Abstract
Background
In recent years, many countries have actively implemented programs and strategies to promote physical education and sports. Despite these efforts, the increase in physical activity has been accompanied by a significant rise in muscle and tendon-ligament injuries, with Achilles tendon rupture being the most prevalent, accounting for 47 % of such injuries. This review aims to summarize all significant factors determining the predisposition of the Achilles tendon to rupture, to develop effective personalized prevention measures.
Objective
To identify and evaluate the risk factors contributing to Achilles tendon rupture and to develop strategies for personalized prevention.
Methods
This review utilized data from several databases, including Elsevier, Global Health, PubMed-NCBI, Embase, Medline, Scopus, ResearchGate, RSCI, Cochrane Library, Google Scholar, eLibrary.ru, and CyberLeninka. Both non-modifiable and modifiable risk factors for Achilles tendon injuries and ruptures were analyzed.
Results
The analysis identified several non-modifiable risk factors, such as genetic predisposition, anatomical and functional features of the Achilles tendon, sex, and age. These factors should be considered when selecting sports activities and designing training programs. Modifiable risk factors included imbalanced nutrition, improper exercise regimens, and inadequate monitoring of Achilles tendon conditions in athletes. Early treatment of musculoskeletal injuries, Achilles tendon diseases, foot deformities, and metabolic disorders is crucial. Long-term drug use and its risk assessment were also highlighted as important considerations. Furthermore, recent clinical advancements in both conventional and surgical methods to treat Achilles tendon injuries were described. The efficacy of these therapies in enhancing functional outcomes in individuals with Achilles injuries was compared. Advancements in cell-based and scaffold-based therapies aimed at enhancing cell regeneration and repairing Achilles injuries were also discussed.
Discussion
The combination of several established factors significantly increases the risk of Achilles tendon rupture. Addressing these factors through personalized prevention strategies can effectively reduce the incidence of these injuries. Proper nutrition, regular monitoring, timely treatment, and the correction of metabolic disorders are essential components of a comprehensive prevention plan.
Conclusion
Early identification of Achilles tendon risk factors allows for the timely development of effective personalized prevention strategies. These measures can contribute significantly to public health preservation by reducing the incidence of Achilles tendon ruptures associated with physical activity and sports. Continued research and clinical advancements in treatment methods will further enhance the ability to prevent and manage Achilles tendon injuries.
The translational potential of this article
This study identifies key modifiable and non-modifiable risk factors for Achilles tendon injuries, paving the way for personalized prevention strategies. Emphasizing nutrition, exercise, and early treatment of musculoskeletal issues, along with advancements in cell-based therapies, offers promising avenues for improving recovery and outcomes. These findings can guide clinical practices in prevention and rehabilitation, ultimately reducing Achilles injuries and enhancing public health.
Keywords: Achilles injuries, Anatomy, Clinical reports, Regenerative medicine, Rupture-risk factors
Graphical abstract
The translational potential of this article
This study identifies key modifiable and non-modifiable risk factors for Achilles tendon injuries, paving the way for personalized prevention strategies. Emphasizing nutrition, exercise, and early treatment of musculoskeletal issues, along with advancements in cell-based therapies, offers promising avenues for improving recovery and outcomes. These findings can guide clinical practices in prevention and rehabilitation, ultimately reducing Achilles injuries and enhancing public health.
1. Introduction
Enhanced injury prevention and clinical guidance for effective regenerative medicine are significant strategies to ameliorate rupture-mediated Achilles injuries in the lower limb. For instance, Physical activity is a cornerstone of promoting and maintaining overall health. Its benefits include improved cardiovascular health, enhanced mental well-being, and increased longevity. As a result, many countries have been actively implementing programs and strategies aimed at the development of physical education and sport [[1], [2], [3], [4], [5]]. This focus is evident in the significant progress in the physical education of children, adolescents, and young people. Furthermore, the popularity of fitness clubs and gyms is rising among the working-age population, reflecting a broader trend towards an active lifestyle. There is also an intensive development of all types of amateur and professional sports, driven by increased awareness of the health benefits of physical activity and advancements in sports science and technology [[1], [2], [3], [4], [5]].
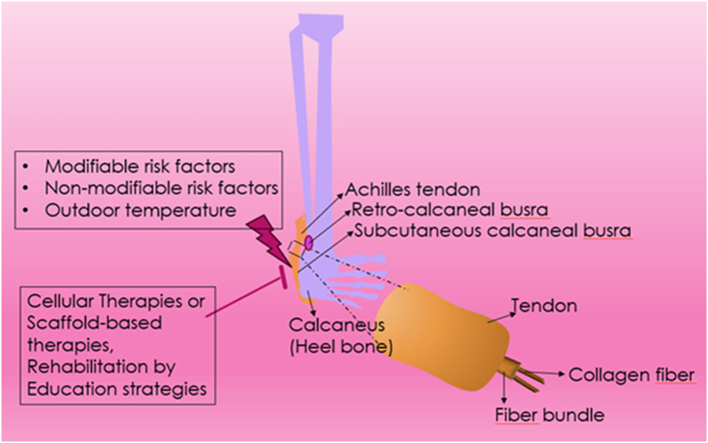
However, the increase in physical activity among the population is accompanied by a corresponding rise in muscle and tendon-ligament injuries, with Achilles tendon rupture taking a leading place. Achilles tendon ruptures account for approximately 47 % of such injuries, highlighting their prevalence and impact [[6], [7], [8]]. Numerous studies indicate that the incidence of Achilles tendon rupture today reaches 25–30 cases per 100,000 population per year and continues to grow. This increase can be attributed to several factors, including higher participation in sports and physical activities, changes in training intensities, and demographic shifts such as an aging yet active population [[6], [7], [8], [9]] (Fig. 1).
Fig 1.
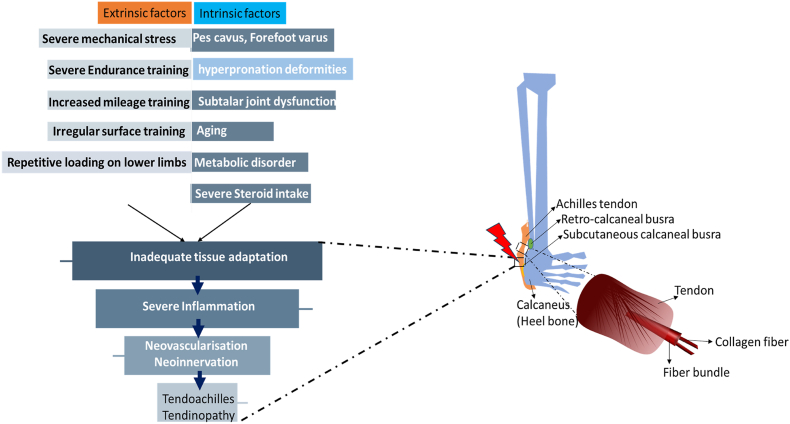
Achilles tendons are subjected to mechanical forces continuously throughout an individual's life. When these forces are of sufficient intensity, such as during physical activity, they can confer various health benefits not only to the tendons but also to the entire musculoskeletal system. Risk factors for Achilles tendon (AT) injuries can be divided into intrinsic and extrinsic categories, which can occur either in isolation or in combination. Intrinsic factors include biomechanical irregularities of the lower limbs. Examples of these irregularities are indifferent leg length, hyperpronation; other irregularities include pes cavus (high arches), forefoot varus deformity, and limited mobility of the subtalar joint. Additionally, systemic conditions like those associated with advanced age, metabolic syndrome, and the use of specific medications, such as corticosteroids, contribute to intrinsic risk factors. Extrinsic factors involve excessive mechanical stress and poor training practices. These include increased interval training, augmented mileage, training on uneven surfaces, repeated loading, and inadequate shock absorption. Achilles tendinopathy typically features a failed healing response, chronic inflammation, and predominant catabolic activity. This complex interplay of intrinsic and extrinsic factors underscores the importance of a comprehensive approach to understanding and mitigating the risk of Achilles tendon injuries.
Achilles tendon is primarily composed of collagen type I (Col I) as well as collagen type III (Col III), with Col I being the dominant structural protein that provides tensile strength, while Col III plays a supportive role, particularly during the healing process [10,11]. The formation of collagen begins when three polypeptide chains undergo enzymatic excision of terminal peptides, leading to the assembly of soluble tropocollagen molecules. These tropocollagen units further align laterally to form collagen fibrils, which aggregate into collagen fibers. Multiple collagen fibers group together into hierarchical structures, forming primary, secondary, and tertiary bundles that contribute to the tendon's overall mechanical properties [12].
In addition to collagen, the extracellular matrix (ECM) of AT is a complex network containing elastin, which imparts elasticity; fibronectin, involved in cell adhesion and wound healing; and proteoglycans such as decorin, biglycan, and fibromodulin, which regulate collagen fibril formation and tendon integrity [13]. These ECM components play critical roles not only in maintaining tendon architecture but also in modulating the response to injury and mechanical load.
Tissue-resident cells within the tendon contribute to its structure and function, displaying considerable heterogeneity. The majority of tendon cells in mature AT are tenocytes, which are elongated, fibroblast-like cells that synthesize and maintain the ECM. In younger tendons, a greater proportion of the cell population consists of tenoblasts, which are more proliferative and responsible for the initial stages of collagen synthesis. Tenocytes and tenoblasts together form approximately 90%–95 % of the cellular content of tendon, with tenoblasts gradually maturing into tenocytes as the tendon ages, playing a vital role in the development, maintenance, and repair of tendon tissue. These cells respond dynamically to mechanical loading, contributing to the tendon's adaptability and resilience [13,14].
Recurrences of this injury also account for a significant percentage, with 35.0 % observed after conservative treatment and 3.5 % after surgery [9]. These recurrences underscore the need for improved treatment protocols and preventive strategies. Achilles tendon rupture is accompanied by serious disorders of support and movement in the ankle joint, leading in some cases to chronic instability and long-term disability. This can significantly impair quality of life and physical function, necessitating comprehensive approaches to treatment and rehabilitation.
Understanding the causes and mechanisms of Achilles tendon injury is crucial for its prevention. Factors such as biomechanical stress, genetic predispositions, and systemic conditions like diabetes or hypercholesterolemia play significant roles. Advances in molecular biology and regenerative medicine have begun to elucidate the underlying pathophysiology of tendon injuries, providing insights that can inform more effective preventive and therapeutic strategies. The role of mesenchymal stromal cells (MSCs) and other regenerative techniques in tendon repair and regeneration is particularly promising, offering the potential to enhance healing outcomes and reduce recurrence rates. Therefore, this review aims to summarize all significant factors determining the Achilles tendon predisposition to rupture, with a focus on developing effective measures for personalized prevention. This includes exploring the latest comparative clinical outcomes of therapeutic modalities, advancements in regenerative medicine, biomechanical analysis, and clinical guidance to provide a holistic approach to injury prevention and management. By integrating these insights, we can enhance clinical outcomes, reduce the incidence of recurrent injuries, and improve the overall quality of life for individuals engaged in physical activities.
1.1. Literature search
To develop a comprehensive understanding of modifiable risk factors for Achilles tendon rupture, we conducted an extensive literature review using various databases. These included PubMed, Medline, eMedicine, Scopus, Google Scholar, the National Library of Medicine (NLM), and ReleMed. Our search focused on published reports and articles examining the impact of metabolic disorders, physical activity, and pharmacological agents on tendon health. Specifically, we investigated the roles of hypercholesterolemia, hyperuricemia, thyroid hormone imbalances, inherited metabolic disorders, and the adverse effects of medications such as fluoroquinolones, statins, corticosteroids, aromatase inhibitors, anabolic steroids, isotretinoin, and drugs affecting the renin-angiotensin system. This review also encompassed studies on the molecular mechanisms involving cholesterol oxidation products, matrix metalloproteinases, and oxidative stress, which contribute to tendon degeneration and increased rupture risk.
1.2. Achilles injuries and clinical manifestations
Despite the identification of numerous risk factors for Achilles injuries, the precise causation remains elusive. A comprehensive evaluation of all potential risk factors and the current clinical reports supporting every risk factor is necessary for developing effective ameliorative strategies. Lower-limb injuries are commonly observed in military personnel and running athletes [15]. Mainly, chronic Achilles tendon injuries, often called tendonitis or tendinopathy; are particularly severe, significantly affecting training and racing schedules [16]. Tendinopathies typically manifest across the middle anatomical region of Achilles, due to the minimal cross-sectional area of tendon [17], or at its insertion point on the calcaneus.
Middle region of the tendon is formed by the integration of collagen fibers form soleus, gastrocnemius (middle or lateral) muscles. These injuries are evident in long-distance runners. For instance, the incidence of chronic Achilles injuries is comparatively higher in middle and long-distance runners [[6], [7], [8],[18], [19], [20]]. Therapeutic strategies to ameliorate clinical manifestations show minimal efficacy as these injuries persist for longer years. Thus, middle-to long-distance runners are at an elevated risk of Achilles injuries, which have significant immediate and long-term health and personal costs. Recurrent running could induce severe force on the lower limbs nearly ninety times greater for a minute [[18], [19], [20]]. The Achilles tendon, along with the calf muscles, bears significant loads early in the absorption process, enduring forces induced by body weight [12]. Furthermore, gait biomechanics adjust according to the training environment to reduce harmful forces on the musculoskeletal system while sustaining performance levels [[21], [22], [23]].
Achilles tendon plays a crucial role in regular locomotive activities by enabling foot contact with the ground and drive force generation and confer to over 50 % of positive work executed at the ankle at the time of running [24]. Achilles functions in concert with gastrocnemius & soleus muscles to foster effective ankle movement during joint movements [[25], [26], [27]]. Hence, the mechanics of tendons are variable due to the intricate multifactorial nature of Achilles injuries. The debate over the mechanisms of Achilles injuries has identified several potential causes, including tensile loading [28], and shearing [29]; In addition, hyperthermia is another mechanism of Achilles injury [24,30]. Mechanisms pertinent to these can lead to ‘non-homologous loading,’ resulting in the deterioration of tissue [31,32]. Insertion region experiences a higher stress ex vivo [33,34]. Strain along tendon's length is variable and lacks a consistent pattern [33]. In chronic tendinopathies, localized damage may result in scar generation or areas of weakened tissue. Repeated loading before complete healing exacerbates this cycle of progressively weakened and dysfunctional tissue, increasing the risk of further injury if loading occurs before the tendon has fully recovered its original strength.
Despite extensive multidisciplinary research, the reasons for Achilles injuries and especially individuals why a few individuals acquire tendon injuries yet require future studies. Multiple mechanisms likely could cause intricate injury physiology which complicates the interpretation of results. Understanding the specific loading patterns that contribute to ‘non-homologous’ loading could shed light on reasons for inducing Achilles overuse injuries. Several risk factors were reported to enhance the likelihood of causing Achilles injuries, including advancing age, male gender, training errors, inappropriate footwear, running on soft surfaces, pronation, and cold weather [35]. Overuse injuries could be due to the multifactorial influence typically due to the multiple risk factors [36]. According to the clinical manifestations, prevention is a significant strategy when compared to treatment, necessitating the identification of causative factors to predict people who are more prone to develop Achilles injuries. Exploring the variations in risk factors among runners with Achilles injuries can reveal areas for further research into potentially adjustable causative factors. An optimal preventive strategy would involve a comprehensive assessment encompassing multiple risk factors to effectively identify athletes at risk [37].
1.3. Non-modifiable Achilles tendon rupture risk factors
1.3.1. Genetic predisposition to Achilles tendon ruptures
Significant advances in human molecular genetics over recent decades, particularly the complete sequencing of the human genome, have provided new insights into predictive personalized medicine. Identification of genetic markers has become a fundamentally new diagnostic vector for detecting individual predispositions to musculoskeletal injuries. Achilles tendon injuries and ruptures are associated with genes encoding extracellular matrix proteins. Mutations in these genes affect not only tendon fiber strength and predisposition to damage but also injury severity and recovery rate [[38], [39], [40], [41], [42]].
Research has shown that the predisposition to Achilles tendon rupture is largely due to polymorphisms in the G1023T gene (rs1800012) at the Sp1 functional binding site in intron-1 of the seventeenth chromosome, involved in type I collagen synthesis. The absence of the TT genotype indicates a possible protective role of these alleles [43]. Of particular importance are changes in the MMP3 gene (STMY1) on the eleventh chromosome, responsible for synthesizing stromelysin I, a matrix metalloproteinase that degrades the extracellular matrix. Polymorphisms in the MMP3 gene (rs679620G, rs591058C, rs650108A) are associated with Achilles tendovaginopathy. The interaction between the G-allele of the MMP3 gene (rs679620) and the T-allele of the COL5A1 gene (rs12722) significantly increases the risk of this pathology [44].
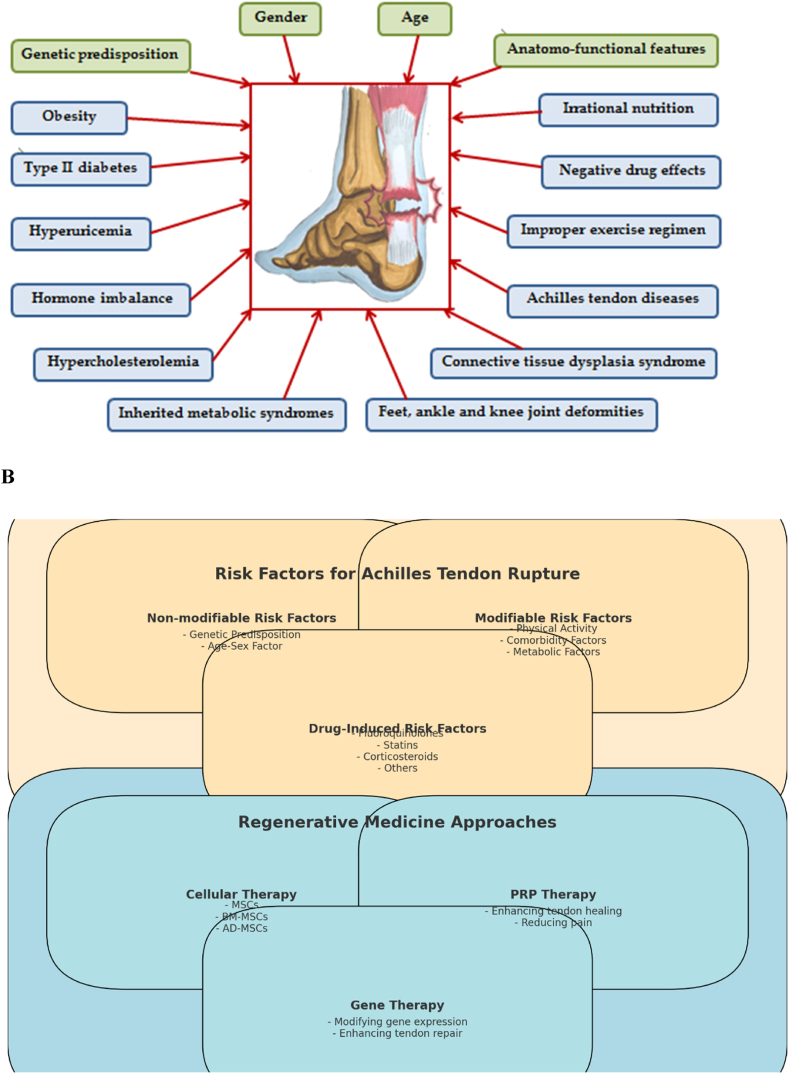
For predicting Achilles tendon rupture, studies have also focused on the TNC gene on the ninth chromosome (9q33), which encodes the extracellular matrix protein tenascin C. This protein inhibits cell adhesion, allowing cells to move. Polymorphisms associated with the twelfth and ITGT repeats in the seventeenth intron are significant markers of high Achilles tendon rupture risk, whereas polymorphisms associated with the thirteenth and seventeenth repeats are protective [45] (Fig. 2A–B, Table 1). Thus, genetic predisposition is a significant factor in Achilles tendon damage.
Fig. 2.
A–B: the risk factors for Achilles tendon rupture and the associated regenerative medicine strategies. The diagram categorizes the risk factors into non-modifiable, modifiable, and drug-induced factors, and outlines different regenerative medicine approaches including cellular therapy, PRP therapy, and gene therapy. This figure enables an understanding of the complex interplay between various risk factors and the emerging therapeutic strategies aimed at improving tendon repair and regeneration.
Table 1.
