Abstract
Infections caused by the Candida species of human fungal pathogens are a significant medical problem because they can disseminate to nearly every organ of the body. In addition, there are only a few classes of antifungal drugs available to treat patients with invasive fungal infections. Candida infections that are associated with biofilms can withstand much higher concentrations of antifungal drugs compared with infections caused by planktonic cells, thus making biofilm infections particularly challenging to treat. Candida albicans is among the most prevalent fungal species of the human microbiota, asymptomatically colonizing several niches of the body, including the gastrointestinal tract, genitourinary tract, mouth, and skin. Immunocompromised health conditions, dysbiosis of the microbiota, or environmental changes, however, can lead to C. albicans overgrowth, causing infections that range from superficial mucosal infections to severe hematogenously disseminated infections. Here, we review the current knowledge of antifungal drug-resistance mechanisms occurring in Candida biofilms.
Introduction
Of the estimated 1.5–5.1 million species of fungi on Earth, only about 400 are known to cause disease in humans [17,36]. These pathogenic fungi are responsible for approximately one billion infections and more than 1.6 million deaths annually worldwide [44]. Infections caused by the Candida species of human fungal pathogens are responsible for the majority of the reported global deaths by fungi [44]. Over the past few decades, concomitant increases in the immunocompromised population and in the use of antifungal drugs have led to a precipitous increase in the number of infections caused by Candida species [7,44].
Candida albicans is one of the most prevalent commensal fungal species of the human microbiota, asymptomatically colonizing numerous body sites, including the gastrointestinal tract, genitourinary tract, and skin of healthy individuals [20,37,45]. Changes in host immunity, stress, resident microbiota, and other factors can lead to C. albicans overgrowth, causing a wide range of infections ranging from superficial mucosal to severe hematogenously disseminated candidiasis. To date, most studies of C. albicans have been carried out in suspension (planktonic) cultures, however, the medical impact of C. albicans depends on its ability to form a biofilm, a closely packed community of cells encased in an extracellular matrix [3]. Biofilms are notorious for growing on implanted medical devices, such as catheters, pacemakers, dentures, and prosthetic joints, which provide a surface and sanctuary for biofilm growth [27]. In addition to these abiotic surfaces, biofilms can also grow on biotic surfaces, such as mucosal epithelial linings [28,48].
Unlike the diversity of antibiotic drug classes available for use against bacterial pathogens, there are only three major classes of antifungal drugs used to treat invasive fungal infections: azoles, echinocandins, and polyenes. Azoles (e.g. fluconazole) are the most prescribed class of antifungal drugs used to treat both systemic and superficial fungal infections, polyenes (e.g. amphotericin B) are the oldest class of antifungal drugs used to treat severe systemic fungal infections, and echinocandins (e.g. caspofungin) are the newest class of antifungal drugs typically used to treat recalcitrant fungal infections.
Drug resistance in Candida biofilms
A large body of research in the field has focused on understanding the mechanisms of acquired antifungal drug resistance and tolerance in planktonic (i.e. free-floating) Candida cells, and several excellent reviews have been recently published on this topic [10,24]. The major planktonic antifungal drug-resistance and tolerance mechanisms include efflux pump overexpression after exposure to antifungal drugs, mutations in genes that encode drug target enzymes (e.g. ERG11), loss-of-function mutations in genes in the ergosterol biosynthesis pathway (e.g. ERG3), and mutations in FKS1, which encodes 1,3-β-glucan synthase. These acquired planktonic-resistance mechanisms can be shared between both planktonic and biofilm states. However, we know that when the same planktonic strains are grown as biofilms, these resistance mechanisms only account for a proportion of the resistance observed in biofilms. Thus, existing as a biofilm affords Candida species with additional resistance and tolerance mechanisms that are unique and specific to the biofilm state. In the following sections of this review, we focus on the mechanisms of antifungal resistance and tolerance that are specific to biofilms.
