Highlights
-
•
Neurophysiological techniques play a key role in the classification of myoclonic phenomena especially EEG plus polymyography.
-
•
Cortico-subcortical epileptic myoclonus occurs in myoclonic seizures, while cortical myoclonus is stimulus and action induced.
-
•
A neurophysiology-based approach to myoclonus is critical as different drugs are effective on distinct subtypes of myoclonus.
Keywords: Cortical myoclonus, Cortico-subcortical myoclonus, Subcortical myoclonus, EEG plus polymyography, EEG correlates and network evaluation, Evoked potentials and reflex responses, High-frequency oscillations, Post-hypoxic myoclonus
Abstract
Myoclonus has multiple clinical manifestations and heterogeneous generators and etiologies, encompassing a spectrum of disorders and even physiological events. This paper, developed from a teaching course conducted by the Neurophysiology Commission of the Italian League against Epilepsy, aims to delineate the main types of myoclonus, identify potential underlying neurological disorders, outline diagnostic procedures, elucidate pathophysiological mechanisms, and discuss appropriate treatments.
Neurophysiological techniques play a crucial role in accurately classifying myoclonic phenomena, by means of simple methods such as EEG plus polymyography (EEG + Polymyography), evoked potentials, examination of long-loop reflexes, and often more complex protocols to study intra-cortical inhibition-facilitation. In clinical practice, EEG + Polymyography often represents the first step to identify myoclonus, acquire signals for off-line studies and plan the diagnostic work-up.
1. Introduction
This paper collects information from a course on the various clinical-pathological expressions, substrates, and implications of myoclonus. The course was conducted in December 2022 in Rome by the Neurophysiology Commission of the Italian League against Epilepsy. It has the following teaching objectives: i) providing a critical review of the available techniques useful to classify myoclonus; ii) identifying the major issues in the differential diagnosis and consequential therapy.
2. The classification of the myoclonus
Myoclonus is defined as a jerky, shock-like involuntary movement, arising from the nervous system that is subtended by a brief synchronous activation or deactivation of antagonist muscles and can thus be distinguished in “positive” and “negative” myoclonus. Positive myoclonus is characterized by sudden, brief, single or repetitive contractions of muscle groups with variable topography (axial, proximal or distal limb muscles), and can occur at rest (Fig. 1, left panel, top trace) or during muscle contraction (Fig. 1, left panel, middle trace). The term “negative myoclonus” (Fig. 1, left panel, bottom trace) defines an interruption of tonic muscular activity for less than 500 ms without evidence of preceding positive jerks (Blume et al., 2001, Fahn, 2002). Negative myoclonus can be overlooked, and a correct diagnosis requires adequate polygraphic investigations, aimed to document the eventual preceding time-locked cortical potential (defining the epileptic negative myoclonus, i.e. ENM). Primary motor, somatosensory and supplementary motor cortex have been shown to generate negative myoclonus (Rubboli et al., 2006). Positive and negative myoclonus can occur in combination in the same patient, and can present with various patterns, i.e. appearing as repetitive and quasi-rhythmic, or isolated and random events.
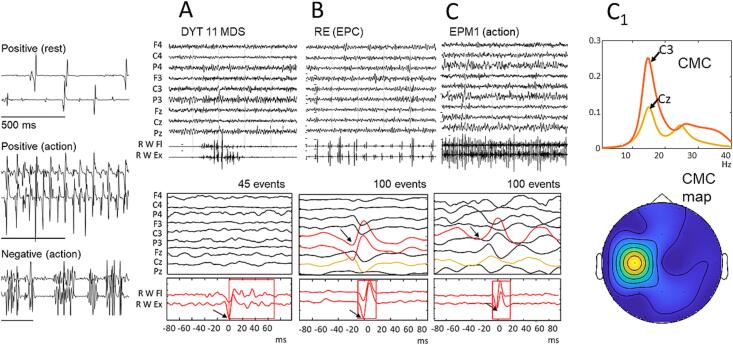
Fig. 1.
The three panels on the left represent three types of myoclonus. In the top panel: positive myoclonus at rest, which can affect the pair of antagonists, but also occur on a single muscle; in the middle panel: typical positive myoclonus, which occurs during voluntary muscle contraction, with an almost rhythmic pattern; in the bottom panel: negative myoclonus, which interrupts the contraction of a pair of antagonist muscles. The traces in A are the average obtained with the jerk-locked back-averaging (JLBA) technique in a patient with subcortical myoclonus, affected with myoclonus-dystonia syndrome due to DYT11 mutation. There is no correlated EEG event, the rectangle identifies the relatively long duration of the averaged myoclonic jerk. The traces in B represent the JLBA obtained on a cortical myoclonus at rest in a patient with EPC. In this case, JLBA identifies the correlated EEG that precedes the myoclonic jerks by about 14 ms. The traces in C and C1 represent the findings in a patient with cortical myoclonus, affected with progressive myoclonus epilepsy due to CSTB expansion mutation (EPM1). Also in this case the JLBA identifies the cortical transient that precedes action myoclonus. The lower panels are samples of the polygraphic signal. The rhythmicity of the positive action myoclonus in C allows an analysis of cortico-muscular coherence (CMC) with a maximum peak on the contralateral central derivation (C3) and its localization in a coherence map is represented in the panel below.
Clinical, anatomo-neurophysiological, and etiological criteria have been used to classify myoclonus (Fahn, 2002, Kojovic et al., 2011, Merchant et al., 2020 Jun 17, Van der Veen et al., 2022). The clinical classification recognizes several forms of myoclonus based on the relation with the conditions in which it appears (action-induced, at rest, reflex), on the topography (generalized, segmental, focal, multifocal), or on the time-based features (rhythmic, irregular, periodic).
The anatomo-neurophysiological classifications derive from the possible sites of origin of myoclonus in the nervous system. The categories most frequently employed are cortical, cortical-subcortical, reticular, spinal, and peripheral myoclonus (Kojovic et al., 2011), however, it is still debated whether this type of classification is sufficient to encompass the large variety of myoclonic manifestations (Carr, 2012 Jan).
The etiological classification defines four main groups: physiological, essential, epileptic, and symptomatic myoclonus, the latter occurring in many neurological disorders, which can be further distinguished into several subgroups (Fahn, 2002). The old definition of essential myoclonus mainly refers at present to the genetically determined myoclonus dystonia syndromes (Marelli et al., 2008).
3. Overviews of applicable techniques to study myoclonus
Myoclonus requires proper neurophysiological investigations to explore the underlying pathological mechanisms and to achieve a correct diagnosis (Merchant et al., 2020 Jun 17, Van der Veen et al., 2021). Although the neurophysiological techniques listed below are all important for the classification of the myoclonic phenomenon, there is no precise information regarding the sensitivity/specificity of each technique (Latorre et al, 2023). In clinical practice, EEG plus polymyography (EEG + Polymyography) may represent the first step for the identification of myoclonus, the acquisition of signals for EEG-EMG correlation studies and the setting of the subsequent diagnostic workup.
3.1. EEG plus polymyography with video recording
Polygraphy involves the simultaneous recording of multiple physiological parameters. In this context, EEG + Polymyography refers to the concurrent acquisition of EEG and EMG signals from muscles involved in jerking movements. EEG + Polymyography is a primary technique for detecting, classifying, and characterizing myoclonic phenomena (Cassim and Houdayer, 2006). It is a relatively simple, inexpensive, and non-invasive method, though careful technical execution is needed to obtain recordings suitable for detailed analysis (see Fig. 1 A-C, top panels and Fig. 2).
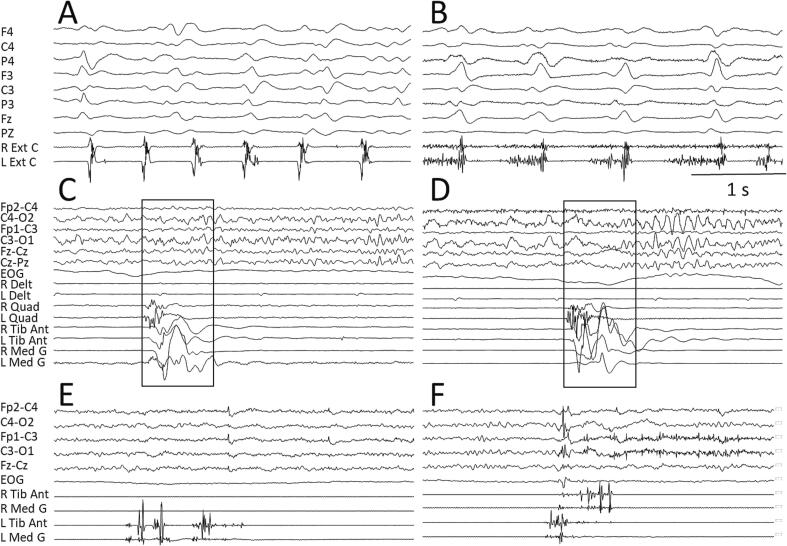
Fig. 2.
Examples of subcortical myoclonus. Brainstem myoclonus characterizes a patient with Creutzfeldt-Jakob disease (CJD), with both positive (A) and positive–negative (B) jerks showing variable temporal association with the typical periodic sharp wave complexes (PSWC). Propriospinal myoclonus is represented in C and D in a patient with a compression of the spinal cord at the lumbar level, starting on the right quadriceps but involving both sides and flowing from proximal to distal muscles. The recordings of jerks considered as psychogenic myoclonus are represented in the panels E and F, showing a scattered muscle activation.
To optimize polygraphic recordings for patients with myoclonic phenomena, several technical considerations are essential. The recording environment should allow patient movement to evoke action-induced myoclonus. Adding synchronized video recording is crucial, with the camera focused on the affected body segment to document the phenomena accurately. The positioning of the electrodes on the single muscle occurs via belly-tendon montage. Since myoclonus involves the synchronous activation of antagonist muscles, surface electrodes must be placed on various pairs of antagonist muscles to collect EMG signals effectively. For accurate post-processing, such as jerk-locked back-averaging (JLBA), the sampling frequency of the digitized polygraphic signal should be sufficiently high (e.g., 512–1024 Hz) to detect the abrupt onset of myoclonus.
During acquisition, optimizing the gain of the EEG and EMG traces is essential for direct online evaluation and further tailoring of the recording.
There is no fixed protocol for polygraphic exams; they must be tailored to each patient. The muscles to be recorded should be chosen based on the localization of the myoclonus, often comparing both sides of the body. Different muscles and body segments should be recorded to define temporal and spatial spread, measuring latencies between phenomena at different locations. For instance, in cases of reticular myoclonus, recording facial and cervical muscles (including orbicularis, masseter, and sternocleidomastoid) helps distinguish cranio-caudal versus caudo-cranial propagation. Additionally, recording proximal and distal limb muscles assesses the conduction velocity of the efferent volley.
Recordings should evaluate different conditions, including rest, motor goal achievement, posture maintenance, and responses to stimuli known to induce reflex myoclonus (e.g., tapping, flashes). Both body sides should be activated separately using simple motor activations. For upper limbs, the classic Mingazzini posture and sustained abduction of the arms with elbows flexed and palms facing downwards (wing-beating) are typical. Postures involving distal segments, such as carpi extension, help preserve EEG quality by minimizing muscle artifacts. For lower limbs, while the patient is sitting, legs or feet can be kept extended, or the patient may be asked to stand if the movement disorder appears only in an upright position. The recording must be precise enough to allow for EEG-EMG post-analyses. For averaging procedures, it is necessary to collect sufficient epochs containing the motor phenomenon, as free from artifacts as possible. The onset of the myoclonic EMG burst must be clear to perform consistent averaging, defining the cortical-EMG relationship and clarifying the spreading and propagation of the motor volley.
3.2. EEG (MEG) correlates and network evaluation
Post-analyses of EEG-EMG or magnetoencephalography (MEG)-EMG signals, for identification of the EEG and MEG correlates typically make use of the jerk-locked back averaging technique (Shibasaki and Kuroiwa, 1975). The procedure, according to Barrett (1992), uses the EMG signal to synchronize the EEG average, by positioning the triggers at the onset of the EMG bursts. The analysis window includes a sufficient period before (at least 100 ms) and after the myoclonus (Fig. 1, A-C, bottom panels).
