performed only by authorized investigators.
Methods of minimizing bias and improving adherence to the intervention
All outcome assessments (primary, secondary) are performed only by trained study personnel. The use of established, standardized, and validated outcome assessment tools helps to minimize bias in outcome assessment. To enhance data quality, we have (i) developed a data monitoring app as a trial governance and steering tool together with the Department of Clinical Research at the University Basel, which displays the completeness of visits and outcome data and enables to send reminders to participating centers. (ii) We regularly contact sites by virtual or in-person meetings. (iii) Data quality and completeness are continuously monitored by data validation checks and (iv) action items are decided in weekly sponsor-investigator team meetings. The inclusion criterion “inpatient rehabilitation required” was explicitly chosen to maximize participant’s adherence to the intervention by supervised and documented (in the clinical information system) application of the study medication by medical staff, as well as type and amount of rehabilitation therapies applied for each patient during in-hospital rehabilitation. (Details in Supplemental Table 1, Post-hoc Fidelity Plan According to NIH Behavior Change Consortium Treatment Fidelity Recommendations 22 ).
Independent on-site and centralized monitoring is performed by the Department of Clinical Research of the University Basel, Switzerland.
ESTREL is registered at clinicaltrials.gov (NCT03735901). No changes to initially planned outcomes were made.
Study intervention
The active treatment (i.e. investigational medicinal product) in the ESTREL trial is levodopa 100 mg/carbidopa 25 mg and is administered orally from day 1 to day 39 after randomization. The comparison group receives matching placebo. The study treatment is administered with (i) a dose escalation phase (1-0-0 on days 1–3; 1-1-0 on days 4–6), (ii) a full dose phase (1-1-1 on days 7–34) and (iii) a tapering phase (1-1-0 on days 35–37; 1-0-0 on days 38–39). Randomization to the study intervention was allowed up to 7 days after stroke onset. The study treatment can be administered via nasogastric tube or percutaneous gastrostomy feeding tube if necessary (e.g. due to a dysphagia).
In addition to the blinded study medication, all ESTREL participants receive the same standardized rehabilitation therapy based on active task-oriented training (previously referred to as the principles of motor learning), irrespective of the treatment group. The process of standardization and characterization of this type of rehabilitation therapy has been developed in collaboration with Interessengemeinschaft Physiotherapie in der Rehabilitation – Neurorehabilitation (IGPTR-N]) 23 (i.e. Interest Group Physiotherapy Rehabilitation - Neurorehabilitation in Switzerland). Further details can be found in Supplement 1. Accordingly, for each participant, the categorized type and the amount (in minutes/hours) of rehabilitation therapies received are recorded for the entire duration of in-hospital rehabilitation. 23
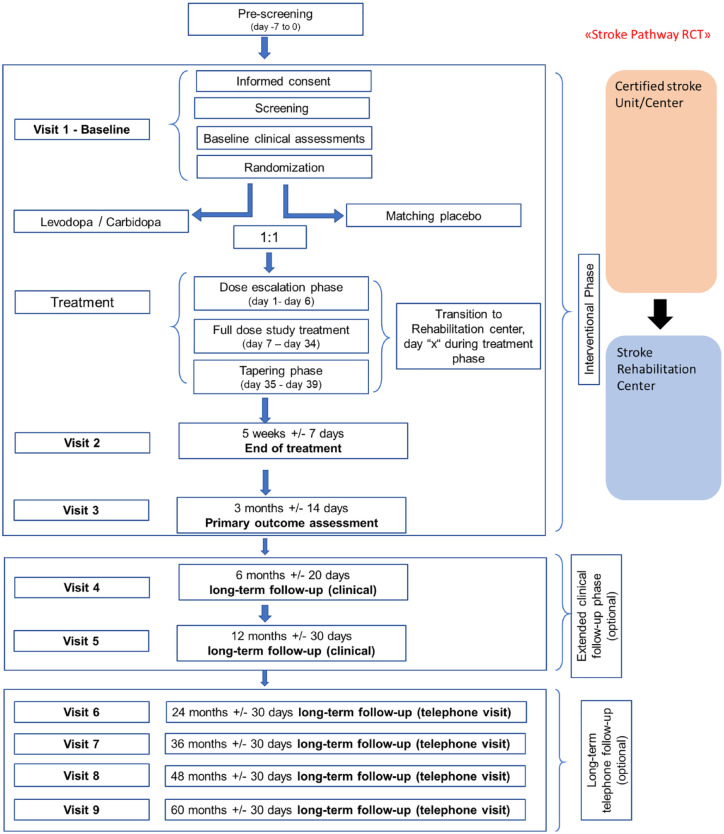
Study schedule: ESTREL consists of a 3-month interventional phase, which includes the administration of the study treatment and ends with the 3 months-visit and the FMMA assessment as the primary outcome measure. Thereafter, an optional extended clinical follow-up phase with in-person follow-up visits at 6 and 12 months after randomization is foreseen. Moreover, participants were encouraged to agree on long-term telephone follow-up interviews, performed annually from 2 to 5 years after randomization.
