Abstract
Background
Recognition of neurovascular variants is crucial for safe endovascular and neurosurgical interventions. We aim to review and highlight various uncommon neurovascular variants and anomalies with a discussion of their relevant embryology and pathology.
Methods
A retrospective review of a prospectively maintained neurovascular database was performed to identify uncommon neurovascular variants and anomalies. A pictorial review of these neurovascular findings is provided along with relevant embryological development, clinical significance, and potential pathological associations.
Results
A pictorial review of selected neurovascular variants and anomalies is presented. These entities, divided between intra- and extra-cranial findings, include infra-optic origin of the anterior cerebral artery, meningo-ophtalmic artery, duplicated posterior cerebral artery, duplicate middle cerebral artery (MCA), MCA fenestration, twig-like MCA, pure arterial malformation, corkscrew basilar artery, persistent hypoglossal artery, persistent trigeminal artery and its variants, direct branches from the common carotid and cervical internal carotid arteries (ICA) (ascending pharyngeal artery from the ICA, thyroidal arteries from the CCA/brachiocephalic, arteria thyroidea ima), and extra-cranial carotid fenestration. The angiographic findings of these entities are presented with relevant 3D reconstruction and multimodal cross-sectional imaging correlation when available.
Conclusions
This pictorial review highlights uncommon neurovascular variants and anomalies that neuroradiologists, interventionalists, and neurosurgeons should be aware of for accurate diagnosis and safe interventions.
Keywords: Neurovascular variants, variant anatomy, angiography, neurointervention, arterial variants
Introduction
Knowledge of neurovascular variants and understanding of their clinical significance is crucial when interpreting head and neck imaging and for ensuring the safety of neuroendovascular and neurosurgical interventions. While the majority of these variants are asymptomatic, certain ones have been associated with pathological processes, including aneurysm formation, intracranial hemorrhage, and ischemic changes, as well as potential implications during endovascular or surgical procedures. Additionally, some described entities may not solely represent congenital variants but could also result from vascular remodeling due to congenital or acquired insults. Therefore, it is essential to accurately diagnose these entities to ensure the safety of endovascular and neurosurgical procedures.
Due to their rarity and often incidental discovery, many of these variants are underreported and, consequently, understudied. In this context, we present a series of uncommon neurovascular variants and anomalies that may be encountered, along with their embryological development and clinical relevance.
Discussion
- A Intracranial:
- Infra-optic anterior cerebral artery
- Meningophthalmic artery
- Duplicated posterior cerebral artery
- Duplicated middle cerebral artery
- Middle cerebral artery fenestration
- Twig-like middle cerebral artery
- Pure Arterial Malformation
- Corkscrew basilar artery
- Persistent trigeminal artery and its variant
- Persistent hypoglossal artery
- Aberrant carotid artery
- B Extracranial:
- Direct branches from the common carotid and cervical internal carotid arteries.
- Ascending pharyngeal artery from the internal carotid artery
- Thyroidal arteries from the common carotid artery
- Arteria thyroidea ima
Intracranial variants
Infra-optic anterior cerebral artery
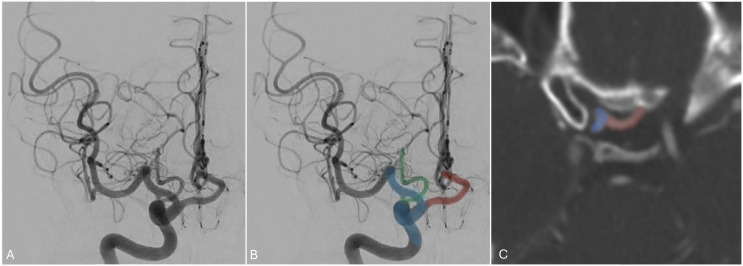
The infra-optic anterior cerebral artery (ACA) is an exceedingly rare neurovascular variant characterized by a horizontal position of the A1 segment arising from the cavernous internal carotid artery (ICA) and then coursing below the optic nerve with a subsequent upward course toward the anterior communicating artery (AComm), as shown in Figure 1. This variant can be associated with AComm aneurysms, so neurointerventionalists and neurosurgeons should also be aware of this variant when navigating their tools intracranially1,2 and before planning endovascular or surgical treatments in the paraclinoid or AComm segment regions.
Figure 1.
Angiogram of the right internal carotid artery and axial CT angiogram demonstrating an infra-optic anterior cerebral artery (red) arising from the anterior genu of the ICA (blue). A posterior communicating artery (blue) is noted arising from its expected location of the supraclinoid ICA.
Embryologically, the ophthalmic artery (OphA) forms as a joining of two separate arteries during development: a ventral ophthalmic artery (VOA), which arises from the ACA, and a dorsal ophthalmic artery (DOA) that arises from the cavernous segment of the internal carotid artery (ICA). This primitive ophthalmic artery becomes definitive when the DOA and VOA regress during development. 3 Any failure of regression may lead to either the ACA arising from the DOA (therefore from the cavernous ICA) which is known as the infra-optic ACA, or the definitive ophthalmic artery arising from the ACA (also termed the VOA) variant. 4
Meningo-ophthalmic artery
The ophthalmic artery (OphA), typically the first intradural branch of the ICA, comes off the ICA at the level of the anterior clinoid process and travels anteriorly through the optic canal inferolateral to the optic nerve. It consists of orbital and ophthalmic branches that arise during development from the supraorbital division of the stapedial artery and the primitive dorsal and ventral ophthalmic arteries (DOA and VOA), respectively. 5 Orbital branches include the lacrimal arteries, supraorbital artery, ethmoid arteries, medial palpebral arteries, and the terminal branches (the frontal artery and the dorsal nasal artery). Ophthalmic branches include the central retinal artery that supplies the inner layer of the retina and the posterior ciliary arteries that supply the posterior uveal tract.
There is considerable variation in OphA anatomy, so familiarity with the embryological mechanisms of the artery and its variants as well as their clinical implications is essential for safe and successful intervention. A common anatomical variant of the OphA is a middle meningeal artery (MMA) origin rather than an ICA one. 6 This is known as the meningo-ophthalmic artery. Rather than coursing through the optic canal like the OphA, the meningo-ophthalmic artery travels through the superior orbital fissure (SOF) or a foramen in the greater sphenoid wing to reach the orbit. 5 In 2.4% of cases, the OphA has a double origin from the ICA and the external carotid artery (ECA) and in 1.2% of cases has a single origin from the MMA 7 (Figure 2).
Figure 2.
A. Angiogram of the right common carotid artery demonstrating no ophthalmic artery from the ICA (blue). The OphA (red) originates from the MMA (green). This is better seen in the selective angiogram of the external carotid artery (C). The choroidal artery blush (black arrow on C) is noted from the selective external carotid artery injection.
Embryologically, the meningo-ophthalmic artery occurs when the supraorbital division of the embryological stapedial artery fully assimilates the primitive OphA during development. 8 The stapedial artery forms from the fusion of the vascular residua of the first branchial arch with the hyoid artery related to the second branchial arch in the 15 mm embryo. It then divides into the maxillofacial and supraorbital divisions in the 20 mm embryo. The latter gives rise to vessels that supply the orbit as well as the intracranial segment of the MMA. At the 20 mm stage, anastomoses between the supraorbital division and the primitive OphA begin to form. Normally, the DOA assimilates the supraorbital division, and the retroorbital branches involute and resorb into the intracranial MMA. In the case of a meningo-ophthalmic artery, the supraorbital division of the stapedial artery assimilates the primitive ophthalmic artery and the retroorbital branches fail to involute. Instead, the proximal portion of the primitive dorsal OphA involutes at its origin at the ICA. 8
On angiography, there will be no visible choroidal blush on ICA injection but choroidal blush will be visible on ECA injection supplied by the meningo-ophhtalmic artery. If this variant is not properly recognized prior to MMA embolization, iatrogenic blindness can occur as the central retinal artery and ciliary arteries receive blood exclusively from MMA 9 (Figure 2). Additionally, it is important to identify any ECA-ICA anastomoses to avoid embolic agent reflux into the OphA from ECA. 10
Duplication of the posterior cerebral artery or “true fetal PCA”
True fetal posterior cerebral artery (PCA), also called “Duplication of the PCA” or “double PComm”, is a rare variant in which the fetal supply of the telencephalon via the anterior choroidal artery (AChoA) persists into adulthood giving the appearance of a duplicated PCA. 11 One PCA consists of a prominent posterior communicating artery (also known as classical “fetal PCA”) and the other is the enlarged AChoA 12 as demonstrated in Figure 3. The reported prevalence of this variant is 1–2%. 13
Figure 3.