A comprehensive table outlining the risk factors and regenerative medicine approaches for Achilles tendon ruptures, organized by modifiable and non-modifiable factors, along with their effects.
| Risk Factors | Modifiable/Non-Modifiable | Effect | References |
|---|---|---|---|
| Genetic Predisposition | Non-modifiable | Polymorphisms in genes encoding extracellular matrix proteins affect tendon fiber strength, predisposition to damage, injury severity, and recovery rate. | [[43], [44], [45]] |
| - G1023T gene (rs1800012) | Affects type I collagen synthesis; absence of TT genotype may be protective. | [43] | |
| - MMP3 gene (rs679620G, rs591058C, rs650108A) | Polymorphisms associated with Achilles tendovaginopathy; interaction with COL5A1 gene increases risk. | [44]. | |
| - TNC gene (9q33) | Polymorphisms in introns associated with high risk and protective markers for Achilles tendon rupture. | [45] | |
| Age-Sex Factor | Non-modifiable | Higher incidence in men due to greater muscle forces; age-related degenerative changes increase rupture risk. | [9,[46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61]] [[62], [63], [64], [65], [66], [67]]. |
| Anatomical Features | Non-modifiable | Structural variations in tendon attachment and fiber architecture influence rupture susceptibility. | [[68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80]] |
| Physical Activity (Sedentary Lifestyle) | Modifiable | Sudden physical activity after a sedentary period increases injury risk. | [46,54,[81], [82], [83], [84], [85], [86], [87]]. |
| Physical Activity (Professional Athletes) | Modifiable | Overtraining and improper training regimens elevate injury risk. | [46,47,[85], [86], [87], [88]]. |
| Comorbidities (Connective Tissue Dysplasia) | Modifiable | Structural abnormalities in connective tissues increase risk. | [89,90] |
| Comorbidities (Foot/Ankle Deformities) | Modifiable | Biomechanical disorders due to deformities predispose to chronic injuries. | [[89], [90], [91], [92], [93], [94], [95]]. |
| Metabolic Disorders (Obesity) | Modifiable | Excessive load on tendons causes pathological changes and reduces strength. | [45,[81], [82], [83]] |
| Metabolic Disorders (Type II Diabetes) | Modifiable | Structural changes and increased tendon thickness observed in diabetic patients. | [[84], [85], [86], [87], [88], [89], [90], [91]] |
| Metabolic Disorders (Hypercholesterolemia) | Modifiable | Cholesterol deposits cause chronic inflammation and tendon degeneration. | [[92], [93], [94], [95]] |
| Metabolic Disorders (Hyperuricemia) | Modifiable | Urate crystal deposition alters tendon structure, increasing rupture risk. | [[96], [97], [98], [99], [100], [101], [102]] |
| Thyroid Hormone Imbalance | Modifiable | Hormone imbalances impair collagen synthesis, increasing injury risk. | [62,82,[103], [104], [105], [106], [107], [108], [109]] |
| Drug-Induced Risk Factors | Modifiable | Certain medications increase the risk of tendinopathy and rupture through various mechanisms. | [[110], [111], [112], [113], [114], [115], [116], [117], [118], [119], [120], [121], [122], [123], [124], [125], [126]] |
| - Fluoroquinolones | Increase MMP expression and collagen degradation; inhibit tenocyte activity. | [[113], [114], [115], [116], [117], [118]] | |
| - Antimicrobial drugs | Linked to tendinopathy and rupture. | [[119], [120], [121]] | |
| - Statins | Weaken tendon structural components and activate apoptosis. | [111,112,122] | |
| - Corticosteroids | Inhibit tenocyte activity and collagen synthesis; increase collagen breakdown. | [111,112,123] | |
| - Aromatase Inhibitors | Decrease estrogen levels, leading to tendon fiber damage. | [[111], [112], [113],124] | |
| - Anabolic Steroids | Cause rapid muscle mass buildup and increased tendon load. | [111,112,125] | |
| - Isotretinoin | Linked to tendon damage. | [126] | |
| - Renin-Angiotensin System Drugs | Statistically associated with tendon rupture. | [119] | |
| - Thiazide Diuretics and Calcium Channel Blockers | Associated with tendinitis and painful ankle swelling. | [111,112,117] |
1.3.2. Anatomo-functional Achilles tendon features
The Achilles, or calcaneus tendon, is the largest and strongest tendon in the human body, formed from the fusion of the flat aponeuroses of the soleus and medial and lateral gastrocnemius muscles that constitute the triceps surae [[68], [69], [70], [71]]. The Achilles tendon structure is characterized by strict architectonics, consisting of a three-dimensional network of dense collagen fibers forming primary bundles, which are grouped into fascicles. These are surrounded by endotenon, a loose connective tissue containing nerves, blood, and lymphatic vessels. All bundles are enveloped by the paratenon, which consists of two sheets: visceral (epitenon) and parietal (peritenon). Thin fluid spaces between all connective tissue layers reduce friction during tendon fiber sliding and prevent damage. The total number of fibrils and bundles, which determines the Achilles tendon strength, varies greatly within the population [70,71,96].
The Achilles tendon attaches to the calcaneus and twists spirally, bending at right angles so that fibers in its proximal part medially are displaced posteriorly in the distal part. This structure allows the tendon to change length during movements, providing biomechanical strength. The degree of tendon fiber twisting is individual and largely determines its predisposition to microtraumas and ruptures [70,72]. The transition of tendon fibers into bone tissue through the hyaline cartilage region is called the enthesis. This complex formation distributes load uniformly, performs shock absorption, and determines the biomechanics of walking, jumping, and running. The bursa subcutanea, located between the enthesis and the skin, and the bursa retrocalcaneale, located between the tendon and the calcaneal bone, reduce friction between moving fibers and surrounding tissues [70,71,96]. The deep tibia fascia acts as a retainer for the Achilles tendon, forming its anterior bend in the distal part and giving stability to the foot during plantar flexion. Additional stabilization is provided by the tight fusion of the tibia fascia with the calcaneal tuberosity, which functions as a guiding block for the Achilles tendon, reducing the load on the enthesis. Studies have shown that congenital anomalies of Achilles tendon attachment to the calcaneus lead to chronic tendon fiber injury, increasing the risk of spontaneous rupture [[70], [71], [72],96]. The cellular fat space along the anterior Achilles tendon surface, known as Keger's fat body, minimizes pressure changes in the bursa retrocalcaneale during ankle movements. It protects blood vessels that feed the Achilles tendon and prevents the tendon from twisting during plantar flexion. Degradation of this fat body during long-term topical corticosteroid application impairs its protective functions [73].
The blood supply peculiarities of the Achilles tendon play a special role in predicting rupture location. The tendon complex receives blood from three sources: the arteries of the musculotendinous transition, the paratenon, and tendon attachment to the bone. Consequently, the middle Achilles tendon part is the least supplied with blood, relying on capillaries in the surrounding connective tissue for reperfusion. Under increased load, these areas are more prone to hypoxia and metabolic disturbances, leading to dystrophic changes. Poorly supplied tendon parts exhibit higher temperature regimes, which increase the elastic component and significantly reduce tendon fiber strength [68,73]. The middle Achilles tendon part is subjected to maximum stress during muscle contraction, explaining why 80.0–88.7 % of primary Achilles tendon acute ruptures occur in this region [68,74,75].
The Achilles tendon is surrounded by a network of nerve fibers in the epitenon and endotenon. Terminal nerve endings include Fater-Pachini corpuscles (sensing acceleration and deceleration of movement), Ruffini corpuscles (pressure and stretch sensors), and nociceptors (pain receptors). The tendinous Golgi organ, located in the proximal tendon zone, serves as a tension receptor. Imbalances in nerve regulation are associated with biomechanical disturbances in the tendon, leading to microtraumas [76]. Asynchronous contraction of the triceps surae or uncoordinated contraction of agonist and antagonist tibia muscles can result in Achilles tendon rupture [77].
The Achilles tendon is primarily composed of dense fibrous connective tissue, with significant fiber predominance over cells and the amorphous substance. The cellular component includes mature fibroblasts (tenocytes) and young forms responsible for extracellular matrix transformation and fiber synthesis, predominantly collagen type I (95 %) and type III (5 %). Tenocytes interact through connexin, a protein allowing them to control collagen fiber synthesis and regulate tendon strength according to load. Tenocytes contain contractile proteins (actin and myosin), enabling the tendon to transfer force from muscle to bone via an active contraction-relaxation mechanism. Regular physical activity increases tendon fiber strength [[78], [79], [80]].
Achilles tendon collagen fibrils are almost entirely type I collagen, which has higher mechanical strength compared to type III collagen. Proteoglycan molecules, predominantly leucine-rich decorin, provide binding between fibers. The interfibrillar space contains numerous glycosaminoglycans and water, with longitudinal and strictly parallel collagen fiber arrangements aligning with mechanical load direction. Collagen molecule tensile strength is maintained by amino acid cross-links, which vary significantly throughout the tendon. The musculotendinous and tendon-bone transitions have the highest number of these contacts, while the medial tendon part has the least, contributing to its reduced mechanical strength. Essential amino acids are crucial for tendon fiber strength, and deficiencies can negatively impact the Achilles tendon. Additionally, the tendon state depends on the content of vitamin C and ions (Ca2⁺, Mg2⁺, Fe2⁺, Mn2⁺, Zn2⁺, Cu⁺), with deficiencies leading to impaired extracellular matrix protein synthesis, decreased fiber cross-linking, and increased fiber degradation, affecting tendon fiber strength [9,46,[97], [98], [99], [100], [101]]. Thus, initial Achilles tendon strength varies greatly within a biologically diverse population and depends on the body's content of micronutrients and essential amino acids required for maintaining tendon homeostasis.
1.3.3. Age-sex factor
Numerous studies have demonstrated that the incidence of Achilles tendon ruptures is significantly higher in men than in women [9,[46], [47], [48], [49], [50]]. This discrepancy is often attributed to the greater male participation in sports activities, which inherently increases the risk of such injuries. Additionally, the male body, due to higher testosterone concentrations, exhibits better muscle hypertrophy, larger muscle cross-sectional areas, more fast-twitch muscle fibers, and consequently greater forces applied to tendons per unit time. These factors collectively increase the functional load on tendons, potentially leading to a higher risk of rupture. In contrast, the female hormone estrogen, which is responsible for the elasticity of ligaments and tendons, is present in very low concentrations in men, rendering their tendons more rigid and less resistant to sudden loads [[51], [52], [53]] (Fig. 2A–B, Table 1).
Interestingly, physiological support for standard leg movements also shows significant sex differences in intermuscular coordination of different muscle groups and the hemodynamic supply of muscles and ligaments, which may contribute to the pathogenesis of Achilles tendon ruptures [9,[54], [55], [56]].
The risk of Achilles tendon rupture varies with age, showing two distinct peaks: one in socio-economically active individuals aged 30–40 years, and the other in older adults aged 60–80 years. In the younger group, injuries are often due to high physical loads and improper training regimens, compounded by potential underlying connective tissue deficiencies [9,[54], [55], [56]]. In older adults, the risk is associated with age-related degenerative changes in the Achilles tendon that significantly reduce its strength, making it susceptible to ruptures even during routine physical activities [9,[54], [55], [56]].
The pathophysiological mechanisms underlying these age-related changes include a decline in blood supply to the Achilles tendon and a slowdown in its physiological regeneration. In tenocytes, there is a decrease in mRNA levels and enzyme activity associated with extracellular matrix protein synthesis. Concurrently, the activity of matrix metalloproteinases, which are involved in collagen fiber degradation, increases significantly [9,54,[57], [58], [59]].
During aging, tendon cell density decreases substantially, with cells changing from a round to a spindle shape [9,54,[57], [58], [59], [60], [61]]. Moreover, aging is associated with a reduction in collagen fiber diameter, a decrease in the degree of fiber twisting, and a shift in the collagen ratio toward an increase in type III collagen, which has significantly lower strength and stress resistance [[62], [63], [64], [65]]. These changes are compounded by an age-related increase in ankle joint stiffness and a decrease in the range of motion, which directly affect the functional capacity and structural integrity of the Achilles tendon [66,67].
Understanding these age and sex differences in Achilles tendon rupture risk factors is crucial for developing targeted injury prevention strategies and clinical guidelines. Enhanced regenerative medicine approaches, tailored to address these specific risk factors, could significantly improve outcomes for individuals at higher risk of Achilles tendon injuries.
1.4. Modifiable Achilles tendon rupture risk factors
1.4.1. Physical activity features
Numerous studies have demonstrated that up to 90 % of Achilles tendon ruptures occur during recreational and amateur sports activities. This injury is particularly common among individuals who lead a sedentary “office” lifestyle and seek to improve their health through periodic visits to fitness clubs and gyms. Tenocytes, the tendon cells, are mechanosensitive and respond to load stress by upregulating the expression of extracellular matrix proteins, primarily collagen and decorin. Insufficient physical activity results in decreased expression of these proteins, leading to a reduction in Achilles tendon strength. Furthermore, the lack of dynamic mechanical tension in the tendons reduces the expression of thyroid receptors, which are crucial for fibrocyte proliferation and anabolic processes. Therefore, irregular physical training and sudden increases in physical activity levels significantly elevate the risk of Achilles tendon injuries [46,54,[81], [82], [83], [84]].
Professional athletes, who account for about 5 % of Achilles tendon injuries, are also at risk due to improper training regimens and tendon overload during exhaustive training. This overload is associated with increased expression of proinflammatory cytokines (IL-1β, TNF-α), prostaglandins, and matrix metalloproteinases, leading to inflammatory and degenerative changes in tendon fibers and a higher risk of spontaneous rupture. Achilles tendon injuries are particularly common in team sports such as basketball, volleyball, tennis, and soccer, where repetitive rapid acceleration, deceleration, and jumps are frequent [46,47,[85], [86], [87], [88]].
1.4.2. Comorbidity factor
Impaired synthesis of collagen fibers, increased degradation, or inflammatory changes in connective tissue dysplasia syndrome significantly affects tendon fiber structure and strength. Research from Sechenov University indicates that a serious risk factor for musculoskeletal injuries, including ligament and tendon ruptures, is the initial connective tissue failure. The prevalence of this pathology in the population is estimated to reach 85 % [55,56]. Clinical pathognomonic markers of predisposition to injuries have significant sex differences: in women, markers include asthenic body type, joint hypermobility, soft ears, hyperelastic skin, telangiectasias, atrophic striae, and varicose veins; in men, markers include dolichostenomelia, arachnodactyly, chest deformities, flat feet, and abdominal muscle diastasis [89,90]. Identification of these markers is crucial for personalized selection of sports activities and training programs to prevent ligament, tendon, and muscle injuries.
Factors increasing Achilles tendon injury risk include various foot, ankle, and knee joint deformities. These anatomical and morphological changes can cause biomechanical disorders that lead to uneven load distribution on tendons, resulting in damage. Congenital foot deformities, such as pronounced flat feet, congenital vertical talus, clubfoot, tarsal coalitions, valgus-adducted foot, varus first toe deformity, neurogenic foot deformities, and hypermobile flat feet with Achilles tendon shortening, significantly alter motor patterns and lead to chronic tendon fiber traumatization [89,90]. Additionally, deformities in ankle and knee joints due to degenerative-dystrophic or inflammatory processes contribute to Achilles tendon injuries and ruptures [[89], [90], [91], [92], [93], [94], [95]].
Pathological changes in the Achilles tendon are a significant risk factor for microtraumas and spontaneous ruptures. These changes can be classified into non-insertional, located in the tendon's middle part, and insertional, located at the lower third near the calcaneus [[102], [103], [104]]. Non-insertional tendinopathy, often manifesting as edema and pain 2–7 cm from the calcaneus attachment, involves tendinosis, characterized by degenerative and atrophic changes without histologic inflammation. Continuous inadequate loads on the tendon can trigger this condition [[102], [103], [104]]. Paratendopathy involves inflammatory changes extending to the paratenon, causing pronounced edema, redness, and crepitation at the lesion site. Histologically, tissue infiltration with inflammatory cells and fibrinous exudate is observed, leading to increased spontaneous rupture risk due to a shift in the collagen type I to type III ratio [[102], [103], [104]].
Insertional tendinopathy, which manifests as edema and pain at the Achilles tendon's attachment to the calcaneus, is often accompanied by bony growths on the calcaneus and within the tendon. Degenerative or inflammatory changes in the bursae, such as retrocalcaneal bursitis (between the tendon and calcaneus) and superficial calcaneal bursitis (between the tendon and skin), further exacerbate the condition [9,[105], [106], [107]]. Chronic corticosteroid use in these areas can lead to tenonecrosis, loss of the tendon's tricuspid structure, and inhibition of reparative processes, significantly increasing the risk of spontaneous tendon rupture [9,[105], [106], [107]].
1.4.3. Metabolic factors
Metabolic disorders associated with various diseases significantly impact tendon structure and function, particularly in the Achilles tendon, leading to tendinopathy and a predisposition to spontaneous rupture under normal mechanical loads [[108], [109], [110]]. Obesity, a significant metabolic factor, places excessive long-term loads on the lower limb joints, ligaments, and tendons, causing pathological changes and impaired movement biomechanics. Adipose tissue cells secrete adipokines, which affect tenocyte activity and alter tendon structure. These bioactive substances increase the production of cytokines, prostaglandins, and matrix metalloproteinases, supporting systemic chronic inflammation of tendons and causing degradation regardless of the load [54,[109], [110], [111]] (Fig. 2A–B, Table 1).
1.4.4. Type II diabetes
Patients with type II diabetes are characterized by increased Achilles tendon thickness and structural changes, such as hypoechogenicity, fibrillar pattern loss, and calcification, detectable via ultrasound [[112], [113], [114], [115], [116]]. Electron microscopy reveals an increased density of disorganized collagen fibrils, a significant decrease in the number of elastic fibers, fibroblasts, and tenocytes per unit area, and a reduced proportion of functioning capillaries, indicating decreased blood supply to the tendon fibers [117,118]. In diabetes mellitus, excess glycation end products form covalent cross-links within collagen fibers, altering their structure and impairing mechanical stability. Collagen cross-linking, tenocyte apoptosis, proinflammatory cytokine release, and chronic inflammation progressively damage the tendon, predisposing it to rupture [114,[117], [118], [119]].
Enhanced injury prevention strategies and clinical guidelines for effective regenerative medicine should focus on these modifiable risk factors. Tailored interventions, such as personalized exercise regimens, early detection of comorbid conditions, and appropriate management of metabolic disorders, can significantly reduce the incidence of Achilles tendon injuries and improve overall tendon health.
1.4.5. Hypercholesterolemia
In long-term hypercholesterolemia, dense, painless subcutaneous nodules known as xanthomas, which contain cholesterol, are found in the Achilles tendon. These deposits initiate and sustain persistent inflammation, leading to degenerative changes in tendon fibers, thickening of the Achilles tendon, and a decrease in its biomechanical strength [[120], [121], [122], [123]]. There is a strong correlation between blood cholesterol levels and Achilles tendon thickness, with men being at higher risk of tendon fiber thickening than women. The pathogenetic mechanisms of Achilles tendon damage in lipid metabolism disorders include the penetration of cholesterol oxidation products into tenocytes, initiating a mitochondrial-dependent pathway of apoptosis [[120], [121], [122], [123]]. Additionally, hypercholesterolemia is associated with increased matrix metalloproteinase activity and more active collagen degradation. Some studies suggest that dyslipidemia is accompanied by hypercoagulation, atherosclerosis, and thrombosis, which significantly worsen blood circulation to the tendon, causing tissue hypoxia and energy metabolism disorders. Collectively, these factors play a significant role in Achilles tendon degeneration and subsequent rupture [[120], [121], [122], [123]] (Fig. 2A–B, Table 1).
1.4.6. Hyperuricemia
The deposition of monosodium urate crystals in tendons and ligaments is a hallmark of chronic hyperuricemia, affecting predominantly the lower extremities and often presenting asymptomatically [[124], [125], [126], [127]]. The crystallization onset depends on triggers such as tendon injury, mechanical stress, low temperature, and long-term elevated serum uric acid concentration. Microtophi formation in the Achilles tendon significantly alters its structure, predisposing it to rupture [[128], [129], [130]].
1.4.7. Thyroid hormone imbalance
Thyroid hormone imbalances can also lead to metabolic disorders and tendinopathies. Thyroxine and triiodothyronine stimulate the proliferation of tenocytes and counteract their apoptosis. They also increase the production of collagen I, biglycan, and cartilage oligomeric matrix protein, which are crucial for tendon mechanical strength. Decreased thyroid hormone levels are predictive of Achilles tendon injuries.
1.4.8. Inherited metabolic disorders
Several inherited metabolic disorders are linked to tendon injuries and ruptures. Alkaptonuria, caused by a deficiency of homogentisic acid oxidase, leads to the accumulation of homogentisic acid in collagen fibers, forming a dark pigment that inhibits collagen cross-linking [84,110,131]. This disrupts structural integrity and increases the likelihood of spontaneous tendon rupture, particularly in the patellar and Achilles tendons. Glycogen storage diseases, due to deficiencies in enzymes responsible for glycogen metabolism, are associated with severe hyperuricemia at a young age, manifesting as gouty tenosynovitis and reduced tendon adaptation to physical activity [84,110,[131], [132], [133], [134], [135], [136], [137]].
1.4.9. Negative drug effects
Several medications are associated with Achilles tendinopathy and rupture [[138], [139], [140]] (Fig. 2A–B, Table 1).
-
•
Fluoroquinolones: These antibiotics inhibit bacterial enzymes topoisomerases II and IV, disrupting DNA synthesis. By inhibiting similar enzymes in connective tissue metabolism, they increase matrix metalloproteinase expression and collagen degradation, reduce cell proliferation, and impair collagen and proteoglycan synthesis. They also act as potent iron chelators, disrupting collagen cross-linking and maturation, necessary for tensile strength, and inducing oxidative stress that leads to tenocyte apoptosis [[141], [142], [143], [144], [145], [146]].
-
•
Several studies have associated Achilles tendinopathy and rupture with the use of such antimicrobial drugs as azithromycin, cephalosporins, and sulfonamides [[147], [148], [149]].
-
•
Statins: Tendon fiber changes are most commonly seen with atorvastatin, with symptoms appearing four to eight months after starting therapy. The pathogenesis involves changes in matrix metalloproteinase activity, weakening of tendon structural components, and activation of apoptosis [139,140,150].
-
•
Corticosteroids: Long-term use, especially in patients with bronchial asthma, allergic, and autoimmune diseases, inhibits tenocyte activity and collagen synthesis, activates collagenase, and increases collagen fiber breakdown [139,140,151].
-
•
Aromatase Inhibitors: Used to treat breast, endometrial, and ovarian cancer, these drugs block androgen conversion to estrogen, significantly decreasing peripheral blood estrogen levels and leading to tendon fiber damage within three months of use [[139], [140], [141],152].
-
•
Anabolic Steroids: These drugs stimulate protein synthesis, causing rapid muscle mass buildup that creates an excessive load on the tendons and increases tendon fiber stiffness [139,140,153].
-
•
Isotretinoin: A synthetic retinoid derived from vitamin A, used to treat cystic nodular acne, has been linked to Achilles tendon damage within two to six weeks of use [154].
-
•
Renin-Angiotensin System Drugs: Especially renin-angiotensin receptor II antagonists that regulate aldosterone levels, are statistically associated with Achilles tendon rupture [147].
-
•
Thiazide Diuretics and Calcium Channel Blockers: Long-term use of these drugs has been associated with Achilles tendinitis and bilateral Achilles tendonitis with painful ankle swelling [139,140,145].
Understanding these modifiable risk factors is crucial for developing enhanced injury prevention strategies and clinical guidelines for effective regenerative medicine. Tailored interventions, such as personalized exercise regimens, early detection of comorbid conditions, and appropriate management of metabolic disorders, can significantly reduce the incidence of Achilles tendon injuries and improve overall tendon health. Identifying and addressing these factors through targeted therapies and lifestyle modifications will enhance tendon resilience and reduce the risk of rupture. The established non-modifiable and modifiable risk factors for Achilles tendon injuries and rupture are summarized in(Fig. 2A–B, Table 1).