Figures 2 and 3 illustrate the study schedule and flow.
Figure 3.
Study flow chart of the ESTREL trial.
Harm
All investigators are trained to report serious adverse events to the sponsor investigator within 24 h according to ICH GCP guidelines. 24 Based on the known side effects of the active treatment in stroke patients from the DARS-trial, 13 pre-specified adverse events are assessed (see Supplemental Table 2). An independent Data Safety Monitoring Committee (DSMC) regularly monitors safety aspects of the trial and the frequency of the safety outcomes in both treatment groups. DSMC meetings are scheduled after the inclusion of 200, 400, and 500 participants.
Sample Size Estimation
We plan to enroll 610 participants. A drop-out rate of 10% is assumed leaving 549 evaluable participants. Assuming, that the FMMA is normally distributed with a standard deviation of 25 points (as based on the FLAME-trial 25 data), 548 participants will allow to detect a mean difference between the levodopa- and the placebo-group in the FMMA total score of 6 points at 3 months (which we assumed to be patient-relevant based on prior research26,27) with a power of 80% (two-sided significance level of 5%).
Statistical analysis
Primary analysis
The main analysis follows the intention-to-treat principle (“treatment policy estimand” 28 ). The FMMA total score at 3 months after randomization is the primary outcome. We considered a difference of 6 points in the FMMA total score at 3 months as patient-relevant based on the following data and considerations. A difference of 5.25 points for the upper extremity 26 and 6 points for the lower extremity part of the score 27 has been described as minimal clinically important difference. For ESTREL, a 6 points difference for the FMMA total score is considered a patient-relevant difference between both treatment groups for the primary endpoint. To illustrate this choice, a 6 points increase in the FMMA total score translates either into a recovery of shoulder function or – independent of any recovery of the shoulder function – into recovery of hip function in the lower extremity. 29
The primary outcome will be analyzed by a linear model, with the FMMA score at baseline as covariate to adjust for differences between the treatment groups at baseline and treatment as two-level factor (“levodopa’’ vs “placebo”) variable of interest. The estimated treatment difference, the P-value and the 95%-confidence interval will be presented. The analysis will be performed using α = 0.05 (two-sided).
Secondary analyses
Secondary endpoints will be analyzed by fitting the measurements by regression models appropriate to the data type. The analyses will be adjusted for the same variables as defined for the main analysis as well as for the baseline measures where available.
Detailed methodology for summaries and statistical analyses of the data collected in this trial is documented in the statistical analysis plan (SAP). The statistical analysis plan will be finalized before database closure and is under version control at the Department of Clinical Research, University Basel, Switzerland.
Study organization and funding
ESTREL is an investigator-initiated clinical trial funded by the Swiss National Science Foundation (IICT 33IC30_179667) and the Swiss Heart Foundation. ESTREL is governed by the ESTREL core team, which are clinical researchers affiliated to the University Department of Geriatric Medicine FELIX PLATTER, the Department of Neurology at the University Hospital Basel, and the Department of Clinical Research of the University Basel, Switzerland. Data management and data monitoring are provided by independent teams of the Department of Clinical Research at the University Basel, Switzerland. The Steering committee oversees the ESTREL study activities.
Trial status
ESTREL is currently recruiting. As of April 19th, 2024, 599 participants (i.e. 98% of the target population) were enrolled.
