Abstract
Purpose
Helicobacter pylori infections differ between children and adults. The Pediatric society practice guidelines recommend against a test-and-treat approach, characterized by the use of non-invasive tests for diagnosis (e.g. urea breath test [UBT] or stool antigen test). However, significant variations exist in clinical practice. This study examined the use of non-invasive testing for the screening and diagnosis of H. pylori infection in children at a tertiary pediatric hospital in Singapore, reviewing both management decisions and patient outcomes.
Methods
A retrospective review was conducted on children between the ages of 0 and 18 years who were tested for H. pylori infection using either a stool antigen test or UBT between January 2018 and June 2020.
Results
Among the 1,397 children tested, 117 (8.4%) had a positive stool H. pylori antigen result, and 5 out of 85 tested (5.9%) had a positive UBT. Abdominal pain was the predominant symptom (n=98; 80.3%). Only 7 treatment-naïve children had biopsy-proven disease. Tissue biopsies for H. pylori culture were sent to 2 children, with 1 negative result. A total of 111 children (91.0%) received treatment, wherein proton pump inhibitor, amoxicillin, and clarithromycin for 14 days was the most common therapeutic regimen. Symptom resolution was observed in 62 children (50.8%).
Conclusion
A test-and-treat strategy was more widely utilized than endoscopy-based testing, showing a low compliance to existing guidelines for the management of H. pylori infections in children at our center and significant false-positive rates.
Keywords: Children, Helicobacter pylori, Diagnosis
INTRODUCTION
Helicobacter pylori is a common chronic bacterial infection worldwide [1] and an important cause of peptic ulcer disease (PUD) and gastric cancer [2]. The incidence of H. pylori infection in Southeast Asia is increasing, with a rising number of adults being tested and treated for this ubiquitous disease. In Singapore, the seroprevalence rate has been reported to be 31% [3]. However, studies on the prevalence and clinical presentation of H. pylori infection in children remain scarce.
Current recommendations for pediatric treatment and management have mostly been adapted from adult practice. Compared to adults, children and adolescents develop PUD and gastric cancer infrequently. Symptoms generally appear only after a long-term infection, and the factors influencing the clearance of acute infection remain largely unknown [4].
The immune mechanisms against infection differ in children. Compared to adults, gastric biopsies obtained from children infected with H. pylori show reduced pathological inflammation [5]. In addition, an increase in the number of immunosuppressive T regulatory cells and anti-inflammatory interleukin (IL)-10 rather than a proinflammatory IL-17 cytokine response has been reported [6].
Therefore, diagnosis and treatment should not be conducted in the same way in children as in adults. The European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) and North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) recommend a test-and-treat approach [7]. Instead, invasive endoscopy-based testing is recommended for valid clinical indications. Studies on children do not support the role of H. pylori infection testing in functional disorders, such as recurrent abdominal pain [8]. The purpose of testing should be to investigate the cause of symptoms and not simply to determine whether H. pylori is present.
Despite the established guidelines, there is significant variation in clinical practice regarding the utilization of empiric treatment based on positive non-invasive H. pylori testing, such as the urea breath test (UBT), stool antigen test, and serology in children [9]. There are clinical challenges in compliance with these guidelines, from advising for invasive endoscopy to obtaining gastric biopsies for culture-directed treatment.
The aim of this clinical audit was to examine the frequency of non-invasive testing as the primary tool for the screening and diagnosis of H. pylori in children at a tertiary pediatric hospital in Singapore and to review management decisions and patient outcomes in patients with positive non-invasive H. pylori tests.
