Abstract
Alcoholic beverage consumption is associated with an increased risk of upper gastrointestinal cancer. Acetaldehyde (AA), the first metabolite of ethanol, is a suspected human carcinogen, but the molecular mechanisms underlying AA carcinogenicity are unclear. In this work, we tested the hypothesis that polyamines could facilitate the formation of mutagenic α-methyl-γ-hydroxy-1,N2-propano-2′-deoxyguanosine (Cr-PdG) adducts from biologically relevant AA concentrations. We found that Cr-PdG adducts could be formed by reacting deoxyguanosine with μM concentrations of AA in the presence of spermidine, but not with either AA or spermidine alone. The identities of the Cr-PdG adducts were confirmed by both liquid and gas chromatography-mass spectrometry. Using a novel isotope-dilution liquid chromatography-mass spectrometry assay, we found that in the presence of 5 mM spermidine, AA concentrations of 100 μM and above resulted in the formation of Cr-PdG in genomic DNA. These AA levels are within the range that occurs in human saliva after alcoholic beverage consumption. We also showed that spermidine directly reacts with AA to generate crotonaldehyde (CrA), most likely via an enamine aldol condensation mechanism. We propose that AA derived from ethanol metabolism is converted to CrA by polyamines in dividing cells, forming Cr-PdG adducts, which may be responsible for the carcinogenicity of alcoholic beverage consumption.
INTRODUCTION
Alcoholic beverage consumption is strongly associated with an increased risk of certain types of cancers, especially cancers of the upper gastrointestinal tract (1). According to the 11th Report on Carcinogens, alcoholic beverage consumption is classified as a known human carcinogen (2). While the mechanisms underlying the effect of alcoholic beverage consumption on cancer risk are not completely understood, it is likely that acetaldehyde (AA), the first metabolite of alcohol, plays an important role. AA is a mutagen (3), a carcinogen in animal models (4), and causes chromosomal damage, including sister-chromatid exchanges (SCEs) and chromosomal aberrations (CAs) (5). AA is currently classified as a suspected human carcinogen (2).
AA is formed from ethanol by the action of alcohol dehydrogenase (ADH), and subsequently converted to acetate by aldehyde dehydrogenase (ALDH) (6). Variants of human ADH or ALDH genes that are associated with increased AA levels after ethanol consumption are protective against the development of alcoholism (7). However, these same alleles increase the risk of developing gastrointestinal cancer as a result of alcoholic beverage consumption (8,9).
While cellular ADHs represent an important source of AA, AA can also be formed in the human oral cavity by the action of microorganisms, including yeasts and bacteria (10,11). Owing in large part to microbial AA production, AA concentrations in human saliva have been measured to be 100 μM under laboratory conditions, and by extrapolation may be as high as 450 μM (10). Importantly, genetic variants of ALDH2 and ADH1C that are associated with an increased risk of cancer are also associated with increased salivary AA levels after alcohol consumption (12,13).
The molecular mechanisms underlying the genotoxic and carcinogenic effects of AA are not yet fully understood. In DNA, AA has been shown to react with 2′-deoxyguanosine (dG) to form N2-ethyl-2′-deoxyguanosine (N2-EtdG) (14). Vaca et al. (15,16) detected elevated levels of this adduct in animal models of alcohol exposure and in white blood cells of human alcoholics. However, this adduct does not appear to have significant mutagenic properties in mammalian cells (17,18), although it is mutagenic when bypassed by a bacterial DNA polymerase (19). Moreover, while some other biological effects of this adduct have been reported (20), it is not clear how this specific adduct could be responsible for the cytogenetic abnormalities (increased SCE and CA formation) (5) that have been observed following AA exposure.
More recent studies have shown that several additional adducts can be formed from AA and DNA. Notable among these are a propano-dG adduct, α-methyl-γ-hydroxy-1,N2-propano-2′-deoxyguanosine (21,22) (Cr-PdG), which results from the interaction of crotonaldehyde (CrA) with dG or DNA. CrA is an environmental pollutant, and this adduct is believed to be responsible for its mutagenic and genotoxic effects (23,24). The Cr-PdG adducts are also endogenous lesions (25), apparently derived from lipid peroxidation (26).
Other cyclic-dG adducts can exist in either of two forms, ring-opened or ring-closed (27,28). In the case of acrolein–PdG adduct, which is most similar to Cr-PdG, a structural NMR study has shown that the ring-closed form predominates in single-stranded DNA, while the ring-opened form is more favorable in the duplex DNA (29). While similar structural analyses have yet to be carried out with the Cr-PdG adduct, the observations that the Cr-PdG adduct can form DNA–DNA interstrand cross-links (ICLs) (30) and DNA–peptide cross-links (31), and that the DNA–peptide cross-links occurred more rapidly in double-stranded versus single-stranded DNA, indicate that this adduct can also undergo ring-opening, and this is likely to be favored in double-stranded DNA.
While these properties of the Cr-PdG adduct and its cross-linked derivatives would be consistent with the observed genotoxic effects of AA, the 40 mM concentration of AA used to generate this lesion in vitro (21) is in excess of what would be generated in the human body from ethanol metabolism. Recently, Sako and co-workers (32,33) have, however, shown that histones or the basic amino acids arginine or lysine can facilitate the formation of Cr-PdG from AA and dG or from AA and DNA, although these studies were also carried using a high AA concentration (≈594 mM) (33). These observations prompted us to ask whether polyamines might also affect the reaction of AA with DNA. Similar to histones, polyamines are highly basic molecules. They are present in millimolar concentrations in mammalian cells (34) and concentrated in the nucleus (35). Polyamines have been implicated in a wide variety of cellular functions, including cell growth, differentiation and regulation of nucleic acid synthesis (36) and more recently in protection against oxidative damage (37,38). With regard to genotoxicity, polyamines can influence chemical carcinogenesis (39,40) and the genotoxic effects of ionizing radiation (34,41). Therefore, in the present work, we tested the hypothesis that polyamines could also facilitate the formation of Cr-PdG from AA and dG or from AA and DNA. We found that under physiologically relevant conditions, polyamines stimulate Cr-PdG formation from AA and dG or from AA and DNA by directly reacting with AA to generate CrA. The implications of these results for understanding the mechanisms by which alcoholic beverage consumption increases the risk of cancer development are discussed.
