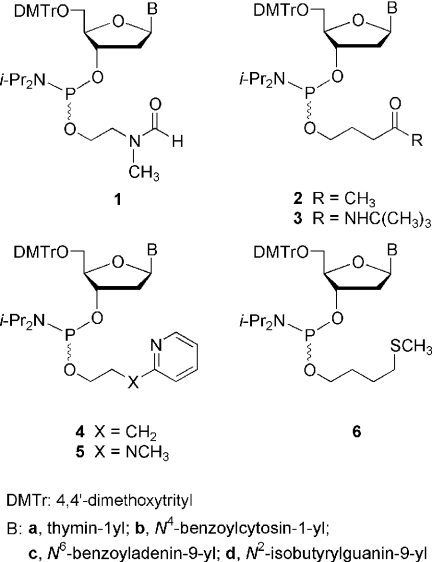
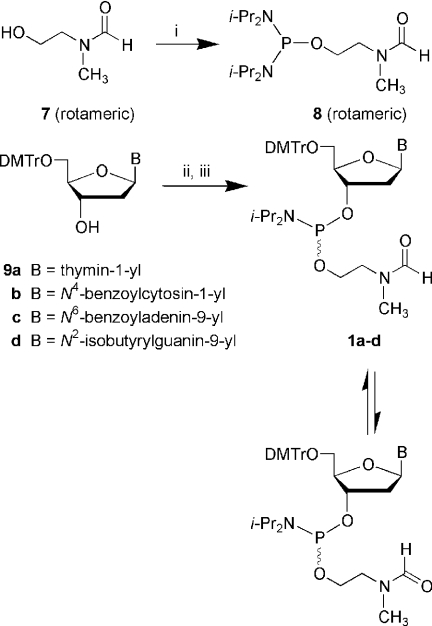
Abstract
A CpG-containing DNA oligonucleotide functionalized with the 2-(N-formyl-N-methyl)aminoethyl thiophosphate protecting group (CpG ODN fma1555) was prepared from phosphoramidites 1a–d using solid-phase techniques. The oligonucleotide behaved as a prodrug by virtue of its conversion to the well-studied immunomodulatory CpG ODN 1555 through thermolytic cleavage of the 2-(N-formyl-N-methyl)aminoethyl thiophosphate protecting group. Such a conversion occurred at 37°C with a half-time of 73 h. The immunostimulatory properties of CpG ODN fma1555 were evaluated in two in vivo assays, one of which consisted of mice challenged in the ear with live Leishmania major metacyclic promastigotes. Local intradermal administration of CpG ODN fma1555 was as effective as that of CpG ODN 1555 in reducing the size of Leishmania lesions over time. In a different infectious model, CpG ODN 1555 prevented the death of Tacaribe-infected mice (43% survival) when administered between day 0 and 3 post infection. Administration of CpG ODN fma1555 three days before infection resulted in improved immunoprotection (60–70% survival). Moreover, co-administration of CpG ODN fma1555 and CpG ODN 1555 in this model increased the window for therapeutic treatment against Tacaribe virus infection, and thus supports the use of thermolytic oligonucleotides as prodrugs in the effective treatment of infectious diseases.
INTRODUCTION
Over the last few years, our research efforts have focused on the design and development of thermolytic groups for 5′-hydroxyl (1) and phosphate (2–7) protection in an attempt to implement a ‘heat-driven’ process (8) for the synthesis of DNA oligonucleotides on microarrays. In sharp contrast to the current methods employed for oligonucleotide synthesis, such a process would involve rapid and efficient thermal removal of protecting groups from oligonucleotides under neutral conditions throughout chain assembly and final deprotection. Specifically, heat-sensitive phosphate/thiophosphate protecting groups exhibiting unique thermolytic properties have been incorporated into oligonucleotides via phosphoramidites 1–6 (Scheme 1) employing solid-phase techniques.
Scheme 1.
Deoxyribonucleoside phosphoramidites functionalized with thermolytic groups for phosphorus protection.
The deprotection mechanism of each thermolytic phosphate protecting group is consistent with a well-studied intramolecular cyclodeesterification reaction (9–12), which has also been observed by others (13–16). Given the relatively rapid phosphate deprotection kinetics of DNA oligonucleotides that have been prepared using phosphoramidites 4, 5 and 6 (6,7), the thermolytic 3-(2-pyridyl)-1-propyl, 2-[N-methyl-N-(2-pyridyl)]aminoethyl, and 4-methythio-1-butyl groups for phosphate protection may indeed find application in the synthesis of oligonucleotides on microarrays considering the mildness of the deprotection conditions used for each group. However, oligonucleotides functionalized with the 2-(N-formyl-N-methyl)aminoethyl group for phosphate/thiophosphate protection require a much higher temperature (90°C) over a considerably longer period of time (∼3 h) to complete the thermolytic cleavage of this protecting group. Although these conditions are not optimal for the preparation of diagnostic oligonucleotide microarrays, oligonucleotides functionalized with the 2-(N-formyl-N-methyl)aminoethyl phosphate/thiophosphate protecting group may serve as potential therapeutic oligonucleotide prodrugs in vivo on the basis of the anticipated sluggish removal of the phosphate/thiophosphate protecting group at 37°C.
Oligonucleotide prodrugs have been the focus of intense scrutiny in recent years in an effort to facilitate cellular uptake of antisense oligonucleotides and provide these biomolecules with increased resistance to hydrolytic nucleases. Thus, masking the phosphodiester groups of oligonucleotides with acylthioethyl (17–21), acyloxymethyl (22) and 4-acyloxybenzyl (23,24) protecting groups or with groups derived from bis(hydroxymethyl)-1,3-dicarbonyl compounds (25,26) that would expectedly be cleaved upon reaction with intracellular enzymes, offers a viable solution to the notoriously poor cellular delivery of negatively charged oligonucleotide drugs. From this perspective, oligonucleoside phosphorothioates functionalized with the 2-(N-formyl-N-methyl)aminoethyl group for thiophosphate protection are likely to exhibit the characteristics of oligonucleotide prodrugs in that they are uncharged and, thus, inherently resistant to the hydrolytic activity of nucleases. A distinctive feature of this class of modified oligonucleotides lies in that esterases or other intracellular enzymes are not required for prodrug to drug conversion. Only a 37°C environment is necessary to thermolytically convert oligonucleoside 2-(N-formyl-N-methyl)aminoethyl phosphorothioate triesters to functional oligonucleoside phosphorothioate diesters.
Synthetic single-stranded DNA oligonucleoside phosphorothioates containing unmethylated CpG motifs (CpG ODNs) have been selected as a model to evaluate whether thermolytic CpG ODNs can be administered as prodrugs in vivo. Typically, CpG ODNs are rapidly internalized by immune cells (B cells, macrophages, dendritic cells and monocytes) (27,28) and localize to endocytic vesicles where they interact with Toll-like receptor 9. This interaction triggers an immunostimulatory cascade that is characterized by B-cell proliferation, dendritic cell maturation, natural killer cell activation, and the secretion of a variety of cytokines, chemokines and polyreactive immunoglobulins (27,29). Such a pro-inflammatory and Th1-biased immune response improves resistance of the host to infectious pathogenic microorganisms. In this regard, numerous studies indicate that administration of CpG ODNs can act alone to improve the response to parasitic, bacterial and viral infections in animal models (30–32). In this study, we report the synthesis and characterization of a CpG ODN functionalized with thermolytic 2-(N-formyl-N-methyl)aminoethyl phosphorothioate triesters and assess for the first time its immunostimulatory and immunoprotective properties in vivo.
MATERIALS AND METHODS
2-(N-Formyl-N-methyl)aminoethan-1-ol (7)
This compound (Scheme 2) was prepared from the reaction of 2-(methylamino)ethanol (Aldrich) with ethyl formate (Aldrich) as described earlier (2).
Scheme 2.
Synthesis of the phosphinylating reagent 8 and preparation of deoxyribonucleoside phosphoramidites (1a–d) from suitably protected deoxyribonucleosides (9a–d). (i) bis(N, N-diisopropylamino)chlorophosphine, N,N-diisopropylethylamine, CH2Cl2, 25°C, 2 h; (ii) 8, 1H-tetrazole, MeCN, 25°C, 2–16 h; (iii) silica gel chromatography.