Other methods should be applied in the case of quasi-rhythmic myoclonus (Grosse et al., 2003 Feb). They make use of the cortico-muscular coherence, allowing evaluation of the relationship between the two signals.
When myoclonus has no identifiable correlate based on visual inspection of raw EEG or MEG signals, identification of a pre-myoclonus cortical potential, using JLBA, or a significant peak of cortico-muscular coherence during rhythmic phases is a fundamental indicator of the cortical origin as typically observed in progressive myoclonic epilepsies (PME). The genetic disorders giving rise to PMEs may present clinically with heterogeneous phenotype and age. Even if “classical” PMEs start in late childhood and adolescence and have been identified for a long time, some other genetic disorders may start later (in juvenile and adult age) or in early childhood (then evolving toward a PME) (Franceschetti et al., 2014, Courage et al., 2021). Furthermore, it is necessary to consider that in some diseases, typically – but not exclusively − in Lafora disease, many myoclonic jerks often occur in association with epileptic paroxysms and should be distinguished from those correlated with action myoclonus, being the latter a symptom which typically identifies the PMEs. The evaluation of the corticomuscular coherence and phase have been largely applied to PMEs, particularly when exhibiting nearly rhythmic myoclonus, often triggered by action (Panzica et al., 2003, Panzica et al., 2014) (see Figure 1, C1). In fact, in the case of recurrent rhythmic jerks it can be difficult to perform an average using JLBA due to the limited pre-myoclonus time. Additionally, cortico-muscular coherence can give a quantitative evaluation, and some research has demonstrated a correlation between the value of the EEG-EMG coherence peaks and the severity of the myoclonus (Canafoglia et al., 2011). Furthermore, studies have revealed that diminished cortico-muscular coherence and alterations in cortico-cortical connectivity in patients with PMEs are associated with the efficacy of the drug treatment (Franceschetti et al., 2021).
Additional studies of the involved brain networks can be performed to further interpret the brain dysfunction and the recruitment of widespread cortical circuitries underlying afferent and efferent cortico-muscular relationships in PME patients with action-induced quasi-rhythmic myoclonus (Panzica et al., 2014).
3.3. Evoked potentials, reflex responses, and the role of high-frequency oscillations
Cortical reflex myoclonus, evoked by electrical shocks delivered to the median nerve at the wrist, is often associated with giant somatosensory evoked potentials (SEPs). The pattern shows an extreme enlargement of P25 and of the subsequent components, whereas the initial component N20 is normal or only slightly enhanced (Avanzini et al., 2016; Fig. 3, A). This is a typical feature occurring in patients with “cortical” myoclonus, and in some patients with “cortico-thalamic” epileptic myoclonus (Horlings et al., 2020 Jul). SEPs should be performed with a low stimulus frequency (e.g. 1 Hz), to avoid the attenuation of the enlarged middle and late components. For further in-depth examination of the topic, see also Latorre et al. (2023).
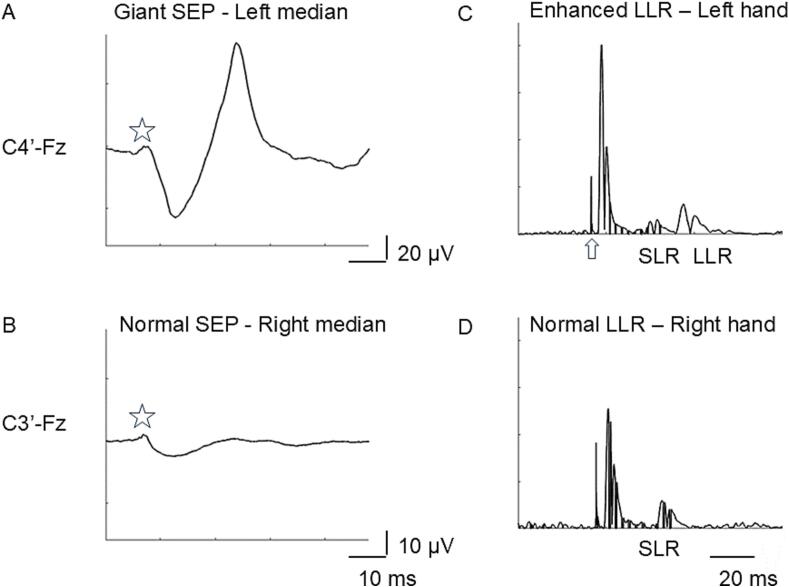
Fig. 3.
Example of unilateral giant somatosensory evoked potential (SEP) in a patient with EPC of the left orbicularis and left upper limb muscles (A). On the other side, SEP is normal (B). The star indicates N20 which is bilaterally normal, while the following components are increased on the right sensory cortex only. In C abnormal amplitude ratio between short latency (SLR) and long latency reflex (LLR), indicating unilateral facilitation of the LLR during moderate muscular activation of the left hand. On the other side, always during moderate motor activation, SLR is well evident, while LLR scarcely defined and their amplitude ratio normal (D). The arrow indicates the artefact due to electrical stimulation.
The high-frequency oscillations (HFO) are small-amplitude waves that appear in the first 20 ms of the SEP, observable after applying filters in the 450 to 750 Hz band. The early burst of HFO, which precedes N20 peak, is probably generated by a presynaptic activity of the final part of the thalamo-cortical tract whereas the late subcomponent, which follows N20 peak, is a post-synaptic response related to the activity of inhibitory GABAergic inhibitory interneurons in the somatosensory cortex. These components are frequently suppressed in patients with cortical myoclonus; more rarely they can be delayed and enhanced. They are not correlated with the amplitude of the cortical components of SEPs. The study of HFO in patients with cortical myoclonus indicates a possible heterogeneity in the pathophysiological mechanisms leading to cortical hyperexcitability, although in most cases it can be considered that hyperexcitability can be generated by an impairment of the inhibitory intracortical circuit (Alegre et al., 2006 Jun, Insola et al., 2019 Apr). Long-latency reflex (LLR) is a physiological motor response usually assessed in the thenar muscles after stimulation of the median nerve (Deuschl and Lücking, 1990). In normal subjects, it can be observed only during muscle contraction, whereas it can be obtained in resting muscles in patients with cortical reflex myoclonus. LLR complex can include three subcomponents (LLR I, II and III). The LLR I (or C reflex) is enhanced in myoclonus (most rarely LLR III) (Cruccu and Deuschl, 2000, Deuschl and Lücking, 1990, Deuschl and Eisen, 1999, Latorre et al., 2023). The afferent pathway of the reflex includes activation of Ia afferents with action potentials travelling through the lemniscus pathway and reaching the primary somatosensory cortex. A transcortical polysynaptic transmission of signals in the sensorimotor cortex is thought to mediate the activation of the efferent pathway through the corticospinal tract. In practice, the LLR evoked by electrical stimulation is the equivalent of the phenomenon defined as “cortical reflex myoclonus”. The LLR should be assessed both at rest and during motor activation. In this latter condition, the amplitude ratio between LLR and short latency response (SLR) is typically enhanced in patients with cortical myoclonus (Fig. 3, C), but more rarely occurs also in some cases of subcortical myoclonus, albeit with atypical characteristics or latency, thus it is insufficient for conclusively discriminating the cortical/subcortical origin of myoclonus.
A pathological change in the excitability of the primary motor cortex (M1) has been identified as the underlying physiological basis in many cases of cortical myoclonus (Shibasaki and Hallett, 2005). In this context, motor-evoked potentials elicited by transcranial magnetic stimulation (TMS) are particularly useful for directly investigating the imbalance between excitatory and inhibitory networks within M1. Most studies demonstrated a reduction of motor thresholds, along with a significant decrease of short intracortical inhibition and a shorter cortical silent period. These results put forward a significant excitatory-inhibitory imbalance within M1 characterized by increased facilitation and/or decreased inhibition involving glutamatergic and GABAergic networks (Shibasaki and Hallett, 2005, Nardone et al., 2018, Dubbioso et al., 2023). Interestingly, another TMS protocol, time-locked with peripheral electrical stimulation, namely short-latency afferent inhibition, consistently shows paradoxical facilitation, suggesting abnormal sensory-motor integration in patients with cortical myoclonus (Shibasaki and Hallett, 2005, Dubbioso et al., 2022).
4. Cortico-subcortical, cortical reflex myoclonus and additional presentations of cortical myoclonus
Cortico-subcortical (probably thalamo-cortical myoclonus) typically occurs in myoclonic seizures and has been defined as “epileptic myoclonus”. The large spectrum of epileptic myoclonus occurs in several distinct epileptic syndromes, namely juvenile myoclonic epilepsy but also in many other epileptic disorders. Examples are some variants of childhood absence epilepsy or epilepsy with eyelid myoclonia and absences; as well, more severe epilepsies in childhood such as Lennox-Gastaut syndrome or myoclonic astatic epilepsy (Guerrini and Takahashi, 2013).
ENM has been observed in a wide range of epileptic conditions with different etiologies (idiopathic, symptomatic, genetic, or unknown) usually associated with other seizure types (Tassinari et al., 1995). ENM may rarely constitute the ictal event accompanying a photoparoxysmal response (PPR) (Gambardella et al., 1996). Polygraphically, it appears as an interruption of tonic muscular activity, time-locked to a spike on the EEG, without evidence of an antecedent myoclonus (Tassinari et al., 1995, Rubboli and Tassinari, 2006). Clinically, ENM can cause dropping of objects from the hands, “tremulousness” of a limb with difficulties in writing and feeding, head nodding, or, at times, gait instability and falls (Capovilla et al., 2000). Despite the relatively short duration, ENM can be so frequent as to lead to a severe motor disturbance resembling motor neglect of the affected limb (Tassinari et al., 1995).
“Epileptic myoclonic seizures” may also occur in neurodegenerative pathologies giving rise to progressive myoclonic epilepsies (PMEs), even if in some of these disorders, “cortical reflex myoclonus”, mainly present on action, dominates the clinical picture and is the most disabling symptom, whereas the epileptic disorder can be quite limited.
Cortical reflex myoclonus, elicited by somatosensory stimuli and by motor activation, typically characterizes the presentation of the PMEs, with multiple etiologies, thus needing an early and specific evaluation. In these cases, the study of both the EEG(MEG)-EMG correlates (see the examples in Fig. 1C and C1), the SEP analysis, and the evaluation of pathological reflexes (namely LLR) is fundamental for the diagnosis and obviously has an important prognostic value. At the onset of the disease, often in adolescence, the correct identification of action and reflex myoclonus is fundamental for the differential diagnosis with respect to a form of non-progressive myoclonic epilepsy.
Cortical reflex myoclonus may also occur as uncommon presentation during the evolution in different neurodegenerative disorders such as Alzheimer’s disease and some presentations of autosomal dominant cerebellar ataxias (Caviness, 2019). Moreover, it can also occur in the chronic stage of post-anoxic myoclonus coexisting with brainstem (reticular) generated myoclonus.
4.1. Cortical tremor
Cortical tremor, unlike other types of tremors, is thought to be generated by the sensorimotor cortex, being actually a type of cortical myoclonus. Cortical tremor typically occurs with rhythmic or near-rhythmic pattern over multiple muscle groups, predominantly in the upper limbs. The frequency of this type of rhythmic myoclonus is often lower than that observed in the classical forms of PMEs, thus inducing the original definition of “tremor”. Cortical tremor is typically seen in familial adult myoclonus epilepsy (FAME), also named benign adult familial myoclonus epilepsy (BAFME) and familial cortical myoclonic tremor and epilepsy (FCMTE), which is an autosomal dominant condition associated with occasional/rare convulsive seizures (Corbett et al., 2019).
Neurophysiological evidence demonstrated a widespread cortical hyperexcitability (Shibasaki and Hallett, 2005, Suppa et al., 2009, Latorre et al., 2020, Dubbioso et al., 2023). Patients with FAME may also show PPR, and EEG polyspikes-wave complexes. As occur in PMEs, JLBA can reveal cortical spikes preceding and time-locked to individual myoclonic jerks and abnormal corticomuscular coherence that demonstrates a cortical drive for involuntary muscle activity (Shibasaki and Hallett, 2005, Suppa et al., 2009, Licchetta et al., 2013, Latorre et al., 2020, Dubbioso et al., 2023).