MATERIALS AND METHODS
2′-Deoxyguanosine, l-Arginine, shrimp alkaline phosphatase, snake venom phosphodiesterase and pyridine were purchased from Sigma Chemical Co (St Louis, MO). Spermidine trihydrochloride and spermine tetrahydrochloride were from Fluka Chemie GmbH (Buchs, Switzerland). AA and CrA were from Supelco (Bellefonte, PA). AA was purchased in multiple 1 ml glass ampoules, and a fresh ampoule was used for each experiment. 2′-Deoxyguanosine-13C10, 15N5 was purchased from Medical Isotopes (Pelham, NH), nuclease P1 was purchased from Roche Diagnostics Corp (Indianapolis, IN), N,O-bis(trimethylsilyl)trifluororacetamide (BSTFA) containing 1% trimethylcholorosilane and dialysis cassettes were obtained from Pierce Chemicals (Rockford, IL). High-performance liquid chromatography (HPLC) grade solvents, acetonitrile from Burdick & Jackson (Muskegon, MI) and water from Omnisolv (EMD Chemical Inc., Gibbstown, NJ) were used as received. Biomax5 ultrafiltration membranes (5 kDa molecular mass cutoff) from Millipore (Bedford, MA) were used to filter the digested DNA samples. Pig liver DNA was isolated using high salt method (42).
HPLC
HPLC analysis was carried out using a Varian HPLC (Prostar, model 210) with a UV-visible detector (Prostar, model 310). A reverse-phase Varian C-18 column (Microsorb 25 × 0.46 cm ID, 5 μm) was used for analytical applications and a Varian C-18 semi-preparative column (Microsorb 25 × 1.0 cm ID, 5 μm) was used for preparative purposes. All analyses were carried out using a water–acetonitrile solvent system (A: 100% water, B: 100% Acetonitrile; 0–25 min: 0–15% B, 25–30 min: 15–70% B, 30–40 min: 70% B, 40–45 min: 70–0% B, 45–60 min: 0% B; Flow rate: 1 ml/min). Analytical injections were performed using an auto sampler (Varian, model 430). In the preparative HPLC, the flow rate was kept at 3.76 ml/min in order to keep the same retention time as in analytical HPLC. The chromatograph was monitored at 254 nm. The Cr-PdG fractions were collected using an automated Varian fraction collector (Prostar, model 701) attached to the preparative HPLC.
Gas chromatography-mass spectrometry (GC-MS)
GC-MS analysis was conducted using a 6890N network GC system with a 5973N Mass Selective Detector (MSD) (Agilent Technologies, Rockville, MD). The column was a J&W Scientific Ultra-2 fused silica capillary, (12.5 m, 0.2 mm ID, 0.33 μm film thickness) purchased from Agilent Technologies. Ultra-high purity helium from Roberts Oxygen Company, Inc. (Rockville, MD) was used as the carrier gas. The carrier gas flow was set at 0.8 ml/min. The temperature at the inlet was maintained at 250°C and the GC-MS interface kept at 280°C. The temperature program of the oven was started at 130°C and held for 2 min followed by a ramp of 8°C/min up to 280°C. The temperature settings of the MSD were 230°C at the ion source and 150°C at the mass filter. The MSD was operated based on electron ionization (EI). The selected-ion monitoring (SIM) mode was applied for the quantification of the adducts using stable isotope-labeled internal standards. The column head pressure was kept at 65 kPa. The samples were lyophilized using a Flexi-Dry MP freeze drier from Kinetics Thermal Systems (Stone Ridge, NY) prior to trimethylsilylation. The lyophilized samples were dissolved in 100 μl of the mixture of BSTFA and pyridine (1:1), and trimethylsilylated at room temperature for 3 h in a closed vial. An aliquot of 3 μl of the derivatized sample was injected into the injection port of the gas chromatograph using an auto sampler (Agilent Technologies, 7683 series injector). The inlet was adjusted to make a split mode injection with 10:1 split ratio.
For the detection of CrA, the column flow was reduced to 0.6 ml/min and the temperature program was started at 60°C with 2 min of holding followed by a ramp of 15°C/min up to 280°C. These samples were injected without derivatization.
Liquid chromatography-mass spectrometry (LC-MS)
LC-MS analysis was carried out using an Agilent Technologies 1100B Series system. The details of the system and methods are described elsewhere (43).
AA reactions with dG
In the reactions shown in Figure 1, 41 mM AA was added to a solution containing 3.45 mM dG and 11 mM spermidine trihydrochloride in phosphate buffer (0.1 M, pH.7.0) in a tightly capped tube and incubated at 37°C for 48 h. In control reactions, either AA or polyamine was omitted. The reaction mixture was analyzed by HPLC at 254 nm. The details of the concentrations used in other experiments are indicated in the text or respective figures.
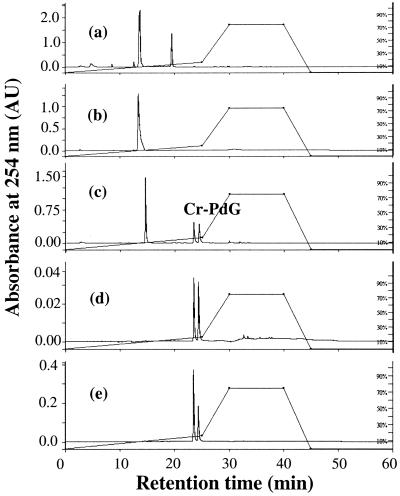
Figure 1.
HPLC chromatograms of (a) reaction of dG and AA; (b) reaction of dG and spermidine; (c) reaction of dG, AA and spermidine. (d) Purified Cr-PdG adducts; and (e) reaction of dG, AA and arginine. The peak eluting at 19.7 min in (a) is most likely a carbinolamine intermediate, with a Schiff base component. The peak eluting at 14 min is dG.
The dependence of Cr-PdG adduct formation on AA or spermidine concentration was analyzed by LC-MS. For AA dependence, the spermidine concentration was kept at 5 mM and the AA concentration varied from 25 to 500 μM (Figure 3a). In the spermidine dependence reactions, the AA concentration was kept at 200 μM and the spermidine concentration was varied from 500 μM to 5 mM (Figure 3b). All these reactions were carried out using 2.61 mM dG in the presence of 0.1 M phosphate buffer (pH 7.0) and an aliquot of Cr-PdG-13C10,15N5 in tightly capped and filled reaction tubes. The LC-MS analysis was carried out after incubating the samples at 37°C for 36 h. After incubation, the reaction tubes were kept open for 90 min at room temperature in a fume hood to evaporate off the unreacted AA.