N,N,N′,N′-Tetraisopropyl-O-2-[(N-formyl-N-methyl)aminoethyl]phosphorodiamidite (8)
This phosphinylating reagent (Scheme 2) was prepared following a procedure that had been modified from its earlier version (2,12). To a stirred solution of 2-(N-formyl-N-methyl)aminoethan-1-ol (3.50 g, 34.0 mmol) and N, N-diisopropylethylamine (35.0 ml, 201 mmol) in anhydrous dichloromethane (20 ml) was added, at 25°C, a solution of bis(N, N-diisopropylamino)chlorophosphine (Aldrich) (9.98 g, 37.4 mmol) in dry dichloromethane (10 ml). Formation of the phosphorodiamidite was monitored by 31P NMR spectroscopy, which revealed over a period of 2 h, complete conversion of bis(N, N-diisopropylamino)chlorophosphine (δP 135.5 p.p.m.) to the desired product as a mixture of rotamers (δP 118.0 and 118.7 p.p.m.). The suspension was filtered and the filtrate was evaporated to an oil under reduced pressure. The material was transferred to a 50 ml round bottom flask, which was then connected to a vacuum-jacketed short path distilling head and a distributing adapter. Vacuum distillation was performed using a heat gun to enable rapid heating without substantial decomposition. A colorless distillate (bp 145°C at 1 mmHg) was obtained in 67% yield (7.58 g, 22.8 mmol). 1H NMR (300 MHz, C6D6): δ 3.56 (m, 2H), 3.53 (sept, J = 6.9 Hz, 2H), 3.49 (sept, J = 6.9 Hz, 2H), 2.30 (m, 2H), 1.80 (s, 3H), 1.63 (m, 4H), 1.23 (d, J = 6.9 Hz, 12H), 1.19 (d, J = 6.9 Hz, 12H). 13C NMR (75 MHz, C6D6): δ 15.2, 24.0, 24.1, 24.7, 24.8, 26.2, 31.1 (d, JPC = 9.6 Hz), 34.2, 44.6, 44.7, 64.1 (d, 2JPC = 21.5 Hz). 31P NMR (121 MHz, C6D6): δ 118.0, 118.7. EI-HRMS: calcd for C16H36N3O2P (M•)+ 333.2545, found 333.2528.
General procedure for the preparation of deoxyribonucleoside phosphoramidites 1a–d
A properly protected deoxyribonucleoside (9a–d, 2 mmol) was dried under high vacuum for 4 h in a 50 ml round-bottom flask and, then, dissolved in anhydrous MeCN (10 ml). To this solution was added N, N, N′, N′-tetraisopropyl-O-2-[(N-formyl-N-methyl)aminoethyl]phosphorodiamidite (8, 730 mg, 2.2 mmol) followed by 0.45 M 1H-tetrazole in MeCN (4.4 ml, 2 mmol), dropwise, over a period of 0.5 h (Scheme 2). Phosphinylation of suitably protected 2′-deoxyribonucleosides was usually complete within 2 h at 25°C with the exception of protected 2′-deoxyguanosine, which was allowed to proceed overnight. The reaction mixture was then concentrated under reduced pressure, dissolved in benzene:triethylamine (9:1 v/v), and chromatographed using a column (4 × 10 cm) containing silica gel 60 (230–400 mesh, ∼20 g) equilibrated in benzene:triethylamine (9:1 v/v). The phosphoramidites were eluted from the column using the equilibration solvent as the eluant. Appropriate fractions were pooled, concentrated, and each of the deoxyribonucleoside phosphoramidites 1a–d was obtained as a white foamy material. The purified product was dissolved in ∼3 ml of benzene and the solution was added to ∼100 ml of cold (−20°C) vigorously stirred hexane. The suspension was allowed to settle and most of the supernatant was carefully decanted. The wet material was pulverized under reduced pressure, and then dissolved in ∼10 ml of benzene. The solution was frozen in a dry-ice/acetone bath, and lyophilized under high vacuum affording triethylamine-free phosphoramidites as white amorphous solids in yields ranging from 70 to 85%.
5′-O-(4,4′-dimethoxytrityl)-3′-O-(N, N-diisopropylamino)[2-(N-formyl-N-methyl)aminoethoxy]phosphinyl-2′-deoxythymidine (1a)
31P NMR (121 MHz, C6D6): δ 148.4, 148.3, 148.2. FAB-HRMS: calcd for C41H53N4O9P (M + Cs)+ 909.2604, found 909.2544.
N4-benzoyl-5′-O-(4,4′-dimethoxytrityl)-3′-O-(N, N-diisopropylamino)[2-(N-formyl-N-methyl)aminoethoxy]phosphinyl-2′-deoxycytidine (1b)
31P NMR (121 MHz, C6D6): δ 149.0, 148.9, 148.5, 148.4. FAB-HRMS: calcd for C47H56N5O9P (M + Na)+ 888.3714, found 888.3745.
N6-benzoyl-5′-O-(4,4′-dimethoxytrityl)-3′-O-(N, N-diisopropylamino)[2-(N-formyl-N-methyl)aminoethoxy]phosphinyl-2′-deoxyadenosine (1c)
31P NMR (121 MHz, C6D6): δ 148.9, 148.8, 148.1. FAB-HRMS: calcd for C48H56N7O8P (M + Na)+ 912.3827, found 912.3843.
N2-isobutyryl-5′-O-(4,4′-dimethoxytrityl)-3′-O-(N, N-diisopropylamino)[2-(N-formyl-N-methyl)aminoethoxy] phosphinyl-2′-deoxyguanosine (1d)
31P NMR (121 MHz, C6D6): δ 149.0, 143.9, 143.7. FAB-HRMS: calcd for C45H58N7O9P (M + Na)+ is 894.3933, found 894.3978.
Proton-decoupled 31P NMR spectra were recorded at 7.05 T (300 MHz for 1H) using deuterated solvents and 85% phosphoric acid in deuterium oxide as an external reference. The NMR spectrometer was run at 25°C and chemical shifts δ are reported in parts per million.
Low- and high-resolution FAB mass spectra were acquired from samples dissolved in either 4-nitrobenzyl alcohol or a mixture of dithiothreitol and dithioerythritol (3:1, v/v), and bombarded with 8 keV fast cesium ions. A mass calibration standard of cesium iodide, or a mixture of cesium iodide and sodium iodide, was used. Accurate mass measurements were performed on [M + H]+ or on [M + Na]+ ions, which were obtained by addition of aqueous sodium iodide to the sample matrix.
Solid-phase oligonucleotide synthesis
Solid phase synthesis of d(GPS(FMA)CPS(FMA)TPS(FMA)APS(FMA)GPS(FMA)APS(FMA)CPS(FMA)GPS(FMA)TPS(FMA)TPS(FMA)APS(FMA)GPS(FMA)CPS(FMA)GPS(FMA)T) [CpG ODN fma1555], d(GPS(FMA)CPS(FMA)TPS(FMA)APS(FMA)GPS(FMA)APS(FMA)GPS(FMA)GPS(FMA)TPS(FMA)TPS(FMA)APS(FMA)GPS(FMA)GPS(FMA)GPS(FMA)T) [ODN fma1556], where PS(FMA) stands for a thermolytic 2-(N-formyl-N-methyl)aminoethyl phosphorothioate triester function, and d(GPSCPSTPSAPSGPSAPSCPSGPSTPSTPSAPSGPSCPSGPST) [CpG ODN 1555], where PS stands for a phosphorothioate diester function, was performed on a scale of 1 μmol using a succinyl long chain alkylamine controlled-pore glass (Succ-LCAA-CPG) support functionalized with 5′-O-DMTr-dT as the leader nucleoside. The syntheses were carried out using an ABI 392 DNA/RNA synthesizer and phosphoramidites 1a–d or commercial 2-cyanoethyl deoxyribonucleoside phosphoramidites as 0.1 M solutions in dry MeCN. The reaction time for each phosphoramidite coupling step was 180 s. With the exception of the deblocking solution, all ancillary reagents necessary for the preparation of oligonucleotides were purchased and utilized as recommended by the instrument's manufacturer. Given that only thioated oligodeoxyribonucleotides were prepared, the iodine oxidation step of the synthesis cycle was replaced with a sulfurization step employing 0.05 M 3H-1,2-benzodithiol-3-one 1,1-dioxide in MeCN, as recommended in the literature (33,34). The sulfurization step was performed before the capping step, and the reaction time for these steps was 120 and 60 s, respectively. The dedimethoxytritylation step of the synthesis cycle was effected over a period of 60 s with a freshly prepared solution of 3% trichloroacetic acid (w/v) in dichloromethane.
