Abstract
Objectives
Carotid artery stenting (CAS) using a 7-F Optimo balloon guide catheter (BGC) via the right radial artery (RA) was performed for stenosis of the right carotid artery. The factors affecting the difficulty in navigating the BGC from the right RA to the right common carotid artery (CCA) were investigated.
Materials and Methods
For 40 cases of stenosis of the right carotid artery, CAS using a 7-F Optimo BGC via the right RA was performed. Pre-operative anatomical length and angle of the access route were retrospectively examined.
Results
The 7-F Optimo BGC successfully reached all lesions; however, navigational difficulties were encountered in seven out of 40 cases (17.5%). One case in the difficult group experienced an ischemic complication. The height from the topmost point of the subclavian artery (SA) to the origin of the SA (SA height) was 44.4 mm versus 28.1 mm (p < 0.01), and the angle between the SA and the CCA (SA–CCA angle) was 21.6° versus 47.9° (p < 0.01) in the difficult and easy groups, respectively. For lesions with difficult navigation, the sensitivity and specificity of the SA height >34 mm were 100% and 82%, and the sensitivity and specificity of the SA–CCA angle <30° were 100% and 82%.
Conclusions
For stenosis of the right carotid artery, transradial-CAS using a 7-F Optimo BGC is a safe procedure. However, navigating the BGC becomes difficult when the SA height is >34 mm and the SA–CCA angle is <30°.
Keywords: Carotid artery stenting, transradial access, proximal protection
Introduction
Transradial (TR) access has several advantages over transfemoral access for neurointervention, including reduced ischemic complications and improved patient compliance.1–3 With the advancement of devices, TR access has become more common in carotid artery stenting (CAS), and transradial (TR)-CAS using a 5–6 French (F) guiding sheath has been reported.4–8 However, CAS using a balloon guide catheter (BGC) has been associated with lower ischemic complications compared to regular guiding catheters. 9
Recent BGCs have improved catheter stiffness, larger inner lumen diameters, and enhanced navigability.10–14 The 7-F Optimo BGC (Tokai Medical Products, Aichi, Japan) has been upgraded to have an inner lumen diameter of 0.071 inch, allowing a 5-F carotid stent system to pass through it. Although the 7-F Optimo BGC has been used in transfemoral CAS, it has also started to be used via the less invasive radial approach. 10 This study analyzed the difficulty of navigating the 7-F Optimo BGC in TR-CAS for stenosis of the right carotid artery.
Materials and methods
Patients
We performed a retrospective review of collected data in a single institute from January 2022 to March 2024. The inclusion criteria were a degree of stenosis >50% in symptomatic patients and >80% in asymptomatic patients. Symptomatic was defined as carotid stenosis occurring within 30 days before the procedure, with ischemic minor stroke (Rankin scale score >2), transient ischemic attack, or retinal artery ischemia. This study was approved by the ethics committee of our hospital and was conducted according to the tenets of the Declaration of Helsinki. Written informed consent was obtained from all patients.
During the period, 86 cases of right CAS were performed, with 40 cases using TR access, 45 cases using transfemoral access, and 1 case using carotid exposure. Pre-operative evaluation of the access route from the aortic arch to the carotid artery was performed using computed tomography angiography or magnetic resonance angiography. The RA was pre-operatively assessed with ultrasound, and TR-CAS was applied to cases with a vessel diameter of 2 mm or more. The access route was chosen by the operator based on the pre-operative evaluation.
Navigational difficulty was defined based on several criteria: kinking of the BGC necessitating repositioning to complete CAS; instability of the BGC in the CCA post-navigation, which required the use of a 0.018-inch guidewire for stabilization in the external carotid artery (ECA); and the risk of BGC displacement during stent guidance, which was mitigated by using the balloon anchoring technique.
CAS procedure
The 7 F Optimo BGC can accommodate a 5 F carotid stent system, such as a 6- to 8-mm Precise stent (Cordis, Miami Lakes, FL, USA). The Casper stent (MicroVention, Tustin, CA, USA) is a 5 F stent system, and all sizes of the Casper stent (6–10 mm) can pass through the 7 F Optimo BGC.
