Abstract
In this study, we clarified the molecular mechanism(s) underlying the regulation of matrix metalloproteinase (MMP)-1 gene by hepatocyte growth factor (HGF) in cultured human dermal fibroblasts. HGF induced MMP-1 protein as well as mRNA at a transcriptional level via extracellular signal-regulated kinase (ERK) signaling pathway. The region in the MMP-1 promoter mediating the inducible responsiveness to HGF, defined by the transient transfection analysis of the serial 5′ deletion constructs, contained an Ets binding site. Mutation of this Ets binding site abrogated the HGF-inducible promoter activity. Ets1 up-regulated the expression of MMP-1 promoter activity, whereas Fli1 had antagonistic effects on them. After HGF treatment, the protein level and the binding activity of Ets1 was increased and those of Fli1 was decreased, which were canceled by PD98059. These results suggest that HGF up-regulates MMP-1 expression via ERK signaling pathway through the balance of Ets1 and Fli1, which may be a novel mechanism of regulating MMP-1 gene expression.
INTRODUCTION
Hepatocyte growth factor (HGF), originally identified as a potent mitogen for hepatocytes and also known as a ‘scatter factor’, is a multifunctional mediator that shows mitogenic and morphogenetic activities in a variety of cells (1–7).
Recently, HGF has been shown to reverse fibrogenic processes, including hepatic fibrosis (8–11). In these reports, HGF inhibited extracellular matrix deposition and successfully reduced the amount of preexisting extracellular matrix constituents, including fibrillar collagens. Most of these reports demonstrated effects of HGF on tissue fibrosis in an animal model, but its effects on normal human cells in vitro are poorly investigated. Thus, the mechanism by which HGF acts against fibrogenesis is not fully understood. However, one of the anti-fibrogenic effects of HGF is thought to be expressed by the induction of matrix metalloproteinases (MMPs) (9–11). Notably, MMP-1, a collagenase which mainly digests interstitial collagens type I and III, is reported to be up-regulated by HGF in several cell types (12,13).
Earlier investigations demonstrated that HGF induces MMP-1 via the transcription factor Ets1 in human hepatic stellate cell line (13). In their study, HGF increases Ets1 protein level and their binding activity. MMP-1 promoter activity is dose-dependently stimulated by the co-transfection of Ets1. The treatment of the HGF-exposed cells with antisense oligonucleotides against Ets1 prevents an HGF-induced increase of Ets1 and MMP-1 mRNA expression, showing that Ets1 was essential for the regulation of MMP-1 expression by HGF in this cell line. In this study, we showed that Fli1, Ets family transcriptional factor same as Ets1, is also involved in this HGF-mediated MMP-1 up-regulation in human dermal fibroblasts. We also demonstrated that the MMP-1 gene expression is controlled by the balance of Ets1 and Fli1 on Ets binding sites (EBS) of this promoter.
MATERIALS AND METHODS
Reagents
Recombinant human HGFs were obtained from R & D systems (Minneapolis, MN). Actinomycin D, cycloheximide and antibody for β-actin were purchased from Sigma (St Louis, MO). LY294002 and PD98059 were purchased from Calbiochem (La Jolla, CA). Anti-phospho-extracellular signal-regulated kinase (ERK), ERK2, Ets1, Fli1 and c-jun antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). FuGENE 6 was obtained from Roche Diagnostics (Indianapolis, IN).
Cell cultures
Fibroblasts were obtained by skin biopsy of healthy donors. All biopsies were obtained with informed consent, institutional review board approval and written informed consent according to the Declaration of Helsinki. Primary explant cultures were established in 25 cm2 culture flasks in MEM supplemented with 10% fetal calf serum (FCS), 2 mM glutamine and 50 μg/ml gentamycin, as described previously (14,15). Monolayer cultures were maintained at 37°C in 5% CO2 in air. Fibroblasts between the third and sixth subpassages were used for experiments.
Immunoblotting
Dermal fibroblasts were cultured until they were confluent. Cells were serum-starved in MEM and 0.1% BSA for 24 h before the cytokine treatment. After incubation with the indicated reagent, the condition medium was collected. Remaining cells were washed twice with cold phosphate-buffered saline and lysed in lysis buffer (10 mM Tris–HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% NP-40, 50 mM sodium fluoride, 1 mM phenylmethylsulfonyl fluoride, 1 mM sodium orthovanadate, 1 μg/ml leupeptin, 1 μg/ml aprotinin and 1 μg/ml pepstatin). Aliquots of conditioned media (normalized for cell numbers) or cell lysates (normalized for protein concentrations as measured by the Bio-Rad reagent) were subjected to electrophoresis on SDS–polyacrylamide gels and transferred to nitrocellulose membranes. The membranes were blocked for 1 h and incubated overnight at 4°C with anti-type I collagen, MMP-1, TIMP-1, TIMP-2, Ets1, Fli1 or β-actin antibody. The membranes were washed in Tris-buffered saline and 0.1% Tween-20, incubated with secondary antibodies and washed again. The detection was performed using the Enhanced Chemiluminescence Detection system (Amersham, Arlington Heights, IL).
For immunoblotting using antibodies against phospho-ERK, membranes were incubated with anti-phospho-ERK monoclonal antibody overnight at 4°C. As a loading control, immunoblotting was also performed using antibodies against ERK2.
MMP-1 activity assay
The active MMP-1 level was determined using the MMP-1 Biotrak activity assay system (Amersham, Arlington Heights, IL), according to the manufacturer's directions (16). Briefly, anti-MMP-1 antibodies were precoated onto microtiter wells. Cultured medium was added to each well, and incubation conducted at 4°C overnight. The wells were washed and the absorbance at 405 nm was measured before and after the incubation with a specific chromogenic peptide substrate for 1.5 h. The concentration of active MMP-1 in each sample was determined by interpolation from a standard curve.
RNA preparation and the northern blot analysis
Total RNA was extracted using an acid guanidinium thiocyanate–phenol–chloroform method and analyzed by the northern blotting, as described previously (14,15). RNA was subjected to electrophoresis on 1% agarose/formaldehyde gels and blotted onto nylon filters (Roche Diagnostics, Indianapolis, IN). The filters were UV cross-linked, prehybridized and sequentially hybridized with cDNA probes. The following cDNA probes were used: human MMP-1 XhoI fragment and GAPDH HindIII–NotI fragment. The membranes were then washed and exposed to X-ray film.
DNA affinity precipitation assay
Oligonucleotides containing biotin on the 5′-nucleotide of the sense strand were used in the assays. The sequence of each oligonucleotide is as follows: (i) MMP1-EBS oligo, 5′-CTATTCATAGCTAATCAAGAGGATGTTATAAAGCATGAGTCAGAC, which corresponds to positions bp −107 to −63 of the human MMP-1 promoter; (ii) MMP1-EBS-M oligo, 5′-CTATTCATAGCTAATCAAGAGTATGTTATAAAGCATGAGTCAGAC, lacking the EBS, which is able to bind the Ets family of transcription factors. These oligonucleotides were annealed to their respective complementary oligonucleotides, and double-stranded oligonucleotides were gel-purified and used. Cell lysates were obtained using lysis buffer (17). Poly (dI–dC) competitor was incubated with the cell lysates, followed by incubation with each double-stranded oligonucleotide. After incubation, streptavidin–agarose (Sigma) was added to the reaction and incubated. The protein–DNA–streptavidin–agarose complex was washed and loaded onto a SDS–polyacrylamide gel. Detection of c-jun, Ets1 or Fli1 was performed with anti-c-jun, Ets1 or Fli1 antibodies.
