Abstract
Alpha/beta interferon (IFN-α/β) triggers antiviral and antiproliferative responses in target cells through modulation of gene expression. The JAK-STAT pathway is the major mediator of these biological effects through the activation of the transcription factors STAT1 and STAT2, and gene ablation studies have demonstrated that both STAT1 and STAT2 are required for most antiviral responses induced by IFN-α/β. However, additional signaling pathways are also activated by IFN. Here, we show that these additional pathways provoke a proliferative response in activated T lymphocytes. While activation of IFN-stimulated gene factor 3 produces a dominant inhibitory signal capable of overriding the mitogenic response, absence of either STAT1 or STAT2 leads to a proliferative response to IFN. Growth stimulation by IFN-α/β is independent of other STAT proteins, particularly of STAT3, since T lymphocytes from STAT1-STAT3 double-knockout mice are growth stimulated by IFN-α/β treatment. IFN-α/β can cooperate with numerous T-cell mitogens, including interleukin-2 (IL-2), IL-4, IL-7, and IL-12, and can contribute to the rapid restoration of the thymus following glucocorticoid-mediated ablation. These results underscore the complexity of the cellular response to IFN and suggest that the ultimate outcome of IFN action results from a balance between growth-inhibitory and -stimulatory effects.
The type I interferon (IFN) cytokines IFN-α and IFN-β trigger well-characterized antiviral responses in target cells as an essential element of innate immunity (32). They produce additional responses in a variety of cells, the best-characterized response being an antiproliferative action that has led to their use as antineoplastic agents (33). Although production of type I IFN is largely confined to infections, these cytokines are also produced constitutively at low levels in hematopoietic tissues and have been ascribed various roles, particularly in the immune system. These additional functions include effects on the proliferation, survival, and differentiation of T lymphocytes, the maturation and responsiveness of B lymphocytes, and the proliferation and differentiation of dendritic cells. These additional responses are less well characterized; they have been reported to be both positive and negative, depending on the cell system; and their molecular mechanisms remain largely unclear (1).
Previous studies have clearly established that the antiviral properties of IFN-α/β depend on the integrity of the JAK-STAT pathway, relying on the transcriptional activities of STAT1 and STAT2 (7, 11, 22, 25). However, involvement of the JAK-STAT pathway in other aspects of IFN function has been less clear. For instance, some type I IFN effects on B-cell proliferation do not require STAT1 (8), and immune responses initiated during inflammatory processes in the central nervous system triggered by IFN-α are largely independent of STAT2 (39). These findings are reminiscent of studies showing STAT1-independent functions for IFN-γ, including antiviral responses, cell proliferation, and gene expression that proceed in the absence of STAT1 (28). As is the case for type I IFN, the mechanisms underlying STAT1-independent signaling by IFN-γ remain unclear, although it is hypothesized that activation of alternative transcription factors is involved. Of particular note in this regard has been the realization of antagonism between STAT1 and STAT3 during signaling that results in augmented STAT3 phosphorylation during IFN-γ responses in the absence of STAT1 and augmented STAT1 phosphorylation during interleukin-6 (IL-6) responses in the absence of STAT3 (3, 27). These findings underscore the complexity and carefully balanced nature of signaling pathways and suggest that alternative transcription factor activation may underlie differential responses observed in the absence of a primary signaling target protein.
In this study, we have investigated the effects of IFN-α/β on lymphocytes in the absence of STAT proteins. We have previously reported biological roles for STAT1 that are independent of IFN-α/β signaling (15, 16, 18). Here, we have explored effects of IFN-α/β that occur in the absence of STAT1, STAT2, or STAT3. We report the surprising finding that not only was the antiproliferative activity of IFN on T lymphocytes lost in the absence of STAT1 or STAT2, but it acquired a novel mitogenic activity that effectively synergized with T-cell mitogens. This novel activity, which was specific to T lymphocytes, could not be accounted for by activation of alternative STAT proteins, in spite of the prominent activation of STAT3 by IFN-α treatment. The mitogenic response to IFN may be of particular importance during infectious processes that cause depletion of STAT1 protein (10) and may contribute to a viral etiology of autoimmune disorders that have been linked to IFN responses (12, 31).
MATERIALS AND METHODS
Animals.
Animals lacking STAT1 (7), STAT2 (25), IFN-α receptor (23), and RAG1/STAT1 (16) have been described previously. Mice with a specific deletion of STAT3 in T cells (CD4-Cre:STAT3-f/f) were generated by interbreeding STAT3-f/f mice (17) with transgenic mice expressing Cre recombinase under the control of CD4 gene regulatory elements (40). Mice doubly deficient for STAT1 and T-cell STAT3 were generated by standard interbreeding. Confirmation of Cre-mediated STAT3 deletion used a PCR genotyping method that distinguishes STAT3, STAT3-flox, and STAT3-deleted alleles (30). Experiments with mutant animals used wild-type mice of the same genetic background as controls (C57BL/6J or 129/SvEv). All animals were housed and handled under specific-pathogen-free conditions in accordance with protocols approved by the Institutional Animal Care and Use Committee of New York University School of Medicine and National Institutes of Health (NIH) guidelines.
Reagents.
Human recombinant IL-2 (rIL-2) was obtained from NIH Research Resources, and rIL4 and rIL7 were from Peprotech, Rocky Hill, NJ. Murine rIL-12 was a gift from Genetics Institute, Cambridge, MA; transforming growth factor β (TGF-β) was a gift from Jeanette Thorbecke; murine natural IFN-α/β was purchased from Access Biomedical (San Diego, CA); and rIFN-γ and rIFN-α6 were from Boehringer Mannheim (Indianapolis, IN). Rapamycin and dexamethasone were obtained from Sigma (St. Louis, MO), and U0126, SB203580, and LY294002 were from Biomol (Plymouth Meeting, PA).
Cell preparation and culture.
Single-cell suspensions prepared from thymuses and spleens of 6- to 8-week-old mice were cultured in RPMI 1640 medium (Gibco, Grand Island, NY) supplemented with 10% heat-inactivated fetal calf serum (FCS), 50 μM β-mercaptoethanol, 100 U/ml penicillin, and 100 μg/ml streptomycin. T-cell blasts were generated by stimulation of splenocytes with 2.5 μg/ml concanavalin A (ConA; Sigma, St. Louis, MO). After 72 h of culture, cells were harvested, purified over Ficoll-Hypaque, and expanded in medium containing human rIL-2 (50 U/ml). After an additional 3 days, cells were washed extensively and rested for 6 to 8 h in medium containing 0.5% heat-inactivated FCS without IL-2.
CD8+ T cells were isolated from the spleen by negative selection using magnetic beads and MACS separation columns (Miltenyi Biotec, Auburn, CA). NK cells were isolated from the spleens of RAG1:STAT1-deficient mice and expanded in medium containing IL-2 (50 U/ml) for 10 days. Mouse embryonic fibroblasts prepared by standard methods from wild-type and STAT1-deficient animals (18) were maintained in Dulbecco modified Eagle medium with 10% calf serum and antibiotics.
Lipopolysaccharide (LPS), ConA, staphylococcal enterotoxin B (SEB), and phorbol 12,13-dibutyrate (PDB) (all from Sigma) were used to stimulate the cells. Specific activation of T and B cells was achieved by stimulation of splenocytes with plate-bound purified anti-CD3 (2C11) and F(ab′)2 goat anti-mouse immunoglobulin M (IgM) antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA), respectively.
Flow cytometry.