C. albicans biofilms are inherently resistant to the majority of known antifungal drugs, making biofilm infections particularly challenging to treat. Resistance and tolerance of C. albicans biofilms to antifungal drugs is multifactorial and mechanistically complex, but is largely attributed to (i) the presence of the extracellular matrix, (ii) the upregulation of drug-efflux pumps (independent of exposure to antifungal drugs), and (iii) the presence of persister cells (Figure 1). We next discuss these three biofilm-specific drug-resistance mechanisms in detail and briefly discuss other mechanisms that are implicated in biofilm-specific drug resistance and tolerance, such as increased cell density and quorum sensing, and the upregulation of the general stress response.
Figure 1.
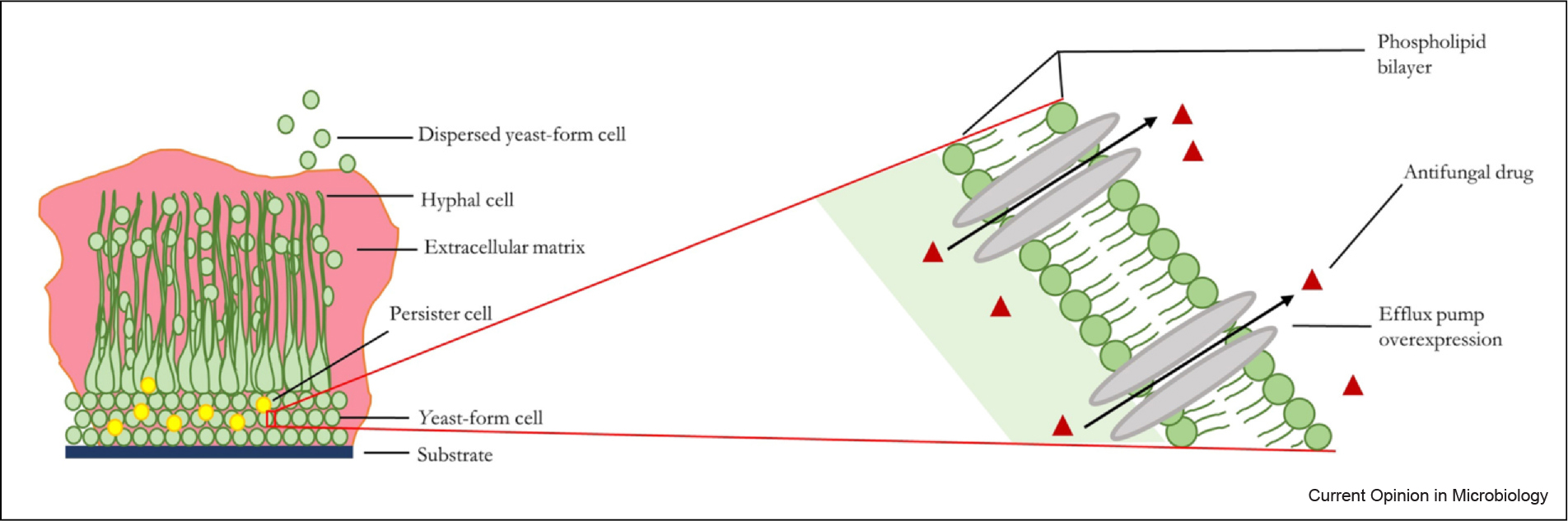
Major drug-resistance and/or tolerance mechanisms in C. albicans biofilms. A schematic representation of the major C. albicans biofilm drug-resistance and/or tolerance mechanisms: the presence of the extracellular matrix, the upregulation of drug-efflux pumps, and the presence of persister cells.
