Abstract
The integrity of eukaryotic translation initiation factor (eIF) interactions in ribosomal preinitiation complexes is critical for the proper regulation of GCN4 mRNA translation in response to amino acid availability. Increased phosphorylation of eIF2 under amino acid starvation conditions leads to a corresponding increase in GCN4 mRNA translation. The carboxyl-terminal domain (CTD) of eIF5 (eIF5-CTD) has been identified as a potential nucleation site for preinitiation complex assembly. To further characterize eIF5 and delineate its role in GCN4 translational control, we isolated mutations leading to temperature sensitivity (Ts− phenotype) targeted at TIF5, the structural gene encoding eIF5 in yeast (Saccharomyces cerevisiae). Nine single point mutations were isolated, in addition to an allele in which the last 15 amino acids were deleted. The nine point mutations clustered in the eIF5-CTD, which contains two conserved aromatic/acidic boxes. Six of the point mutations derepressed GCN4 translation independent of eIF2 phosphorylation (Gcd− phenotype) at a permissive temperature, directly implicating eIF5-CTD in the eIF2/GTP/Met-tRNAiMet ternary complex binding process required for GCN4 translational control. In addition, stronger restriction of eIF5-CTD function at an elevated temperature led to failure to derepress GCN4 translation (Gcn− phenotype) in all of the mutants, most likely due to leaky scanning of the first upstream open reading frame of GCN4 mRNA. This latter result directly implicates eIF5-CTD in the process of accurate scanning for, or recognition of, AUG codons. Taken together, our results indicate that eIF5-CTD plays a critical role in both the assembly of the 43S complex and the postassembly process in the 48S complex, likely during the scanning process.
In eukaryotes, many stress stimuli activate the phosphorylation of eukaryotic translation initiation factors (eIFs) to alter gene expression profiles (12). In the case of the budding yeast Saccharomyces cerevisiae, amino acid starvation selectively induces the translation of GCN4, which, in turn, activates the transcription of at least ∼70 amino acid biosynthetic genes as well as other genes encoding vitamin- or cofactor-biosynthetic enzymes, peroxisomal components, mitochondrial carrier proteins, and autophagy proteins (general control pathway) (33). This unique induction of GCN4 translation is due to regulatory upstream open reading frames (uORFs) in its mRNA. Translation of the first uORF (uORF1) tethers the 40S subunit to GCN4 mRNA (23). The ribosome then migrates downstream as it reacquires at least a subset of eIFs and resumes scanning for start codons. Under nonstarvation conditions, the tethered 40S subunits dissociate from the mRNA without translating GCN4, as they readily reacquire the necessary eIFs and initiate translation at uORF2, uORF3, or uORF4. The accumulation of uncharged tRNA under amino acid starvation conditions activates the kinase Gcn2p, which, in turn, phosphorylates eIF2, a GTP-dependent Met-tRNAiMet binding factor, at a conserved Ser residue (Ser-51) of its α-subunit. This phosphorylation event renders eIF2 into a competitive inhibitor of its guanine nucleotide exchange factor eIF2B, thereby reducing the level of eIF2-GTP and hence its ternary complex (TC) with Met-tRNAiMet. As a result, ribosomes that have translated uORF1 migrate down the GCN4 mRNA leader and, as a result of reduced levels of TC, bypass uORF2-4, setting up initiation complexes instead at the authentic GCN4 start codon.
Translational control of GCN4 expression depends critically on the intact translation initiation machinery (for a review of the translation initiation pathway, see reference 22). The process of eukaryotic translation initiation starts by the assembly of the 40S ribosomal subunit with eIF1A, eIF1, eIF5, eIF3, and eIF2 to form the 43S complex. Prior to ribosome binding, eIF2 forms the TC with GTP and Met-tRNAiMet. eIF4F binds the 5′-m7G cap of the mRNA via its eIF4E subunit, whereas the eIF4G subunit of eIF4F binds a part of the 43S complex to recruit the latter to the mRNA (21). The resultant 48S complex migrates downstream towards the start codon in a process called scanning. Once the 48S complex is positioned at the start codon, which base pairs with the anticodon of the Met-tRNAiMet, eIF2 GTPase is activated by a mechanism that appears to involve the N-terminal domain (NTD) of eIF5 (eIF5-NTD) (11, 36) and a conformational change of the preinitiation complex (28). The assembled factors then dissociate to allow the formation of a 40S initiation complex composed of the 40S subunit, mRNA, and Met-tRNAiMet. The ribosome-dependent GTPase activity of eIF5B facilitates joining of the 40S initiation complex with the 60S subunit to produce the 80S initiation complex, an immediate precursor for the elongation phase of protein synthesis (37, 39).
Yeast strains with deletions of positive regulators of GCN4 expression (e.g., GCN2) are sensitive to 3-amino-1,2,4-triazole (3AT), an inhibitor of the histidine biosynthetic pathway used to induce amino acid starvation. gcn2Δ strains are unable to phosphorylate eIF2 in the presence of 3AT, leading to an inability of ribosomes to bypass inhibitory uORF2-4 on the GCN4 leader to turn on GCN4 translation (general control nonderepressible, or Gcn− phenotype). On the other hand, alterations of any of the three subunits of eIF2 or five subunits of its guanine nucleotide exchange factor eIF2B activate GCN4 translation independent of eIF2 phosphorylation (general control derepressed, or Gcd− phenotype), by constitutively reducing TC levels without altering the integrity of the 48S complex for GCN4 translation (23). Due to the constitutively elevated level of Gcn4p, Gcd− mutants are 3AT resistant even in the absence of GCN2. Interestingly, recent studies indicate that mutants defective in eIF3b (34) and eIF5B (10) display Gcn− phenotypes. The eIF3b mutation appears to slow down migration of the 40S subunit past uORF1 to allow translation of uORF2-4, thus preventing reinitiation at GCN4 (34), whereas the eIF5B mutations appear to increase leaky scanning of uORF1, thereby preventing 40S subunits from bypassing uORF2-4 (10, 39).
In the yeast Saccharomyces cerevisiae, eIF5 is encoded by the single-copy essential gene TIF5 (9). The 165-amino-acid CTD of yeast eIF5 (see Fig. 1A for the primary structure) binds simultaneously to eIF1, the β subunit of eIF2, and the c subunit of eIF3, thereby mediating formation of a multifactor complex (MFC) with eIFs 1, 2, 3, and 5 and with Met-tRNAiMet (2, 41). The eIF5-CTD contains a bipartite motif called aromatic/acidic boxes (AA-boxes) 1 and 2 (also known as W2 or eIF5C domain), a feature that is also conserved in the C-terminal domains of all eukaryotic eIF2Bɛ and mammalian eIF4G proteins (1, 25). Mutational studies have demonstrated the functional importance of these AA-boxes (3, 16, 30). Due to the critical role of eIF5-CTD in factor assembly, eIF5-CTD mutations are expected to impair GCN4 translational control. However, their effect on GCN4 translational control is difficult to predict, because they are expected to impair both the TC binding to the 40S subunit (in favor of the Gcd− phenotype) and the factor linkage in the 48S complex critical for its ability to scan for the start codon (in favor of the Gcn− phenotype). Curiously, the tif5-7A allele, in which seven amino acids of AA-box 2 have been altered, was shown to be defective in Met-tRNAiMet binding to the 40S subunit in cell extracts but prevented translational derepression of GCN4 (Gcn−) (34) rather than constitutively derepressing GCN4 translation (Gcd−) (3, 5).
FIG. 1.
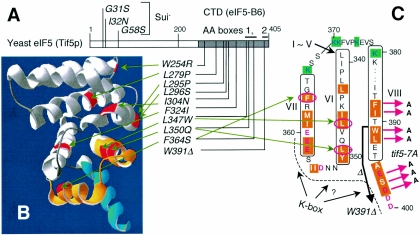
The positions of eIF5 point mutations used in this study. (A) Positions of Sui− and Ts− mutation sites are located in the primary structure of yeast eIF5. Numbers indicate eIF5 amino acid positions. The gray region indicates the CTD. Bars above the box denote AA-boxes 1 and 2. (B) The positions of eIF2Bɛ residues homologous to yeast eIF5-CTD mutation sites in panel A are highlighted in red on the solved structure of eIF2Bɛ-CTD (7). AA-box 1 and 2 amino acids are highlighted in orange and blue, respectively. The three-dimensional structure was drawn with DeepView/Swiss-Pdf Viewer (v. 3.7) (Swiss Institute of Bioinformatics). (C) Yeast eIF5 amino acids from positions 339 to 400 are arranged to show secondary structures predicted from Boesen's eIF2Bɛ-CTD structure (7). Essentially, the entire eIF5-CTD is predicted to form eight α-helices, designated helix I (most N-terminal) to helix VIII (most C-terminal). Residues predicted to participate in α-helices VI through VIII are boxed with the helix numbers in Roman numerals. Highlighted with orange are AA-box amino acids (AA-box 1 in helices VI and VII; AA-box 2 in helix VIII), while highlighted with green in blue letters are lysine residues highly conserved in all eIF5s. Conserved acidic residues are shown in red. The dotted line and question mark indicate a possible K box interface. Red arrows indicate tif5-7A mutation sites. The thick arrow indicates the region deleted by tif5-W391Δ. Circled in red are the residues altered by indicated mutations.
