Abstract
Time-resolved electron paramagnetic resonance (TREPR) spectroscopy was used to study melanin free radicals in human retinal pigment epithelium (RPE) cells and tyrosine-derived synthetic melanin. TREPR signal traces from RPE cells reveal in vivo light-induced melanin free radical photochemistry in more detail than previously known. Electron spin polarization reflecting a non-Boltzmann population within the energy levels of the spin system is observed in RPE cells as the result of the triplet state photoproduction and subsequent disappearance of free radicals in the melanin polymer. In a set of RPE cells cultured from individual sources, differences in optical absorption, continuous wave EPR spectra, and TREPR signals were correlated with apoptosis assays performed by flow cytometry. Continuous wave EPR spectra of RPE cells and TREPR of acidified synthetic melanin suggest that increased melanin aggregation provides an increase in photoprotection in the RPE cells that are relatively less susceptible to blue light-induced apoptosis.
Keywords: continuous wave EPR, electron spin polarization, time-resolved EPR
In the eye, retinal pigment epithelium (RPE) is a monolayer of cuboidal cells between the photoreceptors and choriocapillaris of the eye and is specialized to uptake, phagocytize, and recycle the outer segment of photoreceptors and retinaldehyde, the chromophore of rhodopsin (1). RPE cell death plays a major role in the pathogenesis of age-related macular degeneration, the leading cause of blindness in the population >60 years of age in the developed world (2). Possible reasons given for the degeneration of RPE cells are their exposure to high oxidative stress and damaging irradiation (2–6).
The pigment melanin, found in RPE cells, is a heterogeneous polymer consisting of various monomers believed to be oxidation products of dopa (dihydroxyphenylalinine) derived from tyrosine (7). Melanin contains intrinsic, indolesemiquinone-like doublet-state radicals (8, 9). Extrinsic indolesemiquinone-like radicals are reversibly photogenerated under visible or UV irradiation (9). RPE melanin serves a photoprotective role by absorbing radiation and scavenging free radicals and reactive oxygen species (ROS) (8–11). Evidence also exists for a phototoxic role for melanin in RPE cells, especially in aged cells, including measurable ROS photoproduction (8, 12–18). Electron paramagnetic resonance (EPR) spectroscopy enables a sensitive and nondestructive analysis and differentiation of natural melanin (9, 19, 20). The photophysical, redox, and aggregation properties of melanin as well as its ability to bind metals, proteins, and drugs have stimulated numerous studies in chemical, physical, and medical research (8, 9). The diversity and interrelations of its properties and the lack of chemical structures have hampered a clear understanding of the roles of melanin in RPE cells (8, 9).
Nanosecond time-resolved (TR) EPR studies have not been reported despite the relevance of TREPR to the study of melanin photochemistry (9). Electron spin polarization (ESP), routinely detected by TREPR, is a major tool for establishing the sequence of free radical events in the nanosecond to microsecond timeframes (21–28). To determine whether EPR with nanosecond time resolution can follow the photochemistry of RPE cells and to confirm the suggestion that melanin radicals are generated from the photoexcited triplet state (20), TREPR spectroscopy was used to study human RPE cells and tyrosine-derived synthetic melanin, revealing details of melanin photochemistry. Further, the photochemistry of melanin free radical production and decay was correlated with blue light-induced apoptosis in RPE cells.
Materials and Methods
Isolation and Culture of Human RPE Cells. RPE cells were obtained from human fetal eyes (Advanced Bioscience Resources, Alameda, CA) as described in ref. 29. Eyes were opened by a circumferential incision above the ora serrata near the limbus; the anterior segment and lens were separated. The posterior segment of each eye was cut into four quadrants and placed in a Petri dish containing DMEM (Sigma). The neural retina and remaining vitreous were removed. Sheets of RPE cells were separated from the choroid and placed into a Petri dish containing PBS without Ca2+/Mg2+. After the separation of all four quadrants, the sheets were trypsinized (0.25% trypsin; Sigma) for 15 min. Growth medium consisting of DMEM, 15% FBS, and 1% solution of antibiotics and l-glutamine (Sigma) was added, and the content was centrifuged. The cells isolated were resuspended with growth medium into one well of a 24-well plate (Becton Dickinson) and incubated for one week in 95% air/5% CO2 at 37°C. Cells were trypsinized and resuspended into larger culture f lasks. At conf luence, cells were subcultured by trypsinization. For EPR experiments, cells were trypsinized (5 min), washed with PBS, and centrifuged (212 × g). The color of the pellets ranged from white to dark brown.
Preparation of Synthetic Melanin Samples. Tyrosine-derived synthetic melanin (Sigma) solutions were prepared by dissolving melanin in warm KOH (pH >12). Basic melanin solutions were used without modification for EPR and optical absorption experiments. A separate 5 mg/ml acidified synthetic melanin solution was made with HCl (pH 1.30) in water. Synthetic melanin is soluble in basic solution but insoluble in acidic solution, forming visible aggregates that settle from solution. The optical density (OD) of acidified melanin solutions after precipitation is much less than that of the basic solutions.