1.5. ATR risk factors and surgical site infections
Achilles tendon ruptures are a frequent injury in athletes, often necessitating surgical intervention to restore function and prevent further complications. However, a major postoperative challenge is the development of surgical site infections (SSIs), which significantly impact recovery outcomes. SSIs are a critical concern in tendon repair surgeries, as they can lead to delayed healing, long-term morbidity, and even recurrent tendon injuries, ultimately affecting athletic performance and quality of life [155]. The multifactorial etiology of SSIs following Achilles tendon repair involves a complex interplay of microbial pathogens, patient-specific risk factors, and surgical variables [156].
Microbial pathogens responsible for SSIs in Achilles tendon surgeries commonly include skin-resident bacteria such as Staphylococcus aureus, but antibiotic-resistant organisms prevalent in hospital environments can also play a significant role. S. aureus is a notable pathogen due to its high virulence and ability to form biofilms on implanted materials, increasing resistance to treatment. Patient-specific risk factors for SSIs include underlying conditions such as diabetes, immunosuppression, and prior infection history, all of which impair the body's ability to combat infection [157]. Surgical factors influencing SSI development include the duration of the procedure, the use of specific surgical techniques, and the quality of perioperative care. For instance, prolonged surgeries expose tissues to contaminants for extended periods, increasing the likelihood of infection [158,159].
A critical understanding of the microbial spectrum is essential for effective prevention and treatment strategies in postoperative care. Identifying and addressing specific risk factors that predispose athletes to SSIs can facilitate personalized treatment plans, enhancing surgical outcomes and minimizing complications. Furthermore, tailoring antibiotic prophylaxis based on pathogen profiles and patient risk factors could reduce SSI incidence and improve recovery trajectories in tendon repair surgeries.
Chenhao Guo et al. performed a retrospective cohort analysis [159] to investigate the risk factors and microbial causes of SSIs in athletes undergoing Achilles tendon repair. This study [159] was performed on 75 patients who underwent Achilles tendon repair. The study group included 25 patients with confirmed SSIs (case group) and 50 patients without infections (control group). Inclusion criteria comprised athletes with clinically diagnosed Achilles tendon ruptures treated surgically, while exclusion criteria eliminated those with previous tendon disorders or significant chronic illnesses that could confound the results. SSIs were diagnosed based on clinical symptoms such as elevated body temperature, localized tenderness, and confirmed positive microbiological cultures [159]. Advanced bacterial identification was conducted using VITEK® 2 technology, which provided precise pathogen profiling. The authors of this study employed univariate and multivariate logistic regression to analyze the risk factors contributing to SSIs. The results showed that S. aureus was the primary pathogen isolated in infected patients, consistent with other reports of SSIs in orthopedic surgeries. Significant risk factors identified included the absence of prophylactic antibiotic administration, diabetes, the presence of open wounds, and extended surgical durations. Univariate analysis highlighted stark contrasts in the presence of these factors between the case and control groups, while multivariate analysis confirmed their critical roles in SSI development [159]. The findings of this study [159] elucidate the importance of effective preoperative planning and perioperative care to mitigate infection risks in Achilles tendon repair surgeries. The lack of prophylactic antibiotic use, in particular, emerged as a major modifiable risk factor. Moreover, patients with diabetes and those undergoing longer surgical procedures were at significantly higher risk of developing SSIs. By addressing these factors, surgeons can improve postoperative outcomes, reduce infection rates, and ensure quicker recovery for athletes [159].
1.6. Outdoor temperature - ATR risk factor
ATR is associated with several well-documented risk factors, such as male sex, younger age, higher BMI, certain racial demographics, smoking, corticosteroid intake, previous reports of a Achilles tendinopathy, O-group blood type, comorbid conditions, and recurrent sports activities [17,21,[25], [26], [27], [28]] [65,[160], [161], [162], [163], [164]] [165]. However, due to limitations in claims data in the study by Kwang Hwan Park et al. [165], risk factors include sex, age, household income, regional latitude, and temperature index were ascertained [165]. Through logistic regression modelling, significant variables linked to ATR were identified which include male sex, younger age, higher income, as well as median temperature. Importantly, after controlling for confounding factors, median outdoor temperature emerged as a profound predictor of ATR in the South Korean population, suggesting it may be a novel risk factor within this region [165].
The exact mechanisms linking outdoor temperature to ATR remain poorly understood. However, it is hypothesized that higher outdoor temperatures influence ATR risk through multiple pathways. First, elevated temperatures may encourage increased outdoor physical activity, including sports participation, which is known to contribute to ATR risk [166]. Several studies have established a positive correlation between warmer temperatures and heightened levels of physical activity [[167], [168], [169], [170], [171]], with physical activity demonstrating clear seasonal patterns, often peaking during spring and summer months [[172], [173], [174], [175], [176], [177]]. A high household or recreational activity typically coincide with warmer weather [178,179], while a reduction in physical activity is noted during colder months [172]. Furthermore, studies, such as one conducted in Galveston, TX, where the average July temperature is 29 °C, have shown that extreme heat can also serve as a deterrent to outdoor activity [180]. In addition to this behavioral factor, previous research indicates that higher temperatures are associated with increased risks of sports, recreational, and occupational injuries [166,181], providing a plausible explanation for the observed peak in ATR incidence during May, when the average temperature in the region reaches 17.8 °C.
Beyond behavioral influences, high temperatures may directly contribute to injury risk by inducing physiological changes such as muscle fatigue and dehydration [182]. Dehydration, commonly experienced in hot environments, has been shown to negatively affect both muscular endurance and strength [181,183], which may increase susceptibility to tendon injuries like ATR. In this context, dehydration might impair the tendon's ability to handle repetitive strain, leading to rupture, particularly during periods of intense physical activity in warm conditions. In conclusion, while traditional risk factors such as sex, age, and medical history continue to play a significant role in ATR, environmental factors like outdoor temperature are emerging as important considerations in understanding the overall risk landscape. Future reports are required to fully elucidate the direct and indirect effects of temperature on tendon health, which could inform future prevention strategies, particularly in regions with seasonal temperature variations [165].
1.7. Tissue homeostasis and ATR risk factors
Tendon homeostasis is intricately controlled by the interplay between mechanical loading as well as cellular activity; these are modulated by neuronal and cellular mediators, which can be synthesized locally at the tendon site or distally in other tissues, are subsequently delivered to the tendon through blood circulation or nerve supply [184,185]. Mechanical loading exerts a crucial role in this regulatory process, as appropriate levels of mechanical stress initiate anabolic responses within the tendon, particularly through enhanced expression of collagen genes [186,187]. Therefore, collagen production, a key component of tendon repair and strengthening, typically peaks around 24 h after physical activity and can persist for up to 80 h post-exercise.
However, when the mechanical load becomes excessive, it can disrupt this balance by triggering collagen degradation, leading to a catabolic response. Notably, the catabolic peak characterized by the breakdown of collagen proteins occurs before the anabolic peak, resulting in a net loss of collagen within the first 24–36 h after exercise. This initial collagen degradation is followed by a period of net collagen gain as anabolic processes subsequently dominate [188]. Thus, the timing of physical activity and recovery is critical in maintaining tendon integrity. Sufficient rest intervals between training sessions allow for proper tissue adaptation, minimizing the risk of overuse injuries by preventing a persistent catabolic state.
In this context, the maintenance of a healthy tendon environment depends on balancing the anabolic and catabolic phases. Ensuring that the tendon remains in an anabolic or neutral state for a sufficient period after exercise may help prevent tendon degeneration and injury. Proper management of mechanical loading, combined with periods of adequate recovery, promotes tissue remodeling and enhances tendon resilience. This highlights the importance of structured training regimens that emphasize both activity and recovery to support tendon homeostasis and reduce the likelihood of injury [189].
1.8. Effect of rupture factors before or after treatment
Achilles tendon injuries treated with bone marrow mesenchymal stem cells (BMMSCs) in combination with TGF-β1 have demonstrated faster and complete healing clinical outcomes. TGF-β1 plays a pivotal role by enhancing collagen protein synthesis, promoting the formation of cross-links, and driving matrix remodeling during tendon repair, ultimately boosting the mechanical strength of the tendon [190]. Additionally, low-magnitude, low-frequency vibration training at 10 Hz has been shown to upregulate TGF-β1 expression, leading to higher tenomodulin generation and collagen type I (Col I), as well as enhanced Achilles tendon stiffness in rat models. This treatment modality improves the structural integrity of the tendon and reduces the likelihood of reinjury at the time of rehabilitation [191]. Further supporting the role of TGF-β1 in ATrecovery, a clinical study on patients with AT rupture revealed that TGF-β1 and VEGF expression levels significantly increased three months post-treatment but decreased by six months following surgery. The dynamic expression patterns of TGF-β1 and VEGF suggest that these molecules could serve as key biomarkers for monitoring and predicting clinical outcomes in the patients diagnosed with Achilles tendon rupture [192].
Fibroblast growth factors (FGFs) also have significant implications in tendon repair by regulating critical cellular processes such as migration, proliferation, as well as differentiation. FGFs can indue these effects by activating high-affinity FGF receptors, which consequently trigger downstream signaling cascades essential for tissue homeostasis and regeneration [193]. Usage of fibrin clots and vitamin C during the surgical repair of AT ruptures has been shown to result in superior tendon structure and improved healing quality, potentially due to their ability to stimulate FGF generation at the time of early phases of tendon injury repair [194].
In patients with AT rupture, serum levels of TGF-β1 and VEGF increased significantly at the three-month mark post-surgery and subsequently declined at six months, concluding the improvements in clinical outcomes. These findings further suggest that TGF-β1 and VEGF serve as reliable markers for assessing the efficacy of treatment in such cases [192].
Platelets, a primary source for HMGB1 protein, are essential in tendon healing, particularly in platelet-rich plasma (PRP) therapy. Platelet-derived HMGB1 in PRP has been shown to reduce inflammation, increase local HMGB1 concentrations, and recruit stem cells to the injury site. This mechanism highlights the potential of PRP treatments in tendon repair, although its effectiveness may depend heavily on the concentration and quality of platelets used [195]. In clinical practice, PRP injections have been investigated as a potential treatment for AT ruptures [196]. But other clinical reports have described that PRP showed no significant improvement in clinical outcomes or functional recovery in nonsurgical AT rupture treatments when compared to conventional treatments such as percutaneous fixation [[197], [198], [199]]. This discrepancy in results may be due to variations in treatment protocols. Despite the ongoing debate regarding its efficacy, PRP remains one of the most commonly used biological treatments for tendinopathies [14,199].
1.9. Early stages of recovery after an Achilles tendon rupture-clinical reports
Following an Achilles tendon (AT) injury, the decision to resume full weightbearing activities, including sports or other physically demanding tasks, is traditionally based on clinical assessment alone. However, the use of objective quantitative measures such as tendon stiffness and foot plantar pressure may provide valuable insights to support clinical decision-making. A study by Didier Laurent et al. [200], evaluated these parameters in 15 patients for up to 3 months post-rupture with the aid of shear wave elastography (SWE) and wearable insoles. Additionally, patient-reported outcomes were collected using Achilles Tendon Total Rupture Score (ATRS) to assess the impact on physical activity [200]. At two weeks post-injury, stiffness of the tendon associated with injury has shown variability, with shear wave velocities near the rupture site typically in the distal portion of the tendon. By eight weeks, near-complete recovery of stiffness was evident in both the distal and middle regions, while the proximal region only achieved 65 % recovery relative to the uninjured tendon by week 12. A complementary pre-clinical report in a rat model demonstrated a strong correlation between in vivo tendon stiffness measured using SWE and ex vivo Young's modulus values, further validating the precision of SWE for assessing tendon stiffness [200]. The evaluation of plantar pressure distribution using wearable insoles revealed minor suboptimal function pertinent to the affected foot during walking at week 12, despite significant recovery of ATRS scores [200]. These findings suggest a persistent biomechanical imbalance even as patients reported subjective improvement. Notably, significant correlations were observed between tendon stiffness, plantar pressure variables, as well as specific ATRS activities, concluding the clinical relevance of these measurements for tracking functional recovery.
Didier Laurent et al. highlighted the impact of AT structural changes on daily activities and illustrate the utility of digital biomarkers in monitoring functional recovery over time. The correlation of tendon stiffness and plantar pressure distribution with patient-reported outcomes provides a more comprehensive understanding of the biomechanical and functional recovery process [200]. In conclusion, the study demonstrated the potential of SWE as a reliable diagnostic tool for detecting tendon injuries and monitoring treatment progress aimed at accelerating tendon regeneration. Additionally, the integration of structural and biomechanical data, such as foot plantar pressure, with patient perceptions of their ability to perform physical activities offers a novel approach to understanding how alterations in AT structure affect recovery and daily function. These findings advocate for the inclusion of objective digital biomarkers alongside traditional clinical assessments to improve decision-making and rehabilitation strategies after AT rupture [200].
1.10. AT-patient specific therapies & recent clinical reports
Achilles tendon rupture (ATR) is significantly observed in the middle-aged individuals who are involved in military activities and recreational sports [48,201]. For example, a study in Sweden described the ATR incidence with a steep increase which depicts an upward trend from 2001 to 2012, with rates increasing from 47 to 55.2 per 100,000 person-years for males and from 12 to 14.7 per 100,000 person-years for females [48]. A few other reports described that the rate and measures of returning to play post-ATR found that, on average, about 80 % of individuals return to their previous activity levels after rehabilitation, though the range varies widely from 28 % to 100 % [86,202,203].
Considerable efforts have been made to identify the optimal treatment for ATR, leading to numerous systematic reviews and meta-analyses. However, there remains no consensus on whether surgical or nonsurgical treatment is superior [[204], [205], [206], [207], [208]]. Recent research suggests that nonsurgical methods may be preferable if functional rehabilitation is performed effectively, as surgical treatment carries a higher risk of infection [207,208]. Nonetheless, another systematic review indicated that surgical treatment reduces the risk of rerupture, though it shows no significant differences in terms of deep venous thrombosis, or return to sports [204]. Comparisons using patient-reported outcome measures (PROMS) such as Achilles tendon Total Rupture Score and the Physical Activity Scale found no significant differences between treatment methods [204,209].
Weight-bearing exercises and rehabilitation has been reported to bestow significant efficacy pertinent to functional outcomes in individuals diagnosed with ATR [210,211]. For instance, early rehabilitation with immobilization fostered good results in all categories compared to immobilization, with the combined approach yielding the highest satisfaction levels [210]. However early rehabilitation following nonsurgical treatment failed to produce good functional outcomes in individuals with ATR. According to these studies, there was a notable improvement in health-related quality of life favoring the early weight-bearing group [212].
Health-related rejuvenation after therapy to AT involves an individual's experiences after treatment and rehabilitation in addition to intrinsic factors (Fig. 1). Patient-reported outcome measures (PROMs) are frequently employed to assess these factors; however, qualitative research approaches can offer a deeper insight into ATR, subsequently describing individual's personal experience. These adaptive methods are significantly used to explore the factors that typically influence the individual's ability to return to sports activities by improving the Achilles tendon repair [213] as well as anterior cruciate ligament reconstruction [214,215]; other therapy factors are crucial which can enhance the hip arthroscopy for femoroacetabular impingement [216], and arthroscopic Bankart repair [217].
Another study [213] described that the return to sports post-ATR is solely replies upon selecting a suitable surgeon as well as a physical therapist along with a commitment to adhere to rehabilitation guidelines is significantly required to mediate effective healing. This aligns with findings from another report that interviewed patients and their parents about their experiences with physical therapy following an anterior cruciate ligament reconstruction. The study highlighted the importance of the patient-therapist relationship, emphasizing the therapist's role as a guide, motivator, confidence booster, and care coordinator [218]. Effective communication and making physiotherapy sessions enjoyable were also noted as significant factors [218]. These insights suggest that providing patients with consistent, individualized information throughout the rehabilitation process from various healthcare providers is vital for successful recovery.
In today's digital age, where information is readily accessible, comparisons with others undergoing similar experiences can be both beneficial and detrimental. Relating to others and gaining insights into the rehabilitation process is important, but exposure to extreme stories—both negative and unrealistic positive outcomes—can skew expectations. No specific studies were found related to the influence of external information on recovery from musculoskeletal injuries [219]. on dermatological conditions showed that much online information is financially biased and discourages seeking medical advice [219]. This underscores the need for patients to rely on credible, evidence-based sources and consult healthcare professionals for accurate information.
Fear of reinjury and insecurity about the tendon's performance were frequently mentioned, highlighting the psychological impact on recovery. A study found that individuals who exhibited fear of reinjury had poorer physical improvements and reduced self-reported function compared to those without such fears [220]. The avoidance of activities due to fear of reinjury can be explained by the Fear-Avoidance Model of Musculoskeletal Pain, that describes a cycle where pain leads to fear, avoidance, and ultimately more pain and disability. Some participants in Peterson's study fell into this cycle, avoiding the use of the affected muscle/tendon, resulting in decreased strength and endurance [221,222].
Physiological limitations, such as stiffness, reduced endurance, and strength in the Achilles tendon or calf muscle, were also commonly reported [222]. A study investigating a return to sports and patient satisfaction following nonsurgical treatment for acute Achilles tendon rupture (ATR) revealed that while 94 % of participants expressed satisfaction with their treatment outcomes, the return to preinjury levels of sports participation was less favorable. Specifically, only 70 % of individuals had resumed their preinjury sports activities at the 1-year follow-up, with a modest increase to 73 % at the 5-year mark. These findings highlight a significant gap between patient satisfaction and the actual return to previous levels of athletic performance, suggesting that while nonsurgical treatments may be well-received, they might not fully restore athletic function to preinjury standards. This underscores the need for further research into optimizing rehabilitation protocols and long-term outcomes for individuals undergoing nonsurgical management of ATR. Comparison of activity levels before and after the injury revealed that most participants were less active than before, with a mean score of 2.3 on a scale where 1 indicates being much less active and 5 indicates being much more active [222].
Taken together, these findings emphasize the importance of comprehensive and individualized rehabilitation programs that address both physical and psychological aspects, along with reliable information and strong patient-therapist relationships to enhance recovery outcomes after Achilles tendon injuries.
1.11. Cellular therapy to ameliorate Achilles tendinopathies
Mesenchymal stromal cells (MSCs) possess typically a higher ability to self-renew and confer to differentiate into distinct kinds of mature cell types to foster regenerative medicine for a variety of ailments [223]. MSCs can be identified by the expression of different cell surface markers including cluster differentiation markers and this expression varies by different culture conditions [224,225]. MSCs exert significant influence over their local microenvironment through paracrine and autocrine signaling mechanisms, allowing them to modulate surrounding tissues [226]. Their low immunogenicity makes them suitable for allogenic transplantation, as they generally do not provoke aggressive immune responses. This immune-evasive nature enhances their potential in regenerative therapies, primarily due to their homing and engraftment capabilities in target tissues [[227], [228], [229]]. Functionally, MSCs could foster the proliferation of cells by the influence of paracrine or autocrine secretions [230].
BM niche is composed of a variety of cells and especially [231], hematopoietic stem cells (HSCs) and mesenchymal stromal/stem cells (MSCs) are significantly involved in undergoing differentiation into distinct types of cells. BM-derived MSCs are notable for their potent anti-inflammatory properties, particularly through the secretion of interleukin-1 receptor antagonist (IL-1Ra), which significantly mitigates matrix degradation; in addition, the expression of MMP-3 and TNF-α genes are mitigated during this BM-dived MSCs-mediated inflammation; Furthermore, these BM-derived MSCs could mitigate chondrocyte apoptosis while enhancing deposition of collagen [232,233]. This cytokine's effects are clinically significant, offering pain relief and enhancing the management of chronic tissue inflammation, particularly in tendinopathies. For instance, intratendinous injection of BM-MSCs in Achilles tendon (AT) injuries in mammalian models has shown improvements in biomechanical properties and collagen fiber organization during early tendon healing phases [234]. In rat models, BM-MSCs demonstrate a higher tendon healing efficacy compared to platelet-rich plasma (PRP) in histological, biochemical, and immunohistochemical assessments [235]. The regenerative potential of tendon stem cells (TSCs) may even surpass that of BM-MSCs, as evidenced by increased Tenascin-C expression in treated groups [236]. Clinical applications under ultrasound guidance, such as the administration of autologous BM-aspirate concentrate (BMAC), resulted in mitigating pain and subsequently enhanced the functional outcomes of tendons in the individuals diagnosed with chronic AT, as evidenced by MRI findings [237,238]. Furthermore, BM-MSCs support early rehabilitation, reduce the incidence of re-rupture, and improve tendon structure [[238], [239], [240], [241], [242], [243], [244]]. However, further robust clinical data are necessary to fully endorse the efficacy and safety of BMAC for AT treatment [245].
Adipose tissue has emerged as a prolific source for MSCs substantially higher when compared to bone marrow source [[246], [247], [248]]. Adipose-derived MSCs (AD-MSCs) and stromal vascular fraction (SVF), which includes endothelial cells, preadipocytes, macrophages, T cells, pericytes, and progenitor cells, have shown promising regenerative outcomes in tendinopathy treatments [[249], [250], [251], [252], [253], [254], [255]]. For example, SVF and AD-MSCs enhance tendon fiber organization and facilitate neovasculogenesis, essential for tendon repair [253,256]. Clinical studies have demonstrated faster recovery and fostered and improved tendon matrix composition with SVF injections [252,253]. For instance, AD-MSCs have been shown to promote neovasculogenesis, mitigate inflammation, and enhance AT repair typically in the models of collagenase-induced AT and upregulate tendon repair [256]. Additionally, AD-MSCs influence collagen composition and matrix metalloprotease expression, crucial for tendon healing [257,258]. Tenogenically differentiated AD-MSCs have improved histological scores and collagen fiber organization in Achilles tendon repairs [255]. Compared to PRP, SVF derived from adipose tissue offers good clinical and functional outcomes in AT treatments [259,260]. However, further clinical validation is needed to evaluate the pharmacological efficiency of AD-derived MSCs to foster tendon healing [245] (Table 2).
Table 2.
