Abstract
Histone acetyltransferase (HAT) activities of proteins such as p300, CBP, and P/CAF play important roles in activation of gene expression. We now show that the HAT activity of p300 can also be required for down-regulation of transcription by a DNA binding repressor protein. Promyelocytic leukemia zinc finger (PLZF), originally identified as a fusion with retinoic acid receptor alpha in rare cases of all-trans-retinoic acid-resistant acute promyelocytic leukemia, is a transcriptional repressor that recruits histone deacetylase-containing corepressor complexes to specific DNA binding sites. PLZF associates with p300 in vivo, and its ability to repress transcription is specifically dependent on HAT activity of p300 and acetylation of lysines in its C-terminal C2-H2 zinc finger motif. An acetylation site mutant of PLZF does not repress transcription and is functionally deficient in a colony suppression assay despite retaining its abilities to interact with corepressor/histone deacetylase complexes. This is due to the fact that acetylation of PLZF activates its ability to bind specific DNA sequences both in vitro and in vivo. Taken together, our results indicate that a histone deacetylase-dependent transcriptional repressor can be positively regulated through acetylation and point to an unexpected role of a coactivator protein in transcriptional repression.
Alterations of chromatin structure by covalent modification of nucleosomal histones at specific lysine residues in their amino-terminal tails play a major role in regulation of gene expression (26). Over the past few years, a large number of chromatin-modifying factors and complexes have been identified and characterized (see references 1, 15, and 27) and references therein for reviews). Intrinsic histone acetyltransferase (HAT) activities have been found to be associated with a number of transcriptional coactivator proteins, such as p300 (62) and P/CAF (79) (see also references 12 and 44 for reviews).
The results of these studies have provided an explanation for the direct relationship between histone acetylation and gene transcription. However, in addition to histones, acetylation of gene-specific (see below) and basal transcription factors (36) by specific HATs has also been shown to play a role in regulating gene expression. For example, p53 is acetylated at specific lysine residues by p300 in vitro and in vivo (32). Acetylation of p53 contributes to its activation by facilitating a transition to a conformation with a higher affinity to its target DNA (32). Key hematopoietic transcription factors such as GATA1 (9) and ELKF (83) were also shown to be acetylated in their DNA binding domains leading to enhanced DNA binding and transcriptional activation. Acetylation has also been shown to antagonize the activities of transcriptional repressors by inhibiting their association with corepressor proteins (8, 35, 82). In the case of hypoxia-inducible factor 1α (HIF-1α), acetylation represented a prerequisite for recruitment of the ubiquitin mediated degradation system (39). Proteins that do not participate directly in transcriptional regulation are also targeted by acetylation (5, 77), suggesting that in analogy to phosphorylation, acetylation serves a general and important regulatory role for protein activity.
The promyelocytic leukemia zinc finger gene (PLZF) was identified through the characterization of a rare case of acute promyelocytic leukemia with a variant chromosomal translocation t(11;17) (q23;q21) and resistance to therapy with all-trans-retinoic acid (81). Leukemic cells that harbor the above chromosomal translocation, which fuses the PLZF and the retinoic acid receptor alpha (RARα) genes, express PLZF-RARα and RARα-PLZF fusion proteins (13). The wild-type PLZF protein is a DNA sequence-specific transcription repressor that is characterized by nine Krüppel-like C2-H2 zinc fingers and an N-terminal BTB/POZ domain (33, 47). The BTB/POZ domain is a conserved structural motif found in a number of pox and zinc finger proteins that has been shown to mediate homo/heterodimerization, nuclear localization, and direct binding of corepressors (2, 3, 6, 21, 33, 55). The POZ domain appears to be critical for the function of the PLZF protein as a transcriptional repressor (33, 55, 56). Studies from our laboratories and others have shown that PLZF can repress transcription through recruitment of nuclear receptor corepressors (N-CoR or SMRT)/histone deacetylase (HDAC) complexes via its POZ domain (20, 31, 33, 34, 48). An additional transcriptional repression domain, which binds the ETO protein and lies between the POZ domain and the zinc finger region, has also been identified (58), and the first two zinc fingers of PLZF were found to be important for repression and activity of the chimeric PLZF-RARα protein (14).
We now show that the ability of PLZF to bind DNA and repress transcription is regulated by acetylation of lysines in its most C-terminal zinc finger motif. Mutating the target lysines to residues that cannot be acetylated or inhibiting p300 HAT activity abolishes the ability of PLZF to bind DNA, repress transcription, and suppress cell growth.
MATERIALS AND METHODS
Expression plasmids.
Constructions of PLZF (1), 5′PLZF (N-terminal region of PLZF, amino acids 1 to 455), 3′PLZF (C-terminal region of PLZF, amino acids 455 to 673), PLZFΔPOZ (PLZF deleted for the BTB/POZ domain, amino acids 1 to 120), cDNA expression vectors, and the glutathione S-transferase (GST)-PLZF plasmid have been previously described (14, 21, 33). PLZFΔZnF9 (PLZF deleted for its last zinc finger, amino acids 625 to 673), PLZF/Zn1 to 5 (PLZF deleted of its zinc fingers 6 to 9, amino acids 552 to 673), PLZF/K-to-R, -K-to-A, and -K-to-Q, bearing point mutations (lysine to arginine, alanine and glutamine residues, respectively) at the codons encoding lysine residues 647, 650, and 653, for zinc finger 9, and at lysine residues 562 and 565 for zinc finger 6 have been constructed by PCR amplification using the Quickchange site-directed mutagenesis kit (Stratagene).
Mammalian and in vitro expression vectors for the full-length and partial p300 were previously described (9). The GAL4(UAS)5-tk-luc reporter was derived from pT109luc (60) plasmid by inserting five copies of the GAL4 DNA binding site (upstream activation sequence) upstream of the minimal herpes simplex virus thymidine kinase promoter (33). The HoxB2-thymidine kinase-luciferase reporter contains two PLZF binding sites (38) upstream of the minimal herpes simplex virus thymidine kinase (tk) promoter, GATCCGGCTACTGTACGGCACGTTGATACTGTACGGG sequence (PLZF binding sites italic), cloned into the BamHI and SalI sites of the polylinker in the pT109 plasmid.
Avidin-biotin complex DNA binding assays.
Sense and antisense oligonucleotides were designed with one PLZF target site derived from the HoxB2 r3/r5 enhancer region (38). The sense (biotinylated in the 5′ end) and the antisense strands were 5′biotin-GCCTTACTGTACAGGACTCCCTTC3′; 5′-GAAGGGAGTCCTGTACAGTAAGGC3′, respectively. Radiolabeled PLZFs were translated in vitro using a programmed rabbit reticulocyte or wheat germ lysates in the presence of [35S]methionine. 293T cells were transfected using the calcium phosphate precipitation method (Promega) with a total of 6 μg DNA in 60-mm plates and harvested 24 h later. Whole-cell extracts were prepared using commercial reagents according to the manufacturer's recommendations (Promega) in a total volume of 200 μl, and 30 μl were used for incubation with biotinylated DNA oligonucleotide. Protein-DNA complexes were pulled down using an avidin-biotin complex DNA (ABCD) binding assay (29) as described. [35S]PLZF was incubated with 25 μl of H buffer (20 mM HEPES, pH 7.7, 50 mM KCl, 20% glycerol, 0.1% NP-40), 2.5 μg poly(dI:dC) (2 mg/ml), and 500 fmol of biotinylated oligonucleotides for 30 min on ice. PLZF-DNA complexes were precipitated by addition of 20 μl of streptavidin-agarose, washed three times with 1 ml of ice-cold H buffer, and analyzed by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE).
Electrophoretic mobility shift assays.
Nuclear extracts were prepared as previously described (38) from 293T cells transiently transfected using the calcium phosphate precipitation method (Promega). Cells were transfected with a total of 6 μg DNA in 60-mm plates and harvested 40 h later. Protein expression was analyzed by SDS-PAGE and visualized by Western blotting. For the electrophoretic mobility shift assays (EMSAs), double-stranded oligonucleotide probes (250 fmol) were end-labeled with [γ-32]ATP using T4 polynucleotide kinase. Nuclear extract (1 to 3 μg) was incubated with radiolabeled probe (approximately 5 fmol) for 40 min on ice in binding buffer (50 mM KCl, 2 mM dithiothreitol, 10 mM HEPES, pH 7.9, 10% glycerol, 1 μg poly[dI:dC], 2 mM MgCl2, and 0.2% NP-40). Protein/DNA complexes were electrophoresed on a 5% polyacrylamide gel and visualized by autoradiography. For competition assays, unlabeled oligonucleotides (200-fold molar excess) were included in the binding reaction. The sequence of the oligonucleotide used for these binding studies (sense strand) was as 5′-ggcTACTGTACggcacgttgaTACTGTAC-ggcacgttgaTACTGTAC-3′ (PLZF binding sites [38] are in uppercase letters).
Chromatin immunoprecipitation assay.
293T cells in 10-cm plates were transfected with 5 μg of either GAL4 or PLZF reporter plasmid plus 10 μg of expression vector, using the calcium phosphate precipitation method. Immunoprecipitation of plasmid DNA plus associated histones was carried out approximately 40 h after transfection according to a previously published protocol (10), with the following modifications. Histone/DNA complexes were cross-linked by addition of 1% formaldehyde to the medium and incubation at 37°C for 10 min. After lysis, the chromatin was sonicated to 0.2 to 1.0 kb and diluted 10-fold in IP buffer (0.01% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl, pH 8.0, 150 mM NaCl, plus protease inhibitors). Protein samples for Western blotting were taken prior to dilution; control samples for assaying input DNA were taken after dilution and decross-linked. Anti-acetylated histone H4 or H3 polyclonal antibody (Upstate Biotechnology) was used for the immunoprecipitation, and the DNA/histone complexes were collected overnight with protein A/G-Sepharose beads (Santa-Cruz). After decross-linking of DNA, sequences spanning GAL4 or PLZF binding sites in each reporter plasmid were detected by semiquantitative PCR using primers (derived from sequences common to both reporters) 5′-GGATCCCCACTTAACACCCAA-3′ (forward) and 5′-CTTGGGAAACTGCTCTTAACTAG-3′ (reverse). The number of cycles was determined empirically to give results that fall within the linear range of this particular PCR assay.
In vitro immunoprecipitation.
[35S]methionine-labeled proteins were synthesized in vitro using the TNT coupled transcription-translation system (Promega), following the supplier's directions. Assays were performed in NETN buffer (20 mM Tris, pH 8.0, 100 mM NaCl, 1 mM EDTA, 0.5% NP-40) at 4°C for 60 min with gentle rocking. Immunocomplexes were isolated by further incubation with an appropriate antibody preadsorbed on protein A/G-Sepharose (Pharmacia), washed five times in H buffer (20 mM HEPES, pH 7.7, 50 mM KCl, 20% glycerol, 0.1% NP-40). Bound proteins were eluted in Laemmeli loading buffer and separated on a 5% or 10% SDS-PAGE. Gels were fixed in 25% isopropanol and 10% acetic acid, dried, and exposed to Kodak Biomax film. Anti-p300 (Santa Cruz Biotechnology), anti-Gal4 (Santa Cruz Biotechnology), rabbit polyclonal anti-acetyl-lysine (Upstate Biotechnology, catalog no. 06-933), and anti-Flag (Sigma) antibodies were purchased from the indicated suppliers and used as directed. The monoclonal and polyclonal anti-PLZF antibodies were previously described (33, 43, 66, 80).