EPR Experiments. All EPR experiments were performed on samples in a closed EPR flat cell (WG-814-Q, Wilmad, Buena, NY) of 0.25-mm path length in a Varian TE102 resonance cavity attached to a modified ER041 magnetic resonance bridge (Bruker, Billerica, MA) equipped with a gallium arsenide field effect transistor microwave amplifier operating at X-band with 20-ns time resolution. The third harmonic (355 nm) was provided by a DCR-4 Nd:yttrium/aluminum garnet laser (10 Hz; Quanta Ray, Mountain View, CA). Irradiation power was measured with an ORION/TH meter (Ophir Optronics). For continuous wave (CW) EPR experiments, RPE cell pellets without dilution were used. Magnetic field modulation of 0.4 mT was used in the CWEPR experiments. TREPR experiments were conducted in two modes as follows: (i) sample flowing such that the laser irradiation continuously struck fresh sample, and (ii) sample not flowing where irradiation excites the same sample volume repeatedly, allowing the investigation of melanin photochemistry under conditions of high extrinsic melanin radical concentration, i.e., redox stress. TREPR signal intensity was recorded at single field values as functions of time, creating time profiles. Time profiles were accumulated on a Waverunner Digital Oscilloscope (LeCroy, Chestnut Ridge, NY) programmed to average 800 time profiles at manually set magnetic field settings on and off resonance. The average on-resonance time profile was subtracted from the average off-resonance time profile to eliminate coherent, laser-induced artifacts.
Cells assayed for light-induced apoptosis also were checked for TREPR signals to determine correlation between detectable photochemistry and RPE apoptosis. However, the displayed TREPR data of RPE cells comes from either a combined sample of the following freshly harvested, darkly colored cell pellets [donor (D) 45-passage (P) 5, D43-P6, D42-P7, D39-P8, D37-P8, D42-P7, and D46-P2] or from D39-P8 cells used without flow. The combined sample provided sufficient volume for the flow system without dilution. Because all TREPR signals observed from RPE cells have identical features to those presented, representative time profiles are presented.
Optical Absorption Measurements. RPE cells were pelleted as described above. From each pellet, 10 μl of cells was added to an UV quartz cuvette and diluted to 3 ml with PBS. For normalization purposes, the sample of RPE cells with the lowest absorption spectrum, D44-P6, was subtracted from all RPE absorption spectra before the absorption at 1,100 nm was set to zero. Comparing the resulting spectra with the spectra for synthetic melanin provided information regarding the relative melanin concentrations of different cell samples.
Apoptosis Assays of RPE Cells. Apoptosis of RPE cells was determined by Annexin V–FITC staining (R & D Systems). Annexin V binds to phosphatidyl serine (exposed on the outer leaflet of the plasma membrane), which is one of the earliest indicators of cellular apoptosis. RPE cells were cultured on six-well tissue culture plates (Becton Dickinson Labware) and were exposed to continuous blue light irradiation (440 nm, 4.55 mW/cm2) for 7 days as they grew inside an incubator fitted with a permanently attached blue light source. Cells in the dark (without light exposure) served as a control. Oxygenation of the environment was maintained constant at 21%. After 7 days, RPE cells were washed twice with PBS and were incubated for 20 min at 37°C with Versene (GIBCO/Invitrogen). Then, cells were gently pipetted to detach them from the plate. Cells were centrifuged, and the resulting pellet was washed twice with PBS before resuspension in 1× binding buffer (pH 7.4; BD Pharmingen) at a concentration of 1 million cells per ml. Staining procedures were performed according to the manufacturer's instructions (R & D Systems). Samples then were diluted in Annexin V binding buffer (pH 7.4) and analyzed by flow cytometry (FACSCalibur, Becton Dickinson) with cellquest software (Becton Dickinson).
Results
CWEPR of Synthetic Melanin. The relative signal amplitude of tyrosine-derived synthetic melanin (1, 2.5, and 5 mg/ml) was determined as a function of irradiation power (Fig. 1A). A fast increase in steady-state radical concentration was seen at low irradiation power followed by a slower linear increase at higher laser powers. The amplitudes and slopes of both the fast and slow segments increase with increasing melanin concentration. CWEPR spectra of acidified melanin without irradiation are larger than those spectra of basic melanin, although acidified solutions have visibly less solubilized melanin.
Fig. 1.
CWEPR signal amplitudes as functions of irradiation power (355 nm). (A) Basic synthetic melanin samples of 5.0 mg/ml (•), 2.5 mg/ml (♦), and 1.0 mg/ml (▪). (Inset) Representative CWEPR spectrum in the light. (B) RPE samples as follows: black, D39-P8; yellow, D43-P5; red, D37-P7; blue, D41-P6; cyan, D42-P4; and green, D40-P7. (Inset) Representative dark (red) and light (black) CWEPR spectra.