The extracellular matrix
The C. albicans biofilm extracellular matrix is composed of proteins (55%), carbohydrates (25%), lipids (15%), and extracellular DNA (5%), and is a major contributor to antifungal drug resistance and tolerance [32]. The matrix acts both as a physical barrier to drug penetration and supports the overall architecture of the biofilm. Glucans and mannans in the C. albicans biofilm matrix, for example, are known to form a complex that sequesters antifungal drugs, significantly increasing the drug tolerance of biofilms compared with planktonic cells [8,30]. Glucan synthesis by Fks1 is a known mechanism that contributes to C. albicans biofilm antifungal drug resistance, and it has been shown that increased expression of FKS1 impacts the susceptibility of C. albicans biofilms to antifungal drugs by increasing the levels of biofilm matrix sequestration, thus preventing the antifungal drugs from reaching their cellular targets [33]. Interestingly, this specific Fks1-mediated resistance mechanism appears to be biofilm-specific since modulating FKS1 levels in C. albicans cells grown planktonically, had no impact on antifungal drug susceptibility [33]. We note, however, that several mutations in short conserved regions of FKS1 have been linked to caspofungin resistance in clinical C. albicans isolates grown under planktonic conditions [2]. In addition, glucanases, such as Bgl2 and Xog1, have been shown to be required for assembly of the polysaccharide components of the extracellular matrix and have known roles in antifungal drug sequestration [46]. The importance of matrix glucans and mannans in contributing to biofilm drug tolerance appears to be a conserved mechanism that is also known to occur in biofilms formed by other Candida species, such as Candida glabrata, Candida parapsilosis, Candida tropicalis, and Candida auris [8,9]. Notably, biofilms are typically polymicrobial in host settings, forming complex biofilms with many different species, which ultimately changes the composition of the matrix. C. albicans can form polymicrobial biofilms with several other Candida species as well as with bacteria, such as Staphylococcus aureus, Escherichia coli, and Streptococcus mutans, which can further alter the drug sequestration abilities of these complex biofilms [6,12,16,18,49].
Recent work has shown that Candida species release extracellular vesicles during biofilm formation that transport components of the extracellular matrix, including glucan and mannan, throughout the biofilm structure [30,50–52]. Interestingly, individual vesicle cargo proteins were shown to play roles in drug sequestration via the glucan–mannan complex of the extracellular matrix. For example, the tos1Δ/Δ strain showed enhanced antifungal susceptibility in C. albicans, C. tropicalis, C. parapsilosis, C. glabrata, and C. auris relative to its complemented strain [50,51]. These findings indicate that extracellular vesicles play important roles in contributing to drug tolerance of Candida biofilms, and could be a novel target for future antifungal drug therapies.
Last, the composition of the biofilm matrix is thought to impact how C. albicans interacts with the host innate immune system. For example, a pmr1Δ/Δ strain, which is unable to produce matrix mannan, forms biofilms that are more susceptible to neutrophil killing than the reference strain [15]. In addition, the release of host neutrophil extracellular traps (NETs) was increased in scanning electron micrographs of biofilms formed by the pmr1Δ/Δ strain compared with the reference strain, further supporting the idea that matrix composition contributes to the immune evasion properties of Candida biofilms [15].
Upregulation of drug-efflux pumps
There are two major classes of efflux pumps that modulate drug exportation in C. albicans: the ATP-binding cassette superfamily containing Cdr1/Cdr2 transporters, regulated by the Tac1 transcriptional regulator, and the major facilitator class containing Mdr1 transporters, regulated by the Mrr1 transcriptional regulator. These efflux pumps are typically upregulated in planktonic cells only when they are exposed to antifungal drugs, however, in biofilms, these efflux pumps are highly upregulated during the adherence stage of biofilm formation and remain upregulated as the biofilm matures, irrespective of whether or not an antifungal drug is present [11,34,39]. It seems likely that this early upregulation of C. albicans drug-efflux pumps may play an important role in the establishment of the biofilm within a host niche and may have evolved as an adaptive response to inhibitory compounds produced by competing microorganisms within the host environment.