Patients with FAME usually also display “giant” SEPs and enhanced LLR, similar to that found in typical PMEs (Shibasaki and Hallett, 2005, Suppa et al., 2009, Latorre et al., 2020, Dubbioso et al., 2023). TMS studies demonstrate a reduction of motor thresholds and a significant decrease in short interval intracortical inhibition and cortical silent period, overall pointing to decreased inhibition in the primary motor cortex (Shibasaki and Hallett, 2005, Suppa et al., 2009, Latorre et al., 2020, Dubbioso et al., 2023). Cortico-muscular coherence can provide distinctive elements to differentiate essential tremor and cortical myoclonic tremor (Sharifi et al., 2021) and to define the location of the involved cortical areas (Franceschetti et al., 2023).
4.2. Epilepsia partialis continua
Epilepsia partialis continua (EPC) is a variant of simple focal motor status epilepticus including a peculiar condition in which focal jerks of one part of the body may spontaneously recur with quasi-rhythmic/periodic or irregular course for a prolonged period. Myoclonus has a cortical origin, with possible involvement of subcortical structures (Guerrini, 2009 Dec). Voluntary motor activity or sensory stimuli may enhance the jerks. Rasmussen encephalitis is the most frequent cause of EPC in childhood (Mameniškienė and Wolf, 2017).
EEG usually shows epileptiform and/or slow focal activity congruent with the location of the muscle jerks. EMG polygraphy shows that the bursts are often longer than 50 ms. The EEG transients correlated to the jerks are often, but not always, evident at the visual inspection, therefore in the latter JLBA needs to be performed (an example is reported in the panels of Fig. 1B). In some cases, in EPC, SEP amplitude is unilaterally increased, as well LLR can be enhanced on one side (Fig. 3).
4.3. Photic myoclonus
Photic reflex myoclonus occurs in many types of epilepsy, and it may be associated with spontaneous myoclonus or myoclonus triggered by other stimuli.
Studies from experimental animal models (photosensitive Papio papio, or Guinea baboon) have demonstrated the cortical origin of the myoclonus induced by visual stimuli (Naquet et al., 1987 Oct); the data were confirmed in neurophysiological studies in humans (Rubboli et al., 1999). Syndromes with epilepsy, photosensitivity and photic reflex myoclonus include genetically generalized epilepsies, developmental and epileptic encephalopathies, and PMEs (Hirsch et al., 2022, Riney et al., 2022, Specchio et al., 2022).
The foremost mechanisms to generating photic reflex myoclonus is a hyperexcitable visual cortex to flash stimuli, as revealed by single and paired flash visual evoked potentials (F-VEPs) (Strigaro et al., 2012). According to experimental and clinical studies, photosensitivity is the result of a synchronous recruitment of large neuronal populations within the visual cortex, which, at certain harmonic frequencies, overcomes the inhibitory capability of the network, leading to a synchronous resonant discharge (Fisher et al., 2005). It is assumed that a dysregulation of the occipital-frontal pathways and their connections takes place, in response to the abnormal visual stimulus processing (Parra et al., 2003, Siniatchkin et al., 2007, Moeller et al., 2009, Visani et al., 2010, Varotto et al., 2012, Vaudano et al., 2017) becoming capable to elicit myoclonic jerks.
Additional information comes from flash-evoked high-frequency EEG oscillations (F-HFOs), easily extracted from F-VEPs through appropriate filtering, showing a consistently enhanced spectral power at about 85 Hz, which suggests a pivotal role in the generation of the PPR. TMS also showed an overactive visuomotor integration underlying the fast spread of epileptic activity from posterior to anterior areas of the brain, where epileptic myoclonus originates (Strigaro et al., 2015a, Strigaro et al., 2015b, Suppa et al., 2015, Vaudano et al., 2017).
Intermittent light stimulation may induce the appearance of clinical subjective manifestations and epileptic seizures, which include myoclonus (orbitofrontal, eyelid, focal, generalized), tonic, absence, generalized tonic-clonic, and focal seizures (Kasteleijn-Nolst Trenité et al., 2001). In recent years, a fundamental role has been attributed to genetic factors in predisposing to photosensitivity, through complex inheritance mechanisms. Genes associated with clinical and EEG photosensitivity, with a predominant myoclonic phenotype, have been identified in epilepsy syndromes of varying severity (Galizia et al., 2015, Johannesen et al., 2016, Lo Barco et al., 2021). Photic reflex myoclonus is also a hallmark of various PMEs, which can show PPR even at low frequencies (Canafoglia et al., 2015, Avanzini et al., 2016 Sep 1, Riney et al., 2022, Specchio et al., 2022).
5. Subcortical myoclonus
Subcortical myoclonus typically occurs in neurodegenerative disorders and in genetically determined syndromes, such are those included in the myoclonic dystonia (see the example in Fig. 1A). Brainstem generated myoclonus typically occurs in post-hypoxic damage, and Creutzfeldt-Jakob disease (CJD).
5.1. Propriospinal and spinal myoclonus
Propriospinal myoclonus (PSM) is a rare hyperkinetic movement disorder, which could appear especially during relaxed wakefulness preceding sleep onset (Antelmi and Provini, 2015). PSM consists in repetitive and arrhythmic jerky flexion, or less frequently, extension of the trunk. The neurophysiological pattern of PSM suggests that the disorder arises from a spinal generator, most commonly at the thoracic level with a peculiar slow propagation (from 5 to 15 m/sec) up and down in the spinal cord without any activation of cranial nerve-innervated muscles. Polymyographic evaluation, showing a stereotyped pattern of muscle activation with slow spinal conduction time, is mandatory for the diagnosis (Fig. 2C and D) and represents a differential element with psychogenic movements which show variable patterns of activation (Fig. 2E and F). Differential diagnosis with psychogenic axial jerks can employ detection of Bereitschaftspotential (BP), also called premotor potential, which is a slow midline potential preceding the voluntary motor phenomena, obtained using a procedure similar to JLBA. To appreciate BP, it is necessary to remove the high pass filter. The detection of the BP in patients with axial jerks indicates a psychogenic origin (Erro et al., 2013).
Spinal segmental myoclonus is the expression of a focal activation in one or two contiguous myotomes, can be unilateral or bilateral. It happens at rest and is rarely enhanced by somatosensory stimuli, is often rhythmic, at low frequency and shows a long burst duration > 100 ms. It is typically linked to lesions of the spinal cord that should be investigated by neuroimaging (Apartis and Vercueil, 2016, Zutt et al., 2018).
5.2. Reticular myoclonus
It is a rare condition, due to hypersynchronous activity of neurons in the brainstem reticular formation (Guerrini and Takahashi, 2013).
Polymyography allows evaluating the order of recruitment of the muscles innervated by cranial nerves, where the sternocleidomastoid muscle is the first muscle involved (Apartis and Vercueil, 2016). JLBA typically shows inconsistent findings that exclude a cortical origin but suggest a brainstem pacemaker. In Creutzfeldt-Jakob disease (CJD), there is a variable relationship between the periodic sharp-wave complexes (PSWCs) and the myoclonic events that can be positive or positive–negative (Fig. 2A and B) (Binelli et al., 2010 Dec 15).
5.3. Startle reflex myoclonus (and hyperekplexia syndromes)
The startle response can be elicited by an unexpected auditory, or sometimes other types of stimuli including somaesthetic stimuli applied to the head or face. Habituation of the generalized startle response is rapid, although the blink reflex tends to persist.
Hyperekplexia consists of a pathologically exaggerated response to unexpected stimuli, particularly sounds (Brown, 2002). Hyperekplexia can be genetically determined (Matsumoto et al., 1992 Jul) or symptomatic (Salvi et al., 2000 Nov 14).
It is distinguished from the normal startle reflex by its lower threshold, greater extent, and resistance to habituation. The normal startle response habituates within one to five trials of auditory stimulation repeated every 20 s or so, leaving only an auditory blink reflex, whereas in hyperekplexia generalized jerks persist.
The startle response probably originates in lower brainstem; this is supported by the pattern of the involvement of the cranial muscles. Typically, in auditory startle the earliest EMG activity is in orbicularis oculi, with sternocleidomastoid then masseter following, while in startle due to taps to the face the shortest latency responses are recorded in sternocleidomastoid and later in orbicularis oculi and masseter. Moreover, the EMG activity in trunk and limb muscles follows that in sternocleidomastoid, usually at intervals that are longer than those seen for activation through the pyramidal tract.
Therefore, from a practical point of view, the EMG polygraphy for the study of the startle must always include the orbicularis oculi, masseter and sternocleidomastoid muscles (with attention focused on the sternocleidomastoid-masseter relationship so the earliest muscular response is always in the sternocleidomastoid), and the proximal and distal muscles of the limbs to evaluate the conduction velocity. Furthermore, repeated stimuli must be applied to evaluate habituation, thus distinguishing the forms of hyperekplexia (Brown, 2002).
A simplified startle protocol can also be applied in a diagnostic setting using EEG-EMG recording to exclude significant cortical changes, however for an optimal study it is necessary to consider the need for extensive polygraphic recordings and standardized stimuli for which a dedicated laboratory may be appropriate.
5.4. Sleep myoclonus
Although sleep is traditionally considered a period of quiescence, a wide range of motor events, including myoclonic jerks are commonly observed both during NREM and during REM sleep in physiological conditions (Montagna, 2003). Hypnic jerks (HJ), otherwise known as sleep starts or hypnagogic jerks, constitute a universal component of the sleep-onset process, occurring in normal people and at any age. HJs consist of non-periodic myoclonic movements of one or more body segments, usually involving the trunk and upper and lower limbs (Calandra-Buonaura et al., 2014). HJ are associated with autonomic activation and sometimes with a sensory feeling of “shock” or ‘‘falling into the void”. When particularly frequent and severe, HJs could be the cause of insomnia; occasionally HJs enter the differential diagnosis of epileptic seizures (Serino and Fusco, 2015).
The physiologic hypnic fragmentary myoclonus consists of sudden, arrhythmic asynchronous and asymmetric brief twitches involving various body segments, in particular distal limbs, and face, showing an inverse relationship with the degree of sleep EEG synchronization. Twitches are observed also during REM sleep and are considered physiological until their frequency and severity interfere with sleep continuity (Montagna, 2003).
Neck myoclonus (NM) is a recently recognized motor manifestation and presents as sudden myoclonic dorsal or ventral flexion or version of the head to one side, with varying amplitude. NM is common during REM sleep and more frequent in younger individuals. NM appears as a “short stripe‐shaped movement‐induced artifact” visible vertically over the polysomnographic (PSG) traces. Based on its high frequency in routine PSG recordings and apparently minor impact on sleep, NM is considered as a physiological phenomenon (Frauscher et al., 2010).
Benign sleep myoclonus (BSM) in neonates and infants consists of repetitive myoclonic jerks involving limbs, trunk, or whole body, occurring typically from birth to six months of age. BSM occurs only during sleep, stopping abruptly and consistently when the infant is aroused.
Nocturnal myoclonus is the term used by Lugaresi and colleagues to describe Periodic Leg Movements during Sleep (PLMS) (Ferri et al., 2017). PLMS are involuntary movements typically recorded from the tibialis anterior muscle and closely resemble the triple flexion reflex, which consists of dorsiflexion of the ankle and flexion of the knee and hip. According to the international criteria, PLMS included all leg movements lasting 0.5–10 s, separated by an interval between 4 and 90 s and occurring in a brief series. The presence of PLMS is defined by an index as > 15/h in adults and > 5/h in children (Ferri et al., 2017).
6. Different types of myoclonus in neurodegenerative and in Metabolic-Toxic encephalopathies
Symptomatic myoclonus occurs in a wide range of neurodegenerative diseases; usually unassociated with seizures, including dementia, basal ganglia, spinocerebellar disorders (Table 1) (Caviness, 2019).
Table 1.