MATERIALS AND METHODS
A retrospective clinical audit of non-invasive H. pylori testing was conducted in a tertiary pediatric hospital. Children between the ages of 0 and 18 years who were tested for H. pylori infection using either the stool antigen test (OXOID Rapid Immunochromatographic Assay) or the Urea Breath Test (13C-UBT) between January 2018 and June 2020 were included. Electronic medical and laboratory records of children with abnormal stool antigen and/or 13C-UBT results were accessed, and relevant information on their demographics, presenting complaints, investigations, treatments, and management plans were analyzed. For children who underwent endoscopy, 2 gastric biopsies from the antrum and 2 gastric biopsies from the corpus were obtained for histological evaluation, as recommended by the ESPGHAN/NASPGHAN guidelines for children. Standard hematoxylin and eosin staining of the gastric tissue was performed to detect H. pylori infection. Children diagnosed with H. pylori infection primarily by endoscopy were excluded. The empirical first-line treatment regimen used at our institution is a proton pump inhibitor (PPI), amoxicillin (AMO), and clarithromycin (CLA) for 14 days. Other regimens include PPI, AMO, and metronidazole (MET). Alternatively, in patients with a penicillin allergy, MET or tetracycline can be used with PPI and CLA, with or without bismuth. Ethics approval was obtained from the Centralized Institutional Review Board (CIRB) for this retrospective clinical audit (approval no. 2022/2574).
RESULTS
Patient characteristics and presenting symptoms
A total of 1,397 children who underwent stool H. pylori antigen testing and 85 children with 13C-UBT performed between January 2018 and June 2020 were identified. Among them, 117 (8.4%) had a positive stool H. pylori antigen result, and 5 (5.9%) had a positive 13C-UBT. The main indication for screening was the presence of abdominal pain. The characteristics of the patients with a positive screening test are described in Table 1. The children were aged 15–210 months (median, 132 months). Notably, 4 children had previously positive results from non-invasive H. pylori tests.
Table 1. Baseline patient characteristics of children with positive screening test.
| Baseline patient characteristics | Number of children (percentage of total) | |
|---|---|---|
| Number of children with positive screening test | 122 | |
| Male | 58 (47.5) | |
| Race | ||
| Chinese | 78 (64.0) | |
| Malay | 18 (14.7) | |
| Indian | 19 (15.6) | |
| Others | 7 (5.7) | |
| Significant past medical history | 20 (16.4) | |
| Previous Helicobacter pylori infection | 4 (3.3) | |
| Asthma | 3 (2.5) | |
| Anorexia nervosa | 1 (0.8) | |
| Crohn’s disease | 1 (0.8) | |
| Gitelman syndrome | 1 (0.8) | |
| Positive family history of Helicobacter pylori | 21 (17.2) | |
| Family history of gastric cancer | 4 (3.3) | |
| Presenting symptoms | ||
| Abdominal pain | 98 (80.3) | |
| Nausea | 48 (39.3) | |
| Loss of appetite | 40 (32.8) | |
| Vomiting | 37 (30.3) | |
| Anemia | 30 (24.6) | |
| Loss of weight | 26 (21.3) | |
| Nocturnal awakening | 24 (19.7) | |
| Acid brash | 13 (10.7) | |
| Blood in stool | 10 (8.2) | |
| Heartburn | 8 (6.6) | |
| Hematemesis | 6 (4.9) | |
| Food impaction | 2 (1.6) | |
Abdominal pain was the predominant symptom (n=98, 80.3%), with the duration of pain varying between 1 and 1,095 days (median, 60 days). Other symptoms, such as nausea, vomiting, acid brash, heartburn, food impaction, hematemesis, blood in stools, and nocturnal awakening, were reported less frequently (Table 1). A total of 21 children (17.2%) had a positive family history of H. pylori infection, and 4 (3.3%) had a history of gastric cancer.
