Abstract
The serine/threonine kinase Akt is known to promote cell growth by regulating the cell cycle in G1 phase through activation of cyclin/Cdk kinases and inactivation of Cdk inhibitors. However, how the G2/M phase is regulated by Akt remains unclear. Here, we show that Akt counteracts the function of WEE1Hu. Inactivation of Akt by chemotherapeutic drugs or the phosphatidylinositide-3-OH kinase inhibitor LY294002 induced G2/M arrest together with the inhibitory phosphorylation of Cdc2. Because the increased Cdc2 phosphorylation was completely suppressed by wee1hu gene silencing, WEE1Hu was associated with G2/M arrest induced by Akt inactivation. Further analyses revealed that Akt directly bound to and phosphorylated WEE1Hu during the S to G2 phase. Serine-642 was identified as an Akt-dependent phosphorylation site. WEE1Hu kinase activity was not affected by serine-642 phosphorylation. We revealed that serine-642 phosphorylation promoted cytoplasmic localization of WEE1Hu. The nuclear-to-cytoplasmic translocation was mediated by phosphorylation-dependent WEE1Hu binding to 14-3-3θ but not 14-3-3β or -σ. These results indicate that Akt promotes G2/M cell cycle progression by inducing phosphorylation-dependent 14-3-3θ binding and cytoplasmic localization of WEE1Hu.
Many growth factors transduce signals to promote cell survival and proliferation. The characterization of the survival signals stimulated by growth factors has revealed that phosphatidylinositide-3-OH kinase (PI3K) and Ras are involved in cell survival and cell proliferation (14). Stimulation with growth factors activates PI3K on the plasma membrane, and then PI3K generates the putative second messenger phosphatidylinositol-3,4,5-triphosphate [PtdIns(3,4,5)P3]. The major targets of PtdIns(3,4,5)P3 are pleckstrin (PH) and phox (PX) homology-containing proteins (11, 14, 45). One target of PtdIns(3,4,5)P3 is 3-phosphoinositide-dependent kinase 1 (PDK1) containing the PH domain in the COOH terminus; another target is serine/threonine kinase Akt (also known as protein kinase B and RAC protein kinase) containing the PH domain in the NH2 terminus (45). Thus, PtdIns(3,4,5)P3 leads to the recruitment of PDK1 and Akt to the plasma membrane, and Akt is converted from an inactive to an active form by phosphorylation on Thr308, in a region termed the T loop, by PDK1. Furthermore, Akt is phosphorylated on Ser473 in a region termed the hydrophobic motif (45). The kinase responsible for phosphorylation of Akt on Ser473 was called PDK2. Several investigators suggest that PDK2 is Akt itself, integlin-linked kinase (ILK), or PDK1 bound to a COOH-terminal 77-amino-acid fragment of protein kinase C-related kinase 2 (PRK2) (2, 6, 43). Recently, DNA-dependent protein kinase (DNA-PK) was identified as a Ser473 kinase candidate (13). Then, fully activated Akt transduces many biological responses, including glucose uptake, protein synthesis, apoptosis inhibition, and cell cycle progression.
Eukaryotic cell cycle progression is promoted by the activity of phase-specific kinase complexes composed of cyclins and cyclin-dependent kinases (Cdks). The onset of mitosis is controlled by activation of the complex composed of B-type cyclin and cell division cycle 2 (Cdc2). Regulatory control over Cdc2 is highly conserved in eukaryotic evolution, with its activity being regulated at two levels (10). First, Cdc2 is inactive as a monomer and must bind a cyclin B at the G2/M transition. Second, there is a series of reversible phosphorylations that control the activity of the Cdc2/cyclin B complex. Phosphorylation of Cdc2 on Thr161 by Cdk-activating kinase (CAK) is essential for Cdc2 kinase activity (7). This phosphorylation stabilizes the kinase in the active conformation (7). On the other hand, Cdc2 is subject to inhibitory phosphorylation, which is located in the ATP-binding domain of the kinase. The inhibitory phosphorylation is catalyzed by WEE1Hu (also known as Wee1A, a member of Wee1 family) and Myt1 (another member of the Wee1 family) kinases.
In mammalian cells, Cdc2-inhibitory phosphorylations are catalyzed by WEE1Hu on Tyr15 of Cdc2 and Myt1 and on both Thr14 and Tyr15 of Cdc2 (20, 29, 49). At the G2/M transition, inhibitory phosphorylation on Cdc2 is dephosphorylated by the Cdc25C phosphatase, which leads to an abrupt activation of Cdc2 (18, 25, 42). Thus, the activity of WEE1Hu exceeds that of Cdc25C in G2 phase, and therefore, their regulation is crucial for the G2/M transition. Once initially activated, the Cdc2/cyclin B complex is further activated through feedback loops with Cdc25C or WEE1Hu. However, it is unknown how the dominance between the activities of the WEE1Hu and Cdc25C is initially reversed to activate Cdc2/cyclin B at entry into M phase.
Akt is suggested to function as a G2/M initiator. Akt activation by overexpression of a constitutively active form or by the loss of PTEN (phosphatase and tensin homologue deleted in chromosome 10) could overcome the G2/M arrest that was induced by gamma irradiation (22). In addition, PTEN null embryonic stem (ES) cells were reported to transit faster from the G2/M to the G1 phase of the cell cycle than wild-type ES cells, and LY294002, an inhibitor of PI3K, elicited G2/M arrest in human embryonic kidney (HEK) 293 cells (22). Although these results suggest that the PI3K-Akt signaling pathway strongly promotes G2/M transition in a mammalian cell cycle progression, the direct relationship between the PI3K-Akt signaling pathway and regulation of the G2/M transition is not fully understood. However, in primary oocytes from the starfish Asterina pectinifera, Akt was reported to inhibit Myt1 through Akt-dependent phosphorylation and down-regulation at the G2/M transition (31). As a result, Akt indirectly causes the activation of Cdc2 and promotes the cell cycle progression at the G2/M transition. These findings suggest the possibility that the PI3K-Akt signaling pathway functions as an M-phase initiator in mammals.
Here, we show that, in mammalian cells, Akt promotes cell cycle progression at the G2/M transition through WEE1Hu inactivation. Chemotherapeutic drugs such as etoposide, cisplatin, and 17-allylamino-17-demethoxygeldanamycin were reported to down-regulate Akt kinase activity and induce S or G2/M arrest (1, 8, 12, 15, 30, 36, 39, 44). We found that these arrests were caused by the increase in phosphorylated Cdc2 on Tyr15. In addition, LY294002 increased the Tyr15-phosphorylated Cdc2 level and increased the population of the G2/M fraction. Because WEE1Hu tyrosine kinase is known to phosphorylate Cdc2 on Tyr15 (49), we hypothesized that Akt regulates WEE1Hu kinase activity. We discovered that Akt directly bound to the middle range of WEE1Hu and phosphorylated at Ser642 residue in its COOH-terminal catalytic domain during late S to G2 phase. The Ser642 phosphorylation on WEE1Hu could associate with 14-3-3θ but not with 14-3-3β or -σ. Further analyses revealed that Akt accelerated the cytoplasmic localization of WEE1Hu by associating with 14-3-3θ and that the expression of Akt together with 14-3-3θ abolished the WEE1Hu-induced G2/M arrest. Thus, we identified Akt as an M-phase initiator through the mechanisms of phosphorylation-dependent 14-3-3θ binding to WEE1Hu and cytoplasmic localization.
MATERIALS AND METHODS
Cell culture conditions and reagents.
Human embryonic kidney 293T and human fibrosarcoma HT1080 cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum. Human cervical cancer HeLa cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum. Etoposide (VP-16) and cisplatin (cDDP) were kindly provided by Bristol-Myers Squibb Co., Ltd. (Tokyo, Japan). LY294002 were purchased from Sigma (St. Louis, MO). 17-Allylamino-17-demethoxygeldanamycin (17-AAG) and aphidicolin were purchased from Alomone Labs (Jerusalem, Israel) and Calbiochem (La Jolla, CA), respectively. Inactive and active Akt proteins were purchased from Upstate Biotechnology (Lake Placid, NY).
Plasmids.
Human wild-type and dominant-negative (AAA, K179A/T308A/S473A) akt1 cDNAs in a pFLAG-CMV-2 vector (Sigma) or a pHM6 vector (Roche Molecular Biochemicals, Mannheim, Germany), wild-type and phosphatase-inactive (C124S) PTEN in a pFLAG-CMV-2 vector, 14-3-3θ in a pFLAG-CMV-2 vector or a pHM6 vector, and aggrus (also known as podoplanin or T1α) in a pcDNA3 vector were established in our laboratory (15-17, 24). The NH2-terminal myristoylated (Myr) active mouse akt1 cDNA in a pUSEamp vector was purchased from Upstate Biotechnology, and a pEGFP-C2 vector was purchased from BD Biosciences Clontech (Palo Alto, CA).
The NH2-terminally deleted wee1hu cDNA (ΔN214, amino acids 215 to 646) in a pFLAG-CMV-2 vector was generated by PCR with a RIKEN DNA Bank clone (clone 1204; RIKEN, Tsukuba, Japan) as the template. The sense and antisense primers used for the PCR were 5′-TGGATACAGAAAAATCAGGAAAAAGGG-3′ and 5′-GGAGGTGGGAAAGGAGTAGCTC-3′, respectively. The Δ1-364 (ΔN364-WEE1Hu, amino acids 365 to 646) and Δ1-559 (ΔN559-WEE1Hu, amino acids 560 to 646) deletion mutants of wee1hu cDNA were generated by PCR with ΔN214-wee1hu cDNA as the template. The PCR products were ligated to a pCRII vector (Invitrogen, San Diego, CA). ΔN214-, ΔN364-, and ΔN559-wee1hu in a pCRII vector were digested with EcoRI and cloned into the EcoRI site of a pFLAG-CMV-2 vector.
