Abstract
The purpose of this study was to determine whether the heart in large mammals contains cardiac progenitor cells that regulate organ homeostasis and regenerate dead myocardium after infarction. We report that the dog heart possesses a cardiac stem cell pool characterized by undifferentiated cells that are self-renewing, clonogenic, and multipotent. These clonogenic cells and early committed progeny possess a hepatocyte growth factor (HGF)–c-Met and an insulin-like growth factor 1 (IGF-1)-IGF-1 receptor system that can be activated to induce their migration, proliferation, and survival. Therefore, myocardial infarction was induced in chronically instrumented dogs implanted with sonomicrometric crystals in the region of the left ventricular wall supplied by the occluded left anterior descending coronary artery. After infarction, HGF and IGF-1 were injected intramyocardially to stimulate resident cardiac progenitor cells. This intervention led to the formation of myocytes and coronary vessels within the infarct. Newly generated myocytes expressed nuclear and cytoplasmic proteins specific of cardiomyocytes: MEF2C was detected in the nucleus, whereas α-sarcomeric actin, cardiac myosin heavy chain, troponin I, and α-actinin were identified in the cytoplasm. Connexin 43 and N-cadherin were also present. Myocardial reconstitution resulted in a marked recovery of contractile performance of the infarcted heart. In conclusion, the activation of resident primitive cells in the damaged dog heart can promote a significant restoration of dead tissue, which is paralleled by a progressive improvement in cardiac function. These results suggest that strategies capable of activating the growth reserve of the myocardium may be important in cardiac repair after ischemic injury.
Keywords: cardiac stem cells, myocardial infarction, myocardial regeneration
In the last few years, endothelial progenitor cells, mononuclear bone marrow cells, skeletal myoblasts, and unfractionated bone marrow have been used in the treatment of the postinfarcted heart in animals and humans (1, 2). Although different cell populations were used and the route of injection of the cells was different, consistent positive results have been obtained acutely and chronically. Despite these encouraging preliminary findings, the mechanism responsible for the improvement in cardiac function has been elusive. The possibility of transdifferentiation of these cells in myocytes and coronary vessels and actual myocardial regeneration has been challenged (3, 4), and alternative interpretations have been offered. The most frequent claim involves the ability of the injected cells to exert a paracrine effect on the resident progenitor cells. However, it has recently been shown that bone marrow cells can form myocardium independently of a paracrine component (5). These contrasting observations do not exclude that direct activation of the cardiac stem cell (CSC) pool may have a more powerful and beneficial impact on the repair of the damaged heart (6).
Primitive cells in the bone marrow, some areas of the brain, skeletal muscle, and the heart share in different proportions the stem cell-related antigens c-kit, MDR1, and Sca-1-like (6–11). Additionally, injection of CSCs locally or systemically promotes myocardial regeneration after infarction in rats and mice (6, 7). Although preliminary evidence of primitive cells expressing stem cell antigens has been obtained in the human heart (12, 13), the actual growth potential of these cells and their ability to form de novo myocardium in humans or in large mammals is lacking. Therefore, questions have been raised on the clinical and therapeutic implications that resident CSCs may have in the treatment of ischemic heart disease. Myocardial infarction leads to a cardiomyopathy that accounts for >60% of the cases of heart failure in the patient population (14).
Hepatocyte growth factor (HGF) activates the c-Met receptor, leading to the formation of matrix metalloproteinases (15) that digest collagen and other extracellular components of the interstitium, facilitating cell migration and homing in the brain (16) and other organs. Importantly, HGF enhances vessel growth and favors cell–extracellular matrix interaction, which may be critical during myocardial regeneration after infarction. Stimulation of insulin-like growth factor 1 (IGF-1) receptors by IGF-1 prevents cell death and induces division and differentiation of neural stem cells (17) and cardiomyocytes (18), suggesting that HGF and IGF-1 may have complementary function in the translocation and proliferation of progenitor cells, respectively. Because of these issues, we have isolated and characterized a CSC in the canine heart. Moreover, we have identified that HGF and IGF-1 can activate CSCs and early committed cells (ECCs) located in proximity of infarcted myocardium in chronically instrumented dogs. After activation, these primitive cells reconstitute a large portion of the dead myocardium with the reappearance of contractile function in the infarcted segment of the wall.
