Abstract
Many pathological conditions involve the head and neck organs, which have complicated anatomy and functions. Recent advances in endovascular treatment have enabled clinicians to use it for treating various lesions, including hemorrhagic conditions, hypervascular tumors, and vascular malformations. Head and neck lesions may present with region-specific clinical manifestations, angioarchitecture, and complications, particularly regarding cosmetic, ingestion, respiratory, and neuronal functions. Therefore, the treatment strategy should consider cosmetic concerns and the preservation of critical functions. A detailed understanding of functional vascular anatomy and treatment techniques can help achieve successful management of head and neck lesions. This review summarizes the clinical manifestations of head and neck lesions, treatment strategies, and complications.
Keywords: endovascular embolization, arteriovenous malformation, vessel injury, tumor, epistaxis
Introduction
The head and neck are crucial body regions comprising the cervical spine, visual pathway, airway, digestive tract, and other surrounding soft tissues. The head and neck organs may be affected by various pathological conditions, such as neoplasms, injuries, infections, and vascular anomalies. Many of these lesions can be treated with embolotherapy; however, inappropriate treatment is associated with incomplete treatment and even fatal complications. In particular, neurological complications and airway compromise may result in serious outcomes. To perform safe and effective embolotherapy, physicians must be familiar with the details of imaging and functional anatomy of head and neck vessels, imaging findings of vascular lesions, and treatment techniques. In this article, we comprehensively review the techniques used for embolization of vascular lesions in the head and neck region, including relevant imaging, anatomical, and physiological information.
Bleeding
Severe bleeding in the head and neck region is caused by trauma, infection, iatrogenic causes, and tumors [1]. Severe hemorrhage in the head and neck can cause shock due to blood loss and other adverse effects, such as airway stenosis and cerebrovascular ischemic complications. Transcatheter embolization is a minimally invasive and effective treatment for bleeding in various fields. Its usefulness has also been reported in the head and neck region, but the anatomy of this region is extremely complex; the arteries supply complicated and small organs and may anastomose with many other arteries, including the cerebrospinal and ophthalmic arteries. Therefore, when embolizing bleeding in the head and neck region, careful attention is required to prevent incomplete embolization due to proximal embolization or ischemic complications in the cranial nerve or cerebrospinal region via anastomoses between the target arteries and normal cerebrospinal, ophthalmic, or vasa nervorum vessels. Additionally, in cases of damage to the cerebral blood vessels themselves, clinicians must decide how to protect the normal cerebral blood flow. Therefore, knowledge of the imaging and functional anatomy of cerebral and head and neck blood vessels is important for embolization in this area. Embolotherapy strategies for epistaxis and arterial injury, given their frequency and clinical importance, are discussed in this review.
(a) Epistaxis
Epistaxis may be caused by idiopathic factors, trauma, neoplasm, inflammation, or vascular malformation, including hereditary hemorrhagic telangiectasia. The basic management of epistaxis involves manual compression by sitting upright and pinching the nose, which results in hemostasis in most cases. The few cases in which self-management does not work require medical management, such as gauze packing with or without vasoconstrictor and local anesthetic administration, balloon packing, laser ablation, and transarterial embolization. For effective embolotherapy, the responsible artery must be definitively embolized (Fig. 1).
Figure 1.
A 50-year-old man presenting with repeated epistaxis.
a. Nasal endoscopy shows oozing from the lateral wall of the nasal cavity (arrowheads).
b. Lateral view of the right external carotid arteriogram during the late arterial phase showing a blush-like enhancement in the nasal cavity (arrowheads).
c. A microcatheter was navigated into the sphenopalatine artery. Frontal view of the sphenopalatine arteriogram showing contrast blushes fed by the posterior lateral nasal artery (arrow) and posterior septal artery (double arrow) along with the lateral mucosal wall (arrowheads).
d. The right sphenopalatine artery was embolized using gelatin sponge particles. Frontal view of right maxillary arteriography immediately after embolization showing disappearance of the mucosal contrast blush in the right lateral cavity.
e. Frontal view of contralateral external carotid arteriography showing no collateral flow into the right lateral nasal wall.
Knowledge of the complicated anatomy of the nasal arterial supply will help in performing safe and effective embolization. The sphenopalatine artery arises from the maxillary artery, supplies the nasal mucosa, and gives rise to the posterolateral nasal artery, which supplies the nasal lateral wall, and the posterior septal artery, which supplies the nasal septum [2]. The superior labial artery, which arises from the facial artery, supplies the anteroinferior portion of the septal mucosa through the incisive foramen. The anterior and posterior ethmoidal arteries from the ophthalmic artery supply the superior part of the nasal septum and the lateral wall (Fig. 2). Therefore, the sphenopalatine artery can anastomose with the ophthalmic artery via the anterior-posterior ethmoidal arteries. The greater palatine artery, which arises from the descending palatine artery, runs forward along the palate and anastomoses with the posterior septal and superior labial arteries around the incisive foramen [2]. The arterial plexus formed by these arterial branches at the nasal septum is known as the Kieselbach's plexus. The responsible artery can be identified on the basis of the bleeding point.