Substitution of Arg412 or Arg611 for stop codons in ΔN214-WEE1Hu to generate the COOH-terminal deletion mutants (412STOP-WEE1Hu and 611STOP-WEE1Hu, respectively) was accomplished by converting the appropriate codons to the stop codon TGA. Substitution of Tyr295, Tyr362, Thr257, Thr620, Ser622, Thr624, Ser626, Ser642, or Thr644 with Ala in a pFLAG-CMV-2-ΔN214-wee1hu was accomplished using a QuickChange mutagenesis kit (Stratagene, La Jolla, CA). Full-length wild-type human wee1hu cDNA in a pFLAG-CMV-2 vector was generated by PCR with an I.M.A.G.E clone (clone 6147695; Invitrogen) as the template (27, 41). The sense and antisense primers used for the PCR were 5′-CGGAATTCGATCAGCTTCCTGAGCCGACA-3′ and 5′-CGGAATTCCGGGAGGTGGGAAAGGAGTAG-3′, respectively. The PCR-amplified wild-type wee1hu was digested with EcoRI and cloned into the EcoRI site of a pFLAG-CMV-2 vector. All plasmid DNAs for transfection were purified using a QIAGEN plasmid maxi kit according to the manufacturer's protocol (QIAGEN, Chatsworth, CA).
Transient transfection, immunoprecipitation, and Western blot analysis.
Cells were transfected with appropriate plasmids using Superfect transfection reagent (QIAGEN) or LipofectAMINE 2000 reagent (Gibco Laboratories, Grand Island, NY), according to the manufacturer's instructions. Immunoprecipitation and Western blot analyses were performed as described previously (15, 36, 40). The whole-cell lysates were prepared using lysis buffer containing sodium dodecyl sulfate (SDS) (1% SDS, 10% glycerol, and 100 mM Tris-Cl [pH 7.6]). In some experiments, nuclear and cytoplasmic fractions were separated using an NE-PER extraction kit according to the manufacturer's instruction (Pierce, Rockford, IL).
For immunoprecipitation, we used antibodies to Wee1 (C-20), Akt (C-20), or 14-3-3θ (C-17) (Santa Cruz Biotechnology, Santa Cruz, CA) or antibody to the FLAG tag (clone M2) (Sigma). For Western blot analysis, we used the following: antibodies to phospho-Cdc2 (Tyr15), Akt, phospho-Akt (Ser473), or phospho-(Ser/Thr) Akt substrate (Cell Signaling Technology, Beverly, MA); antibodies to Wee1 (B-11), Cdc2 (clone 17), 14-3-3β (A-6), 14-3-3θ (C-17), or 14-3-3σ (C-18) (Santa Cruz Biotechnology); an antibody to cyclin A (Neo Markers); antibodies to cyclin B1 or DNA topoisomerase IIβ (TopoIIβ; Pharmingen, San Diego, CA); an antibody to the hemagglutinin (HA) tag (clone 3F10; Roche Molecular Biochemicals); and antibodies to the FLAG tag (clone M2) or β-actin (Sigma). Subsequently, membranes were washed and incubated with horseradish peroxidase-conjugated secondary antibody. After washing several times, the membranes were developed with an enhanced chemiluminescence (ECL) system according to the manufacturer's instructions (Amersham Biosciences, Buckinghamshire, United Kingdom).
Small interfering RNA design and transfection.
Three small interfering RNAs (siRNAs) were designed from the human wee1hu sequence (WEE1Hu-1∼3). The coding strands of the siRNAs were GGACAGUGUCGUCGUAGAA (WEE1Hu-1; directed to residues 745 to 763; 3′-dTdG overhang), ACAAGACCUGCUAAGAGAA (WEE1Hu-2; directed to residues 832 to 850; 3′-dTdT overhang), and GGUGAUCUUGGGCAUGUAA (WEE1Hu-3; directed to residues 1384 to 1402; 3′-dTdA overhang). Although each siRNA suppressed human WEE1Hu expression in 293T and HeLa cells, WEE1Hu-3 siRNA showed nonspecific cytotoxicity. Thus, we used WEE1Hu-1 and -2 siRNAs for further analysis. Nonsilencing control siRNA was purchased from QIAGEN. Cells were transfected with siRNAs using the LipofectAMINE 2000 reagent, according to the manufacturer's instructions.
Measurement of Akt and Cdc2 kinase activities.
293T and HeLa cells were treated with VP-16, cDDP, or 17-AAG for 6 h. The cells were solubilized with lysis buffer (0.2% NP-40, 10% glycerol, 137 mM sodium chloride, 20 mM Tris-HCl [pH 7.6], 1.5 mM magnesium chloride, 1 mM EDTA, 50 mM sodium fluoride, 1 mM sodium vanadate, 12 mM β-glycerophosphate, 1 mM phenylmethylsulfonyl fluoride, and 1 mM aprotinin) for an Akt kinase assay. For a Cdc2 kinase assay, nuclear extracts were prepared with an NE-PER extraction kit and diluted with lysis buffer. Cell lysates were reacted with protein G-Sepharose that had been conjugated with an anti-Akt antibody (C-20) or an anti-Cdc2 antibody (clone 17). The beads were washed three times with lysis buffer. Cdc2 or Akt kinase activity was estimated with an Akt kinase assay kit or a cdk1/cdc2 kinase assay kit (Upstate Biotechnology), respectively, in accordance with the manufacturer's instructions.
In vitro phosphorylation of WEE1Hu.
293T cells were transfected with the pFLAG-CMV-2 vector alone or encoding wild-type WEE1Hu. After transfection for 24 h, transfectants were harvested and lysed with lysis buffer for immunoprecipitation, as described previously (40). After immunoprecipitation with anti-FLAG M2-agarose, the proteins were incubated with recombinant inactive Akt (500 ng) or active Akt (500 ng) in 40 μl of kinase reaction buffer (20 mM morpholinepropanesulfonic acid [MOPS], 25 mM β-glycerophosphate, 5 mM EGTA, 1 mM sodium orthovanadate, 1 mM dithiothreitol, 112.5 μM ATP, and 17 mM magnesium chloride) for 30 min at 30°C, in the presence of 15 μCi [γ-32P]ATP. The levels of incorporated radioactivity were visualized and quantified using a BAS1000 Bio-Imaging analyzer (Fuji Film, Tokyo, Japan).
Cell synchronization assay.
293T cells were transfected with ΔN214- or ΔN214/S642A-wee1hu cDNA. After transfection for 24 h, the cells were treated with complete growth medium containing 1 μg/ml of aphidicolin for 24 h to block at early S phase (5, 21). To release the block, cells were rinsed with phosphate-buffered saline (PBS) and then changed to complete growth medium. The cells were harvested at 0 h or every 2 h from 4 h after the release. For flow cytometric analysis, one third of the cells were fixed with 70% ethanol for 30 min at 4°C, incubated with 1 mg/ml of RNase for 30 min at 37°C, and then stained with 50 μg/ml of propidium iodide for 30 min at 4°C. Others were analyzed by immunoprecipitation, following Western blot, as described above.
Immunostaining.
HeLa cells were cotransfected with the pEGFP-C2 empty vector and the pUSEamp vector alone or encoding Myr-Akt together with the pHM6 vector alone or encoding 14-3-3θ. After transfection for 24 h, cells were washed and fixed with 4% paraformaldehyde containing 0.2% Triton X-100 for 10 min at 23°C. The cells were then washed with ice-cold PBS and blocked with 10% bovine serum albumin for 1 h at 23°C. After blocking, we incubated the cells with an anti-Wee1 mouse monoclonal antibody (1:100 dilution; Santa Cruz Biotechnology) for 1 h at 23°C. Then, the cells were washed with PBS and incubated with an Alexa Fluor 568 goat anti-mouse antibody (1:1,000 dilution; Molecular Probes, Eugene, OR) and Hoechst 33342 (Hoechst Japan, Tokyo, Japan). The cells were washed and then visualized using a fluorescence microscope (Olympus IX-70; Olympus, Tokyo, Japan) equipped with a charge-coupled device camera. We counted approximately 100 green fluorescent protein (GFP)-positive cells in the samples.
Flow cytometric analysis of transfected cells.
293T cells were cotransfected with the pFLAG-CMV-2 vector, either empty (mock) or encoding wild-type WEE1Hu, or the pUSEamp vector, empty (mock) or encoding Myr-Akt, and the pHM6 vector, empty (mock) or encoding 14-3-3θ, together with the pcDNA3 vector encoding wild-type Aggrus as a cell surface marker. After transfection for 48 h, the cells were harvested and fixed with 70% ice-cold ethanol for 30 min at 4°C. The cells were washed with PBS and then incubated with a rat monoclonal anti-Aggrus primary antibody (clone 8F11), a fluorescein isothiocyanate-conjugated anti-rat secondary antibody, and propidium iodide, as described previously (23, 24). Analyses were performed using a Cytomics 500 flow cytometer (Beckman Coulter, Miami, FL) with a Cytomics RXP and MultiCycler software. To analyze the transfectants, we set the gate for fluorescein isothiocyanate-positive cells as described previously (23).
RESULTS
Relationship between Akt inactivation and G2/M arrest.