CWEPR of RPE Cells. The CWEPR spectra of melanin free radicals (g ≈ 2.004 with line widths ≈ 0.7 mT) exhibit reversible extrinsic radical photogeneration with accompanying g-value shifts as previously observed (9) (Fig. 1B Inset). The relative amplitude of the melanin radical CWEPR signal varies with RPE cell donor (Fig. 1B). As judged by OD measurements, the upper steady-state limit of the signal amplitude does not reflect the amount of melanin in the RPE cells, even relatively, nor does the CW signal amplitude in the absence of light. Generally, RPE cells with a dark brown-colored cell pellet (D39-P8 and D37-P7) gave larger signal amplitudes, whereas cells with a white/gray-colored pellet (D41-P6, D40-P7, and D42-P4) gave smaller signal amplitudes. An exception to the trend is D43-P5, a light brown-colored pellet with signal amplitudes similar to D37-P7, which was very dark. Radical signals present in the dark also vary among donors, but generally follow the same trend: darker pellets had larger intrinsic radical signals. D42-P4 and D40-P7 had no observable intrinsic radicals. D40-P7 did not show radical photogeneration under low irradiation. The first EPR-detectable free radical signal was found with 6.0-mJ laser pulses, indicating a barrier to radical generation observed for D40-P7 that was not observed in other cells, suggesting an initial barrier to radical photoproduction in some RPE cells. Studies are needed to verify such a barrier as well as the many other differences existing among various donors. Differences in age-related macular degeneration susceptibility among individuals are expected, and differences among populations are well documented, with Caucasian-Americans much more likely to develop age-related macular degeneration than African-Americans (30–32).
No RPE cells showed melanin radical photogeneration in two distinguishable portions as observed for synthetic melanin in basic solutions (Fig. 1 A), suggesting that in vivo radical photogeneration is mechanistically different from radical photogeneration in basic systems. Many studies of melanin photochemistry have been performed using synthetic melanin systems as models of native RPE melanin (9, 33, 34). Our observations suggest limitations of highly basic melanin systems as model systems for native melanin photochemistry. Many contradictory results of melanin studies may have arisen from variations in sample preparation (9).
TREPR of RPE Cells and Synthetic Melanin. Both TREPR signals and strong CWEPR signals were observed for one group of RPE samples comprised of D39-P8, D44-P6, D37-P9, D43-P5, and the combined sample described above. However, for a second group of RPE cells composed of D41-P5, D42-P4, and D40-P7, no TREPR was found and only weak CWEPR signals were observed. At all resonant field values, the TREPR signal goes sharply negative within 250 ns, such that the entire EPR signal is initially in emission as a result of the triplet mechanism, a form of ESP (28, 35) (Fig. 2A). Fig. 3 provides a scheme for interpreting the TREPR. In the triplet mechanism, an excited singlet state 1*Q(Q)n decays to an excited triplet state 3*Q(Q)n with a non-Boltzmann population of the three spin sublevels (Fig. 3, a3). The triplet state 3*Q(Q)n chemically reacts to form a geminate, triplet radical pair (RP) 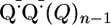 , which captures the non-Boltzmann population of 3*Q(Q)n within the electron spin levels of the RP. Such photochemistry is exemplified by the similar model compound p-benzoquinone (36). As commonly done (7), 5,6-indolequinone of Fig. 3 is used as a guide for interpreting EPR spectra of melanin radicals and their chemistry. For melanin (37) and reference compound p-benzoquinone (36), the initial EPR signal of the RP is emissive. The negative emission signal of the RP returns to the baseline within ≈10 μs by means of the RP spin lattice relaxation (Fig. 2 A Inset).
, which captures the non-Boltzmann population of 3*Q(Q)n within the electron spin levels of the RP. Such photochemistry is exemplified by the similar model compound p-benzoquinone (36). As commonly done (7), 5,6-indolequinone of Fig. 3 is used as a guide for interpreting EPR spectra of melanin radicals and their chemistry. For melanin (37) and reference compound p-benzoquinone (36), the initial EPR signal of the RP is emissive. The negative emission signal of the RP returns to the baseline within ≈10 μs by means of the RP spin lattice relaxation (Fig. 2 A Inset).
Fig. 2.
TREPR time profiles. (A) Three-dimensional emission spectrum of RPE cells, flowing, created by collecting time profiles while changing magnetic field. (Inset) Single time profile at 339.0 mT. (B) RPE cells, not flowing, and three microwave powers as follows: red, 1.99 mW; black, 6.2 mW; and green, 19.9 mW. (Inset) Characteristic f-pair polarization pattern that shows an additional emissive contribution at low field (black, 338.4 mT) changing to enhanced absorption at a higher field position (red, 339.0 mT).
Fig. 3.
Schemes for melanin photochemistry and ESP mechanisms. The superscripts and subscripts 1 and 3 represent singlet and triplet, respectively. The subscripts n–1, x, and y represent arbitrary polymer lengths. The separated RP is partially singlet and partially triplet. Although the reacting geminate RP is configured as pure singlet, when members of any RP become adjacent, quenching c1 can occur to the extent that the RP has singlet character.