Figure 2.
Schematic of the arterial supply to the nasal mucosa.
a. Left anterolateral view with a sagittal section at the level of the lateral wall of the nasal cavity. The posterolateral nasal artery, which is discharged from the sphenopalatine artery, runs anteroinferiorly along the lateral wall to supply the mucosa and submucosa of the lateral wall.
b. Left anterolateral view with sagittal section at the level of the nasal septum. The posterior septal artery discharged from the sphenopalatine artery runs anteroinferiorly to supply the nasal septal mucosa and submucosa. The anterior and posterior ethmoidal arteries discharged from the ophthalmic artery descend with the nasal septum. The branch of the superior labial artery ascends along the anteroinferior portion of the nasal septum. The greater palatine artery from the descending palatine artery runs along the palate and supplies the inferior part of the nasal septum. The anastomotic network area composed of these arteries is the so-called Kiesselbach’s plexus (dotted circle).
When a patient bleeds from a mucosal lesion showing contrast blush-like staining on angiography, embolization using particles is ideal to achieve hemostasis [3-5]. When the responsible artery shows extravasation or pseudoaneurysm, the bleeding point should be embolized using particles, liquid embolic material, or metallic coils. If angiography does not definitively show the bleeding point, the physician should embolize the arteries supplying the bleeding point as previously described. A recent meta-analysis reported a high rate of rebleeding (16%) associated with hereditary hemorrhagic telangiectasia [6]. Therefore, physicians must be aware of this condition.
(b) Large arterial injury
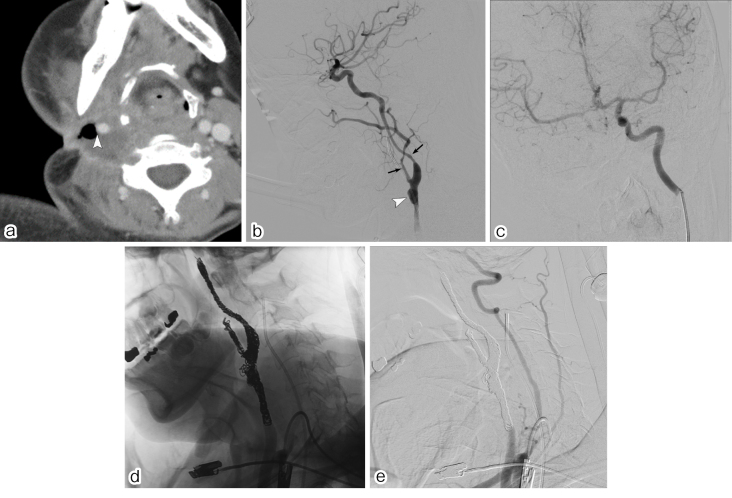
Arterial injury reportedly occurs in 3%-20% of patients with blunt and penetrating trauma in the head and neck [7], which is often caused by a motor vehicle accident, falling, serious bruising, stabbing, gunshot wound, or acute overstretching of the neck. The other causes of arterial injury in this region include infection, tumor invasion, and iatrogenic causes (Fig. 3). Arterial injury may result in contrast extravasation, pseudoaneurysm formation, dissection, occlusion, or arteriovenous fistula (Fig. 3). These lesions were successfully treated with transcatheter embolization.
Figure 3.
A 50-year-old woman with extensive cervical lymph node metastasis from lingual cancer.
a. This patient presented with sudden arterial bleeding from a cervical ulcer while undergoing supportive care. Postcontrast CT image showing deep ulceration surrounded by necrotic tissue. The right common carotid artery is exposed to the ulcer base (arrowhead).
b. A lateral view of the right common carotid arteriogram showing pseudoaneurysm proximal to the segment of the carotid bifurcation (arrowhead). Luminal narrowing with irregular calibers in the proximal internal and external carotid arteries indicates arterial wall injury due to tumor invasion (arrows).
c. A balloon occlusion test was performed to confirm the collateral flow. Contralateral internal carotid and vertebral arterial injections demonstrated sufficient collateral flow via communicating arteries without any circulation delay or neurological symptoms during balloon occlusion of the right common carotid artery.
d. Lateral view of the digital radiograph during coil placement. After navigating the two microcatheters into the carotid arteries, internal trapping was performed from the distal normal segments to the proximal normal segments using detachable and pushable microcoils.
e. Brachiocephalic arteriogram immediately after coil embolization shows no contrast filling in the pseudoaneurysm or other abnormal segments.