Regenerative Medicine Approaches: The regenerative medicine approaches highlight the promising therapies available to enhance tendon repair and recovery.
| Approach | Mechanism | Effect | References |
|---|---|---|---|
| Mesenchymal Stromal Cells (MSCs) | Differentiation into specific cell lineages, modulating local microenvironment through paracrine/autocrine signals | Promote tendon healing, reduce inflammation, and improve structural integrity. | [189,[223], [224], [225], [226], [227], [228], [229], [230]] |
| Bone Marrow-Derived MSCs (BM-MSCs) | Anti-inflammatory properties, IL-1Ra secretion, collagen deposition enhancement | Improved biomechanical properties, collagen fiber organization, and early tendon healing. | [189,[231], [232], [233], [234], [235], [236], [237], [238], [239], [240], [241], [242], [243], [244], [245]] |
| Adipose Tissue-Derived MSCs (AT-MSCs) | Easy isolation, differentiation into multiple cell types, anti-inflammatory properties | Enhance tendon healing and reduce inflammation. | [189,[246], [247], [248], [249], [250], [251], [252], [253], [254], [255], [256], [257], [258], [259], [260]] |
| Platelet-Rich Plasma (PRP) | Concentrated platelets release growth factors and cytokines | Stimulate healing processes, improve pain, and enhance tendon function. | [189,[261], [262], [263]] |
| Gene Therapy | Targeting specific genes involved in tendon repair and regeneration | Potential to correct genetic predispositions and enhance tendon healing processes. | [189,264,265] |
| Scaffold-Based Therapies | Use of biocompatible materials to provide structural support and deliver cells/growth factors | Promote tissue regeneration and structural support for tendon repair. | [189,[266], [267], [268]] |
1.12. Platelet-rich plasma (PRP) mediated amelioration of Achilles injuries
Chronic tendinopathy is characterized by a pro-inflammatory marker expression that hampers the healing process because of limited vascularization and slow cell turnover across the regions of tendons [269]. PRP-based therapy is an acellular therapy. PRP, which concentrates platelets to generate growth factors from alpha granules, has been shown to enhance tissue regeneration in musculoskeletal disorders by promoting neovascularization and stimulating resident stem cells [270,271]. The specific composition of PRP, including leucocytes and chemokines, can regulate inflammatory responses and improve collagen production and cell proliferation [[272], [273], [274]]. Despite varying reports on the effects of PRP on tendon thickness and vascularity, many studies indicate its potential to improve clinical outcomes in AT [189,[261], [262], [263]]. The effectiveness of PRP may be affected by factors including patient age and type of tendinopathy [275,276]. Despite some controversy, PRP remains a promising and safe biological agent for treating AT injuries, offering profound pain relief and functional improvements [197,[277], [278], [279]] (Table 2).
1.13. Future therapeutic modalities of exosomes-based at injury amelioration
Exosomes derived from cellular and noncellular sources, present significant potential in tendon repair by modulating gene expression and promoting anti-inflammatory and regenerative responses [264,265,[280], [281], [282], [283], [284]]. Exosomes from MSCs, for example, enhance tendon-bone interface healing and regulate macrophage polarization, crucial for tissue repair [281,282]. These vesicles can improve collagen organization and tenocyte proliferation, contributing to tendon regeneration [[285], [286], [287], [288], [289], [290], [291], [292]]. However, the production and commercialization of exosomes are challenging due to technical, ethical, and cost-related factors. Advances in engineering “smart exosomes” and tendon-specific scaffolds hold promise for improving tendon repair and regeneration [266,267,[293], [294], [295], [296], [297], [298], [299], [300], [301], [302]]. Emerging concepts like “Smart Exosomes” and engineered tenogenesis offer exciting future directions for AT treatment. These strategies involve manipulating exosome content and combining stem cells with growth factors on scaffolds to enhance tendon regeneration [266,267,[293], [294], [295], [296], [297], [298], [299], [300], [301], [302]]. Additionally, “Extracellular vesicles-educated macrophages” represent a novel approach to inflammatory disorders, including tendon injuries, by leveraging the regenerative potential of macrophages exposed to exosomes [266,302]. These innovative therapies aim to improve tendon healing by modulating the inflammatory environment, enhancing biomechanical properties, and promoting the differentiation and proliferation of tenocytes.
1.14. ATR management by rehabilitation & education strategies
There is well-established evidence that surgical and non-surgical treatments for ATR yield comparable outcomes, yet the debate over the optimal treatment method continues [[303], [304], [305]]. In light of this, the focus has shifted to the importance of rehabilitation strategies in ensuring recovery and functional outcomes [210,303,306]. Despite the growing emphasis on rehabilitation, data on the recovery process following ATR remains limited, which may contribute to suboptimal rehabilitation protocols and inconsistent patient outcomes [307]. The scarcity of research addressing psychosocial factors, return-to-play (RTP) timelines after ATR treatment, and the application of novel imaging methods have been associated with increased re-rupture rates [304,308,309]. Additionally, these gaps in knowledge contribute to unpredictable recovery trajectories and RTP outcomes for athletes [86,[310], [311], [312]]. Several factors, both patient-related (such as BMI, nutritional status, as well as comorbidities) and injury-related (such as delayed presentation, injury mechanism, and tendon gap size), are believed to influence recovery and outcomes after ATR [65,308,313,314].
In case of tendon gap size, a few reports have investigated whether the gap between the ruptured tendon ends affects patient-reported outcomes in ATR cases managed with functional rehabilitation [315,316]. Another report [315] used ultrasound method to measure the tendon gap at the initial presentation and followed patients for at least one year, assessing outcomes using ATRS, plantarflexion strength, and re-rupture rates. Their findings indicated the absence of a significant correlation between tendon gap size and ATRS, suggesting that gap size may not be a major determinant of outcome in non-operative functional rehabilitation. Conversely, Yassin et al. [316] reported that a larger tendon gap, particularly when exceeding 10 mm, can be observed using dynamic ultrasound method, and the results of this report elucidated poorer patient-reported outcomes following functional rehabilitation, as indicated by lower ATRS scores. This highlights the potential impact of tendon gap size on recovery, although further studies are necessary to solidify this relationship.
Current research supports the preference for early weight-bearing and functional rehabilitation over traditional immobilization approaches for ATR management [303,317,318]. Although rehabilitation methods are gaining increasing importance [210,303,306], there remains a lack of conclusive evidence on the most effective rehabilitation regimens, as well as limited guidelines for early rehabilitation protocols [318,319]. Additionally, long-term data on recovery trajectories and athletic performance after ATR are limited [307]. Early weight-bearing as well as functional rehabilitation following both operative and non-operative treatments of ATRs have shown promise in promoting new tendon generation and improving long-term functional clinical outcomes, including faster return to work and sports [[320], [321], [322], [323], [324], [325], [326], [327]]. However, given the absence of universally accepted protocols for early rehabilitation and the timing of weight-bearing, further research is essential to establish evidence-based guidelines for optimal recovery after ATR [326,328].
Modifying activity levels is one of the most effective strategies for managing pain, making it a cornerstone of physiotherapy treatment. Reducing or eliminating activities identified from patient history, such as high-intensity stretch-shortening exercises (e.g., faster running, walking on inclines) and decreasing total activity volume by more than 50 %, is often necessary for two to six weeks if load tolerance is diminished. In cases of severe pain, though rare, complete cessation of provocative activities (100 % reduction) may be necessary. This could involve temporarily stopping sports or wearing a walking boot if regular walking exacerbates the condition. Explaining the rationale behind these modifications is crucial, specifically for individuals who adopt an “activity endurance” approach and persist in exercising despite pain. Providing such patients with clear, shared activity goals can lead to better treatment adherence and outcomes [329].
Conversely, individuals who follow an “activity avoidance” strategy—often driven by fear of worsening their condition [330,331] may benefit from a more structured, gradual reintroduction of physical activity. This should be combined with pain education and strategies for monitoring and balancing pain with physical activity. Behavioral change interventions should also be incorporated to enhance self-management and ensure the success of activity modification strategies. These approaches include addressing patient beliefs as well as outcome expectations, setting mutually agreed-upon goals that align with the patient's motivations, and employing action-intention strategies. Additionally, providing feedback, monitoring progress, and implementing practical strategies such as exercise demonstration videos that can improve exercise self-efficacy and long-term adherence to rehabilitation plans [332].
Regarding exercise interventions, 39 trials (spanning 40 reports) have investigated exercise therapies compared to controls, other exercise regimens, or adjunctive treatments for Achilles tendinopathy [[333], [334], [335], [336], [337], [338], [339], [340], [341], [342], [343], [344], [345], [346], [347]]. However, the majority of these trials were underpowered, with significant variability in interventions and outcome measures. This heterogeneity complicates data pooling and limits the certainty of any conclusions drawn from the available evidence. Predominantly used exercise strategies included the Alfredson eccentric protocol (or its modified versions), Silbernagel program, as well as Stanish and Curwin program, and heavy slow resistance program. Despite the challenges in synthesizing this data, exercise remains a key component in the management of Achilles tendinopathy. Further research with standardized interventions and outcome metrics is necessary to provide clearer insights into the most effective exercise-based therapies for this condition [348].
2. Conclusions and future acknowledgements
There is compelling evidence that non-modifiable risk factors, including genetic predisposition, individual anatomical and functional features of the Achilles tendon, sex, and age, play a significant role in the susceptibility to Achilles tendon injuries and rupture. These factors must be considered when selecting sports activities and designing training programs to mitigate injury risk.
Priority areas for the prevention of Achilles tendon rupture include recommendations for balanced nutrition, screening for connective tissue integrity, implementing proper exercise regimens, and conducting regular monitoring of the Achilles tendon condition in athletes. Additionally, timely treatment of musculoskeletal injuries, Achilles tendon diseases, and deformities of the feet, ankle, and knee joints is crucial. Addressing metabolic disorders and assessing the risks associated with long-term drug use are also important components of a comprehensive prevention strategy.
The interplay of multiple established factors significantly elevates the risk of Achilles tendon rupture. Early identification and intervention regarding these risk factors will enable the development of effective personalized prevention strategies, thereby contributing to the preservation of public health during physical activity and sports.
The primary challenge in translating advanced cellular therapies to clinical practice lies in the lack of evidence from sufficient animal models that accurately simulate chronic Achilles tendinopathy, as most studies focus on acutely injured tendons. Additionally, standardizing the isolation procedures for these biological therapies is crucial before their clinical application can be considered viable. Ensuring robust evidence of efficacy and standardized protocols will be essential steps in advancing these promising treatments from experimental settings to widespread clinical use.
Author contributions
Maria V Sankova (MVS), Narasimha M Beeraka (NMB), Marine V Oganesyan (MVO), Negoriya A Rizaeva (NAR), Aleksey V Sankov (AVS), Olga S Shelestova (OSS), Kirill V Bulygin (KVB), Hemanth Vikram PR (HVPR), Barinov A.N (BAN), Khalimova A.K (KAK), Padmanabha Reddy Y (PRY), Basappa Basappa (BB), Vladimir N Nikolenko (VNN) performed the literature search regarding available databases and drafted the manuscript. NMB, VNN helped in reviewing the relevant literature. NMB and VNN evaluated and reinforced the technical background. NMB and VNN contributed to editing the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Data availability statement
Not applicable.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Not applicable.
Contributor Information
Maria V. Sankova, Email: cankov@yandex.ru.
Narasimha M. Beeraka, Email: biraka_n@staff.sechenov.ru, bnmurthy24@gmail.com.
Marine V. Oganesyan, Email: marine-oganesyan@mail.ru.
Negoriya A. Rizaeva, Email: rizaevan@yandex.ru.
Aleksey V. Sankov, Email: A.V.Sankov@yandex.ru.
Olga S. Shelestova, Email: sh197509@mail.ru.
Kirill V. Bulygin, Email: kirill-bh-red@yandex.ru.
Hemanth Vikram PR, Email: hemanthvikrampr@gmail.com.
A.N. Barinov, Email: mmom-mc@mail.ru.
A.K. Khalimova, Email: assiyakhalimova@gmail.com.
Y. Padmanabha Reddy, Email: ypreddyatp@gmail.com.
Basappa Basappa, Email: salundibasappa@gmail.com.
Vladimir N. Nikolenko, Email: vn.nikolenko@yandex.ru.
References
- 1.Habyarimana JdD., Tugirumukiza E., Zhou K. Physical education and sports: a backbone of the entire community in the twenty-first century. Int J Environ Res Publ Health. 2022;19(12):7296. doi: 10.3390/ijerph19127296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Michael S.L., Wright C., Mays Woods A., van der Mars H., Brusseau T.A., Stodden D.F., et al. Taylor & Francis; 2021. Rationale for the essential components of physical education; pp. 202–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.An R. Policy and physical activity. J Sport Health Sci. 2021;10(3):253. doi: 10.1016/j.jshs.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corbin C.B. Conceptual physical education: a course for the future. J Sport Health Sci. 2021;10(3):308–322. doi: 10.1016/j.jshs.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khairullina A., Rendikova R. Analysis and prospects for the development of the Russian fitness industry market. Sci Sport: Current Trends. 2022;10(2):92–100. [Google Scholar]
- 6.Silbernagel K.G., Hanlon S., Sprague A. Current clinical concepts: conservative management of Achilles tendinopathy. J Athl Train. 2020;55(5):438–447. doi: 10.4085/1062-6050-356-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meulenkamp B., Stacey D., Fergusson D., Hutton B., Mlis R.S., Graham I.D., et al. Protocol for treatment of Achilles tendon ruptures; a systematic review with network meta-analysis. Syst Rev. 2018;7:1–7. doi: 10.1186/s13643-018-0912-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lantto I., Heikkinen J., Flinkkilä T., Ohtonen P., Leppilahti J. Epidemiology of Achilles tendon ruptures: increasing incidence over a 33‐year period. Scand J Med Sci Sports. 2015;25(1) doi: 10.1111/sms.12253. [DOI] [PubMed] [Google Scholar]
- 9.Sereda A. Treatment options for neglected Achilles tendon rupture: whether spontaneous healing is possible? Traumatol Orthop Russ. 2018;24(2):59–69. [Google Scholar]
- 10.Fukuta S., Oyama M., Kavalkovich K., Fu F.H., Niyibizi C. Identification of types II, IX and X collagens at the insertion site of the bovine Achilles tendon. Matrix Biol. 1998;17(1):65–73. doi: 10.1016/s0945-053x(98)90125-1. [DOI] [PubMed] [Google Scholar]
- 11.Im G.-I., Kim T.-K. Stem cells for the regeneration of tendon and ligament: a perspective. Int J Stem Cells. 2020;13(3):335–341. doi: 10.15283/ijsc20091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andarawis‐Puri N., Flatow E.L., Soslowsky L.J. Tendon basic science: development, repair, regeneration, and healing. J Orthop Res. 2015;33(6):780–784. doi: 10.1002/jor.22869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walia B., Huang A.H. Tendon stem progenitor cells: understanding the biology to inform therapeutic strategies for tendon repair. J Orthop Res. 2019;37(6):1270–1280. doi: 10.1002/jor.24156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin M., Li W., Ni X., Sui Y., Li H., Chen X., et al. Growth factors in the treatment of Achilles tendon injury. Front Bioeng Biotechnol. 2023;11 doi: 10.3389/fbioe.2023.1250533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dias Lopes A., Hespanhol Junior L.C., Yeung S.S., Pena Costa L.O. What are the main running-related musculoskeletal injuries? Sports Med. 2012;42(10) doi: 10.1007/BF03262301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vleck V.E., Garbutt G. Injury and training characteristics of male elite, development squad, and club triathletes. Int J Sports Med. 1998;19(1):38–42. doi: 10.1055/s-2007-971877. [DOI] [PubMed] [Google Scholar]
- 17.Muraoka T., Muramatsu T., Fukunaga T., Kanehisa H. Geometric and elastic properties of in vivo human Achilles tendon in young adults. Cells Tissues Organs. 2005;178(4):197–203. doi: 10.1159/000083731. [DOI] [PubMed] [Google Scholar]
- 18.Müller R., Siebert T., Blickhan R. Muscle preactivation control: simulation of ankle joint adjustments at touchdown during running on uneven ground. J Appl Biomech. 2012;28(6):718–725. doi: 10.1123/jab.28.6.718. [DOI] [PubMed] [Google Scholar]
- 19.Novacheck T.F. The biomechanics of running. Gait Posture. 1998;7(1):77–95. doi: 10.1016/s0966-6362(97)00038-6. [DOI] [PubMed] [Google Scholar]
- 20.Zhang S.-N., Bates B.T., Dufek J.S. Contributions of lower extremity joints to energy dissipation during landings. Med Sci Sports Exerc. 2000;32(4):812–819. doi: 10.1097/00005768-200004000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Dixon S.J., Collop A.C., Batt M.E. Surface effects on ground reaction forces and lower extremity kinematics in running. Med Sci Sports Exerc. 2000;32(11):1919–1926. doi: 10.1097/00005768-200011000-00016. [DOI] [PubMed] [Google Scholar]
- 22.Ferris D.P., Louie M., Farley C.T. Running in the real world: adjusting leg stiffness for different surfaces. Proc R Soc Lond B Biol Sci. 1998;265(1400):989–994. doi: 10.1098/rspb.1998.0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Müller R., Grimmer S., Blickhan R. Running on uneven ground: leg adjustments by muscle pre-activation control. Hum Mov Sci. 2010;29(2):299–310. doi: 10.1016/j.humov.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 24.Farris D.J., Trewartha G., McGuigan M.P. Could intra-tendinous hyperthermia during running explain chronic injury of the human Achilles tendon? J Biomech. 2011;44(5):822–826. doi: 10.1016/j.jbiomech.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 25.Maganaris C.N., Narici M.V., Maffulli N. Biomechanics of the achilles tendon. Disabil Rehabil. 2008;30(20–22):1542–1547. doi: 10.1080/09638280701785494. [DOI] [PubMed] [Google Scholar]
- 26.Hintermann B., Nigg B.M., Sommer C. Foot movement and tendon excursion: an in vitro study. Foot Ankle Int. 1994;15(7):386–395. doi: 10.1177/107110079401500708. [DOI] [PubMed] [Google Scholar]
- 27.Wakahara T., Kanehisa H., Kawakami Y., Fukunaga T. Fascicle behavior of medial gastrocnemius muscle in extended and flexed knee positions. J Biomech. 2007;40(10):2291–2298. doi: 10.1016/j.jbiomech.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 28.Lersch C., Grötsch A., Segesser B., Koebke J., Brüggemann G.-P., Potthast W., et al. Influence of calcaneus angle and muscle forces on strain distribution in the human Achilles tendon. Clin Biomech. 2012;27(9):955–961. doi: 10.1016/j.clinbiomech.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Bojsen-Møller J., Hansen P., Aagaard P., Svantesson U., Kjaer M., Magnusson S.P., et al. Differential displacement of the human soleus and medial gastrocnemius aponeuroses during isometric plantar flexor contractions in vivo. J Appl Physiol. 2004;97(5):1908–1914. doi: 10.1152/japplphysiol.00084.2004. [DOI] [PubMed] [Google Scholar]
- 30.Wilson A., Goodship A. Exercise-induced hyperthermia as a possible mechanism for tendon degeneration. J Biomech. 1994;27(7):899–905. doi: 10.1016/0021-9290(94)90262-3. [DOI] [PubMed] [Google Scholar]