Investigation
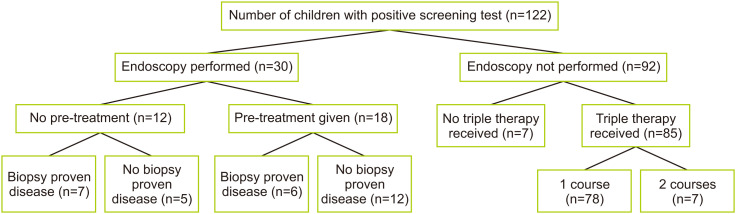
Only 30 children (24.6%) underwent upper gastrointestinal (GI) endoscopy. Among these, 12 underwent endoscopy before the initiation of eradication therapy, 16 underwent at least 1 course of eradication therapy, and 2 underwent ongoing eradication therapy. In order of decreasing prevalence, the most common findings in children after biopsy were stomach erythema, stomach nodularity, duodenal erythema, stomach erosions, mucosal friability, and esophageal erythema. The most common histopathological finding was chronic gastritis, followed by H. pylori inclusion bodies and lymphoid follicles. One child developed active duodenitis. No children had gastric or duodenal ulcers, metaplasia, or carcinomatous changes. The endoscopic and histopathological findings are described in Table 2. In the treatment-naïve group of 12 children, only 7 showed evidence of biopsy-proven disease, with the remainder having false-positive stool antigen test results (Fig. 1). The tissue biopsies for H. pylori culture were sent to 2 children, 1 of whom had a negative result without prior treatment, and the other of which showed H. pylori sensitivity to AMO and MET and resistance to CLA. Among the 6 patients who presented with hematemesis, 3 had previously undergone endoscopy with findings of gastric erosions.
Table 2. Endoscopic and histopathological findings in children who underwent endoscopy.
| Endoscopic findings | Number of children, n=30 (%) | |
|---|---|---|
| Rapid urease test | ||
| Positive | 7 (5.7) | |
| Negative | 16 (12.8) | |
| Not done/unknown | 7 (5.7) | |
| Esophagus erythema | 1 (3.3) | |
| Stomach erythema | 13 (43.3) | |
| Stomach nodularity | 10 (33.3) | |
| Stomach erosions | 2 (6.7) | |
| Mucosal friability | 2 (6.7) | |
| Duodenal erythema | 4 (13.3) | |
| Histopathological findings | ||
| Chronic gastritis | 27 (90.0) | |
| Helicobacter pylori inclusion bodies | 13 (43.3) | |
| Lymphoid follicles | 1 (3.3) | |
| Active duodenitis | 1 (3.3) | |
Fig. 1. Distribution of endoscopic evaluation and treatment of children with positive screening test for Helicobacter pylori.
Treatment
A total of 111 children (91.0%) received triple therapy, 7 (5.7%) were treated with only PPIs, and 4 (3.3%) received no treatment. Overall, 97 children (79.5%) received 1 course, 13 children (10.7%) received 2 courses, and 1 child (0.8%) received 3 courses of triple therapy. The indication for more than 1 course was the persistence of H. pylori infection on non-invasive testing. All 7 treatment-naïve patients with endoscopy-proven disease received only 1 course of AMO/CLA/omeprazole. The various combinations of triple therapies prescribed are detailed in Table 3. One child with Crohn’s received ciprofloxacin/MET/omeprazole, and 1 child with inflammatory abdominal phlegmon received augmentin/CLA/omeprazole.
Table 3. Number of courses and types of triple therapy administered.
| Number of courses of triple therapy | Number of children (%) | |
|---|---|---|
| One course | 97 (79.5) | |
| AMO/CLA/omeprazole | 84 (68.8) | |
| AMO/MET/omeprazole | 7 (5.7) | |
| CLA/MET/omeprazole | 4 (3.3) | |
| Ciprofloxacin/MET/omeprazole | 1 (0.8) | |
| Augmentin/CLA/omeprazole | 1 (0.8) | |
| Two courses | 13 (10.7) | |
| AMO/CLA/omeprazole+AMO/MET/omeprazole | 8 (6.6) | |
| Two courses of AMO/CLA/omeprazole | 4 (3.3) | |
| AMO/CLA/omeprazole+tetracycline/bismuth/MET/omeprazole | 1 (0.8) | |
| Three courses | 1 (0.8) | |
| AMO/CLA/omeprazole+AMO/MET/omeprazole+bismuth/AMO/MET/omeprazole | 1 (0.8) | |
AMO: amoxicillin, CLA: clarithromycin, MET: metronidazole.