To further characterize eIF5 and delineate its role in GCN4 translational control, we isolated 10 mutations leading to temperature sensitivity (Ts− phenotype) targeted at TIF5. Nine of these mutations were due to single-point mutations altering eIF5-CTD while the other resulted in the deletion of the C-terminal 15 amino acids, including a part of AA-box 2. Six of the point mutations conferred a Gcd− phenotype at a permissive temperature, directly implicating eIF5-CTD in the TC binding process required for GCN4 translational control. In addition, all of the eIF5-CTD point mutations conferred Gcn− phenotypes at an elevated temperature. GCN4-lacZ reporter assays under the latter conditions strongly suggest that the Gcn− phenotype is a result of leaky scanning of uORF1, implicating eIF5-CTD in the accurate scanning for AUG codons. None of the eIF5-CTD mutants conferred an increase in non-AUG start codon recognition (the Sui− phenotype; suppressor of initiation codon mutations). Taken together, our results indicate that eIF5-CTD plays a critical role both in the assembly of the 43S complex and in the postassembly process in the 48S complex, likely during the scanning process. We discuss the mechanism of controlled reinitiation of GCN4 translation based on these data. Our results extend and complement the recent work of Valášek et al., who reported that mutations in the eIF3c-NTD, an important partner of eIF5-CTD, display Gcd− and Sui− phenotypes in yeast, implicating eIF3c-NTD in both MFC assembly and stringent AUG recognition (44).
MATERIALS AND METHODS
Plasmid construction.
Plasmids used in this study are listed in Tables 1 and 2. We constructed YCpW-TIF34-HA and YCpW-FL-SUI3 by transferring the AlwNI-SacI segment of YCpL-TIF34-HA (4) and the SalI-HindIII segment of YCpSUI3 (3), respectively, to YCplac22 (15). The TIF34 (eIF3i) or SUI3 (eIF2β) product encoded by these plasmids is epitope tagged with three copies of hemagglutinin (HA) or one copy of FLAG (DYKDDDDK) peptide, respectively.
TABLE 1.
Plasmids employed in this study
| Plasmid | Description | Source or reference |
|---|---|---|
| pKA234 | Single-copy LEU2 TIF5 plasmid | 3 |
| Ep1404 | Single-copy LEU2 tif5-F364S plasmid (Ts−) | This study |
| Ep1412 | Single-copy LEU2 tif5-L295P plasmid (Ts−) | This study |
| Ep1416 | Single-copy LEU2 tif5-F324I plasmid (Ts−) | This study |
| Ep1418 | Single-copy LEU2 tif5-W254R plasmid (Ts−) | This study |
| Ep1444 | Single-copy LEU2 tif5-L350Q plasmid (Ts−) | This study |
| Ep1445 | Single-copy LEU2 tif5-F296S plasmid (Ts−) | This study |
| Ep1446 | Single-copy LEU2 tif5-G58S plasmid (Ts+, Sui−) | C. Curtis/E. Hannig |
| Ep1447 | Single-copy LEU2 tif5-I304N plasmid (Ts−) | This study |
| Ep1448 | Single-copy LEU2 tif5-L347W plasmid (Ts−) | This study |
| Ep1449 | Single-copy LEU2 tif5-W39IΔ plasmid (Ts−) | This study |
| Ep1450 | Single-copy LEU2 tif5-I32N plasmid (Ts+, Sui−) | C. Curtis/E. Hannig |
| Ep1451 | Single-copy LEU2 tif5-G31S plasmid (Ts+, Sui−) | C. Curtis/E. Hannig |
| Ep1476 | Single-copy LEU2 tif5-L279P plasmid (Ts−) | This study |
| pKA758 | Single-copy LEU2 tif5-7A plasmid (Ts−) | 40 |
| YEplac195 | High-copy-number URA3 plasmid | 15 |
| p1780-IMT | High-copy-number SUI2 SUI3 GCD11 IMT URA3 plasmid | 3 |
| YEpU-SUI1 | High-copy-number SUI1 URA3 plasmid | 20 |
| YCpW-TIF34-HA | Single-copy TRP1 TIF34-HA plasmid | This study |
| YCpW-FL-SUI3 | Single-copy TRP1 FL-SUI3 plasmid | This study |
| pGEX-SUI3ΔS | eIF2β (1-140) cloned in a pGEX vector (Pharmacia) | 41 |
| pT7-SUI1 | eIF1 cloned under a T7 promoter | 4 |
| pT7-SUI3ΔS | eIF2β (1-140) cloned under a T7 promoter | 3 |
| pHis-NIP1-N | His6-eIF3c (1-156) cloned under a T7 promoter | 2 |
| pT7-TIF4632ΔS | eIF4G2 (439-914) cloned under a T7 promoter | 5 |
| p180 | Low-copy-number GCN4-lacZ URA3 plasmid | 32 |
| pM199 | p180 derivative with uORF1 only | 17 |
| pM226 | pM199 derivative with uORF1′ overlapping with GCN4 | 17 |
TABLE 2.
Plasmids and yeast strains with different tif5 mutations
| Mutation | GST-eIF5 fusion plasmid | Sc TIF5 plasmid | TIF34-HA gcn2Δ straina | FL-SUI3 gcn2Δ strainb | GCN2+ strainc |
|---|---|---|---|---|---|
| Wild type | pGEX-TIF5d | pKA234 | KAY113 | KAY128 | KAY314 |
| F364S | Ep1521 | Ep1404 | KAY114 | KAY129 | KAY315 |
| L295P | Ep1525 | Ep1412 | KAY115 | KAY130 | KAY316 |
| F324I | Ep1519 | Ep1416 | KAY116 | KAY131 | KAY317 |
| W254R | Ep1526 | Ep1418 | KAY117 | KAY132 | KAY318 |
| L350Q | Ep1528 | Ep1444 | KAY118 | KAY133 | KAY319 |
| F296S | Ep1520 | Ep1445 | KAY119 | KAY134 | KAY320 |
| G58S | N. C.e | Ep1446 | KAY120 | KAY135 | KAY321 |
| I304N | Ep1527 | Ep1447 | KAY121 | KAY136 | KAY322 |
| L347W | Ep1523 | Ep1448 | KAY122 | KAY137 | KAY323 |
| W391Δ | Ep1522 | Ep1449 | KAY123 | KAY138 | KAY324 |
| I32N | N. C.e | Ep1450 | KAY124 | KAY139 | KAY325 |
| G31S | N. C.e | Ep1451 | KAY125 | KAY140 | KAY326 |
| L279P | Ep1524 | Ep1476 | KAY126 | KAY141 | KAY327 |
| tif5-7A | pGEX-TIF5-7Ad | pKA758 | KAY282 | KAY284 | KAY328 |
Isogenic to KAY113 except carrying a tif5 LEU2 CEN plasmid listed in column 3 instead of pKA234.
Isogenic to KAY128 except carrying a tif5 LEU2 CEN plasmid listed in column 3 instead of pKA234.
Isogenic to KAY314 except carrying a tif5 LEU2 CEN plasmid listed in column 3 instead of pKA234.
See reference 3.
N. C., not constructed for this study.
Yeast strains.
S. cerevisiae strains used in this study are listed in Table 2. The new strains were constructed as follows. To create KAY107 and KAY127, the transformants of KAY48 (MATa ura3-52 leu2-3,-112 trp1-Δ63 tif34Δ gcn2Δ tif5Δ p[TIF34-HA LEU2] p[TIF5 URA3]) and KAY112 (MATa ura3-52 leu2-3,-112 trp1-Δ63 sui3Δ gcn2Δ tif5Δ p[TIF5 URA3] p[LEU2 SUI3-His]) carrying YCpW-TIF34-HA and YCpW-FL-SUI3, respectively, were grown for >10 generations in liquid synthetic complete (SC)-Trp medium to allow segregation of the LEU2 plasmid in the original strain. Trp+ Leu− colonies were isolated as KAY107 and KAY127 for plasmid shuffling of different tif5 alleles. KAY113 (TIF34-HA gcn2Δ) and its tif5 mutant derivatives were generated by growing the transformants of KAY107 carrying pKA234 (TIF5) and mutant tif5 plasmids listed in Table 2 on an FOA (5-fluoroorotic acid)-containing medium and selecting for FOAr strains (plasmid shuffling; see reference 6). FOA selects against the URA3 marker in these strains and thus selects for strains that have lost the corresponding plasmid. KAY128 (FL-SUI3 gcn2Δ) or KAY314 (GCN2) and their tif5 mutant derivatives were constructed similarly with KAY127 or EY933 (MATα ura3-52 leu2-3,-112 trp1-Δ63 tif5Δ p[TIF5 URA3]) transformants carrying corresponding TIF5 and tif5 plasmids, respectively.
Isolation of Ts− mutations mapping in eIF5.