Oligonucleotide deprotection and purification
The synthesis column containing the 5′-O-dimethoxytritylated oligonucleotide was placed into a stainless steel pressure vessel and exposed to pressurized ammonia (10 bar at 25°C) for 12 h (35). Upon removal of residual ammonia from the pressure container, the 5′-O-DMTr-oligonucleotide was eluted off the column with 40% MeCN in 0.1 M triethylammonium acetate (TEAA, pH 7.0) (1 ml). The purification of each oligonucleotide was accomplished by reverse phase high performance liquid chromatography (RP-HPLC) employing a UV detection system and a semi-preparative 5 μm Supelcosil LC-18-S column (10 mm × 25 cm). An elution gradient for the purification of 5′-O-DMTr-CpG ODN fma1555 or 5′-O-DMTr-ODN fma1556 was optimized as follows: starting from 5% MeCN in 0.1 M TEAA (pH 7.0), 1.5% MeCN/min was pumped at a flow rate of 3 ml/min for 30 min. A different elution gradient was however used for the purification of 5′-O-DMTr-CpG ODN 1555; thus starting from 0.1 M TEAA (pH 7.0), 1% MeCN/min was pumped at a flow rate of 3 ml/min for 40 min. The product peaks were collected and the eluates were evaporated using a stream of air without heating. The residue was dissolved in 80% acetic acid (1 ml) and the solution was left standing at ambient temperature for 30 min. The acidic solution was also evaporated through the use of a stream of air without a heat source. Each oligonucleotide was then dissolved in a solution of 40% MeCN in 0.1 M TEAA (pH 7.0) (1 ml) and purified by RP-HPLC using the same conditions (column and elution gradient) as those employed for the purification of the respective 5′-O-DMTr-CpG ODN fma1555, 5′-O-DMTr-ODN fma1556 and 5′-O-DMTr-CpG ODN 1555. The pooling and evaporation of eluates containing each purified 5′-O-deprotected oligonucleotide was performed in a manner similar to that described for the respective 5′-O-DMTr-oligonucleotides. After reconstitution of each purified oligonucleotide in ddH2O (1 ml), its concentration was determined by UV spectrophotometry at 260 nm. The recovered yields of CpG ODN fma1555 and ODN fma1556 were 65 and 68 OD260 units, respectively, whereas the recovered yield of CPG ODN 1555 was 95 OD260 units. Each oligonucleotide solution was stored frozen at −20°C.
Oligonucleotide characterization
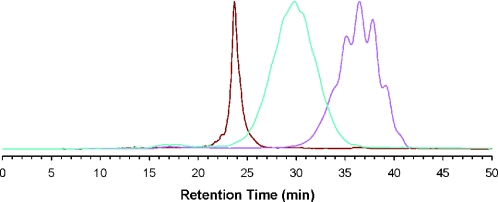
RP-HPLC analysis of purified CpG ODN fma1555 was performed using an analytical 5 μm Supelcosil LC-18S column (4.6 mm × 25 cm) according to the following conditions: starting from 0.1 M TEAA (pH 7.0), a linear gradient of 1% MeCN/min was pumped at a flow rate of 1 ml/min for 40 min. An RP-HPLC profile of the purified oligonucleotide is shown in Figure 1.
Figure 1.
RP-HPLC profiles of d(GPS(FMA)CPS(FMA)TPS(FMA)APS(FMA)GPS(FMA)APS(FMA)CPS(FMA)GPS(FMA)TPS(FMATPS(FMA)APS(FMA)GPS(FMA)CPS(FMA)GPS(FMA)T) [CpG ODN fma1555]. Magenta line: Chromatographic profile of purified CpG ODN fma1555. Brown line: Chromatographic profile of purified CpG ODN fma1555 that was heated in 1× PBS (pH 7.2) for 626 h at 37°C. Turquoise line: Chromatographic profile of purified CpG ODN fma1555 that was heated in 1× PBS (pH 7.2) at 37°C for 73 h. RP-HPLC analyses were performed using a 5 μm Supelcosil LC-18S column (4.6 mm × 25 cm) according to the following conditions: starting from 0.1 M TEAA (pH 7.0), a linear gradient of 1% MeCN/min is pumped at a flow rate of 1 ml/min for 40 min. Peak heights of each profile are normalized to the highest peak, which is set to one arbitrary unit.
Samples of purified CpG ODN fma1555, CpG ODN 1555 and ODN 1556 were analyzed employing a Waters 1525μ binary pump equipped with a Waters 2777 sample manager, which was connected online to an orthogonal Electrospray Ionization Time of Flight (ESI-TOF) mass spectrometer (Micromass LCT Premier, Waters, Milford, ME). The mobile phase for direct introduction of the sample was composed of 0.2% formic acid (40%) and acetonitrile (60%). MS chromatograms were acquired in the positive ion mode using an ESI-MS capillary voltage of 3.5 kV, a sample cone voltage of 60 V, and an MCP detector voltage of 2200 V. Desolvation gas flow rate was maintained at 400 l/h, cone gas flow rate at 50 l/h. Desolvation temperature and source temperature were set to 150 and 80°C, respectively. The acquisition range was set at m/z 50–2000. The 2.1 s scan cycle consisted of a 2 s acquisition time and a 0.1 s interscan delay. Instrument calibration was performed routinely in positive ion mode prior to MS experiments by direct infusion of sodium formate in 2-propanol:water (9:1 v/v) at 10 μl/min. Leu-enkephalin was used as a lock mass and MassLynx was the operating software for the MS system. Raw summed spectra were deconvoluted using the MaxEnt1™ software.
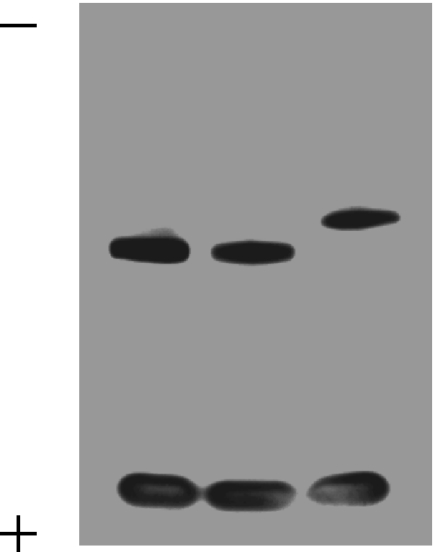
To further characterize CpG ODN fma1555 and ODN fma1556, the purified oligonucleotides (1 OD260 unit each) were dissolved in 1× Phosphate-buffered saline (PBS, pH 7.2) (0.5 ml) and placed in a heat block, pre-heated to 37 ± 2°C, to thermolytically cleave the 2-(N-formyl-N-methyl)aminoethyl thiophosphate protecting group over a period of 626 h. Each fully deprotected oligonucleotide (0.25 OD260 unit) was analyzed by PAGE using a denaturing 20% polyacrylamide–7 M urea gel (40 cm × 20 cm × 0.75 mm), which was prepared as described by Maniatis et al. (36). Electrophoresis was carried out at 350 V until the bromophenol blue dye of the loading buffer traveled ∼80% of the length of the gel. The gel was then stained by soaking in a solution of Stains-all, as reported elsewhere (10). A photograph of the gel is shown in Figure 2.
Figure 2.