Persister cells
Persister cells are metabolically dormant variants of microbial cells that form stochastically in microbial populations and are highly tolerant to antimicrobial drugs [22,25,26]. In C. albicans biofilms, persister cells are thought to form upon surface attachment and exist primarily in the depths of the biofilm basal layer as a small subset of the yeast-form cell population [23,35]. The presence of persister cells within C. albicans biofilms was first discovered when biofilms were treated with amphotericin B, and a biphasic fungal cell death response was observed [22,29]. The antifungal drug tolerance observed for persister cells is due to the metabolically dormant state of the cells and is independent of drug-efflux pump expression and cell membrane or cell wall composition. Interestingly, patients suffering from long-term Candida infections showed significantly higher persister cell frequency than those with intermittent Candida infections, suggesting that persister cells may be important players in the development of recurrent infections [23].
Other mechanisms contributing to drug resistance and/or tolerance in Candida biofilms
In the biofilm environment, cells are densely packed together and communicate with one another via quorum sensing in a cell density-dependent manner. The presence of closely packed cells in a C. albicans biofilm leads to increased drug tolerance because the high number of cells in the community protects cells located deeper within the biofilm architecture from exposure to antifungal drugs. Farnesol and tyrosol are two quorum sensing molecules that exert opposing physiological effects on C. albicans cells. Farnesol has been shown to inhibit both hyphal formation and biofilm development, while tyrosol has been shown to stimulate adherence and hyphal production in the early stages of biofilm formation [1,5,13,40,43]. Treatment of C. albicans cells with farnesol has been reported to induce global gene expression changes, including the activation of drug-resistance genes through the two-component-signaling histidine kinase Chk1 [4,38,43]. Interestingly, treatment of C. parapsilosis cells with tyrosol has been reported to lead to the upregulation of the drug-efflux pump genes CDR1 and MDR1. Despite these findings that farnesol and tyrosol upregulate a subset of genes involved in drug resistance, because farnesol and tyrosol have such significant effects on the physiology of fungal cells, they have nevertheless been explored as potential antifungal therapies [14,31,43]. Currently, the utility of farnesol and tyrosol as antifungal therapies is inconclusive and controversial, and additional studies are needed to mechanistically understand the complex physiological effects of farnesol and tyrosol, and of quorum sensing more generally, on Candida biofilms.
The upregulation of general stress responses in Candida biofilms can result in increased drug resistance in biofilms [41]. Surface contact of C. albicans cells results in the activation and accumulation of Mkc1, the terminal mitogen-activated protein kinase of the protein kinase C pathway that is normally activated in response to cell wall stress [19]. C. albicans biofilms formed by the mkc1Δ/Δ strain are abnormally structured and contain fewer hyphal cells compared with the wild-type strain, and the cells of the mkc1Δ/Δ strain are 100 times more susceptible to fluconazole than the wild-type strain [19,42]. Mkc1 is known to be involved in resistance to azoles and echinocandins, which is mediated by Hsp90 and calcineurin. Indeed, pharmacological inhibition of calcineurin or Hsp90 is synergistic with fluconazole against C. albicans biofilms [21,47]. Based on these findings, the Mkc1 contact-activated signaling pathway may be an interesting pathway to explore in the search for novel antibiofilm drug targets.
Concluding remarks
There are no biofilm-specific drugs in the market that effectively treat biofilm infections in patients. The resistance and tolerance of fungal biofilms to standard antifungal drugs is complex and multifactorial. Biofilms provide physical protection from antifungal drugs (e.g. via the production of the extracellular matrix) and cells in biofilms are intrinsically resistant to antifungal drugs due to their constitutive upregulation of drug-efflux pumps and their altered metabolic states (e.g. via metabolically inactive persister cells). Deeper mechanistic understanding of these and other unique biofilm properties will be important in the future development of antifungal drugs with efficacy against biofilms. In addition, since biofilms are typically polymicrobial in nature, future studies will also need to extensively explore how different microbial species work together to form biofilms that are associated with common infections in patients, and identify novel drug targets for polymicrobial biofilm infections.