Outcomes
Ninety children (73.8%) were referred to a pediatric gastroenterologist for further assessment. The eradication of H. pylori was documented in 56 children (45.9%), among which 12 (9.8%) underwent endoscopy, 28 (23.0%) stool antigen testing, and 16 (13.1%) 13C-UBT. A total of 48 children (39.3%) did not undergo any eradication testing done as they had default follow-up. Symptom resolution was only observed in 62 children (50.8%). Of the 92 children who received treatment based on positive non-invasive testing alone, 32 (34.8%) had documented eradication, mostly via stool antigen test. Fourteen (15.2%) of these children had persistent of symptoms, despite documented eradication in half of them. The majority of the children (n=110, 90.1%) were no longer being followed-up at the time of this publication.
DISCUSSION
This study represents the experience of a single large pediatric tertiary care center in the management of H. pylori infection in Singapore, and is the first known local audit in Singapore to study the non-invasive testing of H. pylori, which has only become readily available and increasingly used in the pediatric population in recent years.
The audit showed that the “test-and-treat” strategy was more widely utilized than endoscopy-based invasive diagnostic testing, with only a small subset of patients having had a gastric biopsy culture. This mirrors the physician management patterns reported in the literature, which highlights a low compliance with existing guidelines [9].
These non-invasive tests may have been favored over endoscopy because they are easily administered with a short turnaround time. The advantages of stool antigen testing include easy sample collection, no need for fasting, and no dependence on operator or clinician experience. There are also fewer risks to patients than endoscopy, which is a highly specialized procedure that requires a referral to a pediatric gastroenterologist and the administration of intravenous sedation for pediatric patients. This makes endoscopy less appealing for caregivers and attending physicians.
Notably, almost half of the treatment-naïve children with a positive screening test who underwent endoscopy did not have biopsy-proven disease, raising concerns about the significantly high false-positive rate of non-invasive screening tests. Different methods of H. pylori stool antigen testing exist and differ in their performance. The conventional stool antigen test using an enzyme-linked immunosorbent assay detects H. pylori antigen using polyclonal or monoclonal antibodies, and the rapid stool antigen test uses immunochromatography [10]. The reported sensitivity ranges from 87% to 100%, with the specificity ranging from 82% to 100% [11,12]. Tests using monoclonal antibodies have been reported to have a higher diagnostic accuracy [13,14].
13C-UBT has a reported sensitivity and specificity of approximately 95% in children [15]. As 13C-UBT easures the isotopic ratio of 13CO2/12 CO2 and endogenous CO2 production differs according to age, sex, body weight, and height, the isotopic ratio may be higher in children despite ingesting an amount of 13C-urea identical to that in adults. Therefore, the 13C-UBT may give false-positive results in children younger than 6 years of age [16,17]: 8.3% in children younger than 6 years of age compared to 0.85% in children older than 6 years [18]. There are also technical difficulties in performing 13C-UBT in young children because they are often unwilling to swallow the substrate.
Abdominal pain was the most common symptom among our cohort of patients who underwent non-invasive H. pylori testing. No association was found between the presence of H. pylori infection and the frequency of functional abdominal pain disorders [19]. Moreover, at present, definitive evidence of the improvement or resolution of symptoms after eradication therapy is lacking [20,21]. This was also evident in the cohort of the present study, in which symptom resolution was observed in only half of patients after H. pylori treatment. Therefore, in cases of abdominal pain in which an organic cause is considered, diagnostic upper endoscopy, rather than non-invasive testing for H. pylori, should be performed.
The “test-and-treat” approach is a reasonable option for adult patients presenting with dyspepsia without alarming features, such as weight loss, dysphagia, overt GI bleeding, abdominal mass, or iron deficiency anemia [22,23,24]. The age threshold depends on the local prevalence of gastric malignancies, ranging from 40 to 60 years [25]. For the evaluation of dyspepsia in adults, this approach showed an economic advantage of cost savings, which was weighed against the relatively lesser benefits of symptom improvement and patient satisfaction with the endoscopy-based strategy [26]. Hence, this “test-and-treat” approach would be more feasible in clinical practice, particularly for primary care physicians who have an important role in the first line management of such adult patients. However, as most children with H. pylori infection remain asymptomatic, empirical treatment is unlikely to outweigh the potential side effects.