Ethical aspects
The study protocol was approved by relevant local authorities in all centers and complied with Swiss regulations concerning ethical approvals and informed consent (Ethikkommission Nordwest- und Zentralschweiz EKNZ [lead ethics committee], BASEC-ID 2018-02021 and Swissmedic). A writing committee consisting of selected members of the steering committee, the sponsor investigator team and participating centers will author the main paper. The main paper will be made publicly available.
Discussion
We designed ESTREL as large-scale stroke recovery trial with the aim to investigate whether levodopa compared to placebo given in addition to standardized rehabilitative therapies results in a patient-relevant enhancement of functional motor recovery after acute stroke.
Previous research suggested potential benefit of this approach and - more importantly - indicators of harm were absent while levodopa is a well-tolerated agent used for other conditions such as Parkinson’s disease.10–13,30
ESTREL takes into account lessons learnt from previous research in this field as follows:
(i) Levodopa is administered three times per day (tid) as preclinical data point toward beneficial effects of continuous levodopa administration on learning abilities. 31 Furthermore, the tid approach minimizes the risk of the study medication being disconnected from the rehabilitation sessions due to taking the medication too early or too late compared to administering a single dose. (ii) Concomitant rehabilitation therapy has been standardized and follows modern treatment approaches (motor learning, active training, task-oriented training). (iii) Inclusion in the study is as early as possible but not later than 7 days post-stroke onset and the duration of the intervention is 5 weeks ± 1 week, which takes into account current knowledge of neuroplasticity, follows recent consensus recommendations on the target timepoint to start rehabilitative measures in stroke patients, 32 and reflects the usual length of in-hospital rehabilitation after stroke in Switzerland. (iv) The timepoint of primary outcome measurement (at 3 months post-stroke), as well as the primary outcome measure (FMMA), is also in line with recent consensus recommendations on outcome measurements in stroke recovery and rehabilitation trials. 33
Existing studies on the use of levodopa in stroke recovery explored a single dose administration of the agent, given − according to the protocol − at least 30 10 or 45–60 min prior to a rehabilitation treatment session, 21 although 0–15 min were also acceptable. 21 The peak effect of levodopa can be expected 0.5–2 h after an oral dose. 21 Animal research demonstrated the beneficial effect of a continuous levodopa administration on learning abilities, suggesting that a tid-administration is preferable regarding neuronal plasticity. 31 In addition, a tid-administration is established to be safe and effective for Parkinson’s disease. Furthermore, it avoids failures in coordinating the timing of medication intake with that of rehabilitation therapy sessions.
The usage of 100 mg (rather than a higher or a lower dose) of levodopa at each single study drug administration, considers the findings of prior studies in humans using three different dosages. Different dosages of levodopa resulted in different plasticity effect in human motor cortex.34,35 In both studies, 100 mg single dosages prolonged facilitatory and inhibitory plasticity, whereas 25 and 200 mg as single dosages, respectively abolished plasticity effects. A dose of 100 mg for each single administration, was also used in studies about enhanced learning in healthy humans36,37 and in a RCTs about stroke motor recovery.10,11,21
We use a between group difference of 6 points on the FMMA total score at 3 months as threshold to statistically compare the levodopa and the placebo group. This choice took into account the literature about the minimal clinical-meaningful difference of the FMMA separately for the upper 26 and for the lower extremity, 27 as it was known, when the ESTREL-protocol was written and which was limited by relative small sample sizes, different populations and separate FMMA data for the upper and the lower extremities. Thus, our choice to apply the 6 point threshold also to the FMMA total score may be criticized as arbitrary. Indeed, other thresholds have been reported more recently. 38 More importantly, the aforementioned thresholds26,27,38 reflect a health care provider rather than the patient view. Therefore, in ESTREL – as a further objective – we aimed to include the patient perspective. In detail – at each FMMA follow-up assessment – participants are asked whether they observed an improvement compared to the last FMMA assessment and if yes, whether they consider this improvement to be clinically relevant.
Furthermore, among the secondary outcomes, ESTREL focuses on outcomes reported by the participants, such as the self-reporting of post-stroke fatigue, which is common and a relevant contributor to functional recovery after stroke. 30
In addition, an individual-patient-data-meta-analysis combining data from ESTREL with those of the DARS-trial 13 is foreseen to explore subgroup effects.
Finally, ESTREL serves as a platform for several nested studies. These studies, albeit considered explorative, allow to increase the evidence and knowledge about stroke recovery and provide important novel insights.
