Abstract
Purpose
This study aimed to establish and characterize patient-derived intestinal organoids (PDOs) from children with Crohn’s disease (CD).
Methods
To generate PDOs, endoscopic biopsy specimens were obtained from non-inflamed duodenal bulbs of normal controls and CD patients. To verify the presence of PDOs, histological staining and quantitative reverse transcription polymerase chain reaction (RT-qPCR) analyses were performed.
Results
PDOs were successfully established in normal controls (n=2) and CD patients (n=2). Hematoxylin and eosin staining of formalin-fixed, paraffin-embedded PDO sections revealed crypt and villus structures, whereas immunofluorescence staining with EpCAM and DAPI confirmed the epithelial-specific architecture of the PDOs. RT-qPCR results revealed a significant increase in Lgr5, Si, and Chga gene expression and a decrease in Olfm4 and Muc2 expression in CD patients compared to normal controls, suggesting altered stem cell activity and mucosal barrier function (p<0.05).
Conclusion
We successfully established and characterized PDOs in children with CD, providing a valuable tool for understanding the pathophysiology of the disease and evaluating potential therapeutic approaches. The differential gene expression of PDOs in CD patients might be caused by the complex interplay between epithelial adaptation and inflammation in the intestinal epithelium.
Keywords: Crohn disease, Organoids, Child, Biopsy, Endoscopy
INTRODUCTION
Crohn’s disease (CD) is a chronic, relapsing-remitting inflammatory disease that affects the entire gastrointestinal tract. Owing to an incomplete understanding of the pathophysiology of the disease, pediatric cases present particular challenges [1]. The main treatment for CD involves biological therapies, such as antitumor necrosis factor agents, which are aimed at suppressing inflammation [2]. However, managing these therapies can be difficult, and success rates vary depending on factors such as disease severity and patient response to previous treatments [3]. In pediatric patients, biological therapies have been shown to induce clinical remission in approximately 40–60% of cases, with approximately 30–50% maintaining remission over time [4]. While further research on the pathophysiology of CD is critical to overcome these challenges, studying pediatric patients presents additional limitations [5]. Thus, there is an urgent need to develop new treatments targeting different pathways to improve patient outcomes [6].
Conventional research models, such as in vitro cell cultures and in vivo animal models, have significant limitations [7]. Animal models have long life cycles, differ from humans in key biological aspects, and are costly, with additional concerns around animal ethics [8]. Similarly, cellular models often lack sufficient representation of human biology [9]. Consequently, more effective, safe, and human-representative cellular models are needed to reduce reliance on animal experiments. Patient-derived intestinal organoids (PDOs) offer a promising alternative because they recapitulate the structures, specific functions, molecular characteristics, and expression profiles of the primary intestinal mucosa [10]. These three-dimensional structures are derived from multipotent epithelial stem cells located at the base of intestinal crypts and are grown in culture from endoscopically obtained mucosal biopsies [11]. PDOs retain important features of the intestinal epithelium, such as self-renewal, self-organization, barrier function, and the ability to differentiate into various epithelial cell types [12]. As a result, PDOs have revolutionized research in inflammatory bowel diseases by providing new, patient-specific models for studying disease mechanisms and developing treatments [13,14].
Pediatric CD often leads to growth retardation, delayed puberty, and follows a different disease course compared to adult-onset CD [15]. These unique clinical features highlight the need for establishing pediatric-specific PDOs. Despite the growing incidence of CD in children, research addressing the specific characteristics of PDOs from pediatric patients remains sparse. This study aimed to establish and characterize PDOs in children with CD, providing a valuable tool for understanding the pathophysiology of the pediatric CD and evaluating potential therapeutic approaches.
MATERIALS AND METHODS
Collection of tissues and ethical statements
To generate PDOs, endoscopic biopsy specimens were obtained from the non-inflamed duodenal bulbs of both normal controls and CD patients at Seoul National University Hospital and Hallym University Kangnam Sacred Heart Hospital between November 2022 and May 2024. Normal controls were patients who exhibited normal mucosal findings during endoscopy. CD patients were diagnosed according to the pediatric CD guidelines [16]. All samples were collected after obtaining informed consent. This study was conducted in compliance with the Declaration of Helsinki and was approved by the Institutional Review Board of Seoul National University (approval no. H-2207-171-1344) and Hallym University Kangnam Sacred Heart Hospital (approval no. 2024-01-013). Written informed consent was obtained from all participants, and for participants under the age of 16 years, consent was obtained from their parents or legal guardians prior to the study.