Local anesthesia was administered to allow continuous monitoring of the level of consciousness and motor function. Systemic anticoagulation was achieved by heparin administration to maintain an activated clotting time of at least 275 s. The 7-F Optimo BGC was navigated with a 5 F SY-3 inner catheter (Medikit, Tokyo, Japan) and a 0.035-inch guidewire from the right radial artery (RA) through a 7 F Glidesheath (Terumo Corp, Tokyo, Japan). A distal filter protection device (FilterWire EZ; Stryker, Fremont, CA, USA or Spider FX; Medtronic, Minneapolis, MN, USA) crossed the stenotic lesions with the common carotid artery (CCA) clamping. After the deployment of the distal filter protection device, clamping of the CCA was released to restore internal carotid artery (ICA) flow. The 7 F Optimo BGC was continuously inflated during the procedure from pre-dilatation to post-dilatation in cases neurologically tolerable. Approximately 20 mL of blood was manually aspirated using a 20 mL syringe after each step of pre-dilatation, stent placement, and post-dilatation. If neurological intolerance occurred, or vital adjustments such as hypotension and bradycardia were needed, the blood flow of the CCA was restored by deflating the balloon of the BGC at each step, that is, balloon dilatation and stent placement. A TR band (Terumo Corp.) was used for the hemostasis of the punctured RA.
Measurement of access route distance and angle
The distance and angle of the access route were measured using a 3D volume-rendering method and a maximum intensity projection method with 3D software from AquariusNET Enterprise 8000 (TeraRecon, Inc., Foster City, CA). The CCA length was also measured.
The definition of each anatomical predictor is as follows (Table 1 and Figure 1):
The aortic arch was categorized into one of three types according to the Casserly classification. 15
Subclavian artery (SA) height: the height of the right SA was defined as the distance between the axial planes drawn at the topmost point of the right SA and the origin of the right SA (Figures 1 and 2).
CCA length: the right CCA length was defined as the distance from the right CCA origin to the carotid bifurcation (Figure 1).
SA–CCA angle: the SA–right CCA angle was defined as the angle between the centerline of the right SA and the right CCA (Figures 1 and 2).
SA topmost angle: the angle between the centerline passing through the SA topmost was defined as the angle between the centerline of the right SA. Each centerline was drawn from the topmost point of the right SA (Figure 1).
Table 1.
Definition of anatomical predictor for right CCA navigation
| SA height | The distance between the axial planes drawn at the topmost point of the right SA and the origin of the right SA |
| CCA length | The distance from the right CCA origin to the carotid bifurcation |
| SA–CCA angle | The angle between the centerline of the right SA and right CCA |
| SA topmost angle | The angle between the centerline of the right SA. Each centerline was drawn from the topmost point of the right SA |
SA: subclavian artery; CCA: common carotid artery.
Figure 1.
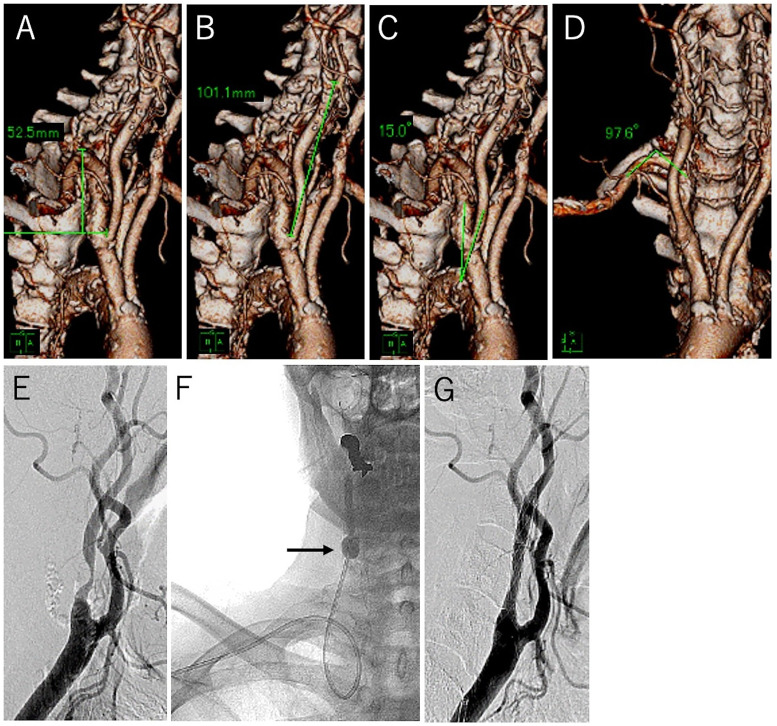
Representative case of difficult navigation. CTA images of the right SA and CCA with an illustration of measurements. (A) SA height is 52.5 mm. (B) CCA length is 101.1 mm. (C) SA–CCA angle is 15.0°. (D) SA topmost angle is 97.6°. (E) Lateral angiographic view revealing stenosis of the right carotid artery. (F) Anterior–posterior X-ray view during balloon dilatation after stent placement. The 7-F Optimo BGC (arrow) is navigated from the right RA to the CCA. The risk of BGC displacement during stent guidance and prevented by using the balloon anchoring technique. (G) Post-procedural lateral angiographic view showing good revascularization.