Surface staining was performed by incubating 1 × 106 cells with appropriate concentrations of different monoclonal antibodies for 30 min on ice in phosphate-buffered saline (PBS) containing 1% bovine serum albumin and 0.1% sodium azide (staining buffer). Cells were washed twice in staining buffer, fixed with 1% paraformaldehyde, and analyzed with a FACScan flow cytometer (Becton Dickinson, Mountain View, CA). The results are presented either as the percentage of cells expressing the defined markers or as the mean fluorescence intensity. Fluorochrome-conjugated antibodies against CD3, IgM, CD4, CD8, and CD25 were obtained from Caltag Laboratories (South San Francisco, CA), and anti-NK1.1 was from Pharmingen (San Diego, CA).
Proliferation assays.
Proliferation was measured either by [3H]thymidine incorporation, adding 0.5 μCi [3H]thymidine per well in 96-well plates during the last 12 to 16 h of culture, or by incorporation of 5-bromo-2-deoxyuridine (BrdU). BrdU (Sigma) was added to the medium during the last 10 to 12 h of 40 to 48 h of culture, and staining for BrdU was done. In brief, after surface staining for relevant markers as described above, cells were fixed with 70% ethanol for 30 min at 4°C, followed by incubation in 1% paraformaldehyde plus 0.01% Tween 20 for 30 min at room temperature. After washing with PBS, cells were treated with 50 U of DNase I (Sigma) in 0.15 M NaC1-4.2 mM MgC12, pH 5, for 10 min at room temperature, washed again, and then stained with anti-BrdU-fluorescein isothiocyanate (Becton Dickinson) in staining buffer. Stained cells were analyzed by flow cytometry.
Protein assays.
Nuclear extracts were prepared from IL-2-deprived T-cell blasts stimulated with IFN-α/β (1,000 U/ml) or left untreated. Gel mobility shift assays employed equal amounts of protein and 32P-labeled double-stranded oligonucleotide probes containing either an IFN-stimulated response element (ISRE) or an IFN-γ-activated sequence (GAS), as previously described (19).
Western blot assays were performed following standard procedures using antibodies against the retinoblastoma protein Rb (Pharmingen), the tyrosine-phosphorylated forms of STAT3 and -5 (Cell Signaling, Beverly, MA), and JAK1 (Upstate Biotechnology, Lake Placid, NY). Ponceau S staining of membranes before antibody probing confirmed equal loading of total cell lysates.
Thymic ablation and reconstitution.
Mice were injected intraperitoneally with 1.5 mg dexamethasone (Sigma) in PBS. After 2 or 4 days, single-cell suspensions were prepared from the thymus, stained for CD4 and CD8, and analyzed by flow cytometry. In some experiments, BrdU (1 mg/mouse) was injected intraperitoneally 48 h after dexamethasone treatment, and the thymus was removed 2 h later and analyzed by flow cytometry using anti-BrdU antibodies, as indicated above.
RESULTS
T lymphocytes lacking STAT1 proliferate in response to IFN-α/β.
Previous studies have demonstrated that the antiviral properties of IFN-α/β rely on the integrity of the JAK-STAT pathway, but antiproliferative mechanisms are less well understood. To delve into this problem, we measured the effect of IFN-α/β on the proliferation of activated T cells in the presence of mitogenic cytokines, comparing cells from wild-type and STAT1-deficient mice. We used a system of competence and progression that separates G0-G1 transition and S-phase entry in order to minimize the contribution of endogenously produced cytokines. In this system, T cells are initially stimulated with the incomplete phorbol ester PDB to stimulate reentry into the cell cycle, but they fail to cycle in the absence of exogenously added cytokines, such as IL-2 (14).
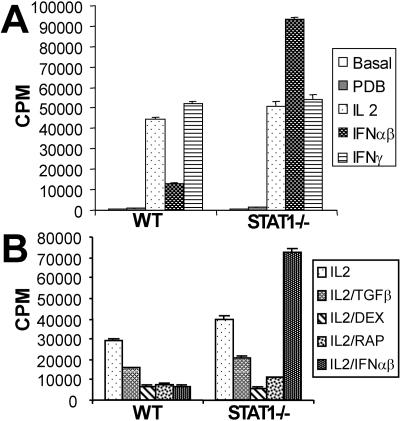
Splenocytes from wild-type mice proliferated vigorously when stimulated with PDB and IL-2 but not in the presence of PDB alone (Fig. 1A). This proliferation was inhibited by treatment with IFN-α/β and was resistant to inhibition by IFN-γ, as expected for T lymphocytes (26). Cells from STAT1-deficient mice proliferated similarly in a PDB- and IL-2-dependent manner. However, these cells failed to arrest in response to IFN-α/β treatment (Fig. 1A, right part). Surprisingly, not only did STAT1-null cells fail to arrest, but their proliferation was significantly augmented by cotreatment with IL-2 and IFN-α/β. This failure to growth arrest in response to IFN-α/β was not due to an intrinsic defect in the ability to respond to antimitogenic treatments, because STAT1-null cells arrested in response to other inhibitory signals in a manner similar to wild-type cells. As shown in Fig. 1B, PDB/IL-2-induced proliferation of cells from STAT1−/− mice was inhibited by such agents as TGF-β, dexamethasone, and rapamycin. The growth-stimulatory effect of IFN-α/β in STAT1-null cells could not override the inhibitory effects of these agents (data not shown). Moreover, this mitogenic effect of IFN-α/β was not due to a contaminating factor in the IFN preparation because the effect was observed with both natural and rIFN preparations and no effect, inhibitory or stimulatory, was observed with splenocytes isolated from IFN receptor-null or STAT1/IFN receptor doubly deficient mice (data not shown).
FIG. 1.
Absence of STAT1 converts IFN-α/β effects from antiproliferative to mitogenic. (A) Splenocytes from wild-type (WT) or STAT1-deficient animals were left untreated or were stimulated with PDB (50 nM) or with combinations of PDB plus IL-2 (50 U/ml), IFN-α/β (1,500 U/ml), or IFN-γ (100 U/ml) as indicated for 60 h, and proliferation was determined by [3H]thymidine incorporation and expressed as mean counts per minute ± the standard error for triplicate cultures. (B) Splenocytes were stimulated as in panel A with PDB plus IL-2, except incubations also included TGF-β (10 ng/ml), 100 nM dexamethasone (DEX), 10 mM of rapamycin (RAP), or IFN-α/β (1,500 U/ml), as indicated.