Secure hemostasis is achieved by endovascular trapping (embolization of the distal and proximal parts of the injured segment) while avoiding incomplete occlusion of the collateral flow. Endovascular trapping is achieved using metallic coils, particles, n-butyl-2-cyanoacrylate (NBCA), or a combination of these agents. When the carotid or vertebral artery is injured, physicians should consider ischemic tolerance to endovascular trapping in these cerebral arteries. Tolerability to endovascular trapping can usually be confirmed using the balloon occlusion test; however, there can be false-negative cases in which the patient develops ischemic complications due to incomplete collateral circulation [8]. Additionally, there are some situations in which the patient's condition prevents balloon occlusion testing from being performed, especially in emergency cases. Effective alternatives to endovascular trapping to preserve cerebral arterial blood flow include bare-stent implantation, stent-assisted coil embolization, and covered-stent implantation [9, 10]; however, these methods carry the risks of rebleeding, clinical off-label use, and thrombogenic events (especially with covered-stent implantation). In Japan, subclavian arterial injury is considered a good indication for endovascular repair using a covered stent.
Tumor Vessel Embolization
Embolization is used for head and neck tumors, particularly as an adjunctive treatment to surgical resection. Various hypervascular tumors, including meningioma, juvenile angiofibroma, paraganglioma, and hypervascular metastatic tumors, can occur in the head and neck region. Embolization of the vascular bed of hypervascular tumors can reduce intraoperative blood loss and facilitate resection (Fig. 4) [11].
Figure 4.
A 10-year-old boy with juvenile angiofibroma.
a. Postcontrast T1WI shows a strongly enhanced tumor in the nasopharyngeal space (arrowheads). This patient underwent presurgical embolotherapy.
b, c. Frontal view of the bilateral external carotid arteriograms (b, right; c, left). A fine contrast blush corresponding to the nasopharyngeal tumor can be seen on external carotid angiograms (arrowheads). This was fed by the right sphenopalatine artery (b, arrow), left sphenopalatine artery (c, arrow), and pharyngeal blanch of the left ascending pharyngeal artery (c, double arrow).
d. Frontal view of the right internal carotid arteriogram showing contrast blush fed by the Vidian artery (arrow) and other branches coming off from the inferolateral trunk (double arrows).
e, f. Frontal views of the right maxillary (e) and left external carotid (f) arteriograms immediately after embolization. Feeding arteries from the bilateral sphenopalatine and left ascending pharyngeal arteries were embolized using microspheres (Embosphere 300–500 μ, Merit Medical, Salt Lake City, UT, USA). The Vidian artery from the right internal carotid artery was also embolized using detachable microcoils. Bilateral external carotid arteriograms showing the disappearance of the contrast blush in the tumor.
Effective treatment comprises embolization of the intratumoral vascular bed and the feeding artery. Therefore, embolization using particles is safe and effective. When the tumor vasculature comprises an arteriovenous fistula or a large blood pool, liquid embolic material (such as NBCA) and metallic coils are also used. Select an appropriate particle size when using particles. The use of particles that are too small, such as 50-150 μm, may lead to their migration into the venous side, causing the hemorrhage to spread into the anastomotic channels to the cerebral artery and vasa nervorum [12]; the use of liquid embolic materials also carries the same risk. To prevent these complications, physicians should consider selective embolization of the feeding artery or combined use of metallic coils, and they should avoid embolization of the arteries with the greatest risk. Knowledge of the functional anatomy, particularly of potential anastomoses and the vasa nervorum, may help avoid such complications.
Arteriovenous Malformation
Arteriovenous malformation (AVM) is a high-flow vascular malformation that occurs via direct communication between arteries and veins rather than via capillary beds. This pathological condition can be treated by embolotherapy. In the head and neck region, AVM frequently involves the scalp, auricle, cheek, nose, lips, and mandible [13]. AVMs in the head and neck present with various symptoms, depending on the location. In low-grade (early) AVM, the lesion may cause cosmetic issues and mass effects. However, progression of AVM causes ulceration, rest pain, organ dysfunction, and hemorrhage. Among these conditions, nasopharyngeal hemorrhage may cause the greatest hemostatic difficulty and respiratory disturbance. Localized AVMs, particularly Cho classification types I and II, can be cured by embolization [14]. Treatment may relieve symptoms and prevent progression in advanced and severe cases of AVM.
AVMs can be effectively treated by blocking arteriovenous fistulas. Generally, transarterial occlusion that is too proximal should be avoided because it may cause incomplete obliteration and loss of access route. Excessive proximal transvenous occlusion carries the risk of ineffectiveness, congestion, or hemorrhage due to pressure overload through the residual shunt.
Cho et al. reported effective treatment strategies based on angiographic classification via endovascular, percutaneous, and combined approaches [14]. For type II AVMs, coil embolization via percutaneous or transvenous approaches combined with ethanol injection is recommended. Maxillomandibular AVM frequently presents as type II AVM with dominant outflow drainage into the maxillary or mandibular veins [15, 16]. Because the dominant outflow veins lie in the maxillary region of the mandibular bone, coil packing is mainly performed via the transvenous approach or direct percutaneous puncture (Fig. 5).