Conclusion
ESTREL will provide evidence to determine whether treatment with levodopa/carbidopa in addition to standardized rehabilitation therapy in acute stroke patients is superior to rehabilitation therapy alone and results in a patient relevant enhancement of functional recovery. The results of ESTREL could have a major impact on the current standard of clinical care and potentially offer a novel pharmacological therapeutic agent widely applicable to a large population of stroke survivors with a clinically relevant benefit.
Supplemental Material
Supplemental material, sj-docx-1-eso-10.1177_23969873241255867 for Enhancement of STroke REhabilitation with Levodopa (ESTREL): Rationale and design of a randomized placebo-controlled, double blind superiority trial by Annaelle Zietz, Josefin E Kaufmann, Karin Wiesner, Sandro Kevin Fischer, Martina Wiegert, Wilma DJ Verhagen-Kamerbeek, Yannik Rottenberger, Anne Schwarz, Nils Peters, Henrik Gensicke, Friedrich Medlin, Jens Carsten Möller, Bartosz Bujan, Leo H Bonati, Marcel Arnold, Sabine Schaedelin, René M. Müri, Lars G Hemkens, Patrik Michel, Philippe A Lyrer, Jeremia P Held, Gary A Ford, Andreas R Luft, Christopher Traenka and Stefan T Engelter in European Stroke Journal
Acknowledgments
We would like to thank every participating ESTREL patient and their next of kin.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: AZ, JK, KW, SKF, MW, WVK, AS, YR, HG, FM, RMM, PL, JCM, BB, LB, MA, SS, JH, GF, AL, and NP report no conflict of interest, via their institutions STE and CT received funding for ESTREL from the Swiss National Science Foundation (IICT 33IC30_179667) the Swiss Heart Foundation and the Science Funds Rehabilitation of the University Geriatric Medicine FELIX PLATTER, University of Basel, Switzerland.
LGH’s institution (RC2NB; Research Center for Clinical Neuroimmunology and Neuroscience Basel) is supported by the Foundation Clinical Neuroimmunology and Neuroscience Basel.
PM received funding outside this trial from the Swiss National Science Foundation, the Swiss Heart Foundation, and Faculty of Biology and Medicine of the Lausanne University.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: ESTREL is an investigator-initiated clinical trial funded by the Swiss National Science Foundation (IICT 33IC30_179667), the Swiss Heart Foundation and supported by the Science Funds Rehabilitation of the University Geriatric Medicine FELIX PLATTER, University of Basel, Switzerland.
Written informed consent: Participants and/or their next-of-kin gave their written informed consent to participate to the trial.
Ethical approval: Obtained in all participating sites.
Guarantor: Professor Stefan Engelter.
Contributorship: STE and CT designed and wrote the study protocol. AZ JK wrote the first draft of the manuscript. The other authors were actively involved in patient recruitment and data management. SS wrote the section about statistical analyses. All authors reviewed and edited the manuscript critically and approved the final version.
Trial Registration: ClinicalTrials.gov Identifier: NCT03735901.
ORCID iDs: Annaelle Zietz  https://orcid.org/0000-0002-4362-2497
https://orcid.org/0000-0002-4362-2497
Josefin E Kaufmann  https://orcid.org/0000-0001-7744-2796
https://orcid.org/0000-0001-7744-2796
Anne Schwarz  https://orcid.org/0000-0001-8943-5673
https://orcid.org/0000-0001-8943-5673
Supplemental material: Supplemental material for this article is available online.
