Abstract
South America has some of the most diverse floras and insect faunas that are known, but its Cenozoic fossil record of insects and insect herbivory is sparse. We quantified insect feeding on 3,599 leaves from the speciose Laguna del Hunco flora (Chubut, Argentina), which dates to the early Eocene climatic optimum (52 million years ago) and compared the results with three well preserved, rich, and identically analyzed early- and middle-Eocene floras from the following sites in North America: Republic, WA; Green River, UT; and Sourdough, WY. We found significantly more damage diversity at Laguna del Hunco than in the North American floras, whether measured on bulk collections or on individual plant species, for both damage morphotypes and feeding groups. An ancient history of rich, specialized plant–insect associations on diverse plant lineages in warm climates may be a major factor contributing to the current biodiversity of South America.
Keywords: paleobotany, Argentina, herbivory, Laguna del Hunco, paleoecology
South America is well known for its highly diverse** floras (1–3) and associated insect faunas (4–7). Observations in South America and elsewhere show strong positive linkage between plant and insect-herbivore diversity (8–14). Proposed mechanisms include dependency of insect diversity on plant diversity (8, 9, 13), coevolution of plants and insects (15–21), herbivore selection against host density (22–25), and herbivore intensification of abiotic factors that select for habitat specialization (26, 27). Paleontological data can be used to test whether plant and herbivore diversity were correlated in the past (28–30), although data bearing on the primary mechanisms are not typically available from fossils. However, throughout South America, insect body fossils have a limited Cenozoic record (31), and insect damage on fossil plants has rarely been reported (32, 33). Here, we seek to determine whether a diverse fossil flora from South America has an elevated level of plant–insect associations. We quantify insect-feeding damage on one of the most speciose known assemblages of fossil plants, from early-Eocene Patagonia and, for comparison, on three well preserved, diverse early- and middle-Eocene floras from comparable absolute latitudes of North America.
Study Areas, Specimens, and Methods
The Patagonian flora comes from tuffaceous lake beds exposed at Laguna del Hunco (LH; S42.5°, W70°; paleolatitude, ≈47°S) in Chubut, Argentina (34–36). These sediments were deposited 52 million years ago (35, 36), during the early Eocene global climatic optimum (37). At this time, thermophilic organisms reached middle and high latitudes of both hemispheres (38–42), and the LH flora contains a large proportion of tropical as well as temperate lineages (34–36, 39). Our unbiased macrofloral sample, in which all material was collected or tallied, totals 6,521 specimens from 25 quarries, of which 4,303 specimens (66%) are identifiable to 186 species of plant organs. These include 152 leaf species, of which 132 are magnoliid or eudicot angiosperms (referred to as “dicots” hereafter, for simplicity) (36). Four major quarries found at distinct horizons through 62 m of stratigraphic section account for 87% of identifiable specimens. Adjusted for sample size by using rarefaction, the LH flora is more speciose than comparable Eocene assemblages known from other continents (35, 36). Relatively rare fossil insects are also preserved, including several phytophagous taxa (43).
Although not previously reported, the LH flora has well preserved insect damage on a broad spectrum of host plants, and some individual leaves bear an extremely diverse array of herbivory (Fig. 1). Individual feeding associations, under separate investigation, show varied phylogenetic and biogeographic affinities for the insect herbivores. These data reveal part of the mostly undocumented heritage of the insect herbivore fauna of South America, and we briefly note three examples. The first example is a distinctive frass-filled blotch mine in an araucarian or podocarpaceous conifer leaf, very similar to damage inflicted today by the basal ditrysian moths Paraectopa (Gracillariidae) and Chrysorthenches (Plutellidae) on the same respective hosts in New Zealand (44, 45). This damage also is similar to feeding by basal chrysomeloid clades on these plant hosts in Australia and South America (19, 46, 47). Collectively, this and biogeographic evidence suggest a Gondwanan interaction that originated in the Mesozoic Era. The second example is a distinctive angulate-serpentine mine on an unidentified dicot produced by a nepticulid moth, almost identical to extant Stigmella (Nepticulidae) mines on Urticaceae in Micronesia and Polynesia (48) and Rubiaceae in southern Africa (49). These mine types also are known from Eurasian Fagaceae, Rosaceae, and Rhamnaceae from a separate Stigmella lineage (50). Third, an occurrence of cecidomyiid galls on a probable Sterculiaceae may indicate tropical affinities.