Acknowledgments
This work was supported by the National Institutes of Health (NIH) National Institute of General Medical Sciences (NIGMS) award R35GM124594 and by the Kamangar Family in the form of an endowed chair to C.J.N. We thank Austin Perry for his assistance with the figure and all members of the Nobile lab for feedback on the paper. J.K. thanks Vāhigurū Jī for insurmountable courage, patience, and strength.
Footnotes
Conflict of interest statement
C.J.N. is a cofounder of BioSynesis Inc., a company developing diagnostics and therapeutics for biofilm infections.
Data availability
No data were used for the research described in the article.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Alem MAS, Oteef MDY, Flowers TH, Douglas LJ: Production of tyrosol by Candida albicans biofilms and its role in quorum sensing and biofilm development. Eukaryot Cell 2006, 5:1770–1779, 10.1128/EC.00219-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balashov SV, Park S, Perlin DS: Assessing resistance to the echinocandin antifungal drug caspofungin in Candida albicans by profiling mutations in FKS1. Antimicrob Agents Chemother 2006, 50:2058–2063, 10.1128/AAC.01653-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barantsevich N, Barantsevich E: Diagnosis and treatment of invasive candidiasis. Antibiotics 2022, 11:718, 10.3390/antibiotics11060718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao Y-Y, Cao Y-B, Xu Z, Ying K, Li Y, Xie Y, Zhu Z-Y, Chen W-S, Jiang Y-Y: cDNA microarray analysis of differential gene expression in Candida albicans biofilm exposed to farnesol. Antimicrob Agents Chemother 2005, 49:584–589, 10.1128/AAC.49.2.584-589.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen H, Fujita M, Feng Q, Clardy J, Fink GR: Tyrosol is a quorum-sensing molecule in Candida albicans. Proc Natl Acad Sci USA 2004, 101:5048–5052, 10.1073/pnas.0401416101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Brucker K, Tan Y, Vints K, De Cremer K, Braem A, Verstraeten N, Michiels J, Vleugels J, Cammue BPA, Thevissen K: Fungal β-1,3-glucan increases ofloxacin tolerance of Escherichia coli in a polymicrobial E. coli/Candida albicans biofilm. Antimicrob Agents Chemother 2015, 59:3052–3058, 10.1128/AAC.04650-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Oliveira Santos GC, Vasconcelos CC, Lopes AJO, de Sousa Cartágenes M, do S, Filho AKDB, do Nascimento FRF, Ramos RM, Pires ERRB, de Andrade MS, Rocha FMG, de Andrade Monteiro C: Candida infections and therapeutic strategies: mechanisms of action for traditional and alternative agents. Front Microbiol 2018, 9:1351, 10.3389/fmicb.2018.01351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dominguez E, Zarnowski R, Sanchez H, Covelli AS, Westler WM, Azadi P, Nett J, Mitchell AP, Andes DR: Conservation and divergence in the Candida species biofilm matrix mannan-glucan complex structure, function, and genetic control. mBio 2018, 9:e00451–18, 10.1128/mBio.00451-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dominguez EG, Zarnowski R, Choy HL, Zhao M, Sanchez H, Nett JE, Andes DR: Conserved role for biofilm matrix polysaccharides in Candida auris drug resistance. mSphere 2019, 4:e00680–18, 10.1128/mSphereDirect.00680-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisher MC, Alastruey-Izquierdo A, Berman J, Bicanic T, Bignell EM, Bowyer P, Bromley M, Brüggemann R, Garber G, Cornely OA, Gurr SJ, Harrison TS, Kuijper E, Rhodes J, Sheppard DC, Warris A, White PL, Xu J, Zwaan B, Verweij PE: Tackling the emerging threat of antifungal resistance to human health. Nat Rev Microbiol 2022, 20:557–571, 10.1038/s41579-022-00720-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gulati M, Nobile CJ: Candida albicans biofilms: development, regulation, and molecular mechanisms. Microbes Infect 2016, 18:310–321, 10.1016/j.micinf.2016.