In vitro acetylation.
To test the in vitro acetylation of PLZF by different HATs we took advantage of the fact that the PLZF protein generated using the wheat germ in vitro translation system was not acetylated. Equal amounts of [35S]methionine labeled PLZF (1 to 2 μl from 25 μl of in vitro translation reaction), derived by in vitro translation using wheat germ extract, were incubated with 1 μl of either in vitro-translated HAT or unprogrammed rabbit reticulocyte lysates in a 30-μl final volume of solution containing 50 mM Tris-HCl, pH 8, 10% glycerol, 1 mM dithiothreitol, 1 mM protease inhibitors (Roche complete protease inhibitor cocktail tablets). This reaction solution was adjusted to contain 10 mM of sodium butyrate and 100 μM of acetyl coenzyme A (CoA), and proteins were incubated for 1.5 h at 30°C. After incubation, the acetylation of PLZF was assessed by immunoprecipitation of [35S]methionine-PLZF using 1 μg of an anti-acetyl lysine antibody (Upstate Biotechnology). Bound proteins were eluted in Laemmli loading buffer and separated on a 10% SDS-PAGE. Gels were fixed in 25% isopropanol and 10% acetic acid, dried, and exposed to Kodak Biomax film.
In vivo immunoprecipitation and immunoblotting.
Nuclear extracts were prepared from transfected 293T cells or KG1 cells as described previously (38) and incubated with 10 μg/ml of anti-acetyl lysine, anti-PLZF, and anti-p300 antibodies in NETN buffer at 4°C for 60 min. Immunocomplexes were isolated by incubation with protein A/G-agarose, washed five times in H buffer, analyzed by SDS-PAGE, and visualized by Western blotting using monoclonal anti-PLZF or commercial anti-p300 (Santa Cruz Biotechnology) antibodies for detection.
Preparation of H3-CoA-20-Tat and Lys-CoA-Tat.
Preparation of H3-CoA-20-Tat (Ac-ARTKQTARKSTGGK[CoA]APRKQL-YGRKKRRQRRR-OH) is described elsewhere (19). Lys-CoA-Tat (Ac-Lys[CoA]-YGRKKRRQRRR-OH) was synthesized by the solid-phase method on a Rainin PS3 peptide synthesizer using the Fmoc (N-[9-fluorenyl]methoxycarbonyl) strategy analogously to previously described methods (45, 64). The epsilon amino group of the lysine corresponding to the Lys14 residue of histone H3 was dimethyldioxocyclohexylidene (Dde) protected whereas other Lys residues of H3 fragment were tert-butoxycarbonyl (Boc) protected. Following amino acid couplings and N-terminal acetylation, the Dde group was removed by mixing the fully protected peptide-resin with 2% hydrazine in dimethylformamide for 3 h at room temperature. The peptide resin was then reacted with five equivalents of bromoacetic acid and 5 equivalents diisopropylcarbodiimide for 16 h at room temperature. Peptides were cleaved from the resin with reagent K (trifluoroacetic acid-phenol-H2O-thioanisole-ethanedithiol-triisopropylsilane [81.5:5:5:5:2.5:1]) for 4 h at room temperature and subsequently precipitated with ice-cold diethyl ether.
Precipitated peptides were dissolved in water, flash-frozen, lyophilized, and purified by preparative reversed-phase (C18) high-pressure liquid chromatography (HPLC) using a gradient of H2O-CH3CN-0.05% trifluoroacetic acid. The bromoacetylated peptide was conjugated with two equivalents of CoA in a minimal volume of aqueous 0.5 M trimethylammonium bicarbonate (pH 8) for about 16 h at room temperature, lyophilized, and purified initially by passage over anion exchange chromatography (Dowex 1x8-100, to remove excess CoA) followed by reversed-phase HPLC in a gradient of H2O-CH3CN-0.05% trifluoroacetic acid. Peptides were >95% pure by HPLC, and their structural identities were confirmed by mass spectrometry. Peptide concentrations were determined by UV absorption (260 nm).
Lys-CoA-Tat and H3-CoA-20-Tat HAT inhibition assays.
Details of H3-CoA-20-Tat inhibition of p300 and PCAF HATs have been reported elsewhere (19). Inhibition studies with Lys-CoA-Tat were carried out with purified recombinant human P/CAF-cat (64), GCN5-cat (64), and p300-cat (amino acids 1284 to 1673 (76) as described previously (45, 64). Assays contained 20 μM acetyl-CoA and 10 μM peptide substrate (H3-20) for P/CAF-cat and GCN5-cat and 20 μM acetyl-CoA and 50 μM peptide substrate (H4-20) for p300. The reaction buffer contained 50 mM Tris-HCl (pH 8), 1 mM dithiothreitol, 0.1 mM EDTA, and 50 μg/ml acetylated bovine serum albumin.
Reactions used purified enzymes at concentrations of 2 nM for the P/CAF-cat and GCN5-cat and 5 nM for p300-cat. Assays were carried out at 30°C with reaction volumes of 30 μl. Reactions were initiated with enzyme after the other components equilibrated at 30°C and quenched after 3 to 6 min with 6 μl 6× Tris-tricine gel loading buffer. Mixtures were separated on 16% SDS-Tris-tricine-polyacrylamide gels and dried, and radioactivity was quantified by PhosphorImage analysis (Molecular Dynamics) by comparing to known quantities of 14C-labeled bovine serum albumin standard (NEN Life Science Products). All assays were performed at least twice and agreed within 20%. Enzyme activities were demonstrated to be linear versus enzyme concentration and time in the concentration ranges used. In inhibition assays, the range of inhibitor concentrations used were varied at least 16-fold around the measured 50% inhibitory concentrations (IC50s) and in all cases were at least fivefold greater than the enzyme concentration used.
Cell culture and transient transfection.
Transient transfections using 293T (24) or HeLa (68) cells, maintained in Dulbecco's modified Eagle's medium with 100 U/ml penicillin, 100 μg/ml streptomycin, 20 mM glutamine, and 10% fetal calf serum, were performed using the calcium phosphate precipitation method (Promega) as previously described (33). KG1 (42) cells were grown in RPMI 1640 media (Life Technologies Ltd, Paisley, United Kingdom) containing 100 U/ml penicillin, 100 μg/ml streptomycin, 20 mM glutamine, and 10% fetal calf serum, in a humidified atmosphere of 95% O2 and 5% CO2 at 37°C. Transfection of KG1 cells was carried out through electroporation, using a Bio-Rad Gene Pulser II at 800 μF and 270 V. Approximately 20 × 106 KG1 cells were transfected with 8 μg of pGAL4-(UAS)5-tk-Luc, 2.2 μg of pGAL4(DBD)-P/CAF, or pGAL4(DBD)-p300, and 2 μg of cytomegalovirus-driven β-galactosidase gene expression plasmid (CMV-lacZ) as a control for transfection efficiency.
HeLa cells were grown and transfected in six-well plates using 3 μg of a given expression vector per well and PolyFect transfection reagent (QIAGEN). All cells were harvested for assaying luciferase and β-galactosidase activities 26 to 48 h after transfection. Luciferase assays were normalized using units of β-galactosidase activity. Both assays were carried out using commercial reagents according to the manufacturer's recommendations (Promega). BlueScribe M+ DNA was used as a carrier to equalize the total amount of transfected DNA. In all cotransfection experiments, the total amount of transfected mammalian expression vector was kept constant. All transfections were performed at least three times.
Colony suppression assays.
For colony suppression assay PLZF was cloned into pcDNA3.1/Myc-His (Invitrogen) and the PLZF K-to-A mutant was introduced into pcDNA3.1(-). Both vectors contain the neomycin resistance gene necessary for the selection of transfected colonies. Experiments were carried out essentially as described by Melnick and colleagues (55); 106 SAOS-2 cells were transfected with 10 μg of DNA using Superfect (QIAGEN) following the manufacturer's instructions. After 24 h the cells were diluted 1:40 and incubated in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.5 mg/ml G418 for 3 weeks, replacing the medium every 3 to 4 days. Finally, the colonies were stained with Giemsa and counted.
Immunofluorescence and confocal microscopy.
For immunofluorescence, cells were fixed in 4% paraformaldehyde for 20 min at room temperature. Slides were then washed twice for 5 min in Ca2+- and Mg2+-free phosphate buffered saline (PBS) solution and cytospun (for KG1, HeLa cells were grown and stained on coverslips) onto polylysine-coated slides and permeabilized with 0.3% Triton in PBS for 5 min at room temperature, washed twice for 5 min in PBS, and incubated in blocking buffer (1% bovine serum albumin in PBS) for 30 min at room temperature. Cells were then incubated with mouse monoclonal anti-PLZF antibody (43) (diluted 1:500 in blocking buffer) for 2 h at room temperature, followed by three 5-min washes in PBS. Secondary fluorescein isothiocyanate (FITC)-conjugated anti-mouse antibody (diluted 1:200 in blocking buffer) (Jackson ImmunoResearch Laboratories) was then applied for 2 h. Cells were subsequently washed twice for 5 min in PBS and then twice for 5 min in PBS plus To-pro3 iodide (dilution, 1:10,000). Cells were mounted in Vectarshield mounting medium, sealed with nail varnish, and visualized using the Leica TCS SP2 true confocal system.
RESULTS
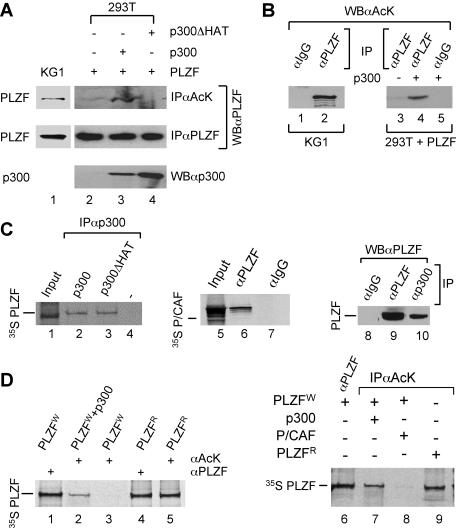
Previous studies have demonstrated that PLZF localizes to specific subnuclear compartments (43, 66). These structures appeared to coincide with those of the promyelocytic leukemia (PML) nuclear bodies (43) suggesting a functional relationship between them and/or PML and PLZF proteins. Given that the PML nuclear bodies may be associated with HAT activity and protein (22), for example in acetylation of p53 (63), we have sought to investigate whether PLZF could be a substrate for acetylation and what effect such a posttranslational modification would have on its function. Initial analysis of PLZF expressed endogenously in KG1 cells indicated that a fraction of the protein could be immunoprecipitated with anti-acetyl-lysine polyclonal antibody, suggesting that it exists in acetylated form (Fig. 1A, lane 1). Following immunoprecipitation with anti-PLZF monoclonal antibody, acetylated PLZF could also be detected by the anti-acetyl-lysine antiserum on a Western blot, confirming that endogenous PLZF is indeed acetylated in KG1 cells (Fig. 1B, lane 2). As expected, no signal was detected when irrelevant immunoglobulin G (IgG) (anti-Gal4) was used as a control in immunoprecipitation step of this experiment (Fig. 1B, lane 1).