A significant proportion of our patients were treated with CLA-based triple therapy based on a positive non-invasive screening test. The disadvantages of empiric therapy for H. pylori include potentially unnecessary treatment, a lack of definitive documentation of the organism and pathology, and most importantly, the use of incorrect antibiotic regimens, with higher treatment failure rates and declining microbial susceptibility [9].
An understanding of the local antibiotic resistance rates is important guideline for empirical therapy. The recommendation includes a 2-week period of first-line therapy, to be guided by antibiotic susceptibility testing or based on local susceptibility data [27,28]. Nonetheless, in Singapore, CLA-based triple therapy has continued to be effective as the first line treatment option, with an eradication rate exceeding 90% reported in adults. Although these numbers seem to favor the use of empirical CLA as a first-line therapy, a lower eradication success rate among native Singapore children (76.7%) has been reported, with non-compliance cited as the most likely contributing factor [29].
Therefore, antibiotic resistance remains a major concern. In many countries, primary CLA resistance has increased to levels above the recommended threshold (15%) for use as a first-line treatment for H. pylori infection [30]. The data indicate that the prevalence of H. pylori infections resistant to CLA in children varies between 10% and 40% depending on the geographical region [31]. Similarly, in Singapore, rising rates of CLA and MET resistance have been reported in both adult and paediatric populations [3]. Immigration factors have also been shown to influence H. pylori antibiotic resistance patterns in children, with non-natives at a higher risk of antimicrobial-resistant strains and poorer eradication outcomes [29].
Singapore has a significant proportion of non-native population, which may come from regions with higher prevalence rates and different antibiotic resistance patterns. This, coupled with the growing H. pylori antibiotic resistance rate, adds to management challenges. Thus, culture-based, susceptibility-guided therapy and a reinforcement of the importance of compliance are becoming crucial in achieving the successful eradication of H. pylori. However, we should also acknowledge possible unique factors, including the inherent differences between patient populations in terms of their acceptance of invasive procedures and their associated healthcare costs, which impact clinical management. Further longitudinal observations and the collection of clinical data on H. pylori infection in children, both regionally and globally, are needed to optimize testing methods for this insidious infection.
The limitations of this study include the inherent verification bias due to the retrospective method of data collection and the high loss-to-follow-up rate, resulting in incomplete data on the patient outcomes, particularly pertaining to eradication rates. In addition, only a small number of gastric biopsy cultures were performed; hence, we were unable to study antibiotic susceptibility in our cohort. However, the findings presented in this study provide an important snapshot of our current real-world practice, allowing us to reflect on the current management challenges and guide the development of future quality improvement processes.
In summary, this study documents the widespread use of the “test-and-treat” approach, highlighting a low compliance to existing guidelines in the management of H. pylori infections in children at our center. More importantly, significant false positive rates were observed in patients who underwent confirmatory endoscopic evaluation, and only modest success was observed in terms of symptom improvement in children with the “test-and-treat” approach based on non-invasive testing. The findings of this audit provide a basis for hospital-wide quality improvement initiatives that seek to improve the testing and treatment of H. pylori infections in children.
Footnotes
Funding: None.
Conflict of Interest: The authors have no financial conflicts of interest.