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harriott MM, Noverr MC: Candida albicans and Staphylococcus aureus form polymicrobial biofilms: effects on antimicrobial resistance. Antimicrob Agents Chemother 2009, 53:3914–3922, 10.1128/AAC.00657-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hornby JM, Jensen EC, Lisec AD, Tasto JJ, Jahnke B, Shoemaker R, Dussault P, Nickerson KW: Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl Environ Microbiol 2001, 67:2982–2992, 10.1128/AEM.67.7.2982-2992.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jakab Á, Toth́ Z, Nagy F, Nemes D, Bacskaý I, Kardos G, Emri T, Pocsí I Majoros L, Kovacś R: Physiological and transcriptional responses of Candida parapsilosis to exogenous tyrosol. Appl Environ Microbiol 2019, 85:e01388–19, 10.1128/AEM.01388-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. •. Johnson CJ, Cabezas-Olcoz J, Kernien JF, Wang SX, Beebe DJ, Huttenlocher A, Ansari H, Nett JE: The extracellular matrix of Candida albicans biofilms impairs formation of neutrophil extracellular traps. PLoS Pathog 2016, 12:e1005884, 10.1371/journal.ppat.1005884. This work shows that the extracellular matrix plays a major role in protecting the C. albicans biofilm from killing by NETs.
- 16.Kim D, Liu Y, Benhamou RI, Sanchez H, Simón-Soro Á, Li Y, Hwang G, Fridman M, Andes DR, Koo H: Bacterial-derived exopolysaccharides enhance antifungal drug tolerance in a cross-kingdom oral biofilm. ISME J 2018, 12:1427–1442, 10.1038/s41396-018-0113-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Köhler JR, Casadevall A, Perfect J: The spectrum of fungi that infects humans. Cold Spring Harb Perspect Med 2014, 5:a019273, 10.1101/cshperspect.a019273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kong EF, Tsui C, Kucharíková S, Andes D, Van Dijck P, Jabra-Rizk MA: Commensal protection of Staphylococcus aureus against antimicrobials by Candida albicans biofilm matrix. mBio 2016, 7:e01365–16, 10.1128/mBio.01365-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. •. Kumamoto CA: A contact-activated kinase signals Candida albicans invasive growth and biofilm development. Proc Natl Acad Sci USA 2005, 102:5576–5581, 10.1073/pnas.0407097102. This work shows that physical contact of C. albicans cells with a surface results in activation of the Mkc1 pathway, and that Mkc1 is important for normal biofilm formation.
- 20.Kumamoto CA, Gresnigt MS, Hube B: The gut, the bad and the harmless: Candida albicans as a commensal and opportunistic pathogen in the intestine. Curr Opin Microbiol 2020, 56:7–15, 10.1016/j.mib.2020.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LaFayette SL, Collins C, Zaas AK, Schell WA, Betancourt-Quiroz M, Gunatilaka AAL, Perfect JR, Cowen LE: PKC signaling regulates drug resistance of the fungal pathogen Candida albicans via circuitry comprised of Mkc1, calcineurin, and Hsp90. PLoS Pathog 2010, 6:e1001069 10.1371/journal.ppat.1001069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LaFleur MD, Kumamoto CA, Lewis K: Candida albicans biofilms produce antifungal-tolerant persister cells. Antimicrob Agents Chemother 2006, 50:3839–3846, 10.1128/AAC.00684-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. •. Lafleur MD, Qi Q, Lewis K: Patients with long-term oral carriage harbor high-persister mutants of Candida albicans. Antimicrob Agents Chemother 2010, 54:39–44, 10.1128/AAC.00860-09. This work shows that patients suffering from long-term Candida infections have higher persister cell frequency than patients with intermittent Candida infections, suggesting that persister cells are important players in the development of recurrent infections.