FIG. 1.
PLZF protein is acetylated by p300 in vivo. (A) To assess PLZF acetylation, PLZF was immunoprecipitated with a rabbit polyclonal anti-acetyl-lysine antibody (αAcK) prior to Western blotting with the monoclonal anti-PLZF antibody (WBαPLZF). Endogenous PLZF in KG1 cells (lane 1); PLZF expressed in transfected 293T cells in the absence of p300 (lane 2) or in the presence of wild-type (lane 3) or the ΔHAT mutant (lane 4) of p300. To assess the level of expression of p300 proteins in each experiment, Western analysis was performed with a polyclonal anti-p300 antibody (WBαp300). Results of protein expression are shown in lanes 3 and 4 for wild-type p300 and p300ΔHAT, respectively. (B) Similar results reflecting the presence of endogenously acetylated PLZF were obtained using anti-PLZF and anti-acetyl-lysine antibodies for immunoprecipitation and Western blotting, respectively. Immunoprecipitation using anti-Gal4 antibody was used as a control (lanes 1 and 5). Acetylation of PLZF was examined in KG1 (lanes 1 and 2) and transfected 293T cells (lanes 3 to 5) as indicated. (C) PLZF interacts with p300 in vivo and in vitro. In vitro 35S-labeled PLZF was translated using the rabbit reticulocyte system (see input lane 1) and incubated with unlabeled p300 (lane 2) and p300ΔHAT (lane 3) proteins or unprogrammed rabbit reticulocyte (lane 4). Anti-p300 antibody (αp300) was used for coimmunoprecipitation to evaluate interactions between the PLZF and p300 proteins. Similarly, 35S-labeled P/CAF was coimmunoprecipitated with PLZF (lane 6) using anti-PLZF antibody (αPLZF) but not anti-Gal4 control (αIgG). Whole-cell extracts from KG1 were subjected to immunoprecipitation with anti-p300 (αp300, lane 10), polyclonal anti-PLZF (αPLZF, lane 9), or an irrelevant anti-Gal4 IgG (αIgG, lane 8) followed by immunoblotting with a monoclonal anti-PLZF antibody (WBαPLZF). (D) In vitro acetylation of PLZF by p300 proteins. In vitro 35S-labeled PLZF was translated using the wheat germ (W) or rabbit reticulocyte (R) system. The acetylation level of PLZF was assessed by immunoprecipitation of 35S-labeled PLZF with an anti-acetyl-lysine antibody (lanes 2, 3, 5, and 7 to 9) followed by SDS gel electrophoresis and autoradiography of the dried gel. To assess the amount of PLZF in a given sample, immunoprecipitations were also performed with an anti-PLZF antibody (lanes 1, 4, and 6). In vitro-translated PLZF (using wheat germ lysate) was incubated with acetyl-CoA and unprogrammed reticulocyte lysate (PLZFw, lane 3) or in vitro-translated p300 (PLZFw+p300, lanes 2 and 7) or P/CAF (lane 8) for 1.5 h at 30°C and assessed for acetylation.
We then examined a number of HATs for their abilities to interact with PLZF in vitro. Although PLZF could interact with several members of the HAT family of proteins, such as p300 and P/CAF (see Fig. 1C, lanes 1 to 4 and 5 to 7, respectively), only p300 appeared to acetylate PLZF in vitro (Fig. 1D). For in vitro acetylation experiments we took advantage of the fact that in contrast to PLZF generated using rabbit reticulocyte lysate (PLZFR), PLZF translated in vitro in the wheat germ lysate (PLZFW) is not acetylated (Fig. 1D, lane 3, and data not shown). When PLZFW was incubated with in vitro-translated p300 protein and acetyl-CoA, acetylated PLZF was readily detected with anti-acetyl-lysine antibodies (Fig. 1D, lanes 2 and 7). However, acetylated PLZFW was not detected after incubation with acetyl-CoA and in vitro-translated P/CAF (Fig. 1D, lane 8) or in equal amounts of unprogrammed reticulocyte lysate (Fig. 1D, lane 3).
Consistent with these in vitro data, coexpression of p300 with PLZF in 293T cells enhanced its acetylation (Fig. 1A, lane 3). Enhancement of PLZF acetylation following overexpression of p300 in 293T cells could also be detected following immunoprecipitation of proteins with anti-PLZF (but not a control anti-Gal4 IgG) and Western blotting with anti-acetyl-lysine antibodies (Fig. 1B, lanes 3 to 5). As expected, coexpression of a p300 mutant lacking its catalytic domain (p300ΔHAT) with PLZF failed to induce PLZF acetylation in this cellular context (Fig. 1A, lane 4). Note that p300ΔHAT was not impaired for interaction with PLZF (Fig. 1C, lane 3). Interaction between p300 and PLZF could also be observed using mammalian two-hybrid assays (data not shown) and endogenous PLZF and p300 proteins readily coimmunoprecipitated from KG1 cells (Fig. 1C, lane 10), indicating that the two proteins can associate with each other in vivo under physiological concentrations.
The results of the above experiments suggest that PLZF is a direct and specific substrate of the p300 HAT. To test this possibility further, we have treated KG1 cells with two different HAT inhibitors, Lys-CoA-Tat and H3-CoA-20-Tat, and examined the levels of acetylated endogenous PLZF protein by immunoprecipitation with anti-acetyl-lysine and immunoblotting with anti-PLZF antibodies. The above HAT inhibitors (see Materials and Methods for details) are previously described Lys-CoA and H3-CoA-20 (46) linked with an 11-amino-acid human immunodeficiency virus (HIV) Tat transduction domain (30, 59) to enable cellular permeability. Lys-CoA-Tat is moderately selective for the p300 HAT, with the IC50 being an order of magnitude higher than that for P/CAF or GCN5. H3-CoA-20-Tat, on the other hand, displays 100-fold higher specificity for P/CAF than for p300 HAT when assayed in vitro (see Table 1).
TABLE 1.
HAT inhibitor valuesa
| Inhibitor | Mean IC50 (μM) ± SD
|
||
|---|---|---|---|
| p300 | PCAF | GCN5 | |
| Lys-CoA-Tat | 0.25 ± 0.05 | 2.2 ± 0.4 | 2.3 ± 0.5 |
| H3-CoA-20-Tat | 12 ± 3 | 0.04 ± 0.01 | N/D |
Assays were performed as described in Materials and Methods. The IC50 values are defined as the concentration of compound necessary to cause 50% inhibition of acetylation reaction. N/D, not done.
Consistent with data indicating that PLZF is a substrate for acetylation by p300, treatment of KG1 cells with Lys-CoA-Tat but not with the P/CAF-specific inhibitor H3-CoA-20-Tat, completely abolished acetylation of the endogenous PLZF protein (Fig. 2A, lane 2). As expected, Lys-CoA-Tat and H3-CoA-20-Tat effectively inhibited the ability of the GAL4 DNA binding domain (DBD)-p300 (Gal4-p300) or P/CAF (Gal4-P/CAF) fusion to activate transcription from a reporter with GAL4 binding sites when cotransfected into KG1 cells (Fig. 2B and C, respectively). Treatment of KG1 cells with trichostatin A (TSA), a compound that inhibits class I and II histone deacetylase (HDAC) activities, stimulated levels of acetylated PLZF (data not shown), suggesting that it may also be a substrate for deacetylation by class I and/or II HDACs.
FIG. 2.
Inhibition of p300 reduces PLZF acetylation in vivo. (A) Whole-cell lysates were prepared from KG1 cells untreated (lane 1) or treated overnight with the specific inhibitor of p300, Lys-CoA-Tat 10 μM (lane 2), or P/CAF, H3-CoA-20-Tat 10 μM (lane 3). Acetylated PLZF and total protein were immunoprecipitated using the anti-acetyl lysine (αAcK) or anti-PLZF (αPLZF) polyclonal antibodies, and immunoprecipitates were analyzed by Western blotting with a monoclonal antibody raised against PLZF (WBαPLZF). Enhanced chemiluminescence was used for detection (both panels). (B and C) Specific inhibition of p300 (B) and P/CAF (C) activities by Lys-CoA-Tat and H3-CoA-20-Tat in KG1 cells was controlled using a luciferase-based reporter with Gal4 binding sites upstream of the herpes simplex virus thymidine kinase minimal promoter and cotransfected Gal4DBD-p300 (B) or Gal4DBD-P/CAF (C) expression vector.
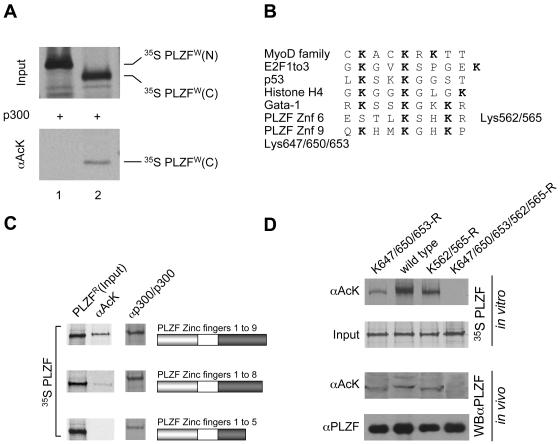
The PLZF protein can be broadly divided into two distinct functional domains: an amino-terminal domain that contains a protein-protein interaction domain (BTB/POZ domain, residues 1 to 120) and a 9-C2-H2 zinc finger carboxy-terminal domain (residues 377 to 673) with sequence-specific DNA binding activity. Initial analysis using isolated amino- and carboxy-terminal regions of PLZF indicated that acetylation by p300 targets the zinc finger region (Fig. 3A). The amino acid sequence comprising the zinc finger region of PLZF contains two clusters of lysine residues, two lysines in zinc finger 6 and three in zinc finger 9, which resemble acetylation sites characterized for histone H4 and some transcription factors such as GATA-1, for example (see Fig. 3B).
FIG. 3.
PLZF is acetylated in the zinc finger DNA binding region. (A) N- and C-terminal parts of PLZF (amino acids 1 to 455 and 455 to 673, respectively) were expressed and labeled in vitro with [35S]methionine using the wheat germ translation system. In vitro-translated proteins were acetylated in the presence of acetyl-CoA and p300. The degree of acetylation was evaluated by immunoprecipitation with anti-acetyl lysine (αAcK) antibodies (lower panel). (B) Alignment of previously described acetylation sites in other proteins and the potential PLZF sites. (C) Mapping of PLZF acetylation sites. Deletion mutants of PLZF were translated in vitro using rabbit reticulocyte lysate and [35S]methionine and were tested for acetylation (PLZFR) by immunoprecipitation with anti-acetyl-lysine antibodies (αAcK). Corresponding deletion mutants are represented by schematics in which the grey box represents the POZ domain of PLZF and the dark box represents its zinc finger region. The input of each PLZF protein is shown in the column labeled PLZFR. Interactions of PLZF proteins with p300 were tested in vitro by immunoprecipitation using p300 protein immobilized on a protein A/G matrix with a p300 polyclonal antibody (αp300/p300) as bait. (D) PLZF lysine mutants assessed for acetylation in vitro and in vivo. Lysine (K) residues of interest were changed to arginines (R). A mutant lacking the zinc finger 9 acetylation site (K647/650/653-R), a mutant lacking the zinc finger 6 acetylation site (K562/565-R), and the double mutant (K647/650/653/562/565-R) were compared to wild-type PLZF (wild type). In vitro and in vivo experiments were performed with in vitro 35S-labeled PLZF proteins and transfected 293T cell extracts, respectively. The acetylation level of PLZF was assessed by immunoprecipitation of 35S-labeled PLZF with an anti-acetyl-lysine antibody (αAcK). Polyclonal anti-PLZF antibody (αPLZF) was used to immunoprecipitate the total protein (input). For in vivo analysis, proteins were immunoprecipitated as above, and immunoprecipitates were subjected to Western blot analysis with monoclonal anti-PLZF antibodies (WBαPLZF). Enhanced chemiluminescence was used for detection.