Crypt isolation and passage
Intestinal crypts were isolated from fresh duodenal endoscopic biopsies using a modified protocol [17]. Briefly, biopsies (~4×7 mm) were thoroughly washed in PBS containing penicillin/streptomycin (Pen/Strep; Gibco) and amphotericin B (Gibco). The washes were repeated until the solution became clear. The cleaned biopsies were then incubated in PBS supplemented with dithiothreitol (DTT; Sigma-Aldrich), ethylenediaminetetraacetic acid (EDTA; Invitrogen), and Pen/Strep for 30 minutes on ice. After incubation, the samples were shaken vigorously, and the supernatant was collected and supplemented with 1 mL of fetal bovine serum (FBS; Sigma-Aldrich). This process was repeated at least five times. All collected fractions were centrifuged at 1,000 rpm for 10 minutes at room temperature to pellet the crypts for further use. The collected cell pellet was resuspended in Matrigel, and 11.5 µL drops of the Matrigel-cell suspension were pipetted into a 6-well plate. The culture medium was composed of advanced DMEM/F12 (Gibco), supplemented with pen/strep (Gibco), GlutaMAX (Gibco), non-essential amino acids (Gibco), Sodium Pyruvate (Gibco), N2 and B27 supplements (Sigma-Aldrich), Epidermal Growth Factor (EGF; PeproTech), Gastrin, N-acetyl cysteine, and insulin (Sigma-Aldrich) The culture medium was refreshed every two days, and the organoids were subjected to mechanical passaging at 7-to-8-day intervals.
Histologic analyses
Mature intestinal organoids were harvested by carefully collecting Matrigel, and the organoid pellet was fixed with 4% paraformaldehyde (Biosesang) and embedded in paraffin. Sections were cut at a thickness of 4 µm for hematoxylin and eosin (H&E) and immunofluorescence (IF) analysis. The sections were deparaffinized with xylene and rehydrated using a graded ethanol series. For H&E staining, deparaffinized sections were stained with a H&E staining kit (Vector Laboratories) following the manufacturer’s guidelines. For IF staining, the deparaffinized slides were subjected to antigen retrieval by heating in citrate buffer (Sigma-Aldrich) for 20 minutes. Endogenous peroxidase was inactivated with 0.3% hydrogen peroxide, followed by permeabilization with 0.15% TritonX-100 (Sigma-Aldrich) in PBS. Non-specific binding was blocked using goat serum (Sigma-Aldrich) before incubating the slides with primary antibody EpCAM (Cell Signaling Technology) overnight at 4°C. Slides were then incubated with a goat anti-mouse IgG (H+L) cross-absorbed secondary antibody, Alexa Fluor 488 (Invitrogen), for 1 hour. The nuclei were counterstained with DAPI (Invitrogen).
Quantitative reverse transcription polymerase chain reaction analyses
Total RNA from the organoids of normal controls and CD patients was isolated using TRIzol reagent (Invitrogen) and Direct-zolTM RNA Miniprep (Zymo Research), according to the manufacturer’s protocol. Complementary DNA was synthesized using Maxima H Minus First Strand cDNA Synthesis Kit (Thermo Fisher Scientific). mRNA expression levels were measured with FastStart Essential DNA Green Master (Roche), which was analyzed by using the Lightcycler® 96 Instrument (Roche). The primer sequences are listed in Table 1. Fold changes in gene expression were calculated by the 2−ΔΔCt method, with normalization to the housekeeping gene (18S ribosomal RNA).
Table 1. Quantitative reverse transcription polymerase chain reaction primer sequences.
| Gene | Forward | Reverse |
|---|---|---|
| 18S | AGAAACGGCTACCACATCCA | CCCTCCAATGGATCCTCGTT |
| Lgr5 | CTCCCAGGTCTGGTGTGTTG | GAGGTCTAGGTAGGAGGTGAAG |
| Olfm4 | ACTGTCCGAATTGACATCATGG | TTCTGAGCTTCCACCAAAACTC |
| Si | TCCAGCTACTACTCGTGTGAC | CCCTCTGTTGGGAATTGTTCTG |
| Muc2 | CAACAACACCCTGCTCAACG | CTTCGGGTCGCTCTTGAAGT |
| Chga | TAAAGGGGATACCGAGGTGATG | TCGGAGTGTCTCAAAACATTCC |
| Lyz | CTTGTCCTCCTTTCTGTTACGG | CCCCTGTAGCCATCCATTCC |
Statistical analysis
Statistical analysis of differences between groups was performed using Student’s t-test from triplicate samples using GraphPad Prism (version 11; GraphPad Software, Inc.). A p-value of less than 0.05 was considered indicative of statistical significance.