A 2-kb BamHI-HindIII fragment containing the yeast TIF5 gene was randomly mutagenized using the intrinsic mutation rate of the Taq DNA polymerase and cloned into pSB32 (LEU2 CEN). The resultant mutant library was introduced into EY920 {MATa ura3-52 leu2-3,-112 trp1-Δ63 his4-306(UUG) tif5Δ p[TIF5 URA3]} by transformation. Leu+ transformants were isolated and replica printed onto two FOA-containing media and incubated at 23 or 37°C. Clones that formed FOAr colonies at 23°C but failed to do so at 37°C were isolated as Ts− mutant candidates. Single colonies were isolated on streak plates and retested to confirm the Ts− phenotype. TIF5 LEU2 plasmids were recovered from the candidates and reintroduced into EY920. If the Ts− phenotype was reproduced, the entire TIF5 coding region of the TIF5 LEU2 plasmid was sequenced to locate the mutation site(s). In cases where plasmids contained multiple mutations, alleles containing corresponding single mutations were constructed either by subcloning or by PCR. Out of 18 independent Ts− alleles which reproduced the phenotype, all were mapped in eIF5-CTD and found to be due to one of 10 unique single-point mutations shown in Fig. 1A. The initial EY920 transformants were also screened for the Sui− phenotype. The his4-306(UUG) allele in this strain alters the HIS4 mRNA start codon from AUG to UUG (14), resulting in a His− phenotype. Sui− alleles of TIF5 are expected to increase the frequency of translation initiation from an in-frame non-AUG start codon in the HIS4 mRNA, suppressing the initiation codon mutation and conferring a His+ phenotype.
RESULTS
Isolation of Ts− mutations mapping in the entire eIF5-CTD.
Figure 1A summarizes the eIF5 mutations employed in this study. They are subdivided into two groups based on their effects on eIF5 function. The first group includes tif5 mutations tif5-G58S, -I32N, and -G31S, which alter the eIF5-NTD implicated in catalysis of the eIF2 GTPase-activating function. These mutations do not alter the growth rate of yeast cells but, rather, relax the stringency of AUG selection and confer a Sui− phenotype (C. Curtis and E. M. Hannig, unpublished results). The Sui− phenotype appears to be associated with higher rates of GTP hydrolysis by eIF2 (24). It has been proposed that as a result of this defect in Sui− strains, preinitiation complexes positioned at non-AUG codons (a UUG codon in many cases) may produce (albeit infrequently) mature 40S initiation complexes, thereby resulting in an increase in translation from inappropriate start codons.
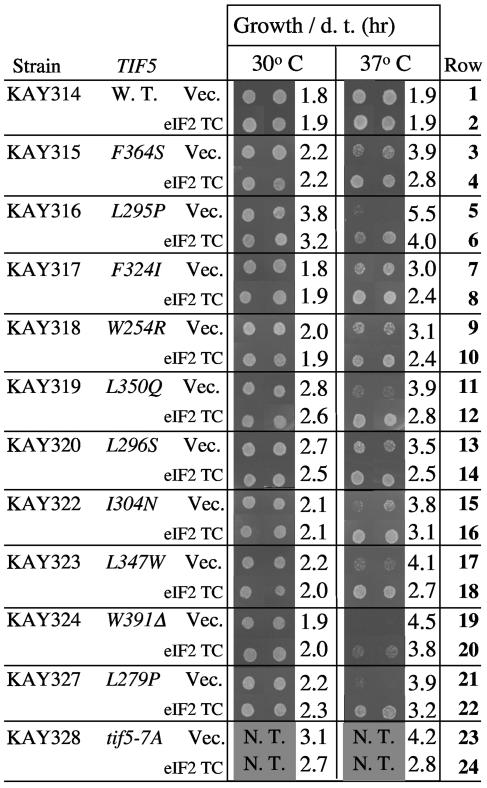
The second group maps in the eIF5-CTD and includes all of the Ts− mutants isolated in this study. None of the new eIF5-CTD mutations were Sui− (Curtis and Hannig, unpublished results). The eIF5-CTD (amino acids 241 to 405) has been shown by deletion analysis to be sufficient for eIF5 binding to MFC partners (3). An additional allele, tif5-7A, alters seven amino acids of AA-box 2 (Fig. 1A and C) and was previously reported (3). Yeast tif5-7A mutants grow slowly at 30°C and more slowly at 36°C (3). At 30°C, the tif5-7A mutant failed to induce GCN4 expression upon amino acid starvation (and hence is 3AT sensitive) both in the presence (34) and in the absence (3) of GCN2 (a Gcn− phenotype). As shown in Fig. 2, the severity of the Ts− growth defect in the new eIF5-CTD point mutants differs in each mutant, with tif5-L295P as the strongest Ts− mutation (row 5). However, all alleles increased the doubling time by >1 h at the restrictive temperature of 37°C (Fig. 2, odd-numbered rows). Three of the point mutations (L347W, L350Q, F364S) altered hydrophobic amino acids in AA-box 1, and the 15 amino-acid truncation allele (W391Δ) removed a part of AA-box 2. The remaining six point mutants altered hydrophobic residues upstream of AA-boxes (Fig. 1A). Consistent with the importance of the altered hydrophobic residues outside of the AA-boxes, these residues are highly conserved among other eukaryotic eIF5 homologues. The first structure of the AA-box-containing domain from yeast eIF2Bɛ, equivalent in length to eIF5-CTD, was recently solved at atomic resolution and found to form an atypical HEAT domain composed of eight α-helices (7). Mapping of yeast eIF2Bɛ residues homologous to the eIF5 mutation sites (based on Fig. 2 of reference 7) indicates that F324, L347, L350, and F364 are located in helices 5, 6, and 7 on the interior side, suggesting that these residues serve as hydrophobic cores of the structure. In contrast, four of the other five mutation sites appear exposed to surfaces or located in a loop of the model, indicating either that the model has an ambiguity or that these residues are important for interaction with specific partners (see Discussion).
FIG. 2.
Suppression of eIF5-CTD Ts− mutations by eIF2 TC overexpression. Two independent transformants of indicated strains carrying an empty vector YEplac195 (Vec.) or p1780-IMT (eIF2 TC) were grown in synthetic dextrose (SD) medium supplemented with tryptophan and diluted to the same density. Five microliters of the diluted culture was spotted onto SD plates containing tryptophan and incubated at the indicated temperatures for 2 days. Doubling time (d.t.) in the liquid medium was measured for two independent transformants, and the average values are indicated beside the growth patterns. N. T., not tested.
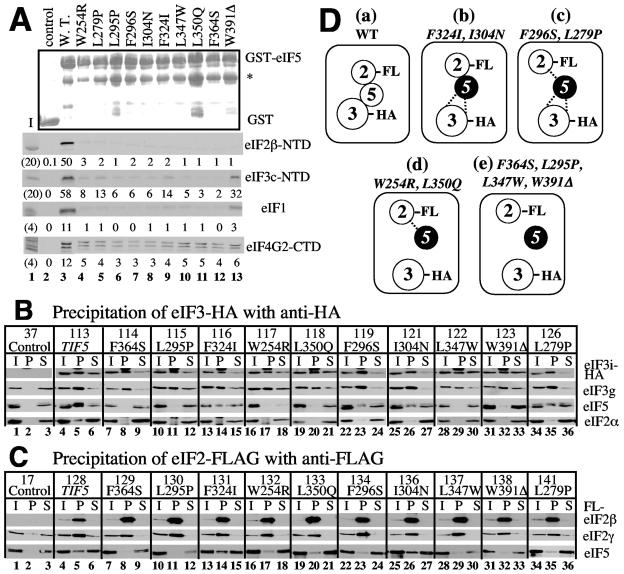
To test the effect of these mutations on eIF5 binding to MFC partners in vitro, we constructed glutathione S-transferase (GST) fusions with the mutant eIF5 proteins and allowed them to bind 35S-eIF1, -eIF2β-NTD, or -eIF3c-NTD, which was expressed in rabbit reticulocyte lysates. As shown in Fig. 3A, all of the point mutations abolished interactions with eIF1, eIF2β, and eIF3c (Fig. 3A, compare lanes 4 to 12 with lane 3). In contrast, tif5-W391Δ abolished the interaction with eIF2β but reduced that with eIF3c only by twofold (second and third panels, lanes 13). In addition, it had the smallest effect on eIF1 binding (fourth panel, lane 13). In general, Ts− mutations tend to unfold hydrophobic cores of the protein structure. Thus, the nine point mutations may abolish MFC partner interactions by unfolding the structure of eIF5-CTD at least in vitro. The results with tif5-W391Δ also suggest that eIF3c and eIF1 bind to eIF5-CTD at interfaces different from (or in addition to) an acidic surface composed of the AA-box 2 amino acids that are removed by this mutation (see Discussion). Finally, all of the mutations reduced but did not abolish the interaction with eIF4G (Fig. 3A, bottom panel), suggesting that this interaction is more resistant to unfolding caused by the mutations and/or that an interaction occurs at an additional site(s). This result is consistent with the idea that the eIF5-CTD binds to the relatively large eIF4G HEAT domain at multiple interfaces (20).