- 24.Lee Y, Puumala E, Robbins N, Cowen LE: Antifungal drug resistance: molecular mechanisms in Candida albicans and beyond. Chem Rev 2021, 121:3390–3411, 10.1021/acs.chemrev.0c00199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewis K: Persister cells. Annu Rev Microbiol 2010, 64:357–372, 10.1146/annurev.micro.112408.134306 [DOI] [PubMed] [Google Scholar]
- 26.Lewis K: Multidrug tolerance of biofilms and persister cells. Curr Top Microbiol Immunol 2008, 322:107–131, 10.1007/978-3-540-75418-3_6 [DOI] [PubMed] [Google Scholar]
- 27.Lohse MB, Gulati M, Johnson AD, Nobile CJ: Development and regulation of single- and multi-species Candida albicans biofilms. Nat Rev Microbiol 2018, 16:19–31, 10.1038/nrmicro.2017.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopes JP, Lionakis MS: Pathogenesis and virulence of Candida albicans. Virulence 2022, 13:89–121, 10.1080/21505594.2021.2019950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mathé L, Van Dijck P: Recent insights into Candida albicans biofilm resistance mechanisms. Curr Genet 2013, 59:251–264, 10.1007/s00294-013-0400-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitchell KF, Zarnowski R, Sanchez H, Edward JA, Reinicke EL, Nett JE, Mitchell AP, Andes DR: Community participation in biofilm matrix assembly and function. Proc Natl Acad Sci USA 2015, 112:4092–4097, 10.1073/pnas.1421437112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monteiro DR, Arias LS, Fernandes RA, Deszo da Silva LF, de Castilho MOVF, da Rosa TO, Vieira APM, Straioto FG, Barbosa DB, Delbem ACB: Antifungal activity of tyrosol and farnesol used in combination against Candida species in the planktonic state or forming biofilms. J Appl Microbiol 2017, 123:392–400, 10.1111/jam.13513 [DOI] [PubMed] [Google Scholar]
- 32.Nett JE, Andes DR: Contributions of the biofilm matrix to Candida pathogenesis. J Fungi 2020, 6:E21 10.3390/jof6010021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nett JE, Crawford K, Marchillo K, Andes DR: Role of Fks1p and matrix glucan in Candida albicans biofilm resistance to an echinocandin, pyrimidine, and polyene. Antimicrob Agents Chemother 2010, 54:3505–3508, 10.1128/AAC.00227-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nobile CJ, Fox EP, Nett JE, Sorrells TR, Mitrovich QM, Hernday AD, Tuch BB, Andes DR, Johnson AD: A recently evolved transcriptional network controls biofilm development in Candida albicans. Cell 2012, 148:126–138, 10.1016/j.cell.2011.10.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nobile CJ, Johnson AD: Candida albicans biofilms and human disease. Annu Rev Microbiol 2015, 69:71–92, 10.1146/annurev-micro-091014-104330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Brien HE, Parrent JL, Jackson JA, Moncalvo J-M, Vilgalys R: Fungal community analysis by large-scale sequencing of environmental samples. Appl Environ Microbiol 2005, 71:5544–5550, 10.1128/AEM.71.9.5544-5550.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pérez JC: Fungi of the human gut microbiota: roles and significance. Int J Med Microbiol IJMM 2021, 311: 151490 10.1016/j.ijmm.2021.151490 [DOI] [PubMed] [Google Scholar]
- 38.Perumal P, Mekala S, Chaffin WL: Role for cell density in antifungal drug resistance in Candida albicans biofilms. Antimicrob Agents Chemother 2007, 51:2454–2463, 10.1128/AAC.01237-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramage G, Bachmann S, Patterson TF, Wickes BL, López-Ribot JL: Investigation of multidrug efflux pumps in relation to fluconazole resistance in Candida albicans biofilms. J Antimicrob Chemother 2002, 49:973–980, 10.1093/jac/dkf049 [DOI] [PubMed] [Google Scholar]
- 40.Ramage G, Saville SP, Wickes BL, López-Ribot JL: Inhibition of Candida albicans biofilm formation by farnesol, a quorum-sensing molecule. Appl Environ Microbiol 2002, 68:5459–5463, 10.1128/AEM.68.11.5459-5463.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robbins N, Caplan T, Cowen LE: Molecular evolution of antifungal drug resistance. Annu Rev Microbiol 2017, 71:753–775, 10.1146/annurev-micro-030117-020345 [DOI] [PubMed] [Google Scholar]
- 42.Robbins N, Uppuluri P, Nett J, Rajendran R, Ramage G, Lopez-Ribot JL, Andes D, Cowen LE: Hsp90 governs dispersion and drug resistance of fungal biofilms. PLoS Pathog 2011, 7:e1002257 10.1371/journal.ppat.1002257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. •. Rodrigues CF, Černáková L: Farnesol and tyrosol: secondary metabolites with a crucial quorum-sensing role in Candida biofilm development. Genes 2020. 11:E444 10.3390/genes11040444. This article provides a useful review of the roles of farnesol and tyrosol in Candida biofilms.