Neurodegenerative and toxic-metabolic disorders associated with myoclonus.
|
|
|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Toxic Alcohol- chronic abuse and withdrawal Aluminum Bismuth Dichloro-diphenyl-trichloroethane Heavy metal poisons Methyl bromide and gasoline sniffing Toluene abuse Drug-induced Psychiatric Medications Cyclic antidepressants Selective serotonin reuptake inhibitors (SSRIs) Monoamine oxidase inhibitors Lithium preparations Antipsychotic agents (including tardive syndrome) Anti-infectious Agents Penicillin Carbapenem classes Cephalosporins |
Narcotics and opioids Morphine Fentanyl Tramadol Antiseizure medications and Anesthetics Phenytoin Carbamazepine Valproic acid Gabapentin Pregabalin Lidocaine Midazolam Cardiac Medications Antiarrhythmics (e.g., amiodarone, flecainide) Calcium Channel Blockers Contrast Media Others Amantadine Levodopa Metoclopramide |
Sporadic CJD accounts for ∼ 90 % of cases with prion disease (Puoti et al., 2012), and it is often associated with myoclonus. The phenotype is influenced by the genotype at codon 129 of the prion protein (PrP) gene and the type (1 or 2) of the scrapie PrP. The disease is characterized by rapidly progressive dementia associated with myoclonus in 84 % of all cases, and in up to 97 % of those with methionine/methionine or methionine/valine genotype at codon 129 (Puoti et al., 2012).
In Alzheimer’s disease (AD) and in the most common degenerative diseases with dementia − Dementia with Lewy Bodies (DLB) and Frontotemporal Dementia spectrum (FTD) − the cumulative probability of developing myoclonus is 42 %, highest in DLB (58.1 %) (Beagle et al., 2017). In addition to DLB, other synucleinopathies with myoclonus include Parkinson’s disease (PD) and PD with dementia, multiple system atrophy (MSA) and neurodegeneration with brain iron accumulation (NBIA). In PD, bilateral and non-synchronous, small amplitude postural/action myoclonus can occur as a complication of L-Dopa/amantadine treatment (Defebvre, 2006). A small-amplitude cortical myoclonus has been documented in ∼ 5 % of PD without dementia (Caviness et al., 2002).
Myoclonus is reported in 55–93 % of corticobasal syndrome (CBS) but in only 27 % of pathologically confirmed corticobasal degeneration (CBD) cases (Armstrong et al., 2013). It is typically action-induced and stimulus-sensitive, with a focal distribution involving the dystonic limb, reflecting the asymmetric cortical and subcortical abnormalities on neuroimaging. Neurophysiological findings show the involvement of a subcortical network (Di Stasio et al., 2019).
Reflex myoclonus triggered at rest by several stimuli has been more frequently reported in the patients with the cerebellar type MSA (cMSA) than in those with the parkinsonian type (pMSA). However, distal postural/action myoclonus has been frequently observed in patients with pMSA and supposed to be of cortical origin (Salazar et al., 2000, Okuma et al., 2005).
6.1. Drug-induced myoclonus
Drug-induced cortical or subcortical myoclonus can be due to a broad spectrum of pharmacological products, including antipsychotics, antibiotics, and anti-seizure medication (ASM). Liver or kidney dysfunction may elevate the levels of certain drugs or their metabolites, contributing to myoclonus. Typical and atypical anti-psychotics, including quetiapine, may induce myoclonus (Velayudhan and Kirchner, 2005). Lithium exposure can cause various types of myoclonus (Rissardo et al., 2022). Among the movement disorders that can be induced by ASM, myoclonus mainly depends on sodium channel blockers, but also from drugs binding to calcium channels, inhibiting gamma-transaminase or acting with multiple mechanisms (Rissardo et al., 2023 Aug 18).
Drug-induced myoclonus needs to be recognized because it is potentially fully treatable with the withdrawal of the causative drug.
Withdrawal of certain medications (i.e. sedatives and ASM) or toxic agents (alcohol in chronic abuse) may also cause myoclonus (Kojovic et al., 2011).
7. Post-hypoxic myoclonus
Post-hypoxic myoclonus (PHM) has different presentations depending on acute or chronic phase.
Acute post-hypoxic myoclonus appearing within 1 day of hypoxic injury is usually generalized, and associated with coma, while chronic post-hypoxic myoclonus appearing few days after the resolution of coma is typically multifocal (Gupta and Caviness, 2016 Sep), and is variably associated with dysarthria, ataxia, seizures, and/or cognitive deficits. This condition is defined as Lance–Adams syndrome (LAS).
PHM is considered a marker of poor prognosis and is transient. Patients usually worsen (with death), continue in a chronic vegetative state, or improve with or without LAS. If acute generalized PHM is continuous (for at least 30 min), it is also called myoclonic status. In the presence of acute PHM, EEG may show: burst suppression, spike-wave activity (both continuous and intermittent), myoclonic status epilepticus, diffuse slow background and waves, and alpha coma. The EEG discharges are variably associated with myoclonus but not time locked to it. According to Hallett et al., 1977 Mar, PHM is originated in the reticular formation of the medulla oblongata and called “reticular reflex myoclonus.” This type of myoclonus is stimulus sensitive and generalized with initial activation of the muscles innervated by lower brainstem nuclei followed by rostral and caudal recruitment spread. EMG discharges in the affected muscles are brief (10–30 ms). Differently from startle, the efferent pathway consists of rapidly conducting fibers, just slightly slower than the most rapid motor fibers.
The observation of epileptiform abnormalities on the EEG, the presence of giant potentials and the association with epileptic seizures have also led to the hypothesis of a possible cortical origin of acute PHM, at least in some cases (Bouwes et al., 2012 Aug). It is possible that the association of cortical and subcortical lesions may lead to variable myoclonic phenomena.
The PHM observed in LAS is multifocal and action-induced and may be associated with giant evoked potentials and LLR facilitation, as in cortical reflex myoclonus. In contrast to generalized myoclonus, chronic PHM is therefore likely to originate in the cortex, but reticular reflex myoclonus may also occur in some cases (Hallett, 2000).
8. Elements for differential diagnosis
Classification of myoclonus is needed to assess generators and hypothesize possible etiologies. In particular, the identification and definition of myoclonus using electrophysiological tests helps to determine its pathophysiology and can be used to orientate symptomatic treatment approaches.
The diagnostic procedure includes basic neurophysiologic testing and more complex techniques, but also imaging techniques that can be essential in some conditions. Imaging may contribute to identifying the origin and cause of myoclonus when it is clearly focal.
The clinical examination should characterize the amplitude, distribution, time course, and activation characteristics of the myoclonus, but the correct classification firstly requires the evidence of synchronous activation of the antagonist muscles (that is clearly demonstrable only by analyzing the corresponding EMG bursts). Various etiologies may have typical findings.
Spinal, propriospinal and reticular myoclonus have quite stereotyped EMG activation; therefore, the investigation needs to be performed to clearly determining the type and origin of the jerks, but generally does not need complex neurophysiological tests.
Rarely, local lesions of the brain structures can be non-progressive or acquired; in that case, imaging techniques add fundamental information, but neurophysiological definitions supply information about pathophysiology.
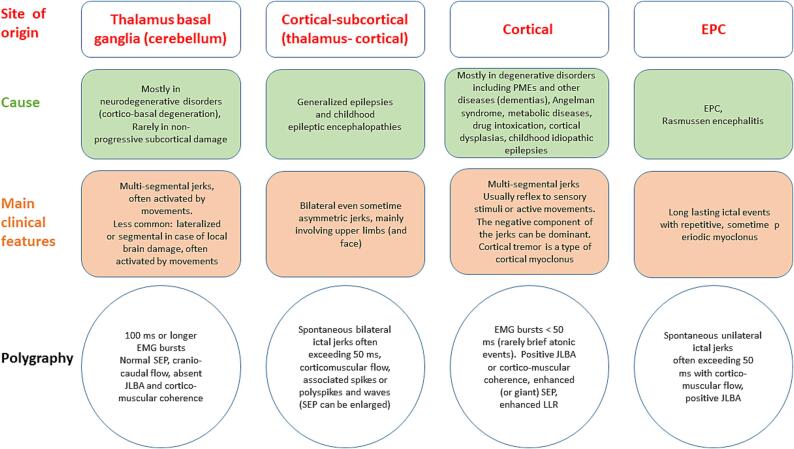
The diagnostic accuracy in case of cortical myoclonus can be increased by combining the results of multiple tests (Latorre et al., 2023). Fig. 4, Fig. 5 summarize the basic tests and simple post-analysis procedures that can help to define the type and origin of myoclonic jerks.
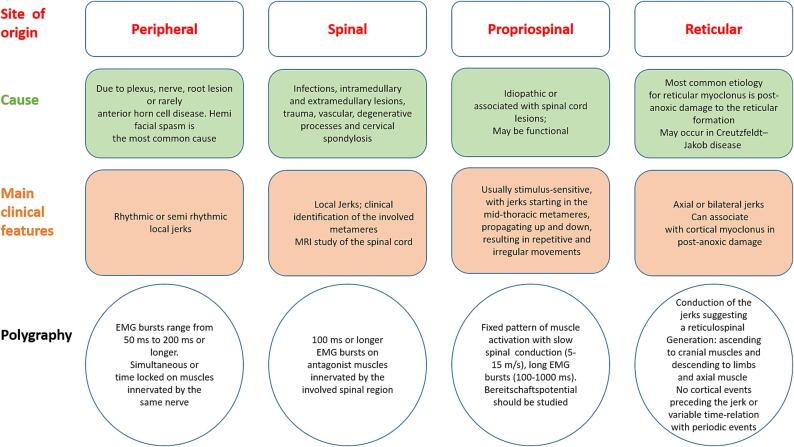
Fig. 4.
The origin, main causes, clinical features, and neurophysiological findings are resumed for peripheral, spinal, propriospinal, and reticular myoclonus.
Fig. 5.
The origin, main causes, clinical features, and neurophysiological findings are resumed for thalamus basal ganglia (cerebellum), cortical-subcortical (thalamus-cortical), cortical myoclonus, and EPC.
9. Controversial issues
Two main critical issues came up during the didactic course and were discussed to better define the problems and propose solutions.
A first issue concerns the use of some definitions; for example, the distinction between “cortical” versus “epileptic” myoclonus is important from a neurophysiological point of view and for the prognosis. Epileptic myoclonus can be bilateral, even sometime asymmetric, when associated with diffuse spike and wave discharges. However, it must be considered that “epileptic” myoclonus is also present, in association with reflex myoclonus, in forms of PMEs and therefore requires a precise and prognostically relevant differential diagnosis.
A second aspect is that the classification of myoclonus, often solely based on clinical presentation, may induce incorrect diagnosis, thus detailed neurophysiological study is needed to take away the errors associated with misclassifying various jerk-like movement disorders. EEG + Polymyography is the most important technique in defining movement disorders, but it must be applied properly. Other neurophysiological techniques are important to distinguish cortical versus subcortical myoclonus. However, they may fail to identify signs of cortical hyperexcitability due to i) the antiepileptic treatment already underway, ii) technical aspects or lack of correct normative data, iii) different excitability of the sensory cortex compared to the motor cortex (Latorre et al., 2023).
10. Treatment
Treatment may be only partially beneficial and limited by side effects, as polytherapy with a combination of multiple drugs at high dosages is often required (Levy and Chen, 2016, Stahl and Frucht, 2019, Pena and Caviness, 2020). To date, evidence-based data about myoclonus management remains scarce and treatment approach is mostly based on expert opinions, results from case reports or small case series, and very few controlled studies (Pena and Caviness, 2020, Caviness, 2019). Treatment options should consider also recent data on teratogenic issues, with consequent restrictions on the use of different ASMs in both females and males (Battino et al., 2024, Hernández-Díaz et al., 2024 Mar 21).
A neurophysiological-based approach to myoclonus is crucial since different drugs may be effective for the different myoclonus subtypes (Pena and Caviness, 2020, Caviness, 2019). Benzodiazepines, especially clonazepam (CNZ) may be useful as monotherapy or add-on treatment in different clinical contexts. However, sedative effect and development of tolerance are common (Levy and Chen, 2016, Caviness, 2019). The drugs and dosages are recapitulated in Table 2.
Table 2.