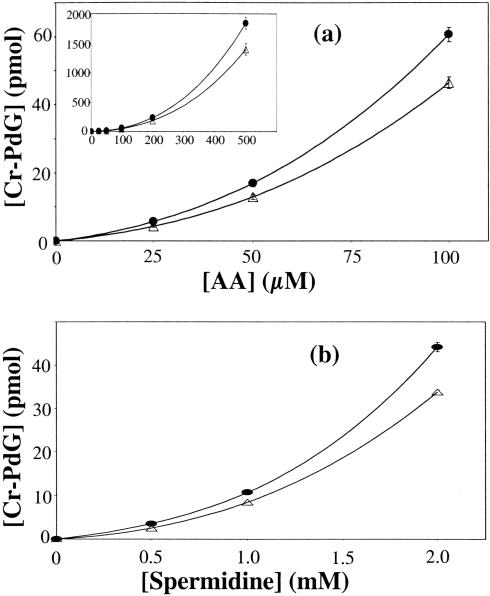
Figure 3.
(a) Dependence of Cr-PdG levels on AA concentration as measured by LC-MS. dG (2.61 mM) was incubated with different concentrations of AA in the presence of 5 mM spermidine, inset: AA dependence up to 500 μM. (b) Dependence of Cr-PdG levels on spermidine concentration as measured by LC-MS. dG (2.61 mM) was incubated with different concentrations of spermidine in the presence of 200 μM AA in 0.1 M phosphate buffer at pH 7.0. Δ, first diastereomer; •, second diastereomer.
AA reactions with DNA
In the reaction of AA with DNA, 200 μg of DNA in 0.1 M phosphate buffer (pH 7.0) was allowed to react with 25 μM–4 mM AA in the presence or absence of 5 mM spermidine. Reactions were carried out in tightly capped tubes for 36 h at 37°C. The DNA was dialyzed against 0.5 M NaCl for 24 h, and then precipitated using cold ethanol. For the mass spectrometric analysis, 50 μg of modified DNA was dissolved in a mixture of 50 μl Tris–HCl (10 mM, pH 7.5) and 1.25 μl of 1 M sodium acetate containing 45 mM ZnCl2. An aliquot of 3 U of nuclease P1, 0.002 U of SVPD and 16 U of alkaline phosphatase were added and incubated at 37°C overnight. Aliquots of isotopically labeled Cr-PdG and N2-EtdG adducts were added to the digestion mixture as internal standards. Digested DNA samples were filtered through an Amicon filter (5 kDa MWCO) and injected into the LC-MS.
The concentration curves shown in Figures 3 and 4 were generated and analyzed using Microsoft Excel.
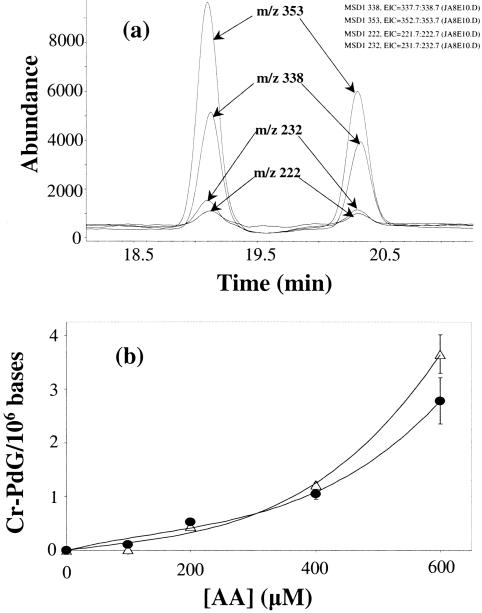
Figure 4.
(a) Ion-current profiles of the BH2+ and MH+ ions of Cr-PdG (m/z 222 and 338, respectively), and Cr-PdG-13C10,15N5 (m/z 232 and 353, respectively) obtained during LC-MS-SIM analysis after enzymic hydrolysis of pig liver DNA (50 μg) treated with AA (1 mM) in the presence of 5 mM spermidine and 0.1 M phosphate buffer at pH 7.0. (b) Dependence of Cr-PdG levels on AA concentration by the reaction of AA with pig liver DNA (50 μg) in the presence of 5 mM spermidine and 0.1 M phosphate buffer at pH 7.0. Δ, first diastereomer; •, second diastereomer.
RESULTS
To determine whether polyamines could facilitate the formation of Cr-PdG, dG was incubated with AA, polyamine, or both in the presence of neutral phosphate buffer. Treatment of dG with AA alone (Figure 1a) resulted in a single peak with a retention time of 19.7 min. This peak is most likely a carbinolamine intermediate (with a Schiff base component) in the reaction of AA with dG since it was unstable upon isolation and reinjection, and could be converted to the stable adduct N2-ethyl dG by reducing agents (data not shown) consistent with previous observations (21). Reaction of dG with polyamine alone resulted in no detectable adduct formation (Figure 1b). However, when the AA reaction was carried out in the presence of spermidine, neither the intermediate nor N2-ethyl dG was formed. Instead, two closely eluting product peaks (retention times: 1st peak, 23.5 min; 2nd peak, 24.5 min) were observed (Figure 1c). The same two peaks were observed when spermine was used instead of spermidine (data not shown). These peaks were consistent with the properties of the diastereomers of Cr-PdG adducts formed from the reaction of AA in the presence of histone (33) and from the reaction of dG with CrA (25,44).
To confirm the identity of the peaks resulting from the reaction of AA, dG and spermidine as Cr-PdG adducts, we analyzed the Cr-PdG adducts prepared by directly reacting dG with CrA. The synthetic Cr-PdG adducts derived from the CrA reaction showed the same elution properties as those from the AA and spermidine reaction (Figure 1d). The areas under both diastereomer peaks were essentially the same (1st peak/2nd peak ratio: 1.2/1). Preparation of Cr-PdG adducts by incubation of dG with arginine and AA (Figure 1e) also resulted in the same two peaks, although the ratio of the peak areas (1st peak/2nd peak ratio: 1.77/1) was clearly different from that observed with dG and CrA or with dG, AA and spermine.