RESULTS
Establishment of PDOs in pediatric CD patients
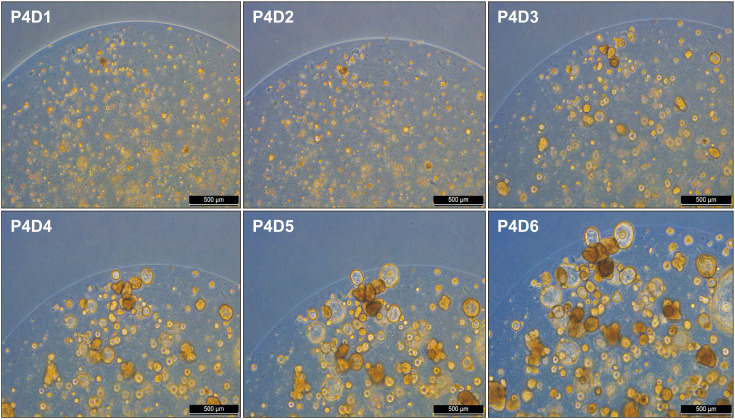
Normal and CD PDOs were established using endoscopic biopsy specimens obtained from non-inflammatory mucosal lesions in the duodenal bulb. Intestinal crypts were isolated from the duodenal biopsies of normal controls (n=2) and CD patients (n=2). Table 2 shows the subject characteristics. Established PDOs from duodenal biopsies of normal controls were stably cultured for 23 weeks, and PDOs from non-inflamed duodenal biopsies of CD patients were stably cultured until 20 weeks before freezing. The PDOs were split once weekly, and the conditioned media were replaced three times a week. On average, a split ratio of 1:3–1:5 was used for the normal control and CD groups. Fig. 1 shows the process of PDOs growth from days one to six in the fourth week of passage in the control group. From days one to three, the intestinal organoids began to recover from dissociation, showing early expansion and initial lumen formation (Fig. 1). By day six, the organoids had grown significantly and matured, with well-defined structures and differentiated cell types (Fig. 1). PDOs in CD patients exhibited the same growth pattern.
Table 2. Clinical characteristics of subjects.
| Subjects | Age (yr)/sex | The reason of endoscopy | Endoscopic findings at the time samples acquisition | Diagnosis |
|---|---|---|---|---|
| Normal 1 | 13/M | Vomiting | NOS | Cyclic vomiting syndrome |
| Normal 2 | 13/F | Poor oral intake | NOS | Anorexia nausea |
| CD 1 | 15/F | Abdominal pain, chronic diarrhea, recurrent oral ulcer, weight loss | Multiple mucosal ulcers in the second portion of duodenum, and multiple cobblestone ulceration in colon. | CD |
| Paris classification (A1b, L3+L4a, B1p, G0) | ||||
| CD 2 | 12/F | Abdominal pain, poor oral intake, weight loss | Aphthous ulcers (jejunum on capsule and terminal ileum). | CD |
| Paris classification (A1b, L1+L4b, B1, G1) |
M: male, F: female, NOS: not otherwise specified, CD: Crohn’s disease.
Fig. 1. Process of intestinal organoid growth from days one to six in fourth week passage in control group.