Subsequent analysis of PLZF lacking zinc fingers 6 to 9 indicated that despite retaining its ability to interact with p300 HAT, this deletion mutant could not be acetylated (Fig. 3C). Deletion of zinc finger 9 alone led to partial loss of PLZF acetylation (>60%), confirming that lysines in both zinc fingers can be acetylated in vitro. To confirm that lysines 562 and 565 (K562/565) in zinc finger 6 and lysines 647, 650, and 653 (K647/650/653) in zinc finger 9 are acetylated, codons encoding these residues were mutated to arginines, which cannot be acetylated. As predicted by the analysis of deletion mutants, the PLZF proteins in which residues K562/565 and K647/650/653 were replaced by arginines were no longer acetylated in vitro and in vivo (Fig. 3D). As expected, mutation of K647/650/653 of zinc finger 9 to arginines resulted only in partial loss of PLZF acetylation, which is consistent with interaction of this mutant with p300 (not shown). Identical results were obtained with the lysines in zinc fingers 9 and/or 6 mutated to alanines (data not shown).
Previous studies have indicated that PLZF functions as a transcriptional repressor. To determine how acetylation affects this function of PLZF we examined the effects of p300 overexpression on PLZF function and the consequences of acetylation site mutants of PLZF on the ability of PLZF to repress transcription from a promoter containing its DNA binding sequences. Surprisingly, PLZF-dependent repression was enhanced by the cotransfection of a p300 expression plasmid but not of a vector expressing the mutant p300ΔHAT that is unable to acetylate PLZF (Fig. 4A). PLZF with lysines in zinc finger 9 mutated to arginines, a mutation that would abolish the ability of p300 to acetylate zinc finger 9, was completely defective in transcriptional repression. Interestingly, when the same mutant was fused to the GAL4 DNA binding domain (DBD) and tested for the ability to repress transcription of a reporter with GAL4 binding sites, it repressed transcription as effectively as the wild-type PLZF-GAL4(DBD) fusion. Furthermore, coexpression of p300 had no effect on the activity of the PLZF-GAL4 fusion. Expression of p300 or mutation of the PLZF protein had no effect on the levels of PLZF (lower panel, Fig. 4A and B).
FIG. 4.
Repression activity of PLZF is regulated by p300 HAT activity. The repression activity of PLZF mutant K647/650/653-R, deficient in acetylation of zinc finger 9 lysines (PLZFmut), was tested in transfection experiments using the luciferase reporter with a herpes simplex virus thymidine kinase promoter and with two upstream PLZF binding sites as identified in the HoxB2 r3/r5-enhancer (38). Expression vectors for PLZF and PLZFmut (A) and their respective GAL4 fusions (B) were cotransfected with the indicated reporter vectors and 50 ng of CMV-lacZ plasmid as a control for transfection efficiency. Where indicated, the expression vector for either p300 or p300ΔHAT was also cotransfected. The activity of the PLZF proteins and GAL4-PLZF fusions were assayed by chromatin immunoprecipitation to determine the level of acetylated histone H4 in the proximity of the PLZF or GAL4 binding and by assaying for luciferase activity; 10% of each cell lysate was used to determine the amount of DNA prior to the immunoprecipitation (input DNA). Levels of transiently expressed proteins were monitored by Western blot analysis using an anti-PLZF or an anti-GAL4(DBD) antibody.
Repression by PLZF involves recruitment of nuclear receptor corepressors and histone deacetylases to its target promoters. The PLZF reporter plasmid used in these experiments (60) contains the simian virus 40 origin of replication and can replicate in 293T cells that express the simian virus 40 large T antigen (24). Transfected plasmids associate with the core histones into chromatinlike structures and replicating plasmids can form regular nucleosomal arrays (25). Therefore, chromatin immunoprecipitation (ChIP) assays were employed to determine whether p300 enhancement of repression by PLZF was reflected by decreases in the levels of acetylated core histone H4 in the proximity of the PLZF binding sites.
The results of this analysis closely reflected those of the experiments assaying reporter gene activity (Fig. 4A and B, upper panel). Expression of the wild-type PLZF protein resulted in decrease of histone H4 acetylation in the region surrounding the PLZF binding sites in the reporter plasmid. Coexpression of p300 but not p300ΔHAT further reduced the levels of acetylated histone H4. Likewise, expression of PLZF lacking zinc finger 9 acetylation sites did not affect the levels of acetylated H4 surrounding the PLZF binding site but as a fusion with GAL4(DBD) effectively reduced H4 acetylation in the vicinity of the GAL4 binding site in the reporter plasmid. It is noteworthy that although the K647/650/653-R PLZF mutant was used in the above experiments, mutation of any one of the three lysines in zinc finger 9 was sufficient to inhibit its ability to repress transcription (see Fig. 5).
FIG. 5.
PLZF lysine mutants assessed for transcriptional activity. Lysine (K) residues in the ninth zinc finger of PLZF were changed to arginines (R) by introducing single point mutation in the PLZF DNA coding sequence. A mutant lacking all the zinc finger 9 acetylation sites (K647/650/653-R) and each mutation separately (K647-R, K650-R or K653-R), were compared to wild-type PLZF (wild type) in its ability to repress transcription using cotransfection experiments in 293T cells. For each mutant, equal amounts of each expression vector and luciferase reporter with a minimal herpes simplex virus thymidine kinase promoter and two PLZF binding sites were cotransfected into 293T cells, and luciferase activity was determined 24 h later. The expression vector for the p300 HAT was cotransfected in each case to enhance PLZF-mediated repression of the luciferase reporter. Each lysine mutation occurring in zinc finger 9 has an impact on the repression activity of the PLZF protein. Levels of transiently expressed proteins were monitored by Western blot analysis using an anti-PLZF antibody (WBαPLZF).
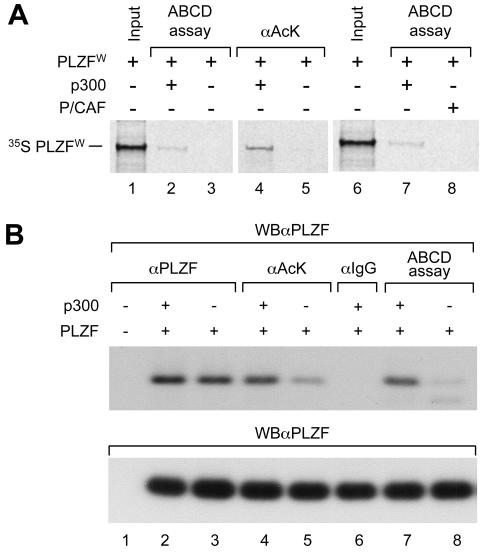
Taken together these results indicated that acetylation of PLZF by p300 does not affect the ability of PLZF to recruit corepressor/HDAC complexes but may affect its ability to bind its DNA response element. Thus, acetylation of lysines in zinc finger 9 would be required for effective DNA binding and hence transcriptional repression by PLZF. Overexpression of p300 would result in higher levels of acetylated PLZF, leading to more DNA-bound PLZF and therefore a further decrease in overall reporter activity. Consistently, using the ABCD binding assay (29) we could demonstrate that unacetylated PLZF derived by in vitro translation in wheat germ lysate (PLZFW) lacked any sequence-specific DNA binding activity unless acetylated by p300 in vitro prior to incubation with DNA (Fig. 6A). Similarly, the ABCD binding activity of PLZF derived from nuclear extract of transfected 293T cells increased when p300 was coexpressed from a cotransfected expression vector (Fig. 6B, compare lanes 7 and 8). As expected from the results of previous experiments, P/CAF failed to stimulate binding of PLZFW to DNA in vitro (Fig. 6A, lane 8).
FIG. 6.
Overexpression of p300 stimulates acetylation of PLZF and its ability to bind DNA. (A) In vitro 35S-labeled PLZF was translated using the wheat germ system ([35S] PLZFW). In vitro-translated PLZF was incubated with acetyl-CoA and in vitro-translated p300 (lanes 2, 4, and 7) or in vitro-translated P/CAF (lane 8) or an equal amount of unprogrammed reticulocyte lysate (lanes 3 and 5) for 1.5 h at 30°C. Levels of PLZF acetylation and PLZF DNA affinity were assessed by immunoprecipitation with an anti-acetyl lysine antibody (αAcK, lanes 4 and 5) or DNA binding assay (ABCD assay, lanes 2, 3, 7, and 8). Samples were electrophoresed, and the dried gel was autoradiographed. (B) PLZF was expressed in 293T cells after transfection in the absence or presence of p300 overexpression. Total PLZF present in each sample was quantified by Western blot analysis (WBαPLZF, lower panel). PLZF-containing cell extracts were subjected to immunoprecipitation with a control antibody (αIgG) an anti-PLZF antibody (αPLZF) or an anti-acetyl lysine antibody (αAcK). The ability of PLZF to bind DNA was assessed using the ABCD binding assay. As shown in lanes 2 and 3, coexpression of p300 does not affect the level of expression of PLZF. However, the level of PLZF acetylation (lane 4 versus lane 5) and the affinity of PLZF for DNA (lane 7 versus lane 8) are sharply increased with overexpression of p300.
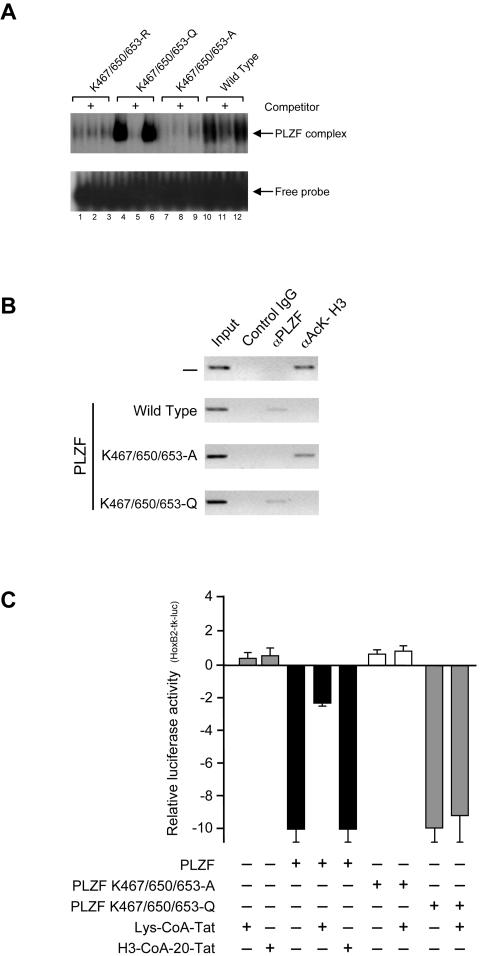
To examine directly the effects of zinc finger 9 acetylation on sequence specific DNA binding by PLZF, we used electrophoretic mobility shift assays (EMSAs) and ChIP to evaluate the ability of PLZF proteins with lysines in zinc finger 9 mutated to alanines (A), arginines (R), or glutamines (Q) to bind DNA. The rationale for generating a glutamine mutation was that through the structural relationship of its side chain, it might mimic the acetylated lysine residue through neutralization of the positive charge (49) and thus behave as an acetylated PLZF independent of p300 activity. Such K-to-Q mutations were shown to behave as acetylated lysines in the context of the p53 protein. As expected, both K647/650/653-R and K647/650/653-A mutants were unable to effectively bind DNA (Fig. 7A, lanes 1 to 3 and 7 to 9) compared to the wild-type PLZF protein (Fig. 7A, lanes 10 to 12).
