Abstract
Purpose
This study aims to evaluate the clinical outcomes of arthroscopic autologous matrix-induced chondrogenesis (A-AMIC) for osteochondral lesions of the talus (OLT) at 24 months and 60 months of follow-up. The secondary aim was to assess whether age, body mass index (BMI), and lesion surface affect outcomes.
Design
Sixty-three patients (32 males, 31 females) with a median age of 37 years [interquartile range (IQR): 25-48] were included. Preoperative and postoperative (24 months and 60 months) clinical outcomes were evaluated using a Visual Analog Score (VAS) for pain during walking, the American Orthopaedic Foot and Ankle Society (AOFAS), Short-Form Survey (SF-12), the Halasi, and the University of California, Los Angeles (UCLA) scores. Patients were categorized according to age, BMI, and lesion surface (1-1.5 cm2 and over 1.5 cm2). The effect of each category was evaluated.
Results
There were significant improvements in the VAS, AOFAS, SF-12, and UCLA, comparing the preoperative scores to the 60-month follow-up scores (P < 0.001). There were no significant differences in the above-mentioned outcomes between the follow-up periods. Patients older than 33 years had lower SF-12, Halasi, and UCLA scores (P = 0.005, 0.004, and <0.001, respectively). Overweight patients had lower VAS, SF-12, Halasi, and UCLA scores (P = 0.006, 0.002, 0.024, and 0.007, respectively). Lesion size was uninfluential.
Conclusion
A-AMIC yielded clinical improvements at a minimum follow-up of 60 months in patients with symptomatic OLTs, with clinical improvement peaking in the first 2 years, followed by a plateau period. Increased age and BMI were significantly associated with inferior outcomes.
Keywords: osteochondral lesion of the talus, arthroscopic autologous matrix-induced chondrogenesis, all-arthroscopic autologous matrix-induced chondrogenesis
Introduction
Osteochondral lesions of the talus (OLTs) are characterized by damage to the cartilaginous and subchondral bone of the talar dome. Up to 75%-100% of the OLTs are associated with underlying traumas,1,2 either by significant traumatic events or recurrent microtrauma. 3 In recent years, advancements in imaging modalities, arthroscopic techniques, and biological-based treatment have extended the surgical indication for OLTs. 1 Currently, joint-preserving surgeries for OLT include fragment fixation, bone marrow stimulation (BMS), osteoperiosteal transfer (OT), or cartilage implantation techniques.1,2
The surgical treatment for OLT varies with lesion size. Microfracture, a bone marrow stimulating technique, is a highly effective treatment for lesions with a diameter of up to 107-150 mm.4 -7 However, the clinical results of microfracture and BMS in more extensive lesions and revision surgeries are less predictable. Therefore, many authors advocate using an alternative treatment modality in larger or nonprimary lesions. 7 Therefore, it is imperative to tailor a patient-specific surgical solution that considers the lesion size.
BMS technique relies on the formation of blood clots, which induces the chondrogenic differentiation of mesenchymal cells and the production of fibrocartilage coverage to the lesion.7 -9 A possible explanation for the reduced efficacy of the BMS technique in larger OLTs stems from the cloth instability in large lesions4,10,11 Over the last two decades, we have witnessed the introduction and widespread use of matrix solutions for focal chondral lesions, either in the form of matrices seeded in vitro before implantation or as acellular matrices for intrinsic seeding in vivo. 12 Autologous matrix-induced chondrogenesis (AMIC) was first introduced to OLT surgery in 2005. 9 It is a single-step procedure that exploits the regenerative potential of autologous bone marrow–derived mesenchymal stem cells. In contrast to the traditional BMS technique, AMIC uses a cell-free membrane to stabilize the bone marrow clot and ensure adequate lesion coverage.8,9,13,14 The original technique required an arthrotomy via medial malleolar osteotomy. Patients treated with AMIC demonstrated improvement in Patient-reported outcome measures (PROMS) and a relatively low failure rate. However, despite producing encouraging clinical results,9,14 this technique is associated with the comorbidities of the medial malleolar osteotomy. In 2015, Usuelli et al.11,13 introduced an all-arthroscopic AMIC (A-AMIC). They reported a significant reduction in the AOFAS, VAS, and lesion volume. However, no long-term follow-up of A-AMIC has been reported. 4
Introducing a new surgical technique should follow a specific, stepwise process including (1) concept/theory formation, (2) procedure development and exploration, (3) procedure assessment, and (4) long-term, ideally evidence-based studies. This study aims to evaluate the clinical outcome of A-AMIC at the 2- and 5-year follow-up. The secondary aim is to assess the influence of patient age, BMI, and lesion size on the clinical outcome.
