Abstract
Objective
The Pipeline Vantage Embolization Device (PVED) is a novel coated flow diverter with reduced wire diameters to improve neoendothelialization and stent porosity. This systematic review evaluates the safety and efficacy of the PVED based on the current literature.
Methods
Following the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines, a thorough literature search was conducted using PubMed, EMBASE, and Cochrane. The random effects model was used to calculate estimates with major neurological complications within 30 days of treatment as the primary safety endpoint and ≤1-year complete occlusion rate as the primary efficacy endpoint.
Results
Six single-arm studies (5 retrospective, 1 prospective) with 392 patients and 439 aneurysms (6.8% ruptured) were included. Antiplatelet regimens varied, but dual antiplatelet therapy was administered in the majority. The pooled technical success rate was 99.0% (95%CI, 98.0%–100%) with an average of 1.2 devices implanted per procedure. Balloon angioplasty was performed in 17.0% (95%CI, 6.4–27.6%) and adjunctive coiling in 28.0% (95%CI, 17.8–38.2%), with significant heterogeneity for both variables. Pooled estimates for major neurological complications were 3.5% (95%CI, 1.7%–5.2%) with total ischemic events in 4.1% (95% CI, 1.6%–6.6%) and hemorrhagic events in 1.0% (95% CI, 0.0%–1.9%). The rate of complete angiographic occlusion was 75.7% (95%CI, 70.7%–80.6%) at a mean follow-up of 7 months, with in-stent stenoses in 8.1% (95%CI, 4.5%–11.8%).
Conclusions
The safety and efficacy profile of the PVED appears comparable to competing devices, with potentially fewer complications than first-generation flow diverters. Long-term and comparative studies are needed to further confirm these results.
Keywords: Aneurysm, angiography, device, flow diverter, intervention
Introduction
The introduction of flow diverters (FDs) has significantly expanded the therapeutic landscape for endovascular treatment, particularly for complex aneurysms with characteristics such as wide-neck, large size and fusiform morphology.1–3 FDs are characterized by high metal coverage and low porosity, which redirect blood flow away from the aneurysm sac, leading to intra-aneurysmal thrombosis. Despite their efficacy, with occlusion rates exceeding 80%, concerns remain about the thrombogenicity associated with the high metal content prior to complete endothelialization, leading to ischemic complications in approximately 6% of patients treated with a FD. 4 To mitigate these risks, long-term dual antiplatelet therapy is required, but with the trade-off of an increased risk of bleeding. 5 To overcome these limitations, the next frontier in FD technology is to reduce the thrombogenic potential of these devices.6,7 The Pipeline Vantage Embolization Device (PVED; Medtronic, Dublin, Ireland) represents the fourth generation of Pipeline flow diversion devices. It has the same coating (“Shield Technology”) as its predecessor, the Pipeline Flex with Shield Technology, but has smaller wire diameters and higher pore density to improve neoendothelialization and aneurysm occlusion. 8 This systematic review and meta-analysis synthesizes the results of published studies and provides a comprehensive assessment of the technical success rate, safety, and efficacy of PVED in the treatment of intracranial aneurysms.
Methods
Study design
A comprehensive review of the scientific literature available in the PubMed/MEDLINE, EMBASE, and Cochrane databases was performed for studies published between January 2020—the year PVED was launched—and January 2024. The following index terms were used with Boolean operators to increase sensitivity: “Vantage,” “pipeline embolization device with shield technology,” “surface modification,” “flow diverter,” and “aneurysm”. Bibliographies of screened full texts were reviewed to identify additional studies. The study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Protocol registration and ethics committee approval were not required by institutional policy.
Study selection
Studies published in English and reporting a series of at least 10 patients with intracranial aneurysms treated with the PVED, with at least 30 days of follow-up and clear documentation of primary outcomes were considered for inclusion. Review articles, guidelines, narrative review or opinion articles, technical notes, animal or in vitro studies were excluded. Titles and abstracts were screened by the first author and reviewed by the senior author. Full-text articles corresponding to included abstracts were obtained and analyzed by the same authors. Potential discrepancies in study selection were resolved by consensus.
Data extraction
For each included study, the following data were collected: country of participating center, study design, age and sex of enrolled population, aneurysm characteristics (unrupted/ruptured, size, neck width, morphology, location), antiplatelet therapy, technical problems encountered, use of adjunctive balloon angioplasty and coiling, procedure-related complications, and mortality. The primary safety endpoint was the rate of major neurological complications within 30 days of treatment, defined as a complication with new neurological deficits lasting at least 7 days. Secondary safety endpoints were the overall rate of ischemic and hemorrhagic events and 30-day treatment-related mortality. The primary efficacy endpoint was 4 to 12-month complete occlusion rate. Complete occlusion was defined as O'Kelly-Marotta (OKM) Grade D or Raymond-Roy Occlusion Classification (RROC) Grade 1, as reported in the studies. Other outcome measures were self-reported technical success rates, and adequate occlusion rates (complete and residual neck) defined as OKM C + D or RROC 1 + 2 and the rate of in-stent stenosis.