FIG. 7.
Acetylated lysine residues are critical for PLZF DNA binding and trans-repressing activities. (A) EMSA shows binding of wild-type (lanes 10 to 12) and mutant PLZF proteins expressed transiently in 293T cells (K-to-A in lanes 7 to 9, K-to-Q in lanes 4 to 6 and K-to-R in lanes 1 to 3) to 32P-end-labeled double-stranded oligonucleotide containing three PLZF binding sites. For the competition assays, a 200-fold molar excess of unlabeled oligonucleotides (lanes 2, 5, 8, and 11) was included in the binding reaction. (B) Expression vectors for wild-type PLZF and the K-to-A and K-to-Q mutants were transiently transfected in 293T cells together with a reporter vector containing PLZF binding sites. Cross-linked chromatin was subjected to immunoprecipitation with an anti-PLZF antibody (αPLZF), an anti-acetylated histone H3 (αAck-H3), and an irrelevant anti-GAL4(DBD) (control IgG) antibodies. Coimmunoprecipitated DNA was analyzed by semiquantitative PCR using primers flanking the PLZF binding sites. The PCR products were electrophoresed on an agarose gel (inverted image shown); 10% of each cell lysate was used to determine the amount of DNA prior to the immunoprecipitation (input). Levels of protein expression from transfected vectors were monitored byWestern blotting using an anti-PLZF antibody (data not shown). (C) Expression vectors for PLZF and the indicated PLZF mutants were cotransfected with a reporter containing PLZF binding sites (HoxB2-tk-luc) and 50 ng of CMV-lacZ plasmid as a control for transfection efficiency. Where indicated, Lys-CoA-Tat (10 μM) and H3-CoA-20-Tat (10 μM) treatment was applied overnight. The levels of expression of transiently expressed proteins were monitored by Western blot analysis using an anti-PLZF antibody (not shown). In experiments leading to the results shown in panels B and C, the expression vector for p300 was cotransfected to enhance and maximize PLZF activity.
Strikingly, the K647/650/653-Q mutant, which is expected to structurally mimic PLZF with all lysines in zinc finger 9 acetylated, bound DNA in vitro (Fig. 7, lanes 4 to 6) even more effectively than the wild-type PLZF. This stronger binding by the K-to-Q PLZF mutant most likely reflects the fact that only a fraction of the wild-type PLZF transiently expressed in 293T cells is acetylated (see Fig. 1A, lane 2 and 6B, lane 5).
These in vitro results were corroborated with ChIP experiments evaluating the ability of the above mutants to bind the PLZF DNA target site in vivo (Fig. 7B). Wild-type PLZF and K647/650/653-Q (Fig. 7B) were detected on the PLZF DNA target site in vivo, while neither K647/650/653-A nor K647/650/653-R (not shown) was found associated with the same DNA sequence (Fig. 7B). Consistent with the DNA binding data, the K647/650/653-A mutant failed to cause hypoacetylation of histone H3 on the chromatin of the PLZF reporter gene, while both the K647/650/653-Q mutant and the wild-type protein caused deacetylation of histone H3 in the vicinity of the PLZF DNA binding site in vivo.
The p300 inhibitor Lys-CoA-Tat blocked the ability of PLZF to repress transcription, presumably through inhibition of its acetylation by p300 proteins. In contrast, the ability of the K647/650/653-Q PLZF mutant, which is predicted to act as a constitutively acetylated protein, was unaffected by Lys-CoA-Tat (Fig. 7C). Consistent with the inability of P/CAF to modify PLZF, the P/CAF-specific inhibitor H3-CoA-20-Tat had no effect on the ability of the wild-type PLZF protein to repress transcription (Fig. 7C). It is worth noting that mutation of K562 and K565 in zinc finger 6 had no effect on PLZF activities (DNA binding and transcriptional repression) (data not shown), indicating the critical importance of zinc finger 9 acetylation in these regulatory processes.
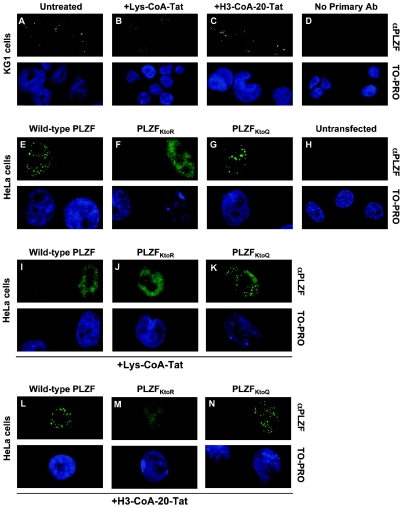
We have previously shown that in KG1 cells PLZF partially localizes in a speckled nuclear pattern (66), which can colocalize with the PML nuclear bodies (43). Given that acetylation has been shown to regulate cellular distribution of transcription factors (28, 72, 73) and in the case of p53 may be linked to the PML nuclear bodies (63), we have investigated whether the acetylation of PLZF associates with its particular nuclear localization status. Immunofluorescence and confocal microscopy analyses indicated that treatment of KG1 cells with p300 (Fig. 8B) but not P/CAF (Fig. 8C) inhibitor disrupted the punctate nuclear localization pattern of PLZF, suggesting that acetylation of PLZF is required for its subnuclear localization. Note the persistence or even enhancement of diffuse nuclear PLZF staining after Lys-CoA-Tat treatment (Fig. 8B), which likely corresponds to redistribution of PLZF in the nucleus and is consistent with Western blot results indicating no change in total PLZF levels following incubation with the p300 inhibitor (Fig. 2A).
FIG. 8.
Acetylated PLZF localizes in specific subnuclear compartments. The nuclear localization pattern of PLZF (and indicated mutants) was analyzed in KG1 (A to D) and transfected HeLa (E to N) cells by indirect immunofluorescence and confocal microscopy. As reported previously (43), punctate nuclear distribution of endogenous PLZF was observed in KG1 cells (A). However, only diffuse nuclear localization was observed when cells were incubated overnight with 10 μM of Lys-CoA-Tat (B), whereas 10 μM of P/CAF inhibitor H3-CoA-20-Tat had no effect on the punctate localization pattern of the PLZF protein (C). No immunofluorescence signal was observed when primary anti-PLZF monoclonal antibody was omitted from the experimental procedure (D). Both the wild-type (E) and K-to-Q mutant (G) of PLZF but not the zinc finger 9 acetylation-deficient K-to-R mutant (F), localized to a speckled nuclear pattern when transiently expressed in HeLa cells. As HeLa cells do not express PLZF, no staining was observed in untransfected controls (H). Consistent with other results, treatment of transfected HeLa cells with the p300 inhibitor Lys-CoA-Tat led to a loss of speckled nuclear localization for wild-type PLZF (I) but not for the K-to-Q mutant (K), which mimics PLZF that is constitutively acetylated in the ninth zinc finger. As before, P/CAF inhibitor had no effect on the nuclear localization of transiently expressed PLZF proteins (L to N). In all cases HeLa cells were transfected with 3 μg of a given expression vector per well in six-well plates, allowed to grow for 48 h, and then treated overnight with 10 μM of p300 and P/CAF inhibitors as indicated.
In order to exclude the possibility that changes in PLZF nuclear localization pattern may be due to the effects of the p300 inhibitor on acetylation of other proteins, we have evaluated effects of the acetyltransferase inhibitors and various mutations in the ninth zinc finger of PLZF on its localization after transient expression in HeLa cells (Fig. 8E to N). Consistent with the above view, both the wild type (Fig. 8E) and the K-to-Q PLZF mutant (Fig. 8G) but not its acetylation-deficient K-to-R mutant (Fig. 8F) displayed punctate nuclear localization patterns. As observed for KG1 cells, inhibition of endogenous p300 activity with Lys-CoA-Tat led to loss of this punctate localization pattern for the wild-type protein (Fig. 8I) but not the K-to-Q mutant (Fig. 8K), which mimics constitutively acetylated PLZF. As before, P/CAF inhibitor had no effect (Fig. 8L-N).
As PLZF is a cell growth suppressor (69, 80) we also analyzed the effect of PLZF acetylation on cell growth using a colony growth suppression assay. We compared the colonies formed in the presence of the wild-type PLZF with those developed in the presence of the PLZF K-to-A mutant (Fig. 9A and B, respectively). Relative to the vector-transfected control, wild-type PLZF suppressed cell growth, while the acetylation-deficient mutant was severely impaired in this activity even though it was as effectively expressed as the wild-type protein (Fig. 9C). These results are consistent with findings that, through direct binding to its DNA target sequences, PLZF can downregulate expression of genes encoding cell growth-promoting factors (see Discussion).
FIG. 9.
PLZF K-to-A fails to inhibit SAOS cell growth. (A and B) At 24 h after transfection, the cells were diluted 1:40 and incubated in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum and 0.5 mg/ml G418 for 3 weeks. After staining with Giemsa, the colonies grown in three plates were counted. Panel B shows the number of colonies counted on the plates transfected with wild-type PLZF (grey). The empty vector was used as a control (black). (B) The number of colonies grown in the presence of PLZF with lysines in the ninth zinc finger mutated to alanines (K-to-A) (grey) compared with the empty vector (pcDNA3.1[-]) (black) is shown. Statistical significance was determined by a two-tailed Student's t test. (C) At 24 h after transfection, cells expressing wild-type PLZF (Pwt) or PLZF with lysines in the ninth zinc finger mutated to alanines (PKtoA) were lysed with 1% NP-40 and the resulting lysates were blotted with antibodies directed against PLZF or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (upper and lower panels, respectively). The expression levels of wild-type PLZF and the PLZF K-to-A mutant were equivalent.
DISCUSSION
Previous studies have indicated that the PLZF protein can bind DNA in a sequence-specific manner and function to repress transcription from promoters containing PLZF binding sites (7, 38, 47, 80). Furthermore, transcriptional repression by PLZF has been linked with recruitment of corepressors and deacetylation of histones surrounding PLZF binding sites (38, 54). We now show that repression by PLZF through its cognate binding site requires p300 HAT activity and acetylation of lysines in its most C-terminal zinc finger motif. The function of p300 in acetylation of PLZF appears to be specific, as other HATs that interact with PLZF, such as P/CAF, neither acetylate PLZF nor augment PLZF function.