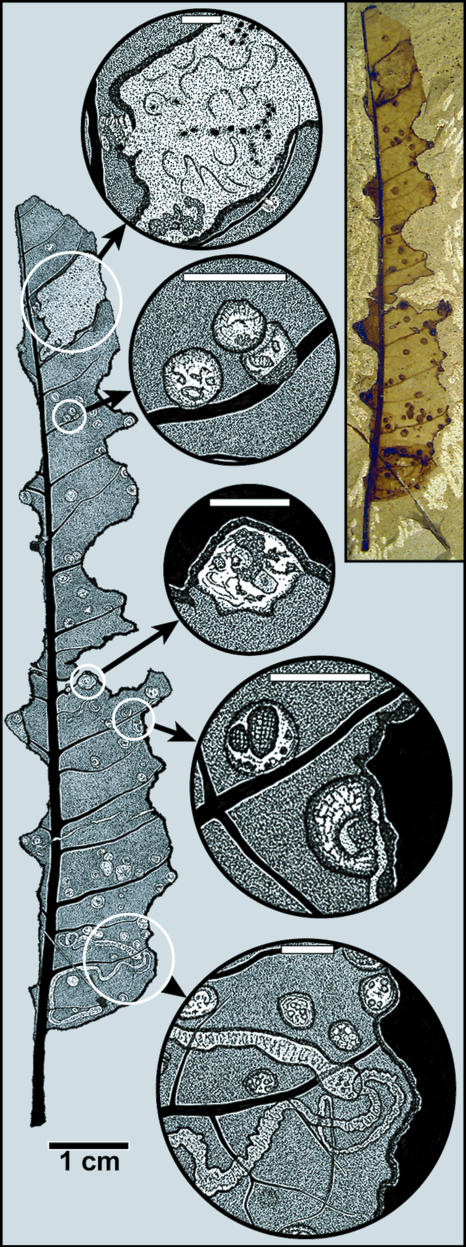
Fig. 1.
Three functional feeding groups (mining, galling, and external feeding), including two types of mines, occurring on a single leaf of “Cupania” grosse-serrata (Sapindaceae; MPEF-Pb 983, from locality LH-2). The photographed specimen at upper right is depicted as a camera lucida drawing at left, with intervening circular Insets. (Scale bars, 2 mm.) Insets show the following details (from top to bottom): an unattributed blotch mine with remnant frass; a cluster of three galls with exit holes along a secondary vein; a gall whose margin has been consumed by an external feeder; two galls, one of which has been consumed by an external feeder; and two linear, probably lepidopteran gracillariid, mines with early sap-feeding and subsequent solid-feeding instars, the latter characterized by serpentine frass trails. Crosscutting feeding relationships and gall placement show the temporal order of herbivory events to be mining, galling, and external foliage feeding.
During macrofloral sampling, all specimens with insect damage were collected to generate an unbiased data set for direct comparison with North American floras that were collected and analyzed by using identical methods. Determination of herbivory, as contrasted to postmortem detritivory or physically induced damage, was based on four explicit criteria, detailed in ref. 51. These criteria were (i) detection of callus or other reaction tissue along or adjacent to a leaf area with removed tissue; (ii) presence of veinal stringers, necrotic tissue flaps and cuspate chewed margins consistent with known patterns of insect feeding; (iii) stereotyped patterns of leaf damage that are nearly identical or identical to known modern feeding; and (iv) distinctive host specificity patterns that would be highly unusual if the causative agent were not biological. Most leaf specimens typically preserved the same insect damage on both part and counterpart, indicating that the capture of damage types from single specimens was robust. All voucher specimens from LH are deposited at the Museo Paleontológico Egidio Feruglio, as described in ref. 36.
We scored 3,599 identified dicot leaves for presence or absence of 52 discrete damage types, belonging to four functional feeding groups: external feeding, mining, galling, and piercing-and-sucking. The damage types are expanded from our previous work on Cretaceous and Paleogene floras from the Western Interior USA (28–30, 51, 52). Some damage types may have been created by multiple biological species, or a single biological species may have produced multiple damage types (53). However, damage-type data record trophic interactions at varying levels of host specialization and thus most directly indicate the diversity of ecological associations, rather than the actual diversity of herbivores (28, 30). Many relatively rare damage types are typically made by host-specialized insects (54) and can be diagnostic of particular herbivore clades when fossilized (55–57); their diversity may have a relatively direct proportional relationship to herbivore diversity. Nonfeeding damage, such as oviposition scars and fungal infections, was excluded from analysis.