- 44.Rokas A: Evolution of the human pathogenic lifestyle in fungi. Nat Microbiol 2022, 7:607–619, 10.1038/s41564-022-01112-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Romo JA, Kumamoto CA: On commensalism of Candida. J Fungi 2020, 6:E16, 10.3390/jof6010016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. •. Taff HT, Nett JE, Zarnowski R, Ross KM, Sanchez H, Cain MT, Hamaker J, Mitchell AP, Andes DR: A Candida biofilm-induced pathway for matrix glucan delivery: implications for drug resistance. PLoS Pathog 2012, 8:e1002848, 10.1371/journal.ppat.1002848. This work shows that the glucanases Bgl2 and Xog1 are critical for assembly of the polysaccharide components of the extracellular matrix of Candida biofilms and play roles in antifungal drug sequestration.
- 47.Uppuluri P, Nett J, Heitman J, Andes D: Synergistic effect of calcineurin inhibitors and fluconazole against Candida albicans biofilms. Antimicrob Agents Chemother 2008, 52:1127–1132, 10.1128/AAC.01397-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Valle Arevalo A, Nobile CJ: Interactions of microorganisms with host mucins: a focus on Candida albicans. FEMS Microbiol Rev 2020, 44:645–654, 10.1093/femsre/fuaa027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vila T, Kong EF, Montelongo-Jauregui D, Van Dijck P, Shetty AC, McCracken C, Bruno VM, Jabra-Rizk MA: Therapeutic implications of C. albicans-S. aureus mixed biofilm in a murine subcutaneous catheter model of polymicrobial infection. Virulence 2021, 12:835–851, 10.1080/21505594.2021.1894834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zarnowski R, Noll A, Chevrette MG, Sanchez H, Jones R, Anhalt H, Fossen J, Jaromin A, Currie C, Nett JE, Mitchell A, Andes DR: Coordination of fungal biofilm development by extracellular vesicle cargo. Nat Commun 2021, 12:6235, 10.1038/s41467-021-26525-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. ••. Zarnowski R, Sanchez H, Jaromin A, Zarnowska UJ, Nett JE, Mitchell AP, Andes D: A common vesicle proteome drives fungal biofilm development. Proc Natl Acad Sci USA 2022, 119:e2211424119, 10.1073/pnas.2211424119. This work shows that extracellular vesicle cargo proteins play important roles in contributing to drug tolerance of Candida biofilms.
- 52.Zarnowski R, Westler WM, Lacmbouh GA, Marita JM, Bothe JR, Bernhardt J, Lounes-Hadj Sahraoui A, Fontaine J, Sanchez H, Hatfield RD, Ntambi JM, Nett JE, Mitchell AP, Andes DR: Novel entries in a fungal biofilm matrix encyclopedia. mBio 2014, 5:e01333–01314, 10.1128/mBio.01333-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data were used for the research described in the article.