Figure 5.
A 10-year-old boy with right maxillary arteriovenous malformation.
a, b. Arterial dominant phase of postcontrast CT (a, axial image; b, right anterior oblique view of volume rendering reconstruction). Dilated feeding arterial branches from the maxillary and facial arteries (white arrowheads) and draining veins emptying into the facial, superficial temporal, and maxillary veins surrounding the right maxillary sinus (black arrowheads). Markedly dilated vein, indicating a dominant outflow vein, is also observed within the right maxillary bone (a, arrow).
c. A lateral view of the right external carotid arteriogram showing an arteriovenous malformation (AVM) fed by branches from the maxillary arterial branches (arrows: infraorbital, descending palatine, and posterior superior dental arteries), transverse facial artery (double arrow), and facial artery (triple arrow). These feeders converge into the dilated vein (dominant outflow vein, white arrowheads) on the inferior wall of the maxillary sinus and drain into the facial, superficial temporal, and maxillary veins (black arrowheads).
d. Lateral view of the digital radiograph during coil placement. A 6-French guiding sheath (Shuttle sheath, Cook Medical, Indianapolis, IN, USA) was placed in the right internal jugular vein, and a 6-French distal access catheter (Cerulean DD6, Medikit Co. Ltd., Tokyo, Japan) was navigated into the distal segment of the right facial vein. Two microcatheters (Excelsior 1018, Stryker Neurovascular, Fremont, CA, USA) were parallelly advanced into the dominant outflow vein (arrows). A 5-French guiding sheath (Axcelguide, Medikit Co. Ltd.) combined with a 5-French balloon catheter (Cello, Santa Rosa, CA, USA) was inserted into the right external carotid artery (black arrowhead). A thin microcatheter (DeFrictor Nano, Medicos Hirata, Tokyo, Japan) was advanced into the infraorbital artery (white arrowhead). Transvenous coil embolization of the dominant outflow vein was performed followed by transarterial glue injection via the right infraorbital and posterior superior dental arteries under arterial flow control.
e. Lateral view of the external carotid artery immediately after transvenous coiling of the dominant outflow vein and transarterial glue embolization of several feeding arteries. A residual arteriovenous shunt is identified (arrowheads).
f, g. Transvenous 25% glue injection via a microcatheter in the coil cage of the distal segment of the dominant outflow vein.
h. A lateral view of the right external carotid arteriogram immediately after embolotherapy showing complete resolution of the arteriovenous malformation.
The treatment results in previous reports are variable. The location, stage, angioarchitecture, treatment strategies, and risks of head and neck AVMs are highly variable; therefore, it is difficult to comprehensively discuss treatment outcomes. In a case series of extracranial AVMs in the head and neck, the overall recurrence rate of patients treated with embolization alone was 98%, whereas that of patients treated with combined embolization and resection was 21% in stage I and 81% overall [17].
Devices
To achieve better treatment outcomes, clinicians must select appropriate devices and use them in appropriate situations. The devices used for head and neck embolotherapy comprise a guiding catheter, distal access catheter (DAC), microcatheter, and embolic materials. The characteristics and procedural applications of these devices are described below.
(a) Guiding catheter
A guiding catheter is required to approach the target via the transarterial or transvenous route. The required characteristics (roles) of the guiding catheter are to support the microcatheter, have a soft tip that will not injure the normal vessels, and have an appropriate inner diameter to flush the heparinized saline and inject sufficient contrast media. For the treatment of high-flow vascular lesions, flow control is essential for safe and effective embolization. A balloon guiding catheter equipped with a compliant balloon at the catheter tip can temporarily stop arterial blood flow at the proximal segment.
(b) Distal access catheter
The DAC is designed to support the microcatheter, especially for embolotherapy of very distal vascular lesions with a tortuous access route [18]. The DAC has a 3.2- to 6-French-sized flexible shaft and supports the distal navigation of the microcatheter along a long and tortuous access route (Fig. 5d). The balloon DAC has a flexible and hydrophilic shaft that can also be used to support the microcatheter with balloon flow control (Fig. 5d) (Cello 5F, Medtronic, Irvine, CA, USA).
(c) Microcatheter
There are many types of microcatheters. The microcatheter was selected on the basis of its appropriate diameter, active length, radiopaque marker, and chemical compatibility with the selected embolic materials. The tip and shaft of the microcatheter are flexible and soft to prevent vasospasm and vessel wall injury, especially when treating head and neck vessels. For the treatment of high-flow arteriovenous fistulas, the catheter profile should be suitable for advancing in the tortuous feeding arteries. In general, 1.2- to 1.5-French-sized flow-directed and/or floppy-type wire-directed microcatheters are favorable.