Study risk of bias assessment
The Newcastle-Ottawa Quality Assessment Scale for cohort studies was used to assess the risk of bias in the studies included in our analysis. 9 Due to the lack of a control (nonexposed) group, the comparability domain was slightly modified to consider subanalysis instead of controlling for confounders between groups. A mean follow-up of at least 6 months was considered as adequate follow-up. Each study was assigned a rating from 0 to 9 points. Studies with ≥6 points were considered “good” quality, 3–5 points were considered “fair” quality, and ≤2 point was considered “poor” quality.
Statistical analysis
For each included study, we calculated the cumulative incidence, expressed as the event rate per procedure or per aneurysm at the end of the study, with 95% confidence intervals. Because substantial heterogeneity in the baseline characteristics of the study populations was observed, a random-effects model was used to aggregate the incidence rates. Statistical heterogeneity between studies was assessed using the I2 statistic, with values of 50% indicating significant heterogeneity. Forest plots were generated for proportions and estimated overall rates. In addition, subgroup analyses were performed to compare core laboratory and self-reported occlusion rates. All statistical analyses were performed using OpenMeta[Analyst], an open-source statistical software available at http://www.cebm.brown.edu/openmeta/.
Results
Systematic search
After removing duplicates, the search yielded 33 articles. After excluding irrelevant articles by title and abstract screening, 7 articles were selected for full-text screening and 6 studies were included in our qualitative and quantitative analysis (Figure 1).8,10–14
Figure 1.
Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow chart for study selection.
Study characteristics
All 6 studies were single-arm case series conducted in Germany, the United Kingdom, and Australia. Five studies were retrospective and one was prospective. Three studies were rated as “good” and 3 as “fair” study quality. The number of patients ranged from 28 to 121. The cumulative population consisted of 392 patients (mean age 57 years, 74.5% female) who underwent PVED treatment in 395 procedures for 439 aneurysms (409 [93.2%] unruptured, 30 [6.8%] ruptured). The average aneurysm size was 7.3 mm with a neck width of 3.5 mm. The characteristics and results of each study are summarized in Tables 1 and 2.
Table 1.
Baseline characteristics and technical experience reported in the included studies. Retro = retrospective; Pro = prospective; F = female; ICA = internal carotid artery; MCA = middle cerebral artery; Acom = anterior communicating artery; ACA = anterior cerebral artery; SUCA = superior cerebellar artery; BA = basilar artery; VA = vertebral artery; PCA = posterior cerebral artery; PICA = posterior inferior cerebellar artery; AICA = anterior inferior cerebellar artery; FD = flow-diverter.
| Study, year | Design/ country/ no. of centers | Age (mean years)/ sex (%F) | Total no. of unruptured/ruptured aneurysms | Aneurysm sizes/neck widths (mean mm) | Fusiform, dissecting, or blister morphology (%) | Locations (%) | Technical experience (%) |
|---|---|---|---|---|---|---|---|
| Vollherbst et al., 2023 | Retro/Germany/10 | 54/68.3 | 67/3 | 8.2/1.9 | 11.4 | 50.0 ICA 24.3 MCA 8.6 Acom 7.1 ACA 4.3 SUCA 5.7 Other |
19.7 hang-up of FD 24.6 balloon angioplasty 1.6 displacement of FD 1.6 high resistance 21.3 coiling |
| Sciacca et al., 2023 11 | Retro/UK/3 | 57/78.8 | 55/5 | 10.6/4.1 | 6.7 | 8.3 ICA cavernous 50.0 ICA paraophthalmic 16.7 ICA posterior communicating 6.7 ICA choroidal 6.7 ICA terminus 1.7 MCA 3.3 Acom 1.7 BA 3.3 SUCA 1.7 AICA |
21.2 balloon angioplasty 3.8 additional stent to improve wall apposition 1.8 vessel perforation during unsheathing of FD 1.8 dissection 38.5 coiling |
| Döring et al., 2023 10 | Retro/Germany/1 | 56/76.7 | 32/0 | 5.1/4.2 | 0 | 50.0 ICA paraophthalmic 18.8 ICA posterior communicating 6.2 MCA 3.1 ACA 6.2 BA 3.1 VA 9.4 SUCA 3.1 PICA |
3.3 fish mouthing |
| Booth et al., 2023 12 | Retro/UK/8 | 57/71.9 | 114/17 | 9.6/4.8 | 9.2 | 0.8 ICA cervical 13.0 ICA cavernous 42.7 ICA paraophthalmic 19.1 ICA posterior communicating 2.3 ICA choroidal 1.5 ICA terminus 6.9 MCA 1.5 ACA 4.6 Acom 2.3 BA 0.8 PCA 0.8 VA 3.8 PICA |
16.6 balloon angioplasty 1.7 additional WEB 5.7 difficulty deploying FD 1.7 dissection 33.9 coiling |
| Goertz et al., 2023 13 | Retro/Germany/3 | 56/67.9 | 26/5 | 5.0/3.2 | 19.4 | 9.7 ICA cavernous 58.1 ICA paraophthalmic 9.7 ICA posterior communicating 9.7 ICA terminus 3.2 BA 9.7 VA |
6.7 balloon angioplasty 10.0 coiling |
| De Villiers et al., 2024 14 | Pro/Australia /1 | 59/80.2 | 115/0 | 5.0/3.0 | N/A | 3.5 ICA cervical 6.1 ICA cavernous 22.6 ICA paraophthalmic 8.7 ICA posterior communicating 1.7 ICA choroidal 7.8 ICA supraclinoid 1.0 ICA terminus 21.7 MCA 3.4 ACA 14.0 Acom 7.8 BA/P1 1.7 VA |
4.0 balloon angioplasty 36.6 coiling |
Table 2.