With a nonflowing sample, the TREPR signal becomes considerably more complex. Both plus and minus amplitudes occur (Fig. 2B). According to CWEPR experiments, the extrinsic radical population builds up in the sample, creating a higher redox stress than when the sample is flowing. Because the frequency of the plus and minus signal is invariant to microwave power (Fig. 2B), Torrey oscillations are ruled out as a source of the change in signal sign (38). The enhanced absorption (Fig. 2B) is produced by a RP ESP mechanism (28) that follows the triplet mechanism. In the RP ESP mechanism two radicals encounter each other, separate, reencounter, etc., as shown in Fig. 3 by two types of RP encounters. The geminate RP mechanism involves the correlated reencounter of the same radicals of the pair that were created by the laser pulse (Fig. 3, top). In the f-pair mechanism (39–43), random or free, pairs of radicals meet, i.e., either random radicals formed by different laser pulses or formed by the same laser pulse but at different reaction sites (Fig. 3, shaded bottom). Because the excited triplet is energetically inaccessible to either case, ultimately the RPs are quenched by means of the singlet manifold (Fig. 3, c1). The free radical quenching steps of Fig. 3 occur to the extent that the reacting RP has electron spins paired as singlet. For nonflowing samples, the extrinsic radical concentration is large because of multiple laser excitations such that f-pair encounters become more probable. The f-pair RP mechanism is consistent with melanin radicals diffusing within the biopolymer by electron-transfer type reactions (labeled kD in Fig. 3). The effective diffusion length depends in part on the concentration of melanin radicals as determined by the laser power and the flow vs. no-flow conditions. However, when the sample is flowed, each laser pulse encounters a fresh sample volume such that reacting geminate RP reencounters as in Fig. 3 c1 do not occur during the TREPR time profile.
No TREPR signals were observed from basic synthetic melanin systems (pH > 12) of concentrations of 1, 2.5, and 5 mg/ml. In contrast, TREPR signals were observed after the synthetic melanin was acidified (see Fig. 4, which is published as supporting information on the PNAS web site). Interestingly, similar systematic changes in the ESP intensities and signs were observed in poly(methacrylic acid) as a function of pH and state of aggregation (44). The time profiles observed from acidified synthetic melanin are analogous to the RPE time profiles, indicating the existence of a different environment for in vivo melanin photochemistry, an environment not present in synthetic melanin under basic conditions.
Apoptosis Assay. The rate of blue light-induced apoptosis in RPE cells was determined (Table 1) and correlated with EPR-detected photochemistry. Cells least susceptible to light-induced apoptosis (D37-P8, D39-P8, and D44-P6) displayed TREPR-detected photochemistry, whereas cells showing relatively high apoptosis (D40-P7, D41-P6, and D42-P5) had no observable TREPR. Cells with larger CWEPR signals (D37-P8 and D39-P8) were less susceptible to apoptosis, whereas cells with smaller CWEPR amplitudes (D40-P7, D41-P6, and D42-P5) underwent more apoptosis. RPE cells D43-P5 were an exception to both trends but were consistent with RPE cells having large CWEPR amplitudes (relatively more melanin radical photoproduction) also having TREPR-detected photochemistry (melanin radical photoproduction from the triplet manifold and f-pair ESP). The optical absorption spectrum of D43-P5 cells is very similar to that of D40-P7 and D42-P5 (see Fig. 5, which is published as supporting information on the PNAS web site).
Table 1. RPE blue light-induced apoptosis assays.
| RPE sample | Average apoptosis, light, % | Average apoptosis, dark, % | Average apoptosis due to light, % |
|---|---|---|---|
| D40-P7 | 94.71 | 26.56 | 68.15 |
| D41-P6 | 94.80 | 29.11 | 65.69 |
| D43-P5 | 84.90 | 19.96 | 64.94 |
| D42-P5 | 70.29 | 24.72 | 45.57 |
| D44-P6 | 76.30 | 39.37 | 36.93 |
| D37-P8 | 66.88 | 36.98 | 29.90 |
| D39-P8 | 58.42 | 31.83 | 26.59 |
Optical Absorption Measurements of Whole RPE Cells. Relative melanin concentrations of individual RPE cell samples were estimated by optical absorption spectroscopy (Fig. 5), with synthetic melanin providing a reference melanin absorption spectrum. Melanin absorbs strongly in the visible and UV. Spectra in the visible region should indicate relative melanin concentrations among samples (45, 46). According to that premise, D40-P7, D43-P5, and D42-P5 had more concentrated melanin than D41-P6, D37-P8, and D39-P8.