Some anticancer drugs have been reported to induce S or G2/M arrest and decrease the kinase activity of Akt in certain human cancer cell lines (1, 8, 12, 15, 30, 36, 39, 44). The PI3K inhibitor LY294002 was also reported to cause G2/M arrest in HEK 293 cells (22). These findings indicate the possibility that PI3K-Akt signaling positively regulates the cell cycle on the G2/M boundary. However, the detailed mechanisms of the relationship between the PI3K-Akt signaling pathway and regulation of the G2/M transition were poorly understood.
In order to clarify these relationships, we first checked anticancer drug-induced S or G2/M arrest and Akt inactivation in 293T and HeLa cells. VP-16, cDDP, and 17-AAG increased the population of the S and G2/M fraction in both cell lines except when HeLa cells were treated with cDDP (Fig. 1A). Although cDDP did not induce S or G2/M arrest within 24 h in HeLa cells, it did increase the population in the S and G2/M phases at 36 h (data not shown). Western blot analysis revealed that all of these drugs increased the phosphorylation of Cdc2 on Tyr15, an indicator of G2/M arrest (Fig. 1B, top panels). Simultaneously, a decrease in the phospho-Akt level, which indicates Akt inactivation, was observed in cells treated with these drugs (Fig. 1B, fourth panels). Furthermore, we checked the change of Akt and Cdc2 kinase activities and found that the drugs suppressed them in 293T and HeLa cells (Fig. 1C and D). These findings suggest that these drugs induce G2/M arrest by promoting Cdc2 phosphorylation and that the decrease in Akt kinase activity correlates with the arrest.
FIG. 1.
Chemotherapeutic drug-induced cell cycle arrest with down-regulation of Akt kinase activity. (A) 293T or HeLa cells were treated with medium alone (cont.) or 10 μM VP-16, 5 μM cDDP, or 100 nM 17-AAG. After treatment for 24 h, cellular DNA content was determined by a flow cytometer. (B) After treatment with the indicated chemotherapeutic drugs as for panel A for 4 h, cell lysates were subjected to immunoblot analysis with the indicated antibodies. (C and D) After treatment with the indicated chemotherapeutic drugs as for panel A for 6 h, endogenous Akt or Cdc2 was immunoprecipitated from cell lysates and was subjected to the Akt or Cdc2 kinase assay as described in Materials and Methods. Each vertical bar represents the mean ± standard deviation of three independent experiments. (E) 293T cells were transfected with nonsilencing control siRNA (cont.) or WEE1Hu siRNAs (WEE1Hu-1 and WEE1Hu-2). After transfection for 48 h, cells were treated with the indicated chemotherapeutic drugs as for panel A for 4 h. The cell lysates were subjected to immunoblot analysis with the indicated antibodies. (F and G) 293T cells were transfected with nonsilencing control siRNA (cont.) or WEE1Hu siRNA (WEE1Hu-1). After transfection for 48 h, cells were treated with the indicated chemotherapeutic drugs as for panel A for 6 h. Endogenous Akt or Cdc2 was immunoprecipitated from cell lysates and was subjected to the Akt or Cdc2 kinase assay as described in Materials and Methods. Each vertical bar represents the mean ± standard deviation of three independent experiments.
Because WEE1Hu tyrosine kinase phosphorylates Cdc2 on Tyr15 (20, 29, 49), we then examined the role of WEE1Hu in anticancer drug-induced Cdc2 phosphorylation. As shown in Fig. 1E (top panel), wee1hu knockdown by two independent WEE1Hu siRNAs completely suppressed this Cdc2 phosphorylation in 293T cells. Similar results were obtained in HeLa cells (data not shown). We further examined Akt and Cdc2 kinase activities in drug-treated 293T cells which had been transfected with WEE1Hu siRNA. Although the drugs decreased Akt kinase activities in both control siRNA- and WEE1Hu siRNA-transfected cells, Cdc2 kinase activities were recovered in wee1hu-knocked-down cells compared with control siRNA-transfected cells (Fig. 1F and G). These results suggest that anticancer drugs may arrest cells at S or G2/M by somehow up-regulating WEE1Hu kinase activity.
In order to clarify the direct relationship between Akt activity and G2/M arrest or WEE1Hu kinase activity, we examined the phospho-Cdc2 (Tyr15) level and the population in G2/M phase in 293T and HeLa cells under specific inhibition of the PI3K-Akt signaling pathway. Flow cytometric and Western blot analyses revealed that the PI3K inhibitor LY294002 increased the population in G2/M phase in a time-dependent manner (Fig. 2A) and the amount of phospho-Cdc2 (Tyr15) in both cell types (Fig. 2B, top panels, lanes 2 and 4). The expression levels of total Cdc2 were not changed under LY294002 treatment (Fig. 2B, second panels). An increase in the phospho-Cdc2 level was not observed in WEE1Hu siRNA-treated cells (Fig. 2C, top panel, lanes 4 and 6). Because Akt is a main downstream kinase of PI3K, Akt may be associated with the regulation of WEE1Hu kinase activity.
FIG. 2.
Down-regulation of PI3K-Akt signaling pathway promotes Cdc2 phosphorylation and G2/M arrest. (A) 293T or HeLa cells were treated with 50 μM LY294002. After treatment for 12 h or 24 h, cellular DNA content was determined by a flow cytometer. The indicated percentages of the population are average of the three independent experiments. cont., no-treatment control. (B) 293T or HeLa cells were treated (+) or not (−) with 50 μM LY294002 for 4 h. Cell lysates were subjected to immunoblot analysis with the indicated antibodies. (C) 293T cells were transfected with nonsilencing control siRNA (cont.) or WEE1Hu siRNAs (WEE1Hu-1 and WEE1Hu-2). After transfection for 48 h, cells were treated (+) or not (−) with 50 μM LY294002 for 4 h. The cell lysates were subjected to immunoblot analysis with the indicated antibodies. (D and E) 293T or HeLa cells were transfected with the pFLAG-CMV-2 vector encoding no PTEN (mock), wild-type PTEN, or C124S-PTEN (D) or wild-type Akt or AAA-Akt (E). After transfection for 24 h, cell lysates were subjected to immunoblot analysis with the indicated antibodies.
To confirm the effect of Akt on the phospho-Cdc2 level, we transfected wild-type PTEN cDNA to suppress Akt kinase activity. In both 293T and HeLa cells, wild-type PTEN expression increased phospho-Cdc2 levels compared with mock and C124S-PTEN transfectants (Fig. 2D, top panels). Consistent with the PTEN transfection assay, dominant negative (AAA)-Akt expression but not wild-type Akt expression also increased the phospho-Cdc2 level (Fig. 2E, top panels). In both experiments, the expression levels of Cdc2 were not affected by transfection (Fig. 2D and E). These results indicate that WEE1Hu kinase activities are negatively regulated by the PI3K-Akt signaling pathway.
Akt directly binds to and phosphorylates WEE1Hu.
A putative Akt consensus phosphorylation sequence is known as RXRXX(S/T), where X is any amino acid, and WEE1Hu contains some Akt consensus sequences. To confirm the WEE1Hu phosphorylation by Akt, we treated 293T and HT1080 cells with LY294002 and checked the phosphorylated form of WEE1Hu. The phosphorylation of WEE1Hu was estimated by immunoblot analysis using an anti-phospho-Ser/Thr Akt substrate antibody that could preferentially recognize the conserved Akt phosphorylation motif RXRXX(S/T) only when Ser or Thr was phosphorylated by Akt (16). As shown in Fig. 3A (top panels), the anti-phospho-Ser/Thr Akt substrate antibody recognized the phosphorylated form of WEE1Hu in all cases, and the phosphorylation was diminished by treating cells with LY294002. Densitometric analysis revealed that the phosphorylation of WEE1Hu was suppressed to approximately 50% by treatment of the cells with LY294002 (data not shown).
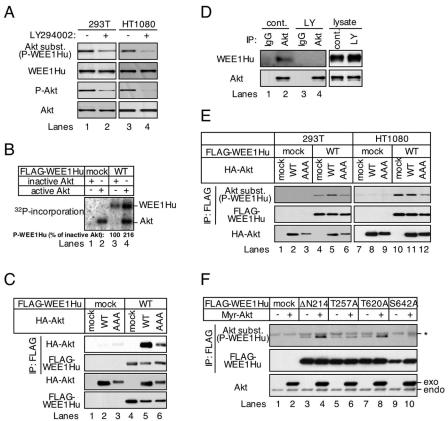
FIG. 3.