CTA: computed tomography angiography; SA: subclavian artery; CCA: common carotid artery; BGC: balloon guide catheter.
Figure 2.
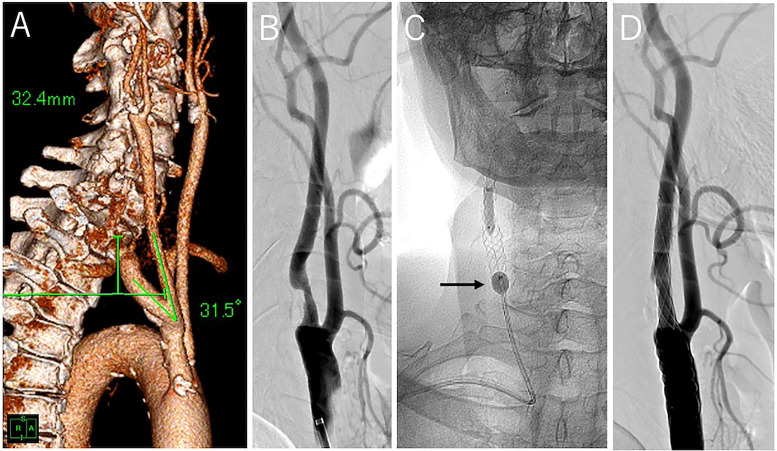
Representative case of easy navigation. (A) CTA image of the right SA and CCA with an illustration of measurements. SA height is 32.4 mm and CCA length is 31.5°. (B) Lateral angiographic view revealing stenosis of the right carotid artery. (C) Anterior–posterior X-ray view during balloon dilatation after stent placement. The 7-F Optimo BGC (black arrow) is navigated from the right RA to the CCA. (D) Post-procedural lateral angiographic view showing good revascularization.
CTA: computed tomography angiography; SA: subclavian artery; CCA: common carotid artery; BGC: balloon guide catheter.
Statistical analysis
Variables are expressed as absolute numbers and percentages or mean ± standard deviation. Comparisons were made using the t-test for unpaired samples or the chi-squared test as appropriate. To identify the SA height and SA–CCA angle that provided the best cutoff for predicting the difficult group, we chose the values in which the sum of the specificity and sensitivity was the highest. This value was obtained using receiver-operating characteristic curve analysis (ROC). Multivariate analysis was performed to identify predictors of antegrade flow at the ICA using factors with a p-value <0.10 in univariate analysis. For all tests, a p-value <0.01 was considered statistically significant. All statistical analyses were performed with the R 3.4.0 statistical software (R Foundation for Statistical Computing, Vienna, Austria).
Results
A summary of the baseline characteristics according to the difficulty in navigating the BGC is shown in Table 2. Representative cases of both difficult and easy guidewire navigation are shown in Figures 1 and 2, respectively. The 7-F Optimo BGC successfully reached all lesions; however, navigational difficulties were encountered in seven out of 40 cases (17.5%). The reasons for difficulty included kinking of the BGC after navigation, necessitating repositioning to complete CAS in three lesions; instability of the BGC in the CCA after navigation, requiring the use of a 0.018-inch guidewire to stabilize it in the ECA in 2 lesions; and the risk of BGC displacement during stent guidance and prevented by using the balloon anchoring technique in two lesions. One case of access difficulty resulted in an embolism in the right middle cerebral artery during the procedure, requiring a thrombectomy with an aspiration catheter. Treatment was completed in all cases using the 7-F Optimo BGC. There were no postoperative neurological symptoms due to cerebral ischemia and no complications at the puncture site.