Discussion
Melanin Photochemistry. The RPE melanin polymer is believed to be primarily composed of indolequinone-like species (Fig. 3, shaded, top), although any of the intermediates produced during melanogenesis may be incorporated into the final polymer (7). For decades melanin has been known to exhibit photoinduced, long-lived semiquinone-like free radicals (7, 9, 47). The creation of long-lived free radicals from an excited state requires special circumstances such as hydrogen abstraction or proton-coupled electron transfer. Because the photoinduced EPR signals of melanin are completely reversible in the absence of O2 (20, 48), we limit the discussion to reversible electron transfer reactions for the production of RPs from excited states. Photoexcited states can readily undergo electron transfer with adjacent molecules (Fig. 3, a1 and b3). Both radical formation and radical quenching require the participating molecules to be adjacent and unless an additional electron transfer step, kD, increases the distance between the two resultant radicals, back reaction e1 occurs very quickly, quenching the free radicals (Fig. 3, c1). The net effect is conversion of absorbed light into heat (Fig. 3, c1, d3, and e1), providing photoprotection by preventing photochemistry.
Short photoexcited state lifetimes increase the difficulty of creating long-lived free radicals. Typically, excited singlet states are short-lived, whereas excited triplet states are relatively long-lived (49). Because of the short singlet lifetime, little reaction time is available for the creation of RPs by means of a singlet state such as 1*Q(Q)n (Fig. 3). Also, because of the conservation of spin angular momentum, singlet RPs are allowed to return to the singlet ground state by means of c1, whereas triplet RPs are forbidden. Thus, singlet RPs normally quench by means of c1 to the singlet ground state unless competing, fast, secondary electron transfer reactions such as kD occur. Consequently, production of long-lived separated RPs from the excited singlet state in melanin is not favored even though a polymer of indolequinone-like molecules may provide for migrations such as kD that increase the distance between radicals. If an excited singlet state does lead to the production of long-lived separated RPs, RPs generated from the singlet manifold (Fig. 3, a1) are born singlet and thus are initially TREPR-silent because singlet states are not observable by EPR.
With its inherently longer lifetime, the excited triplet state 3*Q(Q)n is more suited to the creation of long-lived RPs, i.e., b3 can be much slower than a1 and still remain effective at producing a geminate RP. Also, geminate RPs derived from excited triplet states (Fig. 3, b3) have relatively longer lifetimes because electrons are configured as triplet and thus are spin-forbidden to quench to the singlet ground state. For the RPs born as triplets to quench, some singlet character must develop, a process requiring time of the order of 100 ns. This additional time for the triplet relative to the singlet-born RP permits the diffusive nature of melanin radicals in the biopolymer to increase the distance between the two members of the RP, resulting in the production of long-lived free radicals from the triplet excited state. Conductivity studies (50, 51) also indicate that many electron transfer steps occur in the melanin biopolymer, such that the distance between radicals of RPs can become large, allowing lifetimes exceeding seconds. Because of the indicated photoconductivity, the kinetic scheme of Fig. 3 is illustrated using negatively charged free radicals because the exact free radical state is unknown. As many as four distinct quinone-like free radicals, whose relative abundance is pH dependent, have been implicated in the photoinduced CWEPR of synthetic melanin (52), which is derived from oxidation products of tyrosine.
D41-P6, D42-P4, and D40-P7 CWEPR spectra indicated that melanin radicals were photogenerated in the absence of TREPR signals, suggesting that melanin radicals were dominantly singlet-born in these cells or that their TREPR signals were too weak for detection. Similarly, either the photoproduced radicals observed by CWEPR in basic melanin systems were generated from the singlet manifold or else their TREPR signals were also too weak for detection. Assuming that weak TREPR signals would be detected by our experiments, the observed absence of TREPR signals reveals information about melanin organization within the samples. Despite a singlet birth, f-pair reencounters within the polymer would produce observable TREPR signals. The absence of TREPR signals under all experimental conditions in basic, synthetic melanin and the RPE cells exhibiting relatively high apoptosis indicates that melanin free radicals in those samples did not efficiently migrate to allow f-pair chemistry. Therefore, we suggest that the melanin in highly apoptotic RPE and in basic systems is relatively less aggregated compared with melanin in less apoptotic RPE and acid synthetic systems. The opposite was observed for D39-P8, D37-P9, and D43-P5, whose TREPR signals were mimicked by acidified synthetic melanin, where melanin is insoluble and aggregated. RPE cells under physiological conditions are not likely to be very acidic or very basic; nonetheless, several in vivo factors appear to influence or modulate melanin organization within cells, particularly metal ions (53–56), RPE age (8, 14, 16), and proteins (9, 34). In synthetic systems, pH is a main factor controlling the organization of melanin and determining the type of EPR-observable photochemistry that occurs (52).
The fast rise time and the slow second-order decay of the CWEPR signal (20) is consistent with the scheme of Fig. 3 as can be mimicked using a set of coupled differential equations that describes the populations of the ground, excited triplet, and observed RP states. In the simulations, the rapid rise of the RP states is primarily controlled by a3 and b3, whereas the steady-state RP states result from the net effect of the production steps a3 and b3 and the quenching step c1. When the simulated excitation is turned off, the RP decays slowly by a second-order recombination reaction controlled by kD and c1. Additionally, the dependence of the CWEPR signal intensity vs. laser power as revealed in Fig. 1B is also consistent with the simulations based on the kinetic scheme of Fig. 3.