Material and Method
This study included a single-institution cross-sectional retrospective follow-up of OLT treated by an arthroscopic AMIC procedure. This retrospective study was conducted in accordance with the Declaration of Helsinki and the Guidelines for Good Clinical Practice. Approval by the local medical ethics committee was obtained prior to the start of this study; Helsinki code: 887/20.
Patient Selection and Demographics
Between January 2013 and January 2017, 63 consecutive patients (32 male, 31 female) with a median age of 37 [interquartile range (IQR): 25-48] and a median follow-up time of 84 months [IQR: 60-108] ( Table 1 ) were operated for OLT types III and IV, according to Berndt and Harty’s classification. 15 The inclusion criteria were (1) symptomatic OLT lesion confirmed with either magnetic resonance imaging (MRI) or computed tomography (CT); (2) skeletal maturity; (3) deep ankle pain for more than 6 months, unresponsive to conservative treatment; (4) inability to fixate the OLT fragment; (5) primary treatment; (6) a minimum of 60 months of follow-up; (7) OLT with diameter >107 mm measured with either MRI or CT. The exclusion criteria are (1) ankle osteoarthritis, (2) severe hindfoot malalignment, (3) active infection, (4) metabolic arthropathy, (5) kissing lesions, and (6) revision A-AMIC ( Table 2 ). Patients’ images were extracted from the medical center’s picture archiving and communications system (PACS) database.
Table 1.
Demographic Factors.
| Factor | Results |
|---|---|
| Sex | 32 Males (50.8%) |
| Side | 40 Left (63%), 23 Right (37%) |
| Age (median and IQR) | 37 years [25-48] |
| BMI (median and IQR) | 24.6 kg/m2 [22-28.8] |
| Lesion dimension (median and IQR) | 140 mm2 [130-175] |
| Follow-up time (median and IQR) | 80 months [60-108] |
BMI = body mass index; IQR = interquartile range.
Table 2.
Summarizes the Inclusion and Exclusion Criteria.
| Indications | Contra-indication |
|---|---|
| OLT lesion confirmed with either MRI or CTA | Ankle osteoarthritis |
| Skeletal maturity | Severe hindfoot malalignment |
| Deep ankle pain for more than 6 months, unresponsive to conservative treatment | Active infection |
| Inability to fixate the OLT fragment | Metabolic arthropathy |
| Primary treatment | Kissing lesions |
| A minimum of 60 months follow-up | Revision A-AMIC |
| OLT with a diameter >107 mm measured preoperatively by MRI or CTA |
OLT = osteochondral lesions of the talus; MRI = magnetic resonance imaging; CTA = computed tomography angiography; A-AMIC = all arthroscopic autologous matrix-induced chondrogenesis.
Data Collection
Patient-reported outcomes included the walking Visual Analog Score (VAS), Short-Form Survey (SF-12), Halasi Score, and the University of California, Los Angeles score (UCLA), and American Orthopaedic Foot and Ankle Society Score (AOFAS). All 63 patients were reevaluated in our outpatient clinic postoperatively by fellowship-trained foot and ankle surgeons after 24 months and at least 60 months. The VAS, AOFAS, SF-12, Halasi, and UCLA scores were calculated. In addition, revision surgeries and postoperative complications were documented. Patient demographics were obtained through the electronic medical records scanned for relevant surgical and orthopedic history. Patients were evaluated in our outpatient clinic preoperatively by a foot and ankle surgeon. The clinical data included patients’ age and BMI. Radiographic data included an MRI or CT evaluation of the lesion’s dimensions.
Primary and Secondary Outcome
The primary aim of this study is to compare the preoperative PROMS of A-AMIC to the 2- and 5-year follow-ups. The secondary aim is to assess the influence of patient age, BMI, and lesion size on PROMs at the 5-year follow-up.