Angiogram of the right ICA in the lateral projection demonstrating a true fetal PCA, or “duplicated PCA” (red and green).
True fetal PCA is often confused with “fetal PCA (FPCA)”, the most common class of PCA variants whose estimated prevalence is between 20% and 30%. 11 In contrast to true fetal PCA, FPCA is characterized by a hypoplastic or absent ipsilateral P1 PCA segment and a P2 segment that appears to be a continuation of the posterior communicating artery (PComm). Embryologically, this can be explained by the derivation of the posterior cerebral vascular territory from the caudal trunk of the primitive ICA. However, the so-called “fetal PCA” is a misnomer as it does not truly represent a true fetal origin of the PCA; it is better characterized as an embryological configuration or persistent carotid-basilar anastomosis.11,14 During normal development, the AChoA supplies the telencephalon before regressing and becoming annexed by the developing PCA and MCA. If the AChoA telencephalic supply persists without regressing, it can result in this variant where it maintains the blood supply of the telencephalon, usually the inferior temporo-occipital, and medial occipital vascular territories.
Concurrent true fetal PCA and contralateral FPCA have been reported in the setting of a paradoxical embolic stroke from a stenosed ICA. 11 True fetal PCA alone has also been associated with paradoxical embolic stroke in PCA territory from the ICA, and both variants have been associated with simultaneous PCA-ICA infarcts.15–17
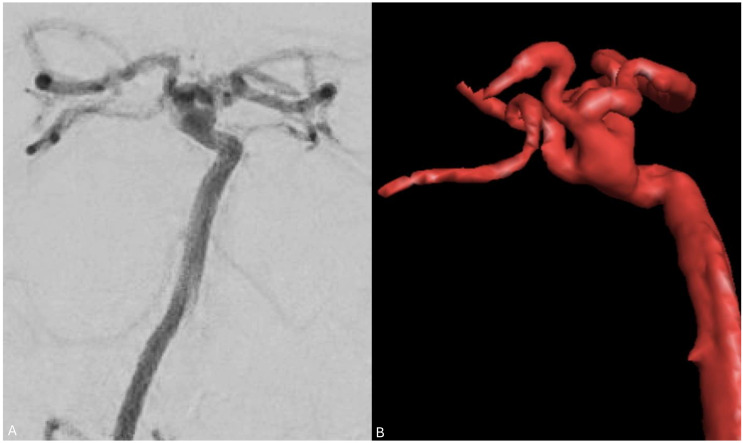
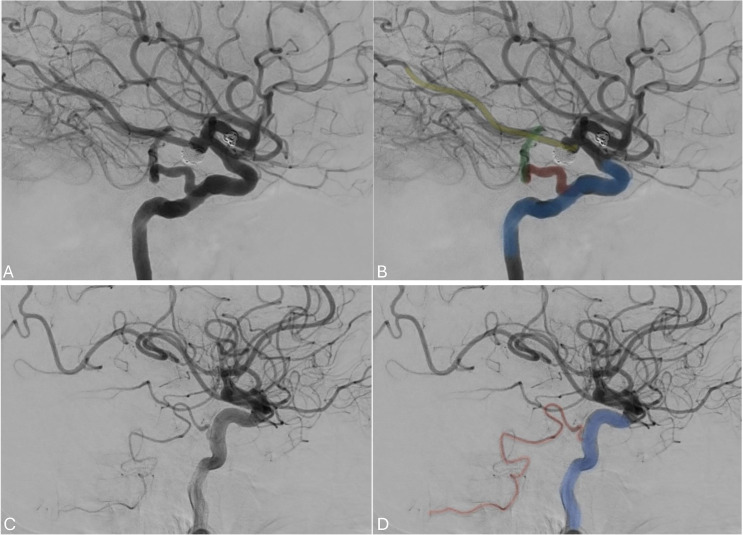
Duplicated middle cerebral artery
A duplicated middle cerebral artery (MCA) is a rare anatomical variation, occurring in less than 1% of cases, where two MCAs originate from the distal end of the ICA. 18 The duplicated MCA typically follows a parallel course and exhibits a similar diameter to the other branch of the MCA, resembling an early bifurcation of the MCA as shown in Figure 4. The best way to understand this variant is by considering the two branches as the two MCA divisions arising separately from the ICA without an intervening M1 trunk. 19 Awareness of the duplicated MCA variation is important when undergoing endovascular treatment for large vessel occlusion.
Figure 4.
(a) angiogram in the anteroposterior projection showing the ICA (blue) giving rise to the left ACA (red) and the two left MCA branches (superior division in green and inferior division in yellow). (b) A 3D reconstruction of the angiogram.
Middle cerebral artery fenestration
Fenestrations of the MCA are infrequent anatomical variations, often incidentally discovered through cross-sectional imaging or cerebral angiography (Figure 5). They manifest as a focal division of the vessel's lumen, resulting in two separate lumens, each lined with its own endothelial wall.20,21
Figure 5.
Anteroposterior and 3D reconstruction of an angiogram showing a right MCA fenestration.
The prevalence of MCA fenestrations falls within the range of 0.1% to 4.4%. 22 While the precise mechanism behind MCA fenestration remains poorly understood, potential factors contributing to this include the presence of an early branching temporo-polar artery or a prominent lenticulo-striate artery, which can hinder the fusion of the MCA twig vessels into a single trunk. 23
Although MCA fenestrations are generally considered benign, numerous reports have linked them to vascular anomalies such as aneurysms and hemorrhagic compilations, largely due to the fragility of the vascular wall at the site of fenestration. 24 Additionally, they can lead to ischemia because of the turbulence in blood flow and possible stagnation caused by the presence of these fenestrations.22,25
From an endovascular perspective, it is crucial for interventionists to be cognizant of this variant, especially since there is a heightened risk of vessel injury when performing navigating endovascular tools through the fenestration limbs. This risk is particularly pronounced in situations when immediate endovascular management is required for acute large vessel occlusion strokes but prior knowledge of the anatomical trajectory is not necessarily available. 26
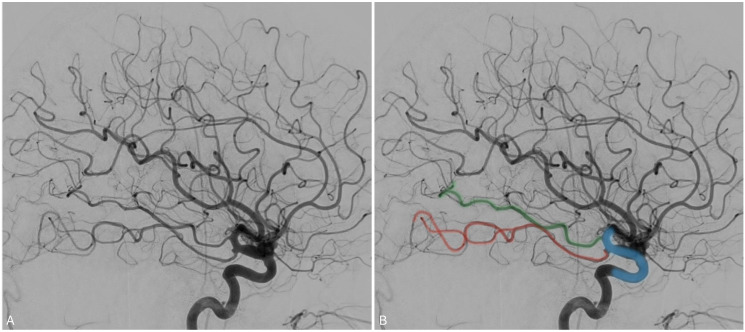
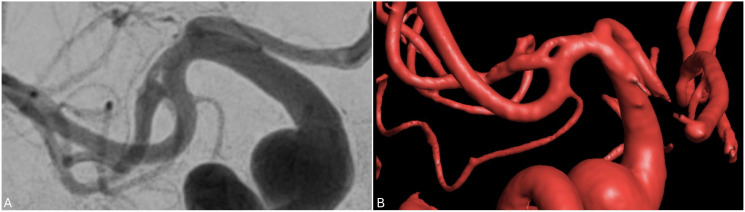
Twig-like middle cerebral artery
Twig-like middle cerebral artery (T-MCA) is a rare vascular anomaly characterized by the replacement of the M1 segment by a plexiform network of horizontal small vessels, known as “twigs”, that run parallel to the M1 axis 27 (Figure 6). It may be found incidentally or present with symptoms secondary to cerebral ischemia, hemorrhage, or aneurysm formation.28,29
Figure 6.