FIG. 3.
Effect of the eIF5-CTD Ts− mutation on factor interactions in vivo and in vitro. (A) Effect of the Ts− mutations on binding of eIF5 to MFC partners in vitro. Lanes 2 to 13, ∼4 μg of GST-eIF5 or its indicated mutant derivatives were incubated with 35S-eIF2β-NTD (second panel), -eIF3c-NTD (third panel), -eIF1 (fourth panel), or -eIF4G-CTD (fifth panel) in 100 μl of binding buffer. The protein complex was isolated using glutathione Sepharose and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis as described previously (40). GST-eIF5 and its mutants were expressed and purified from Escherichia coli BL21(DE3) carrying pGEX-TIF5 or the corresponding pGEX-tif5 derivatives (Table 2). 35S-labeled proteins were expressed in the TnT system (Promega) from pT7-SUI3ΔS, pHis-NIP1-N, pT7-SUI1, and pT7-TIF4632ΔS. Relative amounts of 35S-labeled proteins bound to the resin were determined by phosphorimaging analyses of sodium dodecyl sulfate-polyacrylamide gels and are shown below each panel. Top panel, Coomassie staining pattern; bottom four panels, autoradiography. Asterisk at the top panel indicates the position of a GST-eIF5 cleavage product resulting from bacterial protease activity. Lanes 1, 20% (second and third panels) or 4% (fourth and fifth panels) input amount. (B and C) Two hundred micrograms of whole-cell extract prepared from KAY yeast strains with indicated tif5 mutations, grown in yeast extract-peptone-dextrose medium, was used for immunoprecipitation with anti-HA (panel B) or anti-FLAG (panel C) affinity resin, as described previously (40). The entire pellet fractions (P) were analyzed, along with 10% input (I) and supernatant (S) fractions, by immunoblotting using polyclonal anti-eIF5 (24), anti-eIF3g (38), anti-eIF2α (13), and anti-eIF2γ (18) antibodies as indicated to the right. Monoclonal anti-HA (BabCO) and anti-FLAG (Sigma) antibodies were used to detect eIF3i-HA and FLAG-eIF2β, in panels B and C, respectively. Control, KAY37 (TIF5 TIF34) in panel B and KAY17 (TIF5 SUI3) in panel C; TIF5+ strains encoded untagged eIF3i and eIF2β, respectively (see reference 3 for detailed genotypes). Numbers above the TIF5/tif5 allele designations refer to the relevant KAY yeast strains. In the bottom row of panel B, the bands smaller and larger than eIF2α in lanes 8, 11, 14, and 17 represent cross-reactive species detected by anti-HA antibodies and do not represent eIF2α. (D) Model of MFC assembly in the strains tested. Circles indicate individual eIFs. Filled circles indicate the mutant eIF5. HA and FL refer to HA and FLAG epitope tags introduced to eIF2 and eIF5, respectively. Direct contact indicates strong interactions, equivalent in level to that seen with the respective wild-type strain. The dotted line indicates weak interactions, i.e., ∼30 to 50% of the level seen with the wild type. No line between the factors indicates that the interaction was not detected by the experiments in panels B and C.
As shown in Fig. 2 (even-numbered rows), cooverexpression of all three eIF2 subunits and tRNAiMet partially suppressed the Ts− phenotypes conferred by the eIF5-CTD mutants, suggesting that the mutations result in a defect in binding to eIF2 TC, as observed previously for tif5-7A (3). We recently proposed that the eIF5-eIF2 TC interaction initiates MFC assembly by forming a high-affinity assembly core (40). The observed suppression by TC overexpression supports the idea that all 10 novel CTD mutations impair eIF5 function in mediating the MFC assembly.
The effect of the Ts− mutations on MFC partner interactions in vivo.
To test the effect of the point mutations on the interaction of eIF5 with eIF2 and eIF3 in vivo, we constructed two different derivatives of yeast strains carrying each of the mutations (Table 2), one expressing the HA-tagged eIF3i subunit and the other expressing the FLAG-tagged eIF2β subunit. Cells were grown at the permissive temperature of 30°C prior to the assays. As shown in Fig. 3B, coimmunoprecipitation using anti-HA antibodies indicates that all of the point mutations reduced or abolished the association of eIF5 (visualized in the third panel) with HA-eIF3 (eIF3i and eIF3g subunits visualized in the top and second panels, respectively), with tif5-F324I, -F296S, -I304N, and -L279P having the smallest effects (lanes 14, 23, 26, and 35). Importantly, all of these mutations appeared to abolish coimmunoprecipitation of eIF2 with eIF3i-HA, suggesting that the mutant forms of eIF5 are defective in bridging these factors (Fig. 3B, bottom panel).
Coimmunoprecipitation of FLAG-eIF2 using anti-FLAG antibodies suggested a milder effect overall on eIF5 binding to eIF2 (Fig. 3C, bottom panel). Two mutations, tif5-F324I and -I304N, did not appear to affect this interaction (lanes 14 and 26), and four mutations, tif5-W254R, -L350Q, -F296S, and -L279P, reduced the interaction by two- to fourfold (lanes 17, 20, 23, 35), whereas the remaining four mutations, tif5-F364S, -L295P, -L347W, and -W391Δ, essentially abolished the interaction (lanes 8, 11, 29, 32). None of the eIF5-NTD mutations affected the interaction with FLAG-eIF2 and HA-eIF3, as measured by coimmunoprecipitation (data not shown).
The effect of the Ts− mutations on the ability of eIF5 to link eIF2 and eIF3 in vivo is summarized in Fig. 3D. It is important to note that the impact on eIF3 binding is in general greater than that on eIF2 binding, suggesting that the weakened interaction of eIF2 with the MFC exacerbates the weakening of the interaction of eIF3 with the MFC. This finding is consistent with our recent finding that the eIF3/eIF5 interaction is enhanced by the association of eIF5 with eIF2β K box (40). Based on these results, we conclude that the Ts− mutations impair the ability of eIF5 to bridge eIF2 and eIF3 by impairing either its individual binding to eIF2 or eIF3 or its ability to enhance eIF3 binding upon its association with eIF2β K box.
Six eIF5-CTD point mutants confer Gcd− phenotypes at a permissive temperature.
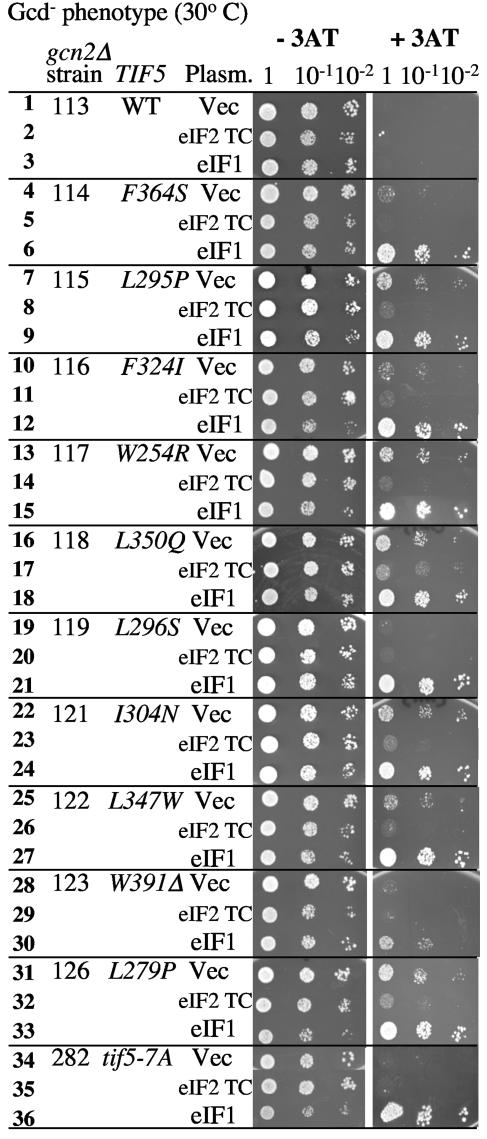
To characterize the role of eIF5-CTD in GCN4 translational control, we investigated the effect of the eIF5 point mutations on the general control phenotype. If these mutations reduce the interaction between eIF2 and eIF3, as observed in vivo in Fig. 3, they are expected to derepress GCN4 translation in the absence of Gcn2p kinase (Gcd− phenotype) by reducing the rate of TC binding to the ribosomes migrating down the GCN4 leader region. To test this idea, we constructed TIF5 mutant derivatives harboring a chromosomal GCN2 deletion allele (gcn2Δ) (Table 2, strains KAY113 to KAY126). The gcn2Δ TIF5 parent strain KAY113 cannot overcome growth inhibition upon 3AT-induced amino acid starvation, as shown in Fig. 4, line 1. As discussed above, this results from an inability to derepress GCN4 translation and stimulate genes under the general control pathway due to the absence of the eIF2α kinase encoded by GCN2. Interestingly, six of the tif5 CTD mutants, i.e., KAY115 (tif5-L295P; line 7), KAY117 (tif5-W254R; line 13), KAY118 (tif5-L350Q; line 16), KAY121 (tif5-I304N; line 22), KAY122 (tif5-L347W; line 25), and KAY126 (tif5-L279P; line 31), conferred 3AT resistance in the gcn2Δ background and are therefore Gcd−, as shown in Fig. 4. The observed Gcd− phenotypes of these six eIF5-CTD mutants were suppressed by eIF2 TC overexpression (Fig. 4, lines 8, 14, 17, 23, 26, 32), in support of the model that the phenotypes are due to reduced eIF2 TC binding to the 40S subunit. Neither the three NTD mutations (data not shown) nor tif5-7A (Fig. 4, line 34) (3) was Gcd−.