In analogy with other posttranslational modifications, the functional consequences of acetylation differ among various target proteins. In the case of the PLZF transcriptional repressor, acetylation appears to stimulate DNA binding; hence, in this context, p300 HAT activity acts to promote repression. Acetylated PLZF also localizes to specific nuclear structures that in immunofluorescence experiments appear in a dotted nuclear pattern. Whether PLZF is actually acetylated in these structures is not clear, but failure of the acetylation-deficient PLZF mutant to localize in a dotted nuclear pattern suggests that acetylation of PLZF is required for its accumulation and/or retention in these subnuclear compartments. As acetylation is required for PLZF binding to DNA (53, 69, 80), such PLZF-containing nuclear complexes may mark loci that are bound and silenced by this repressor.
PLZF contains nine zinc finger motifs, and acetylation of all three lysines in C-terminal zinc finger nine is required for efficient binding of PLZF to DNA. The results from the analysis of PLZF mutants with lysines in zinc finger nine substituted for residues which mimic acetylation are consistent with the notion that zinc finger nine plays an important role in regulation of DNA binding by PLZF. The ability of such mutants to bind more avidly to DNA, as opposed to the decreased DNA binding by the K-to-R or K-to-A mutation, indicate that it is unlikely that the effects of these substitutions may be due solely to change in DNA binding specificity. This is particularly the case in the context of a conservative substitution, such as lysine to arginine, where a wealth of data, derived by phage display, imply that the K-to-R transition should have no effect on sequence-specific DNA binding (18, 37).
To better visualize the potential binding mode of the PLZF zinc finger region, we exploited the fact that zinc fingers often make structurally conserved interactions with DNA, which can be described by a recognition code (17, 74, 75) (Fig. 10). Although such code-based predictions give a valid approximation of the binding surface presented by zinc finger helices, they should be treated with a degree of caution (78); the models are a simplification of the potential geometries of protein-DNA contacts, and the register of the protein-DNA interaction is unlikely to remain in the canonical form shown (Fig. 10A) for more than three consecutive fingers.
FIG. 10.
Code-based prediction of PLZF zinc finger-DNA interactions (17, 74, 75). Zinc fingers are shown aligned with experimental and code-predicted DNA binding sites (boxed). The zinc ion in each finger is highlighted in red. Arrows indicate juxtapositioning between individual amino acids (in the binding helices of the zinc fingers) and DNA bases. Amino acids are denoted by the single-letter code, and heterogeneous DNA bases are written with the universal base degeneracy code. Darker blue shading of individual DNA base boxes denotes a stronger match between the predicted and experimental sequences. Two putative models of DNA-binding are proposed. (A) All nine fingers of PLZF are assumed to be canonical in order to obtain a crude prediction of the overall binding preference. Fingers 2 to 4 and 7 to 9 have the best matches with the minimal DNA subsite 5′-TACTGTAC-3′ (hatched underlining). (B) In a refined prediction, fingers 1 to 4 are discarded as they contribute minimally to binding in EMSA (38) (data not shown). Note that fingers 6 to 8 are linked by canonical TGEKP-type linkers, commonly found in DNA-binding zinc fingers. Accordingly, fingers 6 to 8 give the best fit with the minimal DNA subsite 5′-TACTGTAC-3′ (hatched underlining). The C-terminal acetylated lysine residues (K-Ac; pink) are shown contacting DNA, but it is unlikely that such DNA contacts alone could account for the binding transition observed between unmodified and acetylated PLZF. (C) Models of PLZF homodimerization through the N-terminal POZ/BTB domain (2). Dotted arrows indicate potential dimerization. Applying the binding model in panel B, PLZF dimers might bind to two separate, almost palindromic DNA subsites (dark blue). Note that alternative dimer orientations are also possible by binding to the antiparallel DNA strands.
In fact, the models shown in Fig. 10 reveal several possible modes of binding with the minimal 5′-TACTGTAC recognition sequence. However, sequence analysis and structural considerations suggest that zinc fingers 5 to 8 are most typical DNA binding fingers (containing a canonical TGEKP linker) and zinc fingers 6 to 8 may account best for the minimal PLZF target site, 5′-TACTGTAC, specificity (see Fig. 10B).
Consistent with these considerations, deletion analysis indicated that zinc fingers 1 to 5 of PLZF are not required for sequence-specific DNA binding, although progressive deletion of zinc fingers results in lower DNA binding affinity in vitro (38) (data not shown). Although required for high-affinity DNA binding, zinc finger 9 is not a typical DNA binding finger and is less likely to play a role in sequence-specific protein DNA interactions. Furthermore, DNA contacts from the end of a zinc finger array contribute much less to the overall binding affinity than central contacts (16). The binding energy from the acetylated lysines at the edge of a zinc finger run clearly cannot account for such a sharp switch in DNA binding as observed in our experiments. Acetylation of PLZF by p300 does not affect its stability (see Fig. 4 and 8 for examples), excluding the possibility that degradation of unacetylated PLZF could contribute to the observed decrease in its activities.
Although the exact mechanism by which acetylation of zinc finger 9 enhances the ability of PLZF to bind DNA remains unclear, it is possible that the function of acetylation is to prevent other modifications of the DNA binding domain, which may lead to loss of DNA binding rather than just disruption of intramolecular interaction between zinc finger 9 and other parts of PLZF. This may be analogous to certain mutually exclusive modifications that exist among the histone proteins, such as the lack of phosphorylation of serine 10 of the tail of histone H3 in the presence of lysine 9 methylation (65). This model would explain the requirement of zinc finger 9 for DNA binding but is not consistent with the proposed function of acetylation in p53, namely, to disrupt an intramolecular interaction that blocks the DNA binding activity of the protein (32).
Consistently, PLZF is phosphorylated on threonine and serine residues (4) and, as proposed for C2H2 zinc finger proteins from studies of the lymphoid factor Ikaros (23), it may undergo mitotic inactivation by phosphorylation of the linker sequences in its zinc finger DNA binding domain. Acetylation could serve to counteract this deactivation at other stages of the cell cycle and/or fine tune the mechanism, thus playing a critical regulatory role in DNA binding. Nevertheless, we cannot exclude the possibility that, as in the case of p53, acetylation of PLZF may also lead to conformational changes, potentially affecting its inter- and/or intramolecular interactions, which permit high-affinity sequence-specific DNA binding.
In this respect, it is worth noting that acetylating the three lysines in finger 9 changes the net charge within this subunit from +2 to −1. Although overall charge considerations are important in both protein-protein and protein-DNA interactions, this effect is unlikely to dominate the DNA-binding switch, since the triple Ala mutants (K467/650/653-A; Fig. 7A) do not show increased DNA binding. Acetylation is therefore likely to mediate more specific interaction changes. Notably, switching the residues in zinc finger 9 to acetylated lysines (or Gln) might favor binding to sites with extreme 5′-AT bases (such as in the experimental site used in these studies). However, as discussed above, this effect would not be strong enough to explain the transition to DNA binding due to acetylation.
It is also worth noting that PLZF may form homodimers through its POZ/BTB domain (2) (Fig. 10C). Since dimers have steeper binding curves than monomers, especially if the subunits bind cooperatively, the sharpness of the transition to DNA-binding upon acetylation may be enhanced in PLZF dimers.
The role of p300 and the effects of acetylation on PLZF function differ from the effects of p300 on the activity of BCL-6, another BTB/POZ zinc finger protein. While acetylation of PLZF leads to increased repression via increased DNA binding in vivo, acetylation of BCL-6 leads to dissociation of corepressors from BCL-6 and decreased repression (8). It would appear that each transcription factor interprets its acetylation in a different manner. In an analogous manner, phosphorylation of members of the nuclear receptor family of transcription factors can yield different outcomes, augmenting or inhibiting transcriptional function (41, 70) (see also reference 67 for a review).
Whereas BCL-6 and PLZF both repress transcription, the two proteins have opposing effects on cell growth. BCL-6 can promote cell growth and allow cells to escape from senescence (71), as in its role in B-cell lymphoma. In contrast, PLZF inhibits cell growth through downregulation of genes that promote cell growth such as cyclin A1 (80) and c-myc (54). BCL-6 has been shown to be a substrate for class I HDACs as well as the class III sirtuins (8), which also negatively regulate the growth-controlling function of p53 (50, 51). While active deacetylation of BCL-6 would increase its biological activity in promoting transformation, deacetylation of p53 inhibits its ability to mediate cell cycle arrest (see reference 11 and references therein for review).
Similar to p53, removal of acetyl groups from PLZF inhibits its ability to down-regulate cell growth. For PLZF as well as BCL-6, the physiological signals that lead to modification by acetylation remain to be elucidated. However, opposing functional consequences of PLZF and BCL-6 acetylation are consistent with the opposing effects that these BTB/zinc finger proteins exert on cell growth. The identification of transcription factors that play important roles in cell growth control and oncogenesis as targets for regulation by acetylation has implications for the use of histone deacetylases as therapeutic agents (40, 52, 57). In addition to effects on the constitution of bulk chromatin, HDAC inhibitors seem likely to affect the activity of individual transcription factors, and the net outcome on cell growth may be an integration of general effects as well as specific transcriptional effects.
Although the action of HATs has generally been associated with transcriptional activation (12), our results clearly show the importance of a HAT in transcriptional repression. In an analogous way, HDACs were recently shown to be critical for transcriptional activation by STAT proteins (61). These data indicate that the complexity of transcriptional regulation defies simple rules and suggest that the importance of HDAC/HAT interaction for each transcription factor will need to be explored in an unbiased manner. This study also presents the first description of a cell-permeating, moderately selective p300 HAT inhibitor, which promises to be useful for investigation of HAT activity in a variety of biological contexts.
Acknowledgments
We are grateful to Masashi Suzuki, Sam Waxman, Ari Melnik, and Joan Boyes for comments. We also thank Joan Boyes for providing us with p300 and p300ΔHAT expression vectors, Kevin Petrie for help with the figures, and M. K. Devlin for experimental assistance. We are grateful to Alan Ashworth and the BreakThrough Breast Cancer Research Center for the use of confocal microscope facility.
This work was supported by a program grant from the Leukemia Research Fund of Great Britain (A.Z.), a studentship from the Medical Research Council of Great Britain (S.I.), International Research Fellowship from the Wellcome Trust, United Kingdom (M.I.), Kay Kendall Leukemia Fund (F.G.), FAMRI Foundation and NCI-SPORE Program in Cervical Cancer at Johns Hopkins (R.M.A.), and National Institutes of Health grants CA59936 (A.Z. and J.D.L.) and GM62437 (P.A.C.).