Angiogram in oblique orientation demonstrating the plexiform vessels constituting a left T-MCA with delay filling of the MCA territory compared to the ACA territory.
According to pooled data from case reports of T-MCAs, they are unilateral, occur almost equally in men and women, and are symptomatic in 86% of reported cases. Headache is the most common symptom, followed by weakness contralateral to the defect, and loss of consciousness. 27 Patients with T-MCA can exhibit intracranial hemorrhage, which has been found in 51% of cases. 27 Aneurysms are common among patients with T-MCA. Ischemic infarcts were observed in over one-fifth of cases and additional cerebrovascular anomalies were observed in most cases. 30 Focal and lacunar infarcts occur in 33% of T-MCA cases. 30 Despite the risk of stroke, approximately half of cases are managed conservatively with careful monitoring and close follow-up. Surgery with ECA-MCA bypass or synangioses has been reported whereas endovascular treatment is limited to embolizing concomitant aneurysms. 27
T-MCAs can be misdiagnosed as moyamoya changes, atherosclerotic steno-occlusive disorders of the MCA. The underlying mechanism of T-MCA is likely congenital embryological lack of fusion of the twigs forming the MCA. Defining these lesions as nonprogressive unilateral lesions involving the proximal M1 segment with normal ICA morphology, Ota and Komiyama (2021) have suggested that an embryological explanation is unlikely. This is because the territories supplied by T-MCAs form normally. Therefore, they may arise as collaterals secondary to in utero ischemic insult, such as steno-occlusive disease of the proximal MCA. 31
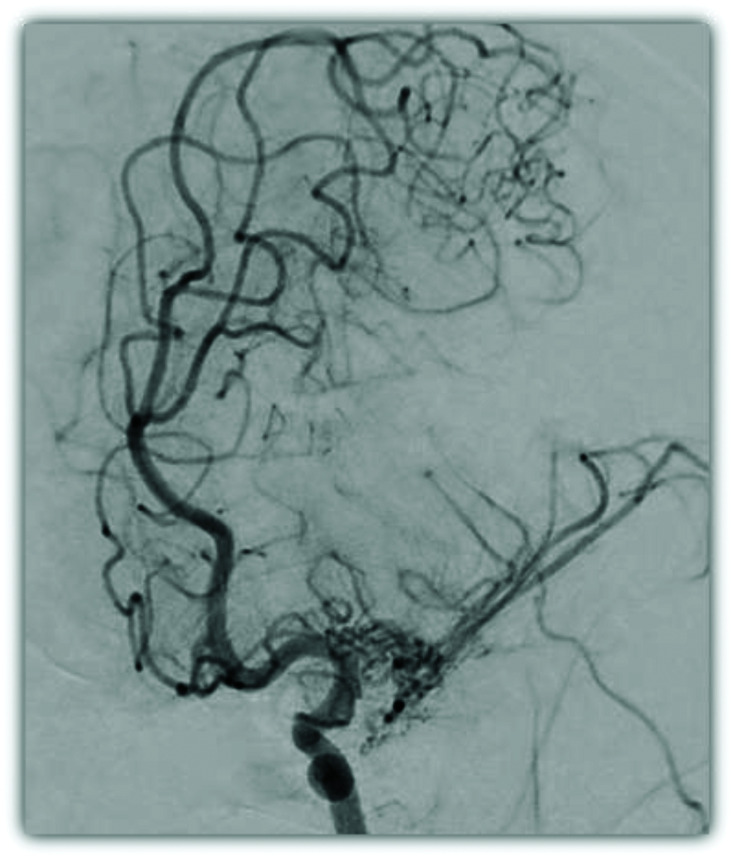
Pure arterial malformation
Pure arterial malformations (PAMs) are defined as dilated, overlapping, and tortuous arteries with the coil-like or arterial loops appearance without any associated arteriovenous shunts 32 (Figure 7). They are often incidental findings in patients undergoing imaging for other reasons. When symptoms arise, headache is most common. 32 In a large cohort by Brinjikji et al., PAMs involved the ACAs in 24% of cases, PComm/PCA in 33.3%, MCAs in 16.6%, and in one case each (8.3% each), the superior cerebellar artery (SCA), basilar artery (BA), and posterior inferior cerebellar artery (PICA). 33 The mean maximum diameter recorded was 7.2 mm ± 5.0 mm with a range of 3–16 mm. 33 The literature does not suggest any obvious site of predilection for PAMs. 32 Though PAMs can be associated with focal aneurysms and/or calcifications, they typically follow a benign natural history and do not require intervention as they rarely grow or change on follow-up imaging. Therefore, management with serial follow-up with MR angiography (MRA) is recommended. 33
Figure 7.
Angiogram of the vertebral artery with 3D volume rendering reconstruction demonstrating a PAM of the distal basilar artery with coiled and tortuous basilar tip involving the PCA and SCA branches.
Certain features of PAM architecture have been associated with their locations. For instance, ACA lesions tend to be ectatic, moderately tortuous, and loosely coiled whereas MCA or PCA/PComm lesions tend to be tightly coiled with superimposed calcifications and aneurysms. SCA and PICA lesions appear tightly coiled but lack significant ectasia or aneurysms. The PAM described here involves a tortuous and coiled basilar tip with its PCA and SCA branches (Figure 7).
Several mechanisms have been proposed for the etiology of PAMs. They include congenital defects or developmental insults resulting in arterial dysplasia,34,35 insults that occur later in life (i.e., viral infection) that affect a vulnerable arterial segment, and a chronic healed dissection. In many cases, PAMs have been shown to occur in regions of aberrant developmental processes, such as cortical dysplasia, hemimegaloencephaly, and agenesis of the corpus callosum. 32 In these cases, PAMs may arise from or alongside the abnormally developed cortical regions they supply. 36 Varicella zoster infection has been associated with supraclinoid ICA or MCA PAMs given the virus’ tropism for these vessels. 37 Arterial dissections may heal in such a way as to produce fusiform dilations, stenosis, and complex aneurysms. 38 The most critical part of recognizing a PAM is that these appear to be benign finding, asymptomatic and stable, without the need of treatment.
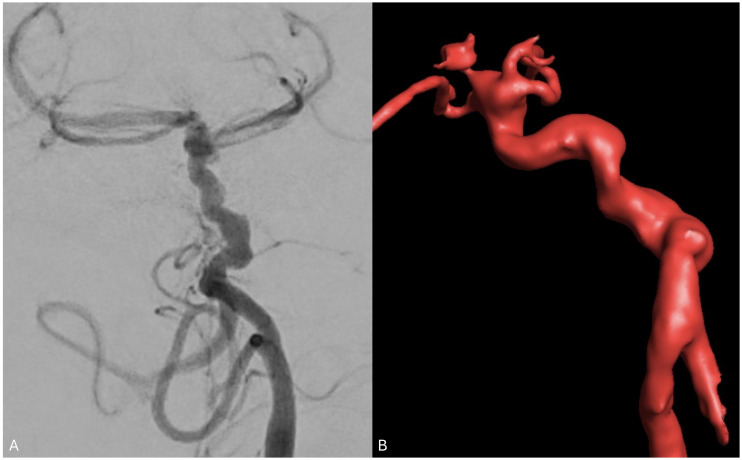
Corkscrew basilar artery
A corkscrew basilar artery (CBA) is defined as an abnormally tortuous coursing of the BA simulating a corkscrew (Figure 8). CBAs are rare with few cases reported in the literature. Moser et al. (2007) describe an incidentally found CBA in a 6-year-old after a workup for head trauma revealing tortuosity with many distal angular turns of the basilar artery. The defect never invaginated into the pons and contained tributaries circumferentially surrounding the brainstem bilaterally. No venous anomalies, aneurysms, or abnormal branching patterns accompanied the defect. 39
Figure 8.