Surgical Technique
High-volume fellowship-trained foot and ankle surgeons performed all the surgical procedures. Patients were positioned in a supine position with an inflated thigh tourniquet. The entire procedure is performed arthroscopically using standard anteromedial and anterolateral portals. Initially, an arthroscopic inspection was performed to assess the lesion size and shape and the presence of concomitant intra-articular injuries. To improve accessibility to the lesion and avoid external traction, a Hintermann spreader (Integra LifeSciences, Plainsboro, NJ) is placed percutaneously. Two 2.5-mm k-wires are positioned over the tibia and talus (medially or laterally, depending on the lesions’ location), and the spreader is placed over them. Opening the spreader distracts the joint and rotates, making the lesion more accessible ( Fig. 1 ). The lesion is then debrided using an arthroscopic curette to remove the damaged cartilage and create smooth-shaped and stable lesion boundaries ( Fig. 2A ). A Chonro Pick (Arthrex, Naples, FL) induces microfractures to penetrate the subchondral bone and promote bleeding. The lesion dimensions are sized preoperatively using CT and remeasured intraoperatively using a probe to confirm the size ( Fig. 2B ). A 5.5-mm cannula is inserted through the portal adjacent to the lesion. The intra-articular liquids are drained, and a Chondro-Gidematrix (Geistlich) Geistlich Pharma AG (Geistlich Surgery, Wolhusen, Switzerland) that matches the size of the lesion is inserted through the cannula and positioned to cover the lesion site ( Fig. 2C ). Slightly downsizing the membrane is advisable since its volume increases once introduced in a wet environment. If needed, an autologous bone graft is harvested from the anterior wall of the tibia, which can fill bone defects below the cartilage layer. Once the membrane fits the lesion, it is glued with fibrin glue (Tisseel; Baxter, Deerfield, IL). The Hintermann spreader is removed, and the stability of the membrane throughout a normal ankle range of motion is checked arthroscopically. Patients were placed in a walker boot for 30 days.
Figure 1.
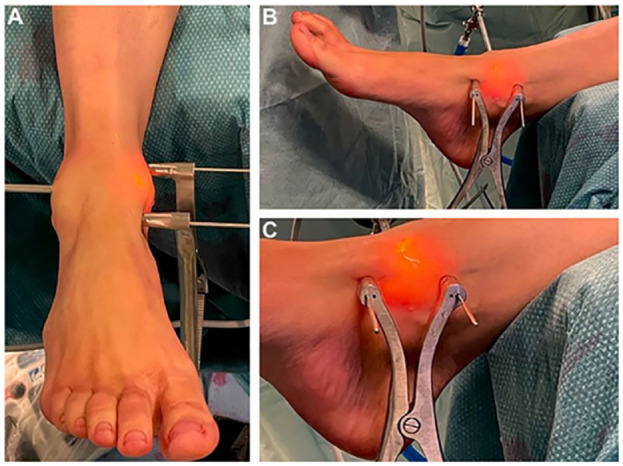
(A) Depicts the positioning of the Hintermann spreader as viewed from the anterior perspective, while (B) and (C) illustrate the placement of the Hintermann spreader from a medial viewpoint.
Figure 2.
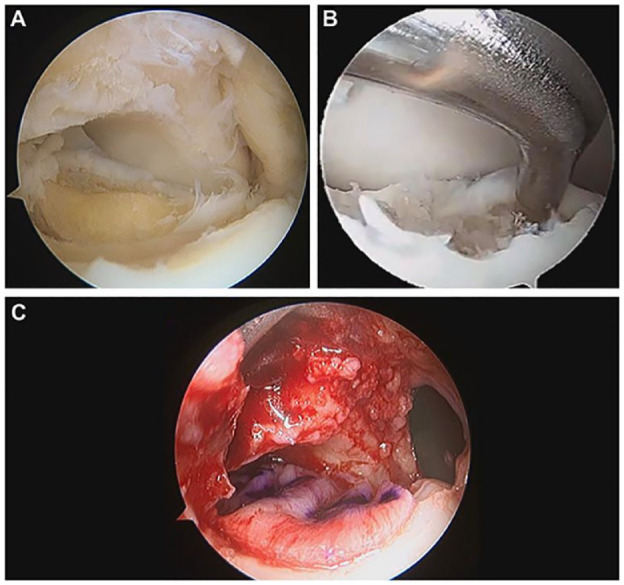
(A) Demonstrates an OLT after a curettage of the damaged cartilage. (B) Displays the intraoperative measurement of the lesion with an arthroscopic probe. (C) Shows the membrane placement over the OLT.