References
- 1.Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, et al. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology. 2017;153:420–429. doi: 10.1053/j.gastro.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 2.Amieva M, Peek RM., Jr Pathobiology of Helicobacter pylori-induced gastric cancer. Gastroenterology. 2016;150:64–78. doi: 10.1053/j.gastro.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ang TL, Fock KM, Ang D, Kwek AB, Teo EK, Dhamodaran S. The changing profile of Helicobacter pylori antibiotic resistance in Singapore: a 15-year study. Helicobacter. 2016;21:261–265. doi: 10.1111/hel.12291. [DOI] [PubMed] [Google Scholar]
- 4.Ernst PB, Peura DA, Crowe SE. The translation of Helicobacter pylori basic research to patient care. Gastroenterology. 2006;130:188–206. doi: 10.1053/j.gastro.2005.06.032. quiz 212-3. [DOI] [PubMed] [Google Scholar]
- 5.Whitney AE, Guarner J, Hutwagner L, Gold BD. Helicobacter pylori gastritis in children and adults: comparative histopathologic study. Ann Diagn Pathol. 2000;4:279–285. doi: 10.1053/adpa.2000.17871. [DOI] [PubMed] [Google Scholar]
- 6.Freire de Melo F, Rocha AM, Rocha GA, Pedroso SH, de Assis Batista S, Fonseca de Castro LP, et al. A regulatory instead of an IL-17 T response predominates in Helicobacter pylori-associated gastritis in children. Microbes Infect. 2012;14:341–347. doi: 10.1016/j.micinf.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Jones NL, Koletzko S, Goodman K, Bontems P, Cadranel S, Casswall T, et al. ESPGHAN, NASPGHAN. Joint ESPGHAN/NASPGHAN guidelines for the management of Helicobacter pylori in children and adolescents (update 2016) J Pediatr Gastroenterol Nutr. 2017;64:991–1003. doi: 10.1097/MPG.0000000000001594. [DOI] [PubMed] [Google Scholar]
- 8.Spee LA, Madderom MB, Pijpers M, van Leeuwen Y, Berger MY. Association between Helicobacter pylori and gastrointestinal symptoms in children. Pediatrics. 2010;125:e651–e669. doi: 10.1542/peds.2010-0941. [DOI] [PubMed] [Google Scholar]
- 9.Bonilla S, Mitchell PD, Mansuri I. Low adherence to society guidelines for the management of Helicobacter pylori among pediatric gastroenterologists. J Pediatr Gastroenterol Nutr. 2021;73:178–183. doi: 10.1097/MPG.0000000000002976. [DOI] [PubMed] [Google Scholar]
- 10.Yang HR. Updates on the diagnosis of Helicobacter pylori infection in children: what are the differences between adults and children? Pediatr Gastroenterol Hepatol Nutr. 2016;19:96–103. doi: 10.5223/pghn.2016.19.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oderda G, Rapa A, Ronchi B, Lerro P, Pastore M, Staiano A, et al. Detection of Helicobacter pylori in stool specimens by non-invasive antigen enzyme immunoassay in children: multicentre Italian study. BMJ. 2000;320:347–348. doi: 10.1136/bmj.320.7231.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ni YH, Lin JT, Huang SF, Yang JC, Chang MH. Accurate diagnosis of Helicobacter pylori infection by stool antigen test and 6 other currently available tests in children. J Pediatr. 2000;136:823–827. [PubMed] [Google Scholar]
- 13.Leal YA, Cedillo-Rivera R, Simón JA, Velázquez JR, Flores LL, Torres J. Utility of stool sample-based tests for the diagnosis of Helicobacter pylori infection in children. J Pediatr Gastroenterol Nutr. 2011;52:718–728. doi: 10.1097/MPG.0b013e3182077d33. [DOI] [PubMed] [Google Scholar]
- 14.Zhou X, Su J, Xu G, Zhang G. Accuracy of stool antigen test for the diagnosis of Helicobacter pylori infection in children: a meta-analysis. Clin Res Hepatol Gastroenterol. 2014;38:629–638. doi: 10.1016/j.clinre.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Leal YA, Flores LL, Fuentes-Pananá EM, Cedillo-Rivera R, Torres J. 13C-urea breath test for the diagnosis of Helicobacter pylori infection in children: a systematic review and meta-analysis. Helicobacter. 2011;16:327–337. doi: 10.1111/j.1523-5378.2011.00863.x. [DOI] [PubMed] [Google Scholar]