Angiogram of the vertebral artery with 3D volume rendering reconstruction demonstrating tortuosity with many distal angular turns of the basilar artery consisting with CBA. This patient presented with multiple intracranial anterior circulation aneurysms.
Nussbaum et al. (2019) revealed a similar incidentally found CBA after a 58-year-old woman presented with a near-syncopal episode after exercising. The CBA was determined to be unrelated to her symptoms given no evidence of ischemic disease or other vascular abnormalities. 40
Alternatively, Lim and Chung (2012) describe a CBA associated with agenesis of the left ICA and absence of the left carotid canal found on CT as well as a ruptured fusiform aneurysm resulting in subarachnoid hemorrhage (SAH).41,42 In Figure 8, we demonstrate an incidentally found CBA in a patient who also presented with aneurysmal SAH found to have both an AComm and PComm aneurysm not directly related to the CBA.
The development of the BA occurs via two mechanisms, the longitudinal and the axial processes. The longitudinal process reflects the contribution of the spinal vascular anatomy and involves the fusion of two paired ventral arteries in the craniocaudal direction. The axial process reflects contribution from the cerebral circulation and involves fusion of the caudal ICA to produce the distal BA. 43 Before and after the longitudinal neural arteries fuse, blood in the BA flows in the rostrocaudal direction through transient vessels that communicate with the ICA, reversing direction after the formation of the Circle of Willis. 39 The quality of the fusion process and the relative contributions from transient segmental artery remnants during development likely influence the formation of anomalies like CBA. Overexpression of an angiogenic growth factor has also been hypothesized to contribute to CBA formation. 41
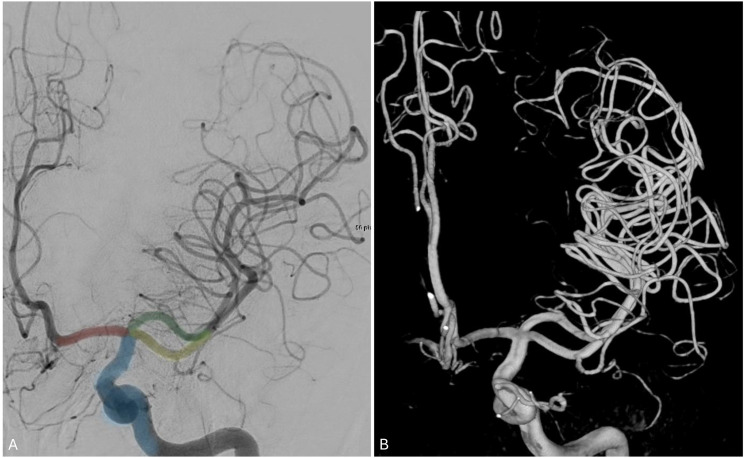
Persistent trigeminal artery and its spectrum
Persistent trigeminal artery (PTA) is a congenital carotid-basilar anastomosis between the cavernous ICA and the basilar artery (Figure 9(a)). PTA variant (PTAV) is a congenital anastomosis between the ICA and the cerebellar artery with no interposing BA segment. 44 PTAs originate in the cavernous ICA, run along the trigeminal nerve, enter the posterior fossa through Meckel's cave or the isolated dural foramen, and directly supply the cerebellum. This can be appreciated in our incidentally found PTAV (Figure 9(b)), where it can be seen supplying the posterior inferior cerebellar artery (PICA). PTAVs occur in 0.1%–0.3% of patients.
Figure 9.
(a) lateral angiogram of the ICA (blue) showing a PTA (red) anastomosing the ICA to the basilar artery. Coil-embolized aneurysms can be seen in the posterior and anterior communicating arteries. (b) Lateral angiogram of the ICA (blue) showing a PTA variant contributing to the anterior inferior cerebellar territory as cerebellar artery (red).
PTA and its variants have been associated with multiple pathologies, such as aneurysms located in the circle of Willis, stenosis, and other variants such as fenestration.45,46 The contribution of PTA to cases of trigeminal neuralgia has also been reported given its proximity to the root entry zone of the trigeminal nerve, which has been identified during microvascular decompressions. 47 It is, therefore, important for neurosurgeons to identify this variant as manipulation of the vessel during parasellar surgery and percutaneous Gasserian ganglion procedures can result in hemorrhage or ischemia. 45
During development, the trigeminal artery arises from the primitive ICA when the embryo is six weeks old. It normally anastomoses with the BA and then involutes after the PComm develops but may persist. 48 The development of PTA likely follows a similar mechanism. Incomplete fusion of the longitudinal neural arteries likely contributes as well. 49
Persistent hypoglossal artery
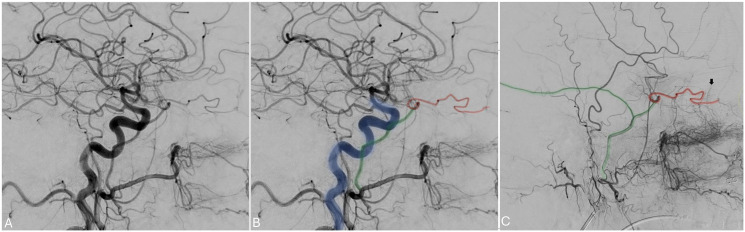
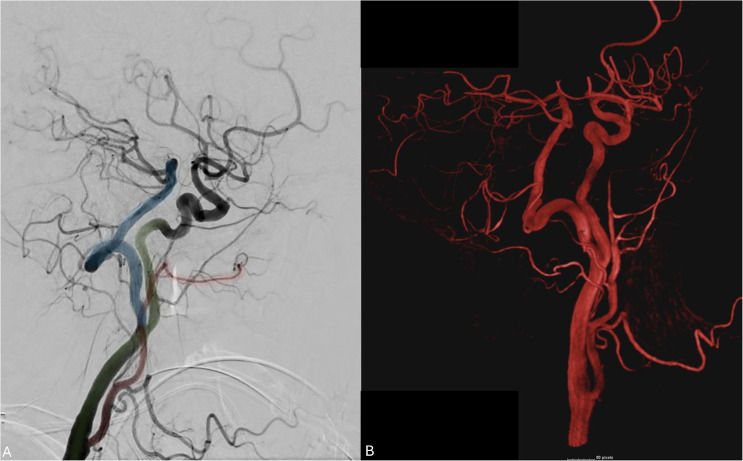
The persistent hypoglossal artery (PHA) is a rare carotid-basilar anastomosis typically arising from the ICA between the C1-C3 levels with reported radiographic incidence of 0.03–0.09%. 50 PHA traverses the hypoglossal canal to serve as the primary blood supply of the vertebrobasilar system, typically associated with hypoplastic vertebral arteries (VAs). This can be appreciated in Figure 10(a) and (b) where the PHA (Figure 10(a); blue) serves as an anastomosis between the right ICA (Figure 10(a); green) and the basilar system. The ipsilateral VA is hypoplastic (Figure 10(a); red).
Figure 10.
Lateral common carotid angiogram and 3D angiogram demonstrating a PHA (blue) persistent anastomosis between the ICA (green) and the BA. The external carotid artery is noted in red.