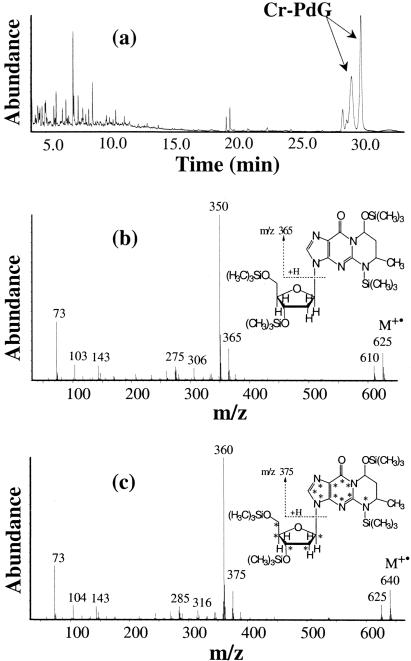
To rigorously confirm their identities, the two adducts resulting from the reaction of AA and polyamine with dG were purified by preparative HPLC and analyzed by GC-MS analysis after trimethylsilylation. Both diastereomers of the Cr-PdG gave identical mass spectra (Figure 2b), which are expected for Cr-PdG with four trimethylsilyl groups. Characteristic ions were observed at m/z 625, 610, 365 and 350. The ion at m/z 625 is the molecular ion (M+•) and the m/z 610 ion is the typical (M-15)+ ion, which results from the loss of methyl radical (•CH3) from M+•. The ion at m/z 365 represents the base moiety after cleavage of the sugar moiety. The most intense peak at m/z 350 results from the loss of •CH3 from the base moiety, which is common in trimethylsilyl derivatives. In the GC column, the second diastereomer of Cr-PdG (in HPLC) elutes earlier than the first diastereomer.
Figure 2.
(a) Total-ion chromatogram obtained during GC-MS analysis of the synthesized Cr-PdG after trimethylsilylation; (b) mass spectrum of the trimethylsilylated Cr-PdG; and (c) mass spectrum of the trimethylsilylated Cr-PdG-13C10,15N5.
For quantification purposes, we synthesized the isotopically labeled Cr-PdG adducts using dG-13C10,15N5 and AA in the presence of arginine. The mass spectrum of the trimethylsilylated Cr-PdG-13C10,15N5 gave the expected corresponding mass spectrum of Cr-PdG with 15 additional mass units for M+• and (M-15)+ ions and 10 additional mass units for ions representing the base moiety (Figure 2c).
Initial attempts to detect the Cr-PdG adduct formation from dG or DNA incubated with lower concentration of AA and spermidine using GC-MS were unsuccessful, due to the partial instability of the Cr-PdG adduct under GC-MS conditions. While others have used either post labeling (25) or LC-MS analysis of free Cr-PdG base (33), we chose to develop an LC-MS assay for the Cr-PdG adducts. Analysis of synthetic Cr-PdG adducts by LC-MS yielded typical ions at m/z 222, 338 and 360. The ion at m/z 222 represents the base moiety with two additional H atoms (BH2+ ion). The ion at m/z 338 corresponds to the protonated molecular ion (MH+) and the ion at m/z 360 is the sodium adduct ion (MNa+). These types of ions are typical of nucleosides in LC-MS analysis. The corresponding ions from the isotopically labeled standard were at m/z 232, 353 and 375, respectively. No ions corresponding to free dG were observed from the purified adduct preparations, indicating that in contrast to GC-MS, the Cr-PdG adducts are stable under LC-MS conditions.
Using LC-MS with isotope dilution, we next assessed the dependence of Cr-PdG lesion formation with dG in response to different, biologically relevant concentrations of AA or spermidine. As shown in Figure 3a, Cr-PdG levels increase with increasing AA concentrations in the range from 25 to 500 μM (using 5.0 mM spermidine). Similarly, in the presence of 200 μM AA, Cr-PdG levels increased with increasing spermidine concentrations in the range 500 μM–2 mM (Figure 3b). In both cases, the concentration curve data could be best fit by a third order polynomial function.
We next investigated the effect of varying concentrations of AA on the formation of Cr-PdG adducts in DNA in the presence or absence of 5 mM spermidine. This value was chosen as the high end of the physiological polyamine concentration range (34). The identification and quantification of Cr-PdG adducts were performed using the SIM mode, monitoring the ions m/z 222, 338 and 360. Figure 4a illustrates a typical SIM analysis of the Cr-PdG adducts with ion-current profiles of the ions at m/z 222 and 338 (Cr-PdG), and m/z 232 and 353 (Cr-PdG-13C10,15N5). The dependence of the Cr-PdG adduct formation in DNA on AA concentration is shown in Figure 4b. In the presence of 5 mM spermidine, Cr-PdG adducts were observed with AA concentrations as low as 100 μM, and increased with increasing AA concentrations. The ratio of the two diastereomers was 1.1:1. As observed in reactions with AA, spermidine and dG (Figure 3), the data were best fit by a third order polynomial function. Under these same conditions, we could not detect any formation of N2-EtdG (data not shown).
Spermidine reacts directly with AA to form CrA
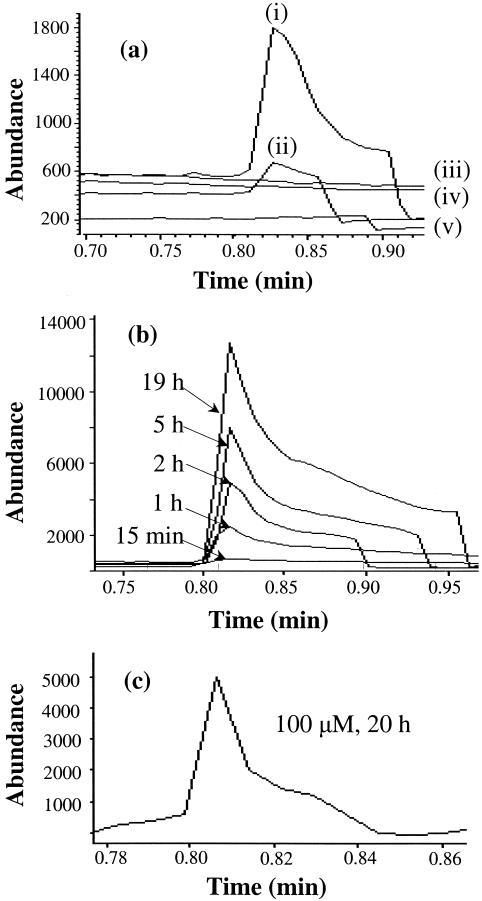
The fact that we observed the same ratio of Cr-PdG diastereomers from combined AA and spermidine treatment as we did from CrA alone (Figure 1) suggested that spermidine could directly react with AA to form CrA. To test this hypothesis, we incubated AA in phosphate buffer with or without spermidine and monitored the formation of CrA by GC-MS. AA elutes at 0.62 min and CrA elutes at 0.82 min under these experimental conditions. CrA was measured using the SIM mode by monitoring the ions at m/z 39, 41, 69 and 70 (molecular ion). As shown in Figure 5a, CrA was produced by incubating AA and spermidine together in the presence of phosphate buffer, but not by incubating either compound alone. CrA formation from AA and spermidine was time dependent (Figure 5b), and CrA formation could be detected from 100 μM AA incubated in the presence of 5 mM spermidine (Figure 5c). Since no DNA or dG was present in these reactions, the results indicate that spermidine directly reacts with AA to generate CrA.