Table 2.
Comparison of baseline characteristics and clinical results between the difficult and easy groups
| Difficult | Easy | P-value | |
|---|---|---|---|
| Age, years | 78.0 ± 5.6 | 74.4 ± 10.7 | 0.39 |
| Female sex | 0 | 5 | 0.57 |
| Symptomatic | 4 | 24 | 0.41 |
| Risk factors | |||
| Hypertension | 1 | 21 | 0.32 |
| Diabetes mellitus | 4 | 18 | 1 |
| Dyslipidemia | 3 | 10 | 0.66 |
| Coronary artery disease | 2 | 16 | 0.42 |
| Stenotic ratio, % | 74.6 ± 16.2 | 69.8 ± 13.0 | 0.4 |
| Aortic arch type III | 0 | 6 | 0.57 |
| SA height, mm | 44.3 ± 9.6 | 28.1 ± 7.9 | <0.01 |
| CCA length, mm | 102.9 ± 10.9 | 94.5 ± 1 3.5 | 0.14 |
| SA topmost angle, degree | 78.2 ± 24.6 | 92 ± 13.5 | 0.11 |
| SA–CCA angle, degree | 21.6 ± 7.6 | 47.9 ± 21.8 | <0.01 |
| Ischemic event | 1 | 0 | 0.17 |
| Puncture site complications | 0 | 0 | 1 |
SA: subclavian artery; CCA: common carotid artery.
Values are presented as the number of patients (%). Mean values are presented as ±standard deviation.
In the ROC curve analysis, an SA height >34 mm and an SA–CCA angle <30° were determined as the most reliable cutoff values for difficult navigation. The diagnostic performance measures for these cutoff values are shown in Table 3. For lesions with difficult navigation, the sensitivity and specificity of the SA height >34 mm were 100% and 82%, and the sensitivity and specificity of the SA–CCA angle <30° were 100% and 82%.
Table 3.
Cutoff values and diagnostic performance measures for difficult navigation.
| Variable | AUC (95% CI) | Cutoff | Sensitivity | Specificity | Positive predictive value | Negative predictive value |
|---|---|---|---|---|---|---|
| SA height | 0.93 (0.86–1) | <34.0 mm | 100% | 82% | 54% | 100% |
| SA–CCA angle | 0.92 (0.84–1) | >30.4° | 100% | 82% | 54% | 100% |
SA: subclavian artery; CCA: common carotid artery; AUC; area under the curve; CI: confidence interval.
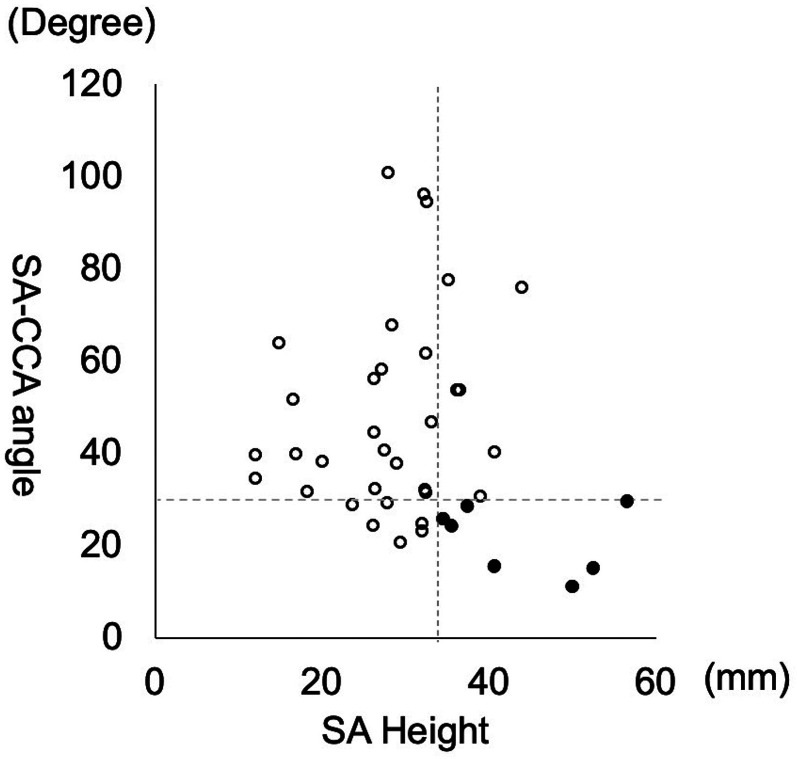
A dot plot of all cases’ SA height and SA–CCA angle is shown in Figure 3. All cases with difficult navigation had an SA height of>34 mm and an SA–CCA angle of <30°.