Histologic verification of PDOs
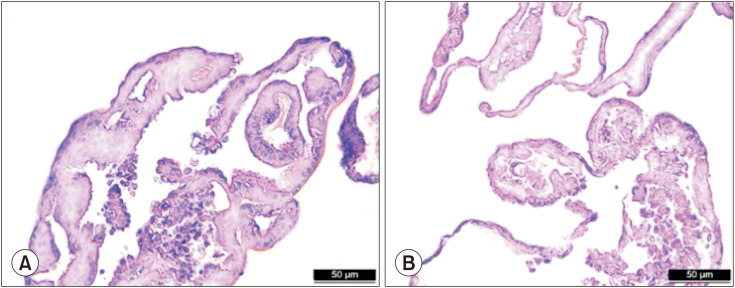
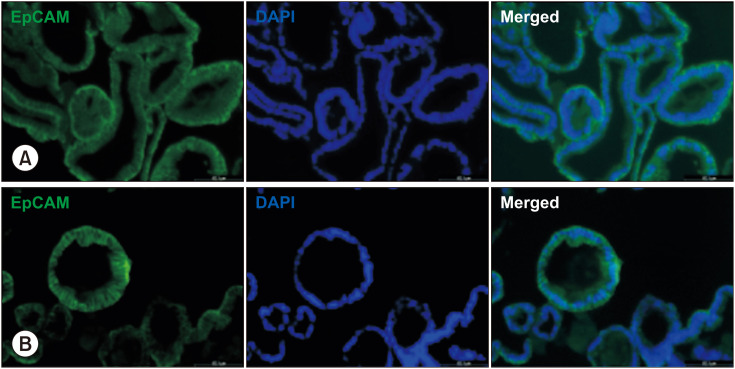
The histological structure of the PDOs on day seven using H&E staining is shown in Fig. 2, which revealed crypt and villus structures. Hematoxylin stains the nuclei of cells dark blue or purple, while eosin stains the cytoplasm and extracellular matrix pink. To confirm the growth of epithelial-specific organoids in our small intestinal crypt culture system, we immunostained the PDOs of normal control and CD patients for EpCAM and DAPI (Fig. 3).
Fig. 2. Distinct crypt and villus structures in normal (A) and CD (B) samples visualized by hematoxylin and eosin staining from paraffin-embedded sections. Scale bars 50 µm. CD: Crohn’s disease.
Fig. 3. Structural analysis of intestinal epithelial cells in human duodenal 3-dimensional organoids from control (A) and CD (B) patients using EpCAM immunofluorescence staining. Representative images show epithelial cell marker with EpCAM (green) and nuclei (blue) with DAPI. Scale bars 60 µm. CD: Crohn’s disease.
Molecular characterization of PDOs in pediatric CD patients
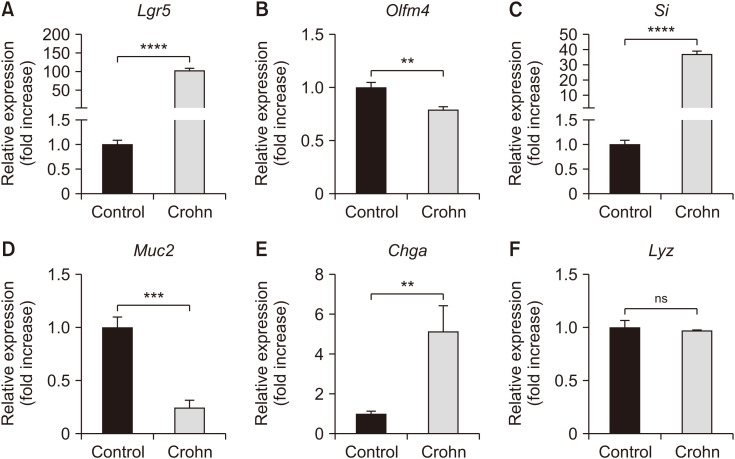
In the RT-qPCR analyses, Lgr5 gene expression was significantly higher in CD patients compared to normal controls (p<0.05, Fig. 4), whereas Olfm4 gene expression was significantly lower (p<0.05, Fig. 4). Additionally, RT-qPCR showed a significant increase in Si and Chga gene expression and a decrease in Muc2 expression in CD patients compared to normal controls (p<0.05, Fig. 4). No significant differences in Lyz expression were observed (Fig. 4).
Fig. 4. mRNA expression of intestine-specific genes between normal and CD patients in human duodenal 3-dimensional organoids. mRNA expression level of stem cell-associated genes (A) Lgr5 and (B) Olfm4, as well as intestinal epithelial maturation-associated genes (C) Si, (D) Muc2, (E) Chga, and (F) Lyz measured using quantitative reverse transcription polymerase chain reaction analyses. Gene expressions presented using 2–ΔΔCt method. Statistical significance differences between groups analyzed by Student’s t-test. ns: not significant, CD: Crohn’s disease. **p<0.01, ***p<0.001, ****p<0.0001.