Figure 5.
(a) Time dependence of CrA formation. Ion-current profiles of the ion at m/z 70 obtained during GC-MS-SIM analysis of the samples from the reaction of spermidine (5 mM) and AA (different concentrations) in the presence of 0.1 M phosphate buffer at pH 7.4. (i) 1 mM AA, (ii) 500 μM AA and (iv) no AA. (iii) No AA or spermidine and (v) 6 mM AA alone in water. (b) GC-MS-SIM (m/z 70) profiles of time-dependent formation of CrA by the reaction of spermidine (5 mM) and AA (5 mM) in presence of 0.1 M phosphate buffer at pH 7.4. (c) GC-MS SIM (m/z 70) profiles of CrA formed from the reaction of spermidine (5 mM) and AA (100 μM) in the presence of 0.1 M phosphate buffer at pH 7.4, 20 h incubation.
DISCUSSION
There is strong evidence to support an important role for AA, the first metabolite of ethanol, in carcinogenesis related to alcoholic beverage consumption. However, the molecular mechanisms underlying this effect are poorly understood. The most commonly studied AA–DNA adduct, N2-ethyl-dG, has not been clearly shown to be mutagenic in mammalian systems. AA has previously been shown to cause the formation of the mutagenic DNA adduct, Cr-PdG, but only at supraphysiological AA concentrations (21,33). Here, we show that polyamines facilitate the formation of the mutagenic Cr-PdG adduct from AA and dG or from AA and DNA. Importantly, this effect was observed at a biologically relevant (34) polyamine concentration, and with AA concentrations overlapping the range that could result in human saliva after ethanol consumption (up to 450 μM) (10). AA concentrations in other gastrointestinal tissues can be even higher; AA levels as high as 2.7 mM have been detected in rodent colon after ethanol exposure (45).
The relative amounts of the different Cr-PdG diastereomers formed from reactions with AA and dG can provide information on the mechanism of lesion formation (21,46). Our observation that the ratio of the different diastereomers formed from AA, spermidine and dG was the same as from CrA and dG (Figure 1), suggesting the hypothesis that CrA, generated from a reaction of spermidine with AA, reacts with dG to form Cr-PdG. The 1.1:1 ratio of Cr-PdG diastereomers observed in reactions with spermidine, AA and DNA was also consistent with this hypothesis. The hypothesis predicted that AA could react with spermidine to form CrA, and this prediction was confirmed by the experiments shown in Figure 5. Thus, based on the results from three different experiments, we conclude that AA reacts with polyamine to form CrA, which then reacts with dG in DNA to generate Cr-PdG adducts. We note that formation of CrA via an aldol condensation reaction of AA has previously been proposed as a mechanism for the formation of AA-derived protein adducts (47).
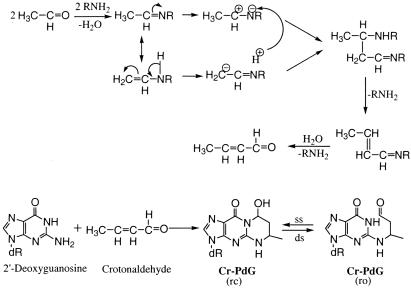
Based on the work of Sako et al. (48,49), the most likely molecular mechanism of CrA formation from AA involves the formation of an imine intermediate, by the reaction between the amino group of the polyamine and the carbonyl group of AA. This imine intermediate also exists in an enamine tautomeric form. These two forms can undergo charge polarization followed by an aldol type condensation reaction and the elimination of two molecules of amine to ultimately form CrA (Figure 6). In this mechanism, the polyamine appears to catalyze the conversion of AA to CrA, and our data are consistent with this possibility. However, more detailed kinetic and mechanistic studies, which are beyond the scope of the present work, would be necessary to formally confirm this. Our observations do raise the possibility that in addition to AA, polyamines might stimulate such reactions with other endogenous or exogenous aldehydes as well.
Figure 6.
Summary diagram to illustrate the mechanisms of AA-derived DNA adduct formation and the proposed relationship of the Cr-PdG adducts to the known cytogenetic effects of AA. The ring-opened (ro) and ring-closed (rc) forms of the Cr-PdG adducts are shown. Other abbreviations: ss, single-stranded DNA; ds, double-stranded DNA.
Polyamines are essential for cell growth, and polyamine synthesis is tightly related to cellular proliferation, with the highest levels being found in rapidly dividing cells (36). Ethanol and AA increase cell proliferation in gastrointestinal mucosa (50), and AA specifically has been shown to increase mucosal levels of ornithine decarboxylase, the rate limiting enzyme for polyamine synthesis (51). Based on our observations, this effect of AA would also make the cells more susceptible to Cr-PdG formation from AA.
Lipid peroxidation has been considered as the main source for the formation of intracellular CrA. While it has been shown that Cr-PdG can be formed in vitro by treating dG with a lipid peroxide-generating mixture (26), this does not necessarily mean that the only mechanism for in vivo formation of the lesion is lipid peroxidation. Polyamines are abundant in cells, and AA is produced in cells during threonine catabolism (52). Also, small amounts of AA may be produced as a byproduct of repair of ethylation damage to DNA (53). Therefore, our observation that AA is converted to CrA in the presence of polyamines provides another potential source for the formation of Cr-PdG lesions and for endogenous CrA.
It has been shown that oligonucleotides containing Cr-PdG can cross-link to peptides, and that this reaction is more rapid in double-stranded than single-stranded DNA (31). DNA–peptide cross-links can be considered as models for DNA–protein cross-links (DPCLs). The Cr-PdG adduct can also react with guanine on the opposite strand of DNA to form a DNA ICL (22,30). These observations indicate that the Cr-PdG adduct can exist in a ring-opened form, and could therefore form DPCLs or ICLs in vivo. AA has been shown to form DPCLs in vitro (54), and these lesions are mechanistically consistent with the observed increases in SCEs observed in mammalian cells after AA treatment (55). The formation of DNA ICLs is also mechanistically consistent with increased CA observed after AA in cultured mammalian cells and in lymphocytes from human alcoholics (56). Moreover, the extent of AA induced CAs were dramatically elevated in cells from a patient with Fanconi's anemia (57), which are known to be highly sensitive to CA formation when exposed to compounds that form DNA ICLs (58).