Individual outcome measures were reported in the included studies. *Not reported.
| Study, year | Technical success (%)/mean number of FDs per procedure | Ischemic/hemorrhagic complications/Mortality (%) | Complete/Adequate occlusion (%) | In-stent stenosis: Overall/>50%/occlusion (%) | Mean length of follow-up (months) |
|---|---|---|---|---|---|
| Vollherbst et al., 2023 | 100/1.3 | 3.3/0/0 | 77.9/95.6 | 19.1/1.5/1.5 | 7 |
| Sciacca et al., 2023 11 | 98.1/1.0 | 3.8/1.9/0 | 62.1/82.8 | 3.4/3.4/0 | 7 |
| Döring et al., 2023 10 | 100/1.0 | 3.3/3.3/0 | 75.0/90.6 | 10.0/10.0/3.3 | 10 |
| Booth et al., 2023 12 | 97.7/* | 8.5/1.5/0.8 | 72.3/90.8 | 7.4/2.8/0 | 6 |
| Goertz et al., 2023 13 | 100/1.2 | 13.3/0/0 | 70.0/80.0 | 5.0/0/0 | 5 |
| De Villiers et al., 2024 14 | 100/1.2 | 3.0/0/0 | 81.7/* | 7.9/5.9/1.0 | 6 |
Technical experience
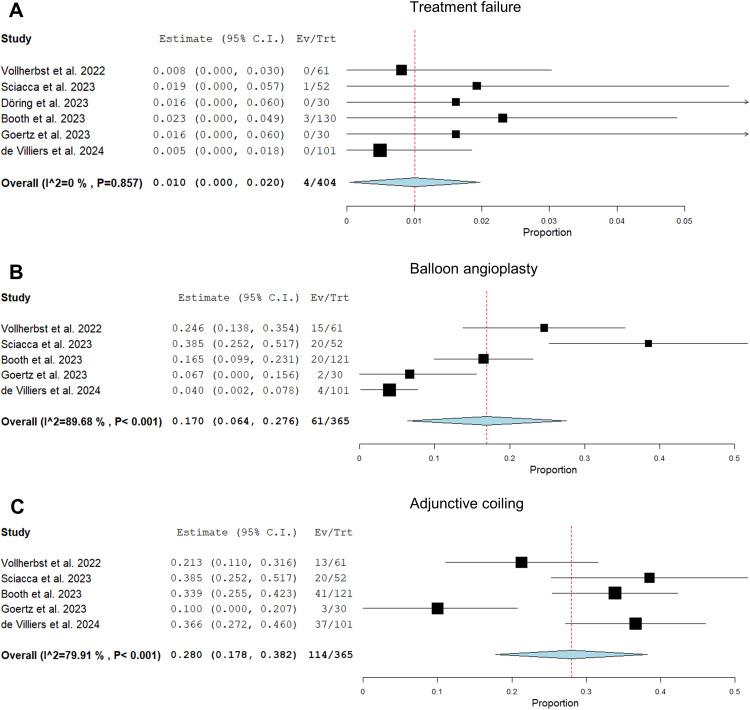
The cumulative technical success rate was 99.0% (95%CI, 98.0%–100%) with a mean of 1.2 (95%CI, 1.1–1.3) flow diverters implanted per procedure (Figure 2). Technical problems were reported in a minority of cases, as detailed in Table 1. Balloon angioplasty and adjunctive coiling were only reported in 5 studies. Balloon angioplasty was performed in 17.0% (95%CI, 6.4–27.6%) and adjunctive coiling in 28.0% (95%CI, 17.8–38.2%). The antiplatelet regimen was inconsistent, and in particular, antiplatelet testing and adherence were poorly documented in the trials. The majority of interventions were performed with dual antiplatelet therapy, as shown in Table 3.
Figure 2.
Forest plots with random effects models showing the rate of treatment failure (A), the rate of adjunctive balloon angioplasty after FD implantation (B), and the rate of adjunctive coiling with FD implantation (C). Ev/Trt indicates event (failure, adjunctive treatment)/treated patient.
Table 3.