Summary of the main pharmacological options for the different forms of myoclonus.
| Drug | Dose | Main adverse events | Type of myoclonus |
|---|---|---|---|
| VPA | 500–3000 mg/day into 2–3 daily doses | Teratogenicity, hepatic failure, thrombocytopenia, pancreatitis, weight gain, hirsutism, hair loss, tremor | Cortical, cortical-subcortical, reticular, propriospinal |
| CLN | 0.5–10 mg/day into 1–3 daily doses | Sedation, cognitive dysfunction, irritability, ataxia | Cortical, subcortical segmental |
| LEV | 1000–3000 mg/day into 2 daily doses | Psychiatric disturbances (irritability, psychosis) | Cortical, cortical-subcortical, propriospinal |
| PIR | 9–45 gr/day into 2–3 daily doses | Diarrhea, weight gain, somnolence, insomnia, nervousness, depression, rash | Cortical, propriospinal |
| ZNS | 100–500 mg/day into 1–2 daily doses | Sedation, fatigue, psychomotor slowing, metabolic acidosis | Cortical, subcortical |
| PER | 2–12 mg/day once daily | Irritability, sedation, dizziness, ataxia, cognitive dysfunction | Cortical, cortical-subcortical |
| PB | 50–200 mg/day once daily | Sedation, cognitive dysfunction, osteoporosis | Cortical, cortical-subcortical |
| LTG | 100–600 mg/day 2 daily doses | Dizziness, headache, blurred or double vision, tremor, insomnia, Steven-Johnson syndrome, toxic epidermal necrolysis | Cortical-subcortical |
| ESM | 500–2000 mg/day 3 daily doses | Anorexia, nausea, abdominal discomfort, headache, ataxia, dizziness, skin rash | Cortical-subcortical ENM in idiopathic partial epilepsies in childhood |
| TPM | 100–400 mg/day 2 daily doses | Sedation, reversible cognitive dysfunction, weight loss, renal lithiasis, closed angle glaucoma | Cortical, cortical-subcortical |
| CBZ | 300–600 mg/day 2–3 daily doses | Dizziness, sedation, ataxia, nausea, vomiting, drowsiness, hyponatremia, PR interval prolongation | Subcortical segmental |
| GBP | 900–1600 mg/day 3 daily doses | Sedation, ataxia, dizziness, drowsiness, fatigue | Subcortical segmental |
| BoNT | BoNT-A: 10–34 U, aboBoNTA: 53–160 U, rBoNT-B: 1250–9000 U | weakness, dysphagia | Segmental |
Legend. aboBoNTA: AbobotulinumtoxinA, BoNT: Botulinum toxin, BoNT-A: OnabotulinumtoxinA, VPA: valproic acid, CBZ: carbamazepine, CLN: clonazepam, ENM: epileptic negative myoclonus, ESM: ethosuximide, GBP: gabapentin, LEV: levetiracetam, LTG: lamotrigine, rBoNT-B: RimabotulinumtoxinB, PB: phenobarbital, PER: perampanel, PIR: piracetam, TPM: topiramate, ZNS: zonisamide.
In EPC: Polytherapy with anti-seizure medications (ASM) is common; phenytoin (PHT) and phenobarbital (PB) may be more effective than valproate (VPA) or carbamazepine (CBZ). Newer ASMs like levetiracetam, brivaracetam, lacosamide, and zonisamide may also be useful. Felbamate may be used in resistant cases. Rasmussen encephalitis has been seen to be responsive to immunosuppressive agents such as azathioprine, rituximab, tacrolimus, intravenous immunoglobulin, and plasma exchange (Pellegrin et al., 2021 Jan 12, Flammer et al., 2023 Feb). Surgical interventions such as hemispherectomy may be considered in cases of Rasmussen encephalitis (Khan et al., 2023).
Cortical myoclonus. Cortical myoclonus is often responsive to ASMs. VPA, levetiracetam (LEV) and CNZ represent the first-line treatment agents. Piracetam (PIR), zonisamide (ZNS), and PB may be also useful (Genton et al., 1999, Levy and Chen, 2016, Ferlazzo et al., 2017, Caviness, 2019, Stahl and Frucht, 2019, Pena and Caviness, 2020). PIR should be used at high dosages (Koskiniemi et al., 1998 Mar). More recently, perampanel (PER) was demonstrated to be effective particularly on action myoclonus in patients with Unverricht-Lundborg disease and other PMEs (Crespel et al., 2017 Apr, Canafoglia et al., 2019 Oct, Assenza et al., 2021 Mar).
Conversely, PHT, CBZ, lamotrigine (LTG), vigabatrin, gabapentin, pregabalin and tiagabine should be avoided since they may exacerbate myoclonus (Michelucci et al., 2016, Caviness, 2019). Alternative treatments aimed at avoiding an excessive pharmacological load have been applied in small groups, including the use of repetitive TMS (Nardone et al., 2018, Rossi Sebastiano et al., 2018).
Effectiveness of ethosuximide in the treatment of ENM in children suffering from idiopathic partial epilepsy has been demonstrated by several studies (Oguni et al., 1998, Capovilla et al., 1999 Jun, Capovilla et al., 2000).
Cortical-subcortical (thalamo-cortical) myoclonus: VPA is considered the drug of choice (Pena and Caviness, 2020). LEV and LTG can be used when VPA is ineffective or contraindicated, although cases of worsening of myoclonus with LTG therapy have been reported (Specchio et al., 2008 Apr). CNZ and ZNS represent adjunctive treatment options (Pena and Caviness, 2020).
Reticular myoclonus. VPA is usually considered as the drug of choice in post-hypoxic myoclonus due to its high effectiveness on myoclonus as well as on seizures and photosensitivity (Caviness, 2019). PIR at high dosages, and LEV can also be useful.
Other types of subcortical myoclonus: In myoclonus-dystonia syndrome, CNZ, ZNS, sodium oxybate, and tetrabenazine may be effective (Priori et al., 2000). Deep brain stimulation targeting globus pallidus or ventralis intermediate nucleus of thalamus should be considered for refractory forms (Roze et al., 2015).
In spinal and propriospinal myoclonus, surgical removal of the causative structural lesion may be effective. ZNS, CNZ, PIR, LEV, VPA, CBZ, oxcarbazepine and baclofen can show some efficacy (Pena and Caviness, 2020). Because of jerks’ extension limited to the muscles of few myotomes, both palatal and spinal myoclonus can be effectively treated with targeted botulinum toxin injections (Pena and Caviness, 2020).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
Thanks to Italian League Against Epilepsy (LICE) and Fondazione Epilessia LICE Onlus, Roma, Italy, for their support.
Contributor Information
Stefano Meletti, Email: stefano.meletti@unimore.it.
Francesca Bisulli, Email: francesca.bisulli@unibo.it.
REFERENCES
- Alegre M., Urriza J., Valencia M., Muruzábal J., Iriarte J., Artieda J. High-frequency oscillations in the somatosensory evoked potentials of patients with cortical myoclonus: pathophysiologic implications. J Clin Neurophysiol. 2006 Jun;23(3):265–272. doi: 10.1097/01.wnp.0000201075.31438.fb. PMID: 16751728. [DOI] [PubMed] [Google Scholar]
- Antelmi E., Provini F. Propriospinal myoclonus: The spectrum of clinical and neurophysiological phenotypes. Sleep Med Rev. 2015;22:54–63. doi: 10.1016/j.smrv.2014.10.007. [DOI] [PubMed] [Google Scholar]
- Apartis E, Vercueil L. To jerk or not to jerk: A clinical pathophysiology of myoclonus. Rev Neurol (Paris). 2016 Aug-Sep;172(8-9):465-476. doi: 10.1016/j.neurol.2016.07.013. Epub 2016 Aug 24. PMID: 27568397. [DOI] [PubMed]
- Armstrong M.J., Litvan I., Lang A.E., Bak T.H., Bhatia K.P., Borroni B., Boxer A.L., Dickson D.W., Grossman M., Hallett M., Josephs K.A., Kertesz A., Lee S.E., Miller B.L., Reich S.G., Riley D.E., Tolosa E., Tröster A.I., Vidailhet M., Weiner W.J. Criteria for the diagnosis of corticobasal degeneration. Neurology. 2013;29;80(5):496–503 doi: 10.1212/WNL.0b013e31827f0fd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assenza G., Nocerino C., Tombini M., Di Gennaro G., D'Aniello A., Verrotti A., Marrelli A., Ricci L., Lanzone J., Di Lazzaro V., Bilo L., Coppola A. Perampanel Improves Cortical Myoclonus and Disability in Progressive Myoclonic Epilepsies: A Case Series and a Systematic Review of the Literature. Front Neurol. 2021 Mar;24(12) doi: 10.3389/fneur.2021.630366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avanzini G., Shibasaki H., Rubboli G., Canafoglia L., Panzica F., Franceschetti S., Hallett M. Epileptic Disord. 2016 Sep 1;18(S2):11–27. doi: 10.1684/epd.2016.0835. [DOI] [PubMed] [Google Scholar]
- Barrett G. Jerk-locked averaging: technique and application. J Clin Neurophysiol. 1992;9(4):495–508. [PubMed] [Google Scholar]
- Battino D, Tomson T, Bonizzoni E, Craig J, Perucca E, Sabers A, Thomas S, Alvestad S, Perucca P, Vajda F; EURAP Collaborators. Risk of Major Congenital Malformations and Exposure to Antiseizure Medication Monotherapy. JAMA Neurol. 2024 May 1;81(5):481-489. doi: 10.1001/jamaneurol.2024.0258. PMID: 38497990; PMCID: PMC10949148. [DOI] [PMC free article] [PubMed]
- Beagle A.J., Darwish S.M., Ranasinghe K.G., La A.L., Karageorgiou E., Vossel K.A. Relative Incidence of Seizures and Myoclonus in Alzheimer's Disease, Dementia with Lewy Bodies, and Frontotemporal Dementia. J Alzheimers Dis. 2017;60(1):211–223. doi: 10.3233/JAD-170031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binelli S., Agazzi P., Canafoglia L., Scaioli V., Panzica F., Visani E., Di Fede G., Giaccone G., Bizzi A., Bugiani O., Avanzini G., Tagliavini F., Franceschetti S. Myoclonus in Creutzfeldt-Jakob disease: polygraphic and video-electroencephalography assessment of 109 patients. Mov Disord. 2010 Dec 15;25(16):2818–2827. doi: 10.1002/mds.23397. PMID: 20939057. [DOI] [PubMed] [Google Scholar]
- Blume W.T., Lüders H.O., Mizrahi E., Tassinari C., van Emde B.W., Engel J., Jr. Glossary of descriptive terminology for ictal semiology: report of the ILAE task force on classification and terminology. Epilepsia. 2001;42(1212–8):25. doi: 10.1046/j.1528-1157.2001.22001.x. [DOI] [PubMed] [Google Scholar]
- Bouwes A., van Poppelen D., Koelman J.H., Kuiper M.A., Zandstra D.F., Weinstein H.C., Tromp S.C., Zandbergen E.G., Tijssen M.A., Horn J. Acute posthypoxic myoclonus after cardiopulmonary resuscitation. BMC Neurol. 2012 Aug;1(12):63. doi: 10.1186/1471-2377-12-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. The startle syndrome. Mov Disord. 2002;17(Suppl 2):S79–S82. doi: 10.1002/mds.10066. PMID: 11836762. [DOI] [PubMed] [Google Scholar]
- Calandra-Buonaura G., Alessandria M., Liguori R., Lugaresi E., Provini F. Hypnic jerks: neurophysiological characterization of a new motor pattern. Sleep Med. 2014;15(6):725–727. doi: 10.1016/j.sleep.2014.01.024. [DOI] [PubMed] [Google Scholar]
- Canafoglia L., Franceschetti S., Uziel G., Ciano C., Scaioli V., Guerrini R., Visani E., Panzica F. Characterization of severe action myoclonus in sialidoses. Epilepsy Res. 2011;94(1–2):86–93. doi: 10.1016/j.eplepsyres.2011.01.013. [DOI] [PubMed] [Google Scholar]