Plasmids
The full-length clone of the human MMP-1 promoter containing fragments of promoter DNA linked to the luciferase reporter was kindly provided by Dr Constance E. Brinckerhoff (18). The deletion constructs were generated by PCR using MMP-1 as a template and substitution mutations were generated using Quick Change site-directed mutagenesis kits (Stratagene) and confirmed by sequencing (18). Expression vector for Ets1 or Fli1 were kindly provided by Dr Maria Trojanowska (19). And Fli/W321R mutants harboring a single amino acid mutation in the Ets domain that abolishes its ability to bind the DNA and a Fli1 dominant interference mutant containing only the Ets domain (Fli/DBD) were also kindly provided by Dr Maria Trojanowska (20–22). Plasmids used in the transient transfection assays were purified twice on cesium chloride gradients, as described previously (15). At least two different plasmid preparations were used for each experiment.
The transient transfection
Fibroblasts were grown to 50% confluence in 100 mm dishes in MEM with 10% FCS. The medium was replaced with serum-free medium, and fibroblasts were transfected with the MMP-1 promoter constructs, expression vectors or corresponding empty constructs, employing FuGENE6 as described previously (23). In order to correct minor variations in transfection efficiency, pSV-β-galactosidase vector (Promega, Madison, WI) was included in all transfections. After 48 h of incubation, the cells were harvested in 0.25 M Tris–HCl (pH 8) and fractured by freeze-thawing. Extracts, normalized for protein content as measured by the Bio-Rad reagent, were incubated with butyl-CoA and [14C]-chloramphenicol for 90 min at 37°C. Butylated chloramphenicol was extracted using an organic solvent (2:1 mixture of tetramethylpentadecane and xylene) and quantitated by scintillation counting. The data were standardized with β-galactosidase activities. Each experiment was performed in duplicate.
Statistical analysis
Statistical analysis was carried out with the Mann–Whitney test for comparison of means. P-values < 0.05 were considered significant.
RESULTS
The effects of HGF on the expression of MMP-1 protein or mRNA in normal dermal fibroblasts
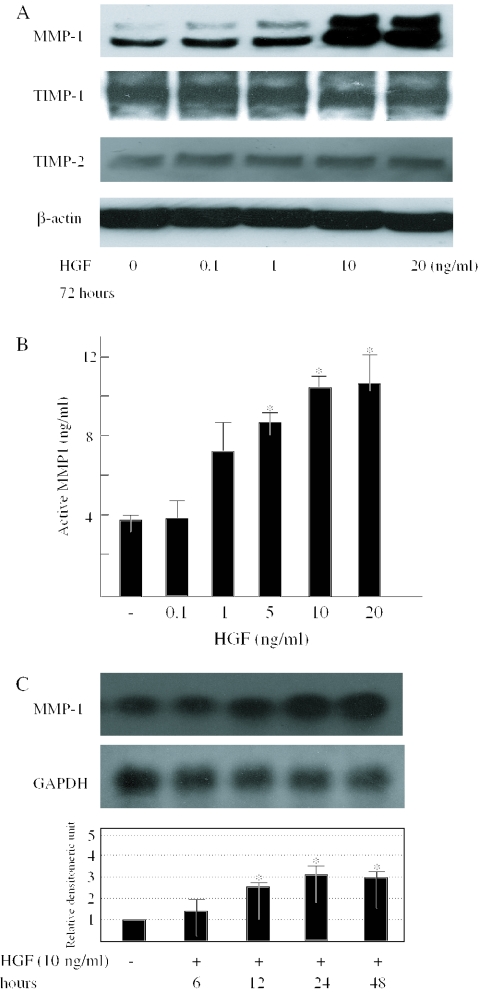
To examine fibrogenic/fibrolytic protein expression by HGF in normal fibroblasts, we determined the dose-dependent effect of HGF on the protein levels of MMP-1, TIMP-1 or TIMP-2. The MMP-1 protein level in cell lysates was up-regulated by the treatment with 10 ng/ml HGF maximally in dermal fibroblasts (Figure 1A). On the other hand, the production of TIMP-1 or -2 protein was not increased by the HGF simulation compared with the levels in untreated cells. Specific enzyme-linked immunosorbent assays revealed that HGF also increased MMP-1 catalytic activity in the cultured media (Figure 1B).
Figure 1.
The effects of HGF on the MMP-1 expression. (A) To examine the dose-dependency of the effect of HGF on the expression of MMP-1 protein, human dermal fibroblasts were cultured until they were confluent, and then incubated for an additional 24 h under conditions of serum starvation. Cells were incubated in serum-free medium for 72 h in the absence or presence of HGF at the indicated doses before the protein extraction. Aliquots were subjected to immunoblotting with anti-MMP-1, -TIMP-1 or -TIMP-2 antibodies. The same membrane was then stripped and reprobed with anti-β-actin antibody as a loading control. (B) Cells were incubated in serum-free medium for 72 h in the presence or absence of HGF at the indicated doses before collection of the medium. Specific enzyme-linked immunosorbent assays for detecting active MMP-1 levels were performed as described in ‘Materials and Methods’. *P < 0.05 as compared with the value in untreated cells. (C) Cultured fibroblasts were incubated in the presence or absence of 10 ng/ml HGF under the same conditions for the indicated time courses, and the northern blot analysis of MMP-1 mRNA expression was performed. Levels of GAPDH mRNA are shown as a loading control. One experiment representative of three independent experiments is shown. MMP-1 mRNA levels quantitated by scanning densitometry and corrected for the levels of GAPDH in the same samples are shown relative to the level in untreated cells (1.0). Data are expressed as the mean ± SD of three independent experiments. *P < 0.05 as compared with the value in untreated cells.
Moreover, the MMP-1 mRNA expression was also elevated significantly after the stimulation with HGF for 12 h, sustained until 48 h later (Figure 1C). Thus, the effect of HGF on the MMP-1 protein level paralleled that on the mRNA level.