References
- 1. Cramer SC. Drugs to enhance motor recovery after stroke. Stroke 2015; 46: 2998–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tritsch NX, Sabatini BL. Dopaminergic modulation of synaptic transmission in cortex and striatum. Neuron 2012; 76: 33–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Abe M, Schambra H, Wassermann EM, et al. Reward improves long-term retention of a motor memory through induction of offline memory gains. Curr Biol 2011; 21: 557–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McAllister TW. Polymorphisms in genes modulating the dopamine system: do they inf luence outcome and response to medication after traumatic brain injury? J Head Trauma Rehabil 2009; 24: 65–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vitrac C, Nallet-Khosrofian L, Iijima M, et al. Endogenous dopamine transmission is crucial for motor skill recovery after stroke. IBRO Neurosci Rep 2022; 13: 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Molina-Luna K, Pekanovic A, Röhrich S, et al. Dopamine in motor cortex is necessary for skill learning and synaptic plasticity. PLoS One 2009; 4: e7082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Leemburg S, Canonica T, Luft A. Motor skill learning and reward consumption differentially affect VTA activation. Sci Rep 2018; 8: 687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Floel A, Hummel F, Breitenstein C, et al. Dopaminergic effects on encoding of a motor memory in chronic stroke. Neurology 2005; 65: 472–474. [DOI] [PubMed] [Google Scholar]
- 9. Flöel A, Breitenstein C, Hummel F, et al. Dopaminergic influences on formation of a motor memory. Ann Neurol 2005; 58: 121–130. [DOI] [PubMed] [Google Scholar]
- 10. Scheidtmann K, Fries W, Müller F, et al. Effect of levodopa in combination with physiotherapy on functional motor recovery after stroke: a prospective, randomised, double-blind study. Lancet 2001; 358: 787–790. [DOI] [PubMed] [Google Scholar]
- 11. Sonde L, Lökk J. Effects of amphetamine and/or L-dopa and physiotherapy after stroke - a blinded randomized study. Acta Neurol Scand 2007; 115: 55–59. [DOI] [PubMed] [Google Scholar]
- 12. Lokk J, Salman Roghani R, Delbari A. Effect of methylphenidate and/or levodopa coupled with physiotherapy on functional and motor recovery after stroke–a randomized, double-blind, placebo-controlled trial. Acta Neurol Scand 2011; 123: 266–273. [DOI] [PubMed] [Google Scholar]
- 13. Ford GA, Bhakta BB, Cozens A, et al. Safety and efficacy of co-careldopa as an add-on therapy to occupational and physical therapy in patients after stroke (DARS): a randomised, double-blind, placebo-controlled trial. Lancet Neurol 2019; 18: 530–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Çelik C, Uzun MK. Inmeli Hastalarda Rehabilitasyon programi ile birlikte levodopa tedavisinin Fonksionel Motor Iyileşme üzerine etkisi. Turk Fiz Tip Idari Rehabil Derg 2004; 70: 18–20. [Google Scholar]
- 15. Acler M, Fiaschi A, Manganotti P. Long-term levodopa administration in chronic stroke patients. A clinical and neurophysiologic single-blind placebo-controlled cross-over pilot study. Restor Neurol Neurosci 2009; 27: 277–283. [DOI] [PubMed] [Google Scholar]
- 16. McDonald MW, Jeffers MS, Issa L, et al. An exercise mimetic approach to reduce poststroke deconditioning and enhance stroke recovery. Neurorehabil Neural Repair 2021; 35: 471–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chan A-W, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 2013; 158: 200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wiesner K, Schwarz A, Meya L, et al. Interrater reliability of the Fugl-Meyer motor assessment in stroke patients: a quality management project within the ESTREL study. Front Neurol 2024; 15: 1335375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Salinas J, Sprinkhuizen SM, Ackerson T, et al. An international standard set of patient-centered outcome measures after stroke. Stroke 2016; 47: 180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hays RD, Spritzer KL, Schalet BD, et al. PROMIS(®)-29 v2.0 profile physical and mental health summary scores. Qual Life Res An Int J Qual Life Asp Treat Care Rehabil 2018; 27: 1885–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bhakta BB, Hartley S, Holloway I, et al. The DARS (dopamine augmented rehabilitation in stroke) trial: protocol for a randomised controlled trial of co-careldopa treatment in addition to routine NHS occupational and physical therapy after stroke. Trials 2014; 15: 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bellg AJ, Borrelli B, Resnick B, et al.; Treatment Fidelity Workgroup of the NIH Behavior Change Consortium. Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH Behavior Change Consortium. Heal Psychol Off J Div Heal Psychol Am Psychol Assoc 2004; 23: 443–451. [DOI] [PubMed] [Google Scholar]
- 23. Christian P. Interessengemeinschaft physiotherapie neurorehabilitation. https://igptr.ch/igptr-n/, 2021.