PAGE analysis of fully deprotected and RP-HPLC-purified oligonucleoside phosphorothioates under denaturing conditions (20% polyacrylamide–7 M urea, 1× TBE buffer, pH 8.3). Left lane: CpG ODN 1555 synthesized from commercial 2-cyanoethyl deoxyribonucleoside phosphoramidites and deprotected by treatment with pressurized ammonia gas (∼10 bar) for 12 h at 25°C. Middle lane: CpG ODN fma1555 synthesized from phosphoramidites 1a–d and deprotected by treatment with pressurized ammonia gas (∼10 bar) for 12 h at 25°C followed by heating in 1× PBS (pH 7.2) for 626 h at 37°C. Right lane: ODN fma1556 synthesized and deprotected under conditions identical to those used for CpG ODN fma1555. Oligonucleotides are visualized as purple bands upon staining the gel with Stains-all. Bromophenol blue is used as a marker and shows as a large band, in each lane, at the bottom of the gel.
Purified CpG ODN fma1555, CpG ODN 1555 and ODN fma1556 were assayed for endotoxins using the Limulus amebocyte lysate assay and were found to contain <0.1 endotoxin unit per mg of oligonucleotide.
Virus and growth conditions
Tacaribe virus (TCRV strain TRVL 11573) was obtained from American Type Culture Collection (ATCC VR-114, Manassas, VA) as a suckling mouse brain desiccate and expanded in Vero cell monolayers in P75 cell culture flasks (Corning) for eight days at 37°C in R5 or R10 medium (RPMI 1640, 5 or 10% heat-inactivated fetal calf serum, 1.5 mM l-glutamine, 100 U penicillin/ml and 100 μg of streptomycin/ml) (Invitrogen) as described (37). Cell-free supernatant was harvested, aliquoted and frozen at −70°C for use in mouse infections. TCRV titers were determined using the Tissue culture ID50 method (38). Briefly, the virus-containing suspension was diluted (10−1 to 10−11) in R5 medium in a 96-well round-bottom plate (Costar). Triplicate to quadruplicate dilutions were subsequently overlaid on Vero cell monolayers cultured in a 96-well flat-bottom plate (Costar). The virus was allowed to adhere 1–2 h at 37°C whereupon the virus suspension was removed from the plate and fresh R5 medium added. Cells were monitored daily for the development of cytopathic effects and were scored after 6–8 days of incubation at 37°C. The virus titer was defined as the last dilution showing mild cytopathic effects in culture in two out of three replicate wells. A similar method was used to titer viruses in tissue homogenates made from infected neonatal brains (38).
Animals and infection protocols
Balb/C mice were obtained from the National Cancer Institute (Frederick, MD), housed in sterile microisolator cages in the CBER-specific pathogen-free animal facility, and bred at 6–12 weeks of age. All experiments were approved by the FDA Animal Care and Use Committee.
Protocol A
To assess immune activation in vivo, healthy 1–3 month old female Balb/C mice were administered CpG ODN 1555 or fma1555 intraperitoneally (IP). Control mice received saline. For experiments assessing the kinetics of immune activation, spleen cells were collected 24, 72 and 120 h after treatment and the number of cytokine-secreting cells enumerated by ELIspot as previously described (39). For studies addressing dose response, spleens were harvested 72 h after treatment with CpG ODN 1555 or fma1555 (30, 100 or 300 μg/mouse, IP). Control mice received saline or ODN fma1556 (100 μg/mouse). Briefly, four dilutions of single cell suspensions were overlaid on 96-well Immunolon H2B plates coated with antibodies to IL-12, IL-6 (BD Pharmingen, San Diego, CA), TNFα (R&D systems, Minneapolis, MN) or anti-IgM (Southern Biotechnology Associates, Birmingham AL). After 5 h at 37°C, the cells were washed away and overlaid with biotin-conjugated antibodies to IL-12, IL-6 (BD Pharmingen), TNF-α (Genzyme, Cambridge, MA) or IgM (Southern Biotechnology Associates) followed by phosphatase-conjugated avidin (BD Pharmingen). After a final wash, spots were visualized by the addition of 5-bromo-4-chloro-3-indolyl phosphate (Kirkegaard and Perry Laboratories, Gaithersburg, MD) in low melt agarose (Sigma, St Louis, MO). Spots generated by individual cytokine secreting cells were counted manually using a 40× magnification and are expressed as per 106 spleen cells.
Protocol B
Infections with Leishmania major were performed as described (40). L.major clone VI (MHOM/IL/80/Friedlin) Promastigotes (kindly provided by Sylvie Bertholet) were grown at 26°C in medium 199 supplemented with 20% Hi-FCE (HyClone), 100 U penicillin/ml, 100 μg of streptomycin/ml, 2 mM L-glutamine, 40 mM HEPES, 0.1 mM adenine in 50 mM HEPES, 5 mg of hemin/ml of 50% triethanolamine and 1 mg of 6-biotin (M199/S)/ml. Infective-stage (metacyclic) promastigotes of L.major were isolated from stationary cultures (4- to 5-days old) by negative selection of infective forms using peanut agglutinin (Vector Laboratories). Three groups of Balb/c mice (6–10 weeks of age, four mice per group) were infected in the ear dermis with 1000 metacyclic promastigotes using a 27.5-gauge needle in a total volume of 5 μl. Each of two groups of animals was treated intradermally at the site with 25 μg of CpG ODN fma1555 or CpG ODN 1555. The third group was left untreated. The number of parasites and total volume was maintained constant for all animals. Cutaneous inoculation of live metacyclic L.major parasites causes a cutaneous lesion that models those observed in human cutaneous leishmaniasis. The size of the lesion was monitored weekly. Statistical analysis was performed by one-way ANOVA (analysis of variance) using the SigmaStat software. The Student–Newman–Keuls test was selected to determine the null hypothesis probability values.
Protocol C
Neonatal BALB/c mice were bred in-house under specific pathogen-free conditions. Newborn mice were infected intraperitoneally with TCRV (2000 TC50/animal) 1–4 days after birth. Mice were treated with CpG ODN fma1555 or CpG ODN 1555 (50 μg/mouse) on the day of infection (n = 9 for each group of mice) or three days before infection (n = 8 or 7, respectively, for each group of mice). Mice were also treated with a combination of CpG ODN fma1555 (25 μg/mouse) and CpG ODN 1555 (25 μg/mouse) on the day of infection (n = 11) or three days before infection (n = 10). Mice that were left untreated (n = 11) or received an ODN lacking a CpG motif (ODN fma1556, 50 μg/mouse, n = 6) three days before the infection were used as negative controls. The mice were monitored daily but infections were allowed to proceed to their natural outcome in order to assess survival. For each condition, survival was determined from 2 to 4 independent experiments using 2–4 mice per group in each experiment. No obvious delay in development or weight loss was observed in uninfected mice treated with the oligonucleotides.
RESULTS AND DISCUSSION
The preparation of the deoxyribonucleoside phosphoramidites 1a–d is a prerequisite to solid-phase synthesis of oligonucleotides functionalized with the thermolytic 2-(N-formyl-N-methyl)aminoethyl thiophosphate protecting group. These phosphoramidites are obtained from the reaction of suitably protected deoxyribonucleosides (9a–d) with the phosphorodiamidite 8 and 1H-tetrazole (Scheme 2) essentially as reported by Lee and Moon (41). The crude phosphoramidites are purified by silica gel chromatography using an eluant containing triethylamine to prevent dedimethoxytritylation and hydrolysis of the phosphoramidite monomers during purification given the inherent acidity of silica gel. It is therefore critically important to remove excess triethylamine from the purified phosphoramidites monomers to avoid neutralization of 1H-tetrazole and poor coupling efficiency during solid-phase oligonucleotide synthesis. The removal of residual triethylamine from purified 1a–d is preferably achieved by dissolving the phosphoramidites in a minimum amount of dry benzene followed by precipitation in a large volume of cold hexane. To ensure complete removal of triethylamine and adventitious moisture, the phosphoramidite precipitate is dissolved in a substantial amount of dry benzene (∼10 ml/g) and then freeze-dried under high vacuum over an extended period of time (16–24 h).