Mechanisms of the HGF-mediated MMP-1 up-regulation
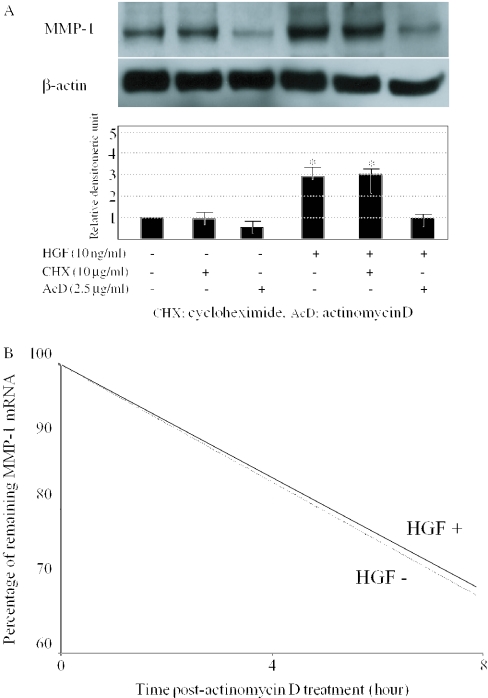
To establish whether the increase in the MMP-1 levels after HGF treatment involves the synthesis of new proteins or the transcriptional activation, we tested the magnitude of MMP-1 induction in the presence of cycloheximide (protein synthesis inhibitor) or actinomycin D (RNA synthesis inhibitor). Cycloheximide did not block the HGF-mediated up-regulation of MMP-1 protein expression, whereas actinomycin D significantly blocked this up-regulation (Figure 2A). In addition, we wished to determine whether HGF increases the stability of MMP-1 mRNA. Following the inhibition of transcription, the loss of MMP-1 mRNA induced by HGF was not significantly different from that observed in the untreated cells (Figure 2B). The failure of HGF to increase the half-life of MMP-1 mRNA suggests that the HGF-mediated induction of the MMP-1 expression is regulated at the level of transcription. Taken together, these results indicate that HGF up-regulates the MMP-1 expression at the transcriptional level and this induction is independent of new protein synthesis.
Figure 2.
Effects of actinomycin D or cycloheximide on HGF-mediated MMP-1 mRNA up-regulation. (A) Human dermal fibroblasts were serum-starved for 24 h and pretreated with 10 μg/ml cycloheximide or 2.5 μg/ml actinomycin D, for 1 h before the addition of 10 ng/ml of HGF for 24 h. Cell lysates were subjected to immunoblotting with anti-MMP-1 antibodies. The same membrane was then stripped and reprobed with anti-β-actin antibody as a loading control. One experiment representative of three independent experiments is shown. MMP-1 protein levels quantitated by scanning densitometry and corrected for the level of β-actin in the same samples are shown relative to the level in untreated cells (1.0). *P < 0.05 as compared with the value in untreated cells. CHX, cycloheximide; AcD, actinomycin D. (B) The Effect of HGF on MMP-1 mRNA half-life was examined. Human dermal fibroblasts were serum-starved for 24 h and incubated in the absence or presence of 10 ng/ml HGF for 12 h before the addition of 2.5 μg/ml actinomycin D. RNA was extracted from the cells at the indicated time after actinomycin D administration. MMP-1 mRNA expression was determined by the northern blot analysis. The corrected density by GAPDH levels was expressed as a percent of the value at time 0 and plotted on a scale. The solid line indicates the HGF-treated levels, and the dotted line indicates control (untreated) levels.
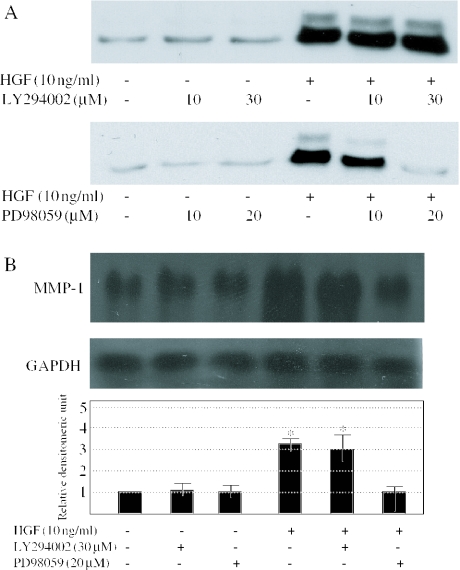
PD98059 inhibits the HGF-mediated up-regulation of MMP-1 protein and mRNA
We investigated whether ERK or phosphoinositide 3-kinase (PI3K) activation is involved in the HGF-mediated MMP-1 protein or mRNA induction. Pretreatment of cells with mitogen-activated protein kinase (MAPK)/ERK inhibitor, PD98059, blocked HGF-mediated up-regulation of MMP-1 protein in a dose-dependent manner, whereas PI3K inhibitor, LY294002 did not (Figure 3A). The pretreatment of cells with PD98059 also blocked the HGF-mediated up-regulation of MMP-1 mRNA (Figure 3B). These results suggest that HGF regulates MMP-1 expression through ERK signaling in human dermal fibroblasts.
Figure 3.
Effects of LY294002 and PD98059 on the HGF-induced MMP-1 expression (A) Human dermal fibroblasts were serum-starved for 24 h and pretreated with 10 or 30 μM LY294002, or 10 or 20 μM PD98059, for 1 h before the addition of 10 ng/ml of HGF for 72 h. Conditioned medium were subjected to immunoblotting with anti-MMP-1 antibodies. (B) The northern blot analysis of MMP-1 mRNA expression was performed. LY294002 (30 μM) or PD98059 (20 μM) was added 1 h before the addition of 10 ng/ml of HGF. After 24 h, cells were collected. Levels of GAPDH mRNA are shown as a loading control. MMP-1 mRNA levels quantitated by scanning densitometry and corrected for the level of GAPDH in the same samples are shown relative to the levels in untreated cells (1.0). One experiment representative of three independent experiments is shown. *P < 0.05 as compared with the value in untreated cells.
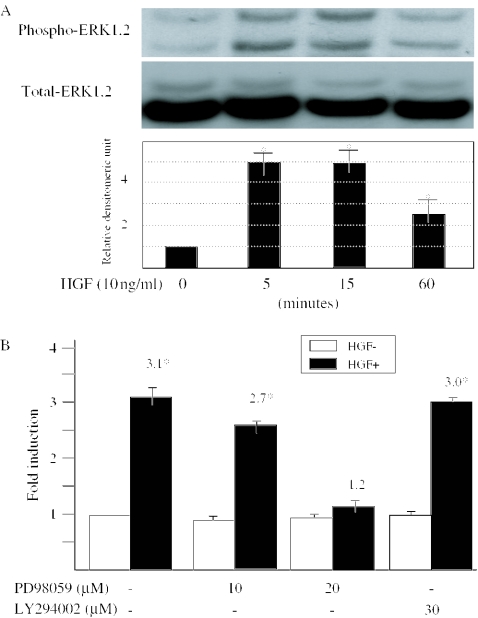
ERK signaling pathway is activated following HGF treatment
We investigated whether HGF treatment induces the ERK phosphorylation in human dermal fibroblasts. Immunoblotting using anti-phospho-ERK antibody revealed a significant phosphorylation of ERK after 5 min of treatment with HGF, and this increase was sustained until 60 min (Figure 4A). Immunoblotting for total ERK protein demonstrated that the amount of ERK did not significantly change in the presence of HGF.
Figure 4.