REFERENCES
- 1.Aalfs, J. D., and R. E. Kingston. 2000. What does ‘chromatin remodeling’ mean? Trends Biochem. Sci. 25:548-555. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad, K. F., C. K. Engel, and G. G. Prive. 1998. Crystal structure of the BTB domain from PLZF. Proc. Natl. Acad. Sci. USA 95:12123-12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmad, K. F., A. Melnick, S. Lax, D. Bouchard, J. Liu, C. L. Kiang, S. Mayer, S. Takahashi, J. D. Licht, and G. G. Prive. 2003. Mechanism of SMRT corepressor recruitment by the BCL6 BTB domain. Mol. Cell 12:1551-1564. [DOI] [PubMed] [Google Scholar]
- 4.Ball, H. J., A. Melnick, R. Shaknovich, R. A. Kohanski, and J. D. Licht. 1999. The promyelocytic leukemia zinc finger (PLZF) protein binds DNA in a high molecular weight complex associated with cdc2 kinase. Nucleic Acids Res. 27:4106-4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bannister, A. J., E. A. Miska, D. Gorlich, and T. Kouzarides. 2000. Acetylation of importin-alpha nuclear import factors by CBP/p300. Curr. Biol. 10:467-470. [DOI] [PubMed] [Google Scholar]
- 6.Bardwell, V. J., and R. Treisman. 1994. The POZ domain: a conserved protein-protein interaction motif. Genes Dev. 8:1664-1677. [DOI] [PubMed] [Google Scholar]
- 7.Barna, M., T. Merghoub, J. A. Costoya, D. Ruggero, M. Branford, A. Bergia, B. Samori, and P. P. Pandolfi. 2002. Plzf mediates transcriptional repression of HoxD gene expression through chromatin remodeling. Dev. Cell 3:499-510. [DOI] [PubMed] [Google Scholar]
- 8.Bereshchenko, O. R., W. Gu, and R. Dalla-Favera. 2002. Acetylation inactivates the transcriptional repressor BCL6. Nat. Genet. 32:606-613. [DOI] [PubMed] [Google Scholar]
- 9.Boyes, J., P. Byfield, Y. Nakatani, and V. Ogryzko. 1998. Regulation of activity of the transcription factor GATA-1 by acetylation. Nature 396:594-598. [DOI] [PubMed] [Google Scholar]
- 10.Braunstein, M., A. B. Rose, S. G. Holmes, C. D. Allis, and J. R. Broach. 1993. Transcriptional silencing in yeast is associated with reduced nucleosome acetylation. Genes Dev. 7:592-604. [DOI] [PubMed] [Google Scholar]
- 11.Brooks, C. L., and W. Gu. 2003. Ubiquitination, phosphorylation and acetylation: the molecular basis for p53 regulation. Curr. Opin. Cell Biol. 15:164-171. [DOI] [PubMed] [Google Scholar]
- 12.Brown, C. E., T. Lechner, L. Howe, and J. L. Workman. 2000. The many HATs of transcription coactivators. Trends Biochem. Sci. 25:15-19. [DOI] [PubMed] [Google Scholar]
- 13.Chen, Z., N. J. Brand, A. Chen, S.-J. Chen, J.-H. Tong, Z.-Y. Wang, S. Waxman, and A. Zelent. 1993. Fusion between a novel Kruppel-like zinc finger gene and the retinoic acid receptor-α locus due to a variant t(11;17) translocation associated with acute promeylocytic leukaemia. EMBO J. 12:1161-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen, Z., F. Guidez, P. Rousselot, A. Agadir, S.-J. Chen, Z.-Y. Wang, L. Degos, A. Zelent, S. Waxman, and C. Chomienne. 1994. PLZF-RARα fusion proteins generated from the variant t(11;17) (q23;21) translocation in acute promyelocytic leukemia inhibit ligand-dependent transactivation of wild-type retinoic acid receptors. Proc. Natl. Acad. Sci. USA 91:1178-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheung, P., C. D. Allis, and P. Sassone-Corsi. 2000. Signaling to chromatin through histone modifications. Cell 103:263-271. [DOI] [PubMed] [Google Scholar]
- 16.Choo, Y. 1998. End effects in DNA recognition by zinc finger arrays. Nucleic Acids Res. 26:554-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choo, Y., and A. Klug. 1997. Physical basis of a protein-DNA recognition code. Curr. Opin. Struct. Biol. 7:117-125. [DOI] [PubMed] [Google Scholar]
- 18.Choo, Y., and A. Klug. 1994. Toward a code for the interactions of zinc fingers with DNA: selection of randomized fingers displayed on phage. Proc. Natl. Acad. Sci. USA 91:11163-11167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cleary, J., K. V. Sitwala, P. S. Roland, P. S. Kwok, N. Mor-Vaknin, M. Cebrat, P. A. Cole, and D. M. Markovitz. Acetylation by P/CAF drives DEK into the nuclear speckle. In preparation.
- 20.David, G., L. Alland, S.-H. Hong, C.-W. Wong, R. DePinho, and A. Dejean. 1998. Histone deacetylase associated with mSin3A mediates repression by the promyelocytic leukemia-associated PLZF protein. Oncogene 16:2549-2556. [DOI] [PubMed] [Google Scholar]
- 21.Dong, S., J. Zhu, A. Reid, P. Strutt, F. Guidez, H.-J. Zhong, Z.-Y. Wang, J. Licht, S. Waxman, C. Chomienne, A. Zelent, and S.-J. Chen. 1996. Amino-terminal protein-protein interaction motif (POZ-domain) is responsible for activities of the promyelocytic leukemia zinc finger-retinoic acid receptor-a fusion protein. Proc. Natl. Acad. Sci. USA 93:3624-3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doucas, V., M. Tini, D. A. Egan, and R. M. Evans. 1999. Modulation of CREB binding protein function by the promyelocytic (PML) oncoprotein suggests a role for nuclear bodies in hormone signaling. Proc. Natl. Acad. Sci. USA 96:2627-2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dovat, S., T. Ronni, D. Russell, R. Ferrini, B. S. Cobb, and S. T. Smale. 2002. A common mechanism for mitotic inactivation of C2H2 zinc finger DNA-binding domains. Genes Dev. 16:2985-2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DuBridge, R. B., P. Tang, H. C. Hsia, P.-M. Leong, J. H. Miller, and M. P. Calos. 1987. Analysis of mutation in human cells by using an Epstein-Barr virus shuttle system. Mol. Cell. Biol. 7:379-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Enver, T., A. C. Brewer, and R. K. Patient. 1985. Simian virus 40-mediated cis induction of the Xenopus beta-globin DNase I hypersensitive site. Nature 318:680-683. [DOI] [PubMed] [Google Scholar]
- 26.Fischle, W., Y. Wang, and C. D. Allis. 2003. Histone and chromatin cross-talk. Curr. Opin. Cell Biol. 15:172-183. [DOI] [PubMed] [Google Scholar]
- 27.Freiman, R. N., and R. Tjian. 2003. Regulating the regulators: lysine modifications make their mark. Cell 112:11-17. [DOI] [PubMed] [Google Scholar]
- 28.Gay, F., D. Calvo, M. C. Lo, J. Ceron, M. Maduro, R. Lin, and Y. Shi. 2003. Acetylation regulates subcellular localization of the Wnt signaling nuclear effector POP-1. Genes Dev. 17:717-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glass, C. K., R. Franco, C. Weinberger, V. R. Albert, R. M. Evans, and M. G. Rosenfeld. 1987. A c-erb-A binding site in rat growth hormone gene mediates trans-activation by thyroid hormone. Nature 329:738-741. [DOI] [PubMed] [Google Scholar]
- 30.Green, M., and P. M. Loewenstein. 1988. Autonomous functional domains of chemically synthesized human immunodeficiency virus tat trans-activator protein. Cell 55:1179-1188. [DOI] [PubMed] [Google Scholar]
- 31.Grignani, F., S. DeMatteis, C. Nervi, L. Tomassoni, V. Gelmetti, M. Cioce, M. Fanelli, M. Ruthardt, F. F. Ferrara, I. Zamir, C. Seiser, F. Grignani, M. A. Lazar, S. Minucci, and P. G. Pelicci. 1998. Fusion proteins of the retinoic acid receptor-alpha recruit histone deacetylase in promyelocytic leukaemia. Nature 391:815-818. [DOI] [PubMed] [Google Scholar]
- 32.Gu, W., and R. G. Roeder. 1997. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell 90:595-606. [DOI] [PubMed] [Google Scholar]
- 33.Guidez, F., S. Ivins, J. Zhu, M. Soderstrom, S. Waxman, and A. Zelent. 1998. Reduced retinoic acid-sensitivities of nuclear receptor corepressor binding to PML- and PLZF-RARα underlie molecular pathogenesis and treatment of acute promyelocytic leukemia. Blood 91:2634-2642. [PubMed] [Google Scholar]
- 34.Hong, S. H., G. David, C. W. Wong, A. Dejean, and M. L. Privalsky. 1997. SMRT corepressor interacts with PLZF and with the PML-retinoic acid receptor a (RARα) and PLZF-RARα oncoproteins associated with acute promyelocytic leukemia. Proc. Natl. Acad. Sci. USA 94:9028-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang, S., Y. Qiu, Y. Shi, Z. Xu, and S. J. Brandt. 2000. P/CAF-mediated acetylation regulates the function of the basic helix-loop-helix transcription factor TAL1/SCL. EMBO J. 19:6792-6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Imhof, A., X. J. Yang, V. V. Ogryzko, Y. Nakatani, A. P. Wolffe, and H. Ge. 1997. Acetylation of general transcription factors by histone acetyltransferases. Curr. Biol. 7:689-692. [DOI] [PubMed] [Google Scholar]
- 37.Isalan, M., A. Klug, and Y. Choo. 1998. Comprehensive DNA recognition through concerted interactions from adjacent zinc fingers. Biochemistry 37:12026-12033. [DOI] [PubMed] [Google Scholar]
- 38.Ivins, S., K. Pemberton, F. Guidez, L. Howell, R. Krumlauf, and A. Zelent. 2003. Regulation of Hoxb2 by APL-associated PLZF protein. Oncogene 22:3685-3697. [DOI] [PubMed] [Google Scholar]
- 39.Jeong, J. W., M. K. Bae, M. Y. Ahn, S. H. Kim, T. K. Sohn, M. H. Bae, M. A. Yoo, E. J. Song, K. J. Lee, and K. W. Kim. 2002. Regulation and destabilization of HIF-1alpha by ARD1-mediated acetylation. Cell 111:709-720. [DOI] [PubMed] [Google Scholar]
- 40.Johnstone, R. W., and J. D. Licht. 2003. Histone deacetylase inhibitors in cancer therapy: is transcription the primary target? Cancer Cell 4:13-18. [DOI] [PubMed] [Google Scholar]
- 41.Kato, S., H. Endoh, Y. Masuhiro, T. Kitamoto, S. Uchiyama, H. Sasaki, S. Masushige, Y. Gotoh, E. Nishida, H. Kawashima, and et al. 1995. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science 270:1491-1494. [DOI] [PubMed] [Google Scholar]
- 42.Koeffler, H. P., and D. W. Golde. 1978. Acute myelogenous leukaemia: a human cell line responsive to colony stimulating activity. Science 200:1153-1154. [DOI] [PubMed] [Google Scholar]
- 43.Koken, M. H. M., A. Reid, F. Quignon, M. K. Chelbi-Alix, J. M. Davies, J. H. S. Kabarowski, J. Zhu, S. Dong, S.-J. Chen, Z. Chen, C. C. Tan, J. Licht, S. Waxman, H. de The, and A. Zelent. 1997. Leukemia associated retinoic acid receptor a fusion partners, PML and PLZF, heterodimerize and colocalize to nuclear bodies. Proc. Natl. Acad. Sci. USA 94:10255-10260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kouzarides, T. 1999. Histone acetylases and deacetylases in cell proliferation. Curr. Opin. Genet. Dev. 9:40-48. [DOI] [PubMed] [Google Scholar]