Though usually an incidental finding, PHA has been associated with increased risk of aneurysm formation, cerebral ischemia, and simultaneous anterior and posterior circulation emboli. 51 The mechanism by which PHA contributes to these pathologies may be related to developmental aberrations, changes in pressure dynamics resulting from the PHA being the only supply to the posterior circulation, or a combination of the two.50,51 Given the PHA courses through the hypoglossal canal, impingement of the hypoglossal nerve and adjacent glossopharyngeal nerve may occur and has been documented. 52
PHA aneurysms are thought to arise from abnormal congenital processes associated with PHA formation rather than from acquired structural weakness of the PHA wall. 53 Specifically, the persistence of immature endothelial cells in the intima of the embryonic artery and congenitally impaired apoptosis may explain the propensity for aneurysm formation. 54 Endovascular coil embolization can successfully treat these entities 54 with one case report describing success with stent-assisted embolization to treat a wide-necked PHA aneurysm causing pulsatile tinnitus. 55
During development, the carotid system supplies the forebrain and is the main contributor to the parallel longitudinal neural arteries via the caudal division as well as multiple anastomoses such as the trigeminal, hypoglossal and proatlantal arteries. The neural arteries then fuse to become the BA. Later, these anastomoses are obliterated with the hypoglossal artery being the second to regress. Failure of regression results in persistent carotid-vertebrobasilar anastomoses with PTA being most common and PHA being second most common. 56 Angiographically, a PHA can be easily mistaken for a type I proatlantal intersegmental artery, but the latter enters the cranium through the foramen magnum while the former enters via the hypoglossal canal which is best appreciated on CTA. 55
Aberrant internal carotid artery
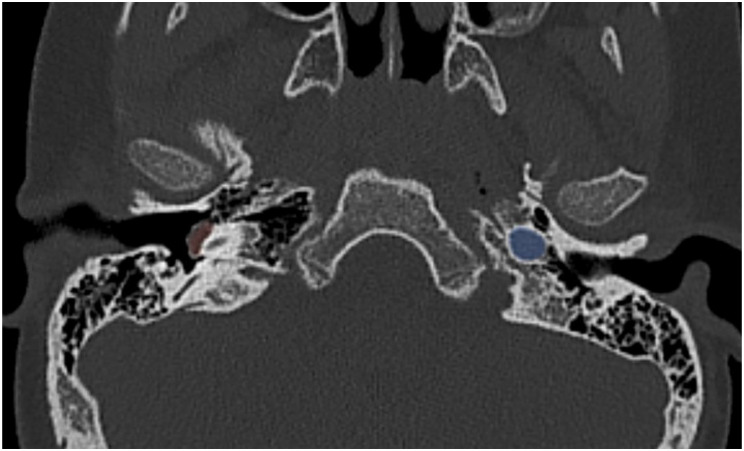
An aberrant internal carotid artery is an ICA variant that results from the involution of the cervical portion (first embryonic segment) of the ICA with subsequent enlargement of a collateral pathway that courses through the middle ear and passes lateral to the cochlear promontory. CT is considered the best way to characterize these lesions with the following classic findings: the ICA enters the floor of the middle ear through an enlarged inferior tympanic canal within the caroticojugular spine and then turns anteriorly to cross the cochlear promontory to continue onto the horizontal portion of the petrous segment. 44 The normal bone covering the artery is absent and the vertical part of the carotid canal may be absent or hypoplastic.44,57 On angiography, aberrant ICA appears to extend laterally beyond the vestibular line of Lapayowker, which is the vertical line drawn along the most lateral aspect of the vestibular apparatus. 58 Our patient presented with right-sided pulsatile tinnitus and was found to have an aberrant ICA on CT scan (Figure 11).
Figure 11.
An axial CT scan showing a right-sided aberrant ICA (highlighted in red) protruding in the tympanic fossa. The left ICA is highlighted in blue for comparison.
Although most cases of aberrant ICAs are asymptomatic, 57 they may present with pulsatile tinnitus, conductive hearing loss, and an anteroinferior red mass in the retrotympanic space on otoscopy. 57 It is crucial to identify this anomaly because of possible hemorrhage, stroke, and/or death that could ensue from middle ear surgery or biopsies. Clinically, There have been cases where aberrant ICA has been mistaken for a glomus tumor with disastrous results. 59 Other pathologies with similar presentation as aberrant ICA include dehiscent jugular bulb, cholesterol granuloma, and petrous carotid aneurysm. 60
Developmentally, aberrant ICA represents a collateral pathway that results from agenesis of the first embryonic segment that gives rise to the cervical ICA. This, in turn, results in the persistence and enlargement of the anastomosis between the inferior tympanic branch of the ascending pharyngeal artery and the caroticotympanic artery of the petrous ICA as a compensatory pathway. Non-developmental mechanisms have also been proposed but are less common. They include trauma, chronic ear infection, thrombophlebitis, and chronic granulomatous disease. 61
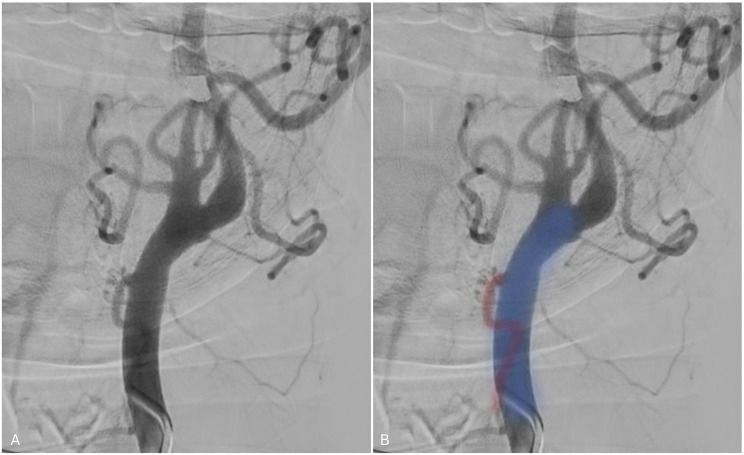
Ascending pharyngeal artery arising from the internal carotid artery
The ascending pharyngeal artery is a small vessel that usually arises from the medial wall of the proximal ECA trunk, but may occasionally arise from the proximal occipital artery, ICA, or ascending cervical artery. It supplies multiple cranial nerves and contributes to multiple anastomoses between anterior and posterior cerebral circulations, dividing into two major trunks: the pharyngeal and neuromeningeal trunks. The former supplies the middle and superior pharyngeal constrictor muscles and stylopharyngeus while the latter supplies cranial nerves VI, IX, X, XI, and XII as well as the posterior fossa meninges. 62
Anomalous origin from the ICA is a rare phenomenon with only one angiographic depiction of an ICA origin ascending pharyngeal artery found in the literature. 62 In our patient (Figure 12), only the pharyngeal trunk of the ascending pharyngeal artery (in red) originated from the cervical ICA (in blue).
Figure 12.
Right ICA angiogram demonstrating the pharyngeal branch of the ascending pharyngeal artery (red) branching off the ICA (blue).
Many pathologies involve the ascending pharyngeal artery making it relevant to the neurointerventionist. Arteriovenous fistulae, meningiomas, glomus jugulare tumors, and adenomatous tumors of the middle ear are commonly fed by the ascending pharyngeal artery. 62 Radiation for high-grade head and neck tumors can cause acute carotid blowout syndrome or pseudoaneurysm involving the ascending pharyngeal artery and/or cervical ICA requiring emergent endovascular embolization. 63 Superselective chemotherapy infusion for cancers involving the palate requires isolation of the ascending pharyngeal artery. 64 Ascending pharyngeal artery can also be accidentally catheterized instead of ICA during endovascular procedures. It is also crucial for the closure of cleft palates following surgery 65 and for the healing of Le Fort fractures. 66 For all these reasons, knowledge of rare variant ascending pharyngeal anatomy is crucial for safe endovascular intervention.
The precise mechanism of this variant is currently unknown. During embryogenesis, the aortic sac sends pairs of branches to each pharyngeal arch. As each new arch develops, the preceding ones regress. The arches undergo rearrangement until they form structures that will supply specific tissues. The third aortic arch gives rise to the common carotid artery (CCA), the ECA, and the proximal ICA. 67 It is therefore possible that an ascending pharyngeal artery originating from the ICA arises due to a combination of the following factors: The proximity of the ECA and ICA origin points on the third aortic arch, the fact that the ascending pharyngeal is usually the second most proximal branch of the ECA, and errors in the rearrangement process.