Anti-platelet protocols as reported in the studies. ASA = acetylsalicylic acid; i.v. = intravenous.
| Study, year | Antiplatelet regimen |
|---|---|
| Vollherbst et al., 2023 | ASA + clopidogrel in 44.3%, prasugrel monotherapy in 34.4%, ASA + prasugrel in 14.8%, ASA + ticagrelor in 4.9%, and ticagrelor monotherapy in 1.6%. |
| Sciacca et al., 2023 11 | Unruptured: starting 7 days before the procedure, ASA 75 mg + clopidogrel 75 mg or prasugrel 50 mg (with 300 mg ASA i.v. during the procedure). DAPT for 6–10 months. Ruptured: i.v. bolus and infusion of tirofiban during the. Then ASA 75 mg (500 mg loading dose) + clopidogrel 75 mg (300 or 600 mg loading dose) for 6 months. |
| Döring et al., 2023 10 | ASA 100 mg (from 1 day before the procedure) + clopidogrel 75 mg for 3 months (loading dose 600 mg the day before the procedure and additional 300 mg on the day of the procedure). |
| Booth et al., 2023 12 | Not specified |
| Goertz et al., 2023 13 | Unruptured: starting 5–7 days before the procedure ASA 100 mg + clopidogrel 75 mg or ticagrelor 2 × 90 mg. DAPT for 4 months. Ruptured: Tirofiban infusion, then loading with ASA 150 mg + clopidogrel 300 mg or ticagrelor 180 mg and same regimen as for unruptured cases. |
| De Villiers et al., 2024 14 | ASA 300 mg loading dose + clopidogrel 300 mg 1 day before and on the day of the procedure. Starting dose of ticagrelor 180 mg in case of non-response to clopidogrel. ASA 100 mg for 9 months + clopidogrel or ticagrelor for 3 months. |
Complications and mortality
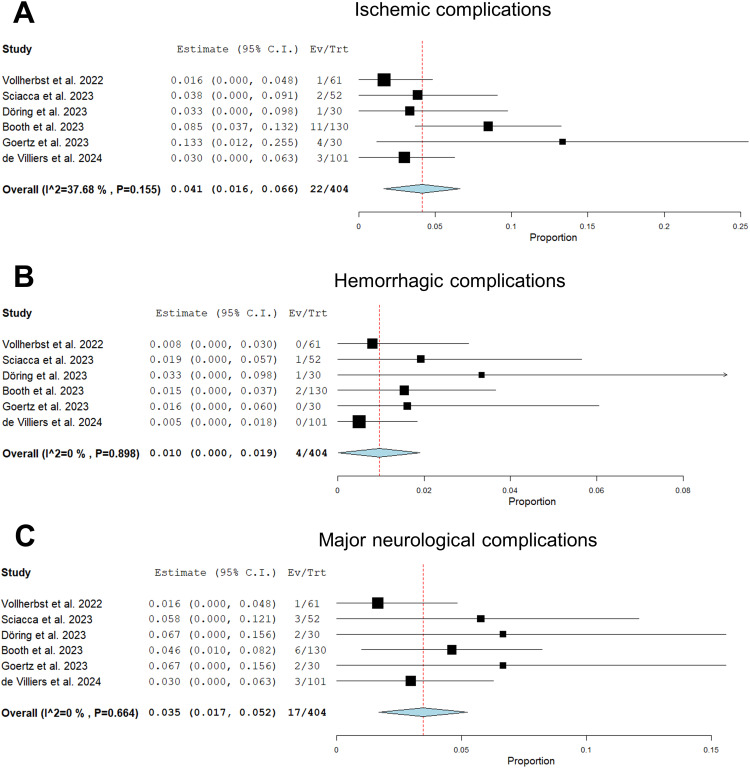
Significant heterogeneity was observed in the definition and reporting of complications. To ensure consistency, we adopted the definitions described in the Materials and Methods section and, where possible, individual events were reclassified according to these adopted definitions. The pooled estimate for major neurological complications was 3.5% (95%CI, 1.7%–5.2%) with an overall ischemic event rate of 4.1% (95% CI, 1.6%–6.6%) and a hemorrhagic event rate of 1.0% (95% CI, 0.0%–1.9%)(Figure 3). Vollherbst et al. reported 1 perforator infarction. Sciacca et al. reported 1 postinterventional thromboembolic stroke. Döring et al. reported 1 basal ganglia infarction on day 2 due to perforator occlusion. Booth et al. reported 5 major and 6 minor strokes within 30 days of treatment. In the study by Goertz et al., there were 2 major and 2 minor postinterventional strokes (3 thromboembolic, 1 perforator occlusion). De Villiers et al. reported 3 ischemic strokes within 30 days in treated MCA bifurcation aneurysms. With regard to hemorrhagic events, there was 1 intraprocedural SAH in the study by Sciacca et al., 1 intracerebral hemorrhage on day 28, presumably related to DAPT, in the study by Döring et al., and 1 hemorrhagic transformation of an asymptomatic infarct on day 24 in the study by Booth et al. There was 1 (0.3%) treatment-related death, which occurred 3 days after embolization of a partially thrombosed fusiform giant aneurysm of the basilar artery due to SAH. Due to the low event rate, a meta-analysis for mortality was not performed.
Figure 3.
Forest plots with random effects models showing procedural and early complications within 30 days of follow-up: the overall rate of ischemic complications (A), the rate of hemorrhagic complications (B), and the rate of major neurological complications (C). Ev/Trt indicates event (complication)/treated patient.