Melanin Phototoxicity and Photoprotection. The RPE cells less susceptible to apoptosis have relatively low optical absorption spectra even though the intensities of the CWEPR signals are relatively high. CWEPR signal amplitudes are larger for acidic synthetic melanin compared with basic melanin (data not shown). Based on the results with the acidic synthetic melanin samples where aggregation and TREPR-observed activity is high, the melanin in these less-susceptible RPE cells is expected to be more aggregated than in RPE cells exhibiting high susceptibility to apoptosis. Accordingly, the physical state of the melanin biopolymer is relevant to the production of free radicals in agreement with photoacoustical studies (57). Our observations indicate that aggregation of melanin could serve a photoprotective role by providing highly diffusive melanin radicals that can quickly scavenge dangerous free radicals including ROS. Independent verification of this aggregation-diffusion hypothesis in vivo may be possible using pulsed EPR to measure rates of radical diffusion (kD in Fig. 3) in aggregated melanin.
On the basis of the work presented here, we therefore suggest that melanin in RPE cells that are less susceptible to apoptosis is more “aggregated” than melanin that is in RPE cells that are highly susceptible to apoptosis. This interpretation has two noteworthy aspects. First, this interpretation can accommodate the studies indicating a phototoxic role of RPE melanin based on measuring O2 uptake and ROS production in melanosomes and RPE. Previous work has shown that melanin radicals can react with O2 to form ROS (8, 12–18). Interestingly, melanin localization into melanosomes and/or melanin-containing aggregates, such as melanolipofuscin granules, increases with aging (1, 8, 16). In addition, measurable O2 consumption and ROS generation from aged RPE has been attributed to the localization of melanin within melanosomes that occurs with aging (1, 8, 16). In this view, if a reaction of melanin with O2 produces ROS, the increased melanin radical photoproduction accompanying melanin aggregation can be expected to lead to an increase in ROS photoproduction in the RPE. Thus, enhanced ROS photoproduction could be expected upon increased melanin aggregation or polymerization, suggesting that melanin aggregation with aging could lead to potentially dangerous chemistry (1, 8, 16).
Second, the correlation between melanin aggregation and increased survival of light-irradiated RPE cells supports a broadly accepted hypothesis of melanin photoprotection. In the protective aspect, we posit that during aging, despite the observation that overall RPE melanin content decreases (12), melanin becomes aggregated into melanosomes and other melanin-containing granules that sequester redox-active metals. ROS produced by means of processes involving sequestered metal ions (8, 9) will be quickly scavenged by melanin radical diffusion before leaving the aggregate, curtailing RPE photodamage.
In both the phototoxic and photoprotective aspects of melanin, free radical diffusion is relevant. In keeping with the observed RP photochemistry of aggregate, polymerized melanin, when a melanin radical is generated by any process, such as radical scavenging, it can diffuse through the polymer by means of electron transfer reactions until it encounters another radical generated separately, for instance, by light absorption. When the radicals encounter each other, they both can be quenched to their singlet ground states through f-pair recombination chemistry, resulting in a refreshed melanin system poised to again serve as a light absorber and an antioxidant. As melanin systems experience increased redox stress and the local melanin radical concentrations increase, f-pair photochemistry will occur more frequently as radical–radical encounters become more rapid. Therefore, radical diffusion and f-pair chemistry could permit polymerized melanin to serve as a radical and a ROS buffer. These TREPR and ESP experiments indicate that increased melanin aggregation correlates with lowered susceptibility to blue light-induced RPE apoptosis. Thus, despite a potential increase of ROS photoproduction, in the RPE cells of this study the overall consequence of increased melanin aggregation is RPE photoprotection, facilitated by diffusion of melanin free radicals within the aggregate. Such photoprotection likely retards age-related macular degeneration pathogenesis.
Supplementary Material
Acknowledgments
B.-L.L.S. was supported by a Richter Grant from the University of Chicago, and Y.K. was supported by a fellowship from the Japan Society for the Promotion of Science. This work was supported in part by Research to Prevent Blindness, Inc.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: ESP, electron spin polarization; CWEPR, continuous wave EPR; ROS, reactive oxygen species; RPE, retinal pigment epithelium; RP, radical pair; TREPR, time-resolved EPR; D, donor; P, passage.