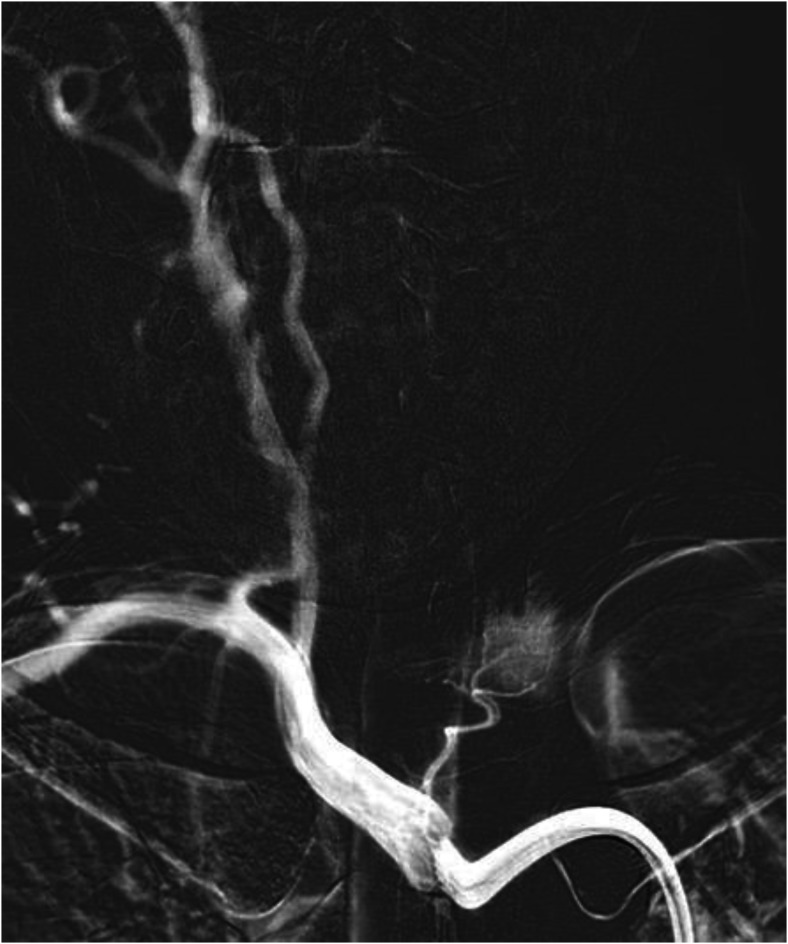
Thyroid artery arising from the common carotid artery
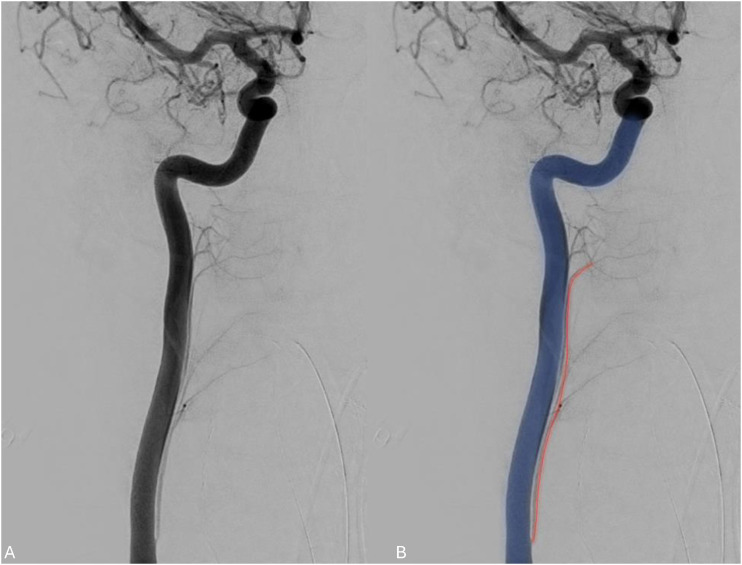
The superior thyroid artery (STA) and the inferior thyroid artery (ITA) are the major contributors to thyroid gland perfusion. They usually arise from the ECA and thyrocervical trunk, respectively. The STA arises from the ECA in 88.33% of cases, the carotid bifurcation in 8.33% of cases, and the CCA in 3.33% of cases according to an anatomic study of sixty cadavers. 68 Results pooled from multiple cadaveric studies found a range of 3.33%-40% for STAs arising from the CCA. 68 It is important to know about this variant for safe endovascular intervention. The aberrant superior thyroidal artery can also be accidentally catheterized instead of CCA during endovascular and surgical procedures (Figure 13). In STAs originating from the CCA proximal to the bifurcation, the external laryngeal nerve courses ventral and parallel to the anomalous STA. It is imperative to know this before thyroidectomy to prevent iatrogenic injury.
Figure 13.
Angiogram demonstrating superior thyroidal artery (red) arising from the CCA (blue) proximal to the carotid bifurcation.
During development, the CCA arises from the third aortic arch and the ECAs arise from the right and left horns of the aortic sac. 69 The developmental mechanism underlying STA origin from CCA is currently unexplored. The clinical importance of understanding this STA variant has to do with its proximity to other structures, such as the superior laryngeal nerve and the carotid bifurcation (CB). In STAs originating from the CCA, the distance between the STA origin and CB varied from 0.1–2.1 cm whereas this distance was 0.1–1.5 cm in ECA origin STAs. 37
Arteria thyroidea ima
The thyroid ima artery is an embryonic artery that may remain patent after birth. The artery is only present in approximately 3–10% of the population. 70 It mostly arises from the brachiocephalic trunk, but may also originate from the aortic arch, the right common carotid, and the subclavian arteries among others. It ascends in the superior mediastinum in front of the trachea to the lower part of the thyroid gland, thymus, or parathyroid glands. Because of its location, it holds significant surgical importance during procedures such as tracheostomy, sternotomy, and thyroidectomy. 71 Its inadvertent damage during surgery can result in severe hemorrhage and significant blood loss. Moreover, it is essential to be aware of its presence during endovascular procedures to prevent unintentional catheterization, which could result in dissection or rupture if large catheters are advanced without prior angiography or roadmap guidance (Figure 14).
Figure 14.
Angiogram of the brachiocephalic artery demonstrating a thyroid ima artery arising from the proximal brachiocephalic trunk.