DISCUSSION
This study describes the methodologies used to establish PDOs from the endoscopic biopsies of normal controls and CD patients. Our study characterized the specific gene expression profiles associated with CD, including increased expression of Lgr5, Si, and Chga, and decreased expression of Olfm4 and Muc2, suggesting alterations in stem cell activity and mucosal barrier function. These findings provide valuable insights into the pathophysiology of CD and a new model for therapeutic research.
We determined that PDOs from both normal controls and CD patients accurately replicated the structural and cellular features of native intestinal tissue, as demonstrated by H&E and IF staining. EpCAM, a marker for epithelial cells, is commonly expressed on the basal or basolateral cell membrane in IF to indicate cell-to-cell adhesion in epithelial tissues, including intestinal organoids [18]. IF staining of EpCAM confirmed that the architecture of the established intestinal organoids closely resembled that of native intestinal tissues.
Additionally, RT-qPCR analyses revealed the expression of various gene markers in both PDOs, including Lgr5 (stem cell marker), Olfm4 (stem cell marker), Si (intestinal epithelial cell marker), Muc2 (goblet cell marker), Chga (neuroendocrine cell marker) and Lyz (Paneth cell marker), indicating that they contain a diverse range of cell types [19].
In PDOs from CD patients, we observed notable differences in gene expression compared to PDOs from normal controls based on RT-qPCR results. Our study shows significantly increased Lgr5 gene expression and decreased Olfm4 gene expression in Crohn’s PDOs compared with normal controls. Several mechanisms can explain these findings. Although Lgr5 and Olfm4 are stem cell markers, they are differentially regulated under different disease conditions. Lgr5 is crucial for maintaining intestinal stem cells and promoting epithelial regeneration, whereas Olfm4 is associated with Paneth cells, which support stem cells but are less involved in their proliferation [20,21]. Chronic inflammation may cause changes in the intestinal epithelium and its microenvironment, thereby affecting stem cell markers. Additionally, increased Lgr5 expression may reflect an adaptive response to damage, whereas decreased Olfm4 expression may indicate impaired Paneth cell function [22]. Thus, the differential expression of Lgr5 and Olfm4 highlights the complex interactions between stem cell biology, inflammation, and tissue regeneration in CD.
A significant increase in Si and Chga gene expression and a significant decrease in Muc2 gene expression were observed in PDOs from CD patients on RT-qPCR. Increased Si expression in CD likely represents an adaptive epithelial response, compensating for digestive function under stress [23]. This upregulation may also indicate changes in epithelial cell differentiation and tissue regeneration during inflammation [24]. Decreased Muc2 expression in CD indicates a compromised mucosal barrier, because Muc2 is crucial for protecting and lubricating the epithelial surface [25]. Lower Muc2 levels suggest that inflammation disrupts the mucosal integrity, exacerbating epithelial damage and vulnerability [26]. Additionally, Chga gene expression analysis revealed increased chromogranin A, indicating a potential rise in neuroendocrine cell populations during inflammation. This upregulation may be an adaptive response of neuroendocrine cells to chronic stress or tissue damage [27]. Overall, these expression profile changes provide insights into the complex alterations in intestinal epithelial cell populations and their potential roles in CD pathology.
CD in children exhibits distinct clinical characteristics that differ significantly from those in adult populations [28]. Pediatric CD often leads to growth retardation, delayed puberty, and a different disease course compared to adult-onset CD [28]. Children with CD frequently present with more aggressive disease behavior, resulting in greater intestinal damage and higher rates of complications compared to adults [29]. Current literature emphasizes the unique pathophysiology and treatment responses in pediatric CD patients, highlighting the need for dedicated pediatric-oriented PDO settings [30]. However, research utilizing PDOs in children is lacking, particularly in Asian cohorts. Our established PDOs in pediatric CD can help advance research and improve clinical outcomes for children with CD.
Our study is the first report from Korea on the establishment of PDOs from mucosal biopsy samples of pediatric CD patients. By successfully generating organoids from patients, this study provides a groundbreaking model for investigating the pathophysiology of CD and predicting drug responses. The ability to perform such studies on organoids rather than directly on patients offers a novel approach for forecasting therapeutic responses. In this study, we performed RT-qPCR using various cell markers, and future experiments could include IF staining for these markers. Additionally, RNA sequencing could be employed to analyze genes and pathways specific to pediatric CD, paving the way for further insights. This study was limited by its small sample size. Our future plans include the creation of a CD organoid bank by collecting mucosal samples from a larger patient cohort.