- Canafoglia L., Gilioli I., Invernizzi F., Sofia V., Fugnanesi V., Morbin M., Chiapparini L., Granata T., Binelli S., Scaioli V., Garavaglia B., Nardocci N., Berkovic S.F., Franceschetti S. Electroclinical spectrum of the neuronal ceroid lipofuscinoses associated with CLN6 mutations. Neurology. 2015;85(4):316–324. doi: 10.1212/WNL.0000000000001784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canafoglia L., Barbella G., Ferlazzo E., Striano P., Magaudda A., d'Orsi G., Martino T., Avolio C., Aguglia U., Sueri C., Giuliano L., Sofia V., Zibordi F., Ragona F., Freri E., Costa C., Nardi Cesarini E., Fanella M., Rossi Sebastiano D., Riguzzi P., Gambardella A., Di Bonaventura C., Michelucci R., Granata T., Bisulli F., Licchetta L., Tinuper P., Beccaria F., Visani E., Franceschetti S. An Italian multicentre study of perampanel in progressive myoclonus epilepsies. Epilepsy Res. 2019 Oct;156 doi: 10.1016/j.eplepsyres.2019.106191. Epub 2019 Aug 16 PMID: 31446282. [DOI] [PubMed] [Google Scholar]
- Capovilla G., Beccaria F., Veggiotti P., Rubboli G., Meletti S., Tassinari C.A. Ethosuximide is effective in the treatment of epileptic negative myoclonus in childhood partial epilepsy. J Child Neurol. 1999 Jun;14(6):395–400. doi: 10.1177/088307389901400609. PMID: 10385848. [DOI] [PubMed] [Google Scholar]
- Capovilla G., Rubboli G., Beccaria F., Meregalli S., Veggiotti P., Giambelli P.M., Meletti S., Tassinari C.A. Intermittent falls and fecal incontinence as a manifestation of epileptic negative myoclonus in idiopathic partial epilepsy of childhood. Neuropediatrics. 2000;31:273–275. doi: 10.1055/s-2000-9237. [DOI] [PubMed] [Google Scholar]
- Carr J. Classifying myoclonus: a riddle, wrapped in a mystery, inside an enigma. Parkinsonism Relat Disord. 2012 Jan;18(Suppl 1):S174–S176. doi: 10.1016/S1353-8020(11)70054-2. PMID: 22166426. [DOI] [PubMed] [Google Scholar]
- Cassim F, Houdayer E. Neurophysiology of myoclonus. Neurophysiol Clin. 2006 Sep-Dec;36(5-6):281-91. doi: 10.1016/j.neucli.2006.10.001. [DOI] [PubMed]
- Caviness J.N. Myoclonus. Continuum (minneap Minn). 2019;25(4):1055–1080. doi: 10.1212/CON.0000000000000750. [DOI] [PubMed] [Google Scholar]
- Caviness J.N., Adler C.H., Beach T.G., Wetjen K.L., Caselli R.J. Small-amplitude cortical myoclonus in Parkinson's disease: physiology and clinical observations. Mov Disord. 2002;17(4):657–662. doi: 10.1002/mds.10177. [DOI] [PubMed] [Google Scholar]
- Corbett M.A., Kroes T., Veneziano L., Bennett M.F., Florian R., Schneider A.L., Coppola A., Licchetta L., Franceschetti S., Suppa A., Wenger A., Mei D., Pendziwiat M., Kaya S., Delledonne M., Straussberg R., Xumerle L., Regan B., Crompton D., van Rootselaar A.F., Correll A., Catford R., Bisulli F., Chakraborty S., Baldassari S., Tinuper P., Barton K., Carswell S., Smith M., Berardelli A., Carroll R., Gardner A., Friend K.L., Blatt I., Iacomino M., Di Bonaventura C., Striano S., Buratti J., Keren B., Nava C., Forlani S., Rudolf G., Hirsch E., Leguern E., Labauge P., Balestrini S., Sander J.W., Afawi Z., Helbig I., Ishiura H., Tsuji S., Sisodiya S.M., Casari G., Sadleir L.G., van Coller R., Tijssen M.A.J., Klein K.M., van den Maagdenberg A.M.J.M., Zara F., Guerrini R., Berkovic S.F., Pippucci T., Canafoglia L., Bahlo M., Striano P., Scheffer I.E., Brancati F., Depienne C., Gecz J. Intronic ATTTC repeat expansions in STARD7 in familial adult myoclonic epilepsy linked to chromosome 2. Nat Commun. 2019;10(1):4920. doi: 10.1038/s41467-019-12671-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courage C., Oliver K.L., Park E.J., Cameron J.M., Grabińska K.A., Muona M., Canafoglia L., Gambardella A., Said E., Afawi Z., Baykan B., Brandt C., di Bonaventura C., Chew H.B., Criscuolo C., Dibbens L.M., Castellotti B., Riguzzi P., Labate A., Filla A., Giallonardo A.T., Berecki G., Jackson C.B., Joensuu T., Damiano J.A., Kivity S., Korczyn A., Palotie A., Striano P., Uccellini D., Giuliano L., Andermann E., Scheffer I.E., Michelucci R., Bahlo M., Franceschetti S., Sessa W.C., Berkovic S.F., Lehesjoki A.E. Progressive myoclonus epilepsies-Residual unsolved cases have marked genetic heterogeneity including dolichol-dependent protein glycosylation pathway genes. Am J Hum Genet. 2021;108(4):722–738. doi: 10.1016/j.ajhg.2021.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespel A., Gelisse P., Tang N.P., Genton P. Perampanel in 12 patients with Unverricht-Lundborg disease. Epilepsia. 2017 Apr;58(4):543–547. doi: 10.1111/epi.13662. Epub 2017 Feb 6 PMID: 28166365. [DOI] [PubMed] [Google Scholar]
- Cruccu G., Deuschl G. The clinical use of brainstem reflexes and hand-muscle reflexes. Clin Neurophysiol. 2000;111:371–387. doi: 10.1016/s1388-2457(99)00291-6. [DOI] [PubMed] [Google Scholar]
- Defebvre L. Myoclonus and extrapyramidal diseases. Neurophysiol Clin. 2006;36(5–6):319–325. doi: 10.1016/j.neucli.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Deuschl G., Eisen A. Long-latency reflexes following electrical nerve stimulation. The Inter national Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol Suppl. 1999;52:263–268. PMID: 10590995. [PubMed] [Google Scholar]
- Deuschl G., Lücking C.H. Physiology and clinical applications of hand muscle reflexes. Electroencephalogr Clin Neurophysiol Suppl. 1990;41:84–101. doi: 10.1016/b978-0-444-81352-7.50012-1. [DOI] [PubMed] [Google Scholar]
- Di Stasio F., Suppa A., Marsili L., Upadhyay N., Asci F., Bologna M., Colosimo C., Fabbrini G., Pantano P., Berardelli A. Corticobasal syndrome: neuroimaging and neurophysiological advances. Eur J Neurol. 2019;26(5):701–e52. doi: 10.1111/ene.13928. [DOI] [PubMed] [Google Scholar]
- Dubbioso R., Striano P., Tomasevic L., Bilo L., Esposito M., Manganelli F., Coppola A. Abnormal sensorimotor cortex and thalamo-cortical networks in familial adult myoclonic epilepsy type 2: pathophysiology and diagnostic implications. Brain Commun. 2022 doi: 10.1093/braincomms/fcac037. Feb 15;4(1):fcac037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubbioso R., Suppa A., Tijssen M.A.J., Ikeda A. Familial adult myoclonus epilepsy: Neurophysiological investigations. Epilepsia. 2023;64(Suppl 1):S39–S46. doi: 10.1111/epi.17553. [DOI] [PubMed] [Google Scholar]
- Erro R., Bhatia K.P., Edwards M.J., Farmer S.F., Cordivari C. Clinical diagnosis of propriospinal myoclonus is unreliable: an electrophysiologic study. Mov Disord. 2013;28(13):1868–1873. doi: 10.1002/mds.25627. [DOI] [PubMed] [Google Scholar]
- Fahn S. Overview, history, and classification of myoclonus. Advances in Neurology. 2002;89:13. [PubMed] [Google Scholar]
- Ferlazzo E., Kasteleijn-Nolst-Trenité D., Haan G.J., Felix Nitschke F., Ahonen S., Gasparini S., Minassian B.A. Update on Pharmacological Treatment of Progressive Myoclonus Epilepsies. Curr Pharm Des. 2017;23(37):5662–5666. doi: 10.2174/1381612823666170809114654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri R., Koo B.B., Picchietti D.L., Fulda S. Periodic leg movements during sleep: phenotype, neurophysiology, and clinical significance. Sleep Med. 2017;31:29–38. doi: 10.1016/j.sleep.2016.05.014. [DOI] [PubMed] [Google Scholar]
- Fisher R.S., Harding G., Erba G., Barkley G.L., Wilkins A. Epilepsy Foundation of America Working Group. Photic- and pattern-induced seizures: a review for the Epilepsy Foundation of America Working Group. Epilepsia. 2005;46(9):1426–1441. doi: 10.1111/j.1528-1167.2005.31405.x. [DOI] [PubMed] [Google Scholar]
- Flammer J., Neziraj T., Rüegg S., Pröbstel A.K. Immune Mechanisms in Epileptogenesis: Update on Diagnosis and Treatment of Autoimmune Epilepsy Syndromes. Drugs. 2023 Feb;83(2):135–158. doi: 10.1007/s40265-022-01826-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschetti S., Michelucci R., Canafoglia L., Striano P., Gambardella A., Magaudda A., Tinuper P., La Neve A., Ferlazzo E., Gobbi G., Giallonardo A.T., Capovilla G., Visani E., Panzica F., Avanzini G., Tassinari C.A., Bianchi A., Zara F. Collaborative LICE study group on PMEs. Progressive myoclonic epilepsies: definitive and still undetermined causes. Neurology. 2014;82(5):405–411. doi: 10.1212/WNL.0000000000000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschetti S., Visani E., Rossi Sebastiano D., Duran D., Granata T., Solazzi R., Varotto G., Canafoglia L., Panzica F. Cortico-muscular and cortico-cortical coherence changes resulting from Perampanel treatment in patients with cortical myoclonus. Clin Neurophysiol. 2021;132(5):1057–1063. doi: 10.1016/j.clinph.2021.01.018. [DOI] [PubMed] [Google Scholar]
- Franceschetti S., Visani E., Panzica F., Coppola A., Striano P., Canafoglia L. Cortico-muscular coherence and brain networks in familial adult myoclonic epilepsy and progressive myoclonic epilepsy. Clin Neurophysiol. 2023;151:74–82. doi: 10.1016/j.clinph.2023.04.009. [DOI] [PubMed] [Google Scholar]
- Frauscher B., Brandauer E., Gschliesser V., Falkenstetter T., Furtner M.T., Ulmer H., Poewe W., Högl B. A descriptive analysis of neck myoclonus during routine polysomnography. Sleep. 2010;33(8):1091–1096. doi: 10.1093/sleep/33.8.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galizia EC, Myers CT, Leu C, de Kovel CG, Afrikanova T, Cordero-Maldonado ML, Martins TG, Jacmin M, Drury S, Krishna Chinthapalli V, Muhle H, Pendziwiat M, Sander T, Ruppert AK, Møller RS, Thiele H, Krause R, Schubert J, Lehesjoki AE, Nürnberg P, Lerche H; EuroEPINOMICS CoGIE Consortium; Palotie A, Coppola A, Striano S, Gaudio LD, Boustred C, Schneider AL, Lench N, Jocic-Jakubi B, Covanis A, Capovilla G, Veggiotti P, Piccioli M, Parisi P, Cantonetti L, Sadleir LG, Mullen SA, Berkovic SF, Stephani U, Helbig I, Crawford AD, Esguerra CV, Kasteleijn-Nolst Trenité DG, Koeleman BP, Mefford HC, Scheffer IE, Sisodiya SM. CHD2 variants are a risk factor for photosensitivity in epilepsy. Brain. 2015; 138(Pt 5):1198-207. doi: 10.1093/brain/awv052. [DOI] [PMC free article] [PubMed]
- Gambardella A., Aguglia U., Oliveri R.L., Pucci F., Zappia M., Quattrone A. Photic-induced epileptic negative myoclonus: a case report. Epilepsia. 1996;37(Pt 5):492–494. doi: 10.1111/j.1528-1157.1996.tb00596.x. [DOI] [PubMed] [Google Scholar]