- 31.Wyndow N., Cowan S.M., Wrigley T.V., Crossley K.M. Neuromotor control of the lower limb in Achilles tendinopathy: implications for foot orthotic therapy. Sports Med. 2010;40:715–727. doi: 10.2165/11535920-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 32.Lyman J., Weinhold P.S., Almekinders L.C. Strain behavior of the distal Achilles tendon: implications for insertional Achilles tendinopathy. Am J Sports Med. 2004;32(2):457–461. doi: 10.1177/0095399703258621. [DOI] [PubMed] [Google Scholar]
- 33.Wren T.A., Yerby S.A., Beaupré G.S., Carter D.R. Mechanical properties of the human Achilles tendon. Clin Biomech. 2001;16(3):245–253. doi: 10.1016/s0268-0033(00)00089-9. [DOI] [PubMed] [Google Scholar]
- 34.Wren T.A., Lindsey D.P., Beaupré G.S., Carter D.R. Effects of creep and cyclic loading on the mechanical properties and failure of human Achilles tendons. Ann Biomed Eng. 2003;31:710–717. doi: 10.1114/1.1569267. [DOI] [PubMed] [Google Scholar]
- 35.Alfredson H., Lorentzon R. Chronic achilles tendinosis. Crit Rev Phys Rehabil Med. 2000;12(2) doi: 10.2165/00007256-200029020-00005. [DOI] [PubMed] [Google Scholar]
- 36.Wen D.Y. Risk factors for overuse injuries in runners. Curr Sports Med Rep. 2007;6(5):307–313. [PubMed] [Google Scholar]
- 37.Lorimer A.V., Hume P.A. Achilles tendon injury risk factors associated with running. Sports Med. 2014;44:1459–1472. doi: 10.1007/s40279-014-0209-3. [DOI] [PubMed] [Google Scholar]
- 38.Ahmetov I.I., Hall E.C., Semenova E.A., Pranckevičienė E., Ginevičienė V. Advances in Clinical Chemistry. Elsevier; 2022. Advances in sports genomics; pp. 215–263. [DOI] [PubMed] [Google Scholar]
- 39.Varillas-Delgado D., Del Coso J., Gutiérrez-Hellín J., Aguilar-Navarro M., Muñoz A., Maestro A., et al. Genetics and sports performance: the present and future in the identification of talent for sports based on DNA testing. Eur J Appl Physiol. 2022;122(8):1811–1830. doi: 10.1007/s00421-022-04945-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Youn B.-Y., Ko S.-G., Kim J.Y. Genetic basis of elite combat sports athletes: a systematic review. Biol Sport. 2021;38(4):667–675. doi: 10.5114/biolsport.2022.102864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kikuchi N., Moreland E., Homma H., Semenova E.A., Saito M., Larin A.K., et al. Genes and weightlifting performance. Genes. 2021;13(1):25. doi: 10.3390/genes13010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Widmann M., Nieß A.M., Munz B. Physical exercise and epigenetic modifications in skeletal muscle. Sports Med. 2019;49(4):509–523. doi: 10.1007/s40279-019-01070-4. [DOI] [PubMed] [Google Scholar]
- 43.Devos H., Zoidakis J., Roubelakis M.G., Latosinska A., Vlahou A. Reviewing the regulators of COL1A1. Int J Mol Sci. 2023;24(12) doi: 10.3390/ijms241210004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo R., Aizezi A., Fan Y., Ji Z., Li W., Li Y., et al. Association between matrix metalloproteinase-3 gene polymorphisms and tendon-ligament injuries: evidence from a meta-analysis. BMC Sports Sci Med Rehabil. 2022;14(1):26. doi: 10.1186/s13102-022-00421-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Teles Filho R.V., Abe GdM., Daher M.T. Genetic influence in disc degeneration-systematic review of literature. Rev Bras Ortop. 2020;55:131–138. doi: 10.1055/s-0039-1692626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scherbak S.G., Makarenko S.V., Shneider O.V., Kamilova T.A., Golota A.S. Regenerative rehabilitation in injuries of tendons. Phys Rehabil Med Med Rehabil. 2021;3(2):192–206. [Google Scholar]
- 47.Vosseller J.T., Ellis S.J., Levine D.S., Kennedy J.G., Elliott A.J., Deland J.T., et al. Achilles tendon rupture in women. Foot Ankle Int. 2013;34(1):49–53. doi: 10.1177/1071100712460223. [DOI] [PubMed] [Google Scholar]
- 48.Huttunen T.T., Kannus P., Rolf C., Felländer-Tsai L., Mattila V.M. Acute Achilles tendon ruptures: incidence of injury and surgery in Sweden between 2001 and 2012. Am J Sports Med. 2014;42(10):2419–2423. doi: 10.1177/0363546514540599. [DOI] [PubMed] [Google Scholar]
- 49.Scott A., Grewal N., Guy P. The seasonal variation of Achilles tendon ruptures in Vancouver, Canada: a retrospective study. BMJ Open. 2014;4(2) doi: 10.1136/bmjopen-2013-004320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sheth U., Wasserstein D., Jenkinson R., Moineddin R., Kreder H., Jaglal S., et al. The epidemiology and trends in management of acute Achilles tendon ruptures in Ontario, Canada: a population-based study of 27,607 patients. Bone Joint Lett J. 2017;99(1):78–86. doi: 10.1302/0301-620X.99B1.BJJ-2016-0434.R1. [DOI] [PubMed] [Google Scholar]
- 51.Fournier G., Bernard C., Cievet‐Bonfils M., Kenney R., Pingon M., Sappey‐Marinier E., et al. Sex differences in semitendinosus muscle fiber‐type composition. Scand J Med Sci Sports. 2022;32(4):720–727. doi: 10.1111/sms.14127. [DOI] [PubMed] [Google Scholar]
- 52.Handelsman D.J., Hirschberg A.L., Bermon S. Circulating testosterone as the hormonal basis of sex differences in athletic performance. Endocr Rev. 2018;39(5):803–829. doi: 10.1210/er.2018-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bassett A.J., Ahlmen A., Rosendorf J.M., Romeo A.A., Erickson B.J., Bishop M.E., et al. The biology of sex and sport. JBJS Rev. 2020;8(3) doi: 10.2106/JBJS.RVW.19.00140. [DOI] [PubMed] [Google Scholar]
- 54.Hoffman J., Gupta S., Amesur A., Anthony T., Winder R.P., Chan H., et al. Achilles tendon rip-stop SpeedBridge repair. Arthrosc Tech. 2021;10(9) doi: 10.1016/j.eats.2021.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nikolenko V., Oganesyan M., Vovkogon A., Sankova M., Rizaeva N. Morphological markers of the post-exercise structural and functional disorders of the locomotor apparatus. Hum Sport Med. 2019;19(3):103–111. [Google Scholar]
- 56.Nikolenko V., Oganesyan M., Vovkogon A., Cao Y., Churganova A., Zolotareva M., et al. Morphological signs of connective tissue dysplasia as predictors of frequent post-exercise musculoskeletal disorders. BMC Musculoskelet Disord. 2020;21:1–7. doi: 10.1186/s12891-020-03698-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kopp L., Kunc V. Acute and chronic Achilles tendon ruptures-current diagnostic and therapeutic options. Rozhl Chir. 2021;100(8):376–383. doi: 10.33699/PIS.2021.100.8.376-383. [DOI] [PubMed] [Google Scholar]
- 58.Thorpe C.T., Peffers M.J., Simpson D., Halliwell E., Screen H.R., Clegg P.D., et al. Anatomical heterogeneity of tendon: fascicular and interfascicular tendon compartments have distinct proteomic composition. Sci Rep. 2016;6(1) doi: 10.1038/srep20455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu T.-Y., Pang J.-H.S., Wu K.P.-H., Chen M.J., Chen C.-H., Tsai W.-C., et al. Aging is associated with increased activities of matrix metalloproteinase-2 and -9 in tenocytes. BMC Musculoskelet Disord. 2013;14:1–7. doi: 10.1186/1471-2474-14-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cury D.P., Dias F.J., Miglino M.A., Watanabe I.-S. Structural and ultrastructural characteristics of bone-tendon junction of the calcaneal tendon of adult and elderly Wistar rats. PLoS One. 2016;11(4) doi: 10.1371/journal.pone.0153568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Svensson R.B., Heinemeier K.M., Couppé C., Kjaer M., Magnusson S.P. Effect of aging and exercise on the tendon. J Appl Physiol. 2016;121(6):1353–1362. doi: 10.1152/japplphysiol.00328.2016. [DOI] [PubMed] [Google Scholar]
- 62.Slane L.C., DeWall R., Martin J., Lee K., Thelen D.G. Middle-aged adults exhibit altered spatial variations in Achilles tendon wave speed. Physiol Meas. 2015;36(7):1485. doi: 10.1088/0967-3334/36/7/1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Slane L.C., Martin J., DeWall R., Thelen D., Lee K. Quantitative ultrasound mapping of regional variations in shear wave speeds of the aging Achilles tendon. Eur Radiol. 2017;27:474–482. doi: 10.1007/s00330-016-4409-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Slane L.C., Thelen D.G. Achilles tendon displacement patterns during passive stretch and eccentric loading are altered in middle-aged adults. Med Eng Phys. 2015;37(7):712–716. doi: 10.1016/j.medengphy.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Claessen F.M., de Vos R.-J., Reijman M., Meuffels D.E. Predictors of primary Achilles tendon ruptures. Sports Med. 2014;44:1241–1259. doi: 10.1007/s40279-014-0200-z. [DOI] [PubMed] [Google Scholar]
- 66.Spink M.J., Fotoohabadi M.R., Wee E., Hill K.D., Lord S.R., Menz H.B., et al. Foot and ankle strength, range of motion, posture, and deformity are associated with balance and functional ability in older adults. Arch Phys Med Rehabil. 2011;92(1):68–75. doi: 10.1016/j.apmr.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 67.Menz H.B. Biomechanics of the ageing foot and ankle: a mini-review. Gerontology. 2015;61(4):381–388. doi: 10.1159/000368357. [DOI] [PubMed] [Google Scholar]
- 68.Winnicki K., Ochała-Kłos A., Rutowicz B., Pękala P.A., Tomaszewski K.A. Functional anatomy, histology and biomechanics of the human Achilles tendon—a comprehensive review. Ann Anat. 2020;229 doi: 10.1016/j.aanat.2020.151461. [DOI] [PubMed] [Google Scholar]
- 69.Kayce J. Gross anatomy: achilles tendon. Clin Podiatr Med Surg. 2022;39(3):405–410. doi: 10.1016/j.cpm.2022.03.003. [DOI] [PubMed] [Google Scholar]
- 70.Mahan J., Damodar D., Trapana E., Barnhill S., Nuno A.U., Smyth N.A., et al. Achilles tendon complex: the anatomy of its insertional footprint on the calcaneus and clinical implications. J Orthop. 2020;17:221–227. doi: 10.1016/j.jor.2019.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dederer K.M., Tennant J.N. Anatomical and functional considerations in Achilles tendon lesions. Foot Ankle Clin. 2019;24(3):371–385. doi: 10.1016/j.fcl.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 72.Pękala P., Henry B., Ochała A., Kopacz P., Tatoń G., Młyniec A., et al. The twisted structure of the Achilles tendon unraveled: a detailed quantitative and qualitative anatomical investigation. Scand J Med Sci Sports. 2017;27(12):1705–1715. doi: 10.1111/sms.12835. [DOI] [PubMed] [Google Scholar]
- 73.Dosal G.C., Schroeder J.D., Oh R.C. Low-volume hydrodissection for the treatment of chronic Achilles tendinopathy. Mil Med. 2023;188(9–10) doi: 10.1093/milmed/usac384. [DOI] [PubMed] [Google Scholar]
- 74.Risch L., Mayer F., Cassel M. Doppler flow response following running exercise differs between healthy and tendinopathic Achilles tendons. Front Physiol. 2021;12 doi: 10.3389/fphys.2021.650507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maffulli N., Via A.G., Oliva F. Chronic Achilles tendon disorders: tendinopathy and chronic rupture. Clin Sports Med. 2015;34(4):607–624. doi: 10.1016/j.csm.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 76.Bjur D., Alfredson H., Forsgren S. The innervation pattern of the human Achilles tendon: studies of the normal and tendinosis tendon with markers for general and sensory innervation. Cell Tissue Res. 2005;320:201–206. doi: 10.1007/s00441-004-1014-3. [DOI] [PubMed] [Google Scholar]
- 77.Maffulli N., Ajis A. Management of chronic ruptures of the Achilles tendon. JBJS. 2008;90(6):1348–1360. doi: 10.2106/JBJS.G.01241. [DOI] [PubMed] [Google Scholar]
- 78.Vasiliadis A.V., Katakalos K. The role of scaffolds in tendon tissue engineering. J Funct Biomater. 2020;11(4):78. doi: 10.3390/jfb11040078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Killian M.L. Growth and mechanobiology of the tendon-bone enthesis. Semin Cell Dev Biol. 2022:64–73. doi: 10.1016/j.semcdb.2021.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Eisner L.E., Rosario R., Andarawis-Puri N., Arruda E.M. The role of the non-collagenous extracellular matrix in tendon and ligament mechanical behavior: a review. J Biomech Eng. 2022;144(5) doi: 10.1115/1.4053086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thermann H. Achilles tendon rupture—Part 1: etiology and diagnostics. Chirurg. 2019;90:863–872. doi: 10.1007/s00104-019-01024-6. [DOI] [PubMed] [Google Scholar]
- 82.Diniz P., Quental C., Pereira H., Lopes R., Kerkhoffs G.M., Ferreira F.C., et al. Progression of partial to complete ruptures of the Achilles tendon during rehabilitation: a study using a finite element model. J Orthop Res. 2024 doi: 10.1002/jor.25827. [DOI] [PubMed] [Google Scholar]
- 83.Oliva F., Piccirilli E., Berardi A.C., Frizziero A., Tarantino U., Maffulli N., et al. Hormones and tendinopathies: the current evidence. Br Med Bull. 2016;117(1) doi: 10.1093/bmb/ldv054. [DOI] [PubMed] [Google Scholar]
- 84.Oliva F., Berardi A., Misiti S., Verga Falzacappa C., Iacone A., Maffulli N., et al. Thyroid hormones enhance growth and counteract apoptosis in human tenocytes isolated from rotator cuff tendons. Cell Death Dis. 2013;4(7) doi: 10.1038/cddis.2013.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Thompson J., Baravarian B. Acute and chronic Achilles tendon ruptures in athletes. Clin Podiatr Med Surg. 2011;28(1):117–135. doi: 10.1016/j.cpm.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 86.Zellers J.A., Carmont M.R., Silbernagel K.G. Return to play post-Achilles tendon rupture: a systematic review and meta-analysis of rate and measures of return to play. Br J Sports Med. 2016;50(21):1325–1332. doi: 10.1136/bjsports-2016-096106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kannus P., Natri A. Etiology and pathophysiology of tendon ruptures in sports. Scand J Med Sci Sports. 1997;7(2):107–112. doi: 10.1111/j.1600-0838.1997.tb00126.x. [DOI] [PubMed] [Google Scholar]
- 88.Mansfield K., Dopke K., Koroneos Z., Bonaddio V., Adeyemo A., Aynardi M., et al. Achilles tendon ruptures and repair in athletes—a review of sports-related Achilles injuries and return to play. Curr Rev Musculoskelet Med. 2022;15(5):353–361. doi: 10.1007/s12178-022-09774-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sankova M.V., Nikolenko V.N., Oganesyan M.V., Vovkogon A.D., Gadzhiakhmedova A.N., Zharikova T.S., et al. Identifying sex-specific injury predictors as a key factor in maintaining optimal physical activity levels. World J Orthoped. 2023;14(3):146. doi: 10.5312/wjo.v14.i3.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sankova M.V., Nikolenko V.N., Oganesyan M.V., Vovkogon A.D., Chirkova E.L., Sinelnikov M.Y., et al. Age pathognomonic indicators of injury predisposition as a basis for public health preservation during physical activity. Int J Environ Res Publ Health. 2021;18(4):1989. doi: 10.3390/ijerph18041989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rampal V., Giuliano F. Forefoot malformations, deformities and other congenital defects in children. Orthop Traumatol Surg Res. 2020;106(1) doi: 10.1016/j.otsr.2019.03.021. [DOI] [PubMed] [Google Scholar]
- 92.Mohan R., Dhotare S.V., Morgan S.S. Hallux varus: a literature review. Foot. 2021;49 doi: 10.1016/j.foot.2021.101863. [DOI] [PubMed] [Google Scholar]
- 93.Tileston K., Baskar D., Frick S.L. What is new in pediatric orthopaedic foot and ankle. J Pediatr Orthop. 2022;42(5) doi: 10.1097/BPO.0000000000002134. [DOI] [PubMed] [Google Scholar]
- 94.Buchhorn T., Polzer H. Arthrose des Sprunggelenks. Unfallchirurg. 2022;125(3):173–174. doi: 10.1007/s00113-021-01132-1. [DOI] [PubMed] [Google Scholar]
- 95.Goldberg A.J., Chowdhury K., Bordea E., Hauptmannova I., Blackstone J., Brooking D., et al. Total ankle replacement versus arthrodesis for end-stage ankle osteoarthritis: a randomized controlled trial. Ann Intern Med. 2022;175(12):1648–1657. doi: 10.7326/M22-2058. [DOI] [PubMed] [Google Scholar]
- 96.Touzell A. The Achilles tendon: management of acute and chronic conditions. Aust J Gen Pract. 2020;49(11):715–719. doi: 10.31128/AJGP-07-20-5506. [DOI] [PubMed] [Google Scholar]
- 97.Ingber D.E. From mechanobiology to developmentally inspired engineering. Philos Trans R Soc Lond B Biol Sci. 2018;373(1759) doi: 10.1098/rstb.2017.0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Master P.B.Z., Macedo R.C.O. Effects of dietary supplementation in sport and exercise: a review of evidence on milk proteins and amino acids. Crit Rev Food Sci Nutr. 2021;61(7):1225–1239. doi: 10.1080/10408398.2020.1756216. [DOI] [PubMed] [Google Scholar]
- 99.Stone M. 2022. Exercise intensity influences plasma and sweat amino acids. [DOI] [PubMed] [Google Scholar]
- 100.Malsagova K.A., Kopylov A.T., Sinitsyna A.A., Stepanov A.A., Izotov A.A., Butkova T.V., et al. Sports nutrition: Diets, selection factors, recommendations. Nutrients. 2021;13(11):3771. doi: 10.3390/nu13113771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kytko O.V., Dydykina I.S., Sankova M.V., Kryuchko P.V., Chilikov V. Pathogenetic aspects of magnesium deficiency in connective tissue dysplasia syndrome. Vopr Pitan. 2020;89(5):35–43. doi: 10.24411/0042-8833-2020-10064. [DOI] [PubMed] [Google Scholar]
- 102.Prudêncio D.A., Maffulli N., Migliorini F., Serafim T.T., Nunes L.F., Sanada L.S., et al. Eccentric exercise is more effective than other exercises in the treatment of mid-portion Achilles tendinopathy: systematic review and meta-analysis. BMC Sports Sci Med Rehabil. 2023;15(1):9. doi: 10.1186/s13102-023-00618-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Paantjens M., Helmhout P., Backx F., Bakker E. Victorian Institute of Sport Assessment—Achilles thresholds for minimal important change and return to presymptom activity level in active soldiers with mid-portion Achilles tendinopathy. BMJ Mil Health. 2023 doi: 10.1136/military-2022-002326. [DOI] [PubMed] [Google Scholar]
- 104.Kim M., Lin C.-I., Henschke J., Quarmby A., Engel T., Cassel M., et al. Effects of exercise treatment on functional outcome parameters in mid-portion Achilles tendinopathy: a systematic review. Front Sports Act Living. 2023;5 doi: 10.3389/fspor.2023.1144484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Persson R., Jick S. Clinical implications of the association between fluoroquinolones and tendon rupture: the magnitude of the effect with and without corticosteroids. Br J Clin Pharmacol. 2019;85(5):949–959. doi: 10.1111/bcp.13879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Arslan İ., Yücel I., Öztürk T.B., Karahan N., Orak M.M., Midi A., et al. The effects of corticosteroid injection in the healthy and damaged Achilles tendon model: Histopathological and biomechanical experimental study in rats. Turk J Pathol. 2020;36(1):39. doi: 10.5146/tjpath.2019.01468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kafa B., Cakmak G., Sahin M.S., Helvacioglu F., Fidan P.A., Demir T., et al. Histological and biomechanical effects of local anesthetics and steroids on Achilles tendon: a study in rats. Am J Sports Med. 2023;51(5):1319–1327. doi: 10.1177/03635465231153640. [DOI] [PubMed] [Google Scholar]
- 108.Via A.G., Oliva F., Padulo J., Oliva G., Maffulli N. Insertional calcific tendinopathy of the Achilles tendon and dysmetabolic diseases: an epidemiological survey. Clin J Sport Med. 2022;32(1) doi: 10.1097/JSM.0000000000000881. [DOI] [PubMed] [Google Scholar]
- 109.Ackermann P.W., Hart D.A. Metabolic Influences on Risk for Tendon Disorders. 2016. General overview and summary of concepts regarding tendon disease topics addressed related to metabolic disorders; pp. 293–298. [DOI] [PubMed] [Google Scholar]
- 110.Oliva F., Marsilio E., Asparago G., Giai Via A., Biz C., Padulo J., et al. Achilles tendon rupture and dysmetabolic diseases: a multicentric, epidemiologic study. J Clin Med. 2022;11(13):3698. doi: 10.3390/jcm11133698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Macchi M., Spezia M., Elli S., Schiaffini G., Chisari E. Obesity increases the risk of tendinopathy, tendon tear and rupture, and postoperative complications: a systematic review of clinical studies. Clin Orthop Relat Res. 2020;478(8):1839–1847. doi: 10.1097/CORR.0000000000001261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Khor B.Y.C., Woodburn J., Newcombe L., Barn R. Plantar soft tissues and Achilles tendon thickness and stiffness in people with diabetes: a systematic review. J Foot Ankle Res. 2021;14(1):35. doi: 10.1186/s13047-021-00475-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dixit R., Singh S., Garg S. Sonoelastographic evaluation of the Achilles tendon in patients with type 2 diabetes mellitus. Ultrasound Med Biol. 2020;46(11):2989–2997. doi: 10.1016/j.ultrasmedbio.2020.07.023. [DOI] [PubMed] [Google Scholar]
- 114.Afolabi B.I., Idowu B.M., Onigbinde S.O. Achilles tendon degeneration on ultrasound in type 2 diabetic patients. J Ultrason. 2021;20(83):291–299. doi: 10.15557/JoU.2020.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Giha H.A., Sater M.S., Alamin O.A. Diabetes mellitus tendino-myopathy: epidemiology, clinical features, diagnosis and management of an overlooked diabetic complication. Acta Diabetol. 2022;59(7):871–883. doi: 10.1007/s00592-022-01860-9. [DOI] [PubMed] [Google Scholar]
- 116.Cramer A., Jacobsen N.C., Hansen M.S., Sandholdt H., Hölmich P., Barfod K.W., et al. Diabetes and treatment with orally administrated corticosteroids negatively affect treatment outcome at follow‐up after acute Achilles tendon rupture. Knee Surg Sports Traumatol Arthrosc. 2021;29(5):1584–1592. doi: 10.1007/s00167-020-06371-0. [DOI] [PubMed] [Google Scholar]