FIG. 4.
Gcd− phenotypes of tif5 CTD mutants and the effect of TC and eIF1 overexpression. Transformants of indicated KAY strains (gcn2Δ and tif5 mutations described under the column TIF5) carrying the multicopy plasmids YEplac195 (Vec), p1780-IMT (eIF2 TC), or YEpU-SUI1 (eIF1) were grown overnight in SC medium lacking uracil. Equal A600 units (undiluted) and 1/10 and 1/100 dilutions were spotted from left to right on SD medium supplemented without or with 30 mM 3AT and incubated at 30°C for 2.5 or 6 days, respectively. WT, wild type. The first column indicates line numbers referred to in the text.
Using a GCN4-lacZ reporter, we found that the Gcd− tif5 mutants constitutively increased GCN4 expression by two- to threefold compared to the wild type (data not shown). These results confirm that the observed Gcd− phenotypes are due to elevated expression of GCN4. However, the enhancement of GCN4 expression by eIF5-CTD mutations was weaker than that exhibited by other canonical gcd mutations, which is typically in the six- to eightfold range.
These results suggest that efficient TC binding to the 40S subunit requires the intact eIF5-CTD. Because eIF5-CTD bridges TC and the 40S subunit-binding factor eIF3 (43), these mutations likely impair TC linkage to the 40S subunit, thereby reducing the rate of TC binding to the 40S subunit and resulting in a Gcd− phenotype. In support of this idea, all of the CTD mutations employed in this study severed the association between eIF2 and eIF3 in vivo (Fig. 3B to D). Thus, our results directly implicate eIF5-CTD in the TC binding process required for GCN4 translational control.
Overexpression of eIF1 confers Gcd− phenotypes to all tif5 CTD mutants.
Four of the new tif5 alleles (F364S, F324I, L296S, and W391Δ), as well as tif5-7A, did not confer obvious Gcd− phenotypes in the gcn2Δ strain KAY113. To test whether MFC formation is still defective in these mutants, we attempted to disturb stoichiometric MFC formation by overexpressing the wild-type eIF1. We recently found that wild-type MFC is resistant to such perturbation, while the mutant MFC in tif5-7A strains is not, suggesting that overexpression of eIF1 is a more sensitive indicator of defective MFC formation. High-copy-number (hc) eIF1 confers a Gcd− phenotype in the tif5-7A mutant, most likely by sequestering TC in an inhibitory complex (40). This sequestration constitutively elevates GCN4 expression by reducing the level of functional TC. Figure 4 demonstrates that hc eIF1 does indeed confer strong Gcd− phenotypes in all the tif5 CTD mutants (lines 6, 9, 12, 15, 18, 21, 24, 27, 30, and 33), as well as in the isogenic tif5-7A mutant KAY282 (line 36), regardless of whether the tif5 mutations alone are Gcd−. In contrast, hc eIF1 did not confer 3AT resistance (in the gcn2Δ background) in the wild-type TIF5 control (line 3) or in eIF5-NTD mutants (data not shown), indicating that this phenotype is specific to the eIF5-CTD mutations. These results are consistent with the observation (Fig. 3B to D) that all of the tif5 CTD mutants, including the Gcd+ alleles, are defective in MFC formation in vivo.
All eIF5-CTD point mutants show Gcn− phenotypes at elevated temperature.
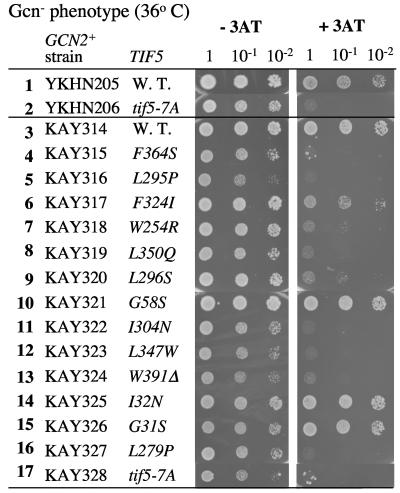
We hypothesized that at least some of the new tif5 CTD mutations might impair ribosomal preinitiation complexes during the process of translation reinitiation on GCN4 mRNA and confer Gcn− phenotypes, as shown previously for the tif5-7A mutant (34). In GCN2+ strains, the latter confers sensitivity to 3AT and is thus impaired in the induction of the general control (wild-type strains are 3AT resistant) (29). To test this notion, we constructed GCN2+ derivatives of the tif5 point mutants and examined their growth on 3AT-containing media. As expected, the GCN2+ tif5-7A strain YKHN206 was partially sensitive to 3AT at the permissive temperature of 30°C (data not shown) and strongly sensitive to 3AT at the more restrictive temperature of 36°C (Fig. 5, line 2), confirming the strain's Gcn− phenotype (34). The tif5-7A mutant KAY328 isogenic to the new eIF5 mutants was also Gcn− at 36°C (Fig. 5, line 17). All of the eIF5-CTD point mutants were somewhat sensitive to 3AT, and thus were Gcn−, when eIF5-CTD function was partially restricted at 36°C (Fig. 5, lines 4 to 9, 11 to 13, and 16). By contrast, none of the eIF5-NTD mutants were Gcn− (lines 10, 14, and 15).
FIG. 5.
Gcn− phenotype test for eIF5 mutants. Growth of the indicated GCN2+ strains harboring wild-type (W.T.) or mutant TIF5 alleles was assayed as for Fig. 4, except that the cells were spotted onto SC medium minus histidine with or without 50 mM 3AT and 40 mM leucine and incubated for 5 days at 36°C.
It is noteworthy that six of the eIF5-CTD point mutants derepressed GCN4 translation in the absence of Gcn2p (Gcd− phenotype) at the permissive temperature of 30°C (Fig. 4) but apparently failed to express GCN4 in the presence of Gcn2p (Gcn− phenotype) at the more restrictive temperature of 36°C (Fig. 5). As discussed below, we propose that the Gcn− phenotypes conferred by the eIF5-CTD mutations at 36°C are due to a defect in a postassembly process of preinitiation complexes at the higher temperature. We reasoned that perhaps at 30°C, but not at 36°C, the mutant eIF5-CTD is folded sufficiently to mediate the postassembly process during scanning or AUG recognition, even though it appears to be defective at the assembly stage, as evidenced by the Gcd− phenotypes at 30°C. To test this idea, we examined the sensitivity of the mutants to 3AT in GCN2+ strains at the more permissive temperature of 30°C. At this temperature, all 10 GCN2+ eIF5-CTD mutants, KAY315 to KAY320, KAY322 to KAY324, and KAY327, as well as TIF5 wild-type strains YKHN205 and KAY314, were 3AT resistant (data not shown), suggesting that the ribosomal preinitiation complexes in the mutants are sufficiently stable to allow GCN4 translation.
Mechanism of Gcn− phenotypes caused by eIF5-CTD mutations.
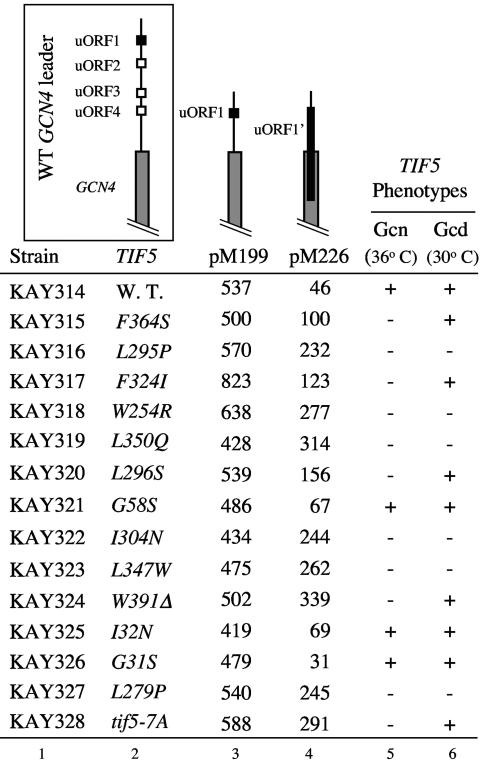
To further investigate the mechanism of Gcn− phenotypes in the tif5 strains, we examined GCN4 expression using GCN4-lacZ reporters containing alterations in the GCN4 uORFs. As shown at the top of Fig. 6, pM199 contains uORF1 only and is used to measure the efficiency of reinitiation of GCN4 translation following uORF1 translation. pM226 is a derivative of pM199 with frameshift mutations in uORF1. The latter extends uORF1 to a site 130 nucleotides downstream of the GCN4 start codon, making the ribosome unable to reinitiate GCN4 translation after translating the altered uORF1. Under these conditions, GCN4 can be translated only by the ribosomes that had failed to initiate translation at uORF1 (e.g., by leaky scanning). Therefore, increased expression from this reporter would indicate an increased frequency of leaky scanning of uORF1. All of the transformants carrying the reporter plasmids were grown at 36°C, the temperature at which the Gcn− phenotype was expressed in the mutants, for β-galactosidase assays (Fig. 5).