- Grosse P., Guerrini R., Parmeggiani L., Bonanni P., Pogosyan A., Brown P. Abnormal corticomuscular and intermuscular coupling in high-frequency rhythmic myoclonus. Brain. 2003 Feb;126(Pt 2):326–342. doi: 10.1093/brain/awg043. [DOI] [PubMed] [Google Scholar]
- Guerrini R. Physiology of epilepsia partialis continua and subcortical mechanisms of status epilepticus. Epilepsia. 2009 Dec;50(Suppl 12):7–9. doi: 10.1111/j.1528-1167.2009.02356.x. PMID: 19941509. [DOI] [PubMed] [Google Scholar]
- Guerrini R., Takahashi T. Myoclonus and epilepsy. Handb Clin Neurol. 2013;111:667–679. doi: 10.1016/B978-0-444-52891-9.00069-5. PMID: 23622214. [DOI] [PubMed] [Google Scholar]
- Gupta H.V., Caviness J.N. Post-hypoxic Myoclonus: Current Concepts, Neurophysiology, and Treatment. Tremor Other Hyperkinet Mov (n y). 2016 Sep;17(6):409. doi: 10.7916/D89C6XM4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M. Physiology of human posthypoxic myoclonus. Mov Disord. 2000;15(Suppl 1):8–13. doi: 10.1002/mds.870150703. PMID: 10755266. [DOI] [PubMed] [Google Scholar]
- Hallett M., Chadwick D., Adam J., Marsden C.D. Reticular reflex myoclonus: a physiological type of human post-hypoxic myoclonus. J Neurol Neurosurg Psychiatry. 1977 Mar;40(3):253–264. doi: 10.1136/jnnp.40.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Díaz S., Straub L., Bateman B.T., Zhu Y., Mogun H., Wisner K.L., Gray K.J., Lester B., McDougle C.J., DiCesare E., Pennell P.B., Huybrechts K.F. Risk of Autism after Prenatal Topiramate, Valproate, or Lamotrigine Exposure. N Engl J Med. 2024 Mar 21;390(12):1069–1079. doi: 10.1056/NEJMoa2309359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch E., French J., Scheffer I.E., Bogacz A., Alsaadi T., Sperling M.R., Abdulla F., Zuberi S.M., Trinka E., Specchio N., Somerville E., Samia P., Riney K., Nabbout R., Jain S., Wilmshurst J.M., Auvin S., Wiebe S., Perucca E., Moshé S.L., Tinuper P., Wirrell E.C. ILAE definition of the Idiopathic Generalized Epilepsy Syndromes: Position statement by the ILAE Task Force on Nosology and Definitions. Epilepsia. 2022;63(6):1475–1499. doi: 10.1111/epi.17236. [DOI] [PubMed] [Google Scholar]
- Horlings C.G.C., Kofler M., Hotter A., Reiter E., Wanschitz J.V., Löscher W.N. The clinical meaning of giant somatosensory evoked potentials of the median nerve. Clin Neurophysiol. 2020 Jul;131(7):1495–1496. doi: 10.1016/j.clinph.2020.03.035. Epub 2020 Apr 23 PMID: 32388474. [DOI] [PubMed] [Google Scholar]
- Insola A., Di Lazzaro V., Assenza G. Cortical inhibitory dysfunction in epilepsia partialis continua: A high frequency oscillation somatosensory evoked potential study. Clin Neurophysiol. 2019 Apr;130(4):439–444. doi: 10.1016/j.clinph.2019.01.005. Epub 2019 Jan 25 PMID: 30769270. [DOI] [PubMed] [Google Scholar]
- Johannesen K., Marini C., Pfeffer S., Møller R.S., Dorn T., Niturad C.E., Gardella E., Weber Y., Søndergård M., Hjalgrim H., Nikanorova M., Becker F., Larsen L.H., Dahl H.A., Maier O., Mei D., Biskup S., Klein K.M., Reif P.S., Rosenow F., Elias A.F., Hudson C., Helbig K.L., Schubert-Bast S., Scordo M.R., Craiu D., Djémié T., Hoffman-Zacharska D., Caglayan H., Helbig I., Serratosa J., Striano P., De Jonghe P., Weckhuysen S., Suls A., Muru K., Talvik I., Talvik T., Muhle H., Borggraefe I., Rost I., Guerrini R., Lerche H., Lemke J.R., Rubboli G., Maljevic S. Phenotypic spectrum of GABRA1: From generalized epilepsies to severe epileptic encephalopathies. Neurology. 2016;87(11):1140–1151. doi: 10.1212/WNL.0000000000003087. [DOI] [PubMed] [Google Scholar]
- Kasteleijn-Nolst Trenité D.G., Guerrini R., Binnie C.D., Genton P. Visual sensitivity and epilepsy: a proposed terminology and classification for clinical and EEG phenomenology. Epilepsia. 2001;42(5):692–701. doi: 10.1046/j.1528-1157.2001.30600.x. [DOI] [PubMed] [Google Scholar]
- Khan Z, Arya K, Bollu PC. Epilepsia Partialis Continua. 2023. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023. [PubMed]
- Kojovic M., Cordivari C., Bhatia K. Myoclonic disorders: a practical approach for diagnosis and treatment. Ther Adv Neurol Disord. 2011;4:47–62. doi: 10.1177/1756285610395653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koskiniemi M., Van Vleymen B., Hakamies L., Lamusuo S., Taalas J. Piracetam relieves symptoms in progressive myoclonus epilepsy: a multicentre, randomised, double blind, crossover study comparing the efficacy and safety of three dosages of oral piracetam with placebo. J Neurol Neurosurg Psychiatry. 1998 Mar;64(3):344–348. doi: 10.1136/jnnp.64.3.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorre A., Rocchi L., Magrinelli F., Mulroy E., Berardelli A., Rothwell J.C., Bhatia K.P. Unravelling the enigma of cortical tremor and other forms of cortical myoclonus. Brain. 2020;143(9):2653–2663. doi: 10.1093/brain/awaa129. PMID: 32417917. [DOI] [PubMed] [Google Scholar]
- Latorre A., Belvisi D., Rothwell J.C., Bhatia K.P., Rocchi L. Rethinking the neurophysiological concept of cortical myoclonus. Clin Neurophysiol. 2023;156:125–139. doi: 10.1016/j.clinph.2023.10.007. [DOI] [PubMed] [Google Scholar]
- Levy A., Chen R. Myoclonus: Pathophysiology and Treatment Options. Curr Treat Options Neurol. 2016;18:21. doi: 10.1007/s11940-016-0404-7. [DOI] [PubMed] [Google Scholar]
- Licchetta L., Pippucci T., Bisulli F., Cantalupo G., Magini P., Alvisi L., Baldassari S., Martinelli P., Naldi I., Vanni N., Liguori R., Seri M., Tinuper P. A novel pedigree with familial cortical myoclonic tremor and epilepsy (FCMTE): clinical characterization, refinement of the FCMTE2 locus, and confirmation of a founder haplotype. Epilepsia. 2013;54(7):1298–1306. doi: 10.1111/epi.12216. [DOI] [PubMed] [Google Scholar]
- Lo Barco T., Kaminska A., Solazzi R., Cancés C., Barcia G., Chemaly N., Fontana E., Desguerre I., Canafoglia L., Hachon Le Camus C., Losito E., Villard L., Eisermann M., Dalla Bernardina B., Villeneuve N., Nabbout R. SYNGAP1-DEE: A visual sensitive epilepsy. Clin Neurophysiol. 2021;132(4):841–850. doi: 10.1016/j.clinph.2021.01.014. [DOI] [PubMed] [Google Scholar]
- Mameniškienė R., Wolf P. Epilepsia partialis continua: A review. Seizure. 2017;44:74–80. doi: 10.1016/j.seizure.2016.10.010. [DOI] [PubMed] [Google Scholar]
- Marelli C., Canafoglia L., Zibordi F., Ciano C., Visani E., Zorzi G., Garavaglia B., Barzaghi C., Albanese A., Soliveri P., Leone M., Panzica F., Scaioli V., Pincherle A., Nardocci N., Franceschetti S. A neurophysiological study of myoclonus in patients with DYT11 myoclonus-dystonia syndrome. Mov Disord. 2008;23(14):2041–2048. doi: 10.1002/mds.22256. [DOI] [PubMed] [Google Scholar]
- Matsumoto J., Fuhr P., Nigro M., Hallett M. Physiological abnormalities in hereditary hyperekplexia. Ann Neurol. 1992 Jul;32(1):41–50. doi: 10.1002/ana.410320108. PMID: 1642471. [DOI] [PubMed] [Google Scholar]
- Merchant S.H.I., Vial-Undurraga F., Leodori G., van Gerpen J.A., Hallett M. Myoclonus: An Electrophysiological Diagnosis. Mov Disord Clin Pract. 2020 Jun 17;7(5):489–499. doi: 10.1002/mdc3.12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelucci R., Pasini E., Riguzzi P., Andermann E., Kälviäinen R., Genton P. Myoclonus and seizures in progressive myoclonus epilepsies: pharmacology and therapeutic trials. Epileptic Disord. 2016;18:145–153. doi: 10.1684/epd.2016.0861. [DOI] [PubMed] [Google Scholar]
- Moeller F., Siebner H.R., Ahlgrimm N., Wolff S., Muhle H., Granert O., Boor R., Jansen O., Gotman J., Stephani U., Siniatchkin M. fMRI activation during spike and wave discharges evoked by photic stimulation. Neuroimage. 2009;48(4):682–95 doi: 10.1016/j.neuroimage.2009.07.019. [DOI] [PubMed] [Google Scholar]
- Montagna P. In: Sleep and Movement Disorders. Chokroverty S., Hening W., Walters A.S., editors. Elsevier Science; Philadelphia: 2003. Physiological body jerks and movements at sleep onset and during sleep; pp. 247–259. [Google Scholar]
- Naquet R., Menini C., Riche D., Silva-Barrat C., Valin A. Photic epilepsy problems raised in man and animals. Ital J Neurol Sci. 1987 Oct;8(5):437–447. doi: 10.1007/BF02334600. [DOI] [PubMed] [Google Scholar]
- Nardone R., Versace V., Höller Y., Sebastianelli L., Brigo F., Lochner P., Golaszewski S., Saltuari L., Trinka E. Transcranial magnetic stimulation in myoclonus of different aetiologies. Brain Res Bull. 2018;140:258–269. doi: 10.1016/j.brainresbull.2018.05.016. [DOI] [PubMed] [Google Scholar]
- Oguni H., Uehara T., Tanaka T., Sunahara M., Hara M., Osawa M. Dramatic effect of ethosuximide on epileptic negative myoclonus: implications for the neurophysiological mechanism. Neuropediatrics. 1998;29:29–34. doi: 10.1055/s-2007-973530. [DOI] [PubMed] [Google Scholar]
- Okuma Y., Fujishima K., Miwa H., Mori H., Mizuno Y. Myoclonic tremulous movements in multiple system atrophy are a form of cortical myoclonus. Mov Disord. 2005;20(4):451–456. doi: 10.1002/mds.20346. [DOI] [PubMed] [Google Scholar]
- Panzica F., Canafoglia L., Franceschetti S., Binelli S., Ciano C., Visani E., Avanzini G. Movement-activated myoclonus in genetically defined progressive myoclonic epilepsies: EEG-EMG relationship estimated using autoregressive models. Clin Neurophysiol. 2003;114(6):1041–1052. doi: 10.1016/s1388-2457(03)00066-x. [DOI] [PubMed] [Google Scholar]
- Panzica F., Canafoglia L., Franceschetti S. EEG-EMG information flow in movement-activated myoclonus in patients with Unverricht-Lundborg disease. Clin Neurophysiol. 2014;125(9):1803–1808. doi: 10.1016/j.clinph.2014.01.005. [DOI] [PubMed] [Google Scholar]
- Parra J., Kalitzin S.N., Iriarte J., Blanes W., Velis D.N., Lopes da Silva F.H. Gamma-band phase clustering and photosensitivity: is there an underlying mechanism common to photosensitive epilepsy and visual perception? Brain. 2003;126(Pt 5):1164–1172. doi: 10.1093/brain/awg109. [DOI] [PubMed] [Google Scholar]
- Pellegrin S., Baldeweg T., Pujar S., D'Arco F., Cantalupo G., Varadkar S., Cross J.H. Immunomodulation With Azathioprine Therapy in Rasmussen Syndrome: A Multimodal Evaluation. Neurology. 2021 Jan 12;96(2):e267–e279. doi: 10.1212/WNL.0000000000011004. [DOI] [PubMed] [Google Scholar]