References
- 1.Schraermeyer, U. & Heimann, K. (1999) Pigm. Cell Res. 12, 219–236. [DOI] [PubMed] [Google Scholar]
- 2.Eperjesi, F. (2004) Nutr. Eyes Ser. 6, 27–30. [Google Scholar]
- 3.Tanito, M., Nishiyama, A., Tanaka, T., Masutani, H., Nakamura, H., Yodoi, J. & Ohira, A. (2002) Invest. Ophthalmol. Visual Sci. 43, 2392–2400. [PubMed] [Google Scholar]
- 4.vanBest, J., Putting, B., Oosterhuis, J., Zweypfenning, R. & Vrensen, G. (1997) Microsc. Res. Tech. 36, 77–88. [DOI] [PubMed] [Google Scholar]
- 5.Dillon, J. (1991) J. Photochem. Photobiol. B 10, 23–40. [DOI] [PubMed] [Google Scholar]
- 6.Handelman, G. & Dratz, E. (1986) Adv. Free Radical Biol. 2, 1–89. [Google Scholar]
- 7.Sealy, R. C., Felix, C. C., Hyde, J. S. & Swartz, H. M. (1980) in Free Radicals in Biology, ed. Pryor, W. A. (Academic, New York), Vol. 4, pp. 209–259. [Google Scholar]
- 8.Boulton, M., Rozanowska, M. & Rozanowski, B. (2001) J. Photochem. Photobiol. B 64, 144–161. [DOI] [PubMed] [Google Scholar]
- 9.Sarna, T. (1992) J. Photochem. Photobiol. B 12, 215–258. [DOI] [PubMed] [Google Scholar]
- 10.Rozanowska, M., Sarna, T., Land, E. & Truscott, T. (1999) Free Radical Biol. Med. 26, 518–525. [DOI] [PubMed] [Google Scholar]
- 11.Korytowski, W., Kalyanaraman, B., Menon, I. A., Sarna, T. & Sealy, R. C. (1986) Biochim. Biophys. Acta 882, 145–153. [DOI] [PubMed] [Google Scholar]
- 12.Sarna, T., Burke, J., Korytowski, W., Rozanowska, M., Skumatz, C., Zareba, A. & Zareba, M. (2003) Exp. Eye Res. 76, 89–98. [DOI] [PubMed] [Google Scholar]
- 13.Rozanowska, M., Korytowski, W., Rozanowski, B., Skumatz, C., Boulton, M., Burke, J. & Sarna, T. (2002) Invest. Ophthalmol. Visual Sci. 43, 2088–2096. [PubMed] [Google Scholar]
- 14.Dayhaw-Barker, P., Davies, S., Shamsi, F., Rozanowska, M., Rozanowski, B. & Boulton, M. (2001) Invest. Ophthalmol. Visual Sci. 42, S755–S755. [Google Scholar]
- 15.Korytowski, W. & Sarna, T. (1990) J. Biol. Chem. 265, 12410–12416. [PubMed] [Google Scholar]
- 16.Boulton, M., Docchio, F., Dayhawbarker, P., Ramponi, R. & Cubeddu, R. (1990) Vision Res. 30, 1291–1303. [DOI] [PubMed] [Google Scholar]
- 17.Kalyanaraman, B., Korytowski, W., Pilas, B., Sarna, T., Land, E. J. & Truscott, T. G. (1988) Arch. Biochem. Biophys. 266, 277–284. [DOI] [PubMed] [Google Scholar]
- 18.Rozanowska, M., Bober, A., Burke, J. & Sarna, T. (1997) Photochem. Photobiol. 65, 472–479. [DOI] [PubMed] [Google Scholar]
- 19.Sealy, R. C., Hyde, J. S., Felix, C. C., Menon, I. A. & Prota, G. (1982) Science 217, 545–547. [DOI] [PubMed] [Google Scholar]
- 20.Felix, C. C., Hyde, J. S. & Sealy, R. C. (1979) Biochem. Biophys. Res. Commun. 88, 456–461. [DOI] [PubMed] [Google Scholar]
- 21.Norris, J. R., Morris, A. L., Thurnauer, M. C. & Tang, J. (1990) J. Chem. Phys. 92, 4239–4249. [Google Scholar]
- 22.Atkins, P. W. & Evans, G. T. (1974) Chem. Phys. Lett. 25, 108–110. [Google Scholar]
- 23.Atkins, P. W. & Evans, G. T. (1974) Mol. Phys. 27, 1633–1644. [Google Scholar]
- 24.Wan, J. K. S., Wong, S. K. & Hutchins, D. A. (1974) Acc. Chem. Res. 7, 58–64. [Google Scholar]
- 25.Jenks, W. S. & Turro, N. J. (1990) Res. Chem. Intermediates 13, 237–300. [Google Scholar]
- 26.Wu, C. H., Jenks, W. S., Koptyug, I. V., Ghatlia, N. D., Lipson, M., Tarasov, V. F. & Turro, N. J. (1993) J. Am. Chem. Soc. 115, 9583–9595. [Google Scholar]
- 27.Turro, N. J. & Khudyakov, I. V. (1999) Res. Chem. Intermediates 25, 505–529. [Google Scholar]
- 28.McLauchlan, K. A. (1997) J. Chem. Soc. Perkin Trans. 2, 2465–2472.