FIG. 6.
GCN4-lacZ reporter assays in eIF5 mutants. Transformants of indicated strains (columns 1 and 2) harboring the GCN4-lacZ-containing plasmids pM119 (column 3) or pM226 (column 4) were grown at 36°C for 6 h and assayed for β-galactosidase activity as described previously (19). Schematics at the top depict the GCN4 leader structure for each of the constructs used. The schematic in the box to the left indicates the structure for wild-type GCN4 leader. Results shown are β-galactosidase activities averaged from the values obtained with at least three independent transformants, where individual measurements had standard deviations within 30% of the average. Columns 5 and 6 summarize Gcn and Gcd phenotypes for each mutant, determined at 36 and 30°C, respectively.
As shown in Fig. 6, GCN4 expression from pM199 was not significantly altered in any of the eIF5 mutants tested, indicating that these mutants are not defective in translation reinitiation (column 3). By contrast, we found that all of the Gcn− eIF5 mutants (but none of the eIF5-NTD Sui− mutants) increased GCN4 expression from pM226, indicating that these mutations increase the frequency of leaky scanning of uORF1 (column 4). Defects observed with pM226 can explain the observed Gcn− phenotype as follows: the preinitiation complexes that had scanned past uORF1 (i.e., demonstrated leaky scanning) would most likely translate any one of the inhibitory uORFs of uORF2-4, thereby allowing the ribosome to dissociate from the mRNA without translating the GCN4 ORF (see Fig. 7B below). As a positive element in the regulation of GCN4 expression, uORF1 has a strong translation initiation signal (17). Leaky scanning of such an ORF could be caused by a significant defect in postassembly process, most likely at scanning. None of the Sui− eIF5-NTD mutants altered GCN4 expression from the two reporter plasmids, suggesting that these mutations do not measurably affect the efficiency of translation from AUG codons, nor do they affect the efficiency of ribosomal scanning. Taken together, our results shown in Fig. 5 and 6 support a role for eIF5-CTD in the maintenance of factor linkage in scanning ribosomal preinitiation complexes.
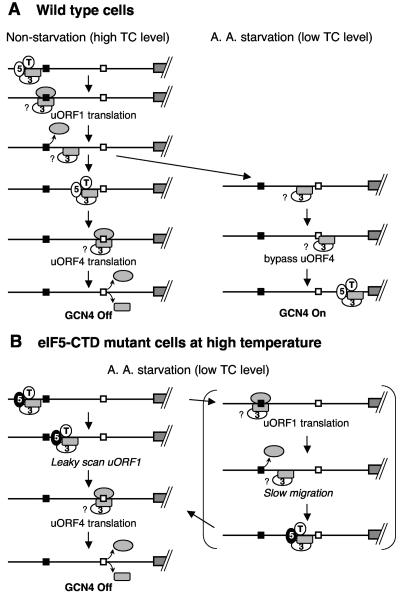
FIG. 7.
Model for GCN4 translational control in wild-type and eIF5-CTD mutant cells. Lines indicate the GCN4 mRNA leader, and the grey boxes to the right (followed by diagonal hashed lines) represent the GCN4 coding region. Two regulatory uORFs (uORFs 1 and 4) have been shown to be necessary and sufficient for regulation of GCN4 expression, and are depicted as filled and open squares, respectively. The figure illustrates the ribosome movement on the leader region with the focus on its association with eIF3 (numeral 3), eIF5 (numeral 5), and eIF2 TC (T). The 40S and 60S subunits are drawn as grey rectangles with rounded corners and as grey ovals, respectively. (A) Control GCN4 translation in wild-type cells. Under nonstarvation conditions (left column), the preinitiation complex scans for and translates uORF1. Evidence suggests that eIF3 is associated with the ribosome at this stage (see the text for details). Question marks indicate uncertainties relative to these associations. Following uORF1 translation, a population of 40S subunits remains associated with the mRNA and resumes scanning after reacquiring TC and other eIFs (third and fourth panels). Subsequent translation of uORF4 dissociates the ribosome, shutting off GCN4 translation. Under amino acid (A. A.) starvation conditions (right column), Gcn2p kinase is activated and phosphorylates eIF2. This phosphorylation renders eIF2 into a competitive inhibitor of GDP/GTP exchange activity (catalyzed by eIF2B; not shown), thereby reducing the level of eIF2/GTP and hence TC levels. uORF4 is bypassed due to low TC levels. TC can be reacquired during scanning of the uORF4-to-GCN4 interval, resulting in translation of GCN4. (B) In eIF5-CTD mutants, impaired eIF5-CTD function at a higher temperature confers a Gcn− phenotype. The figure illustrates possible mechanisms for this observation. Mutant eIF5 (5) is depicted as filled ovals. (Left column) The preinitiation complex with the mutant eIF5 scans past the uORF1 start codon (leaky scanning) and instead initiates at the uORF4 start codon. Translation of uORF4 leads to efficient ribosome dissociation, shutting off GCN4 translation (17). This mechanism differs from that proposed to explain the Gcn−phenotype in prt1-1 mutants. In the latter case (right column), the 40S subunit tethered by uORF1 translation migrates very slowly and receives TC prior to the uORF4 start codon. The slowed migration may compensate for levels of TC reduced by eIF2 phosphorylation (34).
DISCUSSION
Role of eIF5-CTD in TC binding to the 40S subunit.
eIF5 is an integral part of the MFC with eIF1, eIF3, and eIF2 TC, an important intermediate for 43S complex formation (17). For this reason, it has been anticipated that impairing MFC partner linkages by eIF5-CTD mutations would interfere with 43S complex formation and thereby constitutively derepress the general control response (Gcd− phenotype). However, the best-characterized eIF5-CTD mutant, designated tif5-7A (Fig. 1 shows mutation sites), did not show a Gcd− phenotype (3), although it reduced Met-tRNAiMet binding to the 40S subunit in the mutant-cell extracts (5).
In this study, we isolated 10 mutations leading to temperature sensitivity mapping in the eIF5-CTD (Fig. 1). Six of the alleles did indeed show sizable Gcd− phenotypes that were suppressed by eIF2 TC overexpression (Fig. 4), directly implicating eIF5-CTD in the TC binding process. Consistent with a role in TC binding, Ts− phenotypes of all 10 mutations were suppressed by eIF2 TC overexpression (Fig. 2), and all of the Ts− mutations appeared to sever eIF2 from eIF3 in vivo by impairing eIF5 binding to either eIF2 or eIF3 or both (Fig. 3). Although tif5-7A is Gcd+, the MFC formed in this mutant appears to be labile and is rendered into a nonstoichiometric inhibitory complex when eIF1 is overproduced; as a consequence, transformants of tif5-7A mutants overproducing eIF1 demonstrated a strong Gcd− phenotype (40) (Fig. 4). This is also the case for each of the eIF5-CTD mutants isolated in this study (Fig. 4). Based on these results, we suggest that all of the Ts− eIF5-CTD mutations impair 43S complex formation by disrupting its linkage to other MFC partners.
According to the model for GCN4 translational control (Fig. 7A), translation of uORF1 serves to tether the 40S subunit to the GCN4 mRNA leader. Yeast prt1-1 (eIF3b) mutants confer a Gcn− phenotype by impairing the rate of 40S subunit migration at the intercistronic region (34), allowing reacquisition of additional eIFs and subsequent translation of additional inhibitory uORFs (also refer to Fig. 7B, right column). This finding suggests that eIF3 is present on the tethered 40S subunit and increases its rate of migration down the leader sequence (Fig. 7A). While free eIF3 may quickly rebind to an empty 40S subunit tethered to the GCN4 leader region, additional observations support the model that the 40S subunit contains eIF3 during (and subsequent to) uORF1 translation. Examination of diverse reinitiation events found in eukaryotes indicates that relatively short uORFs (2 to ∼20 codons) are required for efficient reinitiation (26, 27, 31). It has been hypothesized that a subpopulation of eIF3 remains tethered to the 40S subunit during the translation of short uORFs, while this interaction would progressively decay after translating a longer uORF of >20 codons. Reinitiation following a longer uORF requires a specific “reinitiation factor” that effectively anchors eIF3 to the ribosome during protein synthesis (35). The identification of an eIF3 binding site at the solvent side of the 40S subunit (42, 43) is in accordance with the idea that eIF3 stays anchored to this side of the ribosome during and subsequent to uORF1 translation.