The thyroid ima artery is thought to arise from disruption between thyroid gland morphogenesis and the extensive angiogenesis of its feeding vessels. The latter provides adequate pathways for thyroid formation and the efficient release of thyroid hormones.72,73 The developmental origin of this variant is supported by the fact that it is 4.5 times more common in fetuses than in adults. 74
Conclusion
We have described uncommon neurovascular variants and anomalies along with their radiographic and clinical manifestations. We also discussed their possible underlying embryological mechanisms. Familiarity with variant anatomy is critical for neuroradiologists and interventionists to successfully treat pathology associated with them.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Mohamad Abdalkader https://orcid.org/0000-0002-9528-301X
Samuel Z Hanz https://orcid.org/0000-0003-4076-8244
Eytan Raz https://orcid.org/0000-0003-2998-8481
Thanh N Nguyen https://orcid.org/0000-0002-2810-1685
References
- 1.Bonasia S, Smajda S, Ciccio G, et al. Embryology of the anterior communicating artery complex: implications on possible adult variants. Surg Radiol Anat SRA 2022; 44: 737–748. [DOI] [PubMed] [Google Scholar]
- 2.Nutik S, Dilenge D. Carotid-anterior cerebral artery anastomosis. Case report. J Neurosurg 1976; 44: 378–382. [DOI] [PubMed] [Google Scholar]
- 3.Tahir RA, Haider S, Kole M, et al. Anterior cerebral artery: variant anatomy and pathology. J Vasc Interv Neurol 2019; 10: 16–22. [PMC free article] [PubMed] [Google Scholar]
- 4.Shapiro M, Sharashidze V, Nossek E, et al. Superior hypophyseal arteries: angiographic re-discovery, comprehensive assessment, and embryologic implications. J NeuroInterventional Surg 2023; jnis-2023-020922. [DOI] [PubMed] [Google Scholar]
- 5.Toma N. Anatomy of the ophthalmic artery: embryological consideration. Neurol Med Chir (Tokyo) 2016; 56: 585–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Louw L. Different ophthalmic artery origins: embryology and clinical significance. Clin Anat N Y N 2015; 28: 576–583. [DOI] [PubMed] [Google Scholar]
- 7.Hayreh SS. Orbital vascular anatomy. Eye Lond Engl 2006; 20: 1130–1144. [DOI] [PubMed] [Google Scholar]
- 8.Dilenge D, Ascherl GF. Variations of the ophthalmic and middle meningeal arteries: relation to the embryonic stapedial artery. AJNR Am J Neuroradiol 1980; 1: 45–54. [PMC free article] [PubMed] [Google Scholar]
- 9.Geibprasert S, Pongpech S, Armstrong Det al. et al. Dangerous extracranial-intracranial anastomoses and supply to the cranial nerves: vessels the neurointerventionalist needs to know. AJNR Am J Neuroradiol 2009; 30: 1459–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loon NW, Gendeh BS, Zakaria R, et al. Ophthalmic artery occlusion following neuro-embolization of the external carotid artery, a case report. BMC Ophthalmol 2017; 17: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masoud H, Nguyen TN, Thatcher J, et al. Duplication of the posterior cerebral artery and the “true fetal” variant. Interv Neurol 2015; 4: 64–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coulier B. Duplication of the posterior cerebral artery (PCA) or “true fetal PCA”: an extremely rare variant. J Belg Soc Radiol 2018; 102: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takahashi S, Suga T, Kawata Yet al. et al. Anterior choroidal artery: angiographic analysis of variations and anomalies. AJNR Am J Neuroradiol 1990; 11: 719–729. [PMC free article] [PubMed] [Google Scholar]
- 14.Gailloud P, Clatterbuck RE, Fasel JHD, et al. Segmental agenesis of the internal carotid artery distal to the posterior communicating artery leading to the definition of a new embryologic segment. AJNR Am J Neuroradiol 2004; 25: 1189–1193. [PMC free article] [PubMed] [Google Scholar]
- 15.Lambert SL, Williams FJ, Oganisyan ZZ, et al. Fetal-type variants of the posterior cerebral artery and concurrent infarction in the major arterial territories of the cerebral hemisphere. J Investig Med High Impact Case Rep 2016; 4: 2324709616665409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Senol MG, Velioğlu M, Toğrol E, et al. Simultaneous posterior and middle cerebral artery infarct. Neurol India 2009; 57: 673–674. [DOI] [PubMed] [Google Scholar]
- 17.Yang JH, Choi HY, Nam HS, et al. Mechanism of infarction involving ipsilateral carotid and posterior cerebral artery territories. Cerebrovasc Dis Basel Switz 2007; 24: 445–451. [DOI] [PubMed] [Google Scholar]
- 18.Chang HY, Kim MS. Middle cerebral artery duplication : classification and clinical implications. J Korean Neurosurg Soc 2011; 49: 102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shapiro M, Raz E, Nossek E, et al. Neuroanatomy of the middle cerebral artery: implications for thrombectomy. J NeuroInterventional Surg 2020; 12: 768–773. [DOI] [PubMed] [Google Scholar]
- 20.Umansky F, Dujovny M, Ausman JI, et al. Anomalies and variations of the middle cerebral artery: a microanatomical study. Neurosurgery 1988; 22: 1023–1027. [DOI] [PubMed] [Google Scholar]
- 21.Sanders WP, Sorek PA, Mehta BA. Fenestration of intracranial arteries with special attention to associated aneurysms and other anomalies. AJNR Am J Neuroradiol 1993; 14: 675–680. [PMC free article] [PubMed] [Google Scholar]
- 22.Abdalkader M, Raftopoulos C, Finet P, et al. Middle cerebral artery fenestration: thromboembolic and hemorrhagic complications. Interv Neuroradiol J Peritherapeutic Neuroradiol Surg Proced Relat Neurosci 2019; 25: 644–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gailloud P, Albayram S, Fasel JHD, et al. Angiographic and embryologic considerations in five cases of middle cerebral artery fenestration. AJNR Am J Neuroradiol 2002; 23: 585–587. [PMC free article] [PubMed] [Google Scholar]
- 24.Finlay HM, Canham PB. The layered fabric of cerebral artery fenestrations. Stroke 1994; 25: 1799–1806. [DOI] [PubMed] [Google Scholar]
- 25.Hudák I, Lenzsér G, Lunenkova Vet al. et al. Cerebral arterial fenestrations: a common phenomenon in unexplained subarachnoid haemorrhage. Acta Neurochir (Wien) 2013; 155: 217–222. [DOI] [PubMed] [Google Scholar]
- 26.Abdalkader M, Siegler JE, Lee JS, et al. Neuroimaging of acute ischemic stroke: multimodal imaging approach for acute endovascular therapy. J Stroke 2023; 25: 55–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Onoue K, Nguyen TN, Mian A, et al. Twig-like middle cerebral arteries: clinical and radiological findings. Clin Imaging 2021; 73: 31–37. [DOI] [PubMed] [Google Scholar]
- 28.Goto Y, Oka H, Hiraizumi S, et al. Aplastic or twig-like middle cerebral artery presenting with intracerebral hemorrhage during pregnancy: report of two cases. World Neurosurg X 2019; 2: 100018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zedde M, Moratti C, Pavone C, , et al. Twig-like Middle Cerebral Artery: Case Series in a European Population. World Neurosurg 2023; S1878875023013906. [DOI] [PubMed] [Google Scholar]
- 30.Seo BS, Lee YS, Lee HG, et al. Clinical and radiological features of patients with aplastic or twiglike middle cerebral arteries. Neurosurgery 2012; 70: 1472–1480. ; discussion 1480. [DOI] [PubMed] [Google Scholar]
- 31.Ota T, Komiyama M. Twig-like middle cerebral artery: embryological persistence or secondary consequences? Interv Neuroradiol J Peritherapeutic Neuroradiol Surg Proced Relat Neurosci 2021; 27: 584–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu TY, Xu N, Wan Z, et al. Diagnosis and treatment of pure arterial malformation: three case reports and literature review. Medicine (Baltimore) 2020; 99: e20229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brinjikji W, Cloft HJ, Flemming KD, et al. Pure arterial malformations. J Neurosurg 2018; 129: 91–99. [DOI] [PubMed] [Google Scholar]
- 34.Uchino A, Abe M, Sawada A, et al. Extremely tortuous superior cerebellar artery. Eur Radiol 2003; 13: L237–L238. [PubMed] [Google Scholar]
- 35.Lalla R, Virmani D, Suchdev K, et al. Intracranial corkscrew angiopathy. Neurol Clin Pract 2022; 12: e228–e231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bautch VL, James JM. Neurovascular development: the beginning of a beautiful friendship. Cell Adhes Migr 2009; 3: 199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lasjaunias P, Santoyo-Vazquez A. Segmental agenesis of the internal carotid artery: angiographic aspects with embryological discussion. Anat Clin 1984; 6: 133–141. [DOI] [PubMed] [Google Scholar]
- 38.Krings T, Choi IS. The many faces of intracranial arterial dissections. Interv Neuroradiol J Peritherapeutic Neuroradiol Surg Proced Relat Neurosci 2010; 16: 151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moser FG, Sarnat HB, Maya MMet al. et al. Corkscrew basilar artery as an incidental finding on neuroimaging. Pediatr Neurol 2007 Nov; 37: 375–377. [DOI] [PubMed] [Google Scholar]
- 40.3D rotational angiography of a rare corkscrew basilar artery.