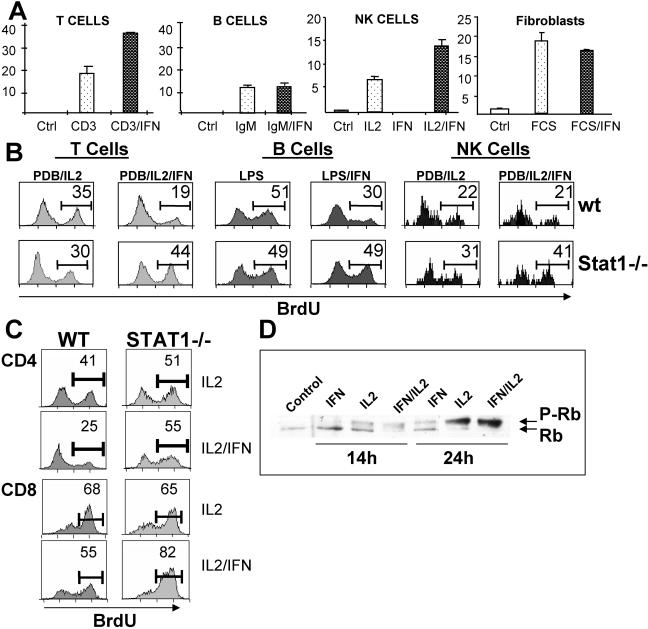
The mitogenic regimen of PDB and IL-2 selectively stimulates T lymphocytes. To confirm the mitogenic effect of IFN-α/β on T cells and investigate the cell type selectivity of this effect, we combined IFN-α/β with other mitogenic treatments of STAT1-null cells. IFN-α/β was also comitogenic on T cells selectively stimulated to proliferate with suboptimal concentrations of anti-CD3ɛ (Fig. 2A). It also displayed comitogenic effects on STAT1-null NK cells (expanded in vitro from spleens of STAT1−/− RAG1−/− animals) stimulated with IL-2. However, neither B lymphocytes stimulated by anti-IgM or with LPS nor fibroblasts stimulated with serum displayed a stimulatory or inhibitory response to IFN-α/β treatment when they were derived from STAT1−/− animals (Fig. 2A and B). T cells and B cells from STAT1-sufficient animals displayed the expected growth-inhibitory responses to IFN-α/β treatment, while NK cell growth was not inhibited (Fig. 2B), as shown previously (24).
FIG. 2.
Mitogenic action of IFN-α/β is limited to T lymphocytes and NK cells. (A) Splenocytes, NK cells, or serum-starved fibroblasts from STAT1-deficient mice were stimulated with anti-CD3 (2.5 μg/ml), anti-IgM (10 μg/ml), IL-2 (20 U/ml), or FCS (10%), respectively, with or without IFN-α/β (1,500 U/ml), and proliferation was measured by [3H]thymidine incorporation, expressed as mean counts per minute ± the standard error for triplicate cultures (÷1,000). (B) Wild-type (WT or wt) or STAT1-deficient splenocytes were stimulated with a combination of PDB and IL-2 or with LPS (25 μg/ml) for 40 h in the presence or absence of IFN-α/β. Proliferation was measured by BrdU incorporation and flow cytometry, using specific antibodies against BrdU plus CD3ɛ, IgM, or NK1.1 and BrdU. The percentage of cells in S phase is indicated. (C) The percentages of CD4 and CD8 cells in S phase were measured as in panel B using splenocytes stimulated with PDB and IL-2 with or without IFN-α/β and antibodies against CD4, CD8, and BrdU. (D) Purified CD8 cells were stimulated with PDB and IL-2, IFN-α/β, or combinations for the indicated times, and Rb phosphorylation was determined by immunoblot analysis of whole-cell lysates. Ctrl, control.
Using BrdU labeling and flow cytometry, we examined separately the proliferative responses of CD4 and CD8 cells (Fig. 2C). Both T-cell subsets proliferated in response to IL-2 and were inhibited by IFN-α/β treatment when they expressed STAT1. Both subsets also responded mitogenically to IFN-α/β treatment in the absence of STAT1, although CD8 cells displayed a more robust proliferative response to IFN-α/β cotreatment. However, this difference between CD4 and CD8 cells was observed only with freshly isolated cells and was lost when the proliferation of activated T cells was examined, in which case both CD4 and CD8 cells responded equally to IFN mitogenesis (data not shown). The limited proliferative response of freshly isolated Stat1−/− CD4 cells may be related to the previous observation of enhanced IFN-γ production by Stat1−/− CD8 T cells (6).
Progression through the cell cycle is regulated by the phosphorylation of regulatory proteins by cyclin/CDK complexes, and an important phosphorylated target is the Rb protein (9). We monitored Rb phosphorylation in purified CD8 cells isolated from STAT1−/− mice. Stimulation with IL-2 resulted in the progressive phosphorylation of Rb (Fig. 2D), and this phosphorylation was augmented by cotreatment with IFN-α/β, as seen by complete loss of the more rapidly migrating nonphosphorylated band (lane 7). Treatment with IFN alone was sufficient to induce a degree of phosphorylation (lane 5). In contrast, IFN treatment of wild-type cells reduces Rb phosphorylation (13).
IFN-α/β is comitogenic with numerous T-cell cytokines.
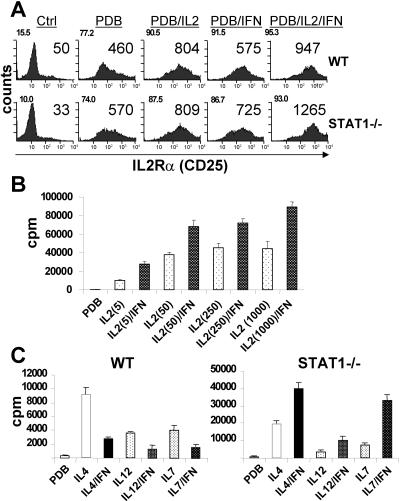
Sensitivity to IL-2 is dependent on expression of the inducible alpha chain of the IL-2 receptor, CD25. We considered whether increased CD25 expression might contribute to the mitogenic effect of IFN-α/β observed in STAT1-deficient cells. CD25 expression was low on resting cells (Fig. 3A, left part) and progressively increased in response to PDB, IL-2, and IFN. Up-regulation of CD25 was observed in both wild-type cells (upper part) that were growth arrested in response to IFN-α/β treatment and in STAT1-deficient cells (lower part) that were costimulated by IFN. While CD25 induction was greater in STAT1-deficient cells, it seems unlikely that this, at least alone, could account for the mitogenic action of IFN, since CD25 was also induced in wild-type cells. It remains unclear whether CD25 induction by IFN results from a direct, STAT1-independent signaling pathway, e.g., from IFN-induced STAT5 phosphorylation (38), or is an indirect effect of cell proliferation.
FIG. 3.
Induction of CD25 is not required for the mitogenic action of IFN-α/β. (A) Wild-type (WT) or STAT1-deficient splenocytes were stimulated with PDB alone or combined with the indicated cytokines for 48 h. Expression of IL-2-Rα (CD25) was measured by flow cytometry on CD3ɛ-gated cells and expressed as a percentage of positive cells (upper left corners) and mean fluorescent intensity. A representative experiment out of three performed is shown. (B) STAT1-deficient splenocytes were stimulated with PDB and increasing doses of IL-2 from 5 to 1,000 U/ml, as indicated, in the presence or absence of IFN-α/β, and proliferation was measured by [3H]thymidine incorporation. (C) Wild-type or STAT1-deficient splenocytes were stimulated with PDB alone or combined with IL-4 (500 U/ml), IL-12 (10 ng/ml), or IL-7 (10 ng/ml), with or without IFN-α/β, and proliferation was evaluated by [3H]thymidine incorporation. Ctrl, control.
To further examine whether induction of CD25 was related to the comitogenic effect of IFN-α/β on STAT1-deficient cells, we tested its dependence on IL-2 concentration. Induction of CD25 results in formation of high-affinity receptors that allow cells to respond to low-dose IL-2 and is dispensable at high concentrations (36). However, we found that IFN was mitogenic on STAT1-null cells even when combined with a high concentration of IL-2 (1,000 U/ml, Fig. 3B), where the presence of high-affinity receptors would not be required. Therefore, induction of CD25 is unlikely to explain the proliferative response of STAT1-null cells to IFN.