Data Analysis and Statistics
The preoperative PROMs were compared to the postoperative 24- and 60-month PROMs. In addition, the 24-month and 60-month PROMS were compared to evaluate if additional improvement or deterioration was documented between the two follow-ups. Treatment failure was defined as no improvement of PROMS or a revision OLT surgery at the 60-month follow-up.
In addition, the patients were divided into groups according to measurements previously described in the medical literature to evaluate the influence of age, BMI, and lesion size.7,10,16 Three groups were created. The group divided the patients into patients younger or older than 33 years. 10 The BMI group divided the patients into overweight and normal-weight patients. The lesion groups divided the patients into sizes from 1 cm to 1.5 cm or over 1.5 cm. We then compare the 60 months of PROMS to evaluate the influence of each group on the clinical outcome.
The normal distribution of the data was tested using the Shapiro-Wilk test since most data are not normally distributed. Continuous data are in the median and IQR. PROMS were considered related samples and were evaluated using Friedman’s two-way analysis of variance (ANOVA). Individual groups were considered independent samples and were assessed using the Mann-Whitney U test. P < 0.05 was considered significant. (IBM Corp. Released 2013. IBM SPSS Statistics, Version 29.0; IBM Corp, Armonk, NY)
Results
Clinical Outcome
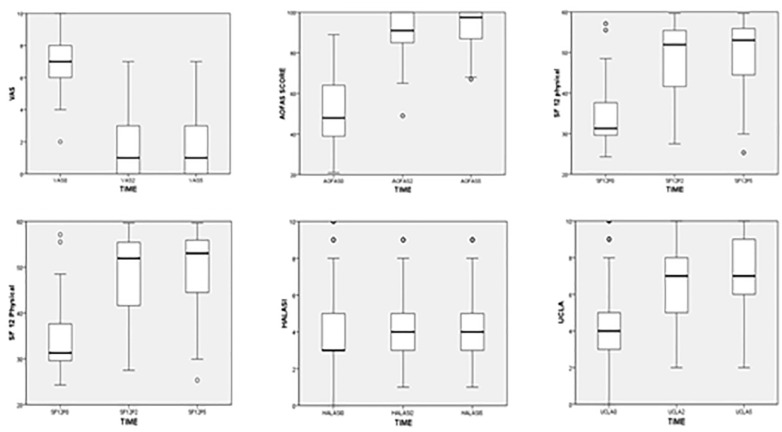
Patient demographics are grouped in Table 2 . There was an improvement of 5.5 points and 5 points in VAS in the 24- and 60-month follow-ups, respectively (P < 0.001). There was an improvement of 47.5 points and 49.5 points in AOFAS in the 24- and 60-month follow-ups, respectively (P < 0.001). There was an improvement of 20 points and 21 points in SF-12 physical status in the 24- and 60-month follow-ups, respectively (P < 0.001). There was an improvement of 9 points and 13 points in SF-12’s mental status in the 24- and 60-month follow-ups, respectively. There was an improvement of 2.5 points and 3.3 points in UCLA’s score in the 24- and 60-month follow-ups, respectively (P < 0.001). There was no significant difference between the 24-month and 60-month follow-ups. There was no significant difference between the preoperative and postoperative Halasi scores ( Table 3 and Fig. 3 ).
Table 3.
Patient-Reported Outcome Scores and AOFAS.
| PROMS | Median Preoperative Score [IQR] | Median 24-Month Score [IQR] | Median 60-Month Score [IQR] | P Value, Preoperative to 60 Months [IQR] | P Value, Preoperative to 60 Months [IQR] |
|---|---|---|---|---|---|
| VAS during walking | 7 [6-8] | 1.5 [0-4] | 2 [0-3] | P < 0.001 | P = 0.7 |
| AOFAS | 46.5 [38-64] | 90 [84-100] | 92 [85-100] | P < 0.001 | P = 0.5 |
| SF-12 physical | 31 [27-37] | 51 [49-58] | 53 [45-56] | P < 0.001 | P = 0.16 |
| SF-12 mental | 43 [40-46] | 51 [41-58] | 56 [44-56] | P < 0.001 | P = 0.1 |
| UCLA | 4 [3-6.5] | 6.5 [5-8] | 7 [6-9] | P < 0.001 | P = 0.19 |
| Halasi | 3 [3-5] | 4 [3-5] | 4 [3-5] | P = 0.7 | P = 0.2 |
Figure 3.