Characterization of the phosphoramidites 1a–d by 31P NMR spectroscopy revealed a noteworthy multiplicity of signals at 25°C. Typically, four signals are observed for 1b and three signals each for 1a, 1c and 1d. However, only two broad 31P NMR signals are seen when either 1b or 1c is heated to 50°C in the NMR probe. These observations are consistent with the notion of 1a–d existing as a mixture of diastereomeric rotamers (Scheme 2) as a consequence of the partial double-bond character of the C–N bond in tertiary amides (11). Likewise, the achiral phosphinylating reagent 8 also exists as a mixture of two rotamers as evidenced by two 31P NMR signals observed at δP 118.0 and 118.7 p.p.m.
The purified deoxyribonucleoside phosphoramidites 1a–d are used as 0.1 M solutions in anhydrous MeCN in the solid-phase synthesis of CpG ODN fma1555 and ODN fma1556 according to a modified synthesis cycle. Commercial 2-cyanoethyl deoxyribonucleoside phosphoramidites are also employed under identical conditions in the preparation of CpG ODN 1555, which serves as a positive immunostimulatory control oligonucleotide in mice. Modifications of the standard solid-phase oligonucleotide synthesis cycle include: (i) extending the phosphoramidite coupling time to 180 s; (ii) replacing the iodine oxidation step with a sulfurization step effected by 0.05 M 3H-1,2-benzodithiol-3-one 1,1-dioxide in MeCN over a period of 120 s; (iii) performing the sulfurization step before the capping step to minimize phosphorus oxygenation (33) and (iv) extending the capping reaction time to 60 s to ensure efficient chain termination of shorter than full-length oligonucleotides.
Upon completion of the last synthesis cycle, the terminal 5′-O-DMTr group is not removed from the oligonucleotide to facilitate its hydrophobic separation from a population of shorter 5′-O-acetylated oligonucleotides that failed quantitative chain extension during oligonucleotide synthesis. The solid support is exposed to pressurized ammonia gas to cleave nucleobase protecting groups and release the oligonucleotide from the support. It is important to note that in the case of CpG ODN fma1555 and ODN fma1556, the standard conditions used for nucleobase deprotection (concentrated NH4OH, 55°C, 10 h) were unsatisfactory, as these caused unwanted thermolytic cleavage of the 2-(N-formyl-N-methyl)aminoethyl thiophosphate protecting group to an unacceptable level and resulted in an intractable mixture of oligonucleotides.
The 5′-O-DMTr-oligonucleotide is eluted off the column along with the shorter oligonucleotides, and is purified by RP-HPLC using a column packed with a C18 resin, which has a higher affinity for the 5′-O-DMTr-oligonucleotide than for the shorter 5′-O-acetylated oligonucleotides. The purified 5′-O-DMTr-DNA oligonucleotide is then dedimethoxytritylated upon treatment with 80% acetic acid and re-purified by RP-HPLC through a C18 column. The chromatographic profile of CpG ODN fma1555 is shown in Figure 1 and exhibits a retention time (tR) of 37 min. The shape of the peak corresponding to the purified oligonucleotide is consistent with that of a complex mixture of rotameric diastereomers (2).
Characterization of the purified CpG ODN fma1555, CpG ODN 1555 and ODN fma1556 by mass spectrometry resulted in the following mass determinations: CpG ODN fma1555 (+ESI-TOF MS): calcd for C203H283N71O89P14S14 (M)+ 6024, found 6023. ODN fma1556 (+ESI-TOF MS): calcd for C205H283N75O89P14S14 (M)+ 6105, found 6103. CpG ODN 1555 (+ESI-TOF MS): calcd for C147H185N57O75P14S14 (M + 14H)+ 4832, found 4832. The experimental mass determination of each oligonucleotide is in close agreement with its expected mass, and thus confirms its identity.
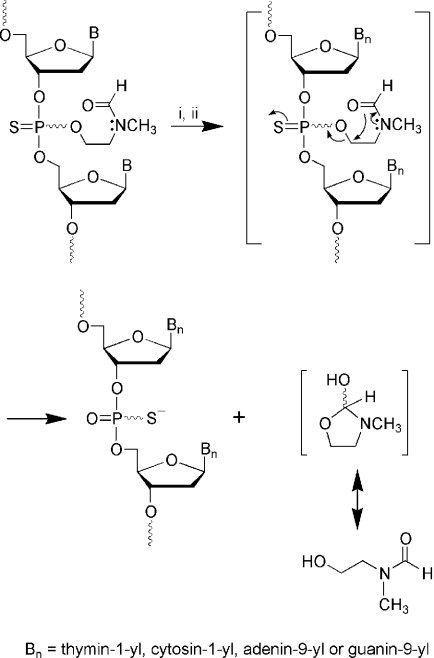
A kinetic profile of the thermolytic cleavage of the 2-(N-formyl-N methyl)aminoethyl thiophosphate protecting group is obtained by heating purified CpG ODN fma1555 and ODN fma1556 to 37 or 90°C in 1× PBS (pH 7.2), and analyzing the deprotection reaction at various time points by RP-HPLC. The half-time of thiophosphate deprotection is estimated to be 73 h at 37°C, and complete deprotection is achieved within 600 h. Fully deprotected CpG ODN fma1555 exhibited a tR (23 min) identical to that of CpG ODN 1555 under identical chromatographic conditions (Figure 1). The thermolytic deprotection mechanism presumably proceeds through a cyclodeesterification mechanism (Scheme 3) that is typically observed with many of the thermosensitive phosphate/thiophosphate protecting groups investigated earlier (2,4,6,7).
Scheme 3.
Tentative mechanism of the thermolytic cleavage of 2-(N-formyl-N-methyl)aminoethyl thiophosphate protecting groups from CpG ODN fma1555. (i) NH3 gas (∼10 bar), 12 h, 25°C; (ii) 0.1 M TEAA (pH 7.0) or 1× PBS (pH 7.2), 90°C, 3 h.
PAGE analysis of purified and fully deprotected CpG ODN fma1555, ODN fma1556 and CpG ODN 1555 showed that each oligonucleotide migrated on the gel as a homogeneous band. A photograph of the stained gel is shown in Figure 2. Although deprotected CpG ODN 1555 and CpG ODN fma1555 exhibited an identical electrophoretic mobility indicative of an identical DNA sequence, deprotected ODN 1556 displayed a slightly different mobility. It should be understood that the electrophoretic mobility of a synthetic oligonucleotide depends more on its base composition than its length (42). Since ODN 1556 has a different DNA sequence than that of CpG ODN 1555, its electrophoretic mobility was expected to differ from that of CpG ODN 1555 in spite of an identical chain length.