A comitogenic effect of IFN independent of CD25 was confirmed by examining cell proliferation stimulated by other cytokines. PDB-stimulated lymphocytes from wild-type and STAT1-null mice were treated with IL-4, IL-12, or IL-7 in the presence or absence of IFN-α/β (Fig. 3C). These cytokines all induced proliferation of wild-type cells that was impaired in the presence of IFN. However, similar to the combined effects of IL-2 and IFN, comitogenic responses were observed for all three cytokines on STAT1-null cells (Fig. 3C, right part). Stat1−/− cells showed enhanced responses to IL-4 and IL-12 even in the absence of IFN, reflecting the enhanced proliferative response of Stat1-deficient lymphocytes noted previously (18). Since none of these cytokines uses CD25 as a receptor, these results reinforce the notion that the mitogenic effect of IFN-α/β on STAT1-null T cells is independent of CD25 induction.
IFN augments proliferation of STAT1-null cells by lowering the threshold for activation.
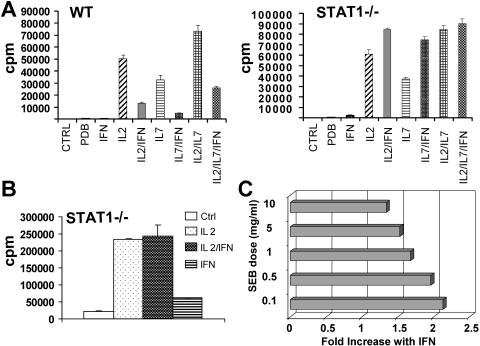
Since the IFN effect on STAT1-null cells appeared to be synergistic with mitogenic cytokines, we examined its action under conditions of saturating mitogen. Thymocytes were stimulated with PDB and the cytokines IL-2 and IL-7, alone or in combination. Both cytokines stimulated proliferation of wild-type cells that was impaired by cotreatment with IFN-α/β (Fig. 4A, left part). Simultaneous stimulation with both IL-2 and IL-7 had an additive effect that remained IFN responsive. STAT1-null cells responded similarly to IL-2 and IL-7 treatment, except that IFN produced a comitogenic effect. However, the additive proliferative response observed in mutant cells cotreated with both IL-2 and IL-7 was resistant to further stimulation of IFN (Fig. 4A, right part). This result suggests that IFN-α/β is incapable of further increasing the proliferation of maximally stimulated T cells.
FIG. 4.
IFN-α/β reduces the activation threshold required to trigger proliferation. (A) Thymocytes from wild-type (WT) or STAT1-deficient mice were stimulated with PDB and the indicated cytokines for 60 h, and proliferation was determined by [3H]thymidine incorporation. (B) Splenocytes were stimulated with ConA (10 μg/ml) for 48 h, washed extensively, rested for 24 h, and then restimulated with IL-2 or IFN-α/β, as indicated, and proliferation was measured after an additional 48 h by [3H]thymidine incorporation. (C) STAT1-deficient splenocytes were incubated with the indicated concentrations of SEB in the absence or presence of IFN-α/β. Proliferation was determined after 60 h and is expressed as the n-fold increase in proliferation in the presence of IFN. CTRL, control.
This notion was further examined by using highly activated T cells. T cells activated by a high dose of ConA and subsequently growth arrested by incubation in the absence of mitogen displayed a robust proliferative response when restimulated with IL-2 (Fig. 4B), but this response could not be further augmented by IFN-α/β. Interestingly, though, IFN-α/β alone was capable of inducing a modest proliferative response in these highly activated cells when added in the absence of exogenous IL-2.
A similar loss of the synergistic effect of IFN on proliferation was observed in cells activated by high doses of superantigen (Fig. 4C). STAT1-null cells activated by SEB displayed enhanced proliferation in the presence of IFN, but this effect was most profound when the dose of SEB was limiting. Increasing concentrations of SEB led to a progressive loss of the synergy with IFN, with cells simulated with high-dose SEB being relatively inured to the added effect of IFN. Taken together, these results suggest that IFN is an effective mitogen for STAT1-null T cells stimulated under suboptimal conditions.
Molecular markers of IFN-stimulated proliferation.
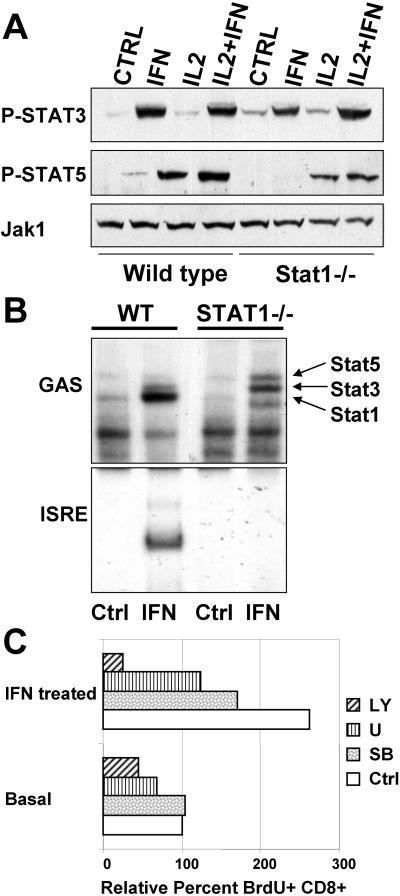
Classic transcriptional responses to IFN-α/β are mediated by phosphorylation-dependent activation of STAT1 homodimers that bind GAS elements and STAT1/STAT2 heterodimers that bind ISRE sequences in conjunction with IRF9 (20). However, other transcription factors have also been implicated in IFN responses, including STAT3 and STAT5 (27, 29, 38). Moreover, the absence of one STAT isoform has been found under some circumstances to affect the cytokine-induced activation of other family members (3, 27), suggesting that abnormal activation of a STAT protein might underlie the mitogenic effect of IFN-α/β in the absence of STAT1. Therefore, we examined STAT3 and STAT5 tyrosyl phosphorylation in T cells from wild-type and STAT1-null animals following IFN and IL-2 stimulation in vitro (Fig. 5).
FIG. 5.
Multiple signaling intermediates are activated by IFN-α/β. (A) T-cell blasts were left untreated or stimulated for 30 min with the indicated cytokines. Activation of STAT3 and STAT5 was measured by immunoblotting with phosphotyrosine-specific antibodies. Levels of Jak1 protein are shown as a loading control. (B) Nuclear extracts from rested T-cell blasts untreated or stimulated for 30 min with IFN-α/β were analyzed for DNA binding activity using the indicated binding site probes. Identities of the different DNA-protein complexes are shown. The more rapidly migrating, IFN-inducible protein complex observed in extracts from Stat1−/− cells is likely due to a truncated form of Stat5. (C) Splenocytes were stimulated with ConA for 48 h, washed, rested for 24 h, treated with a pharmacological inhibitor (SB203580 [SB] at 10 μM, U0126 [U] at 10 μM, or LY294002 [LY] at 5 μM) for 1 h, and stimulated with IFN-α/β for 40 h. Proliferation of CD8 cells was measured by flow cytometry with antibodies against BrdU and CD8 and expressed as the percent proliferation relative to untreated control cells. WT, wild type; Ctrl, control.