(d) Embolic materials
Various embolic materials are available for embolotherapy of vascular lesions of the head and neck. These materials include particles, liquids, sclerosants, coils, and vascular plugs. The embolic materials were selected on the basis of the target vessel diameter, affected segment, flow velocity, and treatment strategy.
(i) Particles
Particles are injected into the target vessel along with blood flow to mix with the contrast agent. This embolic material is suitable for embolizing various vascular beds when the microcatheter cannot reach the lesion. Microspheres are spherical permanent particles with sizes ranging from 40 to 1,200 μm. The optimal size is selected on the basis of the segment of the vascular bed to be embolized.
Polyvinyl alcohol (PVA) is a permanent embolic material used worldwide. Unlike the aforementioned microspheres, PVA has an irregular shape; therefore, care must be taken to avoid recanalization due to “redistribution,” a phenomenon in which PVA forms clumps that occlude the proximal blood vessels and then break apart and distribute to the periphery. Gelfoam (gelatin sponge particles) is also widely used as a temporary embolic material and is absorbed within 4-6 weeks after injection [11].
(ii) Liquid embolic material
Liquid embolic material permanently embolizes the filled segment. This material is mainly used for embolization of vascular injuries, arteriovenous fistulas, and tumor vessels and can be used to alter blood flow or embolize portal and varicose veins. Liquid embolic material is particularly common in the embolization treatment of AVM because of its wide embolization effect from the feeder to the drainage vessels via perifistulous anastomoses.
NBCA has been used in an off-label manner as a liquid embolic agent for many years but was recently approved for health insurance in Japan. NBCA is a monomer-type material that polymerizes and solidifies when it contacts anion (−OH) components in blood [19-36]. NBCA was mixed with the oil-based contrast agent Lipiodol (Guerbet, Paris, France), and the polymerization time, viscosity, and X-ray opacity varied depending on the mixing ratio. Because this material also has adhesive properties, excessive backflow around the catheter increases the risk of catheter sticking. Therefore, the catheter should be removed immediately if backflow is observed around the catheter tip. Furthermore, NBCA has higher thrombogenicity than another liquid embolic agent, ethylene vinyl alcohol (EVOH), which leads to progressive and permanent occlusion [37].
EVOH is a copolymer dissolved in dimethyl sulfoxide and tantalum powder. EVOH solidifies by diffusion of the dimethyl sulfoxide solution after injection through a microcatheter. This material is nonadhesive and can therefore be injected slowly to penetrate deeply into the distal segment. In Japan, EVOH is approved as Onyx (Medtronic, MN, USA) for embolization of intracranial AVMs and dural arteriovenous fistulas. The disadvantages of EVOH are that it has poor thrombogenicity compared with NBCA, the embolic effect is only achieved in fully filled segments, it has an increased radiation dose due to the need for slow injection, and the high radiopacity of tantalum powder creates metallic artifacts that obscure the surrounding vasculature. Furthermore, EVOH is not suitable for embolization of high-flow arteriovenous fistulas because of the risk of migration into the normal venous side.
(iii) Coils and vascular plugs
The metallic coil is an extremely useful embolic material used to occlude large-diameter vessels. The preferred targets for coil placement are the fistulous shunt and the dominant outflow vein close to the shunt. Detachable microcoils are commonly used to embolize head and neck vessels. Various detachable microcoils that allow precise and tight coil placement in target vessels have recently become available. The Amplatzer vascular plug (AVP, St Jude Medical, Plymouth, MN, USA) is used to rapidly occlude extracranial vessels with medium to large diameters. However, the AVP delivery system has a large and stiff profile, which may be unsuitable for treating head and neck lesions. Because metallic materials, coils, and AVPs may cause a mass effect after treatment, the target vessels should be carefully selected to avoid causing cosmetic problems or compression symptoms, especially in the head and neck region.
(iv) Sclerosant
Absolute ethanol is frequently used for embolization (sclerotherapy) of AVMs. Ethanol should be injected via the microcatheter or a needle advanced close to the shunting vessels to avoid necrosis of normal tissue. Polidocanol is another synthetic alcohol used as a sclerosant. This material is often used as a “polidocanol foam” after mixing with contrast media and CO2 or air. However, the thrombogenic effect of polidocanol is milder than that of absolute ethanol; therefore, it is generally used for the treatment of superficial and slow-flowing lesions.
Complications and Their Management
The most common complication of embolization is dermal or mucosal ischemic necrosis. This condition is common when using an ethanol sclerosant, with a reported frequency of approximately 60% [38]. The risk of ischemic necrosis is dependent on the injection volume, injection speed, and embolized vessel. Physicians should not perform embolization that may lead to wide-ranging occlusion of the normal vasculature by injecting a wedged, prolonged, and large volume of liquid embolic material into fine normal vascular beds. When the target vessel is located beneath the skin or mucosal layer, the cast of the liquid embolic material or coil mass may be exposed through the necrotic skin, mucosa, and fragile vessel wall [39-43]. Exposure to these materials can lead to infectious complications. Therefore, the target segment of embolization should be carefully selected.