We recently showed that the association of eIF2β K box peptide with eIF5-CTD strongly enhances the latter's ability to bind eIF3c (40). If so, TC/eIF5 interaction prior to 40S subunit binding would substantially enhance the affinity of eIF5-CTD for eIF3 in the tethered eIF3/40S subunit complex; the eIF5-CTD mutant proteins might fail to bind eIF2 TC for its assembly activation or, alternatively, bind but fail to stably bridge eIF2 and eIF3. Either case could result in derepressed GCN4 translation and hence result in a Gcd− phenotype (Fig. 4). Even if the tethered 40S subunit lacks eIF3, TC binding to the empty 40S subunit would be substantially enhanced by mutual cooperativity effects due to linking eIF2 and eIF3 via the eIF5-CTD.
Gcd− phenotypes in the eIF5-CTD mutants were not as strong as Gcd− mutations that impair eIF2 or eIF2B function at a permissive temperature, as judged by plate tests as well as by measurement of GCN4-lacZ fusion enzyme activity (Fig. 4 and data not shown for eIF2 and eIF2B mutants) (8). Consistently, GCN4 derepression in the eIF5-CTD mutants was not as strong as in these other (canonical) Gcd− mutants (data not shown). These observations can be explained if the reduction in TC binding to the 40S subunit by these mutations is not strong enough to allow the highest level of GCN4 derepression achievable by mutations directly inhibiting eIF2 function or guanine nucleotide exchange. Alternatively, the eIF5 mutations may also destabilize MFC linkage (perhaps as well as other interactions) in the scanning preinitiation complex, thereby causing leaky scanning of uORF1 (albeit to a lesser degree than was observed at the semirestrictive temperature of 36°C, as shown in Fig. 6). In the eIF5-CTD mutants, the defect in scanning appears to be enhanced by growth at higher temperatures. Leaky scanning of uORF1 allows the ribosome to translate uORF2, uORF3, or uORF4, thereby inhibiting GCN4 translation and promoting a Gcn− phenotype (Fig. 7B, left panel; also see below).
Role of eIF5-CTD in scanning.
Our finding of Gcn− phenotypes for all of the eIF5-CTD mutations (Fig. 5) with concomitant increases in the frequencies of uORF1 leaky scanning (Fig. 6) strongly supports the idea that the eIF5-CTD is critical for factor linkage in the scanning preinitiation complex. While it is conceivable that the MFC linkage via the simultaneous eIF5-CTD/eIF1/eIF2β-NTD/eIF3c-NTD interaction that was demonstrated in vitro (40) is retained in the scanning preinitiation complex, evidence suggests that at least a part of such quaternary interaction is resolved prior to AUG recognition. It was hypothesized that the eIF5-CTD/eIF2β interaction gives way to the eIF5-CTD/eIF4G interaction by the time mRNA binds to the 40S subunit and that eIF4G in turn binds eIF1 to help it position at the ribosomal P site (20). Therefore, it is possible that the eIF5-CTD mutations impair its linkage to eIF4G and eIF1 during the scanning process. This model is consistent with reduced eIF5 binding to eIF4G2 in vitro (Fig. 3A). This mechanism may explain why these mutations increased the frequency of leaky scanning: a loose anchoring of eIF1 at the P site or unstable linkage between the factors at this stage may impede coupling of AUG recognition to GTP hydrolysis.
Alternatively, the Gcn− eIF5 mutations may impair eIF3 binding to the 40S subunit because eIF5 stimulates the eIF3a/c subcomplex binding to the 40S subunit (43). The mechanism by which the eIF3/eIF5 complex promotes the 40S subunit's ability to scan mRNA is not clear. One attractive idea is to propose that this eIF3/5 complex plays a role in maintaining the ribosomal conformation in favor of its role in mRNA scanning. In this conformation, the mRNA binding cleft of the 40S subunit might be optimized for rapid sliding of mRNA as well as for precisely matching the tRNAiMet anticodon to mRNA base triplets.
Insights into the structure and function of eIF5-CTD.
The fact that our eIF5 Ts− mutations altered hydrophobic residues located throughout the entire CTD strongly suggests that this entire region is folded. Consistent with the idea that the eIF5-CTD likely forms a HEAT domain (Fig. 1), this region aligns reasonably well with the homologous eIF2Bɛ-CTD, whose structure was recently solved at atomic resolution (7). The alignment proposed by Boesen et al. is especially good at the C-terminal half of eIF5-CTD, which includes the AA-boxes (1), and predicts that this region is folded into four α-helices stacked together (Fig. 1B). It was proposed that the acidic surfaces created by AA-box amino acids are the binding sites for the K boxes of eIF2β, which interacts with both eIF5 and eIF2Bɛ (7). Four Ts− mutations mapping in the C-terminal half of the eIF5-CTD alter hydrophobic residues buried in the structure, consistent with this prediction (Fig. 1). However, the alignment at the N-terminal half is more ambiguous. Four of the Ts− mutations altered hydrophobic residues located on the surface or the loop of the model (Fig. 1B), although they appear to unfold the structure in a manner similar to the effect of the other four alterations, as judged by in vitro binding studies (Fig. 3A). Additional work is required to more fully understand the structure-function relationship of the entire eIF5-CTD. Nevertheless, it is interesting that the region with higher ambiguity at the N-terminal half of the eIF5-CTD contains the two α-helices involved (in the case of eIF2Bɛ) in catalysis mediated by the guanine nucleotide exchange factor eIF2B. The less ambiguous C-terminal half contains an acidic surface made of AA-boxes and a basic surface created by lysine residues conserved specifically in all eukaryotic eIF5 homologs (highlighted in blue in Fig. 1C). Given the overall charged nature of eIF5 binding domains found in different MFC partners, this basic surface might serve as the interface for one or two of these partners. If so, it could be proposed that the current eukaryotic translation initiation system evolved by adding specific functions to a duplicated α-helical HEAT domain, one for eIF2B catalysis and the other for MFC assembly.
Acknowledgments
We are indebted to Alan Hinnebusch for timely advice, the gift of plasmids, and critical comments on the manuscript. We also thank Ashik Srinivasan for initial observation of Gcd− phenotypes caused by some eIF5-CTD mutants, Beth Montelone for critical reading of the manuscript, and Assen Marintchev for inspiring discussion about the structure of eIF5-CTD.
This work was supported by the NIH COBRE awards P20 RR15563 and RR16475, matching support from the State of Kansas and the KSU, and NIH grant R01GM64781 to K.A. and ACS grant RPG-97-061-01-NP to E.M.H.