Akt binds to and directly phosphorylates WEE1Hu on Ser642. (A) 293T or HT1080 cells were treated (+) or not (−) with 50 μM LY294002 for 4 h. Cell lysates were subjected to immunoblot analysis with the indicated antibodies. (B) 293T cells were transfected with the pFLAG-CMV-2 vector alone (mock; lanes 1 and 2) or pFLAG-CMV-2 encoding wild-type WEE1Hu (WT; lanes 3 and 4). The cell lysates were washed extensively and then incubated with an anti-FLAG agarose. Then, the agarose was incubated for 30 min at 30°C with 500 ng of recombinant inactive (lanes 1 and 3) or active (lanes 2 and 4) Akt protein in the presence of [γ-32P]ATP. The reactions were stopped, electrophoresed, and visualized by autoradiography. (C) 293T cells were transfected with the pFLAG-CMV-2 vector alone (lanes 1 to 3) or encoding wild-type WEE1Hu (lanes 4 to 6) together with the pHM6 vector alone (lanes 1 and 4) or encoding wild-type Akt (lanes 2 and 5) or AAA-Akt (lanes 3 and 6). The FLAG-tagged proteins were immunoprecipitated and subjected to immunoblot analysis with an anti-HA antibody (top panel) or an anti-FLAG antibody (second panel). The expression level of transfected Akt and WEE1Hu proteins was confirmed upon immunoblot analysis of the cell lysates with an anti-HA antibody (third panel) or an anti-FLAG antibody (bottom panel). (D) 293T cells were treated (LY) or not (cont.) with 50 μM LY294002 for 20 h. The nuclear fractions were extracted and immunoprecipitated with protein G-Sepharose that had been conjugated with normal goat IgG (IgG) or goat anti-Akt antibody (Akt). The immunoprecipitated proteins were subjected to immunoblot analysis with an anti-Wee1 antibody (left, upper panel) or an anti-Akt antibody (left, lower panel). The expression level of WEE1Hu and Akt proteins was confirmed upon immunoblot analysis of the cell lysates with an anti-Wee1 antibody (right, upper panel) or an anti-Akt antibody (right, lower panel). (E) 293T or HT1080 cells were transfected as for panel C. The FLAG-tagged proteins were immunoprecipitated and subjected to immunoblot analysis with an anti-phospho-Ser/Thr Akt substrate antibody (top panels) or an anti-FLAG antibody (middle panels). The expression level of transfected Akt proteins was confirmed by immunoblot analysis of the cell lysates with an anti-HA antibody (bottom panels). (F) 293T cells were transfected with the pFLAG-CMV-2 vector alone (lanes 1 and 2) or encoding ΔN214-WEE1Hu (lanes 3 and 4), ΔN214/T257A-WEE1Hu (lanes 5 and 6), ΔN214/T620A-WEE1Hu (lanes 7 and 8), or ΔN214/S642A-WEE1Hu (lanes 9 and 10) together with the pUSEamp vector alone (−; lanes 1, 3, 5, 7, and 9) or encoding Myr-Akt (+; lanes 2, 4, 6, 8, and 10). The FLAG-tagged proteins were immunoprecipitated and subjected to immunoblot analysis with an anti-phospho-Ser/Thr Akt substrate antibody (top panel) or an anti-FLAG antibody (middle panel). The expression level of transfected Akt was confirmed by immunoblot analysis of the cell lysates with an anti-Akt antibody (bottom panel). The asterisk indicates background bands. Exo- and endo-Akt indicate exogenous and endogenous Akt, respectively.
We next examined whether WEE1Hu was directly phosphorylated by Akt. When immunoprecipitated FLAG-tagged wild-type WEE1Hu was incubated in vitro with recombinant inactive Akt, WEE1Hu was weakly phosphorylated by itself (Fig. 3B, lane 3). When WEE1Hu was incubated with active Akt, a drastic increase in WEE1Hu phosphorylation was observed (Fig. 3B, lane 4), suggesting that WEE1Hu was phosphorylated by both itself and Akt. Further analyses revealed that WEE1Hu phosphorylated itself at Tyr295 and/or Tyr362 residues (data not shown), allowing its activation. When the autophosphorylation sites were mutated to alanine (Y295A/Y362A), WEE1Hu was only phosphorylated by Akt in vitro (data not shown). These results suggest that WEE1Hu is a substrate of Akt.
We then checked WEE1Hu binding to Akt. FLAG-tagged WEE1Hu was coexpressed with HA-tagged Akt and analyzed by immunoprecipitation with an anti-FLAG agarose. Both wild-type and AAA-Akt were coimmunoprecipitated with WEE1Hu, suggesting that Akt bound to WEE1Hu in cells (Fig. 3C, top panel, lanes 5 and 6). Furthermore, we found that Akt bound to amino acid residues 365 to 412 of WEE1Hu in several WEE1Hu deletion mutants (data not shown). We next checked the interaction of WEE1Hu with Akt in endogenous expression levels in the nucleus. In the normal culture conditions with 293T cells, WEE1Hu was coimmunoprecipitated with Akt (Fig. 3D, lane 2), although coimmunoprecipitation could not be observed in the LY294002-treated 293T cells (Fig. 3D, lane 4) These results suggest that only the active form of Akt is permitted to bind to WEE1Hu in nucleus.
To confirm WEE1Hu phosphorylation by Akt in cells, the FLAG-tagged WEE1Hu plasmid was cotransfected with HA-tagged wild-type or AAA-akt cDNA into 293T and HT1080 cells. As shown in Fig. 3E (top panels, lanes 5 and 11), the phosphorylated form of WEE1Hu was increased only when the cells were cotransfected with wild-type akt cDNA, although some phospho-WEE1Hu was observed in the cells cotransfected with AAA-akt cDNA (Fig. 3E, lanes 6 and 12). These results indicate that Akt directly interacts with and phosphorylates WEE1Hu in cells.
We next sought to determine the phosphorylation sites of WEE1Hu by Akt. To do this, we prepared NH2- and COOH-terminal deletion mutants and transfected them into 293T cells with or without Akt. Although wild-type WEE1Hu and NH2-terminal deletion mutants ΔN214-, ΔN364-, and ΔN559-WEE1Hu were phosphorylated by Akt, none of the COOH-terminal deletion mutants 412STOP-WEE1Hu and 611STOP-WEE1Hu were phosphorylated by Akt (data not shown). These results suggest that the residues around amino acids 612 to 646 of WEE1Hu may contain the phosphorylation sites. Among these residues, WEE1Hu contains six Akt consensus sequences. Thus, we prepared several point mutants in which Thr620, Ser622, Thr624, Ser626, Ser642, or Thr644 was converted to Ala (T620A, S622A, T624A, S626A, S642A, and T644A, respectively). Furthermore, we converted Thr257 of WEE1Hu to Ala (T257A) as a control. Cotransfection of these mutants with Myr-akt cDNA revealed that an anti-phospho-Ser/Thr Akt substrate antibody could not detect only the S642A mutant (Fig. 3F, top panel, lanes 9 and 10, and data not shown), indicating that Ser642 on WEE1Hu was a novel Akt-mediated phosphorylation site.
Akt phosphorylates WEE1Hu during late S to G2 phase.
We next examined when WEE1Hu was phosphorylated by Akt in the cell cycle. WEE1Hu was reported to be phosphorylated on Ser53 and Ser123 by the M-phase kinases polo-like kinase 1 (Plk-1) and Cdc2, respectively, and degraded through phosphorylation-dependent ubiquitination by SCFβ-TrCP (48). Thus, we used ΔN214-WEE1Hu to check the WEE1Hu phosphorylation status because the ΔN214-WEE1Hu expression level might not be regulated by cell cycle-dependent protein phosphorylation and degradation.
FLAG-tagged ΔN214-WEE1Hu was expressed in 293T cells, and synchronization was performed using aphidicolin. After release from the blockade at early S phase, the cells were harvested at 0 h or every 2 h from 4 to 14 h. Although FLAG-tagged ΔN214-WEE1Hu expression levels showed no change at each time point, an anti-phospho-Ser/Thr Akt substrate antibody revealed that the phosphorylated form of WEE1Hu was increased during 4 h to 8 h after release from the blockade (Fig. 4, top and second panels) before degradation of endogenous WEE1Hu (Fig. 4, third panel). In parallel with endogenous WEE1Hu expression levels, Tyr15-phosphorylated Cdc2 was observed (Fig. 4, fourth panel). Flow cytometric analysis (Fig. 4, right panel) and Western blot using anti-cyclin A and B1 antibodies (Fig. 4, sixth and seventh panels) of these cells revealed that WEE1Hu was phosphorylated during late S to G2 phase and degraded during late M to G1 phase. These results indicate that the phosphorylation of WEE1Hu by Akt occurs before its degradation.
FIG. 4.
WEE1Hu phosphorylation at Ser642 during late S to G2 phase. 293T cells were transfected with the pFLAG-CMV-2 vector encoding ΔN214-WEE1Hu. After transfection for 24 h, the cells were treated with 1 μg/ml of aphidicolin for 24 h. Then, the cells were washed with PBS and changed to complete growth medium. Cells were harvested at the indicated time after the release. One-third of the cells were analyzed using a flow cytometer (right panel), and the others were analyzed by immunoprecipitation with an anti-FLAG agarose, following immunoblot analysis with an anti-phospho-Ser/Thr Akt substrate antibody or an anti-FLAG antibody (left, top and second panels, respectively). The expression level of endogenous WEE1Hu (endo-WEE1Hu), phosphorylated Cdc2 (Tyr15), Cdc2, cyclin B1, cyclin A, and β-actin was confirmed by immunoblot analysis with the indicated antibodies (left, from third to bottom panels).
Akt promotes the nuclear export of WEE1Hu.
To clarify the role of Ser642 phosphorylation, we examined the kinase activities of ΔN214-WEE1Hu and ΔN214/S642A-WEE1Hu in vitro. We found that the kinase activity of ΔN214/S642A-WEE1Hu was not changed compared with that of ΔN214-WEE1Hu (data not shown). We then checked the localization of WEE1Hu. 293T cells that had been transfected with the pFLAG-CMV-2 vector encoding ΔN214- or ΔN214/S642A-WEE1Hu were separated into cytoplasm and nucleus. Then, both fractionated samples were immunoblotted with an anti-FLAG antibody.