Activation of ERK signaling pathway by HGF in human dermal fibroblasts. (A) Cell lysates (30 μg of protein/sample) were subjected to immunoblotting with anti-phospho-ERK antibody. That the amounts of ERK proteins were unchanged was confirmed by immunoblotting using anti-ERK2 antibodies. One experiment representative of three independent experiments is shown. The levels of phosphorylated ERK quantitated by scanning densitometry and corrected for the levels of total ERK in the same samples are shown relative to the level of untreated cells (1.0). Data are expressed as the mean ± SD of three independent experiments. (B) Human dermal fibroblasts were transiently transfected with the full-length MMP-1 promoter. After incubation overnight, the cells were pretreated with either vehicle alone (control), 10 or 30 μM LY294002, or 10 or 20 μM PD98059 for 1 h before the addition of HGF. Cells were incubated in the absence (open bars) or presence (closed bar) of 10 ng/ml HGF for additional 24 h. The graph depicts the MMP-1 promoter activities, and the basal promoter activity was arbitrarily set at 1. The number shows the promoter activities stimulated by HGF relative to the promoter without HGF. Data are expressed as the mean ± SD of three independent experiments. *P < 0.05 as compared with the value in untreated cells.
In addition, we investigated the role of ERK signaling pathway in the transcriptional regulation of MMP-1. HGF induced the activity of MMP-1 full-length −4372 to +63 bp promoter construct (3.1-fold). And PD98059 significantly blocked the HGF-mediated MMP-1 promoter activity in a dose-dependent manner (Figure 4B). These results suggest that ERK signaling pathway participates in the regulation of MMP-1 gene by HGF.
Functional analysis of the MMP-1 promoter up-regulation by HGF
To identify potential regulatory elements of the human MMP-1 gene by HGF, we performed the transient transfection assays using a series of 5′-deletions of the MMP-1 promoter linked to the luciferase reporter gene. The full-length bp −4372 to +63 construct and the shorter constructs with deletion end point at bp −1600 to +63 and bp −512 to +63 responded to the HGF stimulation to the same extent. The corresponding empty constructs showed little reactivity to HGF. These data indicate that the HGF-responsive element is localized between bp −512 and +63 in the MMP-1 promoter (Figure 5).
Figure 5.
Identification of the MMP-1 promoter region mediating HGF stimulation. The indicated MMP-1 promoter deletion constructs, the corresponding empty construct or the mutated construct (EBS-mutated) were transfected in the absence or presence of 10 ng/ml HGF. The bar graph and the number on the left represents fold stimulation of the promoter activity stimulated by HGF relative to the promoter activity without HGF, which was arbitrarily set at 1. The number on the right show the basal levels (i.e. without HGF) of each construct relative to the corresponding vector, which was arbitrarily set at 100%. Mean ± SD from four independent experiments are presented. Asterisk indicates statistically significant results compared with the basal promoter activities of each construct (P < 0.05, Mann–Whitney U-test).
This region of MMP-1 promoter has a EBS (from bp −87 to −84) as well as AP-1 binding site, and MMP-1 gene is shown to be regulated by Ets1 as described above (13). The effects of substitution mutations changing GGAT to GTAT in the EBS of MMP-1 gene were investigated (Figure 5). Mutating the EBS resulted in the significant reduction of the promoter activity induced by HGF. Thus, the integrity of the EBS of MMP-1 promoter is critical for the HGF effect on the MMP-1 expression.
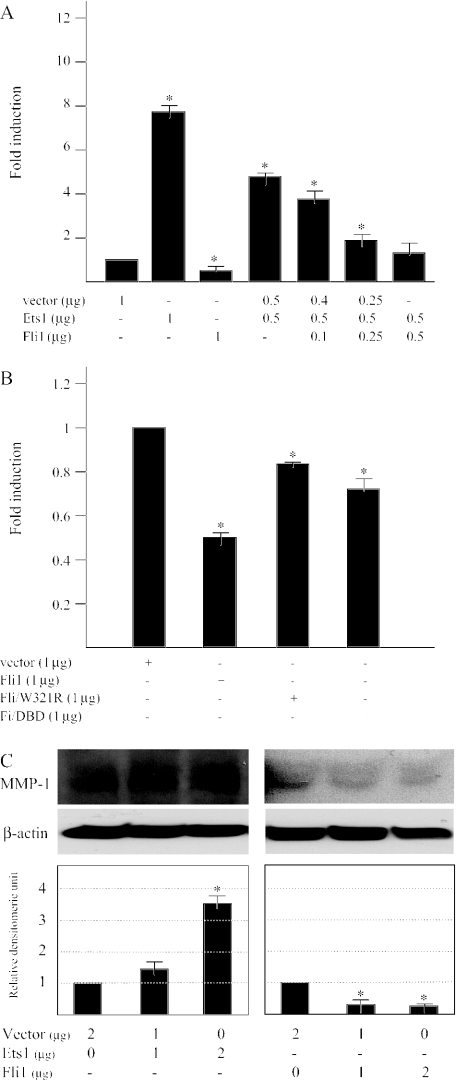
Ets1 and Fli1 have antagonistic effects on the MMP-1 promoter and compete with each other in the regulation of the MMP-1 promoter activity
To explore whether Ets family transcriptional factors are involved in the up-regulated MMP-1 expression by HGF, the bp −4372 to +63 MMP-1 construct was co-transfected with increasing amounts of expression vectors of Ets1 and Fli1. The overexpression of Ets1 remarkably induced the promoter activity. In contrast to Ets1, Fli1 down-regulated the MMP-1 promoter activity and Fli1 abolishes the Ets1-mediated MMP-1 promoter activation in dermal fibroblast, competing with each other (Figure 6A).
Figure 6.
The effects of overexpressed Ets1 or Fli1 on the MMP-1 promoter activity (A) Human dermal fibroblasts were transiently co-transfected with the MMP-1 promoter construct (1.5 μg) and either Fli1 or Ets1 was added individually or with a constant amount of Ets1 expression vector added together with increasing amounts of Fli1 expression vector. To ensure an equal amount of co-transfected expression vectors under each condition, appropriate amounts of corresponding vector were added to individual co-transfections. The bar graph represents the fold induction of the MMP-1 promoter activity co-transfected with Fli1 or Ets1 individually or together relative to the activity of the promoter co-transfected with corresponding vector, which was arbitrarily set at 1. Mean ± SD from four independent experiments are presented. (B) The transient transfections were performed with Fli1 DNA binding mutant (Fli/W321R), Fli1 dominant interference mutant (Fli/DBD) and the MMP-1 promoter construct. The Fli1 DNA binding mutant contains a single amino acid mutation that abolishes DNA binding, and the Fli1 dominant interference mutant contains the Ets domain. (C) The overexpression of the Ets1 or Fli1 in human dermal fibroblasts was performed by the transient transfection as described in ‘Materials and Methods’. The cell lysates were analyzed by immunoblot analysis. The same membrane was then stripped and reprobed with anti-β-actin antibody to show as a loading control. The levels of type I procollagen quantitated by scanning densitometry and corrected for the levels of β-actin are shown relative to the level of cells transfected with vector (1.0). Data are expressed as the mean ± SD of three independent experiments. *P < 0.05 as compared with the value in untreated cells.
Next, the effects of the overexpression of two Fli1 mutants on the MMP-1 promoter were investigated (Figure 6B). In comparison with the wild-type Fli1, both mutants caused less but significant decreases in the MMP-1 promoter activity, suggesting that direct (via DNA binding) and indirect (via protein–protein interaction) mechanisms contribute to the effects of Fli1 on the MMP-1 promoter.