- 41.Lim YC, Chung J. Ruptured aneurysm arising from the corkscrew basilar artery. Acta Neurochir (Wien) 2012; 154: 1153–1155. [DOI] [PubMed] [Google Scholar]
- 42.Chung DY, Abdalkader M, Nguyen TN. Aneurysmal subarachnoid hemorrhage. Neurol Clin 2021; 39: 419–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoh BL, Rabinov JD, Pryor JC, et al. Persistent nonfused segments of the basilar artery: longitudinal versus axial nonfusion. AJNR Am J Neuroradiol 2004; 25: 1194–1196. [PMC free article] [PubMed] [Google Scholar]
- 44.Roll JD, Urban MA, Larson TC, et al. Bilateral aberrant internal carotid arteries with bilateral persistent stapedial arteries and bilateral duplicated internal carotid arteries. AJNR Am J Neuroradiol 2003; 24: 762–765. [PMC free article] [PubMed] [Google Scholar]
- 45.Rhee SJ, Kim MS, Lee CHet al. et al. Persistent trigeminal artery variant detected by conventional angiography and magnetic resonance angiography-incidence and clinical significance-. J Korean Neurosurg Soc 2007; 42: 446–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Watanabe T, Aoki A, Chan SC. [Two cases of persistent trigeminal artery variant]. No Shinkei Geka 1988; 16: 95–100. [PubMed] [Google Scholar]
- 47.Morita A, Fukushima T, Miyazaki S, et al. Tic douloureux caused by primitive trigeminal artery or its variant. J Neurosurg 1989; 70: 415–419. [DOI] [PubMed] [Google Scholar]
- 48.Meckel S, Spittau B, McAuliffe W. The persistent trigeminal artery: development, imaging anatomy, variants, and associated vascular pathologies. Neuroradiology 2013; 55: 5–16. [DOI] [PubMed] [Google Scholar]
- 49.Azab W, Delashaw J, Mohammed M. Persistent primitive trigeminal artery: a review. Turk Neurosurg 2012; 22: 399–406. [DOI] [PubMed] [Google Scholar]
- 50.See AP, Baranoski JF, Flores BC, et al. Basilar stroke from a persistent hypoglossal artery. J Neurointerventional Surg 2017; 9: e30. [DOI] [PubMed] [Google Scholar]
- 51.Jin X, Sun L, Feng Z, et al. Persistent hypoglossal artery as a potential risk factor for simultaneous carotid and vertebrobasilar infarcts. Front Neurol 2018; 9: 837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hikichi H, Ueno T, Iwamura M, et al. Hypoglossal nerve palsy due to compression by a persistent primitive hypoglossal artery: case report. J Stroke Cerebrovasc Dis Off J Natl Stroke Assoc 2020; 29: 104459. [DOI] [PubMed] [Google Scholar]
- 53.Mazighi M, Porter PJ, Rodesch G, et al. Vascular anomalies and the risk of multiple aneurysms development and bleeding. Interv Neuroradiol J Peritherapeutic Neuroradiol Surg Proced Relat Neurosci 2002; 8: 15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Blasi R, Medicamento N, Chiumarullo L, et al. A case of aneurysm on a persistent hypoglossal artery treated by endovascular coiling. Interv Neuroradiol J Peritherapeutic Neuroradiol Surg Proced Relat Neurosci 2009; 15: 175–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baldi S, Zander T, Rabellino Met al. et al. Stent-assisted coil embolization of a wide-neck aneurysm of a persistent primitive hypoglossal artery. Cardiovasc Intervent Radiol 2009; 32: 352–355. [DOI] [PubMed] [Google Scholar]
- 56.Bapuraj JR, Ojili V, Khandelwal N, et al. Basilar artery aneurysm treated with coil embolization via persistent primitive hypoglossal artery. Australas Radiol 2007; 51: B340–B343. [DOI] [PubMed] [Google Scholar]
- 57.Sauvaget E, Paris J, Kici S, et al. Aberrant internal carotid artery in the temporal bone: imaging findings and management. Arch Otolaryngol Head Neck Surg 2006; 132: 86–91. [DOI] [PubMed] [Google Scholar]
- 58.Lapayowker MS, Liebman EP, Ronis MLet al. et al. Presentation of the internal carotid artery as a tumor of the middle ear. Radiology 1971; 98: 293–297. [DOI] [PubMed] [Google Scholar]
- 59.Oates JW, McAuliffe W, Coates HL. Management of pseudo-aneurysm of a lateral aberrant internal carotid artery. Int J Pediatr Otorhinolaryngol 1997; 42: 73–79. [DOI] [PubMed] [Google Scholar]
- 60.Menshawi K, Mohr JP, Gutierrez J. A functional perspective on the embryology and anatomy of the cerebral blood supply. J Stroke 2015; 17: 144–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Anagiotos A, Kazantzi M, Tapis M. Aberrant internal carotid artery in the middle ear: the duplication variant. BMJ Case Rep 2019; 12: e228865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hacein-Bey L, Daniels DL, Ulmer JL, et al. The ascending pharyngeal artery: branches, anastomoses, and clinical significance. AJNR Am J Neuroradiol 2002; 23: 1246–1256. [PMC free article] [PubMed] [Google Scholar]
- 63.Luo CB, Teng MMH, Chang FC. Radiation acute carotid blowout syndromes of the ascending pharyngeal and internal carotid arteries in nasopharyngeal carcinoma. Eur Arch Oto-Rhino-Laryngol Off J Eur Fed Oto-Rhino-Laryngol Soc EUFOS Affil Ger Soc Oto-Rhino-Laryngol - Head Neck Surg 2006; 263: 644–646. [DOI] [PubMed] [Google Scholar]
- 64.Imai S, Kajihara Y, Munemori O, et al. Superselective cisplatin (CDDP)-carboplatin (CBDCA) combined infusion for head and neck cancers. Eur J Radiol 1995; 21: 94–99. [DOI] [PubMed] [Google Scholar]
- 65.Siebert JW, Angrigiani C, McCarthy JGet al. et al. Blood supply of the Le fort I maxillary segment: an anatomic study. Plast Reconstr Surg 1997; 100: 843–851. [DOI] [PubMed] [Google Scholar]
- 66.Mercer NS, MacCarthy P. The arterial supply of the palate: implications for closure of cleft palates. Plast Reconstr Surg 1995; 96: 1038–1044. [DOI] [PubMed] [Google Scholar]
- 67.Khalid N, Bordoni B. Embryology, great vessel. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2023. [cited 2023 Nov 6]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK545254/ [PubMed] [Google Scholar]
- 68.Sreedharan R, Krishna L, Shetty A. Origin of superior thyroid artery: under the surgeon’s knife. J Vasc Bras 2018; 17: 290–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rosen RD, Bordoni B. Embryology, aortic arch. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2023. [cited 2023 Nov 6]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK553173/ [PubMed] [Google Scholar]
- 70.Moore KL, Dalley AF. Clinically oriented anatomy. Philadelphia: Lippincott Williams & Wilkins, 1999. [Google Scholar]
- 71.Yilmaz E, Celik HH, Durgun B, et al. Arteria thyroidea ima arising from the brachiocephalic trunk with bilateral absence of inferior thyroid arteries: a case report. Surg Radiol Anat SRA 1993; 15: 197–199. [DOI] [PubMed] [Google Scholar]
- 72.Totlis T, Natsis K, Achlatis V, et al. Thyroidea ima artery multiple branching pattern over the trachea. Surg Radiol Anat SRA 2023; 45: 813–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Novakov SS, Delchev SD. Two cases of variations in inferior thyroid arterial pattern and their clinical implications. Folia Morphol 2023; 82: 396–399. [DOI] [PubMed] [Google Scholar]
- 74.Yurasakpong L, Nantasenamat C, Janta S, et al. The decreasing prevalence of the thyroid ima artery: a systematic review and machine learning assisted meta-analysis. Ann Anat Anat Anz Off Organ Anat Ges 2022; 239: 151803. [DOI] [PubMed] [Google Scholar]