Boxplot comparing the preoperative score to the 24- and 60-month follow-up showing a significant difference in the VAS, AOFAS, SF-12, and UCLA scores.
Subgroup Analysis
At the 60-month follow-up, There is a significant difference between the patients younger than 33 years and those older than 33 years in the SF-12 physical, Halasi, and the UCLA scores (P = 0.005, 0.004, and <0.001, respectively). At the same time, there was no significant difference between the groups’ AOFAS, VAS, and SF mental status. There is a significant difference between the patients with normal BMI and overweight in the VAS, SF12 physical status, Halasi, and the UCLA scores (P = 0.006, 0.002, 0.024, and 0.007, respectively). Lesion size did not influence the PROMS ( Table 4 ).
Table 4.
Clinical Outcomes Divided by Subgroups at the 60-Month Follow-Up.
| Age |
BMI |
Lesion Dimension |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| PROMS | Under 33 | Over 33 | P Value | Normal Weight | Over Weight | P Value | 1-1.5 cm2 | Over 1.5 cm2 | P Value |
| VAS | 1 [0,2] |
2 [0,4] |
0.1 | 1 [0,2] |
2 [0,4] |
0.06 | 2 [0,7] |
2 [0,4] |
0.7 |
| AOFAS | 98 [90,100] |
90 [85,100] |
0.15 | 98 [90,100] |
90 [85,100] |
0.56 | 90 [85,100] |
97 [87,100] |
0.65 |
| SF-12 physical | 56 [52,57] |
52 [38,55] |
0.005 | 56 [52,57] |
52 [38,55] |
0.002 | 54 [44,57] |
52 [44,57] |
0.4 |
| SF-12 mental | 58 [53,60] |
55 [49,59] |
0.08 | 58 [53,60] |
55 [49,59] |
0.59 | 58 [50,61] |
59 [50,61] |
0.4 |
| UCLA | 9 [7,10] |
6 [4,8] |
<0.01 | 9 [7,10] |
6 [4,8] |
0.007 | 8 [4,10] |
8 [6,10] |
0.6 |
| Halasi | 5 [4,8] |
3 [2,5] |
0.004 | 5 [4,8] |
3 [2,5] |
0.02 | 4 [3,8] |
4 [3,8] |
0.97 |
Failure of Treatment
There was a 9% treatment failure rate. Two patients (4%) underwent revision surgery: one patient for hypertrophic tissue underwent arthroscopic removal of the hypertrophic tissue at 6 months postoperatively and showed clinical improvement. The other patient’s lesion was only partially covered by the fibrocartilage layer; subsequently, he underwent revision arthroscopic microfracture surgery. Due to the progression of symptoms and signs of advanced osteoarthritis, he had a total ankle replacement. Three patients (5%) showed deterioration in their PROMs.
Discussion
This is the first study that evaluates the clinical outcome of A-AMIC with a minimum 60-month follow-up period. The principal finding of this study is a significant improvement in the VAS, AOFAS, SF12, and UCLA scores and only 9% failed treatment. No significant clinical improvement or deterioration was recorded between the 24- and 60-month follow-up. Being over the age of 33 years and having a higher body weight adversely affected the surgical outcomes, while the size of the lesion had no significant impact. Only 2 patients had a revision surgery (4%), and only 3 (5%) did not improve their PROMS.