- 117.Bhardwaj N.K., Thakur P., Grover N., Gupta H., Kaur P. Limited joint mobility in type I diabetes mellitus. Indian J Pediatr. 2021;88:415–416. doi: 10.1007/s12098-020-03364-2. [DOI] [PubMed] [Google Scholar]
- 118.Nichols A.E., Oh I., Loiselle A.E. Effects of type II diabetes mellitus on tendon homeostasis and healing. J Orthop Res. 2020;38(1):13–22. doi: 10.1002/jor.24388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ren B., Li B., Pan T., Zhao E., Ju S., Li X., et al. Risk factors for at-risk foot and peripheral artery disease among the population with diabetes: a multicommunity-based cross-sectional study. Diabetes Res Clin Pract. 2023;203 doi: 10.1016/j.diabres.2023.110869. [DOI] [PubMed] [Google Scholar]
- 120.Yamashita S., Masuda D., Harada-Shiba M., Arai H., Bujo H., Ishibashi S., et al. Effectiveness and safety of lipid-lowering drug treatments in Japanese patients with familial hypercholesterolemia: familial Hypercholesterolemia Expert Forum (FAME) study. J Atheroscler Thromb. 2022;29(5):608–638. doi: 10.5551/jat.62764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Murugan A., Kanakaraju K., Sidesh R., Mishra V.S., Sidhesh R. Achilles tendon softness and thickness in patients with hypercholesterolemia. Cureus. 2022;14(8) doi: 10.7759/cureus.28340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dakin S.G., Newton J., Martinez F.O., Hedley R., Gwilym S., Jones N., et al. Chronic inflammation is a feature of Achilles tendinopathy and rupture. Br J Sports Med. 2018;52(6):359–367. doi: 10.1136/bjsports-2017-098161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yang Y.-P., Tao L.-Y., Gao J.-N., Wang P., Jiang Y.-F., Zheng L.-M., et al. Elevated lipid levels in patients with Achilles tendon ruptures: a retrospective matching study. Ann Transl Med. 2020;8(5) doi: 10.21037/atm.2020.01.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liang J., Chen B., Li Y., Nie D., Lei C., Yang Q., et al. Asymptomatic hyperuricemia is associated with Achilles tendon rupture through disrupting the normal functions of tendon stem/progenitor cells. Stem Cells Int. 2022;2022 doi: 10.1155/2022/6795573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang Y., Deng X.-R., Ji L.-L., Zhang X.-H., Geng Y., Zhang Z.-L., et al. Risk factors and diagnostic value for ultrasound-detected tendon monosodium urate crystal deposition in patients with gout. J Peking Univ Health Sci. 2020;53(1):143–149. doi: 10.19723/j.issn.1671-167X.2021.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mülkoğlu C., Nacır B., Genç H. A rare presentation of gout: achilles tendinopathy, ultrasonographic assessment. Am J Med Sci. 2020;359(4):245–246. doi: 10.1016/j.amjms.2020.01.016. [DOI] [PubMed] [Google Scholar]
- 127.Peng Z., Ding Y., Pei L., Yao H., Zhang X.-W., Tang S., et al. Clinical characteristics of crystal deposits in joints and tendons in patients with gout. J Peking Univ Health Sci. 2021;53(6):1067–1071. doi: 10.19723/j.issn.1671-167X.2021.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dalbeth N., Gosling A.L., Gaffo A., Abhishek A. Gout. Lancet. 2021;397(10287):1843–1855. doi: 10.1016/S0140-6736(21)00569-9. [DOI] [PubMed] [Google Scholar]
- 129.Keysser G. Gout arthritis: pathogenesis, diagnostics and treatment. Dtsch Med Wochenschr. 2020;145(14):991–1005. doi: 10.1055/a-1036-8348. [DOI] [PubMed] [Google Scholar]
- 130.Otter S., Payne C., Jones A.-M., Webborn N., Watt P. Differences in Achilles tendon stiffness in people with gout: a pilot study. BMC Musculoskelet Disord. 2020;21:1–9. doi: 10.1186/s12891-020-03598-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Berardi A.C., Oliva F., Berardocco M., La Rovere M., Accorsi P., Maffulli N., et al. Thyroid hormones increase collagen I and cartilage oligomeric matrix protein (COMP) expression in vitro human tenocytes. Muscles Ligaments Tendons J. 2014;4(3):285. [PMC free article] [PubMed] [Google Scholar]
- 132.Baca E., Kural A., Ziroğlu N., Kural C. Alkaptonuria: spontaneous Achilles tendon rupture: case report. Joint Dis Relat Surg. 2019;30(3):325–328. doi: 10.5606/ehc.2019.66155. [DOI] [PubMed] [Google Scholar]
- 133.Zatkova A., Ranganath L., Kadasi L. Alkaptonuria: current perspectives. Appl Clin Genet. 2020:37–47. doi: 10.2147/TACG.S186773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Davison A.S., Norman B.P. Alkaptonuria—Past, present and future. Adv Clin Chem. 2023;114:47–81. doi: 10.1016/bs.acc.2023.02.005. [DOI] [PubMed] [Google Scholar]
- 135.Yucetas S.C., Ucler N. Black-colored ligamentum flavum due to alkaptonuria. J Neurol Surg Cent Eur Neurosurg. 2019;80(2):131–133. doi: 10.1055/s-0038-1675784. [DOI] [PubMed] [Google Scholar]
- 136.Jiang L., Cao L., Fang J., Yu X., Dai X., Miao X., et al. Ochronotic arthritis and ochronotic Achilles tendon rupture in alkaptonuria: a 6 years follow-up case report in China. Medicine. 2019;98(34) doi: 10.1097/MD.0000000000016837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wu K., Bauer E., Myung G., Fang M.A. Musculoskeletal manifestations of alkaptonuria: a case report and literature review. Eur J Rheumatol. 2019;6(2):98. doi: 10.5152/eurjrheum.2018.18116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bolon B. Mini-review: Toxic tendinopathy. Toxicol Pathol. 2017;45(7):834–837. doi: 10.1177/0192623317711614. [DOI] [PubMed] [Google Scholar]
- 139.Kirchgesner T., Larbi A., Omoumi P., Malghem J., Zamali N., Manelfe J., et al. Drug-induced tendinopathy: from physiology to clinical applications. Joint Bone Spine. 2014;81(6):485–492. doi: 10.1016/j.jbspin.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 140.Goswami R.P., Ghosh P. Drug-induced musculoskeletal syndromes and soft-tissue rheumatism. Indian J Rheumatol. 2019;14(Suppl 1) [Google Scholar]
- 141.Bisaccia D.R., Aicale R., Tarantino D., Peretti G.M., Maffulli N. Biological and chemical changes in fluoroquinolone-associated tendinopathies: a systematic review. Br Med Bull. 2019;130(1):39–49. doi: 10.1093/bmb/ldz006. [DOI] [PubMed] [Google Scholar]
- 142.Alves C., Mendes D., Marques F.B. Fluoroquinolones and the risk of tendon injury: a systematic review and meta-analysis. Eur J Clin Pharmacol. 2019;75:1431–1443. doi: 10.1007/s00228-019-02713-1. [DOI] [PubMed] [Google Scholar]
- 143.Morales D.R., Slattery J., Pacurariu A., Pinheiro L., McGettigan P., Kurz X., et al. Relative and absolute risk of tendon rupture with fluoroquinolone and concomitant fluoroquinolone/corticosteroid therapy: population-based nested case–control study. Clin Drug Invest. 2019;39:205–213. doi: 10.1007/s40261-018-0729-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Shu Y., Zhang Q., He X., Liu Y., Wu P., Chen L., et al. Fluoroquinolone-associated suspected tendonitis and tendon rupture: a pharmacovigilance analysis from 2016 to 2021 based on the FAERS database. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.990241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cohen P.R. Cephalexin-associated Achilles tendonitis: case report and review of drug-induced tendinopathy. Cureus. 2018;10(12) doi: 10.7759/cureus.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Fox A.J., Schär M.O., Wanivenhaus F., Chen T., Attia E., Binder N.B., et al. Fluoroquinolones impair tendon healing in a rat rotator cuff repair model: a preliminary study. Am J Sports Med. 2014;42(12):2851–2859. doi: 10.1177/0363546514545858. [DOI] [PubMed] [Google Scholar]
- 147.Nyyssönen T., Lantto I., Lüthje P., Selander T., Kröger H. Drug treatments associated with Achilles tendon rupture. A case‐control study involving 1118 Achilles tendon ruptures. Scand J Med Sci Sports. 2018;28(12):2625–2629. doi: 10.1111/sms.13281. [DOI] [PubMed] [Google Scholar]
- 148.Baik S., Lau J., Huser V., McDonald C.J. Association between tendon ruptures and use of fluoroquinolone, and other oral antibiotics: a 10-year retrospective study of 1 million US senior Medicare beneficiaries. BMJ Open. 2020;10(12) doi: 10.1136/bmjopen-2019-034844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tran P.T., Antonelli P.J., Winterstein A.G. Quinolone ear drops and Achilles tendon rupture. Clin Infect Dis. 2023;76(3) doi: 10.1093/cid/ciac709. [DOI] [PubMed] [Google Scholar]
- 150.Deren M.E., Klinge S.A., Mukand N.H., Mukand J.A. Tendinopathy and tendon rupture associated with statins. JBJS Rev. 2016;4(5) doi: 10.2106/JBJS.RVW.15.00072. [DOI] [PubMed] [Google Scholar]
- 151.Spoendlin J., Meier C., Jick S.S., Meier C.R. Bisphosphonate therapy start may transiently increase the risk of tendon rupture in patients with glucocorticoid co‐medication: a population‐based observational study. Pharmacoepidemiol Drug Saf. 2016;25(10):1116–1123. doi: 10.1002/pds.4042. [DOI] [PubMed] [Google Scholar]
- 152.Mitsimponas N., Klouva E., Tryfonopoulos D., Grivas A., Demiri S., Koumakis G., et al. Aromatase inhibitor-associated tendinopathy and muscle tendon rupture: report of three cases of this exceedingly rare adverse event. Case Rep Oncol. 2018;11(2):557–561. doi: 10.1159/000491874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jones I.A., Togashi R., Hatch G.F.R., Weber A.E., Vangsness C.T., Jr. Anabolic steroids and tendons: a review of their mechanical, structural, and biologic effects. J Orthop Res. 2018;36(11):2830–2841. doi: 10.1002/jor.24116. [DOI] [PubMed] [Google Scholar]
- 154.Beytemür O., Yüksel S., Tetikkurt Ü.S., Genç E., Olcay E., Güleç A., et al. Isotretinoin-induced Achilles tendinopathy: histopathological and biomechanical evaluation on rats. Acta Orthop Traumatol Turcica. 2018;52(5):387–391. doi: 10.1016/j.aott.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Dombrowski M., Murawski C.D., Yasui Y., Chen A.F., Ewalefo S.O., Fourman M.S., et al. Medical comorbidities increase the rate of surgical site infection in primary Achilles tendon repair. Knee Surg Sports Traumatol Arthrosc. 2019;27(9):2840–2851. doi: 10.1007/s00167-018-5295-6. [DOI] [PubMed] [Google Scholar]
- 156.Pean C.A., Christiano A., Rubenstein W.J., Konda S.R., Egol K.A. Risk factors for complications after primary repair of Achilles tendon ruptures. J Orthop. 2018;15(1):226–229. doi: 10.1016/j.jor.2018.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Egger A.C., Berkowitz M.J. Achilles tendon injuries. Curr Rev Musculoskelet Med. 2017;10:72–80. doi: 10.1007/s12178-017-9386-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Pajala A., Kangas J., Ohtonen P., Leppilahti J. Rerupture and deep infection following treatment of total Achilles tendon rupture. JBJS. 2002;84(11):2016–2021. doi: 10.2106/00004623-200211000-00017. [DOI] [PubMed] [Google Scholar]
- 159.Guo C., Zhang Y., Dong W., Huang B., Liu Y. Risk factors and clinical characteristics of surgical site infections in athletes undergoing Achilles tendon repair surgery. Int Wound J. 2024;21(3) doi: 10.1111/iwj.14666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Davis J.J., Mason K.T., Clark D.A. Achilles tendon ruptures stratified by age, race, and cause of injury among active duty US military members. Mil Med. 1999;164(12):872–873. [PubMed] [Google Scholar]
- 161.Suchak A.A., Bostick G., Reid D., Blitz S., Jomha N. The incidence of Achilles tendon ruptures in Edmonton, Canada. Foot Ankle Int. 2005;26(11):932–936. doi: 10.1177/107110070502601106. [DOI] [PubMed] [Google Scholar]
- 162.Gwynne-Jones D.P., Sims M. Epidemiology and outcomes of acute Achilles tendon rupture with operative or nonoperative treatment using an identical functional bracing protocol. Foot Ankle Int. 2011;32(4):337–343. doi: 10.3113/FAI.2011.0337. [DOI] [PubMed] [Google Scholar]
- 163.Flood L., Harrison J.E. Epidemiology of basketball and netball injuries that resulted in hospital admission in Australia, 2000–2004. Med J Aust. 2009;190(2):87–90. doi: 10.5694/j.1326-5377.2009.tb02285.x. [DOI] [PubMed] [Google Scholar]
- 164.Noback P.C., Jang E.S., Cuellar D.O., Seetharaman M., Malagoli E., Greisberg J.K., et al. Risk factors for Achilles tendon rupture: a matched case-control study. Injury. 2017;48(10):2342–2347. doi: 10.1016/j.injury.2017.08.050. [DOI] [PubMed] [Google Scholar]
- 165.Park K.H., Park J.H., Yoon Y.K., Kwon J.B., Kim J.H., Lee E., et al. Association between outdoor temperature and Achilles tendon repair: a 14-years nationwide population-based cohort study. PLoS One. 2022;17(3) doi: 10.1371/journal.pone.0265041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tobías A., Casals M., Saez M., Kamada M., Kim Y. Impacts of ambient temperature and seasonal changes on sports injuries in Madrid, Spain: a time-series regression analysis. BMJ Open Sport Exerc Med. 2021;7(4) doi: 10.1136/bmjsem-2021-001205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Togo F., Watanabe E., Park H., Shephard R.J., Aoyagi Y. Meteorology and the physical activity of the elderly: the Nakanojo Study. Int J Biometeorol. 2005;50:83–89. doi: 10.1007/s00484-005-0277-z. [DOI] [PubMed] [Google Scholar]
- 168.Chan C.B., Ryan D.A., Tudor-Locke C. Relationship between objective measures of physical activity and weather: a longitudinal study. Int J Behav Nutr Phys Act. 2006;3:1–9. doi: 10.1186/1479-5868-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Carson V., Spence J.C., Cutumisu N., Boule N., Edwards J. Seasonal variation in physical activity among preschool children in a northern Canadian city. Res Q Exerc Sport. 2010;81(4):392–399. doi: 10.1080/02701367.2010.10599699. [DOI] [PubMed] [Google Scholar]
- 170.Harrison F., Goodman A., van Sluijs E.M., Andersen L.B., Cardon G., Davey R., et al. Weather and children's physical activity; how and why do relationships vary between countries? Int J Behav Nutr Phys Act. 2017;14:1–13. doi: 10.1186/s12966-017-0526-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Beghin L., Vanhelst J., Drumez E., Migueles J., Manios Y., Moreno L.A., et al. Influence of meteorological conditions on physical activity in adolescents. J Epidemiol Community Health. 2020;74(4):395–400. doi: 10.1136/jech-2019-212459. [DOI] [PubMed] [Google Scholar]
- 172.Dannenberg A.L., Keller J.B., Wilson P.W., Castelli W.P. Leisure time physical activity in the Framingham Offspring Study: description, seasonal variation, and risk factor correlates. Am J Epidemiol. 1989;129(1):76–88. doi: 10.1093/oxfordjournals.aje.a115126. [DOI] [PubMed] [Google Scholar]
- 173.Bergstralh E.J., Offord K.P., Sinaki M., Wahner H.W., Melton L.J. Effect of season on physical activity score, back extensor muscle strength, and lumbar bone mineral density. J Bone Miner Res. 1990;5(4):371–377. doi: 10.1002/jbmr.5650050410. [DOI] [PubMed] [Google Scholar]
- 174.Levin S., Jacobs D.R., Jr., Ainsworth B.E., Richardson M.T., Leon A.S. Intra-individual variation and estimates of usual physical activity. Ann Epidemiol. 1999;9(8):481–488. doi: 10.1016/s1047-2797(99)00022-8. [DOI] [PubMed] [Google Scholar]
- 175.Plasqui G., Westerterp K.R. Seasonal variation in total energy expenditure and physical activity in Dutch young adults. Obes Res. 2004;12(4):688–694. doi: 10.1038/oby.2004.80. [DOI] [PubMed] [Google Scholar]
- 176.Westerterp K.R., Plasqui G., Goris A.H. Water loss as a function of energy intake, physical activity and season. Br J Nutr. 2005;93(2):199–203. doi: 10.1079/bjn20041310. [DOI] [PubMed] [Google Scholar]
- 177.Tucker P., Gilliland J. The effect of season and weather on physical activity: a systematic review. Publ Health. 2007;121(12):909–922. doi: 10.1016/j.puhe.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 178.Matthews C.E., Freedson P.S., Hebert J.R., Stanek E.J., III, Merriam P.A., Rosal M.C., et al. Seasonal variation in household, occupational, and leisure time physical activity: longitudinal analyses from the seasonal variation of blood cholesterol study. Am J Epidemiol. 2001;153(2):172–183. doi: 10.1093/aje/153.2.172. [DOI] [PubMed] [Google Scholar]
- 179.Chan C.B., Ryan D.A. Assessing the effects of weather conditions on physical activity participation using objective measures. Int J Environ Res Publ Health. 2009;6(10):2639–2654. doi: 10.3390/ijerph6102639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Baranowski T., Thompson W.O., Durant R.H., Baranowski J., Puhl J. Observations on physical activity in physical locations: age, gender, ethnicity, and month effects. Res Q Exerc Sport. 1993;64(2):127–133. doi: 10.1080/02701367.1993.10608789. [DOI] [PubMed] [Google Scholar]
- 181.Im Kampe E.O., Kovats S., Hajat S. Impact of high ambient temperature on unintentional injuries in high-income countries: a narrative systematic literature review. BMJ Open. 2016;6(2) doi: 10.1136/bmjopen-2015-010399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Spector J.T., Masuda Y.J., Wolff N.H., Calkins M., Seixas N. Heat exposure and occupational injuries: review of the literature and implications. Curr Environ Health Rep. 2019;6:286–296. doi: 10.1007/s40572-019-00250-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kraft J.A., Green J.M., Bishop P.A., Richardson M.T., Neggers Y.H., Leeper J.D., et al. The influence of hydration on anaerobic performance: a review. Res Q Exerc Sport. 2012;83(2):282–292. doi: 10.1080/02701367.2012.10599859. [DOI] [PubMed] [Google Scholar]
- 184.Ackermann P.W. Neuronal regulation of tendon homeostasis. Int J Exp Pathol. 2013;94(4):271–286. doi: 10.1111/iep.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Killian M.L., Cavinatto L., Galatz L.M., Thomopoulos S. The role of mechanobiology in tendon healing. J Shoulder Elbow Surg. 2012;21(2):228–237. doi: 10.1016/j.jse.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Guzzoni V., Selistre-de-Araújo H.S., de Cássia Marqueti R. Tendon remodeling in response to resistance training, anabolic androgenic steroids and aging. Cells. 2018;7(12):251. doi: 10.3390/cells7120251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Magnusson S.P., Kjaer M. The impact of loading, unloading, ageing and injury on the human tendon. J Physiol. 2019;597(5):1283–1298. doi: 10.1113/JP275450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Magnusson S.P., Langberg H., Kjaer M. The pathogenesis of tendinopathy: balancing the response to loading. Nat Rev Rheumatol. 2010;6(5):262–268. doi: 10.1038/nrrheum.2010.43. [DOI] [PubMed] [Google Scholar]
- 189.Ramires L.C., Jeyaraman M., Muthu S., Shankar A.N., Santos G.S., da Fonseca L.F., et al. Application of orthobiologics in Achilles tendinopathy: a review. Life. 2022;12(3):399. doi: 10.3390/life12030399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hou Y., Mao Z., Wei X., Lin L., Chen L., Wang H., et al. Effects of transforming growth factor-β1 and vascular endothelial growth factor 165 gene transfer on Achilles tendon healing. Matrix Biol. 2009;28(6):324–335. doi: 10.1016/j.matbio.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 191.Chen C.-H., Lin Y.-H., Chen C.-H., Wang Y.-H., Yeh M.-L., Cheng T.-L., et al. Transforming growth factor beta 1 mediates the low-frequency vertical vibration enhanced production of tenomodulin and type I collagen in rat Achilles tendon. PLoS One. 2018;13(10) doi: 10.1371/journal.pone.0205258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Cui J., Chen Z., Wu W. Expression of TGF-β1 and VEGF in patients with Achilles tendon rupture and the clinical efficacy. Exp Ther Med. 2019;18(5):3502–3508. doi: 10.3892/etm.2019.7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Xie Y., Su N., Yang J., Tan Q., Huang S., Jin M., et al. FGF/FGFR signaling in health and disease. Signal Transduct Targeted Ther. 2020;5(1):181. doi: 10.1038/s41392-020-00222-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Çelik M., Bayrak A., Duramaz A., Başaran S.H., Kızılkaya C., Kural C., et al. The effect of fibrin clot and C vitamin on the surgical treatment of Achilles tendon injury in the rat model. Foot Ankle Surg. 2021;27(6):681–687. doi: 10.1016/j.fas.2020.09.006. [DOI] [PubMed] [Google Scholar]
- 195.Zhang J., Li F., Augi T., Williamson K.M., Onishi K., Hogan M.V., et al. Platelet HMGB1 in platelet-rich plasma (PRP) promotes tendon wound healing. PLoS One. 2021;16(9) doi: 10.1371/journal.pone.0251166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Padilla S., Sánchez M., Vaquerizo V., Malanga G.A., Fiz N., Azofra J., et al. Platelet-rich plasma applications for Achilles tendon repair: a bridge between biology and surgery. Int J Mol Sci. 2021;22(2):824. doi: 10.3390/ijms22020824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Boesen A.P., Hansen R., Boesen M.I., Malliaras P., Langberg H. Effect of high-volume injection, platelet-rich plasma, and sham treatment in chronic midportion Achilles tendinopathy: a randomized double-blinded prospective study. Am J Sports Med. 2017;45(9):2034–2043. doi: 10.1177/0363546517702862. [DOI] [PubMed] [Google Scholar]