Based on these considerations, the Cr-PdG adducts appear to be good candidates for mediating the effects of AA on alcoholic beverage related cancer. If this is the case, it follows that in addition to genetic variation in ADH and ALDH genes, polymorphisms in the genes encoding DNA repair proteins that repair Cr-PdG adducts and their derivatives (DPCLs and ICLs) would also affect susceptibility to cancer from alcoholic beverage consumption. The observation (57) that cells from a Fanconi's anemia patient are susceptible to CA formation after AA exposure would suggest that investigating the role of sequence variation in the Fanconi genes, including BRCA2 (59), on alcoholic beverage related carcinogenesis might be a fruitful line of research.
Acknowledgments
The authors thank Katie Krone for isolating the genomic DNA used in this work, Katie Krone and Cheryl Marietta for helpful comments on the manuscript, and Cheryl Marietta for preliminary experiments. The authors also thank two anonymous reviewers for several helpful comments. Certain commercial equipment or materials are identified in this paper in order to specify adequately the experimental procedure. Such identification neither does it imply recommendation or endorsement by the National Institute of Standards and Technology nor does imply that the materials or equipment identified are necessarily the best available for the purpose. Funding to pay the Open Access publication charges for this article was provided by NIAAA.
Conflict of interest statement. None declared.
REFERENCES
- 1.Poschl G., Seitz H.K. Alcohol and cancer. Alcohol Alcohol. 2004;39:155–165. doi: 10.1093/alcalc/agh057. [DOI] [PubMed] [Google Scholar]
- 2. http://ntp.niehs.nih.gov/ntp/roc/eleventh/profiles/s007alco.pdf Report on Carcinogens, 11th edn. 2005. U.S. Department of Health and Human Services, Public Health Service, National Toxicology Program.
- 3.Dellarco V.L. A mutagenicity assessment of acetaldehyde. Mutat. Res. 1988;195:1–20. doi: 10.1016/0165-1110(88)90013-9. [DOI] [PubMed] [Google Scholar]
- 4.Woutersen R.A., Appelman L.M., Van Garderen-Hoetmer A., Feron V.J. Inhalation toxicity of acetaldehyde in rats. III. Carcinogenicity study. Toxicology. 1986;41:213–231. doi: 10.1016/0300-483x(86)90201-5. [DOI] [PubMed] [Google Scholar]
- 5.Obe G., Anderson D. International Commission for Protection against Environmental Mutagens and Carcinogens. ICPEMC Working Paper No. 15/1. Genetic effects of ethanol. Mutat. Res. 1987;186:177–200. doi: 10.1016/0165-1110(87)90003-0. [DOI] [PubMed] [Google Scholar]
- 6.Ramchandani V.A., Bosron W.F., Li T.K. Research advances in ethanol metabolism. Pathol. Biol. (Paris) 2001;49:676–682. doi: 10.1016/s0369-8114(01)00232-2. [DOI] [PubMed] [Google Scholar]
- 7.Chen C.C., Lu R.B., Chen Y.C., Wang M.F., Chang Y.C., Li T.K., Yin S.J. Interaction between the functional polymorphisms of the alcohol-metabolism genes in protection against alcoholism. Am. J. Hum. Genet. 1999;65:795–807. doi: 10.1086/302540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yokoyama A., Muramatsu T., Ohmori T., Yokoyama T., Okuyama K., Takahashi H., Hasegawa Y., Higuchi S., Maruyama K., Shirakura K., Ishii H. Alcohol-related cancers and aldehyde dehydrogenase-2 in Japanese alcoholics. Carcinogenesis. 1998;19:1383–1387. doi: 10.1093/carcin/19.8.1383. [DOI] [PubMed] [Google Scholar]
- 9.Yokoyama A., Omori T. Genetic polymorphisms of alcohol and aldehyde dehydrogenases and risk for esophageal and head and neck cancers. Jpn. J. Clin. Oncol. 2003;33:111–121. doi: 10.1093/jjco/hyg026. [DOI] [PubMed] [Google Scholar]
- 10.Homann N., Jousimies-Somer H., Jokelainen K., Heine R., Salaspuro M. High acetaldehyde levels in saliva after ethanol consumption: methodological aspects and pathogenetic implications. Carcinogenesis. 1997;18:1739–1743. doi: 10.1093/carcin/18.9.1739. [DOI] [PubMed] [Google Scholar]
- 11.Salaspuro M.P. Acetaldehyde, microbes, and cancer of the digestive tract. Crit. Rev. Clin. Lab. Sci. 2003;40:183–208. doi: 10.1080/713609333. [DOI] [PubMed] [Google Scholar]
- 12.Vakevainen S., Tillonen J., Agarwal D.P., Srivastava N., Salaspuro M. High salivary acetaldehyde after a moderate dose of alcohol in ALDH2-deficient subjects: strong evidence for the local carcinogenic action of acetaldehyde. Alcohol Clin. Exp. Res. 2000;24:873–877. [PubMed] [Google Scholar]
- 13.Visapaa J.P., Gotte K., Benesova M., Li J., Homann N., Conradt C., Inoue H., Tisch M., Horrmann K., Vakevainen S., et al. Increased cancer risk in heavy drinkers with the alcohol dehydrogenase 1C*1 allele, possibly due to salivary acetaldehyde. Gut. 2004;53:871–876. doi: 10.1136/gut.2003.018994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vaca C.E., Fang J.L., Schweda E.K. Studies of the reaction of acetaldehyde with deoxynucleosides. Chem. Biol. Interact. 1995;98:51–67. doi: 10.1016/0009-2797(95)03632-v. [DOI] [PubMed] [Google Scholar]
- 15.Fang J.L., Vaca C.E. Development of a 32P-postlabelling method for the analysis of adducts arising through the reaction of acetaldehyde with 2′-deoxyguanosine-3′-monophosphate and DNA. Carcinogenesis. 1995;16:2177–2185. doi: 10.1093/carcin/16.9.2177. [DOI] [PubMed] [Google Scholar]
- 16.Fang J.L., Vaca C.E. Detection of DNA adducts of acetaldehyde in peripheral white blood cells of alcohol abusers. Carcinogenesis. 1997;18:627–632. doi: 10.1093/carcin/18.4.627. [DOI] [PubMed] [Google Scholar]