The clinical outcome of OLT surgery depends on the lesion dimension and depth. 7 Most studies demonstrate that lesions with a diameter between 1 and 1.5 cm2 could be treated with BMS.7,17 However, BMS showed unpredictable clinical results for larger lesions. Guelfi et al. 6 conducted an international survey among foot and ankle surgeons and found that most surgeons (78%) advocate performing BMS for small lesions. However, there is a considerable variation in management protocols for more extended lesions. The surgeon’s treatment protocols were divided between BMS, BMS with scaffold (e.g., AMIC), bone transfer, and cartilage implantation. Since each technique currently has its advantages and disadvantages, there needs to be more support in the medical literature for a specific technique. 6 BMS techniques are relatively simple; however, this technique relies on fibrocartilage regeneration, which has inferior shock-absorbing quality compared to hyaluronic cartilage. 18 In addition, since they rely on clot formation and stability, they are less effective in treating large lesions.18,19 Bone transfer techniques are effective in large lesions and lesions with extensive bone loss18,19; however, they require graft harvesting and are associated with donor-site morbidity.11,20 First- through third-generation chondrocyte implantation techniques are expensive, requiring two-step procedures. Alternatively, fourth-generation ACI, which utilizes cartilage fragments, offers a 1-stage solution.11,21
The AMIC technique is a single-step procedure that exploits the regenerative potential of autologous bone marrow–derived mesenchymal stem cells. This technique uses a cell-free membrane to stabilize the bone marrow clot and ensure adequate fibrocartilage coverage of the OLT. It is a relatively new technique, and long-term follow-up is still needed to validate t its efficacy compared to other techniques. A recent meta-analysis by Migliorini et al. 22 compared the surgical outcomes of AMIC, Osteochondral Autolog0us transfer surgery (OATS), microfracture, mosaicplasty, and chondrocyte transplantation. 22 They found superior clinical results at the 4-year follow-up for AMIC. Götze et al., 9 in a prospective study, evaluated the clinical outcome of open AMIC in the 24- and 60-month follow-ups. 9 They found a decrease in Foot Function Index (FFI) of 22.5% in the 24-month follow-up and an additional 1.3% in the 60-month follow-up. In addition, they found an improvement in the AOFAS score of 17.2% at the 24-month follow-up and an additional 3.4% at the 60-month follow-up. Walther et al. 14 meta-analysis encompassed 492 patients, and they compared their preoperative clinical status with outcomes at 1-2 years and 3-5 years after surgery. The findings revealed significant improvements at the 3- to 5-year follow-up, with increases of 30.9 points in the FFI and of 32.4 points in the AOFAS scores, respectively.
The original AMIC technique suffers from a significant disadvantage: It requires osteotomy of the medial malleolus, and therefore, it is subjected to comorbidities, such as intra-articular damage to the tibial plafond, nonunion, malunion, and hardware irritation. In contrast, A-AMIC does not violate the medial malleolus, and therefore, it is not subjected to the disadvantages of the classical technique. However, the long-term outcome of this technique has not been reported. The current study is the first one to evaluate A-AMIC’s short-term and midterm results. The current cohort of primary OLT patients with a median follow-up of 84 months and a minimum follow-up of 60 months showed a significant improvement in the VAS, AOFAS, SF-12, and UCLA scores comparable to the open technique indicating that A-AMIC could be used to treat OLT lesions. In addition, we found that there has not been a significant change in the clinical outcome between the 24 and 60 months. This finding reinforces the finding reported by Gottschalk et al., 23 who evaluated the clinical outcome of AMIC. They reported that the patient should expect to reach the peak of their improvement after the first two years and to maintain this improvement for at least five years.
Hollander et al. 24 evaluated the frequency and severity of the complication rate of OLT surgery across 6,962 patients. 24 They found that 3% of patients treated with matrix-induced BMS suffered from postoperative complications. Migliorini et al. 22 conducted a systematic review concentrating on patients treated with AMIC. They reported that 7.8% (44 of 564) of the patients had a revision surgery. At the same time, 6.2% (32 of 515 patients) were considered a failure. Waltenspül et al. 25 analyzed the complication rate across 130 open AMIC patients. They reported that 28% of the patients had revision surgeries due to AMIC-related complications. In addition, 36% of patients underwent a revision surgery for hardware removal. In the current cohort, only 4% of the patients had revision surgeries related to AMIC-related issues; one patient had hypertrophic tissue and was treated with arthroscopic debridement, and one had had graft thinning and was treated with microfracture. In addition, only 5% of the patients did not improve their PROMS score. The finding in this study confirms that A-AMIC can be used to reduce operative complications while not sacrificing the clinical outcome.
The study’s secondary outcome was to assess the influence of age, BMI, and lesion size on postoperative outcomes. D’Ambrosi et al. 10 evaluated the impact of age on postoperative outcomes of AMIC surgery. They found a significant difference in PROMS between patients younger and older than 33 years. Gottschalk et al. 23 found a negative correlation between BMI and postoperative outcome. The current study found that patients older than 33 years had lower SF-12 scores, and overweight patients had significantly lower SF-12 and VAS score improvement than normal-weight patients. In addition, we evaluated the postoperative activity level using the Halasi and UCLA scores. 25 As expected, we found that overweight patients and patients older than 33 years have significantly lower improvement rates. The lesion size did not influence the clinical outcome in the current study.