As shown in Fig. 5A, ΔN214-WEE1Hu was observed in both the cytoplasmic and nuclear fractions (lanes 3 and 4), although ΔN214/S642A-WEE1Hu could only be detected in the nuclear fraction (lanes 5 and 6). This observation suggests that the phosphorylation of WEE1Hu on Ser642 plays an important role in the cytoplasmic localization of WEE1Hu. To confirm whether Akt promotes WEE1Hu translocation from nucleus to cytoplasm, we further examined the localization of WEE1Hu in 293T cells that had been transfected with HA-tagged wild-type or AAA-akt. The cytoplasmic localization of WEE1Hu was increased in wild-type akt but not in AAA-akt transfectants (Fig. 5B, top panel). These results suggest that Akt-dependent WEE1Hu phosphorylation on Ser642 leads to its nuclear export in a phosphorylation-dependent manner.
FIG. 5.
14-3-3θ binds to the phosphorylated form of WEE1Hu. (A) 293T cells were transfected with the pFLAG-CMV-2 vector alone (mock; lanes 1 and 2) or encoding ΔN214-WEE1Hu (lanes 3 and 4) or ΔN214/S642A-WEE1Hu (lanes 5 and 6). The cytoplasmic (C) and nuclear (N) fractions were separated, electrophoresed, and immunoblotted with an anti-FLAG antibody (upper panel). To validate the fractionation, the cell lysates were immunoblotted with an anti-DNA topoisomerase IIβ antibody (TopoIIβ; lower panel). (B) 293T cells were transfected with the pHM6 vector alone (mock; lanes 1 and 2) or encoding wild-type Akt (lanes 3 and 4) or AAA-Akt (lanes 5 and 6). The cytoplasmic (C) and nuclear (N) fractions were separated, electrophoresed, and immunoblotted with the indicated antibodies. Exo- and endo P-Akt indicate phosphorylated exogenous Akt and phosphorylated endogenous Akt, respectively. (C) 293T cells were cotransfected with the pFLAG-CMV-2 vector alone (mock; lanes 1 to 4) or encoding ΔN214-WEE1Hu (ΔN214; lanes 5 to 8) or ΔN214/S642A-WEE1Hu (S642A; lanes 9 to 12) and the pHM6 vector encoding wild-type 14-3-3β (β; lanes 1, 2, 5, 6, 9, and 10) or wild-type 14-3-3θ (θ; lanes 3, 4, 7, 8, 11, and 12) together with the pUSEamp vector alone (−; lanes 1, 3, 5, 7, 9, and 11) or encoding Myr-Akt (+; lanes 2, 4, 6, 8, 10, and 12). The FLAG-tagged WEE1Hu proteins were immunoprecipitated, and coimmunoprecipitated proteins were subjected to immunoblot analysis with the indicated antibodies (from top to third panels). The expression level of transfected Akt or 14-3-3 protein was confirmed by immunoblot analysis (fourth and bottom panels). The asterisk indicates the background band. Exo- and endo-Akt indicate exogenous Akt and endogenous Akt, respectively. (D) 293T cells were transfected with the pFLAG-CMV-2 vector encoding ΔN214- or ΔN214/S642A-WEE1Hu. After transfection for 24 h, the cells were treated with 1 μg/ml of aphidicolin for 24 h. Then, the cells were washed with PBS, cultured in complete growth medium, and harvested at the indicated times after the release. The cells were analyzed by immunoprecipitation with an anti-FLAG agarose, following immunoblot analysis with an anti-14-3-3θ antibody, an anti-phospho-Ser/Thr Akt substrate antibody, or an anti-FLAG antibody (from top to third panels, respectively). The expression level of endogenous 14-3-3θ, -β, cyclin A, cyclin B1, and β-actin was confirmed by immunoblot analysis with the indicated antibodies (from fourth to bottom panels). (E) 293T cells were treated (LY) or not (cont.) with 50 μM LY294002 for 20 h. The nuclear fractions were extracted and immunoprecipitated with protein G-Sepharose that had been conjugated with normal rabbit IgG (IgG) or rabbit anti-14-3-3θ antibody (14-3-3θ). The immunoprecipitated proteins were subjected to immunoblot analysis with an anti-Wee1 antibody (left, upper panel) or an anti-14-3-3θ antibody (left, lower panel). The expression level of WEE1Hu and 14-3-3θ proteins was confirmed upon immunoblot analysis of the cell lysates with an anti-Wee1 antibody (right, upper panel) or an anti-14-3-3θ antibody (right, lower panel). (F) 293T cells were cotransfected with the pFLAG-CMV-2 vector encoding ΔN214-WEE1Hu and the pHM6 vector alone (mock; lanes 1 and 2) or encoding wild-type 14-3-3θ (WT; lanes 3 and 4) or the indicated mutant form of 14-3-3θ (lanes 5 to 10) together with the pUSEamp vector alone (−; lanes 1, 3, 5, 7, and 9) or encoding Myr-Akt (+; lanes 2, 4, 6, 8, and 10). The FLAG-tagged WEE1Hu proteins were immunoprecipitated, and coimmunoprecipitated proteins were subjected to immunoblot analysis with the indicated antibodies (from top to third panels). The expression level of transfected Akt or 14-3-3θ protein was confirmed by immunoblot analysis (fourth and bottom panels). Exo- and endo Akt indicate exogenous Akt and endogenous Akt, respectively.
14-3-3 binding is known to alter the localization, stability, phosphorylation state, activity, and/or molecular interactions of the target proteins (19). The identified phosphorylation site (RSVpS642LT) of WEE1Hu has high homology to 14-3-3 binding motifs (RSXpSXP and RXXXpSXP, where pS represents phosphoserine and X is any amino acid) (9). Furthermore, 14-3-3β and -σ were reported to bind to phosphorylated Ser642 and increase the stability of WEE1Hu (34, 47).
We then examined the ΔN214- and ΔN214/S642A-WEE1Hu binding to an exogenous level of 14-3-3β or -θ. Although ΔN214/S642A-WEE1Hu could not bind to 14-3-3 proteins (Fig. 5C, top panel, lanes 9 to 12), ΔN214-WEE1Hu bound to both 14-3-3β and -θ through Akt-dependent phosphorylation (Fig. 5C, top panel, lanes 6 and 8). This suggests that exogenous 14-3-3 proteins bind to the phosphorylated form of WEE1Hu on Ser642.
We further examined WEE1Hu binding to endogenous levels of 14-3-3 proteins. FLAG-tagged WEE1Hu was immunoprecipitated from 293T cells that had been transfected with ΔN214- or ΔN214/S642A-wee1hu cDNA and synchronized using aphidicolin. As shown in Fig. 5D (top panel, lanes 2 to 6), endogenous 14-3-3θ was coimmunoprecipitated with ΔN214-WEE1Hu but not with ΔN214/S642A-WEE1Hu, suggesting that this binding depends on the Ser642 phosphorylation of WEE1Hu. However, we could not observe WEE1Hu binding to endogenous 14-3-3β or -σ, and the expression levels of 14-3-3β and -σ were down-regulated during late S to G2 phase (Fig. 5D, fifth panel, and data not shown). These results suggest that 14-3-3θ but not 14-3-3β and -σ binds to WEE1Hu in a Ser642 phosphorylation-dependent manner in the cell cycle progression.
We then checked the interaction of WEE1Hu with 14-3-3θ protein in the nuclear fraction of 293T cells. In endogenous expression levels of WEE1Hu and 14-3-3θ, WEE1Hu was coimmunoprecipitated with 14-3-3θ (Fig. 5E, lane 2). On the other hand, coimmunoprecipitation could not be detected in LY294002-treated cells (Fig. 5E, lane 4). These results suggest that the nonphosphorylated form of WEE1Hu cannot bind to 14-3-3θ. Although wild-type 14-3-3θ could bind to WEE1Hu, the 14-3-3θ mutants (R56A/R60A, R127A, and K49E), which lose their ligand binding ability (16), failed to bind to WEE1Hu (Fig. 5F, top panel, lanes 5 to 10). Therefore, WEE1Hu binding to 14-3-3θ becomes specific.
To clarify the role of 14-3-3θ-binding, we checked the localization of endogenous WEE1Hu using immunofluorescence staining. HeLa cells were cotransfected with the pUSEamp vector alone or encoding Myr-Akt and the pHM6 vector alone or encoding 14-3-3θ together with the pEGFP-C2 vector. After transfection for 24 h, the cells were fixed and stained with an anti-Wee1 monoclonal antibody and Hoechst 33342 to detect nuclei. As shown in Fig. 6A (top panels), some WEE1Hu expression was observed in the nucleus when the cells were transfected with the pHM6 vector alone. In contrast, we could partially observe the translocation of WEE1Hu to the cytoplasm in the cells transfected with the akt or 14-3-3θ cDNA (Fig. 6A, second and third panels). Moreover, almost all of the WEE1Hu proteins were localized in the cytoplasm or intermediate in cells transfected with akt in addition to 14-3-3θ (Fig. 6A, bottom panels).
FIG. 6.