- 24. International Council for Harmonisation (ICH). ICH harmonised guideline: integrated addendum to ICH E6(R1): guideline for good clinical practice E6(R2). 2016. International Council for Harmonisation. [Google Scholar]
- 25. Chollet F, Tardy J, Albucher J-F, et al. Fluoxetine for motor recovery after acute ischaemic stroke (FLAME): a randomised placebo-controlled trial. Lancet Neurol 2011; 10: 123–130. [DOI] [PubMed] [Google Scholar]
- 26. Page SJ, Fulk GD, Boyne P. Clinically important differences for the upper-extremity Fugl-Meyer scale in people with minimal to moderate impairment due to chronic stroke. Phys Ther 2012; 92: 791–798. [DOI] [PubMed] [Google Scholar]
- 27. Pandian S, Arya KN, Kumar D. Minimal clinically important difference of the lower-extremity Fugl-Meyer assessment in chronic-stroke. Top Stroke Rehabil 2016; 23: 233–239. [DOI] [PubMed] [Google Scholar]
- 28. Keene ON, Lynggaard H, Englert S, et al. Why estimands are needed to define treatment effects in clinical trials. BMC Med 2023; 21: 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fugl-Meyer AR, Jääskö L, Leyman I, et al. The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand J Rehabil Med 1975; 7: 13–31. [PubMed] [Google Scholar]
- 30. Delbari A, Salman-Roghani R, Lokk J. Effect of methylphenidate and/or levodopa combined with physiotherapy on mood and cognition after stroke: a randomized, double-blind, placebo-controlled trial. Eur Neurol 2011; 66: 7–13. [DOI] [PubMed] [Google Scholar]
- 31. Hosp JA, Pekanovic A, Rioult-Pedotti MS, et al. Dopaminergic projections from midbrain to primary motor cortex mediate motor skill learning. J Neurosci Off J Soc Neurosci 2011; 31: 2481–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bernhardt J, Hayward KS, Kwakkel G, et al. Agreed definitions and a shared vision for new standards in stroke recovery research: the Stroke Recovery and Rehabilitation Roundtable taskforce. Int J Stroke Off J Int Stroke Soc 2017; 12: 444–450. [DOI] [PubMed] [Google Scholar]
- 33. Kwakkel G, Lannin NA, Borschmann K, et al. Standardized measurement of sensorimotor recovery in stroke trials: consensus-based core recommendations from the Stroke Recovery and Rehabilitation Roundtable. Int J Stroke Off J Int Stroke Soc 2017; 12: 451–461. [DOI] [PubMed] [Google Scholar]
- 34. Monte-Silva K, Liebetanz D, Grundey J, et al. Dosage-dependent non-linear effect of L-dopa on human motor cortex plasticity. J Physiol 2010; 588: 3415–3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Thirugnanasambandam N, Grundey J, Paulus W, et al. Dose-dependent nonlinear effect of L-DOPA on paired associative stimulation-induced neuroplasticity in humans. J Neurosci Off J Soc Neurosci 2011; 31: 5294–5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Weis T, Puschmann S, Brechmann A, et al. Effects of L-dopa during auditory instrumental learning in humans. PLoS One 2012; 7: e52504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Knecht S, Breitenstein C, Bushuven S, et al. Levodopa: faster and better word learning in normal humans. Ann Neurol 2004; 56: 20–26. [DOI] [PubMed] [Google Scholar]
- 38. Mattke S, Cramer SC, Wang M, et al. Estimating minimal clinically important differences for two scales in patients with chronic traumatic brain injury. Curr Med Res Opin 2020; 36: 1999–2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-eso-10.1177_23969873241255867 for Enhancement of STroke REhabilitation with Levodopa (ESTREL): Rationale and design of a randomized placebo-controlled, double blind superiority trial by Annaelle Zietz, Josefin E Kaufmann, Karin Wiesner, Sandro Kevin Fischer, Martina Wiegert, Wilma DJ Verhagen-Kamerbeek, Yannik Rottenberger, Anne Schwarz, Nils Peters, Henrik Gensicke, Friedrich Medlin, Jens Carsten Möller, Bartosz Bujan, Leo H Bonati, Marcel Arnold, Sabine Schaedelin, René M. Müri, Lars G Hemkens, Patrik Michel, Philippe A Lyrer, Jeremia P Held, Gary A Ford, Andreas R Luft, Christopher Traenka and Stefan T Engelter in European Stroke Journal