The three North American samples are as follows: 1,019 dicot leaf specimens from the early Eocene, lacustrine Republic flora, Klondike Mountain Formation, northeastern Washington (58, 59); 894 specimens from the middle Eocene, lacustrine Green River flora, Green River Formation, northeastern Utah (29, 60); and 792 specimens from the early Eocene, fluvial Sourdough flora, Wasatch Formation, southwestern Wyoming (28, 29, 61), which constitute a total of 2,705 North American identified dicot leaves. All are unbiased, quantitative samples from single stratigraphic horizons. Republic insect-damage data are from a collection made by K.R.J. at the Denver Museum of Nature and Science (locality 2130), as discussed in refs. 35 and 36. Although no North American site that is an exact match exists, Republic is the most similar to LH in terms of volcanic setting, age, depositional environment, and distance from coastline (35). Adjusted for sample size, Republic has the most speciose dicot-leaf flora known from Eocene North America (36), as well as diverse feeding damage, based on a qualitative assessment (52). Insect-damage data for the Green River and Sourdough floras have been reported in refs. 28 and 29. The Green River assemblage represents a seasonally dry climate, which had characteristic effects on herbivory discussed in ref. 29, and the Sourdough sample is from or very near the beginning of the early Eocene climatic optimum (61, 62). The Sourdough flora, by comparison with the late Paleocene in the same area of Wyoming, records a diversification of plants and herbivory with warming temperatures (28).
We analyzed the insect damage data for bulk collections from single quarries (Fig. 2, including all four major quarries at LH) and for individual host species (Fig. 3). To maximize sample size and decrease noise in the species-level analyses, each species was lumped over all 25 LH quarries. The data were tabulated and processed separately for total damage types and functional feeding groups (Figs. 2 and 3). Analysis of damage types treats all kinds of damage equally, including the damage that is often made by unspecialized insects (such as hole and margin feeding), but it is sensitive to the full morphological spectrum of insect damage. Analysis of feeding groups, because only four categories are scored, is more sensitive to relatively rare damage typically inflicted by host-specialized insects in the mining, galling, and piercing-and-sucking categories (54). We analyzed the data by both including and excluding undamaged leaves, and we found that confining analyses to damaged leaves decreases noise and is a major analytical improvement, although sample size is reduced as a consequence. From each data matrix of herbivorized specimens by damage types, we generated resampling curves for insect damage richness at individual quarries, by using all host species combined (see legend to Fig. 2 for details). For individual host species with ≥10 total specimens with insect damage, we show the resampled mean numbers of damage types and feeding groups for 10 herbivorized specimens (see legend to Fig. 3 for details). These host species means were also combined into floral grand means (Fig. 4).
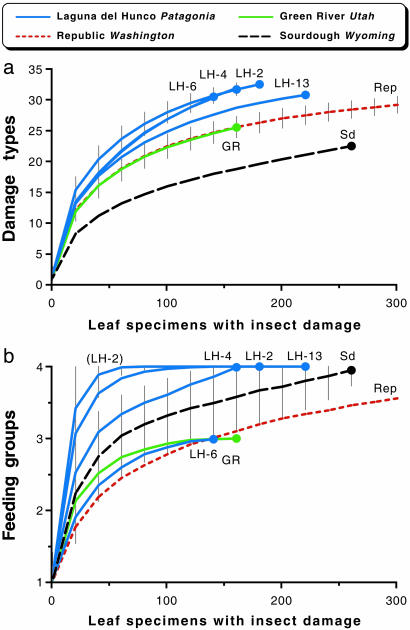
Fig. 2.
Resampling curves for insect-damage diversity on herbivorized dicot leaves for bulk floras, from the LH (four major quarries at distinct stratigraphic levels: LH-2, LH-4, LH-6, and LH-13; see text), Republic (Rep), Green River (GR), and Sourdough (Sd) floras. The curves show the means of 5,000 randomized resamples, with replacement, of the number of damage types (a) or functional feeding groups (external feeding, galling, mining, and piercing-and-sucking) (b) found on the number of herbivorized dicot leaves indicated on the horizontal axis (horizontal spacing of 20 specimens), up to the total number of collected herbivorized specimens. Curve endpoints are enlarged to increase visibility of separate curves. Curves for Republic have no endpoint depicted because this sample has 507 herbivorized specimens, terminating outside the graphed area. Error bars of ±1σ are placed on the most diverse North and South American curves in each graph. The percentage of leaves with insect damage was 33.1%, 13.1%, 29.5%, and 29.3% for LH-2, LH-4, LH-6, and LH-13, respectively, suggesting a taphonomic bias at LH-4, and 49.8%, 19.4%, and 34.8% for Republic, Green River, and Sourdough, respectively.