- 45.Lau, O. D., A. D. Courtney, A. Vassilev, L. A. Marzilli, R. J. Cotter, Y. Nakatani, and P. A. Cole. 2000. p300/CBP-associated factor histone acetyltransferase processing of a peptide substrate. Kinetic analysis of the catalytic mechanism. J. Biol. Chem. 275:21953-21959. [DOI] [PubMed] [Google Scholar]
- 46.Lau, O. D., T. K. Kundu, R. E. Soccio, S. Ait-Si-Ali, E. M. Khalil, A. Vassilev, A. P. Wolffe, Y. Nakatani, R. G. Roeder, and P. A. Cole. 2000. HATs off: selective synthetic inhibitors of the histone acetyltransferases p300 and PCAF. Mol. Cell 5:589-595. [DOI] [PubMed] [Google Scholar]
- 47.Li, J. Y., M. A. English, H. J. Ball, P. L. Yeyati, S. Waxman, and J. D. Licht. 1997. Sequence-specific DNA binding and transcriptional regulation by the promyelocytic leukemia zinc finger protein. J. Biol. Chem. 272:22447-22455. [DOI] [PubMed] [Google Scholar]
- 48.Lin, R., L. Nagy, S. Inoue, W. Shao, W. Miller Jr., and R. Evans. 1998. Role of histone deacetylase complex in acute promyelocytic lekaemia. Nature 391:811-814. [DOI] [PubMed] [Google Scholar]
- 49.Luo, J., M. Li, Y. Tang, M. Laszkowska, R. G. Roeder, and W. Gu. 2004. Acetylation of p53 augments its site-specific DNA binding both in vitro and in vivo. Proc. Natl. Acad. Sci. USA 101:2259-2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luo, J., A. Y. Nikolaev, S. Imai, D. Chen, F. Su, A. Shiloh, L. Guarente, and W. Gu. 2001. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell 107:137-148. [DOI] [PubMed] [Google Scholar]
- 51.Luo, J., F. Su, D. Chen, A. Shiloh, and W. Gu. 2000. Deacetylation of p53 modulates its effect on cell growth and apoptosis. Nature 408:377-381. [DOI] [PubMed] [Google Scholar]
- 52.Marks, P., R. A. Rifkind, V. M. Richon, R. Breslow, T. Miller, and W. K. Kelly. 2001. Histone deacetylases and cancer: causes and therapies. Nat. Rev Cancer. 1:194-202. [DOI] [PubMed] [Google Scholar]
- 53.McConnell, M., N. Chevallier, J. Giltnane, G. Carlile, L. Staudt, and J. Licht. 2000. C-myc is a target for regulation by PLZF: two alternative mechanism for down-regulation of gene expression. Blood 96(Suppl. 1):457a. [Google Scholar]
- 54.McConnell, M. J., N. Chevallier, W. Berkofsky-Fessler, J. M. Giltnane, R. B. Malani, L. M. Staudt, and J. D. Licht. 2003. Growth suppression by acute promyelocytic leukemia-associated protein PLZF is mediated by repression of c-myc expression. Mol. Cell. Biol. 23:9375-9388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Melnick, A., K. F. Ahmad, S. Arai, A. Polinger, H. Ball, K. L. Borden, G. W. Carlile, G. G. Prive, and J. D. Licht. 2000. In-depth mutational analysis of the promyelocytic leukemia zinc finger BTB/POZ domain reveals motifs and residues required for biological and transcriptional functions. Mol. Cell. Biol. 20:6550-6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Melnick, A., G. Carlile, K. F. Ahmad, C. L. Kiang, C. Corcoran, V. Bardwell, G. G. Prive, and J. D. Licht. 2002. Critical residues within the BTB domain of PLZF and Bcl-6 modulate interaction with corepressors. Mol. Cell. Biol. 22:1804-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Melnick, A., and J. D. Licht. 1999. Deconstructing a disease: RARalpha, its fusion partners, and their roles in the pathogenesis of acute promyelocytic leukemia. Blood 93:3167-3215. [PubMed] [Google Scholar]
- 58.Melnick, A. M., J. J. Westendorf, A. Polinger, G. W. Carlile, S. Arai, H. J. Ball, B. Lutterbach, S. W. Hiebert, and J. D. Licht. 2000. The ETO protein disrupted in t(8;21)-associated acute myeloid leukemia is a corepressor for the promyelocytic leukemia zinc finger protein. Mol. Cell. Biol. 20:2075-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nagahara, H., A. M. Vocero-Akbani, E. L. Snyder, A. Ho, D. G. Latham, N. A. Lissy, M. Becker-Hapak, S. A. Ezhevsky, and S. F. Dowdy. 1998. Transduction of full-length TAT fusion proteins into mammalian cells: TAT-p27Kip1 induces cell migration. Nat. Med. 4:1449-1452. [DOI] [PubMed] [Google Scholar]
- 60.Nordeen, S. K. 1988. Luciferase reporter gene vectors for analysis of promoters and enhancers. BioTechniques 6:454-458. [PubMed] [Google Scholar]
- 61.Nusinzon, I., and C. M. Horvath. 2003. Interferon-stimulated transcription and innate antiviral immunity require deacetylase activity and histone deacetylase 1. Proc. Natl. Acad. Sci. USA 100:14742-14747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ogryzko, V. V., R. L. Schiltz, V. Russanova, B. H. Howard, and Y. Nakatani. 1996. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell 87:953-959. [DOI] [PubMed] [Google Scholar]
- 63.Pearson, M., R. Carbone, C. Sebastiani, M. Cioce, M. Fagioli, S. Saito, Y. Higashimoto, E. Appella, S. Minucci, P. P. Pandolfi, and P. G. Pelicci. 2000. PML regulates p53 acetylation and premature senescence induced by oncogenic Ras. Nature 406:207-210. [DOI] [PubMed] [Google Scholar]
- 64.Poux, A. N., M. Cebrat, C. M. Kim, P. A. Cole, and R. Marmorstein. 2002. Structure of the GCN5 histone acetyltransferase bound to a bisubstrate inhibitor. Proc. Natl. Acad. Sci. USA 99:14065-14070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rea, S., F. Eisenhaber, D. O'Carroll, B. D. Strahl, Z. W. Sun, M. Schmid, S. Opravil, K. Mechtler, C. P. Ponting, C. D. Allis, and T. Jenuwein. 2000. Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature 406:593-599. [DOI] [PubMed] [Google Scholar]
- 66.Reid, A., A. Gould, N. Brand, M. Cook, P. Strutt, J. Li, J. Licht, S. Waxman, R. Krumlauf, and A. Zelent. 1995. Leukemia translocation gene, PLZF, is expressed with a speckled nuclear pattern in early hematopoietic progenitors. Blood 86:4544-4552. [PubMed] [Google Scholar]
- 67.Rochette-Egly, C. 2003. Nuclear receptors: integration of multiple signalling pathways through phosphorylation. Cell. Signal. 15:355-366. [DOI] [PubMed] [Google Scholar]
- 68.Scherer, W. F., J. T. Syverton, and G. O. Gey. 1953. Studies on the propagation in vitro of poliomyelitis viruses. IV. Viral multiplication in a stable strain of human malignant epithelial cells (strain HeLa) derived from an epidermoid carcinoma of the cervix. J. Exp. Med. 97:695-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shaknovich, R., P. L. Yeyati, S. Ivins, A. Melnick, C. Lempert, S. Waxman, A. Zelent, and J. D. Licht. 1998. The promyelocytic leukemia zinc finger protein affects myeloid cell growth, differentiation, and apoptosis. Mol. Cell. Biol. 18:5533-5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shao, D., S. M. Rangwala, S. T. Bailey, S. L. Krakow, M. J. Reginato, and M. A. Lazar. 1998. Interdomain communication regulating ligand binding by PPAR-gamma. Nature 396:377-380. [DOI] [PubMed] [Google Scholar]
- 71.Shvarts, A., T. R. Brummelkamp, F. Scheeren, E. Koh, G. Q. Daley, H. Spits, and R. Bernards. 2002. A senescence rescue screen identifies BCL6 as an inhibitor of anti-proliferative p19(ARF)-p53 signaling. Genes Dev. 16:681-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Soutoglou, E., N. Katrakili, and I. Talianidis. 2000. Acetylation regulates transcription factor activity at multiple levels. Mol. Cell 5:745-751. [DOI] [PubMed] [Google Scholar]
- 73.Sterner, D. E., and S. L. Berger. 2000. Acetylation of histones and transcription-related factors. Microbiol. Mol. Biol. Rev. 64:435-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Suzuki, M., M. Gerstein, and N. Yagi. 1994. Stereochemical basis of DNA recognition by Zn fingers. Nucleic Acids Res. 22:3397-3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Suzuki, M., and N. Yagi. 1994. DNA recognition code of transcription factors in the helix-turn-helix, probe helix, hormone receptor, and zinc finger families. Proc. Natl. Acad. Sci. USA 91:12357-12361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thompson, P. R., D. Wang, L. Wang, M. Fulco, N. Pediconi, D. Zhang, W. An, Q. Ge, R. G. Roeder, J. Wong, M. Levrero, V. Sartorelli, R. J. Cotter, and P. A. Cole. 2004. Regulation of the p300 HAT domain via a novel activation loop. Nat. Struct. Mol Biol. 11:308-315. [DOI] [PubMed] [Google Scholar]
- 77.Wolf, D., M. Rodova, E. A. Miska, J. P. Calvet, and T. Kouzarides. 2002. Acetylation of beta-catenin by CREB-binding protein (CBP). J. Biol. Chem. 277:25562-25567. [DOI] [PubMed] [Google Scholar]
- 78.Wolfe, S. A., R. A. Grant, M. Elrod-Erickson, and C. O. Pabo. 2001. Beyond the “recognition code”: structures of two Cys2His2 zinc finger/TATA box complexes. Structure (Cambridge) 9:717-723. [DOI] [PubMed] [Google Scholar]
- 79.Yang, X. J., V. V. Ogryzko, J. Nishikawa, B. H. Howard, and Y. Nakatani. 1996. A p300/CBP-associated factor that competes with the adenoviral oncoprotein E1A. Nature 382:319-324. [DOI] [PubMed] [Google Scholar]
- 80.Yeyati, P. L., R. Shaknovich, S. Boterashvili, J. Li, H. J. Ball, S. Waxman, K. Nason-Burchenal, E. Dmitrovsky, A. Zelent, and J. D. Licht. 1999. Leukemia translocation protein PLZF inhibits cell growth and expression of cyclin A. Oncogene 18:925-934. [DOI] [PubMed] [Google Scholar]
- 81.Zelent, A., F. Guidez, S. Waxman, and J. D. Licht. 2001. Translocations of the RARα gene in acute promyelocytic leukemia. Oncogene 20:7186-7203. [DOI] [PubMed] [Google Scholar]
- 82.Zhang, Q., H. Yao, N. Vo, and R. H. Goodman. 2000. Acetylation of adenovirus E1A regulates binding of the transcriptional corepressor CtBP. Proc. Natl. Acad. Sci. USA 97:14323-14328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang, W., and J. J. Bieker. 1998. Acetylation and modulation of erythroid Kruppel-like factor (EKLF) activity by interaction with histone acetyltransferases. Proc. Natl. Acad. Sci. USA 95:9855-9860. [DOI] [PMC free article] [PubMed] [Google Scholar]