STAT3 was tyrosyl phosphorylated in T cells in response to IFN-α/β treatment, as previously reported for other cell types (29). However, its ability to be phosphorylated was not influenced by cotreatment with IL-2 (Fig. 5A, lane 4). Moreover, its phosphorylation was not affected by the presence or absence of STAT1 (compare lanes 4 and 8). STAT5 was minimally phosphorylated in response to IFN-α/β and was robustly phosphorylated in response to IL-2 (Fig. 5A, middle part). Again, STAT5 phosphorylation was not significantly affected by the absence of STAT1. If anything, STAT5 phosphorylation was reduced rather than augmented in the absence of STAT1, although this difference is small when protein loading differences are considered, as judged by the levels of Jak1 protein (lower part). Therefore, it seems unlikely that the proliferative capacity of IFN-α/β on STAT1-deficent cells can be explained by inappropriate phosphorylation of other STAT family members, although it remains possible that proliferative responses from normal phosphorylation of STAT5 (or other STAT proteins) become dominant in the absence of the inhibitory effects of STAT1 and STAT2.
Activated protein binding to DNA enhancer elements was also compared for extracts from wild-type and STAT1-deficient T cells. STAT1 homodimers were the major inducible GAS-binding activity present in extracts from wild-type cells, with minor amounts of STAT3 (Fig. 5B, upper part), while STAT3 was the major activity detected in extracts from IFN-treated STAT1-deficient cells, with minor amounts of STAT5. Protein identity was confirmed by antibody supershifts, demonstrating the binding of STAT3 and lesser amounts of STAT5 to the GAS probe in the absence of STAT1 (data not shown). The identity of the noninducible binding activities is unknown. IFN-stimulated gene factor 3 (ISGF3) was the major ISRE-binding activity detected in extracts from stimulated wild-type cells (Fig. 5B, lower part). No inducible binding to an ISRE probe was detected in extracts from STAT1-deficient cells, even though phosphorylated STAT2 was present in these extracts (data not shown). Therefore, the presence of novel binding complexes at the two major IFN-dependent enhancer elements is unlikely to explain IFN-stimulated proliferation of STAT1-null cells.
The involvement of non-STAT signaling pathways for proliferation of STAT1-deficient T cells was examined by using pharmacological inhibitors (Fig. 5C). We compared the sensitivities of basal and IFN-stimulated cell proliferation to various kinase inhibitors in cells previously activated by ConA. As shown above, ConA activation renders STAT1-deficient T cells sensitive to the mitogenic effects of IFN-α/β, even in the absence of added cytokines. As has been previously reported for IL-2-induced proliferation of wild-type cells (2), both basal and IFN-stimulated proliferation of STAT1-deficient T cells was dependent on the activity of several serine/threonine and lipid kinases. Specifically, phosphatidylinositol 3-kinase (PI3K), ERK1/2, and p38 activities were implicated in IFN-stimulated proliferation, with inhibition of PI3K providing the most significant contribution to cell growth, possibly due to its additional role in cell survival. However, this analysis did not uncover any significant differences between the known signaling requirements of wild-type cells in response to IL-2 and those for IFN-stimulated growth of STAT1-deficient cells.
STAT2 but not STAT3 affects T-cell proliferation in the presence of IFN-α/β.
STAT2 and STAT3 were the most highly phosphorylated STAT proteins in response to IFN-α/β treatment in the absence of STAT1 (Fig. 5 and data not shown). Therefore, we considered the possibility that these proteins were involved in the mitogenic action of IFN and employed a genetic approach to assess their potential role. Lymphocytes isolated from STAT2-deficient mice (25) responded to IL-2 and IFN-α/β stimulation in a manner very similar to STAT1-deficient cells. Whereas the proliferation of cells from wild-type mice stimulated with IL-2 was markedly impaired by cotreatment with IFN-α/β (Fig. 6A, left part), similar treatment of STAT2-deficient cells induced robust proliferation (Fig. 6A, right part). This result suggests that ISGF3, formed from a dimer of phosphorylated STAT1 and STAT2, is critical for the antiproliferative effects of IFN-α/β and for counteracting the underlying proliferative potential of these cytokines. However, neither STAT1 nor STAT2 is required for the concealed comitogenic action of IFN.
FIG. 6.
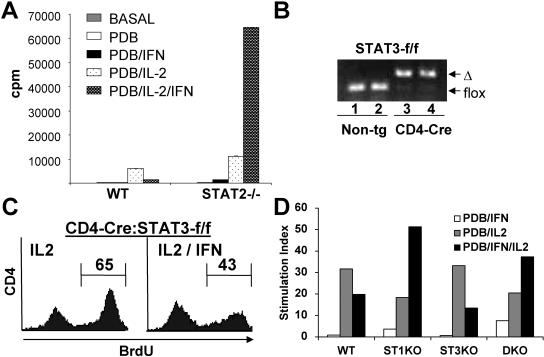
STAT2 but not STAT3 regulates T-cell proliferation in response to IFN-α/β. (A) Wild-type (WT) or STAT2-deficient thymocytes were stimulated with PDB and IL-2 for 60 h, and proliferation was measured by [3H]thymidine incorporation. (B) DNA prepared from thymocytes (lanes 1 and 3) or T-cell blasts (lanes 2 and 4) from STAT3-f/f (lanes 1 and 2) or CD4-Cre:STAT3-f/f (lanes 3 and 4) mice was analyzed by PCR for conversion of the floxed STAT3 allele (flox) to the deleted form (Δ). Non-tg, nontransgenic. (C) Splenocytes from CD4-Cre:STAT3-f/f mice were stimulated with ConA (2 μg/ml) and IL-2 (20 U/ml) with or without IFN-α/β (1,500 U/ml) for 48 h. Proliferation of CD4 cells was determined by BrdU incorporation by flow cytometry. The number of cells in S phase is indicated. (D) Wild-type, STAT1−/− (ST1KO), CD4-Cre:STAT3-f/f (ST3KO), and STAT1−/−:CD4-Cre:STAT3-f/f (DKO) splenocytes were stimulated for 48 h as indicated, and proliferation was determined by [3H]thymidine incorporation.
STAT3 would be a likely a priori participant in IFN-induced proliferation, since it is activated by IFN treatment and is believed to have the capacity to contribute to cell proliferation and survival in some cells (21). To examine this question, we isolated thymocytes from STAT3-conditional mice (17) expressing Cre recombinase driven by the CD4 promoter (40), referred to as CD4-Cre:STAT3-f/f mice. This system induces efficient gene deletion of the STAT3 gene in nearly all thymocytes and T lymphocytes, since CD4-Cre is expressed in some double-negative cells, and in the double-positive cells that are the precursors of single-positive CD4 and CD8 thymocytes and of the vast majority of peripheral T lymphocytes (40). Near complete deletion of STAT3 was confirmed by genotyping thymocyte genomic DNA by PCR (Fig. 6B) and by immunoblotting thymocyte extracts and extracts from T cells expanded in vitro using an antibody to STAT3 (data not shown).
Cells from CD4-Cre:STAT3-f/f mice were stimulated with ConA and IL-2, with or without IFN-α/β, and cell proliferation was assessed by BrdU labeling. Efficient cell proliferation was observed in response to IL-2 that was significantly impaired by IFN-α/β treatment (Fig. 5C), similar to the responses observed for wild-type cells. This result suggests that STAT3 is not required for the antiproliferative properties of IFN-α/β in T cells. To further examine the potential role of STAT3 in the covert proliferative capacity of IFN, we crossed CD4-Cre:STAT3-f/f mice with STAT1−/− animals to obtain doubly deficient T cells lacking both STAT1 and STAT3. Proliferation assays were performed on PDB- and IL-2-stimulated cells in the presence or absence of added IFN-α/β, and the mitogenic responses of wild-type, STAT1-deficient, STAT3-deficient, and STAT1-STAT3 doubly deficient cells were measured (Fig. 6D). As seen previously, proliferation of wild-type cells and STAT3-deficient cells was significantly impaired by IFN-α/β treatment. In contrast, STAT1-deficient cell proliferation was stimulated in response to added IFN-α/β, and the presence or absence of STAT3 had only a marginal effect on this mitogenic response. Therefore, STAT3 is not required for mitogenic responses to IFN-α/β that occur in the absence of STAT1 or STAT2.