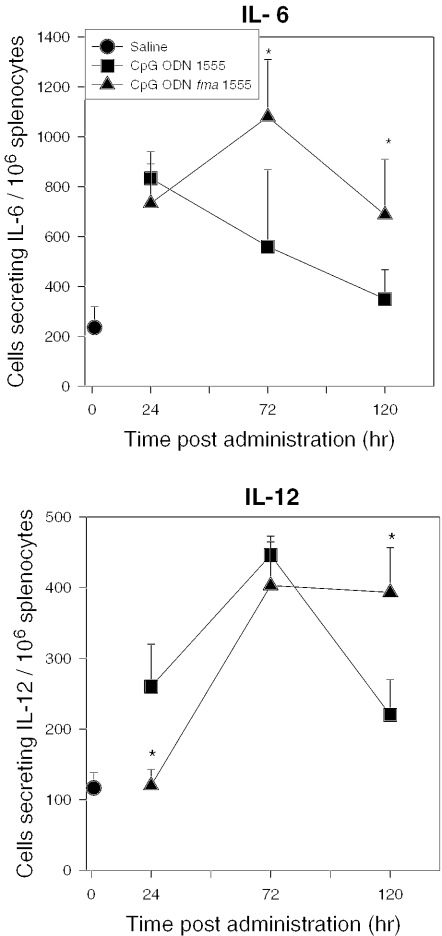
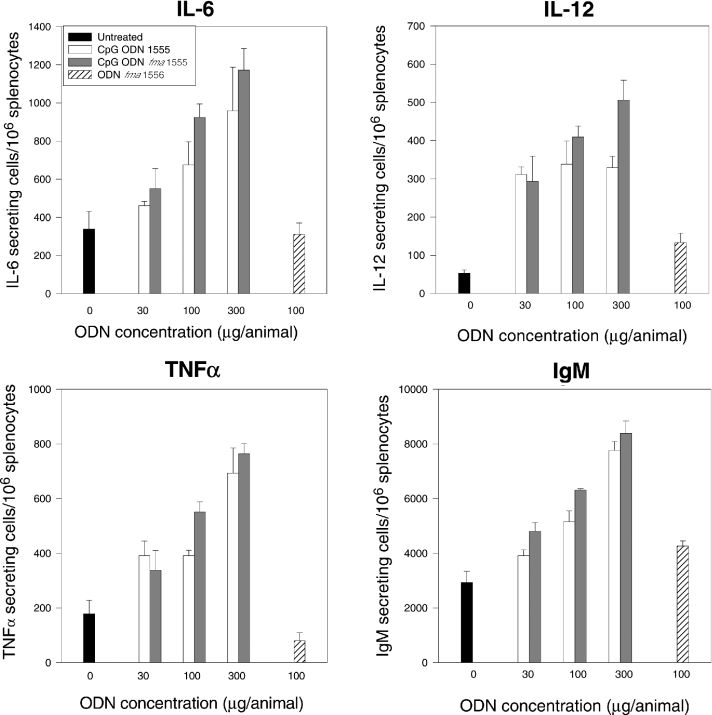
The immunostimulatory properties of CpG ODN fma1555 were evaluated in vivo in mice and compared with those of CpG ODN 1555, which were described earlier (43). Prior studies had shown that treatment with CpG ODNs induced an increase in the number of cytokine secreting cells (44). Given that the thermolytic deprotection of CpG ODN fma1555 proceeds with a half-time of 73 h at 37°C, expression of its immunoprotective properties should be delayed relative to that of CpG ODN 1555. As shown in Figure 3, splenocytes from Balb/c mice treated with CpG ODN fma1555 had a delayed increase in IL-6 and IL-12 secreting cells when compared to mice that received CpG ODN 1555. Importantly, the number of IL-12 secreting cells was still increased 120 h after treatment, as compared to mice receiving CpG ODN 1555. To assess whether the number of cytokine secreting cells was dose dependent, mice were treated intraperitoneally with CpG ODN 1555 or fma1555 (30, 100 or 300 μg/mouse, 3 mice/group) and euthanized 72 h after treatment. As shown in Figure 4, there is a direct correlation between the dose of CpG ODN fma1555 and the number of cytokine- and immunoglobulin-secreting cells in spleen. Mice treated with ODN fma1556 did not elicit cytokine secretion.
Figure 3.
Kinetics of cytokine secretion in mice treated with CpG ODN fma1555. Female Balb/c mice (6–12 weeks of age, 3–6/group) were administered CpG ODN 1555 or CpG ODN fma1555 (100 μg/mouse) intraperitoneally and then euthanized 24, 72 or 120 h later. Shown in this graph are the number of cells secreting IL-6 and IL-12 (p40/p70) ex vivo, as determined by ELIspot for each time point.
Figure 4.
Dose response in mice treated with CpG ODN fma1555 72 h post administration. Female Balb/c mice (3–6 mice/group) received CpG ODN 1555 or CpG ODN fma1555 (30, 100 or 300 μg/mouse) intraperitoneally. Splenocytes were collected 72 h after treatment. Shown in this figure are the numbers of cytokine and IgM secreting cells per 106 splenocytes.
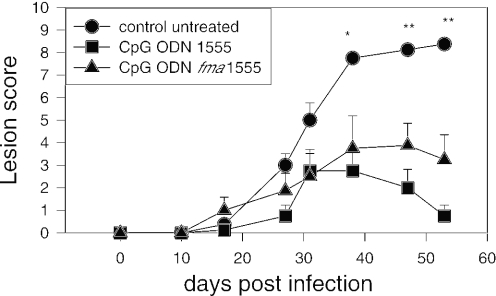
CpG ODNs have been shown to activate the innate immune system to improve the clinical outcome in several infectious models. Specifically, studies assessing the immunoprotective effects of CpG ODNs show that administration of CpG-containing DNA oligonucleotides alone (but not control oligonucleotides lacking CpG motifs) reduces the severity of an intradermal L.major challenge in mice when administered as early as three days prior to infection or as late as three weeks post infection (45). To assess the immunoprotective properties of CpG ODN fma1555, adult female Balb/c mice were challenged with live L.major metacyclic promastigotes intradermally in the ear and left either untreated or treated in situ with 25 μg of CpG ODN fma1555 or CpG ODN 1555 (Figure 5). As reported earlier, untreated Balb/c mice developed an ulcerative skin lesion that ultimately led to the loss of the outer ear 10 weeks after infection (40). In contrast, Balb/c mice that were treated with CpG ODN fma1555 or CpG ODN 1555 showed a significant (P < 0.05) reduction in dermal pathology by comparison to that of untreated mice, as assessed by one-way ANOVA. Although the lesions tended to be smaller in CpG ODN 1555-treated mice than in CpG ODN fma1555-treated mice (Figure 5), the difference was not statistically significant. This study demonstrated that the thermolytic CpG-ODN fma1555 is biologically active and can elicit a protective immune response in mice infected with an intracellular pathogen.
Figure 5.
Immunoprotection of mice treated with CpG ODN fma1555 against progressive L.major infection. Balb/c mice (4 mice/group) were challenged in the ear with 103 live L.major metacyclic promastigotes. The mice were either left untreated or received a single treatment at the site (ID) with CpG ODN 1555 or CpG ODN fma1555. The lesion size was monitored weekly. Note the reduction in lesion size in treated versus untreated mice. Statistical analysis was performed by one-way ANOVA (* indicates P < 0.05, ** indicates P < 0.01). The difference between mice treated with CpG ODN 1555 and CpG ODN fma1555 was not statistically significant.
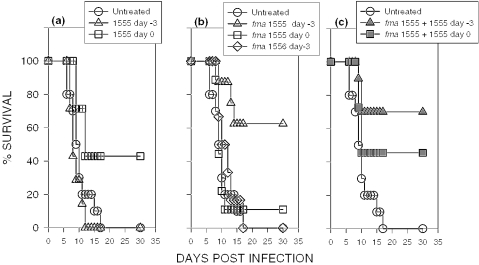
To further assess whether CpG ODN fma1555 behaves as a prodrug, a model of TCRV infection in neonatal mice was used. The TCRV is a New World arena virus that causes a lethal meningoencephalitis in newborn mice. We showed that treatment with CpG-ODN 1555 (50 μg/mouse) protected 43% of the newborn mice from death. As shown in several murine models, the therapeutic window for protection is narrow; treatment was effective when the oligonucleotides were administered at the time, or up to three days after TCRV infection. Mice that received CpG ODN 1555 three days prior to infection were not protected and died from lethal meningoencephalitis at the same rate as untreated mice (Figure 6a). As shown in Figure 6b, mice treated with CpG ODN fma1555 (50 μg/mouse) at the time of TCRV infection were not protected from death, but mice that received the treatment three days before infection had improved survival. The protective effect of ODN fma1555 was CpG-mediated, as mice treated with ODN fma1556 lacking CpG motifs were not protected. These findings indicate that the 2-(N-formyl-N-methyl)aminoethyl (fma) thiophosphate protecting group and/or its thermolytically cleaved counterpart [tentatively, 2-(N-formyl-N-methyl)aminoethanol] do not impart significant immunoprotective properties to CpG ODNs fma1555. Interestingly, when CpG ODN fma1555 and CpG ODN 1555 were co-administered (25 μg of each ODN/mouse) three days prior to or on the same day of the TCRV challenge, the survival rate averaged better than 50% (Figure 6c), thereby suggesting that the combination of CpG ODN 1555 and CpG ODN fma1555 widened the window of therapeutic treatment against the disease. Future experiments will be necessary to determine the mechanism and kinetics of the local and systemic immunostimulation elicited by CpG ODN fma1555. However, our results are consistent with the notion that CpG ODN fma1555 behaves as a prodrug in vivo through an unprecedented thermolytic process. These findings thus pave the way to the development of new thermolytic CpG-containing oligonucleotide prodrugs exhibiting various half-time of thiophosphate deprotection to enable the preparation of more efficient immunotherapeutic oligonucleotide formulations for the treatment of asthma, cancer, autoimmune and various infectious diseases.