Cerebral infarction is caused by injecting embolic materials into the neural arteries or nutrient vessels of the cranial nerves, known as the vasa nervorum. These complications can result in severe neurological impairment. To prevent cerebral infarction, physicians must pay meticulous attention to the angiographic findings of the visualization of the neural arteries. However, the anastomotic channels between the target arteries and the neural and ophthalmic arteries are not always shown in conventional angiography. Moreover, the vasa nervorum is difficult to identify because of the small diameter of the vessels. These anastomotic channels and vasa nervorum branching off target arteries may be obscured, particularly in patients with high flow due to the steal phenomenon. Therefore, it is extremely important to have detailed knowledge of the functional anatomy of the head and neck arteries, which can have anastomotic channels with the cerebral arteries, ophthalmic arteries, and vasa nervorum (Fig. 6) [2, 12, 44, 45]. Clinicians should avoid targeting the arteries with these anastomoses.
Figure 6.

Schematic drawing of potential anastomoses between the external carotid branches and cerebral arteries at middle and posterior cranial fossa (left superolateral view of cranial base).
Maxillary arterial branches and ascending pharyngeal branches have potential anastomoses with the ophthalmic artery and branches from the internal carotid artery (dotted circles). Branches from the ascending pharyngeal and occipital artery anastomoses and branches from the internal carotid and vertebral arteries (dotted circles).
OphA, ophthalmic artery; Rec MA, recurrent meningeal artery; ILT, inferolateral trunk; MHT, meningohypophyseal trunk; AMbr, anteromedial branch; ALbr, anterolateral branch; PMbr, posteromedial branch; PLbr, posterolateral branch; AFR, artery of the foramen rotundum; Vid a, Vidian artery; MMA ant br, anterior branch of the middle meningeal artery; AMA ant br, anterior branch of the accessory meningeal artery; pet br, petrosal branch; CSbr, cavernous sinus branch; APA, ascending pharyngeal artery; OA, occipital artery
The use of absolute ethanol as a sclerosant can result in pulmonary arterial spasm, hemolysis, and cardiocirculatory insufficiency. The recommended amount of ethanol for one injection is <0.14 mL/kg, and the total dose should be <0.5 mL/kg or 30 mL. The pulmonary arteries should also be monitored during ethanol injection [46].
Conclusions
Embolotherapy for head and neck lesions remains challenging. It is extremely important to perform a detailed assessment of the angioarchitecture and ideal treatment endpoint setting and select appropriate devices. The risks of complications and their management should also be considered. Furthermore, understanding the functional anatomy of the head and neck vessels is essential.
Conflict of Interest
None
Author Contribution
ST, MK, AK, TK, and JR were involved in the study design and data interpretation. ST, SM, MS, NF, NT, and TA were involved in the data analysis. All authors critically revised the report, commented on drafts of the manuscript, and approved the final report.
Disclaimer
Shuichi Tanoue is an Editorial Board member of Interventional Radiology. This author was not involved in the peer review or decision-making process for this paper.
Acknowledgments
None.
References
- 1.Cooke D, Ghodke B, Natarajan SK, Hallam D. Embolization in the head and neck. Semin Intervent Radiol. 2008; 25: 293-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tanoue S, Kiyosue H, Mori H, Hori Y, Okahara M, Sagara Y. Maxillary artery: functional and imaging anatomy for safe and effective transcatheter treatment. RadioGraphics. 2013; 33: e209-e224. [DOI] [PubMed] [Google Scholar]
- 3.Robinson AE, McAuliffe W, Phillips TJ, Phatouros CC, Singh TP. Embolization for the treatment of intractable epistaxis: 12 month outcomes in a two centre case series. Br J Radiol. 2017; 90: 20170472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wojak JC. Endovascular treatment of epistaxis. Semin Intervent Radiol. 2020; 37: 150-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Willems PW, Farb RI, Agid R. Endovascular treatment of epistaxis. AJNR Am J Neuroradiol. 2009; 30: 1637-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffman H, Ashok Kumar A, Raventhiranathan N, Masoud HE, Gould GC. Endovascular embolization for the treatment of epistaxis: systematic review and meta-analysis. Interv Neuroradiol. 2023; 29: 172-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ssenyonga PK, Le Feuvre D, Taylor A. Head and neck neurovascular trauma: clinical and angiographic correlation. Interv Neuroradiol. 2015; 21: 108-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lesley WS, Chaloupka JC, Weigele JB, Mangla S, Dogar MA. Preliminary experience with endovascular reconstruction for the management of carotid blowout syndrome. AJNR Am J Neuroradiol. 2003; 24: 975-981. [PMC free article] [PubMed] [Google Scholar]