Figure 3.
Dot plot of the SA height and SA–CCA angle. The vertical dashed line indicates a cutoff value of 34 mm for SA height, and the horizontal dashed line indicates a cutoff value of 30° for the SA–CCA angle. Black dots represent difficult navigation cases, and white circles represent easy navigation cases.
SA: subclavian artery; CCA: common carotid artery.
Discussion
This study indicated that an SA height of >34 mm and an SA–CCA angle of <30° were factors associated with difficulty in guiding the 7-F Optimo BGC in TR right CAS. All cases with difficult navigation had these characteristics. Reports of TR neurointervention are increasing.1–3 Anatomically, the right carotid artery is generally easier to navigate than the left; however, if the access route is highly tortuous, navigation can be difficult for lesions on either side. Anatomical factors associated with difficulty in navigating the left side have been reported. Choi et al. 16 reported that successful navigation of the left ICA during TR neurointervention was significantly linked to a taller right SA, a sharper turnoff angle from the left CCA, a greater distance between the innominate artery and left CCA and more pronounced angulation of both the right SA and the left CCA. However, there are no previous papers on difficult factors for right lesions.
In this study, univariate analysis showed that an SA height of >34 mm and an SA–CCA angle of <30° were factors associated with difficulty in navigation, but the multivariate analysis did not yield valid data due to the small number of cases. This suggests that the combination of arteriosclerosis-related SA height of >34 mm and SA–CCA angle of <30° may have been a contributing factor.
TR-CAS with a regular guiding catheter is easier to navigate due to its simpler structure compared to a BGC. Recently, CAS with a BGC by occluding the CCA without ECA occlusion has yielded good results. Regarding trackability and stability, the new BGCs have better catheter stiffness and trackability than previous BGCs, and they also have a wider lumen. Existing BGCs such as the Concentric BGC (Concentric Medical, Mountain View, CA, USA), FlowGate2 (Stryker, Fremont, CA, USA), and Walrus (QApel Medical Inc., Fremont, CA, USA) have better catheter stiffness, larger inner lumen diameters, and improved navigability.11–14 However, they require an 8–9F sheath.
Harada et al. 10 reported that CAS with a 7-F Optimo and a distal filter was safe in 100 cases. Of these, 15 out of 100 were performed transradially. One case showed kinking of the Optimo BGC, but all cases were completed successfully. The 30-day major adverse event rate was 1%; one patient had cerebral hemorrhage the day after the procedure, and there were no ischemic complications. 10 TR-CAS is less invasive than transfemoral CAS, and the use of BGCs in TR-CAS is likely to become safer in the future.
This study has several limitations. This is a retrospective study from a single institution with a small number of cases, and the criteria for selecting the TR approach were not clear. There have been no large-scale clinical trials comparing CAS with and without BGC use. Also, the advantages of combining a distal filter with a BGC are unclear, as there are only a small number of studies that have reported on CAS combined with BGC use. In this series, multivariate analysis using SA height and SA–CCA angle was performed, but the small number of cases meant that it did not result in valid statistical analysis.
Conclusion
In TR right CAS using a 7-F Optimo BGC, for lesions with an SA height of >34 mm and an SA–CCA angle of <30°, navigation of the catheter is difficult and unstable, requiring special maneuvers.