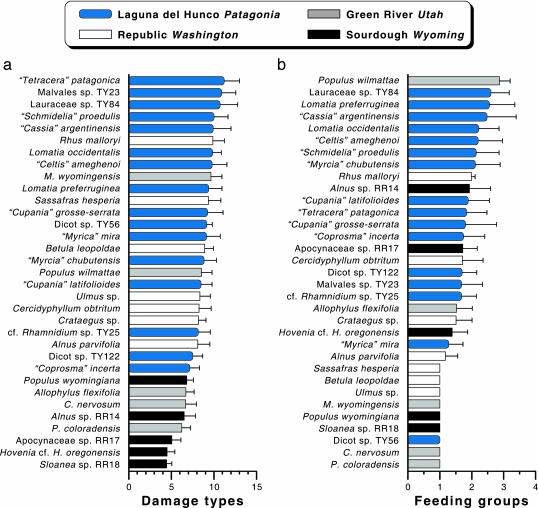
Fig. 3.
Resampled, rank-ordered insect-feeding diversity on individual dicot host plant species with ≥10 damaged specimens. For each species, the mean is shown (+1σ) of 5000 randomized resamples, with replacement, of 10 herbivorized leaves per resample. (a) Damage types. (b) Functional feeding groups. Abbreviated genera are Cedrelospermum, Macginitiea, and Parvileguminophyllum. Genera in quotations were assigned from the historic literature (32, 34) and are in need of taxonomic revision.
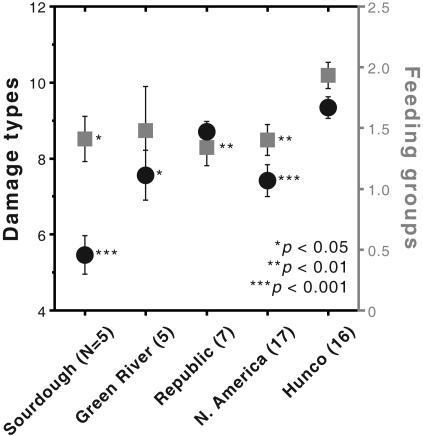
Fig. 4.
Grand means for the resampled species means (shown in Fig. 3) of insect-damage diversity, ±1 standard error of the mean, including single and pooled (second from right) North American samples. n, Number of species in sample. Black circles and left scale, damage types; gray squares and right scale, functional feeding groups. Asterisks next to North American grand means indicate significance (one-way ANOVA) of comparisons with corresponding LH grand means.
Results and Discussion
The resampled number of damage types at each of the four major LH quarries significantly exceeds all three North American samples (Fig. 2a). The number of functional feeding groups is greater than all North American samples for three of the four LH quarries (Fig. 2b; piercing-and-sucking damage was not found at LH-6 or Green River). These LH samples saturate quickly on the resampling curves with respect to feeding groups (Fig. 2b): LH-2 reaches 3.95 of the 4 possible feeding groups at 61, LH-13 at 101, and LH-4 at 161 leaf specimens, whereas Sourdough reaches this value at 261 and Republic at 481 leaf specimens. Notably, the quarry with the greatest plant diversity, LH-2 (36), also has the maximum richness of both damage types and feeding groups.
Elevated diversity of both damage types and feeding groups is also clear on individual hosts (Fig. 3), where LH species occupy most of the highest values. The two measures are not well correlated among hosts (Spearman rank-order correlation coefficient, 0.46), showing variance in herbivore accommodation within vs. among herbivore guilds (damage type vs. feeding group richness). Only two species, both from LH, rank in the top five in both categories: Lauraceae sp. and “Cassia” argentinensis, a legume (Fig. 3). Among the heavily herbivorized species at LH are both typically tropical (Lauraceae, Fabaceae, Malvales, Myrtaceae, and Sapindaceae) and temperate or tropical montane (two species of Lomatia) lineages. The conspicuous exception to the LH hosts ranking highest in damage diversity is Populus wilmattae from the Green River flora, which has the maximum feeding group diversity (Fig. 3); elevated herbivory on P. wilmattae may result from its adaptations to moist, riparian habitats within the seasonally dry Green River climate, as described in ref. 29.