IFN-α/β can contribute to thymic reconstitution in the absence of STAT1.
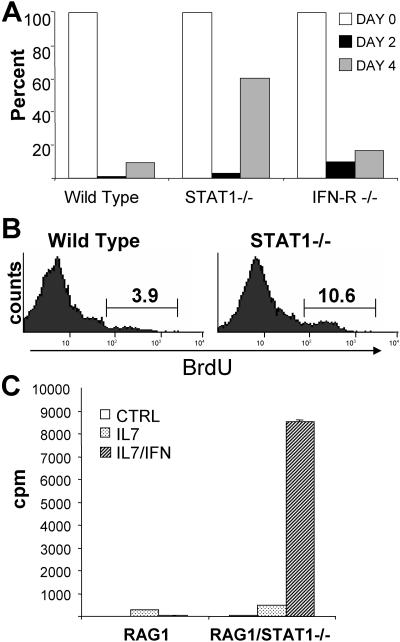
Low levels of type I IFN are present constitutively in vivo and have been implicated as homeostatic regulators of lymphocytes (37). However, no major defects in lymphocyte subsets have been observed in STAT1-deficient or type I IFN receptor-deficient mice (7, 22, 23). To further assess a possible role for IFN-α/β in lymphocyte proliferation, we examined thymic reconstitution following glucocorticoid-induced ablation. Dexamethasone treatment of mice caused a near total ablation of double-positive thymocytes, with significant repopulation beginning after approximately 4 days (Fig. 7A). This repopulation was much more robust in STAT1-deficient mice, resulting in a greater-than-fivefold increase in reconstitution by 4 days. A similar result was previously observed in dexamethasone-treated STAT2-deficient mice (25) and was interpreted as evidence that IFN contributes to growth suppression of repopulating thymocytes in wild-type mice. However, our results that the absence of STAT1 or STAT2 uncovers a covert mitogenic potential of IFN-α/β suggested a different interpretation. That is, accelerated thymic reconstitution in the absence of STAT1 or STAT2 could reflect an acquired proliferative response to IFN rather than the loss of normal inhibitory IFN effects. To resolve this question, we examined thymic reconstitution in type I IFN receptor-deficient mice. These animals, which cannot respond either positively or negatively to IFN, displayed a reconstitution response that more closely resembled wild-type animals than STAT1−/− mice (Fig. 7A, right). They did display a modest reconstitution advantage relative to wild-type mice, suggesting that the bulk of the difference observed in STAT1-deficient mice can be ascribed to uncovering the covert mitogenic action of IFN, with only a small contribution coming from the loss of normal negative growth control.
FIG. 7.
IFN-α/β promotes the proliferation of thymic progenitors. (A) Wild-type, STAT1-deficient, or IFN-α/β receptor-deficient mice (three per group) were injected with dexamethasone (1.5 mg), and the percentage of CD4+ CD8+ double-positive cells measured by flow cytometry at the indicated times. Results are expressed as the percentage of DP cells present at a particular time point relative to the initial percentage at day 0. Variation within groups was less than 15%, and results representative of two experiments are presented. (B) Wild-type or STAT1-deficient mice treated with dexamethasone for 48 h were injected with BrdU, and thymocytes were recovered after 2 h. Cells incorporating BrdU were quantified by flow cytometry. (C) Thymocytes from RAG1−/− or RAG1−/− STAT1−/− mice were treated with PDB (control [CTRL]) alone or combined with IL-7 and/or IFN-α/β. Proliferation was determined after 48 h by [3H]thymidine incorporation.
Proliferation of reconstituting thymocytes was directly measured by BrdU labeling (Fig. 7B). Two days postablation, STAT1-deficient thymocytes exhibited enhanced proliferation relative to wild-type cells, consistent with the higher rates of reconstitution. The increase in thymic cellularity that occurs postablation comes from proliferation of double-negative cells. We measured the proliferative potential of an enriched population of these cells derived from RAG1−/− thymus that lacks mature T cells. RAG1-deficient thymocytes proliferated modestly when stimulated with IL-7, and this proliferation was effectively impaired by IFN-α/β treatment (Fig. 7C). However, RAG1/STAT1 doubly deficient thymocytes were robustly growth stimulated by IFN-α/β in the presence of IL-7 (Fig. 7C, ight). Thus, immature thymocytes are exquisitely sensitive to the mitogenic action of IFN in the absence of STAT1, explaining the profound reconstitution response observed in STAT1−/− animals driven by the relatively low levels of constitutive endogenous type I IFN.
DISCUSSION
This study revealed that type I IFN has the potential to elicit two contradictory responses in T lymphocytes. The classically defined antiproliferative response to IFN-α/β is dominant and is mediated by both STAT1 and STAT2, suggesting that it depends on the induction of classical IFN-stimulated genes targeted by the ISGF3 transcription factor. However, the covert mitogenic potential of IFN-α/β is revealed by the absence of either transcription factor. The effect of IFN-α/β in the absence of STAT1 or STAT2 appears to be substantially mitogenic rather than prosurvival, since our BrdU incorporation assays were designed to measure primarily entry of resting cells into an initial cell cycle. This mitogenic property relies on normal type I IFN receptors but does not appear to require the activation of other STAT proteins. We provide definitive evidence by gene ablation that STAT3 is not required for either the antiproliferative or the mitogenic effects of IFN-α/β. Moreover, activation of STAT5, which occurs normally whether or not STAT1 is present, is unlikely to be responsible for this novel effect.
A conundrum in our understanding of signaling pathways is how a limited set of signaling intermediates can mediate diverse responses to distinct ligands. For instance, IFN receptors activate many of the same signaling cascades as growth-stimulatory receptors, in spite of their opposite biology. Here, we found that the same signaling pathways that have been implicated in T-cell proliferation, as well as in IFN action, such as mitogen-activated protein kinase, PI3K, and p38, contribute as well to IFN-dependent mitogenesis in the absence of the dominant growth-inhibitory effects of STAT1 and STAT2. These pathways were implicated by inhibition studies using pharmacologic agents (Fig. 5C). Similar conclusions were derived by examining stimulation of Rb phosphorylation, Ras-GTP charging, and ERK1/2 phosphorylation by IFN-α/β (Fig. 2D and data not shown). These data suggest that STAT1 and STAT2, presumably through the induction of ISGF3 target genes, exert an overriding growth-inhibitory response that obscures the underlying growth-stimulatory potential of these pathways. We speculate that an underlying growth-promoting signal may be advantageous to cells during an IFN response, possibly by protecting them from adverse effects of viral infections that are better withstood by cycling cells.
It is of note that it is specifically ISGF3 and not simply activated STAT1 that mediates the growth-inhibitory action of IFN-α/β. Perhaps this explains why type I IFN is a more potent growth-inhibitory cytokine than type II IFN, which activates only STAT1 dimers, and why type I IFN is an effective antineoplastic drug, at least for some hematological malignancies. While many ISGF3 target genes have documented antiviral functions, it will be of interest to define the subset of IFN-stimulated genes that provides the growth-suppressive action capable of counteracting not only the covert mitogenic potential of IFN but also the mitogenic action of T-cell cytokines.