In conclusion, we successfully established and characterized PDOs from pediatric CD patients, offering a valuable model for understanding the pathophysiology of the disease and evaluating potential therapeutic approaches. The differential gene expression of PDOs in CD patients might be caused by the complex interplay between epithelial adaptation and inflammation in the intestinal epithelium.
Footnotes
Funding: This research was supported and funded by SNUH Lee Kun-hee Child Cancer & Rare Disease Project, Republic of Korea (22C-017-0200), Hallym University Medical Center Research Fund, and the Korean Society of Pediatric Gastroenterology, Hepatology and Nutrition Research Fund. The funding bodies had no role in the study design, collection, analysis, or interpretation of the data or writing of the manuscript.
Conflict of Interest: The authors have no financial conflicts of interest.
References
- 1.Long D. Crohn’s disease and ulcerative colitis: from pathophysiology to novel therapeutic approaches. Biomedicines. 2024;12:689. doi: 10.3390/biomedicines12030689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Orfanoudaki E, Foteinogiannopoulou K, Theodoraki E, Koutroubakis IE. Recent advances in the optimization of anti-TNF treatment in patients with inflammatory bowel disease. J Clin Med. 2023;12:2452. doi: 10.3390/jcm12072452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al Meslamani AZ. Understanding patient variability to biologic treatments in inflammatory bowel disease. Expert Opin Biol Ther. 2024;24:317–319. doi: 10.1080/14712598.2024.2359021. [DOI] [PubMed] [Google Scholar]
- 4.Wlazło M, Meglicka M, Wiernicka A, Osiecki M, Kierkuś J. Dual biologic therapy in moderate to severe pediatric inflammatory bowel disease: a retrospective study. Children (Basel) 2022;10:11. doi: 10.3390/children10010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scheurlen KM, Parks MA, Macleod A, Galandiuk S. Unmet challenges in patients with Crohn’s disease. J Clin Med. 2023;12:5595. doi: 10.3390/jcm12175595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clinton JW, Cross RK. Personalized treatment for Crohn’s disease: current approaches and future directions. Clin Exp Gastroenterol. 2023;16:249–276. doi: 10.2147/CEG.S360248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Urzì O, Gasparro R, Costanzo E, De Luca A, Giavaresi G, Fontana S, et al. Three-dimensional cell cultures: the bridge between in vitro and in vivo models. Int J Mol Sci. 2023;24:12046. doi: 10.3390/ijms241512046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiani AK, Pheby D, Henehan G, Brown R, Sieving P, Sykora P, et al. International Bioethics Study Group. Ethical considerations regarding animal experimentation. J Prev Med Hyg. 2022;63(2 Suppl 3):E255–E266. doi: 10.15167/2421-4248/jpmh2022.63.2S3.2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rudolph JE, Zhong Y, Duggal P, Mehta SH, Lau B. Defining representativeness of study samples in medical and population health research. BMJ Med. 2023;2:e000399. doi: 10.1136/bmjmed-2022-000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ollivier A, Mahe MM, Guasch G. Modeling gastrointestinal diseases using organoids to understand healing and regenerative processes. Cells. 2021;10:1331. doi: 10.3390/cells10061331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim J, Koo BK, Knoblich JA. Human organoids: model systems for human biology and medicine. Nat Rev Mol Cell Biol. 2020;21:571–584. doi: 10.1038/s41580-020-0259-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tian CM, Yang MF, Xu HM, Zhu MZ, Yue NN, Zhang Y, et al. Stem cell-derived intestinal organoids: a novel modality for IBD. Cell Death Discov. 2023;9:255. doi: 10.1038/s41420-023-01556-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niklinska-Schirtz BJ, Venkateswaran S, Anbazhagan M, Kolachala VL, Prince J, Dodd A, et al. Ileal derived organoids from Crohn’s disease patients show unique transcriptomic and secretomic signatures. Cell Mol Gastroenterol Hepatol. 2021;12:1267–1280. doi: 10.1016/j.jcmgh.2021.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laudadio I, Carissimi C, Scafa N, Bastianelli A, Fulci V, Renzini A, et al. Characterization of patient-derived intestinal organoids for modelling fibrosis in Inflammatory bowel disease. Inflamm Res. 2024;73:1359–1370. doi: 10.1007/s00011-024-01901-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gasparetto M, Guariso G. Crohn’s disease and growth deficiency in children and adolescents. World J Gastroenterol. 2014;20:13219–13233. doi: 10.3748/wjg.v20.i37.13219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Rheenen PF, Aloi M, Assa A, Bronsky J, Escher JC, Fagerberg UL, et al. The medical management of paediatric Crohn’s disease: an ECCO-ESPGHAN guideline update. J Crohns Colitis. 2020:jjaa161. doi: 10.1093/ecco-jcc/jjaa161. [DOI] [PubMed] [Google Scholar]