Floral grand means of the host data in Fig. 3 show the 16 LH species to have more damage types and feeding groups than either the single or pooled North American samples (Fig. 4). The difference is highly significant for the pooled sample and significant for four of six single North American samples, despite the low numbers of species available in the single samples (Fig. 4). Among the North American floras, Green River and especially Sourdough are low in damage-type diversity but high in feeding-group diversity, whereas Republic has a relatively high number of damage types; these results from individual host species are mostly similar to their encompassing bulk floras (Fig. 2).
The LH flora has the maximum scores for both damage types and feeding groups, whether scored on bulk floras from several distinct stratigraphic levels or on individual host species (Figs. 2, 3, 4). The bulk floral results (Fig. 2), especially for feeding groups, are consistent with the pronounced host-specificity of extant herbivorous insects (20, 48, 54) and the possible accumulation of herbivore diversity from distinct component communities (63, 64) of herbivore species on many different plant hosts. Separately, herbivory data from individual hosts (Figs. 3 and 4) indicate greater richness within component communities at LH than at the North American sites.
Our results reinforce modern observations that plant and insect-herbivore diversity are positively linked (8–10, 13, 14) and demonstrate an elevated richness of plant–insect associations within the Patagonian Eocene ecosystem. This diversity is an ancient legacy for the dominant ecological pattern seen in extant South American tropical and subtropical forests: highly diverse, specialized insect herbivores on speciose floras. To test the generality of our results temporally and spatially, comparable data are needed from other South American sites with well preserved paleofloras, especially at low paleolatitudes.
Acknowledgments
We thank L. Canessa, B. Cariglino, I. Escapa, M. Gandolfo, C. González, R. Horwitt, F. Marsh, D. Meade-Hunter, S. Passmore, P. Puerta, M. Reynolds, E. Ruigomez, and S. Wing for exceptional assistance in the field and laboratory; P. Coley, R. Horwitt, D. Royer, two anonymous reviewers, and the Editor for comments on drafts that greatly improved the manuscript; and the Nahueltripay family for land access. This work was supported by National Geographic Society Grant 7337-02, National Science Foundation Grant DEB-0345750, the University of Pennsylvania Research Foundation, the Andrew W. Mellon Foundation, Petroleum Research Fund Grant 35229-G2, and the Penn State Institutes of the Environment.
Author contributions: P.W., C.C.L., K.R.J., and N.R.C. designed research; P.W., C.C.L., K.R.J., and N.R.C. performed research; P.W. and C.C.L. analyzed data; P.W. and C.C.L. wrote the paper; and P.W., C.C.L., K.R.J., and N.R.C. obtained funding for the research.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviation: LH, Laguna del Hunco.
Footnotes
In this article, “richness” and “diversity” have the same, traditional meaning: the number of species or other biological entities.
References
- 1.Gentry, A. H. (1988) Proc. Natl. Acad. Sci. USA 85, 156–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis, S. D., Heywood, V. H., Herrera MacBryde, O. & Hamilton, A. C. (1997) Centres of Plant Diversity: A Guide and Strategy for their Conservation (World Wide Fund for Nature, London), Vol. 3.
- 3.Phillips, O. L. & Miller, J. S. (2002) Global Patterns of Plant Diversity: Alwyn H. Gentry's Forest Transect Data Set (Missouri Botanical Garden Press, St. Louis).
- 4.Erwin, T. L. (1982) Coleopt. Bull. 36, 74–75. [Google Scholar]
- 5.Price, P. W., Diniz, I. R., Morais, H. C. & Marques, E. S. A. (1995) Biotropica 27, 468–478. [Google Scholar]
- 6.Robbins, R. K., Lamas, G., Mielke, O. H. H., Harvey, D. J. & Casagrande, M. M. (1996) in Manu: The Biodiversity of Southeastern Peru, eds. Wilson, D. E. & Sandoval, A. (Smithsonian Institution, Washington, DC), pp. 217–252.
- 7.Jolivet, P. & Verma, K. K. (2002) Biology of Leaf Beetles (Intercept, Andover, U.K.).