- Pena A.B., Caviness J.N. Physiology-Based Treatment of Myoclonus. Neurotherapeutics. 2020;17:1665–1680. doi: 10.1007/s13311-020-00922-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priori A., Bertolasi L., Pesenti A., Cappellari A., Barbieri S. Gammahydroxybutyric acid for alcohol-sensitive myoclonus with dystonia. Neurology. 2000;54:1706. doi: 10.1212/wnl.54.8.1706. [DOI] [PubMed] [Google Scholar]
- Puoti G., Bizzi A., Forloni G., Safar J.G., Tagliavini F., Gambetti P. Sporadic human prion diseases: molecular insights and diagnosis. Lancet Neurol. 2012;11(7):618–628. doi: 10.1016/S1474-4422(12)70063-7. PMID: 22710755. [DOI] [PubMed] [Google Scholar]
- Riney K., Bogacz A., Somerville E., Hirsch E., Nabbout R., Scheffer I.E., Zuberi S.M., Alsaadi T., Jain S., French J., Specchio N., Trinka E., Wiebe S., Auvin S., Cabral-Lim L., Naidoo A., Perucca E., Moshé S.L., Wirrell E.C., Tinuper P. International League Against Epilepsy classification and definition of epilepsy syndromes with onset at a variable age: position statement by the ILAE Task Force on Nosology and Definitions. Epilepsia. 2022;63(6):1443–1474. doi: 10.1111/epi.17240. [DOI] [PubMed] [Google Scholar]
- Rissardo J.P., Caprara A.L.F., Durante Í., Rauber A. Lithium-associated movement disorder: A literature review. Brain Circ. 2022;8(2):76–86. doi: 10.4103/bc.bc_77_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissardo J.P., Vora N., Mathew B., Kashyap V., Muhammad S., Fornari Caprara A.L. Overview of Movement Disorders Secondary to Drugs. Clin Pract. 2023 Aug 18;13(4):959–976. doi: 10.3390/clinpract13040087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi Sebastiano D., Magaudda A., Quartarone A., Brizzi T., Visani E., Capovilla G., Beccaria F., Anversa P., Franceschetti S., Canafoglia L. Effect of repetitive transcranial magnetic stimulation on action myoclonus: A pilot study in patients with EPM1. Epilepsy Behav. 2018;80:33–36. doi: 10.1016/j.yebeh.2017.11.031. [DOI] [PubMed] [Google Scholar]
- Roze E., Vidailhet M., Hubsch C., Navarro S., Grabli D. Pallidal stimulation for myoclonus-dystonia: ten years’ outcome in two patients. Mov Disord. 2015;30(6):871–872. doi: 10.1002/mds.26215. [DOI] [PubMed] [Google Scholar]
- Rubboli G., Mai R., Meletti S., Francione S., Cardinale F., Tassi L., Lo Russo G., Stanzani-Maserati M., Cantalupo G., Tassinari C.A. Negative myoclonus induced by cortical electrical stimulation in epileptic patients. Brain. 2006;129:65–81. doi: 10.1093/brain/awh661. [DOI] [PubMed] [Google Scholar]
- Rubboli G., Tassinari C.A. Negative myoclonus. An overview of its clinical features, pathophysiological mechanisms, and management. Neurophysiol Clin. 2006;36:337–343. doi: 10.1016/j.neucli.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Rubboli G., Meletti S., Gardella E., Zaniboni A., d'Orsi G., Dravet C., Tassinari C.A. Photic reflex myoclonus: a neurophysiological study in progressive myoclonus epilepsies. Epilepsia. 1999;40(Suppl 4):50–58. doi: 10.1111/j.1528-1157.1999.tb00907.x. [DOI] [PubMed] [Google Scholar]
- Salazar G., Valls-Solé J., Martí M.J., Chang H., Tolosa E.S. Postural and action myoclonus in patients with parkinsonian type multiple system atrophy. Mov Disord. 2000;15(1):77–83. doi: 10.1002/1531-8257(200001)15:1<77::aid-mds1013>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Salvi F., Mascalchi M., Bortolotti C., Meletti S., Plasmati R., Rubboli G., Stecchi S., Villari N., Calbucci F., Tassinari C.A. Hypertension, hyperekplexia, and pyramidal paresis due to vascular compression of the medulla. Neurology. 2000 Nov 14;55(9):1381–1384. doi: 10.1212/wnl.55.9.1381. PMID: 11087786. [DOI] [PubMed] [Google Scholar]
- Serino D., Fusco L. Epileptic hypnagogic jerks mimicking repetitive sleep starts. Sleep Med. 2015;16(8):1014–1016. doi: 10.1016/j.sleep.2015.04.015. [DOI] [PubMed] [Google Scholar]
- Sharifi S., Luft F., Potgieter S., Heida T., Mugge W., Schouten A.C., Bour L.J., van Rootselaar A.F. Directionality of corticomuscular coupling in essential tremor and cortical myoclonic tremor. Clin Neurophysiol. 2021;132(8):1878–1886. doi: 10.1016/j.clinph.2021.04.011. [DOI] [PubMed] [Google Scholar]
- Shibasaki H., Hallett M. Electrophysiological studies of myoclonus. Muscle Nerve. 2005;31(2):157–174. doi: 10.1002/mus.20234. [DOI] [PubMed] [Google Scholar]
- Shibasaki H., Kuroiwa Y. Electroencephalographic correlates of myoclonus. Electroencephalogr Clin Neurophysiol. 1975;39(5):455–463. doi: 10.1016/0013-4694(75)90046-2. [DOI] [PubMed] [Google Scholar]
- Siniatchkin M., Groppa S., Jerosch B., Muhle H., Kurth C., Shepherd A.J., Siebner H., Stephani U. Spreading photoparoxysmal EEG response is associated with an abnormal cortical excitability pattern. Brain. 2007;130(Pt 1):78–87. doi: 10.1093/brain/awl306. [DOI] [PubMed] [Google Scholar]
- Specchio N., Boero G., Michelucci R., Gambardella A., Giallonardo A.T., Fattouch J., Di Bonaventura C., de Palo A., Ladogana M., Lamberti P., Vigevano F., La Neve A., Specchio L.M. Effects of levetiracetam on EEG abnormalities in juvenile myoclonic epilepsy. Epilepsia. 2008 Apr;49(4):663–669. doi: 10.1111/j.1528-1167.2007.01523.x. Epub 2008 Feb 5 PMID: 18266754. [DOI] [PubMed] [Google Scholar]
- Specchio N., Wirrell E.C., Scheffer I.E., Nabbout R., Riney K., Samia P., Guerreiro M., Gwer S., Zuberi S.M., Wilmshurst J.M., Yozawitz E., Pressler R., Hirsch E., Wiebe S., Cross H.J., Perucca E., Moshé S.L., Tinuper P., Auvin S. International League Against Epilepsy classification and definition of epilepsy syndromes with onset in childhood: Position paper by the ILAE Task Force on Nosology and Definitions. Epilepsia. 2022;63(6):1398–1442. doi: 10.1111/epi.17241. [DOI] [PubMed] [Google Scholar]
- Stahl C.M., Frucht S.J. An Update on Myoclonus Management. Expert Rev Neurother. 2019;19:325–331. doi: 10.1080/14737175.2019.1592676. [DOI] [PubMed] [Google Scholar]
- Strigaro G., Prandi P., Varrasi C., Monaco F., Cantello R. Defective visual inhibition in photosensitive idiopathic generalized epilepsy. Epilepsia. 2012;53(4):695–704. doi: 10.1111/j.1528-1167.2012.03411.x. [DOI] [PubMed] [Google Scholar]
- Strigaro G., Falletta L., Cerino A., Pizzamiglio C., Tondo G., Varrasi C., Cantello R. Abnormal motor cortex plasticity in juvenile myoclonic epilepsy. Seizure. 2015;30:101–105. doi: 10.1016/j.seizure.2015.06.004. [DOI] [PubMed] [Google Scholar]
- Strigaro G., Falletta L., Varrasi C., Rothwell J.C., Cantello R. Overactive visuomotor connections underlie the photoparoxysmal response. A TMS Study. Epilepsia. 2015;56(11):1828–1835. doi: 10.1111/epi.13190. [DOI] [PubMed] [Google Scholar]
- Suppa A., Berardelli A., Brancati F., Marianetti M., Barrano G., Mina C., Pizzuti A., Sideri G. Clinical, neuropsychological, neurophysiologic, and genetic features of a new Italian pedigree with familial cortical myoclonic tremor with epilepsy. Epilepsia. 2009;50(5):1284–1288. doi: 10.1111/j.1528-1167.2008.01976.x. [DOI] [PubMed] [Google Scholar]
- Suppa A., Rocchi L., Li Voti P., Papazachariadis O., Casciato S., Di Bonaventura C., Giallonardo A.T., Berardelli A. The Photoparoxysmal Response Reflects Abnormal Early Visuomotor Integration in the Human Motor Cortex. Brain Stimul. 2015;8(6):1151–1161. doi: 10.1016/j.brs.2015.05.013. [DOI] [PubMed] [Google Scholar]
- Tassinari CA, Rubboli G, Parmeggiani L, F Valzania, R Plasmati, P Riguzzi, R Michelucci, L Volpi, D Passarelli, S Meletti, Fontana E. Dalla Bernardina B. Epileptic negative myoclonus. Adv Neurol. Vol.67; Lippincott-Raven Publishers, Philapdelphia. 1995; pp. 181-97. [PubMed]
- Van der Veen S., Klamer M.R., Elting J.W.J., Koelman J.H.T.M., van der Stouwe A.M.M., Tijssen M.A.J. The diagnostic value of clinical neurophysiology in hyperkinetic movement disorders: A systematic review. Parkinsonism Relat Disord. 2021;89:176–185. doi: 10.1016/j.parkreldis.2021.07.033. [DOI] [PubMed] [Google Scholar]
- Van der Veen S., Caviness J.N., Dreissen Y.E.M., Ganos C., Ibrahim A., Koelman J.H.T.M., Stefani A., Tijssen M.A.J. Myoclonus and other jerky movement disorders. Clin Neurophysiol Pract. 2022;6(7):285–316. doi: 10.1016/j.cnp.2022.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varotto G., Visani E., Canafoglia L., Franceschetti S., Avanzini G., Panzica F. Enhanced frontocentral EEG connectivity in photosensitive generalized epilepsies: a partial directed coherence study. Epilepsia. 2012;53(2):359–367. doi: 10.1111/j.1528-1167.2011.03352.x. [DOI] [PubMed] [Google Scholar]
- Vaudano A.E., Ruggieri A., Avanzini P., Gessaroli G., Cantalupo G., Coppola A., Sisodiya S.M., Meletti S. Photosensitive epilepsy is associated with reduced inhibition of alpha rhythm generating networks. Brain. 2017;140(4):981–997. doi: 10.1093/brain/awx009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velayudhan L., Kirchner V. Quetiapine-induced myoclonus. Int Clin Psychopharmacol. 2005;20(2):119–120. doi: 10.1097/00004850-200503000-00011. [DOI] [PubMed] [Google Scholar]
- Visani E., Varotto G., Binelli S., Fratello L., Franceschetti S., Avanzini G., Panzica F. Photosensitive epilepsy: spectral and coherence analyses of EEG using 14Hz intermittent photic stimulation. Clin Neurophysiol. 2010;121(3):318–324. doi: 10.1016/j.clinph.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Zutt R, Elting JW, van Zijl JC, van der Hoeven JH, Roosendaal CM, Gelauff JM, Peall KJ, Tijssen MAJ. Electrophysiologic testing aids diagnosis and subtyping of myoclonus. Neurology. 2018 Feb 20;90(8):e647-e657. doi: 10.1212/WNL.0000000000004996. Epub 2018 Jan 19. PMID: 29352095; PMCID: PMC5818165. [DOI] [PMC free article] [PubMed]
- Genton P, Guerrini R, Remy C. Piracetam in the treatment of cortical myoclonus. Pharmacopsychiatry. 1999 Mar;32 Suppl 1:49-53. doi: 10.1055/s-2007-979237. PMID: 10338109. [DOI] [PubMed]
Further reading
- Strigaro G., Gori B., Varrasi C., Fleetwood T., Cantello G., Cantello R. Flash-evoked high-frequency EEG oscillations in photosensitive epilepsies. Epilepsy Res. 2021;172 doi: 10.1016/j.eplepsyres.2021.106597. [DOI] [PubMed] [Google Scholar]
- Fujimoto A., Enoki H., Hatano K., Sato K., Okanishi T. Replacement of Valproic Acid with New Anti-Seizure Medications in Idiopathic Generalized Epilepsy. J Clin Med. 2022 Aug 5;11(15):4582. doi: 10.3390/jcm11154582. [DOI] [PMC free article] [PubMed] [Google Scholar]