In addition, we determined whether the forced overexpression of Ets1 or Fli1 can enhance the MMP-1 protein induction. Immunoblotting revealed that the transient transfection of Ets1 led to the induction of the MMP-1 protein expression (Figure 6C), whereas those of Fli1 had the opposite effect on the MMP-1 protein expression. These data confirmed that both Ets1 and Fli1 can regulate the MMP-1 expression.
The balance of Ets1 and Fli1 is associated with MMP-1 up-regulation by HGF
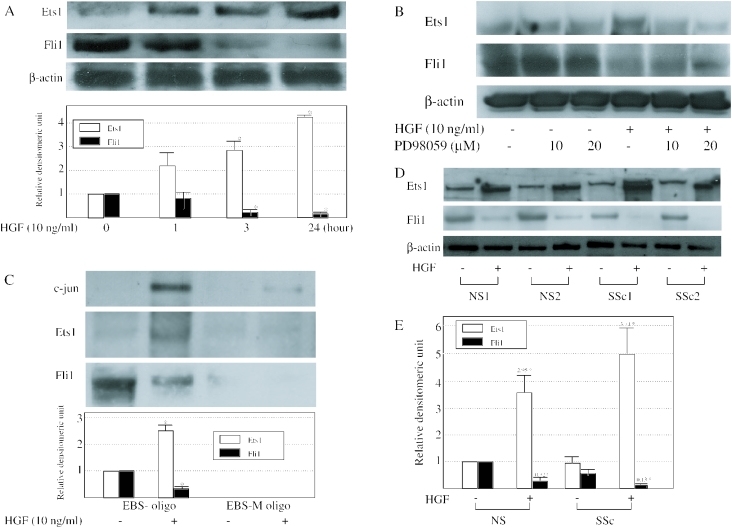
First, we examined whether HGF altered the amounts of Ets1 or Fli1 in cell lysates. Immunoblotting revealed that the amounts of Ets1 were increased by the HGF stimulation, whereas Fli1 proteins were decreased after the stimulation with HGF for 3 h, compared with the level in control cells (Figure 7A). Furthermore, we investigated whether the ERK activation is involved in the HGF-mediated Ets1 or Fli1 protein induction or reduction. Pretreatment of cells with PD98059 blocked the HGF-mediated regulation of Ets1 or Fli1 protein in a dose-dependent manner (Figure 7B). Taken together, these results indicate that HGF up-regulates the MMP-1 expression via the alteration of the levels of Ets1 and Fli1 through ERK signaling pathway.
Figure 7.
The binding activity of Ets1 or Fli1 is altered by HGF (A) To determine the amounts of Ets1 or Fli1 in cell lysates, human dermal fibroblasts were serum-starved for 24 h and treated with 10 ng/ml HGF for the indicated times. Immunoblotting were performed using anti-Ets1 or Fli1 antibodies. The same membrane was then stripped and reprobed with anti-β-actin antibody to show as a loading control. The levels of Ets1 (open bars) and Fli1 (closed bars) quantitated by scanning densitometry and corrected for the levels of β-actin in the same samples are shown relative to those in untreated cells without HGF stimulation (1.0). Data are expressed as the mean ± SD of four experiments. (B) Human dermal fibroblasts were serum-starved for 24 h and pretreated with 10 or 20 μM PD98059 for 1 h before the addition of 10 ng/ml of HGF for 24 h. Cell lysates were subjected to immunoblotting with anti-Ets1 or Fli1 antibodies. (C) Nuclear extracts were prepared from dermal fibroblast and incubated with biotin-labeled oligonucleotide as described under ‘Materials and Methods’. Proteins bound to each nucleotide were isolated with streptavidin–agarose beads, and c-jun, Ets1 or Fli1 was detected by immunoblotting. The levels of Ets1 (open bars) and Fli1 (closed bars) quantitated by scanning densitometry are shown relative to the level of untreated cells (1.0). (D) Normal and SSc fibroblasts were incubated in serum-free medium for 24 h in the presence or absence of 10 ng/ml of HGF prior to collection of the cell lysates. Cell lysates (normalized for protein concentrations as measured with the Bio-Rad reagent) were subjected to immunoblotting with anti-Ets1 or Fli1 antibody. The same membrane was then stripped and reprobed with anti-β-actin antibody as a loading control. The representative results for two normal and two SSc fibroblasts are shown. (E) Ets1 (open bars) or Fli1 (close bars) levels quantitated by scanning densitometry and corrected for the levels of β-actin in the same samples are shown relative to those in normal fibroblasts without HGF stimulation (1.0). Data are expressed as the mean ± SD of independent experiments. The number shows fold-stimulation with HGF relative to those without HGF in each cell type.
Furthermore, we performed DNA affinity precipitation assay using MMP1-EBS oligo, containing the EBS of the MMP-1 promoter. As a negative control, we used MMP1-EBS-M oligo. The results showed that only the MMP1-EBS oligo bound endogenous Ets1 strongly after HGF treatment for 3 h, whereas Fli1 binding to EBS was decreased (Figure 7C). MMP1-EBS-M oligo did not bind these transcriptional factors. MMP1-EBS oligo also contains AP-1 binding sequence (bp −72 to −66). To note, our result showed that the binding activity of c-jun, which is one of the AP-1 transcription factors regulating MMP-1 expression and was reported to be induced by HGF (13,24,25), was also inhibited by the mutation of EBS. These results suggest that the exchange of Fli1 with Ets1 on the promoter by HGF regulates the induction of MMP-1 promoter activity.
We have recently reported that HGF induces MMP-1 expression and activity in fibroblasts from both normal subjects and scleroderma (SSc) patients, but that the HGF-treated MMP-1 level in SSc fibroblasts was overexpressed more apparently than in normal fibroblasts, which is probably a result of the overexpression of HGF receptor (c-met) in SSc fibroblasts (26). Finally, we investigated whether Ets1 or Fli1 also contribute to the hyperreactivity of MMP-1 expression to HGF in SSc fibroblasts. As shown in Figure 7D and E, basal Fli1 protein expression is consistently down-regulated in SSc dermal fibroblasts, whereas there is no difference in Ets1 protein expression between normal and SSc fibroblasts, which was consistent with a previous report (27). On the other hand, the HGF-treated Ets1 or Fli1 level in SSc fibroblasts was up- or down-regulated more dramatically (3.71- and 0.13-fold) than in normal fibroblasts (2.95- and 0.32-fold), respectively. This result suggested that the EBS is equally important for the hyperreactivity of MMP-1 expression to HGF stimulation in SSc fibroblasts.
DISCUSSION
Our study showed that HGF induced the MMP-1 expression at the transcriptional level. Treatment of the cells with PD98059 inhibited HGF effect on MMP-1 expression. The region in the MMP-1 promoter mediating the inducible responsiveness to HGF, defined by the transient transfection analysis of the serial 5′ deletion constructs, contained the EBS.
The Ets transcription factor family was originally identified as a human homolog of viral oncogene, which was identified in E26 avian erythroleukemia virus (28), including >30 currently known members. All posses a conserved region termed the Ets domain, which recognizes and binds to GGA(A/T) purine-rich core sequences that can function as the EBS (29).