- 16.Guarner J, Kalach N, Elitsur Y, Koletzko S. Helicobacter pylori diagnostic tests in children: review of the literature from 1999 to 2009. Eur J Pediatr. 2010;169:15–25. doi: 10.1007/s00431-009-1033-x. [DOI] [PubMed] [Google Scholar]
- 17.Pacheco SL, Ogata SK, Machado RS, Patrício FR, Pardo ML, Kawakami E. Diagnosis of Helicobacter pylori infection by means of reduced-dose ¹³C-urea breath test and early sampling of exhaled breath. J Pediatr Gastroenterol Nutr. 2013;57:607–611. doi: 10.1097/MPG.0b013e3182a02608. [DOI] [PubMed] [Google Scholar]
- 18.Yang HR, Seo JK. Diagnostic accuracy of the 13C-urea breath test in children: adjustment of the cut-off value according to age. J Gastroenterol Hepatol. 2005;20:264–269. doi: 10.1111/j.1440-1746.2004.03541.x. [DOI] [PubMed] [Google Scholar]
- 19.Jaime F, Villagrán A, Hernández C, Ortiz M, Serrano C, Harris PR. Functional gastrointestinal disorders in children from low socio-economic status and Helicobacter pylori infection. Child Care Health Dev. 2018;44:319–325. doi: 10.1111/cch.12486. [DOI] [PubMed] [Google Scholar]
- 20.Farrell S, Milliken I, Murphy JL, Wootton SA, McCallion WA. Nonulcer dyspepsia and Helicobacter pylori eradication in children. J Pediatr Surg. 2005;40:1547–1550. doi: 10.1016/j.jpedsurg.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 21.Bektas M, Soykan I, Altan M, Alkan M, Ozden A. The effect of Helicobacter pylori eradication on dyspeptic symptoms, acid reflux and quality of life in patients with functional dyspepsia. Eur J Intern Med. 2009;20:419–423. doi: 10.1016/j.ejim.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 22.Sugano K, Tack J, Kuipers EJ, Graham DY, El-Omar EM, Miura S, et al. faculty members of Kyoto Global Consensus Conference. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64:1353–1367. doi: 10.1136/gutjnl-2015-309252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fallone CA, Chiba N, van Zanten SV, Fischbach L, Gisbert JP, Hunt RH, et al. The Toronto consensus for the treatment of Helicobacter pylori infection in adults. Gastroenterology. 2016;151:51–69.e14. doi: 10.1053/j.gastro.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 24.Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol. 2017;112:212–239. doi: 10.1038/ajg.2016.563. [DOI] [PubMed] [Google Scholar]
- 25.Chew CA, Lye TF, Ang D, Ang TL. The diagnosis and management of H. pylori infection in Singapore. Singapore Med J. 2017;58:234–240. doi: 10.11622/smedj.2017037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ford AC, Qume M, Moayyedi P, Arents NL, Lassen AT, Logan RF, et al. Helicobacter pylori “test and treat” or endoscopy for managing dyspepsia: an individual patient data meta-analysis. Gastroenterology. 2005;128:1838–1844. doi: 10.1053/j.gastro.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 27.Kato S, Shimizu T, Toyoda S, Gold BD, Ida S, Ishige T, et al. Japanese Society for Pediatric Gastroenterology, Hepatology and Nutrition. The updated JSPGHAN guidelines for the management of Helicobacter pylori infection in childhood. Pediatr Int. 2020;62:1315–1331. doi: 10.1111/ped.14388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ang TL, Ang D. Helicobacter pylori treatment strategies in Singapore. Gut Liver. 2021;15:13–18. doi: 10.5009/gnl19308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang JG, Lim SYS, Aw MM, Quak SH. Antibiotic resistance patterns and therapeutic outcomes of pediatric Helicobacter pylori infection in a high-migrant Singaporean cohort. Helicobacter. 2022;27:e12868. doi: 10.1111/hel.12868. [DOI] [PubMed] [Google Scholar]
- 30.Graham DY, Lee YC, Wu MS. Rational Helicobacter pylori therapy: evidence-based medicine rather than medicine-based evidence. Clin Gastroenterol Hepatol. 2014;12:177–86.e3. doi: 10.1016/j.cgh.2013.05.028. Discussion e12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiong LJ, Tong Y, Wang Z, Mao M. Detection of clarithromycin-resistant Helicobacter pylori by stool PCR in children: a comprehensive review of literature. Helicobacter. 2013;18:89–101. doi: 10.1111/hel.12016. [DOI] [PubMed] [Google Scholar]