- 8.Murdoch, W. W., Evans, F. C. & Peterson, C. H. (1972) Ecology 53, 819–829. [Google Scholar]
- 9.Siemann, E., Tilman, D., Haarstad, J. & Ritchie, M. (1998) Am. Nat. 152, 738–750. [DOI] [PubMed] [Google Scholar]
- 10.Wright, M. G. & Samways, M. J. (1998) Oecologia 115, 427–433. [DOI] [PubMed] [Google Scholar]
- 11.Knops, J. M. H., Tilman, D., Haddad, N. M., Naeem, S., Mitchell, C. E., Haarstad, J., Ritchie, M. E., Howe, K. M., Reich, P. B., Siemann, E. & Groth, J. (1999) Ecol. Lett. 2, 286–293. [DOI] [PubMed] [Google Scholar]
- 12.Leigh, E. G., Jr., (1999) Tropical Forest Ecology: A View from Barro Colorado Island (Oxford Univ. Press, New York).
- 13.Haddad, N. M., Tilman, D., Haarstad, J., Ritchie, M. & Knops, J. M. H. (2001) Am. Nat. 158, 17–35. [DOI] [PubMed] [Google Scholar]
- 14.Hawkins, B. A. & Porter, E. E. (2003) Am. Nat. 161, 40–49. [DOI] [PubMed] [Google Scholar]
- 15.Ehrlich, P. R. & Raven, P. H. (1964) Evolution (Lawrence, Kans.) 18, 586–608. [Google Scholar]
- 16.Berenbaum, M. (1983) Evolution (Lawrence, Kans.) 37, 163–179. [DOI] [PubMed] [Google Scholar]
- 17.Farrell, B. D. & Mitter, C. (1998) Biol. J. Linn. Soc. 63, 553–577. [Google Scholar]
- 18.Mitter, C., Farrell, B. D. & Futuyma, D. J. (1991) Trends Ecol. Evol. 6, 290–293. [DOI] [PubMed] [Google Scholar]
- 19.Farrell, B. D. (1998) Science 281, 555–559. [DOI] [PubMed] [Google Scholar]
- 20.Becerra, J. X. (2003) Proc. Natl. Acad. Sci. USA 100, 12804–12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cornell, H. V. & Hawkins, B. A. (2003) Am. Nat. 161, 507–522. [DOI] [PubMed] [Google Scholar]
- 22.Janzen, D. H. (1970) Am. Nat. 104, 501–528. [Google Scholar]
- 23.Connell, J. H. (1971) in Dynamics of Populations, eds. Den Boer, P. J. & Gradwell, G. (Wageningen, Center for Agricultural Publishing and Documentation, New York), pp. 298–312.
- 24.Wills, C., Condit, R., Foster, R. B. & Hubbell, S. P. (1997) Proc. Natl. Acad. Sci. USA 94, 1252–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wills, C. & Condit, R. (1999) Proc. R. Soc. London Ser. B 266, 1445–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Louda, S. M. & Rodman, J. E. (1996) J. Ecol. 84, 229–237. [Google Scholar]
- 27.Fine, P. V. A., Mesones, I. & Coley, P. D. (2004) Science 305, 663–665. [DOI] [PubMed] [Google Scholar]
- 28.Wilf, P. & Labandeira, C. C. (1999) Science 284, 2153–2156. [DOI] [PubMed] [Google Scholar]
- 29.Wilf, P., Labandeira, C. C., Johnson, K. R., Coley, P. D. & Cutter, A. D. (2001) Proc. Natl. Acad. Sci. USA 98, 6221–6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Labandeira, C. C., Johnson, K. R. & Wilf, P. (2002) Proc. Natl. Acad. Sci. USA 99, 2061–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petrulevicius, J. F. & Martins-Neto, R. G. (2000) Acta Geol. Hisp. 35, 135–147. [Google Scholar]
- 32.Berry, E. W. (1938) Geol. Soc. Am. Spec. Pap. 12, 1–149. [Google Scholar]
- 33.Martins-Neto, R. G. (1989) Rev. Bras. Geociências 19, 375–386. [Google Scholar]
- 34.Berry, E. W. (1925) Johns Hopkins Stud. Geol. 6, 183–251. [Google Scholar]
- 35.Wilf, P., Cúneo, N. R., Johnson, K. R., Hicks, J. F., Wing, S. L. & Obradovich, J. D. (2003) Science 300, 122–125. [DOI] [PubMed] [Google Scholar]
- 36.Wilf, P., Johnson, K. R., Cúneo, N. R., Smith, M. E., Singer, B. S. & Gandolfo, M. A. (2005) Am. Nat. 165, 634–650. [DOI] [PubMed] [Google Scholar]
- 37.Zachos, J. C., Pagani, M., Sloan, L. C., Thomas, E. & Billups, K. (2001) Science 292, 686–693. [DOI] [PubMed] [Google Scholar]
- 38.Estes, R. & Hutchison, J. H. (1980) Palaeogeogr. Palaeoclimatol. Palaeoecol. 30, 325–347. [Google Scholar]
- 39.Romero, E. J. (1986) Ann. Mo. Bot. Gard. 73, 449–461. [Google Scholar]
- 40.Romero, E. J. (1993) in Biological Relationships Between Africa and South America, ed. Goldblatt, P. (Yale Univ. Press, New Haven, CT), pp. 62–85.