Many MMP genes including MMP-1 and MMP-13 contain the EBS in their regulatory region, often combined with an AP-1-binding site to form a responsive complex. Multiple EBS have been identified in the MMP-1 promoter region (30–32), but the EBS that is important in regulating MMP-1 expression is not known. Furthermore, Ets1 has been shown to modulate transcription of these MMP genes, including MMP-1 (24,32); there has been no report that discussed the involvement of Fli1 in the gene regulation of MMP-1. Our results suggested that HGF increases MMP-1 promoter activity through increased expression and binding activity of Ets1 and decreased those of Fli1, which was canceled by PD98059, and that MMP-1 gene expression is regulated by the balance of Ets1 and Fli1. Interestingly, Ets1 and Fli1 have opposite effects on the α2(I) collagen gene expression, and Fli1 inhibits the α2(I) collagen promoter activity by competing with Ets1 (33), although the exchange of Fli1 with Ets1 on the promoter is not examined. Similar phenomenon was shown in the MMP-1 promoter in this study. Taken together, the balance of Ets1 and Fli1 may be a novel mechanism in regulating the MMP-1 gene expression. Furthermore, a previous study reported that Ets transcription factors mostly act in concert with AP-1 to regulate MMP-1 expression, and that the binding activity of c-jun to AP-1 binding sequence in the human MMP-1 promoter was increased by HGF stimulation (13). Our study indicated that effect of HGF on the binding activity of c-jun was inhibited by the mutation of EBS. Thus, EBS may be also important for the gene regulation by AP-1, probably through the modification of chromatin structure.
The association between HGF and ERK, between ERK and Ets family, between ERK and MMP-1 or between Ets family and MMP-1 has been well described previously (13,33–38). However, our study clarified the overall regulatory mechanism of MMP-1 by HGF in human dermal fibroblasts.
HGF is expected to express anti-fibrotic effect in various organs. SSc is an acquired disorder, which typically results in fibrosis of the skin and internal organs (39). Fibroblasts from the affected SSc skin cultured in vitro produce excessive amounts of various collagens, mainly type I and type III collagens (40,41), and display increased transcriptional activities of the corresponding genes (42,43). The basal expression of TIMP-1 was increased whereas those of MMP-1 as well as MMP-2 and MMP-3 is reduced in SSc fibroblasts compared with fibroblasts of normal subjects (44,45). Thus, the balance of the synthesis and decomposition of collagen is thought to play an important role in the pathogenesis of this disease, and the induction of MMP-1 may be a reliable approach to the treatment of SSc. On the other hand, despite its anti-fibrotic properties, the serum level of HGF is reported to be markedly increased in SSc (46). Immunocytochemical staining or immunoblotting revealed that c-met was overexpressed in SSc fibroblasts (26,47). These findings are inconsistent with MMP-1 down-regulation in SSc fibroblasts. This contradiction may be explained by the finding that serum HGF levels in SSc were much lower than those used in our study, suggesting that the increase in serum HGF levels is insufficient to regulate MMP-1 expression in SSc fibroblasts (47). We have recently reported that HGF had stronger effects on MMP-1 induction in SSc fibroblasts than in normal fibroblasts, probably due to the overexpression of c-met in SSc fibroblasts (26). In this study, the HGF-treated Ets1 or Fli1 level in SSc fibroblasts was up- or down-regulated more dramatically than in normal fibroblasts, respectively, which may be also a result of the overexpression of c-met in SSc fibroblasts. This result suggested that the EBS plays a role in the hyperreactivity of MMP-1 expression to HGF stimulation in SSc fibroblasts.
Currently, several investigators have reported a therapeutic effect of cyclophosphamide, prednisolone or methotrexate therapy on the fibrosis of SSc (48,49). However, their approach seems to be initiated at the early stage of SSc, before the fibrosis begins to develop. Our study raises the possibility of the clinical use of HGF that it can improve dermal sclerosis in the chronic stage. Further investigation of the effects of HGF on collagen metabolism may contribute to the treatment of fibrosis in SSc.
Acknowledgments
The authors thank Dr Constance E. Brinckerhoff or Dr Maria Trojanowska for kindly providing the full-length 4372 bp clone of the human collagenase (MMP-1) promoter or the expression vectors for Ets1, Fli1, Fli/W321R and Fli/DBD, respectively. This study is supported in part by a grant for scientific research from Japanese Ministry of Education, Science, Sports and Culture, by project research for progressive systemic sclerosis from the Japanese Ministry of Health and Welfare. Funding to pay the Open Access publication charges for this article was provided by a grant for scientific research from the Japanese Ministry of Education.
Conflict of interest statement. None declared.
REFERENCES
- 1.Nakamura T., Nawa K., Ichihara A. Partial purification and characterization of hepatocyte growth factor from serum of hepatectomized rats. Biochem. Biophys. Res. Commun. 1984;122:1450–1459. doi: 10.1016/0006-291x(84)91253-1. [DOI] [PubMed] [Google Scholar]
- 2.Gohda E., Tsubouchi H., Nakayama H., Hirono S., Sakiyama O., Takahashi K., Miyazaki H., Hashimoto S., Daikuhara Y. Purification and partial characterization of hepatocyte growth factor from plasma of a patient with fulminant hepatic failure. J. Clin. Invest. 1988;81:414–419. doi: 10.1172/JCI113334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zarnegar R., Michalopoulos G. Purification and biological characterization of human hepatopoietin A, a polypeptide growth factor for hepatocytes. Cancer Res. 1989;49:3314–3320. [PubMed] [Google Scholar]
- 4.Nakamura T., Nishizawa T., Hagiya M., Seki T., Shimonishi M., Sugimura A., Tashiro K., Shimizu S. Molecular cloning and expression of human hepatocyte growth factor. Nature. 1989;342:440–443. doi: 10.1038/342440a0. [DOI] [PubMed] [Google Scholar]
- 5.Stoker M., Gherardi E., Perryman M., Gray J. Scatter factor is a fibroblast-derived modulator of epithelial cell mobility. Nature. 1987;327:239–242. doi: 10.1038/327239a0. [DOI] [PubMed] [Google Scholar]