Footnotes
KA was the main operator of the endovascular treatment, designed the research, and drafted the manuscript. KH, MK, RK, DB, and TO were the main assistants of the endovascular treatment and reviewed the manuscript.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval statement/IRB approval number: This study was approved by the ethics committee of our hospital (No. 00199). This study was conducted according to the tenets of the Declaration of Helsinki. Written informed consent was obtained from all the patients.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Kei Arakawa https://orcid.org/0000-0002-0586-8832
Kei Harada https://orcid.org/0000-0003-2293-3509
References
- 1.Brunet MC, Chen SH, Peterson EC. Transradial access for neurointerventions: Management of access challenges and complications. J Neurointerv Surg 2020; 12: 82–86. [DOI] [PubMed] [Google Scholar]
- 2.Hanaoka Y, Koyama JI, Yamazaki D, et al. Transradial approach as the primary vascular access with a 6-Fr Simmons guiding sheath for anterior circulation interventions: A single-center case series of 130 consecutive patients. World Neurosurg 2020; 138: e597–e606. [DOI] [PubMed] [Google Scholar]
- 3.Tanoue S, Ono K, Toyooka T, et al. Feasibility and challenges of transradial approach in neuroendovascular therapy: A retrospective observational study. J Neuroendovasc Ther 2023; 18: 10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruzsa Z, Nemes B, Pintér L, et al. A randomised comparison of transradial and transfemoral approach for carotid artery stenting: RADCAR (RADial access for CARotid artery stenting) study. EuroIntervention 2014; 10: 381–391. [DOI] [PubMed] [Google Scholar]
- 5.Du M, Hu Y, Zhu D, et al. Systematic review and meta-analysis of transradial access for carotid artery stenting. Angiology 2024; 75: 517–526. [DOI] [PubMed] [Google Scholar]
- 6.Hanaoka Y, Koyama J-I, Nakamura T, et al. Smaller diameter sheaths are required to safely perform transradial neurointerventions. Interv Neuroradiol 2023: 15910199231201517. doi: 10.1177/15910199231201517 [DOI] [PubMed] [Google Scholar]
- 7.Batista S, Oliveira LdB, Diniz JBC, et al. Transradial versus transfemoral approach in cerebral angiography: A meta-analysis. Interv Neuroradiol 2023: 15910199231212520. doi: 10.1177/15910199231212520 [DOI] [PubMed] [Google Scholar]
- 8.Rentiya ZS, Kuhn AL, Shazeeb MS, et al. Transradial access for cerebral angiography and neurointerventional procedures: A meta-analysis and systematic review. J Neurointerv Surg 2024; 30: 404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harada K, Morioka J, Higa T, et al. Significance of combining distal filter protection and a guiding catheter with temporary balloon occlusion for carotid artery stenting: Clinical results and evaluation of debris capture. Ann Vasc Surg 2012; 26: 929–936. [DOI] [PubMed] [Google Scholar]
- 10.Harada K, Fujimura H, Kajihara M, et al. Carotid artery stenting using a 7 French Optimo balloon guide catheter combined with a distal filter. Interv Neuroradiol 2023. doi: 10.1177/15910199231162493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dargazanli C, Mahmoudi M, Cappucci M, et al. Angiographic patterns and outcomes achieved by proximal balloon occlusion in symptomatic carotid artery stenosis stenting. Clin Neuroradiol 2020; 30: 363–372. [DOI] [PubMed] [Google Scholar]
- 12.Gabrielli R, Castrucci T, Siani A, et al. Common carotid artery endovascular clamping for neuroprotection during carotid stenting: Flow-gate system as an innovative treatment approach. Catheter Cardiovasc Interv 2021; 97: E71–E78. [DOI] [PubMed] [Google Scholar]
- 13.Salem MM, Kvint S, Baig AA, et al. Carotid artery revascularization using the Walrus balloon guide catheter: Safety and feasibility from a US multicenter experience. J Neurointerv Surg 2022; 14: 709–717. [DOI] [PubMed] [Google Scholar]
- 14.Moldovan K, Yaeger KA, Al-Kawaz M, et al. Transradial carotid artery stenting using walrus balloon guide catheter: Technical aspects and clinical outcome. Oper Neurosurg (Hagerstown) 2023; 25: 28–32. [DOI] [PubMed] [Google Scholar]
- 15.Casserly IP, Sachar R, Yadav JS. Manual of peripheral vascular intervention. Philadelphia: Lippincott Williams & Wilkins, 2005, pp. 214–2.
- 16.Choi SW, Kim S, Kim H, et al. Anatomical predictors of difficult left internal carotid artery navigation in transradial access for neurointervention. J Neurosurg 2022; 139: 157–164. [DOI] [PubMed] [Google Scholar]