- 17.Senger S, Ingano L, Freire R, Anselmo A, Zhu W, Sadreyev R, et al. Human fetal-derived enterospheres provide insights on intestinal development and a novel model to study necrotizing enterocolitis (NEC) Cell Mol Gastroenterol Hepatol. 2018;5:549–568. doi: 10.1016/j.jcmgh.2018.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ouchi T, Morimura S, Dow LE, Miyoshi H, Udey MC. EpCAM (CD326) regulates intestinal epithelial integrity and stem cells via rho-associated kinase. Cells. 2021;10:256. doi: 10.3390/cells10020256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Basak O, Beumer J, Wiebrands K, Seno H, van Oudenaarden A, Clevers H. Induced quiescence of Lgr5+ stem cells in intestinal organoids enables differentiation of hormone-producing enteroendocrine cells. Cell Stem Cell. 2017;20:177–190.e4. doi: 10.1016/j.stem.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 20.Sakahara M, Okamoto T, Srivastava U, Natsume Y, Yamanaka H, Suzuki Y, et al. Paneth-like cells produced from OLFM4+ stem cells support OLFM4+ stem cell growth in advanced colorectal cancer. Commun Biol. 2024;7:27. doi: 10.1038/s42003-023-05504-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsai YH, Wu A, Wu JH, Capeling MM, Holloway EM, Huang S, et al. Acquisition of NOTCH dependence is a hallmark of human intestinal stem cell maturation. Stem Cell Reports. 2022;17:1138–1153. doi: 10.1016/j.stemcr.2022.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santos AJM, Lo YH, Mah AT, Kuo CJ. The intestinal stem cell niche: homeostasis and adaptations. Trends Cell Biol. 2018;28:1062–1078. doi: 10.1016/j.tcb.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tucci SA, Boyland EJ, Halford JC. The role of lipid and carbohydrate digestive enzyme inhibitors in the management of obesity: a review of current and emerging therapeutic agents. Diabetes Metab Syndr Obes. 2010;3:125–143. doi: 10.2147/dmsott.s7005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rath E, Haller D. Intestinal epithelial cell metabolism at the interface of microbial dysbiosis and tissue injury. Mucosal Immunol. 2022;15:595–604. doi: 10.1038/s41385-022-00514-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dorofeyev AE, Vasilenko IV, Rassokhina OA, Kondratiuk RB. Mucosal barrier in ulcerative colitis and Crohn’s disease. Gastroenterol Res Pract. 2013;2013:431231. doi: 10.1155/2013/431231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grondin JA, Kwon YH, Far PM, Haq S, Khan WI. Mucins in intestinal mucosal defense and inflammation: learning from clinical and experimental studies. Front Immunol. 2020;11:2054. doi: 10.3389/fimmu.2020.02054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eissa N, Hussein H, Tshikudi DM, Hendy GN, Bernstein CN, Ghia JE. Interdependence between chromogranin-A, alternatively activated macrophages, tight junction proteins and the epithelial functions. A human and in-vivo/in-vitro descriptive study. Int J Mol Sci. 2020;21:7976. doi: 10.3390/ijms21217976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin HY, Lim JS, Lee Y, Choi Y, Oh SH, Kim KM, et al. Growth, puberty, and bone health in children and adolescents with inflammatory bowel disease. BMC Pediatr. 2021;21:35. doi: 10.1186/s12887-021-02496-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benchimol EI, Fortinsky KJ, Gozdyra P, Van den Heuvel M, Van Limbergen J, Griffiths AM. Epidemiology of pediatric inflammatory bowel disease: a systematic review of international trends. Inflamm Bowel Dis. 2011;17:423–439. doi: 10.1002/ibd.21349. [DOI] [PubMed] [Google Scholar]
- 30.Lahad A, Weiss B. Current therapy of pediatric Crohn’s disease. World J Gastrointest Pathophysiol. 2015;6:33–42. doi: 10.4291/wjgp.v6.i2.33. [DOI] [PMC free article] [PubMed] [Google Scholar]