Angiographic outcomes
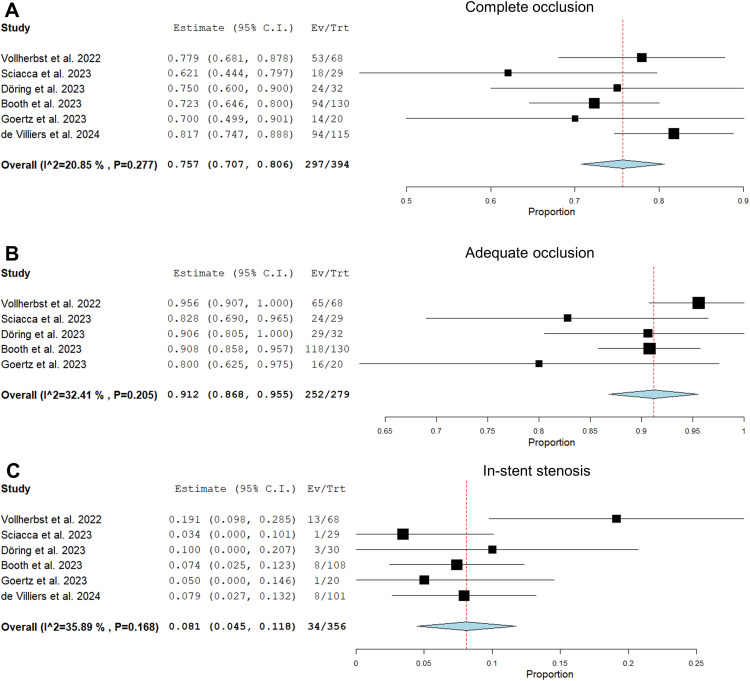
There was considerable variability in follow-up, imaging technique, occlusion assessment and imaging volume among the studies. Angiographic follow-up was available for 394 (89.7%) aneurysms at a mean follow-up of 7 months. The RROC scale was used to assess aneurysm occlusion in 4 studies, while Goertz et al. used the OKM scale and de Villiers et al. reported only complete occlusion rates without reference to a specific scale. The complete occlusion rate was 75.7% (95%CI, 70.7%–80.6%) in 6 studies and the adequate occlusion rate was 91.2% (95%CI, 86.8%–95.5%) in 5 studies (Figure 4). Occlusion rates were assessed by core lab in the Booth et al. study and by an independent interventionalist in the de Villiers et al. study, while the other studies used self-reported results. Complete occlusion rates were comparable between studies with independent (76.3%) and self-reported (73.2%) data (OR: 1.2, 95%CI, 0.2–6.1, p = 0.91).
Figure 4.
Forest plots with random effects models showing the mid-term angiographic outcome: complete occlusion rate (OKM D or RROC 1; A), adequate occlusion rate (OKM C + D or RROC 1 + 2; B), in-stent stenosis rate (C). Ev/Trt indicates event (occlusion, stenosis)/treated patient.
The overall rate of in-stent stenosis was 8.1% (95%CI, 4.5%–11.8%), and moderate or severe stenosis (>50%) accounted for 5.0% (95%CI, 2.0%–8.0%). One asymptomatic in-stent occlusion was reported in each of the studies by Vollherbst et al., Döring et al., and de Villiers et al.
Discussion
Pooling the available evidence on PVED, our results suggest that the device has acceptable rates of periprocedural major neurological complications (3.5%) and low mortality (0.7%). The antiplatelet medication and adherence was poorly documented in the studies, but most interventions followed a dual antiplatelet regimen for 3–6 months after therapy. Syntheses of study results, such as our meta-analysis, play a critical role in the evaluation of treatment options, especially with the rapid emergence of new technologies in the endovascular field.
For the first generation PED, the pooled analysis of the 3 benchmark studies (IntrePED, PUFS, Aspire) showed a major neurological morbidity rate of 5.7% and a neurological mortality rate of 3.3%. 4 For Pipeline Flex, the second generation PED, the Bhatia et al. meta-analysis reported a major complication rate of 1.8% and a periprocedural morbidity rate of 0.8%. 15 For the Pipeline Flex with Shield technology, which represents the third generation of Pipeline flow diverters, the meta-analysis by Luo et al. reported a peri- and post-operative complication rate of 11.1% and a mortality rate of 0.7%. 16
In a meta-analysis of the Derivo Embolization Device (DED; Acandis, Pforzheim, Germany), Monteiro et al. reported a periprocedural ischemic and hemorrhagic complication rate of 4.9% and a mortality rate of 2.1%. 6 For the first-generation Flow Redirection Endoluminal Device (FRED; Microvention, Aliso Viejo, CA, USA), a meta-analysis by Wagas et al. calculated a procedural morbidity of 3.9%, with 3.8% ischemic complications, 1.5% hemorrhagic complications, and 1.4% procedure-related mortality. 17 In the meta-analysis of Silk (Balt Extrusion, Montmorency, France), Florez et al. reported rates of thromboembolic and hemorrhagic complications of 6.1% and 1.6%, respectively, with a mortality rate of 2.8%. 18
Li et al. performed a meta-analysis of surface-modified flow diverters, including the Pipeline Shield and the DED, and reported a morbidity rate of 6.0% and a mortality rate of 1.3%. The total ischemia rate was 6.7% with 1.8% severe ischemia. 19 For the FRED X, another coated flow diverter, Goertz et al. reported ischemic complications in 3.6% and morbidity in 1.7% in a meta-analysis. 20
A comparison of the cited studies shows that the major complication rates of PVED are in the lower range of the studies. In particular, mortality was low with only 1 death following treatment of a giant aneurysm. Cerebral hemorrhage remains the most common cause of mortality in FD treatment. Concomitantly, the rate of hemorrhagic complications in this meta-analysis was only 1.0%, demonstrating the clinical safety of PVED.