Even in the absence of STAT1 or STAT2, IFN-α/β is not a complete mitogen and is incapable of stimulating the growth of resting naive T cells. However, it is a potent comitogen for numerous T-cell cytokines, including IL-2, IL-4, IL-7, and IL-12, and endogenous levels of IFN are capable of cooperating with endogenous levels of other cytokines to significantly accelerate thymic reconstitution following ablation. It is also capable of acting as a complete mitogen for highly activated cells, such as ConA-activated T cells deprived of IL-2. IFN appears to act by lowering the signaling threshold for a mitogenic response, allowing cells to respond productively to otherwise insufficient signals. This notion supports a model in which the ultimate outcome of IFN stimulation (or presumably of other cytokine signals) is dependent on the final balance of growth-stimulatory and -inhibitory signals imparted by the summation of stimuli impinging on the cell.
The growth-stimulatory properties of IFN-α/β were revealed by responses of gene-deleted cells. Determining the function of this pathway in wild-type cells remains speculative. Perhaps IFN would have a particularly detrimental effect on cells incapable of responding mitogenically due to an unopposed growth-inhibitory signal. For instance, IFN-α/β is not normally proapoptotic, but it exerts profound apoptotic effects on virally infected cells, possibly because viral infection disrupts normal growth-promoting pathways (35). The covert mitogenic action of IFN may be revealed in other circumstances as well, since gene ablation is not the only condition that alters the abundance of STAT proteins. For instance, mutations of STAT1 have been characterized in human populations and in human malignancies (4, 5), and it is conceivable that IFN would invoke unexpected proliferative responses in such situations, which could be of therapeutic importance. Moreover, STAT1 and STAT2 are common targets for degradation by a variety of RNA viruses, leading to loss of IFN-induced antiviral responses in cells infected by these viruses (10). We would speculate that T cells infected with such viruses would retain a mitogenic response to IFN-α/β while losing both its antiproliferative and antiviral benefits. IFN might promote the proliferation of virally infected T cells, leading to further dissemination of the virus, as well as possibly to expansion of these T cells. Perhaps this effect could contribute to links between viral infection and the development of autoimmune disease.
While this report was in preparation, a brief report was published describing IFN-β effects on STAT1-deficient T cells (34). Similar to our results, Tanabe et al. also observed IFN-stimulated proliferation of STAT1-null T cells that could not be ascribed to STAT5; however, they attributed this effect to the action of phosphorylated STAT3, based on inhibition studies employing a membrane-permeable peptide described as a STAT3 inhibitor. No data were presented concerning the efficacy or specificity of this inhibitor. Clearly, our results employing STAT3-null T cells provide definitive evidence that IFN-stimulated proliferation does not require the presence of STAT3. Our result is consistent with similar conclusions that myeloid and erythroid cell proliferation is largely independent of STAT protein activation but rather proceeds through alternative receptor-dependent signaling pathways (41), although a role for unknown GAS-binding proteins cannot be excluded. It will be of interest to further evaluate the target of the inhibitory peptide employed in the study of Tanabe et al. (34).
Acknowledgments
We thank Giorgio Inghirami for helpful discussions and for advice and assistance on analysis of STAT3 function in T cells, Alan Frey for helpful discussions, Isabelle Marié for comments on the manuscript, and Esther Casas and Rachel Gertner for technical assistance with animal maintenance and analysis.
This work was supported by a fellowship from the Spanish Ministry of Science and grants from the NIH and the American Heart Association.
REFERENCES
- 1.Biron, C. A. 2001. Interferons alpha and beta as immune regulators—a new look. Immunity 14:661-664. [DOI] [PubMed] [Google Scholar]
- 2.Cantrell, D. A. 2003. Regulation and function of serine kinase networks in lymphocytes. Curr. Opin. Immunol. 15:294-298. [DOI] [PubMed] [Google Scholar]
- 3.Costa-Pereira, A. P., S. Tininini, B. Strobl, T. Alonzi, J. F. Schlaak, H. Is'harc, I. Gesualdo, S. J. Newman, I. M. Kerr, and V. Poli. 2002. Mutational switch of an IL-6 response to an interferon-gamma-like response. Proc. Natl. Acad. Sci. USA 99:8043-8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunn, G. P., L. J. Old, and R. D. Schreiber. 2004. The immunobiology of cancer immunosurveillance and immunoediting. Immunity 21:137-148. [DOI] [PubMed] [Google Scholar]
- 5.Dupuis, S., E. Jouanguy, S. Al-Hajjar, C. Fieschi, I. Z. Al-Mohsen, S. Al-Jumaah, K. Yang, A. Chapgier, C. Eidenschenk, P. Eid, A. Al Ghonaium, H. Tufenkeji, H. Frayha, S. Al-Gazlan, H. Al-Rayes, R. D. Schreiber, I. Gresser, and J. L. Casanova. 2003. Impaired response to interferon-alpha/beta and lethal viral disease in human STAT1 deficiency. Nat. Genet. 33:388-391. [DOI] [PubMed] [Google Scholar]
- 6.Durbin, J. E., A. Fernandez-Sesma, C.-K. Lee, T. D. Rao, A. B. Frey, T. M. Moran, S. Vukmanovic, A. García-Sastre, and D. E. Levy. 2000. Type I IFN modulates innate and specific antiviral immunity. J. Immunol. 164:4220-4228. [DOI] [PubMed] [Google Scholar]
- 7.Durbin, J. E., R. Hackenmiller, M. C. Simon, and D. E. Levy. 1996. Targeted disruption of the mouse Stat1 gene results in compromised innate immunity to viral disease. Cell 84:443-450. [DOI] [PubMed] [Google Scholar]
- 8.Gongora, R., R. P. Stephan, R. D. Schreiber, and M. D. Cooper. 2000. Stat-1 is not essential for inhibition of B lymphopoiesis by type I IFNs. J. Immunol. 165:2362-2366. [DOI] [PubMed] [Google Scholar]
- 9.Hatakeyama, M., and R. A. Weinberg. 1995. The role of RB in cell cycle control. Prog. Cell Cycle Res. 1:9-19. [DOI] [PubMed] [Google Scholar]
- 10.Horvath, C. M. 2004. Silencing STATs: lessons from paramyxovirus interferon evasion. Cytokine Growth Factor Rev. 15:117-127. [DOI] [PubMed] [Google Scholar]
- 11.Horvath, C. M., and J. E. Darnell. 1996. The antiviral state induced by alpha interferon and gamma interferon requires transcriptionally active Stat1 protein. J. Virol. 70:647-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ivashkiv, L. B. 2003. Type I interferon modulation of cellular responses to cytokines and infectious pathogens: potential role in SLE pathogenesis. Autoimmunity 36:473-479. [DOI] [PubMed] [Google Scholar]