- 9.Choi HC, Park SE, Choi DS, et al. Ruptured extracranial carotid artery: endovascular treatment with covered stent graft. J Neuroradiol. 2018; 45: 217-223. [DOI] [PubMed] [Google Scholar]
- 10.Liu C, Shen Y, Qian K, Hu Y, Hu X, Wu X. Application of covered stent graft in the treatment of complex carotid artery lesions: A single center experience. Vascular. 2022; 30: 1034-1043. [DOI] [PubMed] [Google Scholar]
- 11.Lazzaro MA, Badruddin A, Zaidat OO, Darkhabani Z, Pandya DJ, Lynch JR. Endovascular embolization of head and neck tumors. Front Neurol. 2011; 2: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geibprasert S, Pongpech S, Armstrong D, Krings T. Dangerous extracranial-intracranial anastomoses and supply to the cranial nerves: vessels the neurointerventionalist needs to know. AJNR Am J Neuroradiol. 2009; 30: 1459-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanoue S, Tanaka N, Koganemaru M, et al. Head and neck arteriovenous malformations: clinical manifestations and endovascular treatments. Interv Radiol (Higashimatsuyama). 2023; 8: 23-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cho SK, Do YS, Shin SW, et al. Arteriovenous malformations of the body and extremities: analysis of therapeutic outcomes and approaches according to a modified angiographic classification. J Endovasc Ther. 2006; 13: 527-538. [DOI] [PubMed] [Google Scholar]
- 15.Lee A, Patel NA. Systematic review of pediatric mandibular arteriovenous malformations. Int J Pediatr Otorhinolaryngol. 2021; 150: 110942. [DOI] [PubMed] [Google Scholar]
- 16.Monteiro JLGC, de Arruda JAA, Figueiredo Leal JL, Batista LL, Célia de Aguiar Soares Carneiro S, do Egito Vasconcelos BC. Embolization as the primary treatment for mandibular arteriovenous malformations: an analysis of 50 literature reports and of an illustrative case. J Oral Maxillofac Surg. 2018; 76: 1695-1707. [DOI] [PubMed] [Google Scholar]
- 17.Liu AS, Mulliken JB, Zurakowski D, Fishman SJ, Greene AK. Extracranial arteriovenous malformations: natural progression and recurrence after treatment. Plast Reconstr Surg. 2010; 125: 1185-1194. [DOI] [PubMed] [Google Scholar]
- 18.Binning MJ, Yashar P, Orion D, et al. Use of the Outreach Distal Access Catheter for microcatheter stabilization during intracranial arteriovenous malformation embolization. AJNR Am J Neuroradiol. 2012; 33: E117-E119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inagawa S, Isoda H, Kougo H, Isogais S, Sakahara H. In-vitro simulation of NBCA embolization for arteriovenous malformation. Interv Neuroradiol. 2003; 9: 351-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tamatani S, Koike T, Ito Y, Tanaka R. Embolization of arteriovenous malformation with diluted mixture of NBCA. Interv Neuroradiol. 2000; 6(suppl 1): 187-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sinha KR, Duckwiler G, Rootman DB. Urticarial reaction following endovascular embolization of an orbital arteriovenous malformation (AVM) with n-butyl cyanoacrylate (nBCA) glue. Interv Neuroradiol. 2017; 23: 666-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han MH, Seong SO, Kim HD, Chang KH, Yeon KM, Han MC. Craniofacial arteriovenous malformation: preoperative embolization with direct puncture and injection of n-butyl cyanoacrylate. Radiology. 1999; 211: 661-666. [DOI] [PubMed] [Google Scholar]
- 23.Benndorf G, Campi A, Hell B, Hölzle F, Lund J, Bier J. Endovascular management of a bleeding mandibular arteriovenous malformation by transfemoral venous embolization with NBCA. AJNR Am J Neuroradiol. 2001; 22: 359-362. [PMC free article] [PubMed] [Google Scholar]
- 24.Miyachi S, Izumi T, Satow T, et al. Effectiveness of preradiosurgical embolization with NBCA for arteriovenous malformations - Retrospective outcome analysis in a Japanese registry of 73 patients (J-REAL study). NeuroIntervention. 2017; 12: 100-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lv X, Wu Z, Li Y. Arteriovenous malformation in the brain: a theoretical study explaining the behavior of liquid embolic agents during endovascular treatment. Neuroradiol J. 2013; 26: 661-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tamatani S, Ito Y, Koike T, et al. Efficacy of diluted NBCA mixture for embolization of arteriovenous malformations. Interv Neuroradiol. 1999; 5: 161-165. [DOI] [PubMed] [Google Scholar]