The 99.0% technical success rate supports the manufacturers’ claims of improved apposition and deliverability. First-generation FDs have reported technical success rates between 90% and 95%. 4 In PVED, there were no significant adverse outcomes in cases of technical failure, as the stents were successfully re-coated and removed, or issues related to improper expansion were resolved with alternative endovascular techniques. An average of 1.2 devices were implanted per aneurysm, following the trend of fewer device implantations per aneurysm. In contrast, the IntrePED study reported multiple PEDs in 34% of cases, with an average of 1.4 devices per aneurysm. 21 A higher number of stents may increase the risk of thromboembolic events because more metal is introduced into the blood vessels and the duration of treatment is longer.22–24 Across PVED studies, a single device was sufficient in the majority of cases, indicating good deployability. However, additional balloon angioplasty, typically performed to improve wall apposition, was required in an average of 17%, ranging from 4% to 25%. This average rate is higher than that reported in large series for previous Pipeline versions, such as 11% in the ASPIRe study and 3% in the PEDESTRIAN registry.25,26 Possible reasons for this comparatively high rate of balloon support could not be deduced in the current review and may range from anatomical factors to problems with device deployment to differences in standards of care. However, the fact that balloon angioplasty was used in only 4% of cases in the only prospective and second-largest PVED study speaks against problems with device deployment or inadequate wall apposition.
The use of adjunctive coiling was also heterogeneous across the PVED trials, with a mean of 28% and a range of 10% to 39%, which may also be explained by differences in standards of care. This compares to 17% in the ASPIRe trial and 9% in the PEDESTRIAN trial of previous Pipeline versions.25,26 While adjunctive coiling is generally not necessary to achieve progressive aneurysm occlusion, it may maximize immediate aneurysm occlusion, which may be beneficial in ruptured aneurysms to prevent rebleeding or in large aneurysms.
Flow diverters are designed to induce hemodynamic remodeling of the parent artery, resulting in gradual occlusion of the aneurysm and progressive occlusion beyond a mid-term follow-up period. High pore density of the flow diverter is critical to promote effective blood stagnation within the aneurysm sac. 27 The PVED has a higher pore density than its predecessor while maintaining the same overall metal coverage due to the reduced wire diameter. In a preclinical study by Starke et al., the PVED features were associated with higher complete occlusion rates and better preservation of perforator and branch vessel patency. 28 In the current meta-analyses, the complete occlusion rate at 7 months was 76%, which is comparable to previous meta-analyses of the first generation PED (75%), Pipeline Shield (74%), FRED (74%), and DED (81%).4,16,17 Long-term follow-up analysis will determine whether the PVED structure has a long-term beneficial effect on occlusion rates.
In-stent stenosis is another concern with stent grafts, which typically occurs within the first 6 months after treatment and can lead to delayed ischemic stroke. The reduced thickness of implant wires of the PVED correlated with improved neoendothelialization and reduced platelet adhesion to its surface in the animal study by Starke et al. 28 The present meta-analysis found an overall mid-term in-stent stenosis rate of 8.1% with only 3 asymptomatic stent occlusion. Cohen et al. reported in-stent stenosis rates of 39% for the first-generation PED and 38% for the Silk, and symptomatic stenosis accounted for 8%, indicating improved neoendothelial healing for the PVED. 29
Although the literature suggests improved safety and comparable efficacy of advanced FDs such as the PVED for the treatment of intracranial aneurysms, concerns about their clinical utility remain because intra- and inter-study comparisons are hampered by significant heterogeneity in baseline aneurysm characteristics, varying treatment experience and standards of care, and inconsistent reporting of adverse events. In this context, some authors report overall thromboembolic or ischemic events, while others focus on major complications, with varying definitions of these events. To gain further insight into the benefit of coated FDs, the Coating to Optimize Aneurysm Treatment in the New Flow Diverter Generation (Coating) trial was initiated, randomizing patients to receive either p64 HPC (phenox, Bochum, Germany) treatment with single antiplatelet therapy or uncoated p64 treatment with dual antiplatelet therapy. 30 The results of this study will provide further insight into the clinical benefit of flow diverter coatings and whether single antiplatelet therapy is a safe option for these devices. However, further comparative studies are needed to evaluate the full potential of coated flow diverter coatings for different devices, including the PVED.