- 29.Rezai, K., Semnani, R., Patel, S., Ernest, J. & vanSeventer, G. (1997) Invest. Ophthalmol. Vision Sci. 38, 2662–2671. [PubMed] [Google Scholar]
- 30.Friedman, D., West, S., Munoz, B., Park, W., Deremeik, J., Massof, R., Frick, K., Broman, A., McGill, W., Gilbert, D. & German, P. (2004) Arch. Ophthalmol. 122, 1019–1024. [DOI] [PubMed] [Google Scholar]
- 31.Congdon, N., O'Colmain, B., Klaver, C., Klein, R., Munoz, B., Friedman, D., Kempen, J., Taylor, H., Mitchell, P. & Hyman, L. (2004) Arch. Ophthalmol. 122, 477–485. [DOI] [PubMed] [Google Scholar]
- 32.Stock, C. J., Canter, L. A., Puklin, J. E. & Frank, R. N. (1995) Invest. Ophthalmol. Visual Sci. 36, S10–S10. [Google Scholar]
- 33.Haywood, R. & Linge, C. (2004) Laser Surg. Med. 35, 77–83. [DOI] [PubMed] [Google Scholar]
- 34.Haywood, R. & Linge, C. (2004) J. Photochem. Photobiol. B 76, 19–32. [DOI] [PubMed] [Google Scholar]
- 35.Wong, S. K., Hutchison, D. A. & Wan, J. K. S. (1973) J. Chem. Phys. 58, 985–989. [Google Scholar]
- 36.Jager, M., Yu, B. C. & Norris, J. R. (2002) Mol. Phys. 100, 1323–1331. [Google Scholar]
- 37.Felix, C. C., Hyde, J. S., Sarna, T. & Sealy, R. C. (1978) Biochem. Biophys. Res. Commun. 84, 335–341. [DOI] [PubMed] [Google Scholar]
- 38.Torrey, H. C. (1949) Phys. Rev. 76, 1059–1068. [Google Scholar]
- 39.Savitsky, A. N. & Paul, H. (1997) Appl. Magn. Reson. 12, 449–464. [Google Scholar]
- 40.Savitsky, A. N., Paul, H. & Shushin, A. I. (2000) J. Phys. Chem. A 104, 9091–9100. [Google Scholar]
- 41.Bussandri, A. & van Willigen, H. (2001) J. Phys. Chem. A 105, 4669–4675. [Google Scholar]
- 42.Ichino, T. & Fessenden, R. W. (2003) J. Phys. Chem. A 107, 9257–9268. [Google Scholar]
- 43.Monchick, L. & Adrian, F. J. (1978) J. Chem. Phys. 68, 4376–4383. [Google Scholar]
- 44.Maliakal, A., Weber, M., Turro, N. J., Green, M. M., Yang, S. Y., Pearsall, S. & Lee, M. J. (2002) Macromolecules 35, 9151–9155. [Google Scholar]
- 45.Powell, B. J., Baruah, T., Berstein, N., Brake, K., McKenzie, R. H., Meredith, P. & Penderson, M. R. (2004) J. Chem. Phys. 120, 8608–8615. [DOI] [PubMed] [Google Scholar]
- 46.Stark, K. B., Gallas, J. M., Zajac, G. W., Eisner, M. & Golab, J. T. (2003) J. Phys. Chem. B 107, 3061–3067. [Google Scholar]
- 47.Blois, M. S., Maling, J. E. & Zahlan, A. B. (1964) Biophys. J. 4, 471–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Felix, C. C., Hyde, J. S., Sarna, T. & Sealy, R. C. (1978) J. Am. Chem. Soc. 100, 3922–3926. [Google Scholar]
- 49.McClure, D. S. (1949) J. Chem. Phys. 17, 905–913. [Google Scholar]
- 50.Crippa, P. R., Cristofoletti, V. & Romeo, N. (1978) Biochim. Biophys. Acta 538, 164–170. [DOI] [PubMed] [Google Scholar]
- 51.Jastrzebska, M., Kocot, A. & Tajber, L. (2002) J. Photochem. Photobiol. B 66, 201–206. [DOI] [PubMed] [Google Scholar]
- 52.Pasenkiewiczgierula, M. & Sealy, R. C. (1986) Biochim. Biophys. Acta 884, 510–516. [DOI] [PubMed] [Google Scholar]
- 53.Stainsack, J., Mangrich, A. S., Maia, C. M. B. F., Machado, V. G., dos Santos, J. C. P. & Nakagaki, S. (2003) Inorg. Chim. Acta 356, 243–248. [Google Scholar]
- 54.Chodurek, E., Pilawa, B., Dzierzega-Lecznar, A., Kurkiewicz, S., Swiatkowska, L. & Wilczok, T. (2003) J. Anal. Appl. Pyrol. 70, 43–54. [Google Scholar]
- 55.Szpoganicz, B., Gidanian, S., Kong, P. & Farmer, P. (2002) J. Inorg. Biochem. 89, 45–53. [DOI] [PubMed] [Google Scholar]
- 56.Gallas, J., Littrell, K., Seifert, S., Zajac, G. & Thiyagarajan, P. (1999) Biophys. J. 77, 1135–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nofsinger, J. B., Forest, S. E. & Simon, J. D. (1999) J. Phys. Chem. B 103, 11428–11432. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