- 41.Greenwood, D. R. & Wing, S. L. (1995) Geology 23, 1044–1048. [Google Scholar]
- 42.Pole, M. S. & MacPhail, M. K. (1996) Rev. Palaeobot. Palynol. 92, 55–67. [Google Scholar]
- 43.Petrulevicius, J. F. & Nel, A. D. (2003) J. Nat. Hist. 37, 2909–2917. [Google Scholar]
- 44.Wise, K. A. J. (1962) Trans. R. Soc. N. Z. Zool. 31, 373–375. [Google Scholar]
- 45.Dugdale, J. S. (1996) N. Z. J. Zool. 23, 33–59. [Google Scholar]
- 46.Zimmerman, E. C. (1994) Australian Weevils (Coleoptera: Curculionoidea). Volume 1. Orthoceri: Anthribidae to Attelabidae: The Primitive Weevils (Commonwealth Scientific and Industrial Research Organization, Melbourne).
- 47.Labandeira, C. C. (2000) Paleontol. Soc. Pap. 6, 233–269. [Google Scholar]
- 48.van Nieukerken, E. J. & van den Berg, C. (2003) Invert. Syst. 17, 27–37. [Google Scholar]
- 49.Scoble, M. J. (1980) Ann. Transvaal Mus. 32, 197–229. [Google Scholar]
- 50.Steuer, H. (1995) Rudolst. Naturhist. Schriften Suppl. 1, 1–175. [Google Scholar]
- 51.Labandeira, C. C., Johnson, K. R. & Lang, P. (2002) Geol. Soc. Am. Spec. Pap. 361, 297–327. [Google Scholar]
- 52.Labandeira, C. C. (2002) Rocky Mount. Geol. 37, 31–59. [Google Scholar]
- 53.Coulson, R. N. & Witter, J. A. (1984) Forest Entomology: Ecology and Management (Wiley, New York).
- 54.Bernays, E. A. & Chapman, R. F. (1994) Host–Plant Selection by Phytophagous Insects (Chapman and Hall, New York).
- 55.Hickey, L. J. & Hodges, R. W. (1975) Science 189, 718–720. [DOI] [PubMed] [Google Scholar]
- 56.Labandeira, C. C., Dilcher, D. L., Davis, D. R. & Wagner, D. L. (1994) Proc. Natl. Acad. Sci. USA 91, 12278–12282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wilf, P., Labandeira, C. C., Kress, W. J., Staines, C. L., Windsor, D. M., Allen, A. L. & Johnson, K. R. (2000) Science 289, 291–294. [DOI] [PubMed] [Google Scholar]
- 58.Wolfe, J. A. & Wehr, W. C. (1987) U. S. Geol. Surv. Bull. 1597, 1–25. [Google Scholar]
- 59.Radtke, M. G., Pigg, K. B. & Wehr, W. C. (2005) Int. J. Plant Sci. 166, 347–356. [Google Scholar]
- 60.MacGinitie, H. D. (1969) Univ. Calif. Publ. Geol. Sci. 83, 1–140. [Google Scholar]
- 61.Wilf, P. (2000) Geol. Soc. Am. Bull. 112, 292–307. [Google Scholar]
- 62.Smith, M. E., Singer, B. & Carroll, A. (2003) Geol. Soc. Am. Bull. 115, 549–565. [Google Scholar]
- 63.Root, R. B. (1973) Ecol. Monogr. 43, 95–124. [Google Scholar]
- 64.Futuyma, D. J. & Mitter, C. (1996) Philos. Trans. R. Soc. London B 351, 1361–1366. [Google Scholar]