Limitations
The major limitation of the included studies was their primarily retrospective, uncontrolled, nonrandomized design, lacking long-term outcomes beyond one year. Despite these limitations in study design, the authors demonstrated a commitment to high quality research. Angiographic outcome was core lab graded in only one study and graded by an independent interventionalist in another study, while the other studies used self-reported outcomes. This discrepancy introduces a potential power bias; however, the subanalysis showed comparable occlusion rates between independently assessed and self-reported data. The majority of patients enrolled had unruptured, small, anterior circulation saccular aneurysms, which are easier to treat and may introduce an ecological bias leading to more favorable reported outcomes than those seen in real-world clinical practice. The effect of dual antiplatelet therapy emerged as a major confounder for ischemic and hemorrhagic events, but details of regimen, compliance, and platelet function testing were poorly reported across trials. There was substantial heterogeneity in endovascular techniques, including adjunctive coiling, follow-up and imaging protocols, and definitions of success and complications.
Conclusions
The present meta-analysis demonstrated low procedural and early complications, potentially exceeding first-generation device rates and comparable to other advanced flow diverters. Mid-term angiographic results are comparable to previous flow diverter studies, but long-term outcomes have not been evaluated. To address the significant heterogeneity in baseline characteristics and outcome reporting in flow diverter studies, larger prospective studies with standardized antiplatelet regimens and consistent follow-up protocols and more comprehensive reporting of patient, aneurysm, and treatment characteristics are essential. In particular, comparative studies of coated and uncoated FDs are needed to determine the true clinical benefit of surface modifications. Such studies would facilitate the identification of aneurysm subsets that benefit most from these newer devices and the prediction of potential treatment failures.
Footnotes
Data statement: All data will be made available upon request in an anonymized manner.
SH received travel support by Medtronic (Dublin, Ireland). DZ is on the speaker's bureau of Philips (Amsterdam, the Netherlands) and lecturer for Amboss GmbH (Cologne, Germany). TL serves or previously served as proctor for MicroVention Inc./Sequent Medical (Aliso Viejo, CA, USA), CERUS Endovascular (Fremont, CA, USA), Phenox (Bochum, Germany), Stryker (Kalamazoo, MI, USA), and Medtronic (Dublin, Ireland). CK serves as consultant for Acandis GmbH (Pforzheim, Germany) and as proctor for MicroVention Inc./Sequent Medical (Aliso Viejo, CA, USA). The other authors declare that they have no competing interests.
Ethics approval: According to institutional guidelines, no ethics committee approval was required for this retrospective study. The manuscript does not contain any details that might disclose the identity of the patients.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Lukas Goertz https://orcid.org/0000-0002-2620-7611
Sophia Hohenstatt https://orcid.org/0000-0003-0951-3948
David Zopfs https://orcid.org/0000-0001-9978-7453
References
- 1.Awad AJ, Mascitelli JR, Haroun RR, et al. Endovascular management of fusiform aneurysms in the posterior circulation: the era of flow diversion. Neurosurg Focus 2017; 42: E14. [DOI] [PubMed] [Google Scholar]
- 2.Chalouhi N, Tjoumakaris S, Starke RM, et al. Comparison of flow diversion and coiling in large unruptured intracranial saccular aneurysms. Stroke 2013; 44: 2150–2154. [DOI] [PubMed] [Google Scholar]
- 3.Goertz L, Zopfs D, Kottlors J, et al. Treatment of intracranial aneurysms with large-diameter (≥ 5.5 mm) derivo embolization devices, with particular focus on 7 and 8 mm diameter devices. Interv Neuroradiol 2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kallmes DF, Brinjikji W, Cekirge S, et al. Safety and efficacy of the pipeline embolization device for treatment of intracranial aneurysms: a pooled analysis of 3 large studies. J Neurosurg 2017; 127: 775–780. [DOI] [PubMed] [Google Scholar]
- 5.Berger PB, Bhatt DL, Fuster V, et al. Bleeding complications with dual antiplatelet therapy among patients with stable vascular disease or risk factors for vascular disease: results from the clopidogrel for high atherothrombotic risk and ischemic stabilization, management, and avoidance (CHARISMA) trial. Circulation 2010; 121: 2575–2583. [DOI] [PubMed] [Google Scholar]
- 6.Monteiro A, Burke SM, Baig AA, et al. A systematic review and meta-analysis of the derivo embolization device: a novel surface-modified flow diverter for intracranial aneurysm treatment. J Neurointerv Surg 2022; 14: 1125–1129. [DOI] [PubMed] [Google Scholar]
- 7.White TG, Santhumayor BA, Turpin J, et al. Flow diverter surface modifications for aneurysm treatment: a review of the mechanisms and data behind existing technologies. Interv Neuroradiol 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vollherbst DF, Cekirge HS, Saatci I, et al. First clinical multicenter experience with the new pipeline vantage flow diverter. J Neurointerv Surg 2023; 15: 63–69. [DOI] [PubMed] [Google Scholar]