Akt and 14-3-3θ promote cytoplasmic localization of endogenous WEE1Hu. (A) HeLa cells were cotransfected with the pEGFP-C2 vector alone and the pHM6 vector alone (top and second panels) or encoding 14-3-3θ (third and bottom panels) together with the pUSEamp vector alone (top and third panels) or encoding Myr-Akt (second and bottom panels). The localization of endogenous WEE1Hu proteins was detected by staining with an anti-Wee1 antibody, following treatment with an Alexa Fluor 568 goat anti-mouse antibody. Nuclei were also detected by staining with a Hoechst 33342. Cells were visualized using a fluorescence microscope. The transfected cells were observed as green (GFP), endogenous WEE1Hu proteins were red (WEE1Hu), and the nuclei of the cells were blue (Hoechst). The WEE1Hu staining and the Hoechst staining were merged (merge). (B) The percentage of GFP-positive cells exhibiting cytoplasmic (black bar) or nuclear (white bar) localization of WEE1Hu was determined for panel A. Data were obtained from 70 to 100 GFP-positive cells in each experiment. (C) 293T cells were transfected with the pHM6 vector alone (mock; lanes 1 to 4) or encoding wild-type 14-3-3β (β; lanes 5 to 8) or wild-type 14-3-3θ (θ; lanes 9 to 12) together with the pUSEamp vector alone (−; lanes 1, 2, 5, 6, 9, and 10) or encoding Myr-Akt (+; lanes 3, 4, 7, 8, 11, and 12). The cytoplasmic (C) and nuclear (N) fractions were separated, electrophoresed, and immunoblotted with the indicated antibodies. Exo- and endo-Akt indicate exogenous Akt and endogenous Akt, respectively. (D) The expression level of WEE1Hu was measured as for panel C using a densitometer. Each vertical bar represents the mean ± standard deviation of three independent experiments. NS, not significant.
We then determined the number of cells that expressed WEE1Hu protein in the cytoplasmic fraction among 70 to 100 GFP-positive cells (Fig. 6B). Cytoplasmic localization of WEE1Hu was observed in about 30% of the cells transfected with the vector alone (Fig. 6B). Akt or 14-3-3θ alone increased the populations of cells in which WEE1Hu were localized in the cytoplasm to approximately 50% (Fig. 6B). Cotransfection of akt and 14-3-3θ further promoted the cytoplasmic localization of WEE1Hu to approximately 80% (Fig. 6B).
To examine the localization of endogenous WEE1Hu further, we performed Western blot analysis in which the pHM6 vector alone or encoding 14-3-3β or -θ was cotransfected with or without Myr-akt cDNA into 293T cells. After transfection, nuclear and cytoplasmic fractions were separated. Western blot analysis with an anti-Wee1 antibody showed that 14-3-3θ cotransfected with Myr-akt significantly promoted the cytoplasmic localization of endogenous WEE1Hu (Fig. 6C, top panel, lanes 11 and 12 compared with the other lanes, and Fig. 6D), indicating that Akt-mediated phosphorylation on Ser642 promotes its binding to 14-3-3θ and participates in the cytoplasmic localization of WEE1Hu in cells. While exogenous 14-3-3β also bound to WEE1Hu through Akt-dependent phosphorylation (Fig. 5C), it could not affect the translocation of WEE1Hu (Fig. 6C and Fig. 6D). These results suggest that Akt promotes the cytoplasmic localization of WEE1Hu through inducing WEE1Hu binding to 14-3-3θ.
Akt abolishes the WEE1Hu-induced G2/M arrest in cooperation with 14-3-3θ.
The previous studies have shown that WEE1Hu-induced G2/M arrest is enhanced by cotransfection with 14-3-3β or -σ in HEK293 or HeLa cells because these proteins increase the stability of WEE1Hu in the nucleus (34, 47). However, our data showed that Akt promoted the cytoplasmic localization of WEE1Hu through binding to endogenous 14-3-3θ but not 14-3-3β or -σ in the cell cycle progression (Fig. 5D and Fig. 6), suggesting that WEE1Hu might be inactivated and the WEE1Hu-induced G2/M arrest might be extinguished by Akt and 14-3-3θ.
To check these hypotheses, we performed flow cytometric analysis of 293T cells transfected with wee1hu cDNA alone or in combination with akt or 14-3-3θ or both. The cDNA encoding an Aggrus cell surface marker was cotransfected and used to identify the transfected cells. Flow cytometric analysis showed that transfection of wee1hu caused an increase in the G2/M population from 19.7% to 42.0% (Fig. 7A, compare a with b). In comparison, cotransfection with wee1hu, akt, and 14-3-3θ significantly suppressed it up to 28.1% (Fig. 7A, compare b with e). Although cotransfection with other combinations slightly decreased the G2/M fraction, these changes were not significant (Fig. 7A, c and d). To check the events further, we performed a Western blot analysis under the same conditions. An anti-phospho-Cdc2 (Tyr15) antibody used as a monitor of G2/M arrest revealed that coexpression of Akt and 14-3-3θ suppressed the increase in phospho-Cdc2 induced by WEE1Hu (Fig. 7B, top panel, compare b with e). These results suggest that Akt suppresses the kinase activity of WEE1Hu through 14-3-3θ binding and translocation to the cytoplasm.
FIG. 7.
Overexpression of Akt together with 14-3-3θ overcomes the WEE1Hu-induced G2/M arrest. (A) 293T cells were cotransfected with the pFLAG-CMV-2 vector alone (a) or encoding wild-type WEE1Hu (b to e) and the pHM6 vector alone (a to c) or encoding 14-3-3θ (d and e) together with the pUSEamp vector not encoding Myr-Akt (a, b, and d) or encoding Myr-Akt (c and e). To monitor the transfected cells, all cells were simultaneously transfected with the pcDNA3 vector encoding Aggrus as a cell surface marker. The cells were harvested 48 h posttransfection. The Aggrus proteins were detected by staining with a rat monoclonal anti-Aggrus antibody and a fluorescein isothiocyanate-conjugated anti-rat antibody. Nuclei were also detected by staining with propidium iodide, and the cells were analyzed using a flow cytometer. (B) 293T cells were transfected as for panel A. Cell lysates were subjected to immunoblot analysis with the indicated antibodies.
DISCUSSION
The PI3K-Akt signaling pathway controls many cellular functions, such as cell survival, cell cycle progression, cell proliferation, and glucose metabolism. A large number of cancers were reported to aberrantly activate this pathway, resulting in cell proliferation. Although proliferation is strictly regulated by the cell cycle, the aberrant activation of the PI3K-Akt pathway breaks this regulation in proliferating cancer cells. For example, the PI3K-Akt signaling pathway was reported to promote G1 progression through activation of the cyclin D1/Cdk4 complex (4). Cyclin D1 phosphorylation at Thr286 by glycogen synthase kinase 3 (GSK-3) is associated with the translocation of cyclin D1 from the nucleus to the cytoplasm (4). Rapid degradation induced by GSK-3 is inhibited by activation of the PI3K-Akt pathway because Akt directly phosphorylates and inactivates GSK-3 (4). By contrast, Akt is known to inactivate the Cdk inhibitors p21Waf1/Cip1 and p27Kip1 (4, 16, 28, 37, 38, 46, 50). Akt phosphorylates p21Waf1/Cip1 on Thr145 and p27Kip1 on Thr157 and Thr198, resulting in cytoplasmic translocation and suppression of the functions (4, 16, 28, 37, 38, 46, 50). These results indicate that the PI3K-Akt signaling pathway promotes cell cycle progression in G1 phase by activating positive regulators and inactivating negative ones. However, only G1 progression by the PI3K-Akt signaling pathway is not enough to account for cell proliferation because the cell cycle is strictly regulated at each checkpoint.
Several previous studies suggested that some anticancer drugs inhibit the kinase activity of Akt (15, 30, 33, 36, 44). The 90-kDa heat shock protein Hsp90 is reported to control the PDK1-Akt signaling pathway because it binds to PDK1 through its kinase domain and protects against the proteasome-dependent degradation of PDK1 (15, 44). 17-AAG, which is a derivative of geldanamycin, was reported as an Hsp90 inhibitor that protects against Hsp90 binding to PDK1, resulting in inactivation of the PDK1-Akt signaling pathway (3, 15, 44). UCN-01 (7-hydroxystaurosporine), a drug now in clinical trials and with a unique fingerprint pattern, is also known to be a direct inhibitor of PDK1 because UCN-01 binds to PDK1 in an ATP-binding motif and inhibits its kinase activity, resulting in turnoff of survival signaling and apoptosis induction (36, 44). Furthermore, cisplatin, etoposide, adriamycin, and camptothecin were reported to down-regulate the PI3K-Akt pathway in some cell lines (30, 33).
Although it is clear that some anticancer drugs induce S or G2/M arrest (1, 8, 12, 39), the relationship between the arrest and down-regulation of Akt kinase has not been clarified. Thus, we first elucidated the relationship between anticancer drug-induced G2/M arrest and Akt inactivation in 293T and HeLa cells. We found that VP-16, cDDP, and 17-AAG could induce S or G2/M arrest accompanying down-regulation of Akt (Fig. 1). Simultaneously, inhibitory phosphorylation of Cdc2 (Tyr15) was observed and Cdc2 kinase activities were decreased under these conditions, and this phosphorylation depended on WEE1Hu activation (Fig. 1). Moreover, the PI3K inhibitor LY294002 also increased the population in the G2/M phase and the WEE1Hu-dependent inhibitory phosphorylation of Cdc2 (Fig. 2). These findings indicate the possibility that Akt negatively regulates WEE1Hu in a phosphorylation-dependent manner.
Because WEE1Hu has some putative Akt consensus phosphorylation sequences, we examined the phosphorylation of WEE1Hu by Akt. Actually, Akt directly bound to and phosphorylated WEE1Hu at the Ser642 residue in the COOH terminus during the S to G2 phase (Fig. 3 and 4). Although Thr161 on Cdc2, which is known to be essential for Cdc2 kinase activity (7), has homology to a putative Akt consensus sequence, Akt could not phosphorylate Cdc2 at the site in vitro and in vivo (data not shown). Moreover, Cdc25C, which also has a putative Akt consensus sequence, could not be phosphorylated by Akt in vitro (data not shown). These results indicate that Akt regulates the cell cycle at the G2/M transition, mainly by phosphorylating WEE1Hu.