REFERENCES
- 1.Aravind, L., and E. V. Koonin. 2000. Eukaryote-specific domains in translation initiation factors: implications for translation regulation and evolution of the translation system. Genome Res. 10:1172-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asano, K., J. Clayton, A. Shalev, and A. G. Hinnebusch. 2000. A multifactor complex of eukaryotic initiation factors eIF1, eIF2, eIF3, eIF5, and initiator tRNAMet is an important translation initiation intermediate in vivo. Genes Dev. 14:2534-2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asano, K., T. Krishnamoorthy, L. Phan, G. D. Pavitt, and A. G. Hinnebusch. 1999. Conserved bipartite motifs in yeast eIF5 and eIF2Bɛ, GTPase-activating and GDP-GTP exchange factors in translation initiation, mediate binding to their common substrate eIF2. EMBO J. 18:1673-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asano, K., L. Phan, J. Anderson, and A. G. Hinnebusch. 1998. Complex formation by all five homologues of mammalian translation initiation factor 3 subunits from yeast Saccharomyces cerevisiae. J. Biol. Chem. 273:18573-18585. [DOI] [PubMed] [Google Scholar]
- 5.Asano, K., A. Shalev, L. Phan, K. Nielsen, J. Clayton, L. Valasek, T. F. Donahue, and A. G. Hinnebusch. 2001. Multiple roles for the carboxyl terminal domain of eIF5 in translation initiation complex assembly and GTPase activation. EMBO J. 20:2326-2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boeke, J. D., J. Trueheart, G. Natsoulis, and G. R. Fink. 1987. 5-Fluoroorotic acid as a selective agent in yeast molecular genes. Methods Enzymol. 154:164-175. [DOI] [PubMed] [Google Scholar]
- 7.Boesen, T., S. S. Mohammad, G. D. Pavitt, and G. R. Andersen. 2004. Structure of the catalytic fragment of translation initiation factor 2B and identification of a critically important catalytic residue. J. Biol. Chem. 279:10584-10592. [DOI] [PubMed] [Google Scholar]
- 8.Bushman, J. L., A. I. Asuru, R. L. Matts, and A. G. Hinnebusch. 1993. Evidence that GCD6 and GCD7, translational regulators of GCN4, are subunits of the guanine nucleotide exchange factor for eIF-2 in Saccharomyces cerevisiae. Mol. Cell. Biol. 13:1920-1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chakravarti, D., and U. Maitra. 1993. Eukaryotic translation initiation factor 5 from Saccharomyces cerevisiae: cloning, characterization, and expression of the gene encoding the 45,346-Da protein. J. Biol. Chem. 268:10524-10533. [PubMed] [Google Scholar]
- 10.Choi, S. K., J. H. Lee, W. L. Zoll, W. C. Merrick, and T. E. Dever. 1998. Promotion of Met-tRNAiMet binding to ribosomes by yIF2, a bacterial IF2 homolog in yeast. Science 280:1757-1760. [DOI] [PubMed] [Google Scholar]
- 11.Das, S., R. Ghosh, and U. Maitra. 2001. Eukaryotic translation initiation factor 5 functions as a GTPase activating protein. J. Biol. Chem. 276:6720-6726. [DOI] [PubMed] [Google Scholar]
- 12.Dever, T. E. 2002. Gene-specific regulation by general translation factors. Cell 108:545-556. [DOI] [PubMed] [Google Scholar]
- 13.Dever, T. E., W. Yang, S. Åström, A. S. Byström, and A. G. Hinnebusch. 1995. Modulation of tRNAiMet, eIF-2, and eIF-2B expression shows that GCN4 translation is inversely coupled to the level of eIF-2 · GTP · Met-tRNAiMet ternary complexes. Mol. Cell. Biol. 15:6351-6363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donahue, T. F., and A. M. Cigan. 1988. Genetic selection for mutations that reduce or abolish ribosomal recognition of the HIS4 translational initiator region. Mol. Cell. Biol. 8:2955-2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gietz, R. D., and A. Sugino. 1988. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene 74:527-534. [DOI] [PubMed] [Google Scholar]
- 16.Gomez, E., S. S. Mohammad, and G. P. Pavitt. 2002. Characterization of the minimal catalytic domain within eIF2B: the guanine-nucleotide exchange factor for translation initiation. EMBO J. 21:5292-5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grant, C. M., and A. G. Hinnebusch. 1994. Effect of sequence context at stop codons on efficiency of reinitiation in GCN4 translational control. Mol. Cell. Biol. 14:606-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hannig, E. M., A. M. Cigan, B. A. Freeman, and T. G. Kinzy. 1992. GCD11, a negative regulator of GCN4 expression, encodes the γ subunit of eIF-2 in Saccharomyces cerevisiae. Mol. Cell. Biol. 13:506-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hannig, E. M., and A. G. Hinnebusch. 1988. Molecular analysis of GCN3, a translational activator of GCN4: evidence for posttranslational control of GCN3 regulatory function. Mol. Cell. Biol. 8:4808-4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He, H., T. von der Haar, R. C. Singh, M. Ii, B. Li, J. E. G. McCarthy, A. G. Hinnebusch, and K. Asano. 2003. The yeast eukaryotic initiation factor 4G (eIF4G) HEAT domain interacts with eIF1 and eIF5 and is involved in stringent AUG selection. Mol. Cell. Biol. 23:5431-5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hentze, M. W. 1997. eIF4G: a multipurpose ribosome adapter. Science 275:500-501. [DOI] [PubMed] [Google Scholar]
- 22.Hershey, J. W. B., and W. C. Merrick. 2000. Pathway and mechanism of initiation of protein synthesis, p. 33-88. In N. Sonenberg, J. W. B. Hershey, and M. B. Mathews (ed.), Translational control of gene expression. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 23.Hinnebusch, A. G. 1997. Translational regulation of yeast GCN4: a window on factors that control initiator-tRNA binding to the ribosome. J. Biol. Chem. 272:21661-21664. [DOI] [PubMed] [Google Scholar]
- 24.Huang, H., H. Yoon, E. M. Hannig, and T. F. Donahue. 1997. GTP hydrolysis controls stringent selection of the AUG start codon during translation initiation in Saccharomyces cerevisiae. Genes Dev. 11:2396-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koonin, E. V. 1995. Multidomain organization of eukaryotic guanine nucleotide exchange translation initiation factor eIF-2B subunits revealed by analysis of conserved sequence motifs. Protein Sci. 4:1608-1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kozak, M. 1987. Effects of intercistronic length on the efficiency of reinitiation by eukaryotic ribosomes. Mol. Cell. Biol. 7:3438-3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kozak, M. 1999. Initiation of translation in prokaryotes and eukaryotes. Gene 234:187-208. [DOI] [PubMed] [Google Scholar]
- 28.Maag, D., C. A. Fekete, Z. Gryczynski, and J. R. Lorsch. 2005. A conformational change in the eukaryotic translation preinitiation complex and release of eIF1 signal recognition of the start codon. Mol. Cell 17:265-275. [DOI] [PubMed] [Google Scholar]
- 29.Moehle, C. M., and A. G. Hinnebusch. 1991. Association of RAP1 binding sites with stringent control of ribosomal protein gene transcription in Saccharomyces cerevisiae. Mol. Cell. Biol. 11:2723-2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morino, S., H. Imataka, Y. V. Svitkin, T. V. Pestova, and N. Sonenberg. 2000. Eukaryotic translation initiation factor 4E (eIF4E) binding site and the middle one-third of eIF4GI constitute the core domain for cap-dependent translation, and the C-terminal one-third functions as a modulatory region. Mol. Cell. Biol. 20:468-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morris, D. R., and A. P. Geballe. 2000. Upstream open reading frames as regulators of mRNA translation. Mol. Cell. Biol. 20:8635-8642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mueller, P. P., and A. G. Hinnebusch. 1986. Multiple upstream AUG codons mediate translational control of GCN4. Cell 45:201-207. [DOI] [PubMed] [Google Scholar]
- 33.Natarajan, K., M. R. Meyer, B. M. Jackson, D. Slade, C. Roberts, A. G. Hinnebusch, and M. J. Marton. 2001. Transcriptional profiling shows that Gcn4p is a master regulator of gene expression during amino acid starvation in yeast. Mol. Cell. Biol. 21:4347-4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nielsen, K. H., B. Szamecz, L. Valasek, A. Jivotovskaya, B. S. Shin, and A. G. Hinnebusch. 2004. Functions of eIF3 downstream of 48S assembly impact AUG recognition and GCN4 translational control. EMBO J. 23:1166-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park, H.-S., A. Himmelbach, K. S. Browning, T. Hohn, and L. A. Ryabova. 2001. A plant viral “reinitiation” factor interacts with the host translational machinery. Cell 106:723-733. [DOI] [PubMed] [Google Scholar]
- 36.Paulin, F. E., L. E. Campbell, K. O'Brien, J. Loughlin, and C. G. Proud. 2001. Eukaryotic translation initiation factor 5 (eIF5) acts as a classical GTPase-activator protein. Curr. Biol. 11:55-59. [DOI] [PubMed] [Google Scholar]
- 37.Pestova, T. V., I. B. Lomakin, J. H. Lee, S. K. Choi, T. E. Dever, and C. U. T. Hellen. 2000. The joining of ribosomal subunits in eukaryotes requires eIF5B. Nature 403:332-335. [DOI] [PubMed] [Google Scholar]
- 38.Phan, L., L. W. Schoenfeld, L. Valasek, K. Nielsen, and A. G. Hinnebusch. 2001. A subcomplex of three eIF3 subunits binds eIF1 and eIF5 and stimulates ribosome binding of mRNA and tRNAiMet. EMBO J. 20:2954-2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shin, B. S., D. Maag, A. Roll-Mecak, M. S. Arefin, S. K. Burley, J. R. Lorsch, and T. E. Dever. 2002. Uncoupling of initiation factor eIF5B/IF2 GTPase and translational activities by mutations that lower ribosome affinity. Cell 111:1015-1025. [DOI] [PubMed] [Google Scholar]
- 40.Singh, C. R., Y. Yamamoto, and K. Asano. 2004. Physical association of eukaryotic initiation factor 5 (eIF5) carboxyl terminal domain with the lysine-rich eIF2β segment strongly enhances its binding to eIF3. J. Biol. Chem. 279:49644-49655. [DOI] [PubMed] [Google Scholar]
- 41.Singh, C. R., H. Hui, M. Ii, Y. Yamamoto, and K. Asano. 2004. Efficient incorporation of eIF1 into the multifactor complex is critical for formation of functional ribosomal preinitiation complexes in vivo. J. Biol. Chem. 279:31910-31920. [DOI] [PubMed] [Google Scholar]
- 42.Srivastava, S., A. Verschoor, and J. Frank. 1992. Eukaryotic initiation factor 3 does not prevent association through physical blockage of the ribosomal subunit-subunit interface. J. Mol. Biol. 220:301-304. [DOI] [PubMed] [Google Scholar]
- 43.Valášek, L., A. A. Mathew, B. S. Shin, K. H. Nielsen, B. Szamecz, and A. G. Hinnebusch. 2003. The yeast eIF3 subunits TIF32/a, NIP1/c, and eIF5 make critical connections with the 40S ribosome in vivo. Genes Dev. 17:786-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valášek, L., K. H. Nielsen, F. Zhang, C. A. Fekete, and A. G. Hinnebusch. 2004. Interactions of eukaryotic translation initiation factor 3 (eIF3) subunit NIP1/c with eIF1 and eIF5 promote preinitiation complex assembly and regulate start codon selection. Mol. Cell. Biol. 24:9437-9455. [DOI] [PMC free article] [PubMed] [Google Scholar]