- 198.Kearney R.S., Ji C., Warwick J., Parsons N., Brown J., Harrison P., et al. Effect of platelet-rich plasma injection vs sham injection on tendon dysfunction in patients with chronic midportion Achilles tendinopathy: a randomized clinical trial. JAMA. 2021;326(2):137–144. doi: 10.1001/jama.2021.6986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kirschner J.S., Cheng J., Hurwitz N., Santiago K., Lin E., Beatty N., et al. Ultrasound‐guided percutaneous needle tenotomy (PNT) alone versus PNT plus platelet‐rich plasma injection for the treatment of chronic tendinosis: a randomized controlled trial. PM&R. 2021;13(12):1340–1349. doi: 10.1002/pmrj.12583. [DOI] [PubMed] [Google Scholar]
- 200.Laurent D., Walsh L., Muaremi A., Beckmann N., Weber E., Chaperon F., et al. Relationship between tendon structure, stiffness, gait patterns and patient reported outcomes during the early stages of recovery after an Achilles tendon rupture. Sci Rep. 2020;10(1) doi: 10.1038/s41598-020-77691-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Clayton R.A., Court-Brown C.M. The epidemiology of musculoskeletal tendinous and ligamentous injuries. Injury. 2008;39(12):1338–1344. doi: 10.1016/j.injury.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 202.Maffulli N., Longo U.G., Maffulli G.D., Khanna A., Denaro V. Achilles tendon ruptures in elite athletes. Foot Ankle Int. 2011;32(1):9–15. doi: 10.3113/FAI.2011.0009. [DOI] [PubMed] [Google Scholar]
- 203.Motta P., Errichiello C., Pontini I. Achilles tendon rupture: a new technique for easy surgical repair and immediate movement of the ankle and foot. Am J Sports Med. 1997;25(2):172–176. doi: 10.1177/036354659702500205. [DOI] [PubMed] [Google Scholar]
- 204.Deng S., Sun Z., Zhang C., Chen G., Li J. Surgical treatment versus conservative management for acute Achilles tendon rupture: a systematic review and meta-analysis of randomized controlled trials. J Foot Ankle Surg. 2017;56(6):1236–1243. doi: 10.1053/j.jfas.2017.05.036. [DOI] [PubMed] [Google Scholar]
- 205.Reda Y., Farouk A., Abdelmonem I., El Shazly O.A. Surgical versus non-surgical treatment for acute Achilles' tendon rupture: a systematic review of literature and meta-analysis. Foot Ankle Surg. 2020;26(3):280–288. doi: 10.1016/j.fas.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 206.Seow D., Yasui Y., Calder J.D., Kennedy J.G., Pearce C.J. Treatment of acute Achilles tendon ruptures: a systematic review and meta-analysis of complication rates with best-and worst-case analyses for rerupture rates. Am J Sports Med. 2021;49(13):3728–3748. doi: 10.1177/0363546521998284. [DOI] [PubMed] [Google Scholar]
- 207.Wu Y., Lin L., Li H., Zhao Y., Liu L., Jia Z., et al. Is surgical intervention more effective than non-surgical treatment for acute Achilles tendon rupture? A systematic review of overlapping meta-analyses. Int J Surg. 2016;36:305–311. doi: 10.1016/j.ijsu.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 208.Zhang H., Tang H., He Q., Wei Q., Tong D., Wang C., et al. Surgical versus conservative intervention for acute Achilles tendon rupture: a PRISMA-compliant systematic review of overlapping meta-analyses. Medicine. 2015;94(45) doi: 10.1097/MD.0000000000001951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ochen Y., Beks R.B., van Heijl M., Hietbrink F., Leenen L.P., van der Velde D., et al. Operative treatment versus nonoperative treatment of Achilles tendon ruptures: systematic review and meta-analysis. BMJ. 2019;364 doi: 10.1136/bmj.k5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Brumann M., Baumbach S.F., Mutschler W., Polzer H. Accelerated rehabilitation following Achilles tendon repair after acute rupture–development of an evidence-based treatment protocol. Injury. 2014;45(11):1782–1790. doi: 10.1016/j.injury.2014.06.022. [DOI] [PubMed] [Google Scholar]
- 211.De la Fuente C., Lillo R.P., Carreño G., Marambio H. Prospective randomized clinical trial of aggressive rehabilitation after acute Achilles tendon ruptures repaired with Dresden technique. Foot. 2016;26:15–22. doi: 10.1016/j.foot.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 212.Barfod K.W., Bencke J., Lauridsen H.B., Ban I., Ebskov L., Troelsen A., et al. Nonoperative dynamic treatment of acute Achilles tendon rupture: the influence of early weight-bearing on clinical outcome: a blinded, randomized controlled trial. JBJS. 2014;96(18):1497–1503. doi: 10.2106/JBJS.M.01273. [DOI] [PubMed] [Google Scholar]
- 213.Peterson J.G., Tjong V.K., Mehta M.P., Goyette B.N., Patel M., Kadakia A.R., et al. A qualitative assessment of return to sport following Achilles tendon repair. J Orthop. 2021;23:46–51. doi: 10.1016/j.jor.2020.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Burland J.P., Toonstra J., Werner J.L., Mattacola C.G., Howell D.M., Howard J.S., et al. Decision to return to sport after anterior cruciate ligament reconstruction, part I: a qualitative investigation of psychosocial factors. J Athl Train. 2018;53(5):452–463. doi: 10.4085/1062-6050-313-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Tjong V.K., Murnaghan M.L., Nyhof-Young J.M., Ogilvie-Harris D.J. A qualitative investigation of the decision to return to sport after anterior cruciate ligament reconstruction: to play or not to play. Am J Sports Med. 2014;42(2):336–342. doi: 10.1177/0363546513508762. [DOI] [PubMed] [Google Scholar]
- 216.Tjong V.K., Cogan C.J., Riederman B.D., Terry M.A. A qualitative assessment of return to sport after hip arthroscopy for femoroacetabular impingement. Orthop J Sports Med. 2016;4(11) doi: 10.1177/2325967116671940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Tjong V.K., Devitt B.M., Murnaghan M.L., Ogilvie-Harris D.J., Theodoropoulos J.S. A qualitative investigation of return to sport after arthroscopic Bankart repair: beyond stability. Am J Sports Med. 2015;43(8):2005–2011. doi: 10.1177/0363546515590222. [DOI] [PubMed] [Google Scholar]
- 218.Paterno M.V., Schmitt L.C., Thomas S., Duke N., Russo R., Quatman-Yates C.C., et al. Patient and parent perceptions of rehabilitation factors that influence outcomes after anterior cruciate ligament reconstruction and clearance to return to sport in adolescents and young adults. J Orthop Sports Phys Ther. 2019;49(8):576–583. doi: 10.2519/jospt.2019.8608. [DOI] [PubMed] [Google Scholar]
- 219.Pithadia D.J., Reynolds K.A., Lee E.B., Wu J.J. A cross-sectional study of YouTube videos as a source of patient information about topical psoriasis therapies. J Dermatol Treat. 2019 doi: 10.1080/09546634.2019.1597247. [DOI] [PubMed] [Google Scholar]
- 220.Hsu C.J., Meierbachtol A., George S.Z., Chmielewski T.L. Fear of reinjury in athletes: implications for rehabilitation. Sports Health. 2017;9(2):162–167. doi: 10.1177/1941738116666813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Vlaeyen J.W., Linton S.J. Fear-avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain. 2000;85(3):317–332. doi: 10.1016/S0304-3959(99)00242-0. [DOI] [PubMed] [Google Scholar]
- 222.Leeuw M., Goossens M.E., Linton S.J., Crombez G., Boersma K., Vlaeyen J.W., et al. The fear-avoidance model of musculoskeletal pain: current state of scientific evidence. J Behav Med. 2007;30:77–94. doi: 10.1007/s10865-006-9085-0. [DOI] [PubMed] [Google Scholar]
- 223.Rebolj K., Veber M., Drobnič M., Maličev E. Hematopoietic stem cell and mesenchymal stem cell population size in bone marrow samples depends on patient's age and harvesting technique. Cytotechnology. 2018;70:1575–1583. doi: 10.1007/s10616-018-0250-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Devine S.M. Mesenchymal stem cells: will they have a role in the clinic? J Cell Biochem. 2002;85(S38):73–79. doi: 10.1002/jcb.10046. [DOI] [PubMed] [Google Scholar]
- 225.Papathanasopoulos A., Giannoudis P.V. Biological considerations of mesenchymal stem cells and endothelial progenitor cells. Injury. 2008;39 doi: 10.1016/S0020-1383(08)70012-3. [DOI] [PubMed] [Google Scholar]
- 226.Squillaro T., Peluso G., Galderisi U. Clinical trials with mesenchymal stem cells: an update. Cell Transplant. 2016;25(5):829–848. doi: 10.3727/096368915X689622. [DOI] [PubMed] [Google Scholar]
- 227.Sordi V. Mesenchymal stem cell homing capacity. Transplantation. 2009;87(9S) doi: 10.1097/TP.0b013e3181a28533. [DOI] [PubMed] [Google Scholar]
- 228.de Witte S.F., Luk F., Sierra Parraga J.M., Gargesha M., Merino A., Korevaar S.S., et al. Immunomodulation by therapeutic mesenchymal stromal cells (MSC) is triggered through phagocytosis of MSC by monocytic cells. Stem Cell. 2018;36(4):602–615. doi: 10.1002/stem.2779. [DOI] [PubMed] [Google Scholar]
- 229.Kondelková K., Vokurková D., Krejsek J., Borská L., Fiala Z., Ctirad A., et al. Regulatory T cells (TREG) and their roles in immune system with respect to immunopathological disorders. Acta Med (Hradec Kralove). 2010;53(2):73–77. doi: 10.14712/18059694.2016.63. [DOI] [PubMed] [Google Scholar]
- 230.Caplan A.I., Dennis J.E. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98(5):1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 231.Lana J.F., da Fonseca L.F., Azzini G., Santos G., Braga M., Cardoso Junior A.M., et al. Bone marrow aspirate matrix: a convenient ally in regenerative medicine. Int J Mol Sci. 2021;22(5):2762. doi: 10.3390/ijms22052762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Cassano J.M., Kennedy J.G., Ross K.A., Fraser E.J., Goodale M.B., Fortier L.A., et al. Bone marrow concentrate and platelet-rich plasma differ in cell distribution and interleukin 1 receptor antagonist protein concentration. Knee Surg Sports Traumatol Arthrosc. 2018;26:333–342. doi: 10.1007/s00167-016-3981-9. [DOI] [PubMed] [Google Scholar]
- 233.Thampatty B.P., Li H., Im H.J., Wang J.H. EP4 receptor regulates collagen type-I, MMP-1, and MMP-3 gene expression in human tendon fibroblasts in response to IL-1β treatment. Gene. 2007;386(1–2):154–161. doi: 10.1016/j.gene.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Chong A.K., Ang A.D., Goh J.C., Hui J.H., Lim A.Y., Lee E.H., et al. Bone marrow-derived mesenchymal stem cells influence early tendon-healing in a rabbit Achilles tendon model. JBJS. 2007;89(1):74–81. doi: 10.2106/JBJS.E.01396. [DOI] [PubMed] [Google Scholar]
- 235.Yuksel S., Guleç M.A., Gultekin M.Z., Adanır O., Caglar A., Beytemur O., et al. Comparison of the early period effects of bone marrow-derived mesenchymal stem cells and platelet-rich plasma on the Achilles tendon ruptures in rats. Connect Tissue Res. 2016;57(5):360–373. doi: 10.1080/03008207.2016.1189909. [DOI] [PubMed] [Google Scholar]
- 236.Al-Ani M.K., Xu K., Sun Y., Pan L., Xu Z., Yang L., et al. Study of bone marrow mesenchymal and tendon-derived stem cells transplantation on the regenerating effect of Achilles tendon ruptures in rats. Stem Cells Int. 2015;2015(1) doi: 10.1155/2015/984146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.McKenna R., Riordan N. Minimally invasive autologous bone marrow concentrate stem cells in the treatment of the chronically injured Achilles tendon: a case report. CellR4. 2014;2(4) [Google Scholar]
- 238.Stein B.E., Stroh D.A., Schon L.C. Outcomes of acute Achilles tendon rupture repair with bone marrow aspirate concentrate augmentation. Int Orthop. 2015;39:901–905. doi: 10.1007/s00264-015-2725-7. [DOI] [PubMed] [Google Scholar]
- 239.Thueakthong W., de Cesar Netto C., Garnjanagoonchorn A., Day J., Friedman G., Auster H., et al. Outcomes of iliac crest bone marrow aspirate injection for the treatment of recalcitrant Achilles tendinopathy. Int Orthop. 2021;45:2423–2428. doi: 10.1007/s00264-021-05112-3. [DOI] [PubMed] [Google Scholar]
- 240.Farina K.A., Kandah B.A., Sowers N.M., Moore G.A. Bone marrow aspirate concentrate injection of the Achilles tendon in a competitive distance runner. J Musculoskelet Res. 2021;24(1) [Google Scholar]
- 241.Rodas G., Soler R., Balius R., Alomar X., Peirau X., Alberca M., et al. Autologous bone marrow expanded mesenchymal stem cells in patellar tendinopathy: protocol for a phase I/II, single-centre, randomized with active control PRP, double-blinded clinical trial. J Orthop Surg Res. 2019;14:1–11. doi: 10.1186/s13018-019-1477-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Rodas G., Soler-Rich R., Rius-Tarruella J., Alomar X., Balius R., Orozco L., et al. Effect of autologous expanded bone marrow mesenchymal stem cells or leukocyte-poor platelet-rich plasma in chronic patellar tendinopathy (with gap > 3 mm): preliminary outcomes after 6 months of a double-blind, randomized, prospective study. Am J Sports Med. 2021;49(6):1492–1504. doi: 10.1177/0363546521998725. [DOI] [PubMed] [Google Scholar]
- 243.Okamoto N., Kushida T., Oe K., Umeda M., Ikehara S., Iida H., et al. Treating Achilles tendon rupture in rats with bone-marrow-cell transplantation therapy. JBJS. 2010;92(17):2776–2784. doi: 10.2106/JBJS.I.01325. [DOI] [PubMed] [Google Scholar]
- 244.Sheikhani-Shahin H., Mehrabani D., Ashraf M.J., Rajabi H., Norouzian M., Rahmanifar F., et al. The healing effect of bone marrow-derived stem cells and aquatic activity in Achilles tendon injury. J Hellenic Vet Med Soc. 2019;70(1):1373–1380. [Google Scholar]
- 245.Van den Boom N.A.C., Winters M., Haisma H.J., Moen M.H. Efficacy of stem cell therapy for tendon disorders: a systematic review. Orthop J Sports Med. 2020;8(4) doi: 10.1177/2325967120915857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Boquest A.C., Noer A., Collas P. Epigenetic programming of mesenchymal stem cells from human adipose tissue. Stem Cell Rev. 2006;2:319–329. doi: 10.1007/BF02698059. [DOI] [PubMed] [Google Scholar]
- 247.Huang T., He D., Kleiner G., Kuluz J.T. Neuron-like differentiation of adipose-derived stem cells from infant piglets in vitro. J Spinal Cord Med. 2007;30(Suppl 1) doi: 10.1080/10790268.2007.11753967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Kern S., Eichler H., Stoeve J., Klüter H., Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cell. 2006;24(5):1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 249.Tremolada C., Colombo V., Ventura C. Adipose tissue and mesenchymal stem cells: state of the art and Lipogems® technology development. Curr Stem Cell Rep. 2016;2(3):304–312. doi: 10.1007/s40778-016-0053-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Han S., Sun H.M., Hwang K.C., Kim S.W. Adipose-derived stromal vascular fraction cells: update on clinical utility and efficacy. Crit Rev Eukaryot Gene Expr. 2015;25(2) doi: 10.1615/critreveukaryotgeneexpr.2015013057. [DOI] [PubMed] [Google Scholar]
- 251.Guo J., Nguyen A., Banyard D.A., Fadavi D., Toranto J.D., Wirth G.A., et al. Stromal vascular fraction: a regenerative reality? Part 2: mechanisms of regenerative action. J Plast Reconstr Aesthet Surg. 2016;69(2):180–188. doi: 10.1016/j.bjps.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 252.Usuelli F.G., Grassi M., Maccario C., Viganò M., Lanfranchi L., Alfieri Montrasio U., et al. Intratendinous adipose-derived stromal vascular fraction (SVF) injection provides a safe, efficacious treatment for Achilles tendinopathy: results of a randomized controlled clinical trial at a 6-month follow-up. Knee Surg Sports Traumatol Arthrosc. 2018;26:2000–2010. doi: 10.1007/s00167-017-4479-9. [DOI] [PubMed] [Google Scholar]
- 253.Palumbo Piccionello A., Riccio V., Senesi L., Volta A., Pennasilico L., Botto R., et al. Adipose micro-grafts enhance tendinopathy healing in ovine model: an in vivo experimental perspective study. Stem Cells Transl Med. 2021;10(11):1544–1560. doi: 10.1002/sctm.20-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Khoury M., Tabben M., Rolón A.U., Levi L., Chamari K., D'Hooghe P., et al. Promising improvement of chronic lateral elbow tendinopathy by using adipose-derived mesenchymal stromal cells: a pilot study. J Exp Orthop. 2021;8:1–10. doi: 10.1186/s40634-020-00320-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Norelli J.B., Plaza D.P., Stal D.N., Varghese A.M., Liang H., Grande D.A., et al. Tenogenically differentiated adipose-derived stem cells are effective in Achilles tendon repair in vivo. J Tissue Eng. 2018;9 doi: 10.1177/2041731418811183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Kokubu S., Inaki R., Hoshi K., Hikita A. Adipose-derived stem cells improve tendon repair and prevent ectopic ossification in tendinopathy by inhibiting inflammation and inducing neovascularization in the early stage of tendon healing. Regen Ther. 2020;14:103–110. doi: 10.1016/j.reth.2019.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Ferracini R., Artiaco S., Daghino W., Falco M., Gallo A., Garibaldi R., et al. Microfragmented adipose tissue (M-FATS) for improved healing of surgically repaired Achilles tendon tears: a preliminary study. Foot Ankle Spec. 2022;15(5):472–478. doi: 10.1177/1938640020974557. [DOI] [PubMed] [Google Scholar]
- 258.Viganò M., Lugano G., Perucca Orfei C., Menon A., Ragni E., Colombini A., et al. Autologous microfragmented adipose tissue reduces the catabolic and fibrosis response in an in vitro model of tendon cell inflammation. Stem Cells Int. 2019;2019(1) doi: 10.1155/2019/5620286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.de Girolamo L., Grassi M., Viganò M., Orfei C.P., Montrasio U.A., Usuelli F., et al. Treatment of Achilles tendinopathy with autologous adipose-derived stromal vascular fraction: results of a randomized prospective clinical trial. Orthop J Sports Med. 2016;4(7_suppl4) [Google Scholar]
- 260.Usuelli F.G., Grassi M., Montrasio U.A., De Girolamo L., Boga M. Freshly isolated adipose-derived stem cells for the treatment of Achilles tendinopathy: a randomized prospective clinical trial. Foot Ankle Orthop. 2016;1(1) [Google Scholar]
- 261.Hammond J.W., Hinton R.Y., Curl L.A., Muriel J.M., Lovering R.M. Use of autologous platelet-rich plasma to treat muscle strain injuries. Am J Sports Med. 2009;37(6):1135–1142. doi: 10.1177/0363546508330974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Krogh T.P., Ellingsen T., Christensen R., Jensen P., Fredberg U. Ultrasound-guided injection therapy of Achilles tendinopathy with platelet-rich plasma or saline: a randomized, blinded, placebo-controlled trial. Am J Sports Med. 2016;44(8):1990–1997. doi: 10.1177/0363546516647958. [DOI] [PubMed] [Google Scholar]
- 263.Albano D., Messina C., Usuelli F.G., De Girolamo L., Grassi M., Maccario C., et al. Magnetic resonance and ultrasound in Achilles tendinopathy: predictive role and response assessment to platelet-rich plasma and adipose-derived stromal vascular fraction injection. Eur J Radiol. 2017;95:130–135. doi: 10.1016/j.ejrad.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 264.Shen H., Yoneda S., Abu‐Amer Y., Guilak F., Gelberman R.H. Stem cell‐derived extracellular vesicles attenuate the early inflammatory response after tendon injury and repair. J Orthop Res. 2020;38(1):117–127. doi: 10.1002/jor.24406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Shi Z., Wang Q., Jiang D. Extracellular vesicles from bone marrow-derived multipotent mesenchymal stromal cells regulate inflammation and enhance tendon healing. J Transl Med. 2019;17:1–12. doi: 10.1186/s12967-019-1960-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Aktas E., Chamberlain C.S., Saether E.E., Duenwald‐Kuehl S.E., Kondratko‐Mittnacht J., Stitgen M., et al. Immune modulation with primed mesenchymal stem cells delivered via biodegradable scaffold to repair an Achilles tendon segmental defect. J Orthop Res. 2017;35(2):269–280. doi: 10.1002/jor.23258. [DOI] [PubMed] [Google Scholar]
- 267.Shen W., Chen J., Yin Z., Chen X., Liu H., Heng B.C., et al. Allogenous tendon stem/progenitor cells in silk scaffold for functional shoulder repair. Cell Transplant. 2012;21(5):943–958. doi: 10.3727/096368911X627453. [DOI] [PubMed] [Google Scholar]
- 268.Chen J.L., Yin Z., Shen W.L., Chen X., Heng B.C., Zou X.H., et al. Efficacy of hESC-MSCs in knitted silk-collagen scaffold for tendon tissue engineering and their roles. Biomaterials. 2010;31(36):9438–9451. doi: 10.1016/j.biomaterials.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 269.Boswell S.G., Cole B.J., Sundman E.A., Karas V., Fortier L.A. Platelet-rich plasma: a milieu of bioactive factors. Arthroscopy. 2012;28(3):429–439. doi: 10.1016/j.arthro.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 270.Galliera E., Corsi M., Banfi G. Platelet rich plasma therapy: inflammatory molecules involved in tissue healing. J Biol Regul Homeost Agents. 2012;26(2 Suppl 1):35S–42S. [PubMed] [Google Scholar]
- 271.Bielecki T., Gazdzik T., Arendt J., Szczepanski T., Krol W., Wielkoszynski T., et al. Antibacterial effect of autologous platelet gel enriched with growth factors and other active substances: an in vitro study. J Bone Joint Surg Br. 2007;89(3):417–420. doi: 10.1302/0301-620X.89B3.18491. [DOI] [PubMed] [Google Scholar]
- 272.Yoshida R., Murray M.M. Peripheral blood mononuclear cells enhance the anabolic effects of platelet‐rich plasma on anterior cruciate ligament fibroblasts. J Orthop Res. 2013;31(1):29–34. doi: 10.1002/jor.22183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Dos Santos R.G., Santos G.S., Alkass N., Chiesa T.L., Azzini G.O., da Fonseca L.F., et al. The regenerative mechanisms of platelet-rich plasma: a review. Cytokine. 2021;144 doi: 10.1016/j.cyto.2021.155560. [DOI] [PubMed] [Google Scholar]
- 274.Zhang J., Wang J.H. Platelet-rich plasma releasate promotes differentiation of tendon stem cells into active tenocytes. Am J Sports Med. 2010;38(12):2477–2486. doi: 10.1177/0363546510376750. [DOI] [PubMed] [Google Scholar]