- 6.Weidner K.M., Arakaki N., Hartmann G., Vandekerckhove J., Weingart S., Rieder H., Fonatsch C., Tsubouchi H., Hishida T., Daikuhara Y., et al. Evidence for the identity of human scatter factor and human hepatocyte growth factor. Proc. Natl Acad. Sci. USA. 1991;88:7001–7005. doi: 10.1073/pnas.88.16.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boros P., Miller C.M. Hepatocyte growth factor: a multifunctional cytokine. Lancet. 1995;345:293–295. doi: 10.1016/s0140-6736(95)90279-1. [DOI] [PubMed] [Google Scholar]
- 8.Yasuda H., Imai E., Shiota A., Fujise N., Morinaga T., Higashio K. Antifibrogenic effect of a deletion variant of hepatocyte growth factor on liver fibrosis in rats. Hepatology. 1996;24:636–642. doi: 10.1053/jhep.1996.v24.pm0008781336. [DOI] [PubMed] [Google Scholar]
- 9.Matusuda Y., Matsumoto K., Ichida T., Nakamura T. Hepatocyte growth factor suppresses the onset of liver cirrhosis and abrogates lethal dysfunction in rats. J. Biochem. 1995;118:643–649. doi: 10.1093/oxfordjournals.jbchem.a124958. [DOI] [PubMed] [Google Scholar]
- 10.Matsuda Y., Matsumoto K., Yamada A., Ichida T., Asakura H., Komoriya Y., Nishiyama E., Nakamura T. Preventive and therapeutic effects in rats of hepatocyte growth factor infusion on liver fibrosis/cirrhosis. Hepatology. 1997;26:81–89. doi: 10.1053/jhep.1997.v26.pm0009214455. [DOI] [PubMed] [Google Scholar]
- 11.Ueki T., Kaneda Y., Tsutsui H., Nakanishi K., Sawa Y., Morishita R., Matsumoto K., Nakamura T., Takahashi H., Okamoto E., et al. Hepatocyte growth factor gene therapy of liver cirrhosis in rats. Nature Med. 1999;5:226–230. doi: 10.1038/5593. [DOI] [PubMed] [Google Scholar]
- 12.Brummer O., Bohmer G., Hollwitz B., Flemming P., Petry K.U., Kuhnle H. MMP-1 and MMP-2 in the cervix uteri in different steps of malignant transformation—an immunohistochemical study. Gynecol. Oncol. 2002;84:222–227. doi: 10.1006/gyno.2001.6413. [DOI] [PubMed] [Google Scholar]
- 13.Ozaki I., Zhao G., Mizuta T., Ogawa Y., Hara T., Kajihara S., Hisatomi A., Sakai T., Yamamoto K. Hepatocyte growth factor induces collagenase (matrix metalloproteinase-1) via the transcription factor Ets-1 in human hepatic stellate cell line. J. Hepatol. 2002;36:169–178. doi: 10.1016/s0168-8278(01)00245-8. [DOI] [PubMed] [Google Scholar]
- 14.Ihn H., Ohnishi K., Tamaki T., LeRoy E.C., Trojanowska M. Transcriptional regulation of the human α2(I) collagen gene. Combined action of upstream stimulatory and inhibitory cis-acting elements. J. Biol. Chem. 1996;271:26717–26723. doi: 10.1074/jbc.271.43.26717. [DOI] [PubMed] [Google Scholar]
- 15.Ihn H., LeRoy E.C., Trojanowska M. Oncostatin M stimulates transcription of the human α2(I) collagen gene via the Sp1/Sp3-binding site. J. Biol. Chem. 1997;272:24666–24672. doi: 10.1074/jbc.272.39.24666. [DOI] [PubMed] [Google Scholar]
- 16.Verheijen J.H., Nieuwenbroek N.M., Beekman B., Hanemaaijer R., Verspaget H.W., Ronday H.K., Bakker A.H. Modified proenzymes as artificial substrates for proteolytic enzymes: colorimetric assay of bacterial collagenase and matrix metalloproteinase activity using modified pro-urokinase. Biochem. J. 1997;323:603–609. doi: 10.1042/bj3230603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yagi K., Furuhashi M., Aoki H., Goto D., Kuwano H., Sugamura K., Miyazono K., Kato M. c-myc is a downstream target of the Smad pathway. J. Biol. Chem. 2002;277:854–861. doi: 10.1074/jbc.M104170200. [DOI] [PubMed] [Google Scholar]
- 18.Rutter J.L., Benbow U., Coon C.I., Brinckerhoff C.E. Cell-type specific regulation of human interstitial collagenase-1 gene expression by interleukin-1β (IL-1β) in human fibroblasts and BC-8701 breast cancer cells. J. Cell. Biochem. 1997;66:322–336. [PubMed] [Google Scholar]
- 19.Shirasaki F., Makhluf H.A., LeRoy C., Watson D.K., Trojanowska M. Ets transcription factors cooperate with Sp1 to activate the human tenascin-C promoter. Oncogene. 1999;18:7755–7764. doi: 10.1038/sj.onc.1203360. [DOI] [PubMed] [Google Scholar]
- 20.Sementchenko V.I., Schweinfest C.W., Papas T.S., Watson D.K. ETS2 function is required to maintain the transformed state of human prostate cancer cells. Oncogene. 1998;17:2883–2888. doi: 10.1038/sj.onc.1202220. [DOI] [PubMed] [Google Scholar]
- 21.Bailly R.A., Bosselut R., Zucman J., Cormier F., Delattre O., Roussel M., Thomas G., Ghysdael J. DNA-binding and transcriptional activation properties of the EWS-FLI-1 fusion protein resulting from the t(11;22) translocation in Ewing sarcoma. Mol. Cell. Biol. 1994;14:3230–3241. doi: 10.1128/mcb.14.5.3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Czuwara-Ladykowska J., Shirasaki F., Jackers P., Watson D.K., Trojanowska M. Fli-1 inhibits collagen type I production in dermal fibroblasts via an Sp1-dependent pathway. J. Biol. Chem. 2001;276:20839–20848. doi: 10.1074/jbc.M010133200. [DOI] [PubMed] [Google Scholar]
- 23.Ihn H., Yamane K., Asano Y., Kubo M., Tamaki K. IL-4 up-regulates the expression of tissue inhibitor of metalloproteinase-2 in dermal fibroblasts via the p38 mitogen-activated protein kinase dependent pathway. J. Immunol. 2002;168:1895–1902. doi: 10.4049/jimmunol.168.4.1895. [DOI] [PubMed] [Google Scholar]
- 24.Westermarck J., Seth A., Kahari V.-M. Differential regulation of interstitial collagenase (MMP-1) gene expression by ETS transcription factors. Oncogene. 1997;14:2651–2660. doi: 10.1038/sj.onc.1201111. [DOI] [PubMed] [Google Scholar]
- 25.Schroen D.G., Brinckerhoff C.E. Inhibition of rabbit collagenase (matrix metalloproteinase-1: MMP-1) transcription by retinoid receptors: evidence for binding of RARs/RXRs to the −77 AP-1 site through interactions with c-jun. J. Cell. Physiol. 1996;169:320–332. doi: 10.1002/(SICI)1097-4652(199611)169:2<320::AID-JCP11>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 26.Jinnin M., Ihn H., Mimura Y., Asano Y., Yamane K., Tamaki K. Effects of hepatocyte growth factor on the expression of type I collagen and matrix metalloproteinase-1 in normal and scleroderma dermal fibroblasts. J. Invest. Dermatol. 2005;24:324–330. doi: 10.1111/j.0022-202X.2004.23601.x. [DOI] [PubMed] [Google Scholar]