Figure 6.
Survival of TCRV-infected mice treated with CpG ODN fma1555. Newborn Balb/c mice (1–4 days old) were infected with TCRV (2000 TC50/mouse). (a) CpG ODN 1555 (50 μg/mouse IP) protects newborn mice from infection when administered at the time of infection (open squares, n = 9), but cannot protect mice if administered three days prior to infection (open triangles, n = 7). (b) CpG ODN fma1555 (50 μg/mouse) administration protected mice when administered three days prior to infection (open triangles, n = 8), but not when administered at the time of infection (open squares, n = 9). Control mice received ODN fma1556 lacking CpG motifs on day −3 (open diamonds, n = 6). (c) Combination of CpG ODN 1555 (25 μg/mouse) and CpG ODN fma1555 (25 μg/mouse) administered on day −3 (closed triangles, n = 10) or at the time of infection (closed squares, n = 11) extends the period of treatment against the disease.
Acknowledgments
This research was supported in part by an appointment to the Postgraduate Research Participation Program at the Center for Drug Evaluation and Research administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the US Department of Energy and the US Food and Drug Administration. Funding to pay the Open Access publication charges for this article was provided by the US Food and Drug Administration.
Conflict of interest statement. None declared.
REFERENCES
- 1.Chmielewski M.K., Marchán V., Cieślak J., Grajkowski A., Livengood V., Münch U., Wilk A., Beaucage S.L. Thermolytic carbonates for potential 5′-hydroxyl protection of deoxyribonucleosides. J. Org. Chem. 2003;68:10003–10012. doi: 10.1021/jo035089g. [DOI] [PubMed] [Google Scholar]
- 2.Grajkowski A., Wilk A., Chmielewski M.K., Phillips L.R., Beaucage S.L. The 2-(N-formyl-N-methyl)aminoethyl group as a potential phosphate/thiophosphate protecting group in solid-phase oligodeoxyribonucleotide synthesis. Org. Lett. 2001;3:1287–1290. doi: 10.1021/ol0156852. [DOI] [PubMed] [Google Scholar]
- 3.Wilk A., Chmielewski M.K., Grajkowski A., Phillips L.R., Beaucage S.L. The 4-oxopentyl group as a labile phosphate/thiophosphate protecting group for synthetic oligodeoxyribonucleotides. Tetrahedron Lett. 2001;42:5635–5639. [Google Scholar]
- 4.Wilk A., Chmielewski M.K., Grajkowski A., Phillips L.R., Beaucage S.L. The 3-(N-tert-butylcarboxamido)-1-propyl group as an attractive phosphate/thiophosphate protecting group for solid-phase oligodeoxyribonucleotide synthesis. J. Org. Chem. 2002;67:6430–6438. doi: 10.1021/jo0258608. [DOI] [PubMed] [Google Scholar]
- 5.Wilk A., Chmielewski M.K., Grajkowski A., Phillips L.R., Beaucage S.L. The 3-(N-tert-butylcarboxamido)-1-propyl and 4-oxopentyl groups for phosphate/thiophosphate protection in oligodeoxyribonucleotide synthesis. In: Beaucage S.L., Bergstrom D.E., Glick G.D., Jones R.A., editors. Current Protocols in Nucleic Acid Chemistry. Vol. I. New York: John Wiley & Sons, Inc.; 2002. pp. 3.9.1–3.9.16. [Google Scholar]
- 6.Cieślak J., Beaucage S.L. Thermolytic properties of 3-(2-pyridyl)-1-propyl and 2-[N-methyl-N-(2-pyridyl)aminoethyl phosphate/thiophosphate protecting groups in solid-phase synthesis of oligodeoxyribonucleotides. J. Org. Chem. 2003;68:10123–10129. doi: 10.1021/jo0354490. [DOI] [PubMed] [Google Scholar]
- 7.Cieślak J., Grajkowski A., Livengood V., Beaucage S.L. Thermolytic 4-methylthio-1-butyl group for phosphate/thiophosphate protection in solid-phase synthesis of DNA oligonucleotides. J. Org. Chem. 2004;69:2509–2515. doi: 10.1021/jo035861f. [DOI] [PubMed] [Google Scholar]
- 8.Grajkowski A., Cieślak J., Chmielewski M.K., Marchán V., Phillips L.R., Wilk A., Beaucage S.L. Conceptual “Heat-Driven” approach to the synthesis of DNA oligonucleotides on microarrays. Ann. N.Y. Acad. Sci. 2003;1002:1–11. doi: 10.1196/annals.1281.003. [DOI] [PubMed] [Google Scholar]
- 9.Wilk A., Srinivasachar K., Beaucage S.L. N-Trifluoroacetylamino alcohols as phosphodiester protecting groups in the synthesis of oligodeoxyribonucleotides. J. Org. Chem. 1997;62:6712–6713. [Google Scholar]
- 10.Wilk A., Grajkowski A., Phillips L.R., Beaucage S.L. The 4-[N-methyl-N-(2,2,2-trifluoroacetyl)amino]butyl group as an alternative to the 2-cyanoethyl group for phosphate protection in the synthesis of oligodeoxyribonucleotides. J. Org. Chem. 1999;64:7515–7522. [Google Scholar]
- 11.Wilk A., Grajkowski A., Phillips L.R., Beaucage S.L. Deoxyribonucleoside cyclic N-acylphosphoramidites as a new class of monomers for the stereocontrolled synthesis of oligothymidylyl- and oligocytidylyl-phosphorothioates. J. Am. Chem. Soc. 2000;122:2149–2156. [Google Scholar]
- 12.Wilk A., Grajkowski A., Chmielewski M.K., Phillips L.R., Beaucage S.L. Deoxyribonucleoside phosphoramidites. In: Beaucage S.L., Bergstrom D.E., Glick G.D., Jones R.A., editors. Current Protocols in Nucleic Acid Chemistry. Vol. I. New York: John Wiley & Sons, Inc.; 2001. pp. 2.7.1–2.712. [DOI] [PubMed] [Google Scholar]
- 13.Van Houten K.A., Heath D.C., Pilato R.S. Rapid luminescent detection of phosphate esters in solution and the gas phase using (dppe)Pt{S2C2(2-pyridyl)(CH2CH2OH)} J. Am. Chem. Soc. 1998;120:12359–12360. [Google Scholar]
- 14.Guzaev A.P., Manoharan M. A novel phosphate protection for oligonucleotide synthesis: the 2-[(1-naphthyl)carbamoyloxy]ethyl (NCE) group. Tetrahedron Lett. 2000;41:5623–5626. [Google Scholar]
- 15.Guzaev A.P., Manoharan M. 2-Benzamidoethyl group—A novel type of phosphate protecting group for oligonucleotide synthesis. J. Am. Chem. Soc. 2001;123:783–793. doi: 10.1021/ja0016396. [DOI] [PubMed] [Google Scholar]
- 16.Zhang S.-W., Swager T.M. Fluorescent detection of chemical warfare agents: functional group specific ratiometric chemosensors. J. Am. Chem. Soc. 2003;125:3420–3421. doi: 10.1021/ja029265z. [DOI] [PubMed] [Google Scholar]
- 17.Barber I., Rayner B., Imbach J.-L. The prooligonucleotide approach. I: Esterase-mediated reversibility of dithymidine-S-alkyl-phosphorothiolates to dithymidine phosphorothioates. Bioorg. Med. Chem. Lett. 1995;5:563–568. [Google Scholar]