- 27.Lanza E, Gennaro N, Poretti D, et al. Full recovery after non-target cerebral embolization of n-butyl-cyanoacrylate occurred during emergency treatment of a facial arteriovenous malformation. CVIR Endovasc. 2019; 2: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fiani B, Soula M, Sarhadi K, et al. Direct N-butyl-2-cyanoacrylate injections to the head and neck for percutaneous embolized devascularization. Surg Neurol Int. 2021; 12: 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu HM, Huang YC, Wang YH. Embolization of cerebral arteriovenous malformations with n-butyl-2-cyanoacrylate. J Formos Med Assoc. 2000; 99: 906-913. [PubMed] [Google Scholar]
- 30.Wu EM, El Ahmadieh TY, McDougall CM, et al. Embolization of brain arteriovenous malformations with intent to cure: a systematic review. J Neurosurg. 2019; 132: 388-399. [DOI] [PubMed] [Google Scholar]
- 31.See See AP, Mohammaden MH, Rizko M, et al. Morbidity and mortality associated with sequential flow reduction embolization technique of cerebral arteriovenous malformations using n-butyl cyanoacrylate. J Neurointerv Surg. 2021; 13: 237-241. [DOI] [PubMed] [Google Scholar]
- 32.Velat GJ, Reavey-Cantwell JF, Sistrom C, et al. Comparison of N-butyl cyanoacrylate and onyx for the embolization of intracranial arteriovenous malformations: analysis of fluoroscopy and procedure times. Neurosurgery. 2008; 63: ONS73-8; discussion ONS78-80. [DOI] [PubMed] [Google Scholar]
- 33.Li TL, Fang B, He XY, et al. Complication analysis of 469 brain arteriovenous malformations treated with N-butyl cyanoacrylate. Interv Neuroradiol. 2005; 11: 141-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elsenousi A, Aletich VA, Alaraj A. Neurological outcomes and cure rates of embolization of brain arteriovenous malformations with n-butyl cyanoacrylate or Onyx: a meta-analysis. J Neurointerv Surg. 2016; 8: 265-272. [DOI] [PubMed] [Google Scholar]
- 35.Loh Y, Duckwiler GR, Onyx Trial Investigators. A prospective, multicenter, randomized trial of the Onyx liquid embolic system and N-butyl cyanoacrylate embolization of cerebral arteriovenous malformations. Clinical article. J Neurosurg. 2010; 113: 733-741. [DOI] [PubMed] [Google Scholar]
- 36.Labby ZE, Chaudhary N, Gemmete JJ, Pandey AS, Roberts DA. Dosimetric measurements of an n-butyl cyanoacrylate embolization material for arteriovenous malformations. Med Phys. 2015; 42: 1739-1744. [DOI] [PubMed] [Google Scholar]
- 37.Hashimoto K, Komuro T, Isaka F. A case of venous anomaly of diploic origin successfully treated by preoperative direct puncture sclerotherapy. J Neuroendovascular Ther. 2018; 12: 341-347. [Google Scholar]
- 38.Pekkola J, Lappalainen K, Vuola P, Klockars T, Salminen P, Pitkäranta A. Head and neck arteriovenous malformations: results of ethanol sclerotherapy. AJNR Am J Neuroradiol. 2013; 34: 198-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hetts SW, Mong S, Sincic R, English JD, Wilson MW. Delayed transcutaneous extrusion of embolic coils after embolization of facial artery pseudoaneurysm. Interv Neuroradiol. 2012; 18: 353-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chow MW, Chan DT, Boet R, Poon WS, Sung JK, Yu SC. Extrusion of a coil from the internal carotid artery through the middle ear. Hong Kong Med J. 2004; 10: 215-216. [PubMed] [Google Scholar]
- 41.Kiyosue H, Okahara M, Tanoue S, et al. Dispersion of coils after parent-artery occlusion of radiation-induced internal carotid artery pseudoaneurysm. AJNR Am J Neuroradiol. 2004; 25: 1080-1082. [PMC free article] [PubMed] [Google Scholar]
- 42.Lin HW, Tierney HT, Richmon JD, Mark EJ, Deschler DG. Extrusion of embolization coils through the carotid artery in a radiated neck. Auris Nasus Larynx. 2010; 37: 390-393. [DOI] [PubMed] [Google Scholar]
- 43.Shin BS, Do YS, Cho HS, et al. Effects of repeat bolus ethanol injections on cardiopulmonary hemodynamic changes during embolotherapy of arteriovenous malformations of the extremities. J Vasc Interv Radiol. 2010; 21: 81-89. [DOI] [PubMed] [Google Scholar]
- 44.Kiyosue H, Tanoue S, Hongo N, Sagara Y, Mori H. Artery of the superior orbital fissure: an undescribed branch from the pterygopalatine segment of the maxillary artery to the orbital apex connecting with the anteromedial branch of the inferolateral trunk. AJNR Am J Neuroradiol. 2015; 36: 1741-1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hayreh SS. Orbital vascular anatomy. Eye (Lond). 2006; 20: 1130-1144. [DOI] [PubMed] [Google Scholar]
- 46.Burrows PE, Mason KP. Percutaneous treatment of low flow vascular malformations. J Vasc Interv Radiol. 2004; 15: 431-445. [DOI] [PubMed] [Google Scholar]