This study has a few limitations. It is a retrospective study with a relatively small cohort. We did not evaluate the postoperative radiological outcome of this procedure. Finally, this technique included only primary OLT patients. Future studies should assess the clinical results of A-AMIC for revision surgeries.
Conclusion
A-AMIC yielded clinical improvements at a minimum follow-up duration of 60 months in patients with symptomatic OLTs, with clinical improvement peaking in the first two years, followed by a plateau period. Increased age and BMI were significantly associated with inferior outcomes.
Footnotes
Acknowledgments and Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The lead author is currently working in Sackler School of Medicine, Tel Aviv University, Tel Aviv, Israel, and Division of Orthopedic Surgery Tel Aviv Medical Center, Tel Aviv, Israel.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iDs: Agustin Barbero  https://orcid.org/0000-0002-8590-6383
https://orcid.org/0000-0002-8590-6383
Jari Dahmen  https://orcid.org/0000-0002-6849-1008
https://orcid.org/0000-0002-6849-1008
References
- 1. Bruns J, Habermann C, Werner M. Osteochondral lesions of the talus: a review on talus osteochondral injuries, including osteochondritis dissecans. Cartilage. 2021;13(Suppl. 1):1380S-401S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rikken QGH, Kerkhoffs GMMJ. Osteochondral lesions of the talus: an individualized treatment paradigm from the Amsterdam perspective. Foot Ankle Clin. 2021;26(1):121-36. [DOI] [PubMed] [Google Scholar]
- 3. Zengerink M, Struijs PA, Tol JL, van Dijk CN. Treatment of osteochondral lesions of the talus: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2010;18(2):238-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Usuelli FG, Grassi M, Manzi L, Guarrella V, Boga M, DE Girolamo L. Treatment of osteochondral lesions of the talus with autologous collagen-induced chondrogenesis: clinical and magnetic resonance evaluation at one-year follow-up. Joints. 2016;4(2):80-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barbier O, Amouyel T, de l’Escalopier N, Cordier G, Baudrier N, Benoist J, et al. Osteochondral lesion of the talus: what are we talking about. Orthop Traumatol Surg Res. 2021;107(8S):103068. [DOI] [PubMed] [Google Scholar]
- 6. Guelfi M, DiGiovanni CW, Calder J, Malagelada F, Cordier G, Takao M, et al. Large variation in management of talar osteochondral lesions among foot and ankle surgeons: results from an international survey. Knee Surg Sports Traumatol Arthrosc. 2021;29(5):1593-603. [DOI] [PubMed] [Google Scholar]
- 7. Ramponi L, Yasui Y, Murawski CD, Ferkel RD, DiGiovanni CW, Kerkhoffs GMMJ, et al. Lesion size is a predictor of clinical outcomes after bone marrow stimulation for osteochondral lesions of the talus: a systematic review. Am J Sports Med. 2017;45(7):1698-705. [DOI] [PubMed] [Google Scholar]
- 8. Ayyaswamy B, Salim M, Sidaginamale R, Elsayed M, Karpe P, Limaye R. Early to medium term outcomes of osteochondral lesions of the talus treated by autologous matrix induced chondrogenesis (AMIC). Foot Ankle Surg. 2021;27(2):207-12. [DOI] [PubMed] [Google Scholar]
- 9. Götze C, Nieder C, Felder H, Peterlein CD, Migliorini F. AMIC for traumatic focal osteochondral defect of the talar shoulder: a 5 years follow-up prospective cohort study. BMC Musculoskelet Disord. 2021;22(1):638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. D’Ambrosi R, Maccario C, Serra N, Liuni F, Usuelli FG. Osteochondral lesions of the talus and autologous matrix-induced chondrogenesis: is age a negative predictor outcome. Arthroscopy. 2017;33(2):428-35. [DOI] [PubMed] [Google Scholar]