- 18.Tosquellas G., Alvarez K., Dell'Aquilla C., Morvan F., Vasseur J.-J., Imbach J.-L., Rayner B. The pro-oligonucleotide approach: solid phase synthesis and preliminary evaluation of model pro-dodecathymidylates. Nucleic Acids Res. 1998;26:2069–2074. doi: 10.1093/nar/26.9.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bologna J.-C., Morvan F., Imbach J.-L. The prooligonucleotide approach: synthesis of mixed phosphotriester and SATE phosphotriester prooligonucleotides using H-phosphonate and phosphoramidite chemistries. Eur. J. Org. Chem. 1999:2353–2358. [Google Scholar]
- 20.Bologna J.-C., Vivès E., Imbach J.-L., Morvan F. Uptake and quantification of intracellular concentration of lipophilic pro-oligonucleotides in HeLa cells. Antisense Nucleic Acid Drug Dev. 2002;12:33–41. doi: 10.1089/108729002753670247. [DOI] [PubMed] [Google Scholar]
- 21.Iyer R.P., Yu D., Agrawal S. Stereospecific bio-reversibility of dinucleoside S-alkyl phosphorothiolates to dinucleoside phosphorothioates. Bioorg. Med. Chem. Lett. 1994;4:2471–2476. [Google Scholar]
- 22.Iyer R.P., Yu D., Agrawal S. Prodrugs of oligonucleotides—the acyloxyalkyl esters of oligodeoxyribonucleoside phosphorothioates. Bioorg. Chem. 1995;23:1–21. [Google Scholar]
- 23.Iyer R.P., Yu D., Devlin T., Ho N.-H., Agrawal S. Acyloxyaryl prodrugs of oligonucleoside phosphorothioates. Bioorg. Med. Chem. Lett. 1996;6:1917–1922. [Google Scholar]
- 24.Iyer R.P., Ho N.-H., Yu D., Agrawal S. Bioreversible oligonucleotide conjugates by site-specific derivatization. Bioorg. Med. Chem. Lett. 1997;7:871–876. [Google Scholar]
- 25.Ora M., Mäki E., Poijärvi P., Neuvonen K., Oivanen M., Lönnberg H. Hydrolytic stability of nucleoside phosphotriesters derived from bis(hydroxymethyl)-1,3-dicarbonyl compounds and their congeners: toward a novel pro-drug strategy for antisense oligonucleotides. J. Chem. Soc. Perkin Trans. 2. 2001:881–885. [Google Scholar]
- 26.Poijärvi P., Mäki E., Tomperi J., Ora M., Oivanen M., Lönnberg H. Towards nucleotide prodrugs derived from 2,2-bis(hydroxymethyl)malonate and its congeners: hydrolytic cleavage of 2-cyano-2-(hydroxymethyl)-3-methoxy-3-oxopropyl and 3-(alkylamino)-2-cyano-2-(hydroxymethyl)-3-oxopropyl protections from the internucleosidic phosphodiester and phosphorothioate linkages. Helv. Chim. Acta. 2002;85:1869–1876. [Google Scholar]
- 27.Krieg A.M. CpG motifs in bacterial DNA and their immune effects. Annu. Rev. Immunol. 2002;20:709–760. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 28.Hemmi H., Takeuchi O., Kawai T., Kaisho T., Sato S., Sanjo H., Matsumoto M., Hoshino K., Wagner H., Takeda K., Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 29.Klinman D.M., Takeshita F., Gursel I., Leifer C., Ishii K.J., Verthelyi D., Gursel M. CpG DNA: recognition by and activation of monocytes. Microbes Infect. 2002;4:897–901. doi: 10.1016/s1286-4579(02)01614-3. [DOI] [PubMed] [Google Scholar]
- 30.Klinman D.M. Immunotherapeutic uses of CpG oligodeoxynucleotides. Nature Rev. Immunol. 2004;4:249–259. doi: 10.1038/nri1329. [DOI] [PubMed] [Google Scholar]
- 31.Klinman D.M., Verthelyi D., Takeshita F., Ishii K.J. Immune recognition of foreign DNA: a cure for bioterrorism? Immunity. 1999;11:123–129. doi: 10.1016/s1074-7613(00)80087-4. [DOI] [PubMed] [Google Scholar]
- 32.Agrawal S., Kandimalla E.R. Antisense and siRNA as agonists to Toll-like receptors. Nat. Biotechnol. 2004;22:1533–1537. doi: 10.1038/nbt1042. [DOI] [PubMed] [Google Scholar]
- 33.Iyer R.P., Phillips L.R., Egan W., Regan J.B., Beaucage S.L. The automated synthesis of sulfur-containing oligodeoxyribonucleotides using 3H-1,2-Benzodithiol-3-one-1,1-dioxide as a sulfur-transfer reagent. J. Org. Chem. 1990;55:4693–4699. [Google Scholar]
- 34.Regan J.B., Phillips L.R., Beaucage S.L. Large-scale Preparation of the Sulfur-Transfer Reagent 3H-1,2-benzodithiol-3-one 1,1-dioxide. Org. Prep. Proc. Int. 1992;24:488–492. [Google Scholar]
- 35.Boal J.H., Wilk A., Harindranath N., Max E.E., Kempe T., Beaucage S.L. Cleavage of oligodeoxyribonucleotides from controlled-pore glass supports and their rapid deprotection by gaseous amines. Nucleic Acids Res. 1996;24:3115–3117. doi: 10.1093/nar/24.15.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maniatis T., Fritsch E.F., Sambrook J. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1982. pp. 173–185. [Google Scholar]
- 37.Borden E.C., Nathanson N. Tacaribe virus infection of the mouse: an immunopathologic disease model. Lab. Invest. 1974;30:465–473. [PubMed] [Google Scholar]
- 38.Damonte E.B., D'Aiutola A.C., Coto C.E. Persistent infection of Vero cells with Tacaribe virus. J. Gen. Virol. 1981;56:41–48. doi: 10.1099/0022-1317-56-1-41. [DOI] [PubMed] [Google Scholar]
- 39.Verthelyi D.I., Ansar Ahmed S. Estrogen increases the number of plasma cells and enhances their autoantibody production in nonautoimmune C57BL/6 mice. Cell. Immunol. 1998;189:125–134. doi: 10.1006/cimm.1998.1372. [DOI] [PubMed] [Google Scholar]
- 40.Mendez S., Tabbara K., Belkaid S., Bertholet S., Verthelyi D., Klinman D.M., Seder R.A., Sacks D.L. Coinjection with CpG-containing immunostimulatory oligodeoxynucleotides reduces the pathogenicity of a live vaccine against cutaneous Leishmaniasis but maintains its potency and durability. Infect. Immun. 2003;71:5121–5129. doi: 10.1128/IAI.71.9.5121-5129.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee H.-J., Moon S.-H. Bis-(N,N-dialkylamino)-alkoxyphosphines as a new class of phosphate coupling agent for the synthesis of oligonucleotides. Chem. Lett. 1984:1229–1232. [Google Scholar]
- 42.Wu R., Wu N.-H., Hanna Z., Georges F., Narang S. Purification and sequence analysis of synthetic oligodeoxy ribonucleotides. In: Gait M.J., editor. Oligonucleotide Synthesis—A Practical Approach. Oxford: IRL Press; 1984. pp. 135–151. [Google Scholar]
- 43.Elkins K.L., Rhinehart-Jones T.R., Stibitz S., Conover J.S., Klinman D.M. Bacterial DNA containing CpG motifs stimulates lymphocyte-dependent protection of mice against lethal infection with intracellular bacteria. J. Immunol. 1999;162:2291–2298. [PubMed] [Google Scholar]
- 44.Klinman D.M., Currie D., Gursel I., Verthelyi D. Use of CpG oligodeoxynucleotides as immune adjuvants. Immunol. Rev. 2004;199:201–216. doi: 10.1111/j.0105-2896.2004.00148.x. [DOI] [PubMed] [Google Scholar]
- 45.Zimmermann S., Egeter O., Hausmann S., Lipford G.B., Röcken M., Wagner H., Heeg K. Cutting edge: CpG oligodeoxynucleotides trigger protective and curative Th1 responses in lethal murine Leishmaniasis. J. Immunol. 1998;160:3627–3630. [PubMed] [Google Scholar]