- 17.Matsuda T., Terashima I., Matsumoto Y., Yabushita H., Matsui S., Shibutani S. Effective utilization of N2-ethyl-2′-deoxyguanosine triphosphate during DNA synthesis catalyzed by mammalian replicative DNA polymerases. Biochemistry. 1999;38:929–935. doi: 10.1021/bi982134j. [DOI] [PubMed] [Google Scholar]
- 18.Perrino F.W., Blans P., Harvey S., Gelhaus S.L., McGrath C., Akman S.A., Jenkins G.S., LaCourse W.R., Fishbein J.C. The N2-ethylguanine and the O6-ethyl- and O6-methylguanine lesions in DNA: contrasting responses from the ‘bypass’ DNA polymerase eta and the replicative DNA polymerase alpha. Chem. Res. Toxicol. 2003;16:1616–1623. doi: 10.1021/tx034164f. [DOI] [PubMed] [Google Scholar]
- 19.Terashima I., Matsuda T., Fang T.W., Suzuki N., Kobayashi J., Kohda K., Shibutani S. Miscoding potential of the N2-ethyl-2′-deoxyguanosine DNA adduct by the exonuclease-free Klenow fragment of Escherichia coli DNA polymerase I. Biochemistry. 2001;40:4106–4114. doi: 10.1021/bi002719p. [DOI] [PubMed] [Google Scholar]
- 20.Antony S., Theruvathu J.A., Brooks P.J., Lesher D.T., Redinbo M., Pommier Y. Enhancement of camptothecin-induced topoisomerase I cleavage complexes by the acetaldehyde adduct N2-ethyl-2′-deoxyguanosine. Nucleic Acids Res. 2004;32:5685–5692. doi: 10.1093/nar/gkh902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang M., McIntee E.J., Cheng G., Shi Y., Villalta P.W., Hecht S.S. Identification of DNA adducts of acetaldehyde. Chem. Res. Toxicol. 2000;13:1149–1157. doi: 10.1021/tx000118t. [DOI] [PubMed] [Google Scholar]
- 22.Lao Y., Hecht S.S. Synthesis and properties of an acetaldehyde-derived oligonucleotide interstrand cross-link. Chem. Res. Toxicol. 2005;18:711–721. doi: 10.1021/tx0497292. [DOI] [PubMed] [Google Scholar]
- 23.Fernandes P.H., Kanuri M., Nechev L.V., Harris T.M., Lloyd R.S. Mammalian cell mutagenesis of the DNA adducts of vinyl chloride and crotonaldehyde. Environ. Mol. Mutagen. 2005;45:455–459. doi: 10.1002/em.20117. [DOI] [PubMed] [Google Scholar]
- 24.Eder E., Schuler D., Budiawan . IARC Sci. Publ. 1999. Cancer risk assessment for crotonaldehyde and 2-hexenal: an approach; pp. 219–232. [PubMed] [Google Scholar]
- 25.Nath R.G., Chung F.L. Detection of exocyclic 1,N2-propanodeoxyguanosine adducts as common DNA lesions in rodents and humans. Proc. Natl Acad. Sci. USA. 1994;91:7491–7495. doi: 10.1073/pnas.91.16.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan J., Chung F.L. Formation of cyclic deoxyguanosine adducts from omega-3 and omega-6 polyunsaturated fatty acids under oxidative conditions. Chem. Res. Toxicol. 2002;15:367–372. doi: 10.1021/tx010136q. [DOI] [PubMed] [Google Scholar]
- 27.Mao H., Schnetz-Boutaud N.C., Weisenseel J.P., Marnett L.J., Stone M.P. Duplex DNA catalyzes the chemical rearrangement of a malondialdehyde deoxyguanosine adduct. Proc. Natl Acad. Sci. USA. 1999;96:6615–6620. doi: 10.1073/pnas.96.12.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu W., Feng Z., Eveleigh J., Iyer G., Pan J., Amin S., Chung F.L., Tang M.S. The major lipid peroxidation product, trans-4-hydroxy-2-nonenal, preferentially forms DNA adducts at codon 249 of human p53 gene, a unique mutational hotspot in hepatocellular carcinoma. Carcinogenesis. 2002;23:1781–1789. doi: 10.1093/carcin/23.11.1781. [DOI] [PubMed] [Google Scholar]
- 29.de los Santos C., Zaliznyak T., Johnson F. NMR characterization of a DNA duplex containing the major acrolein-derived deoxyguanosine adduct gamma -OH-1,-N2-propano-2′-deoxyguanosine. J. Biol. Chem. 2001;276:9077–9082. doi: 10.1074/jbc.M009028200. [DOI] [PubMed] [Google Scholar]
- 30.Kozekov I.D., Nechev L.V., Moseley M.S., Harris C.M., Rizzo C.J., Stone M.P., Harris T.M. DNA interchain cross-links formed by acrolein and crotonaldehyde. J. Am. Chem. Soc. 2003;125:50–61. doi: 10.1021/ja020778f. [DOI] [PubMed] [Google Scholar]
- 31.Kurtz A.J., Lloyd R.S. 1,N2-deoxyguanosine adducts of acrolein, crotonaldehyde, and trans-4-hydroxynonenal cross-link to peptides via Schiff base linkage. J. Biol. Chem. 2003;278:5970–5976. doi: 10.1074/jbc.M212012200. [DOI] [PubMed] [Google Scholar]
- 32.Sako M., Inagaki S., Esaka Y., Deyashiki Y. Histones accelerate the cyclic 1,N2-propanoguanine adduct-formation of DNA by the primary metabolite of alcohol and carcinogenic crotonaldehyde. Bioorg. Med. Chem. Lett. 2003;13:3497–3498. doi: 10.1016/s0960-894x(03)00800-x. [DOI] [PubMed] [Google Scholar]
- 33.Inagaki S., Esaka Y., Goto M., Deyashiki Y., Sako M. LC-MS study on the formation of cyclic 1,N2-propano guanine adduct in the reactions of DNA with acetaldehyde in the presence of histone. Biol. Pharm. Bull. 2004;27:273–276. doi: 10.1248/bpb.27.273. [DOI] [PubMed] [Google Scholar]
- 34.Chiu S., Oleinick N.L. Radioprotection of cellular chromatin by the polyamines spermine and putrescine: preferential action against formation of DNA–protein crosslinks. Radiat. Res. 1998;149:543–549. [PubMed] [Google Scholar]
- 35.Sarhan S., Seiler N. On the subcellular localization of the polyamines. Biol. Chem. Hoppe-Seyler. 1989;370:1279–1284. doi: 10.1515/bchm3.1989.370.2.1279. [DOI] [PubMed] [Google Scholar]