With DNA damage (e.g., radiation or UV irradiation), Chk1 is activated and induces G2/M arrest through phosphorylation of Cdc25C and WEE1Hu. Cdc25C, which is a phosphatase for the phosphorylated Tyr15 of Cdc2 and which activates it, is phosphorylated on Ser216 by Chk1 and the phosphorylated form of Cdc25C binds to 14-3-3 and eliminates the functions through translocation to cytoplasm (25, 32, 35). Moreover, Chk1 may also phosphorylate WEE1Hu. Although there is no report of this in humans, Lee et al. found that in frog egg extract, Chk1 phosphorylated Xenopus Wee1 on Ser549, corresponding to WEE1Hu on Ser642 (26). Ser642 of WEE1Hu is also conserved in mouse and rat Wee1 homologues. Phosphorylated Ser642 is reported to increase the stability of WEE1Hu in the nucleus by binding to 14-3-3β or -σ (34, 47). As a result, Cdc2 is continuously phosphorylated at Tyr15 and the cell cycle arrests at the G2/M transition.
Since Akt also phosphorylated WEE1Hu on Ser642 (Fig. 3E) and overexpression of wild-type Akt decreased the phosphorylation of Tyr15 of Cdc2 (Fig. 2E), we questioned how WEE1Hu kinase activity was regulated through phosphorylated Ser642. To answer this question, we looked at whether 14-3-3 proteins bound to WEE1Hu through the phosphorylation of Ser642 by Akt and changed the localization of WEE1Hu. Although exogenous 14-3-3β and -θ could bind to WEE1Hu through phosphorylated Ser642 (Fig. 5C), only 14-3-3θ could bind to it at the endogenous expression level of 14-3-3 because the endogenous 14-3-3β and -σ expression levels were down-regulated during late S to G2 phase (Fig. 5D and data not shown). 14-3-3θ binding promoted the translocation of WEE1Hu from the nucleus to the cytoplasm (Fig. 6) and abolished the WEE1Hu-induced G2/M arrest (Fig. 7). Thus, Akt promotes cell cycle progression at the G2/M transition through Akt-dependent WEE1Hu phosphorylation on Ser642 and its binding to 14-3-3θ. On the other hand, the Akt-dependent phosphorylation state of the point-mutated form of Thr257 (T257A) on WEE1Hu was slightly decreased compared with wild-type WEE1Hu, suggesting that Thr257 on WEE1Hu may also be a site phosphorylated by Akt (Fig. 3). However, the T257A mutation had no effect on 14-3-3 binding and translocation of WEE1Hu (data not shown). Thus, we need to examine the effects of phosphorylation on Thr257 by Akt in the future.
Although 14-3-3β and -σ were reported to bind to WEE1Hu through phosphorylated Ser642 (34, 47), we could not observe that they bound to WEE1Hu at endogenous expression levels (Fig. 5D). These different results could be explained by considering two points. First, many antibodies to 14-3-3 cross-react with other 14-3-3 isotypes. Second, overexpression of 14-3-3 proteins, except 14-3-3θ, was not fully down-regulated through the cell cycle progression, resulting in cell cycle-independent binding to WEE1Hu. We also observed exogenous 14-3-3β binding to WEE1Hu (Fig. 5C). At endogenous 14-3-3 levels, only 14-3-3θ formed a complex with WEE1Hu. Moreover, the expression levels of the 14-3-3 proteins, except 14-3-3θ, were changed through the cell cycle progression (Fig. 5D). Actually, the expression levels of 14-3-3β and -σ were down-regulated during late S to G2 phase (Fig. 5D and data not shown). Thus, under physiological conditions, the localization of Ser642-phosphorylated WEE1Hu may only be regulated by binding to 14-3-3θ.
In summary, we discovered that Akt-mediated WEE1Hu phosphorylation on Ser642 induced WEE1Hu binding to 14-3-3θ and cytoplasmic localization upon growth factor stimulation. Thus, Akt eliminates WEE1Hu functions through translocation to the cytoplasm by 14-3-3θ. Okumura et al. reported that Akt-dependent phosphorylation of Myt1, another member of the Wee1 family, led to meiotic G2/M transition in starfish oocytes (31), and now we identify WEE1Hu as a new target of Akt and demonstrate that Akt functions as an M-phase initiator in mammalian cells.
Acknowledgments
We thank RIKEN DNA Bank for proving the pCD-WEE1Hu (ΔN214) plasmid. We also thank M. Naito and A. Tomida for helpful discussions.
This study was supported in part by a special grant for Advanced Research on Cancer from the Ministry of Education, Culture, Sports, Science and Technology, Japan (to T.T. and N.F.), by a grant from Uehara Memorial Fundation (to N.F.), and by a grant for Research Fellowships of the Japanese Society for the Promotion of Science for Young Scientists (to K.K.).
REFERENCES
- 1.Alhasan, S. A., O. Aranha, and F. H. Sarkar. 2001. Genistein elicits pleiotropic molecular effects on head and neck cancer cells. Clin. Cancer Res. 7:4174-4181. [PubMed] [Google Scholar]
- 2.Balendram, A., A. Casamayor, M. Deak, A. Paterson, P. Gaffney, R. Currie, C. P. Downes, and D. R. Alessi. 1999. PDK1 acquires PDK2 activity in the presence of a synthetic peptide derived from the carboxy terminus of PRK2. Curr. Biol. 9:393-404. [DOI] [PubMed] [Google Scholar]
- 3.Basso, A. D., D. B. Solit, G. Chiosis, B. Giri, P. Tsichlis, and N. Rosen. 2002. Akt forms an intracellular complex with heat shock protein 90 (Hsp90) and Cdc37 and is destabilized by inhibitors of Hsp90 function. J. Biol. Chem. 277:39858-39866. [DOI] [PubMed] [Google Scholar]
- 4.Chang, F., J. T. Lee, P. M. Navolanic, L. S. Steelman, J. G. Shelton, W. L. Blalock, R. A. Franklin, and J. A. McCubrey. 2003. Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: a target for cancer chemotherapy. Leukemia 17:590-603. [DOI] [PubMed] [Google Scholar]
- 5.Chen, M. S., J. Hurov, L. S. White, T. Woodford-Thomas, and H. Piwnica-Worms. 2001. Absence of apparent phenotype in mice lacking Cdc25C protein phosphatase. Mol. Cell. Biol. 21:3853-3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delcommenne, M., C. Tan, V. Gray, L. Rue, J. Woodgett, and S. Dedhar. 1998. Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proc. Natl. Acad. Sci. USA 95:11211-11216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desai, D., H. C. Wessling, R. P. Fisher, and D. O. Morgan. 1995. Effects of phosphorylation by CAK on cyclin binding by CDC2 and CDK2. Mol. Cell. Biol. 15:345-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doi, T., T. Morita, N. Wakabayashi, T. Sumi, S. A. Iwai, S. Amekawa, M. Sakuda, and Y. Nishimune. 2002. Induction of instability of p34(cdc2) expression by treatment with cisplatin (CDDP) in mouse teratocarcinoma F9 cells. Cancer Lett. 176:75-80. [DOI] [PubMed] [Google Scholar]
- 9.Dougherty, M. K., and D. K. Morrison. 2004. Unlocking the code of 14-3-3. J. Cell Sci. 117:1875-1884. [DOI] [PubMed] [Google Scholar]
- 10.Dunphy, W. G. 1994. The division to enter mitosis. Trends Cell Biol. 4:202-207. [DOI] [PubMed] [Google Scholar]
- 11.Ellson, C. D., S. Andrews, L. R. Stephens, and P. T. Hawkins. 2002. The PX domain: a new phosphoinositide-binding module. J. Cell Sci. 115:1099-1105. [DOI] [PubMed] [Google Scholar]
- 12.Facompre, M., N. Wattez, J. Kluza, A. Lausiaux, and C. Bailly. 2000. Relationship between cell cycle changes and variations of the mitochondrial membrane potential induced by etoposide. Mol. Cell. Biol. Res. Commun. 4:37-42. [DOI] [PubMed] [Google Scholar]
- 13.Feng, J., J. Park, P. Cron, D. Hess, and B. A. Hemmings. 2004. Identification of a PKB/Akt hydrophobic motif Ser-473 kinase as DNA-dependent protein kinase. J. Biol. Chem. 279:41189-41196. [DOI] [PubMed] [Google Scholar]
- 14.Franke, T. F., D. R. Kaplan, and L. C. Cantley. 1997. PI3K: downstream AKTion blocks apoptosis. Cell 88:435-437. [DOI] [PubMed] [Google Scholar]
- 15.Fujita, N., S. Sato, A. Ishida, and T. Tsuruo. 2002. Involvement of Hsp90 in signaling and stability of 3-phosphoinositide-dependent kinase-1. J. Biol. Chem. 277:10346-10353. [DOI] [PubMed] [Google Scholar]
- 16.Fujita, N., S. Sato, K. Katayama, and T. Tsuruo. 2002. Akt-dependent phosphorylation of p27Kip1 promotes binding to 14-3-3 and cytoplasmic localization. J. Biol. Chem. 277:28706-28713. [DOI] [PubMed] [Google Scholar]