- 11. Usuelli FG, de Girolamo L, Grassi M, D’Ambrosi R, Montrasio UA, Boga M. All-arthroscopic autologous matrix-induced chondrogenesis for the treatment of osteochondral lesions of the talus. Arthrosc Tech. 2015;4(3):e255-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gille J, Kunow J, Boisch L, Behrens P, Bos I, Hoffmann C, et al. Cell-laden and cell-free matrix-induced chondrogenesis versus microfracture for the treatment of articular cartilage defects: a histological and biomechanical study in sheep. Cartilage. 2010;1(1):29-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Usuelli FG, D’Ambrosi R, Maccario C, Boga M, de Girolamo L. All-arthroscopic AMIC® (AT-AMIC®) technique with autologous bone graft for talar osteochondral defects: clinical and radiological results. Knee Surg Sports Traumatol Arthrosc. 2018;26(3):875-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Walther M, Valderrabano V, Wiewiorski M, Usuelli FG, Richter M, Baumfeld TS, et al. Is there clinical evidence to support autologous matrix-induced chondrogenesis (AMIC) for chondral defects in the talus? a systematic review and meta-analysis. Foot Ankle Surg. 2021;27(3):236-45. [DOI] [PubMed] [Google Scholar]
- 15. Lopes R, Geffroy L, Padiolleau G, Ngbilo C, Baudrier N, Mainard D, et al. Proposal of a new CT arthrographic classification system of osteochondral lesions of the talus. Orthop Traumatol Surg Res. 2021;107(6):102890. [DOI] [PubMed] [Google Scholar]
- 16. Usuelli FG, Maccario C, Ursino C, Serra N, D’Ambrosi R. The impact of weight on arthroscopic osteochondral talar reconstruction. Foot Ankle Int. 2017;38(6):612-20. [DOI] [PubMed] [Google Scholar]
- 17. Rikken QGH, Dahmen J, Stufkens SAS, Kerkhoffs GMMJ. Satisfactory long-term clinical outcomes after bone marrow stimulation of osteochondral lesions of the talus. Knee Surg Sports Traumatol Arthrosc. 2021;29(11):3525-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Becher C, Malahias MA, Ali MM, Maffulli N, Thermann H. Arthroscopic microfracture vs. arthroscopic autologous matrix-induced chondrogenesis for the treatment of articular cartilage defects of the talus. Knee Surg Sports Traumatol Arthrosc. 2019;27(9):2731-6. [DOI] [PubMed] [Google Scholar]
- 19. Dahmen J, Hurley ET, Shimozono Y, Murawski CD, Stufkens SAS, Kerkhoffs GMMJ, et al. Evidence-based treatment of failed primary osteochondral lesions of the talus: a systematic review on clinical outcomes of bone marrow stimulation. Cartilage. 2021;13(Suppl. 1):1411S-21S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Powers RT, Dowd TC, Giza E. Surgical treatment for osteochondral lesions of the talus. Arthroscopy. 2021;37(12):3393-6. [DOI] [PubMed] [Google Scholar]
- 21. Hu M, Li X, Xu X. Efficacy and safety of autologous chondrocyte implantation for osteochondral defects of the talus: a systematic review and meta-analysis. Arch Orthop Trauma Surg. 2023;143(1):71-9. [DOI] [PubMed] [Google Scholar]
- 22. Migliorini F, Maffulli N, Bell A, Hildebrand F, Weber CD, Lichte P. Autologous matrix-induced chondrogenesis (AMIC) for osteochondral defects of the talus: a systematic review. Life. 2022;12(11):1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gottschalk O, Altenberger S, Baumbach S, Kriegelstein S, Dreyer F, Mehlhorn A, et al. Functional medium-term results after autologous matrix-induced chondrogenesis for osteochondral lesions of the talus: a 5-year prospective cohort study. J Foot Ankle Surg. 2017;56(5):930-6. [DOI] [PubMed] [Google Scholar]
- 24. Hollander JJ, Dahmen J, Emanuel KS, Stufkens SAS, Kennedy JG, Kerkhoffs GMMJ. The frequency and severity of complications in surgical treatment of osteochondral lesions of the talus: a systematic review and meta-analysis of 6,962 lesions. Cartilage. 2023;14(2):180-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Waltenspül M, Meisterhans M, Ackermann J, Wirth S. Typical complications after cartilage repair of the ankle using autologous matrix-induced chondrogenesis (AMIC). Foot Ankle Orthop. 2023;8(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]