- 36.Tabor C.W., Tabor H. Polyamines. Annu. Rev. Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- 37.Ha H.C., Sirisoma N.S., Kuppusamy P., Zweier J.L., Woster P.M., Casero R.A., Jr The natural polyamine spermine functions directly as a free radical scavenger. Proc. Natl Acad. Sci. USA. 1998;95:11140–11145. doi: 10.1073/pnas.95.19.11140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chattopadhyay M.K., Tabor C.W., Tabor H. Polyamines protect Escherichia coli cells from the toxic effect of oxygen. Proc. Natl Acad. Sci. USA. 2003;100:2261–2265. doi: 10.1073/pnas.2627990100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gerner E.W., Meyskens F.L., Jr Polyamines and cancer: old molecules, new understanding. Nature Rev. Cancer. 2004;4:781–792. doi: 10.1038/nrc1454. [DOI] [PubMed] [Google Scholar]
- 40.Wallon U.M., O'Brien T.G. Polyamines modulate carcinogen-induced mutagenesis in vivo. Environ. Mol. Mutagen. 2005;45:62–69. doi: 10.1002/em.20086. [DOI] [PubMed] [Google Scholar]
- 41.Newton G.L., Aguilera J.A., Ward J.F., Fahey R.C. Polyamine-induced compaction and aggregation of DNA—a major factor in radioprotection of chromatin under physiological conditions. Radiat. Res. 1996;145:776–780. [PubMed] [Google Scholar]
- 42.Jaruga P., Theruvathu J., Dizdaroglu M., Brooks P.J. Complete release of (5′S)-8,5′-cyclo-2′-deoxyadenosine from dinucleotides, oligodeoxynucleotides and DNA, and direct comparison of its levels in cellular DNA with other oxidatively induced DNA lesions. Nucleic Acids Res. 2004;32:e87. doi: 10.1093/nar/gnh087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jaruga P., Birincioglu M., Rodriguez H., Dizdaroglu M. Mass spectrometric assays for the tandem lesion 8,5′-cyclo-2′-deoxyguanosine in mammalian DNA. Biochemistry. 2002;41:3703–3711. doi: 10.1021/bi016004d. [DOI] [PubMed] [Google Scholar]
- 44.Chung F.L., Zhang L. Deoxyguanosine adducts of tert-4-hydroxy-2-nonenal as markers of endogenous DNA lesions. Methods Enzymol. 2002;353:523–536. doi: 10.1016/s0076-6879(02)53074-3. [DOI] [PubMed] [Google Scholar]
- 45.Visapaa J.P., Jokelainen K., Nosova T., Salaspuro M. Inhibition of intracolonic acetaldehyde production and alcoholic fermentation in rats by ciprofloxacin. Alcohol Clin. Exp. Res. 1998;22:1161–1164. [PubMed] [Google Scholar]
- 46.Nechev L.V., Kozekov I., Harris C.M., Harris T.M. Stereospecific synthesis of oligonucleotides containing crotonaldehyde adducts of deoxyguanosine. Chem. Res. Toxicol. 2001;14:1506–1512. doi: 10.1021/tx0100690. [DOI] [PubMed] [Google Scholar]
- 47.Tuma D.J., Hoffman T., Sorrell M.F. The chemistry of acetaldehyde–protein adducts. Alcohol Alcohol. 1991;1(Suppl.):271–276. [PubMed] [Google Scholar]
- 48.Sako M., Yaekura I. A convenient preparative method for the 1,N2-cyclic adducts of guanine nucleosides and nucleotides with crotonaldehyde. Tetrahedron. 2002;58:8413–8416. [Google Scholar]
- 49.Sako M., Yaekura I., Deyashiki Y. Smooth and selective formation of the cyclic 1,N2-propano adducts in the reactions of guanine nucleosides and nucleotides with acetaldehyde. Tetrahedron Lett. 2002;43:6701–6703. [Google Scholar]
- 50.Simanowski U.A., Stickel F., Maier H., Gartner U., Seitz H.K. Effect of alcohol on gastrointestinal cell regeneration as a possible mechanism in alcohol-associated carcinogenesis. Alcohol. 1995;12:111–115. doi: 10.1016/0741-8329(94)00091-3. [DOI] [PubMed] [Google Scholar]
- 51.Seitz H.K., Simanowski U.A., Garzon F.T., Rideout J.M., Peters T.J., Koch A., Berger M.R., Einecke H., Maiwald M. Possible role of acetaldehyde in ethanol-related rectal cocarcinogenesis in the rat. Gastroenterology. 1990;98:406–413. doi: 10.1016/0016-5085(90)90832-l. [DOI] [PubMed] [Google Scholar]
- 52.Ogawa H., Gomi T., Fujioka M. Serine hydroxymethyltransferase and threonine aldolase: are they identical? Int. J. Biochem. Cell Biol. 2000;32:289–301. doi: 10.1016/s1357-2725(99)00113-2. [DOI] [PubMed] [Google Scholar]
- 53.Duncan T., Trewick S.C., Koivisto P., Bates P.A., Lindahl T., Sedgwick B. Reversal of DNA alkylation damage by two human dioxygenases. Proc. Natl Acad. Sci. USA. 2002;99:16660–16665. doi: 10.1073/pnas.262589799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kuykendall J.R., Bogdanffy M.S. Reaction kinetics of DNA-histone crosslinking by vinyl acetate and acetaldehyde. Carcinogenesis. 1992;13:2095–2100. doi: 10.1093/carcin/13.11.2095. [DOI] [PubMed] [Google Scholar]
- 55.Hengstler J.G., Bogdanffy M.S., Bolt H.M., Oesch F. Challenging dogma: thresholds for genotoxic carcinogens? The case of vinyl acetate. Annu. Rev. Pharmacol. Toxicol. 2003;43:485–520. doi: 10.1146/annurev.pharmtox.43.100901.140219. [DOI] [PubMed] [Google Scholar]
- 56.Obe G., Gobel D., Engeln H., Herha J., Natarajan A.T. Chromosomal aberrations in peripheral lymphocytes of alcoholics. Mutat. Res. 1980;73:377–386. doi: 10.1016/0027-5107(80)90202-x. [DOI] [PubMed] [Google Scholar]
- 57.Obe G., Natarajan A.T., Meyers M., Hertog A.D. Induction of chromosomal aberrations in peripheral lymphocytes of human blood in vitro, and of SCEs in bone-marrow cells of mice in vivo by ethanol and its metabolite acetaldehyde. Mutat. Res. 1979;68:291–294. doi: 10.1016/0165-1218(79)90160-5. [DOI] [PubMed] [Google Scholar]
- 58.Wang X., D'Andrea A.D. The interplay of Fanconi anemia proteins in the DNA damage response. DNA Repair (Amst.) 2004;3:1063–1069. doi: 10.1016/j.dnarep.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 59.Howlett N.G., Taniguchi T., Olson S., Cox B., Waisfisz Q., De Die-Smulders C., Persky N., Grompe M., Joenje H., Pals G., et al. Biallelic inactivation of BRCA2 in Fanconi anemia. Science. 2002;297:606–609. doi: 10.1126/science.1073834. [DOI] [PubMed] [Google Scholar]