- 275.Salini V., Vanni D., Pantalone A., Abate M. Platelet rich plasma therapy in non-insertional Achilles tendinopathy: the efficacy is reduced in 60-years old people compared to young and middle-age individuals. Front Aging Neurosci. 2015;7:228. doi: 10.3389/fnagi.2015.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Townsend C., Von Rickenbach K.J., Bailowitz Z., Gellhorn A.C. Post‐procedure protocols following platelet‐rich plasma injections for tendinopathy: a systematic review. Pharm Manag PM R. 2020;12(9):904–915. doi: 10.1002/pmrj.12347. [DOI] [PubMed] [Google Scholar]
- 277.Liu C.J., Yu K.L., Bai J.B., Tian D.H., Liu G.L. Platelet-rich plasma injection for the treatment of chronic Achilles tendinopathy: a meta-analysis. Medicine. 2019;98(16) doi: 10.1097/MD.0000000000015278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Zou J., Mo X., Shi Z., Li T., Xue J., Mei G., et al. A prospective study of platelet‐rich plasma as biological augmentation for acute Achilles tendon rupture repair. BioMed Res Int. 2016;2016(1) doi: 10.1155/2016/9364170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Owens RF Jr, Ginnetti J., Conti S.F., Latona C. Clinical and magnetic resonance imaging outcomes following platelet-rich plasma injection for chronic midsubstance Achilles tendinopathy. Foot Ankle Int. 2011;32(11):1032–1039. doi: 10.3113/FAI.2011.1032. [DOI] [PubMed] [Google Scholar]
- 280.Trouski F.F., Parham A. Exosomes derived from mesenchymal stem cells in the treatment of animal tendon injuries: a review on their isolation and application. Iran J Vet Med. 2021;15(3) [Google Scholar]
- 281.Shi Y., Kang X., Wang Y., Bian X., He G., Zhou M., et al. Exosomes derived from bone marrow stromal cells (BMSCs) enhance tendon-bone healing by regulating macrophage polarization. Med Sci Monit. 2020;26 doi: 10.12659/MSM.923328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Wang Y., He G., Guo Y., Tang H., Shi Y., Bian X., et al. Exosomes from tendon stem cells promote injury tendon healing through balancing synthesis and degradation of the tendon extracellular matrix. J Cell Mol Med. 2019;23(8):5475–5485. doi: 10.1111/jcmm.14430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Li M., Jia J., Li S., Cui B., Huang J., Guo Z., et al. Exosomes derived from tendon stem cells promote cell proliferation and migration through the TGF β signal pathway. Biochem Biophys Res Commun. 2021;536:88–94. doi: 10.1016/j.bbrc.2020.12.057. [DOI] [PubMed] [Google Scholar]
- 284.Xu T., Xu M., Bai J., Lin J., Yu B., Liu Y., et al. Tenocyte-derived exosomes induce the tenogenic differentiation of mesenchymal stem cells through TGF-β. Cytotechnology. 2019;71:57–65. doi: 10.1007/s10616-018-0264-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Filardo G., Di Matteo B., Kon E., Merli G., Marcacci M. Platelet-rich plasma in tendon-related disorders: results and indications. Knee Surg Sports Traumatol Arthrosc. 2018;26:1984–1999. doi: 10.1007/s00167-016-4261-4. [DOI] [PubMed] [Google Scholar]
- 286.Liu H., Zhang M., Shi M., Zhang T., Lu W., Yang S., et al. Adipose-derived mesenchymal stromal cell-derived exosomes promote tendon healing by activating both SMAD1/5/9 and SMAD2/3. Stem Cell Res Ther. 2021;12(1):338. doi: 10.1186/s13287-021-02410-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Zhang M., Liu H., Cui Q., Han P., Yang S., Shi M., et al. Tendon stem cell-derived exosomes regulate inflammation and promote the high-quality healing of injured tendon. Stem Cell Res Ther. 2020;11:1–15. doi: 10.1186/s13287-020-01918-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Clancy R., Gomez P., Abramson S. Nitric oxide sustains nuclear factor kappaB activation in cytokine-stimulated chondrocytes. Osteoarthritis Cartilage. 2004;12(7):552–558. doi: 10.1016/j.joca.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 289.Yu Y., Sun B., Wang Z., Yang M., Cui Z., Lin S., et al. Exosomes from M2 macrophage promote peritendinous fibrosis posterior tendon injury via the MiR-15b-5p/FGF-1/7/9 pathway by delivery of circRNA-Ep400. Front Cell Dev Biol. 2021;9 doi: 10.3389/fcell.2021.595911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Cui H., He Y., Chen S., Zhang D., Yu Y., Fan C., et al. Macrophage-derived miRNA-containing exosomes induce peritendinous fibrosis after tendon injury through the miR-21-5p/Smad7 pathway. Mol Ther Nucleic Acids. 2019;14:114–130. doi: 10.1016/j.omtn.2018.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Wellings E.P., Huang T.C., Li J., Peterson T.E., Hooke A.W., Rosenbaum A., et al. Intrinsic tendon regeneration after application of purified exosome product: an in vivo study. Orthop J Sports Med. 2021;9(12) doi: 10.1177/23259671211062929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Yu H., Cheng J., Shi W., Ren B., Zhao F., Shi Y., et al. Bone marrow mesenchymal stem cell-derived exosomes promote tendon regeneration by facilitating the proliferation and migration of endogenous tendon stem/progenitor cells. Acta Biomater. 2020;106:328–341. doi: 10.1016/j.actbio.2020.01.051. [DOI] [PubMed] [Google Scholar]
- 293.Fang W.H., Agrawal D.K., Thankam F.G. “Smart exosomes”: a smart approach for tendon regeneration. Tissue Eng Part B Rev. 2022;28(3):613–625. doi: 10.1089/ten.TEB.2021.0075. [DOI] [PubMed] [Google Scholar]
- 294.Yang Z., Cao H., Gao S., Yang M., Lyu J., Tang K., et al. Effect of tendon stem cells in chitosan/β-glycerophosphate/collagen hydrogel on Achilles tendon healing in a rat model. Med Sci Monit. 2018;24:4633. doi: 10.12659/MSM.906747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Chailakhyan R.K., Kon E., Shekhter A.B., Ivannikov S.V., Telpukhov V.I., Grosheva A.G., et al. Autologous bone marrow-derived mesenchymal stem cells provide complete regeneration in a rabbit model of the Achilles tendon bundle rupture. Int Orthop. 2021;45:3263–3276. doi: 10.1007/s00264-021-05168-1. [DOI] [PubMed] [Google Scholar]
- 296.Guo X., Lv H., Fan Z., Duan K., Liang J., Zou L., et al. Effects of hypoxia on Achilles tendon repair using adipose tissue-derived mesenchymal stem cells seeded small intestinal submucosa. J Orthop Surg Res. 2021;16:1–13. doi: 10.1186/s13018-021-02713-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Zhang J., Li B., Wang J.H. The role of engineered tendon matrix in the stemness of tendon stem cells in vitro and the promotion of tendon-like tissue formation in vivo. Biomaterials. 2011;32(29):6972–6981. doi: 10.1016/j.biomaterials.2011.05.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Tan C., Lui P.P.Y., Lee Y.W., Wong Y.M. Scx-transduced tendon-derived stem cells (tdscs) promoted better tendon repair compared to mock-transduced cells in a rat patellar tendon window injury model. PLoS One. 2014;9(5) doi: 10.1371/journal.pone.0097453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Yao Z., Li J., Xiong H., Cui H., Ning J., Wang S., et al. MicroRNA engineered umbilical cord stem cell-derived exosomes direct tendon regeneration by mTOR signaling. J Nanobiotechnol. 2021;19(1):169. doi: 10.1186/s12951-021-00906-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Zhang Z., Li Y., Zhang T., Shi M., Song X., Yang S., et al. Hepatocyte growth factor-induced tendon stem cell conditioned medium promotes healing of injured Achilles tendon. Front Cell Dev Biol. 2021;9 doi: 10.3389/fcell.2021.654084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Hsieh C.F., Alberton P., Loffredo-Verde E., Volkmer E., Pietschmann M., Müller P., et al. Scaffold-free Scleraxis-programmed tendon progenitors aid in significantly enhanced repair of full-size Achilles tendon rupture. Nanomedicine. 2016;11(9):1153–1167. doi: 10.2217/nnm.16.34. [DOI] [PubMed] [Google Scholar]
- 302.Chamberlain C.S., Clements A.E., Kink J.A., Choi U., Baer G.S., Halanski M.A., et al. Extracellular vesicle-educated macrophages promote early Achilles tendon healing. Stem Cell. 2019;37(5):652–662. doi: 10.1002/stem.2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Mark-Christensen T., Troelsen A., Kallemose T., Barfod K.W. Functional rehabilitation of patients with acute Achilles tendon rupture: a meta-analysis of current evidence. Knee Surg Sports Traumatol Arthrosc. 2016;24:1852–1859. doi: 10.1007/s00167-014-3180-5. [DOI] [PubMed] [Google Scholar]
- 304.Holm C., Kjaer M., Eliasson P. Achilles tendon rupture–treatment and complications: a systematic review. Scand J Med Sci Sports. 2015;25(1) doi: 10.1111/sms.12209. [DOI] [PubMed] [Google Scholar]
- 305.Westin O., Sjögren T., Svedman S., Horvath A., Hamrin Senorski E., Samuelsson K., et al. Treatment of acute Achilles tendon rupture–a multicentre, non-inferiority analysis. BMC Musculoskelet Disord. 2020;21:1–10. doi: 10.1186/s12891-020-03320-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Wang K.C., Cotter E.J., Cole B.J., Lin J.L. Rehabilitation and return to play following Achilles tendon repair. Oper Tech Sports Med. 2017;25(3):214–219. [Google Scholar]
- 307.Dams O.C., van den Akker-Scheek I., Diercks R.L., Wendt K.W., Bosma E., van Raaij T.M., et al. The recovery after Achilles tendon rupture: a protocol for a multicenter prospective cohort study. BMC Musculoskelet Disord. 2019;20:1–8. doi: 10.1186/s12891-019-2437-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Carmont M.R., Silbernagel K.G., Edge A., Mei-Dan O., Karlsson J., Maffulli N., et al. Functional outcome of percutaneous Achilles repair: improvements in Achilles tendon total rupture score during the first year. Orthop J Sports Med. 2013;1(1) doi: 10.1177/2325967113494584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Metz R., van der Heijden G.J., Verleisdonk E.J.M., Kolfschoten N., Verhofstad M.H., van der Werken C., et al. Effect of complications after minimally invasive surgical repair of acute Achilles tendon ruptures: report on 211 cases. Am J Sports Med. 2011;39(4):820–824. doi: 10.1177/0363546510392012. [DOI] [PubMed] [Google Scholar]
- 310.Horstmann T., Lukas C., Merk J., Brauner T., Mündermann A. Deficits 10-years after Achilles tendon repair. Int J Sports Med. 2012;33(6):474–479. doi: 10.1055/s-0032-1301932. [DOI] [PubMed] [Google Scholar]
- 311.Trofa D.P., Miller J.C., Jang E.S., Woode D.R., Greisberg J.K., Vosseller J.T., et al. Professional athletes' return to play and performance after operative repair of an Achilles tendon rupture. Am J Sports Med. 2017;45(12):2864–2871. doi: 10.1177/0363546517713001. [DOI] [PubMed] [Google Scholar]
- 312.Fox G., Gabbe B., Richardson M., Oppy A., Page R., Edwards E., et al. Twelve-month outcomes following surgical repair of the Achilles tendon. Injury. 2016;47(10):2370–2374. doi: 10.1016/j.injury.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 313.Lawrence J., Nasr P., Fountain D., Berman L., Robinson A. Functional outcomes of conservatively managed acute ruptures of the Achilles tendon. Bone Joint Lett J. 2017;99(1):87–93. doi: 10.1302/0301-620X.99B1.BJJ-2016-0452.R1. [DOI] [PubMed] [Google Scholar]
- 314.Carmont M.R., Zellers J.A., Brorsson A., Olsson N., Nilsson-Helander K., Karlsson J., et al. Functional outcomes of Achilles tendon minimally invasive repair using 4- and 6-strand nonabsorbable suture: a cohort comparison study. Orthop J Sports Med. 2017;5(8) doi: 10.1177/2325967117723347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Mubark I., Abouelela A., Arya S., Buchanan D., Elgalli M., Parker J., et al. Achilles tendon rupture: can the tendon gap on ultrasound scan predict the outcome of functional rehabilitation program? Cureus. 2020;12(9) doi: 10.7759/cureus.10298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Yassin M., Myatt R., Thomas W., Gupta V., Hoque T., Mahadevan D., et al. Does size of tendon gap affect patient-reported outcome following Achilles tendon rupture treated with functional rehabilitation? Bone Joint Lett J. 2020;102(11):1535–1541. doi: 10.1302/0301-620X.102B11.BJJ-2020-0908.R1. [DOI] [PubMed] [Google Scholar]
- 317.Braunstein M., Baumbach S.F., Boecker W., Carmont M.R., Polzer H. Development of an accelerated functional rehabilitation protocol following minimal invasive Achilles tendon repair. Knee Surg Sports Traumatol Arthrosc. 2018;26:846–853. doi: 10.1007/s00167-015-3795-1. [DOI] [PubMed] [Google Scholar]
- 318.Oliva F., Bernardi G., De Luna V., Farsetti P., Gasparini M., Marsilio E., et al. IS Mu. LT Achilles tendon ruptures guidelines. Muscles Ligaments Tendons J. 2018;3(8):310–363. [Google Scholar]
- 319.Frankewycz B., Krutsch W., Weber J., Ernstberger A., Nerlich M., Pfeifer C.G., et al. Rehabilitation of Achilles tendon ruptures: is early functional rehabilitation daily routine? Arch Orthop Trauma Surg. 2017;137:333–340. doi: 10.1007/s00402-017-2627-9. [DOI] [PubMed] [Google Scholar]
- 320.Kim U., Choi Y.S., Jang G.C., Choi Y.R. Early rehabilitation after open repair for patients with a rupture of the Achilles tendon. Injury. 2017;48(7):1710–1713. doi: 10.1016/j.injury.2017.04.050. [DOI] [PubMed] [Google Scholar]
- 321.Schepull T., Aspenberg P. Early controlled tension improves the material properties of healing human Achilles tendons after ruptures: a randomized trial. Am J Sports Med. 2013;41(11):2550–2557. doi: 10.1177/0363546513501785. [DOI] [PubMed] [Google Scholar]
- 322.Metz R., Verleisdonk E.J.M., van der Heijden G.J.M.G., Clevers G.J., Hammacher E.R., Verhofstad M.H., et al. Acute Achilles tendon rupture: minimally invasive surgery versus nonoperative treatment with immediate full weightbearing—a randomized controlled trial. Am J Sports Med. 2008;36(9):1688–1694. doi: 10.1177/0363546508319312. [DOI] [PubMed] [Google Scholar]
- 323.Kangas J., Pajala A., Siira P., Hämäläinen M., Leppilahti J. Early functional treatment versus early immobilization in tension of the musculotendinous unit after Achilles rupture repair: a prospective, randomized, clinical study. J Trauma Acute Care Surg. 2003;54(6):1171–1180. doi: 10.1097/01.TA.0000047945.20863.A2. [DOI] [PubMed] [Google Scholar]
- 324.Kangas J., Pajala A., Ohtonen P., Leppilahti J. Achilles tendon elongation after rupture repair: a randomized comparison of 2 postoperative regimens. Am J Sports Med. 2007;35(1):59–64. doi: 10.1177/0363546506293255. [DOI] [PubMed] [Google Scholar]
- 325.Suchak A.A., Bostick G.P., Beaupré L.A., D'Arcy C.D., Jomha N.M. The influence of early weight-bearing compared with non-weight-bearing after surgical repair of the Achilles tendon. JBJS. 2008;90(9):1876–1883. doi: 10.2106/JBJS.G.01242. [DOI] [PubMed] [Google Scholar]
- 326.Zhao J., Guo W., Zeng X., Kan S. Research progress of early postoperative rehabilitation for acute Achilles tendon rupture after surgical repair. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2019;33(3):382–386. doi: 10.7507/1002-1892.201807146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Lu J., Liang X., Ma Q. Early functional rehabilitation for acute Achilles tendon ruptures: an update meta-analysis of randomized controlled trials. J Foot Ankle Surg. 2019;58(5):938–945. doi: 10.1053/j.jfas.2018.12.014. [DOI] [PubMed] [Google Scholar]
- 328.Tarantino D., Palermi S., Sirico F., Corrado B. Achilles tendon rupture: mechanisms of injury, principles of rehabilitation and return to play. J Funct Morphol Kinesiol. 2020;5(4):95. doi: 10.3390/jfmk5040095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Hasenbring M.I., Verbunt J.A. Fear-avoidance and endurance-related responses to pain: new models of behavior and their consequences for clinical practice. Clin J Pain. 2010;26(9):747–753. doi: 10.1097/AJP.0b013e3181e104f2. [DOI] [PubMed] [Google Scholar]
- 330.Mc Auliffe S., Synott A., Casey H., Mc Creesh K., Purtill H., O'Sullivan K., et al. Beyond the tendon: experiences and perceptions of people with persistent Achilles tendinopathy. Musculoskelet Sci Pract. 2017;29:108–114. doi: 10.1016/j.msksp.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 331.Turner J., Malliaras P., Goulis J., Mc Auliffe S. “It's disappointing and it's pretty frustrating, because it feels like it's something that will never go away.” A qualitative study exploring individuals' beliefs and experiences of Achilles tendinopathy. PLoS One. 2020;15(5) doi: 10.1371/journal.pone.0233459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Bassett S.F. Bridging the intention-behaviour gap with behaviour change strategies for physiotherapy rehabilitation non-adherence. N Z J Physiother. 2015;43(3):105–111. [Google Scholar]
- 333.Rompe J.D., Nafe B., Furia J.P., Maffulli N. Eccentric loading, shock-wave treatment, or a wait-and-see policy for tendinopathy of the main body of tendo Achillis: a randomized controlled trial. Am J Sports Med. 2007;35(3):374–383. doi: 10.1177/0363546506295940. [DOI] [PubMed] [Google Scholar]
- 334.Rabusin C.L., Menz H.B., McClelland J.A., Evans A.M., Malliaras P., Docking S.I., et al. Efficacy of heel lifts versus calf muscle eccentric exercise for mid-portion Achilles tendinopathy (HEALTHY): a randomised trial. Br J Sports Med. 2021;55(9):486–492. doi: 10.1136/bjsports-2019-101776. [DOI] [PubMed] [Google Scholar]
- 335.Gatz M., Schweda S., Betsch M., Dirrichs T., de la Fuente M., Reinhardt N., et al. Line-and point-focused extracorporeal shock wave therapy for Achilles tendinopathy: a placebo-controlled RCT study. Sports Health. 2021;13(5):511–518. doi: 10.1177/1941738121991791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Mansur N.S.B., Matsunaga F.T., Carrazzone O.L., Dos Santos B.S., Nunes C.G., Aoyama B.T., et al. Shockwave therapy plus eccentric exercises versus isolated eccentric exercises for Achilles insertional tendinopathy: a double-blinded randomized clinical trial. JBJS. 2021;103(14):1295–1302. doi: 10.2106/JBJS.20.01826. [DOI] [PubMed] [Google Scholar]
- 337.Pinitkwamdee S., Laohajaroensombat S., Orapin J., Woratanarat P. Effectiveness of extracorporeal shockwave therapy in the treatment of chronic insertional Achilles tendinopathy. Foot Ankle Int. 2020;41(4):403–410. doi: 10.1177/1071100719898461. [DOI] [PubMed] [Google Scholar]
- 338.Rompe J.D., Furia J., Maffulli N. Eccentric loading versus eccentric loading plus shock-wave treatment for midportion Achilles tendinopathy: a randomized controlled trial. Am J Sports Med. 2009;37(3):463–470. doi: 10.1177/0363546508326983. [DOI] [PubMed] [Google Scholar]
- 339.Vahdatpour B., Forouzan H., Momeni F., Ahmadi M., Taheri P. Effectiveness of extracorporeal shockwave therapy for chronic Achilles tendinopathy: a randomized clinical trial. J Res Med Sci. 2018;23(1):37. doi: 10.4103/jrms.JRMS_413_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Knobloch K., Schreibmueller L., Longo U.G., Vogt P.M. Eccentric exercises for the management of tendinopathy of the main body of the Achilles tendon with or without the AirHeel™ Brace: a randomized controlled trial. A: effects on pain and microcirculation. Disabil Rehabil. 2008;30(20–22):1685–1691. doi: 10.1080/09638280701786658. [DOI] [PubMed] [Google Scholar]
- 341.Koszalinski A., Flynn T., Hellman M., Cleland J. Trigger point dry needling, manual therapy and exercise versus manual therapy and exercise for the management of Achilles tendinopathy: a feasibility study. J Man Manip Ther. 2020;28(4):212–221. doi: 10.1080/10669817.2020.1719299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Herrington L., McCulloch R. The role of eccentric training in the management of Achilles tendinopathy: a pilot study. Phys Ther Sport. 2007;8(4):191–196. [Google Scholar]
- 343.Kedia M., Williams M., Jain L., Barron M., Bird N., Blackwell B., et al. The effects of conventional physical therapy and eccentric strengthening for insertional Achilles tendinopathy. Int J Sports Phys Ther. 2014;9(4):488. [PMC free article] [PubMed] [Google Scholar]
- 344.Rompe J.D., Furia J., Maffulli N. Eccentric loading compared with shock wave treatment for chronic insertional Achilles tendinopathy: a randomized, controlled trial. JBJS. 2008;90(1):52–61. doi: 10.2106/JBJS.F.01494. [DOI] [PubMed] [Google Scholar]
- 345.Roos E.M., Engström M., Lagerquist A., Söderberg B. Clinical improvement after 6 weeks of eccentric exercise in patients with mid-portion Achilles tendinopathy–a randomized trial with 1‐year follow‐up. Scand J Med Sci Sports. 2004;14(5):286–295. doi: 10.1111/j.1600-0838.2004.378.x. [DOI] [PubMed] [Google Scholar]
- 346.Wiedmann M., Drews B., Huth J., Burkhardt P., Mauch F. Die Behandlung der Midportion-Achillessehnen-Tendinopathie mit exzentrischem Krafttraining und dessen Auswirkung auf die Neovaskularisation. Sport Orthop Traumatol. 2016;2(32):206. [Google Scholar]
- 347.Benli M.D., Tatari H., Balcı A., Peker A., Şimşek K., Yüksel O., et al. A comparison between the efficacy of eccentric exercise and extracorporeal shock wave therapy on tendon thickness, vascularity, and elasticity in Achilles tendinopathy: a randomized controlled trial. Turk J Phys Med Rehabil. 2022;68(3):372. doi: 10.5606/tftrd.2022.8113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Malliaras P. Physiotherapy management of Achilles tendinopathy. J Physiother. 2022;68(4):221–237. doi: 10.1016/j.jphys.2022.09.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.