- 17.Fujita, N., S. Sato, and T. Tsuruo. 2003. Phosphorylation of p27Kip1 at threonine 198 by p90 ribosomal protein S6 kinases promotes its binding to 14-3-3 and cytoplasmic localization. J. Biol. Chem. 278:49254-49260. [DOI] [PubMed] [Google Scholar]
- 18.Gautier, J., M. J. Solomon, R. N. Booher, J. F. Bazan, and M. W. Kirschner. 1991. cdc25 is a specific tyrosine phosphatase that directly activates p34cdc2. Cell 67:197-211. [DOI] [PubMed] [Google Scholar]
- 19.Hermeking, H. 2003. The 14-3-3 cancer connection. Nat. Rev. Cancer 3:931-943. [DOI] [PubMed] [Google Scholar]
- 20.Igarashi, M., A. Nagata, S. Jinno, K. Suto, and H. Okayama. 1991. Wee1(+)-like gene in human cells. Nature 353:80-83. [DOI] [PubMed] [Google Scholar]
- 21.Jackson, D. A. 1995. S-phase progression in synchronized human cells. Exp. Cell Res. 220:62-70. [DOI] [PubMed] [Google Scholar]
- 22.Kandel, E. S., J. Skeen, N. Majewski, A. D. Cristofano, P. P. Pandolfi, C. S. Feliciano, A. Gartel, and N. Hay. 2002. Activation of Akt/protein kinase B overcomes a G2/M cell cycle checkpoint induced by DNA damage. Mol. Cell. Biol. 22:7831-7841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katayama, K., Y. Dobashi, M. Kitagawa, S. Kamekura, M. Kawai, Y. Kadoya, and T. Kameya. 2001. Overexpression of cdk4/cyclin D1 induces apoptosis in PC12 cells in the presence of trophic support. FEBS Lett. 509:382-388. [DOI] [PubMed] [Google Scholar]
- 24.Kato, Y., N. Fujita, A. Kunita, S. Sato, M. Kaneko, M. Osawa, and T. Tsuruo. 2003. Molecular identification of Aggrus /T1α as a platelet aggregation-inducing factor expressed in colorectal tumors. J. Biol. Chem. 278:51599-51605. [DOI] [PubMed] [Google Scholar]
- 25.Kumagai, A., and W. G. Dunphy. 1991. The cdc25 protein controls tyrosine dephosphorylation of the cdc2 protein in a cell-free system. Cell 64:903-914. [DOI] [PubMed] [Google Scholar]
- 26.Lee, J., A. Kumagai, and W. G. Dunphy. 2001. Positive regulation of Wee1 by Chk1 and 14-3-3 proteins. Mol. Biol. Cell. 12:551-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lennon, G., C. Auffray, M. Polymeropoulos, and M. B. Soares. 1996. The I.M.A.G.E. consortium: an integrated molecular analysis of genomes and their expression. Genomics 33:151-152. [DOI] [PubMed] [Google Scholar]
- 28.Liang, J., J. Zubovitz, T. Petrocelli, R. Kotchetkov, M. K. Connor, K. Han, J. H. Lee, S. Ciarallo, C. Catzavelos, R. Beniston, E. Franssen, and J. M. Slingerland. 2002. PKB/Akt phosphorylates p27, impairs nuclear import of p27 and opposes p27-mediated G1 arrest. Nat. Med. 8:1153-1160. [DOI] [PubMed] [Google Scholar]
- 29.Mitra, J., and R. M. Schultz. 1996. Regulation of the acquisition of meiotic competence in the mouse: changes in the subcellular localization of cdc2, cyclin B1, cdc25C and wee1, and in the concentration of these proteins and their transcripts. J. Cell Sci. 109:2407-2415. [DOI] [PubMed] [Google Scholar]
- 30.Nakashio, A., N. Fujita, S. Rokudai, S. Sato, and T. Tsuruo. 2000. Prevention of phosphatidylinositol-3′-kinase-Akt survival signaling pathway during topotecan-induced apoptosis. Cancer Res. 60:5303-5309. [PubMed] [Google Scholar]
- 31.Okumura, E., T. Fukuhara, H. Yoshida, S. Hanada, R. Kozutsumi, M. Mori, K. Tachibana, and T. Kishimoto. 2002. Akt inhibits Myt1 in the signalling pathway that leads to meiotic G2/M-phase transition. Nat. Cell Biol. 4:111-116. [DOI] [PubMed] [Google Scholar]
- 32.Peng, C. Y., P. R. Graves, R. S. Thoma, Z. Wu, A. S. Shaw, and H. Piwnica-Worms. 1997. Mitotic and G2 checkpoint control: regulation of 14-3-3 protein binding by phosphorylation of Cdc25C on serine-216. Science 277:1501-1505. [DOI] [PubMed] [Google Scholar]
- 33.Rokudai, S., N. Fujita, O. Kitahara, Y. Nakamura, and T. Tsuruo. 2002. Involvement of FKHR-dependent TRADD expression in chemotherapeutic drug-induced apoptosis. Mol. Cell. Biol. 22:8695-8708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rothblum-Oviatt, C. J., C. E. Ryan, and H. Piwnica-Worms. 2001. 14-3-3 binding regulates catalytic activity of human Wee1 kinase. Cell Growth Differ. 12:581-589. [PubMed] [Google Scholar]
- 35.Sanchez, Y., C. Wong, R. S. Thoma, R. Richman, Z. Wu, H. Piwnica-Worms, and S. J. Elledge. 1997. Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25. Science 277:1497-1501. [DOI] [PubMed] [Google Scholar]
- 36.Sato, S., N. Fujita, and T. Tsuruo. 2002. Interference with PDK1-Akt survival signaling pathway by UCN-01 (7-hydroxystaurosporine). Oncogene 21:1727-1738. [DOI] [PubMed] [Google Scholar]
- 37.Sekimoto, T., M. Fukumoto, and Y. Yoneda. 2004. 14-3-3 suppresses the nuclear localization of threonine 157-phosphorylated p27Kip1. EMBO J. 23:1934-1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shin, I., F. M. Yakes, F. Rojo, N. Y. Shin, A. V. Bakin, J. Baselga, and C. L. Arteaga. 2002. PKB/Akt mediates cell-cycle progression by phosphorylation of p27Kip1 at threonine 157 and modulation of its cellular localization. Nat. Med. 8:1145-1152. [DOI] [PubMed] [Google Scholar]
- 39.Sleiman, R. J., and B. W. Stewart. 2000. Early caspase activation in leukemic cells subject to etoposide-induced G2-M arrest: evidence of commitment to apoptosis rather than mitotic cell death. Clin. Cancer Res. 6:3756-3765. [PubMed] [Google Scholar]
- 40.Smith, J. J., D. A. Richardson, J. Kopf, M. Yoshida, R. E. Hollingsworth, and S. Kornbluth. 2002. Apoptotic regulation by the Crk adaptor protein mediated by interaction with Wee1 and Crm1/exportin. Mol. Cell. Biol. 22:1412-1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strausburg, R. L., E. A. Feingold, R. D. Klausner, and F. S. Collins. 1999. The mammalian gene collection. Science 286:455-457. [DOI] [PubMed] [Google Scholar]
- 42.Strausfeld, U., J. C. Labbe, D. Fesquet, J. C. Cavadore, A. Picard, K. Sadhu, P. Russell, and M. Doree. 1991. Dephosphorylation and activation of a p34cdc2/cyclin B complex in vitro by human CDC25 protein. Nature 351:242-245. [DOI] [PubMed] [Google Scholar]
- 43.Toker, A., and A. C. Newton. 2000. Akt/protein kinase B is regulated by autophosphorylation at the hypothetical PDK-2 site. J. Biol. Chem. 275:8271-8274. [DOI] [PubMed] [Google Scholar]
- 44.Tsuruo, T., M. Naito, A. Tomida, N. Fujita, T. Mashima, H. Sakamoto, and N. Haga. 2003. Molecular targeting therapy of cancer: drug resistance, apoptosis and survival signal. Cancer Sci. 94:15-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vanhaesebroeck, B., and D. R. Alessi. 2000. The PI3K-PDK1 connection: more than just a road to PKB. Biochem. J. 346:561-576. [PMC free article] [PubMed] [Google Scholar]
- 46.Viglietto, G., M. L. Motti, P. Bruni, R. M. Melillo, A. D'Alessio, D. Califano, F. Vinci, G. Chiappetta, P. Tsichlis, A. Bellacosa, A. Fusco, and M. Santoro. 2002. Cytoplasmic relocalizaiton and inhibition of the cyclin-dependent kinase inhibitor p27Kip1 by PKB/Akt-mediated phosphorylation in breast cancer. Nat. Med. 8:1136-1144. [DOI] [PubMed] [Google Scholar]
- 47.Wang, Y., C. Jacobs, K. E. Hook, H. Duan, R. N. Booher, and Y. Sun. 2000. Binding of 14-3-3β to the carboxyl terminus of Wee1 increased Wee1 stability, kinase activity, and G2-M cell population. Cell Growth Differ. 11:211-219. [PubMed] [Google Scholar]
- 48.Watanabe, N., H. Arai, Y. Nishihara, M. Taniguchi, N. Watanabe, T. Hunter, and H. Osada. 2004. M-phase kinases induce phospho-dependent ubiquitination of somatic Wee1 by SCFβ-TrCP. Proc. Natl. Acad. Sci. USA 101:4419-4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Watanabe, N., M. Broome, and T. Hunter. 1995. Regulation of the human WEE1Hu CDK tyrosine 15-kinase during the cell cycle. EMBO J. 14:1878-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou, B. P., Y. Liao, W. Xia, B. Spohn, M. H. Lee, and M. C. Hung. 2001. Cytoplasmic localization of p21Cip1/Waf1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat. Cell Biol. 3:245-252. [DOI] [PubMed] [Google Scholar]