- 27.Kubo M., Czuwara-Ladykowska J., Moussa O., Markiewicz M., Smith E., Silver R.M., Jablonska S., Blaszczyk M., Watson D.K., Trojanowska M. Persistent down-regulation of Fli1, a suppressor of collagen transcription, in fibrotic scleroderma skin. Am. J. Pathol. 2003;163:571–581. doi: 10.1016/S0002-9440(10)63685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watson D.K., McWilliams M.J., Lapis P., Lautenberger J.A., Schweinfest C.W., Papas T.S. Mammalian ets-1 and ets-2 genes encode highly conserved proteins. Proc. Natl Acad. Sci. USA. 1988;85:7862–7866. doi: 10.1073/pnas.85.21.7862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tymms M.J., Kola I. Regulation of gene expression by transcription factors Ets-1 and Ets-2. Mol. Reprod. Dev. 1994;39:208–214. doi: 10.1002/mrd.1080390214. [DOI] [PubMed] [Google Scholar]
- 30.Westermarck J., Kahari V.-M. Regulation of matrix metalloproteinase expression in tumor invasion. FASEB J. 1999;13:781–792. [PubMed] [Google Scholar]
- 31.Goldberg G.I., Wilhelm S.M., Kronberger A., Bauer E.A., Grant G.A., Eisen A.Z. Human fibroblast collagenase: complete primary structure and homology to an oncogene transformation-induced rat protein. J. Biol. Chem. 1986;261:6600–6605. [PubMed] [Google Scholar]
- 32.Wasylyk C., Gutman A., Nicholson R., Wasylyk B. The c-Ets oncoprotein activates the stromelysin promoter through the same elements as several non-nuclear oncoporteins. EMBO J. 1991;10:1127–1134. doi: 10.1002/j.1460-2075.1991.tb08053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Czuwara-Ladykowska J., Sementchenko V.I., Watson D.K., Trojanowska M. Ets1 is an effector of the transforming growth factor β (TGF-β) signaling pathway and an antagonist of the profibrotic effects of TGF-β. J. Biol. Chem. 2002;277:20399–20408. doi: 10.1074/jbc.M200206200. [DOI] [PubMed] [Google Scholar]
- 34.Jones M.K., Sasaki E., Halter F., Pai R., Nakamura T., Arakawa T., Kuroki T., Tarnawski A.S. HGF triggers activation of the COX-2 gene in rat gastric epithelial cells: action mediated through the ERK2 signaling pathway. FASEB J. 1999;13:2186–2194. doi: 10.1096/fasebj.13.15.2186. [DOI] [PubMed] [Google Scholar]
- 35.Nakagami H., Morishita R., Yamamoto K., Taniyama Y., Aoki M., Matsumoto K., Nakamura T., Kaneda Y., Horiuchi M., Ogihara T. Mitogenic and antiapoptotic actions of hepatocyte growth factor through ERK, STAT3, and AKT in endothelial cells. Hypertension. 2001;37:581–586. doi: 10.1161/01.hyp.37.2.581. [DOI] [PubMed] [Google Scholar]
- 36.Silvany R.E., Eliazer S., Wolff N.C., Ilaria R.L., Jr Interference with the constitutive activation of ERK1 and ERK2 impairs EWS/FLI-1-dependent transformation. Oncogene. 2000;19:4523–4530. doi: 10.1038/sj.onc.1203811. [DOI] [PubMed] [Google Scholar]
- 37.Wan Y., Belt A., Wang Z., Voorhees J., Fisher G. Transmodulation of epidermal growth factor receptor mediates IL-1β-induced MMP-1 expression in cultured human keratinocytes. Int. J. Mol. Med. 2001;7:329–334. [PubMed] [Google Scholar]
- 38.Tower G.B., Coon C.C., Benbow U., Vincenti M.P., Brinckerhoff C.E. Erk 1/2 differentially regulates the expression from the 1G/2G single nucleotide polymorphism in the MMP-1 promoter in melanoma cells. Biochim. Biophys. Acta. 2002;1586:265–274. doi: 10.1016/s0925-4439(01)00105-3. [DOI] [PubMed] [Google Scholar]
- 39.LeRoy E.C., Black C., Fleischmajer R., Jablonska S., Krieg T., Medsger T.A., Jr Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J. Rheumatol. 1988;15:202–206. [PubMed] [Google Scholar]
- 40.LeRoy E.C. Increased collagen synthesis by scleroderma skin fibroblasts in vitro: a possible defect in the regulation or activation of the scleroderma fibroblast. J. Clin. Invest. 1974;54:880–889. doi: 10.1172/JCI107827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jimenez S.A., Feldman G., Bashey R.I., Bienkowski R., Rosenbloom J. Co-ordinate increase in the expression of type I and type III collagen genes in progressive systemic sclerosis. Biochem. J. 1986;237:837–843. doi: 10.1042/bj2370837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kikuchi K., Smith E.A., LeRoy E.C., Trojanowska M. Direct demonstration of transcriptional activation of collagen gene expression in systemic sclerosis fibroblasts. Biochem. Biophys. Res. Commun. 1992;187:45–50. doi: 10.1016/s0006-291x(05)81456-1. [DOI] [PubMed] [Google Scholar]
- 43.Hitraya E.G., Jimenez S.A. Transcriptional activation of the α(I) procollagen gene in systemic sclerosis dermal fibroblasts. Role of intronic sequences. Arthritis Rheum. 1996;39:1347–1354. doi: 10.1002/art.1780390812. [DOI] [PubMed] [Google Scholar]
- 44.Takeda K., Hatamochi A., Ueki H., Nakata M., Oishi Y. Decreased collagenase expression in cultured systemic sclerosis fibroblasts. J. Invest. Dermatol. 1994;103:359–363. doi: 10.1111/1523-1747.ep12394936. [DOI] [PubMed] [Google Scholar]
- 45.Kuroda K., Shinkai H. Gene expression of types I and III collagen, decorin, matrix metalloproteinases and tissue inhibitors of metalloproteinases in skin fibroblasts from patients with systemic sclerosis. Arch. Dermatol. Res. 1997;289:567–572. doi: 10.1007/s004030050241. [DOI] [PubMed] [Google Scholar]
- 46.Kawaguchi Y., Harigai M., Fukasawa C., Hara M. Increased levels of hepatocyte growth factor in sera of patients with systemic sclerosis. J. Rheumatol. 1999;26:1012–1013. [PubMed] [Google Scholar]
- 47.Kawaguchi Y., Harigai M., Hara M., Fukasawa C., Takagi K., Tanaka M., Tanaka E., Nishimagi E., Kamatani N. Expression of hepatocyte growth factor and its receptor (c-met) in skin fibroblasts from patients with systemic sclerosis. J. Rheumatol. 2002;29:1877–1883. [PubMed] [Google Scholar]
- 48.Apras S., Ertenli I., Ozbalkan Z., Kiraz S., Ozturk M.A., Haznedaroglu I.C., Cobankara V., Pay S., Calguneri M. Effects of oral cyclophosphamide and prednisolone therapy on the endothelial functions and clinical findings in patients with early diffuse systemic sclerosis. Arthritis Rheum. 2003;48:2256–2261. doi: 10.1002/art.11081. [DOI] [PubMed] [Google Scholar]
- 49.Pope J.E., Bellamy N., Seibold J.R., Baron M., Ellman M., Carette S., Smith C.D., Chalmers I.M., Hong P., O'Hanlon D., et al. A randomized, controlled trial of methotrexate versus placebo in early diffuse scleroderma. Arthritis Rheum. 2001;44:1351–1358. doi: 10.1002/1529-0131(200106)44:6<1351::AID-ART227>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]