- 9.Wells G, Shea B, O’Connell D, et al. Newcastle-Ottawa quality assessment scale cohort studies. Ottawa, Ontario, Canada: University of Ottawa; 2014. [Google Scholar]
- 10.Döring K, Aburub A, Krauss JK, et al. Early clinical experience with the new generation pipeline vantage flow diverter in the treatment of unruptured saccular aneurysms using short-term dual antiplatelet therapy. Interv Neuroradiol 2023. [DOI] [PubMed] [Google Scholar]
- 11.Sciacca S, Bassiouny A, Mansoor N, et al. Early outcomes of the pipeline vantage flow diverter: a multicentre study. Clin Neuroradiol 2023; 33: 887–896. [DOI] [PubMed] [Google Scholar]
- 12.Booth TC, Bassiouny A, Lynch J, et al. Outcome study of the pipeline vantage embolization device (second version) in unruptured (and ruptured) aneurysms (PEDVU (R) study). J Neurointerv Surg 2023. DOI:https://doi.org/10.1136/jnis-2023-020754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goertz L, Pflaeging M, Gronemann C, et al. Aneurysm treatment with the pipeline vantage embolization device in retrospective evaluation: periprocedural results from the pipe-VADER study. World Neurosurg 2023; 183: e210–e217. [DOI] [PubMed] [Google Scholar]
- 14.de Villiers L, do Nascimento VC, Domitrovic L, et al. Vanguard study: initial experience with the new fourth generation pipeline vantage flow diverter (PVFD): 6-month results, technical and clinical considerations. J Neurointerv Surg 2024. DOI:https://doi.org/10.1136/jnis-2023-021182 [DOI] [PubMed] [Google Scholar]
- 15.Bhatia KD, Kortman H, Orru E, et al. Periprocedural complications of second-generation flow diverter treatment using pipeline flex for unruptured intracranial aneurysms: a systematic review and meta-analysis. J Neurointerv Surg 2019; 11: 817–824. [DOI] [PubMed] [Google Scholar]
- 16.Luo C, Jin L, Dong J, et al. Clinical outcomes of pipeline embolization devices with shield technology for treating intracranial aneurysms. Front Neurol 2022; 13: 971664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waqas M, Dossani RH, Alkhaldi M, et al. Flow redirection endoluminal device (FRED) for treatment of intracranial aneurysms: a systematic review. Interv Neuroradiol 2022; 28: 347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Florez WA, Garcia-Ballestas E, Quiñones-Ossa GA, et al. Silk® flow diverter device for intracranial aneurysm treatment: a systematic review and meta-analysis. Neurointervention 2021; 16: 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y-L, Roalfe A, Chu E-L, et al. Outcome of flow diverters with surface modifications in treatment of cerebral aneurysms: systematic review and meta-analysis. AJNR Am J Neuroradiol 2021; 42: 327–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goertz L, Styczen H, Siebert E, et al. FRED X flow diverter for the treatment of intracranial aneurysms: two-center experience and mini-review of the literature. Interv Neuroradiol 2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kallmes DF, Hanel R, Lopes D, et al. International retrospective study of the pipeline embolization device: a multicenter aneurysm treatment study. AJNR Am J Neuroradiol 2015;36:108–115. [published Online First: 2014/10/31] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brinjikji W, Lanzino G, Cloft HJ, et al. Risk factors for ischemic complications following pipeline embolization device treatment of intracranial aneurysms: results from the IntrePED study. AJNR Am J Neuroradiol 2016;37:1673–1678. [published Online First: 2016/04/23] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jabbour P, Chalouhi N, Tjoumakaris S, et al. The pipeline embolization device: learning curve and predictors of complications and aneurysm obliteration. Neurosurgery 2013; 73: 113–120. [DOI] [PubMed] [Google Scholar]
- 24.Tan LA, Keigher KM, Munich SA, et al. Thromboembolic complications with pipeline embolization device placement: impact of procedure time, number of stents and pre-procedure P2Y12 reaction unit (PRU) value. J Neurointerv Surg 2015; 7: 217–221. [DOI] [PubMed] [Google Scholar]
- 25.Kallmes DF, Brinjikji W, Boccardi E, et al. Aneurysm study of pipeline in an observational registry (ASPIRe). Interv Neurol 2016; 5: 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lylyk I, Scrivano E, Lundquist J, et al. Pipeline embolization devices for the treatment of intracranial aneurysms, single-center registry: long-term angiographic and clinical outcomes from 1000 aneurysms. Neurosurgery 2021; 89: 443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maragkos GA, Dmytriw AA, Salem MM, et al. Overview of different flow diverters and flow dynamics. Neurosurgery 2020; 86: S21–S34. [DOI] [PubMed] [Google Scholar]
- 28.Starke RM, Thompson J, Pagani A, et al. Preclinical safety and efficacy evaluation of the pipeline vantage embolization device with shield technology. J Neurointerv Surg 2020; 12: 981–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen JE, Gomori JM, Moscovici S, et al. Delayed complications after flow-diverter stenting: reactive in-stent stenosis and creeping stents. J Clin Neurosci 2014; 21: 1116–1122. [DOI] [PubMed] [Google Scholar]
- 30.Pierot L, Lamin S, Barreau X, et al. Coating (coating to optimize aneurysm treatment in the new flow diverter generation) study. The first randomized controlled trial evaluating a coated flow diverter (p64 MW HPC): study design. J Neurointerv Surg 2022; 15(7): 684–688. [DOI] [PMC free article] [PubMed] [Google Scholar]