- 13.Iwase, S., Y. Furukawa, J. Kikuchi, M. Nagai, Y. Terui, M. Nakamura, and H. Yamada. 1997. Modulation of E2F activity is linked to interferon-induced growth suppression of hematopoietic cells. J. Biol. Chem. 272:12406-12414. [DOI] [PubMed] [Google Scholar]
- 14.Kumagai, N., S. H. Benedict, G. B. Mills, and E. W. Gelfand. 1987. Requirements for the simultaneous presence of phorbol esters and calcium ionophores in the expression of human T lymphocyte proliferation-related genes. J. Immunol. 139:1393-1399. [PubMed] [Google Scholar]
- 15.Lee, C.-K., R. Gimeno, and D. E. Levy. 1999. Differential regulation of constitutive major histocompatibility complex class I expression in T and B lymphocytes. J. Exp. Med. 190:1451-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee, C.-K., D. T. Rao, R. Gertner, R. Gimeno, A. B. Frey, and D. E. Levy. 2000. Distinct requirements for IFNs and STAT1 in NK cell function. J. Immunol. 165:3571-3577. [DOI] [PubMed] [Google Scholar]
- 17.Lee, C.-K., R. Raz, R. Gimeno, R. Gertner, B. Wistinghausen, K. Takeshita, R. A. DePinho, and D. E. Levy. 2002. STAT3 is a negative regulator of granulopoiesis but is not required for G-CSF-dependent differentiation. Immunity 17:63-72. [DOI] [PubMed] [Google Scholar]
- 18.Lee, C.-K., E. Smith, R. Gimeno, R. Gertner, and D. E. Levy. 2000. STAT1 affects lymphocyte survival and proliferation partially independent of its role downstream of IFN-gamma. J. Immunol. 164:1286-1292. [DOI] [PubMed] [Google Scholar]
- 19.Levy, D. E. 1998. Analysis of interferon-regulated proteins binding the interferon-alpha-stimulated response element. Methods 15:167-174. [DOI] [PubMed] [Google Scholar]
- 20.Levy, D. E., and J. E. Darnell, Jr. 2002. STATs: transcriptional control and biological impact. Nat. Rev. Mol. Cell. Biol. 3:651-662. [DOI] [PubMed] [Google Scholar]
- 21.Levy, D. E., and C.-K. Lee. 2002. What does Stat3 do? J. Clin. Investig. 109:1143-1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meraz, M. A., M. J. White, K. C. F. Sheehan, E. A. Bach, S. J. Rodig, A. S. Dighe, D. H. Kaplan, J. K. Riely, A. C. Greenlund, D. Campbell, K. Carver-Moore, R. N. DuBois, R. Clark, M. Aguet, and R. D. Schreiber. 1996. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell 84:431-442. [DOI] [PubMed] [Google Scholar]
- 23.Müller, U., U. Steinhoff, L. F. L. Reis, S. Hemmi, J. Pavlovic, R. M. Zinkernagel, and M. Aguet. 1994. Functional role of type I and type II interferons in antiviral defense. Science 264:1918-1921. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen, K. B., T. P. Salazar-Mather, M. Y. Dalod, J. B. Van Deusen, X. Q. Wei, F. Y. Liew, M. A. Caligiuri, J. E. Durbin, and C. A. Biron. 2002. Coordinated and distinct roles for IFN-α/β, IL-12, and IL-15 regulation of NK cell responses to viral infection. J. Immunol. 169:4279-4287. [DOI] [PubMed] [Google Scholar]
- 25.Park, C., S. Li, E. Cha, and C. Schindler. 2000. Immune response in Stat2 knockout mice. Immunity 13:795-804. [DOI] [PubMed] [Google Scholar]
- 26.Pernis, A., S. Gupta, K. J. Gollob, E. Garfein, R. L. Coffman, C. Schindler, and P. Rothman. 1995. Lack of interferon gamma receptor beta chain and the prevention of interferon gamma signaling in TH1 cells. Science 269:245-247. [DOI] [PubMed] [Google Scholar]
- 27.Qing, Y., and G. R. Stark. 2004. Alternative activation of STAT1 and STAT3 in response to interferon-gamma. J. Biol. Chem. 279:41679-41685. [DOI] [PubMed] [Google Scholar]
- 28.Ramana, C. V., M. P. Gil, R. D. Schreiber, and G. R. Stark. 2002. Stat1-dependent and -independent pathways in IFN-γ-dependent signaling. Trends Immunol. 23:96-101. [DOI] [PubMed] [Google Scholar]
- 29.Raz, R., J. E. Durbin, and D. E. Levy. 1994. Acute phase response factor and additional members of the interferon-stimulated gene factor 3 family integrate diverse signals from cytokines, interferons, and growth factors. J. Biol. Chem. 269:24391-24395. [PubMed] [Google Scholar]
- 30.Raz, R., C.-K. Lee, L. A. Cannizzaro, P. d'Eustachio, and D. E. Levy. 1999. Essential role of STAT3 for embryonic stem cell pluripotency. Proc. Natl. Acad. Sci. USA 96:2846-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ronnblom, L., and G. V. Alm. 2001. An etiopathogenic role for the type I IFN system in SLE. Trends Immunol. 22:427-431. [DOI] [PubMed] [Google Scholar]
- 32.Sen, G. C. 2001. Viruses and interferons. Annu. Rev. Microbiol. 55:255-281. [DOI] [PubMed] [Google Scholar]
- 33.Stark, G. R., I. M. Kerr, B. R. Williams, R. H. Silverman, and R. D. Schreiber. 1998. How cells respond to interferons. Annu. Rev. Biochem. 67:227-264. [DOI] [PubMed] [Google Scholar]
- 34.Tanabe, Y., T. Nishibori, L. Su, R. M. Arduini, D. P. Baker, and M. David. 2005. Role of STAT1, STAT3, and STAT5 in IFN-α/β responses in T lymphocytes. J. Immunol. 174:609-613. [DOI] [PubMed] [Google Scholar]
- 35.Tanaka, N., M. Sato, M. S. Lamphier, H. Nozawa, E. Oda, S. Noguchi, R. D. Schreiber, Y. Tsujimoto, and T. Taniguchi. 1998. Type I interferons are essential mediators of apoptotic death in virally infected cells. Genes Cells 3:29-37. [DOI] [PubMed] [Google Scholar]
- 36.Teshigawara, K., H. M. Wang, K. Kato, and K. A. Smith. 1987. Interleukin 2 high-affinity receptor expression requires two distinct binding proteins. J. Exp. Med. 165:223-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tovey, M. G., M. Streuli, I. Gresser, J. Gugenheim, B. Blanchard, J. Guymarho, F. Vignaux, and M. Gigou. 1987. Interferon mRNA is produced constitutively in the organs of normal individuals. Proc. Natl. Acad. Sci. USA 84:5038-5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uddin, S., F. Lekmine, A. Sassano, H. Rui, E. N. Fish, and L. C. Platanias. 2003. Role of Stat5 in type I interferon-signaling and transcriptional regulation. Biochem. Biophys. Res. Commun. 308:325-330. [DOI] [PubMed] [Google Scholar]
- 39.Wang, J., N. Pham-Mitchell, C. Schindler, and I. L. Campbell. 2003. Dysregulated Sonic hedgehog signaling and medulloblastoma consequent to IFN-α-stimulated STAT2-independent production of IFN-γ in the brain. J. Clin. Investig. 112:535-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolfer, A., T. Bakker, A. Wilson, M. Nicolas, V. Ioannidis, D. R. Littman, P. P. Lee, C. B. Wilson, W. Held, H. R. MacDonald, and F. Radtke. 2001. Inactivation of Notch 1 in immature thymocytes does not perturb CD4 or CD8 T cell development. Nat. Immunol. 2:235-241. [DOI] [PubMed] [Google Scholar]
- 41.Zang, H., K. Sato, H. Nakajima, C. McKay, P. A. Ney, and J. N. Ihle. 2001. The distal region and receptor tyrosines of the Epo receptor are nonessential for in vivo erythropoiesis. EMBO J. 20:3156-3166. [DOI] [PMC free article] [PubMed] [Google Scholar]