Materials and Methods
Cardiac Progenitor Cells. Cardiac c-kit, MDR1, or Sca-1-positive cells were isolated, and lineage-negative cells (i.e., CSCs) were obtained. Subsequently, CSCs were sorted for clonal analysis and migration and invasion assays. The effects of HGF and IGF-1 on cell death and growth were then determined. (See Supporting Materials and Methods, which is published as supporting information on the PNAS web site.)
Surgery and Hemodynamics. Sonomicrometers were implanted in the subendocardial region supplied by the occluded left descending coronary artery, and HGF and IGF-1 were injected in the myocardium adjacent to the infarct. Echocardiographic, hemodynamic, and structural parameters were obtained at different time points after infarction (see Supporting Materials and Methods).
Statistics. All data were collected blindly, and the code was broken at the end of the experiment (see Supporting Materials and Methods).
Results
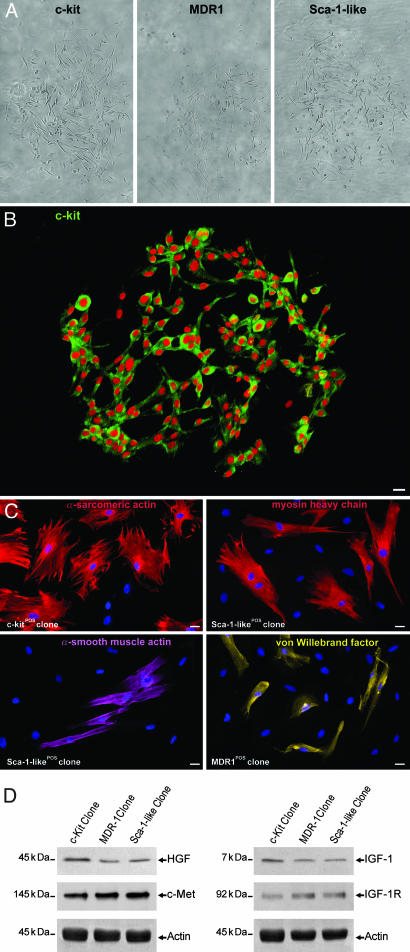
Identification and Cloning of CSCs. Canine CSCs expressed in different proportions the stem cell-related antigens, c-kit, MDR1, and Sca-1-like (Fig. 7, which is published as supporting information on the PNAS web site). These CSCs were negative for markers of hematopoietic cell lineages, cardiac and skeletal muscle transcription factors, and specific cardiac cell cytoplasmic proteins. Most primary antibodies were directly labeled with fluorochromes to avoid unspecific staining and cross-reactivity (Table 1, which is published as supporting information on the PNAS web site). Only ≈5% of CSCs were positive for c-kit, MDR1, or Sca-1-like epitope; >60% possessed the three antigens, and ≈8% had two. There was an average of one CSC per 18,000 myocytes. After enzymatic dissociation, cardiac c-kitPOS, MDR1POS, and Sca-1-likePOS cells were separated by immunomagnetic microbeads and FACS sorting (Fig. 8, which is published as supporting information on the PNAS web site). Single cell cloning (6) was performed by dilution technique and FACS sorting (Fig. 9 A–C, which is published as supporting information on the PNAS web site). Together, from ≈22,000 cell depositions, 216 small clones (automated cloning = 64 and dilution cloning = 152) were obtained. After fixation, 52 clones were analyzed by immunocytochemistry. Most of the clones (42 of 52) were made of undifferentiated cells expressing only stem cell antigens (Fig. 1 A and B). The remaining 10 had primitive cells and cells positive for transcription factors and cytoplasmic proteins specific of cardiomyocytes, smooth muscle cells (SMCs), and endothelial cells (ECs).
Fig. 1.
Cardiac progenitor cells. (A and B) Individual progenitor cells positive for c-kit, MDR1, or Sca-1-like, when placed in single wells, generate multicellular clones (A, phase contrast microscopy). A clone generated by a single c-kit-positive cell (B, green) is also illustrated. (C) Clones expanded in differentiating medium give rise to myocytes, SMCs, and ECs. (Scale bars, 10 μm.) (D) Cardiac progenitor cells express HGF, c-Met, IGF-1, and IGF-1 receptors by Western blotting. Actin shows loading conditions.
Differentiation of Clonogenic CSCs. Of the original 216 clones, 38 were characterized (13 for c-kit, 13 for MDR1, and 12 for Sca-1-like; automated cloning = 21 and dilution cloning = 17). After 1 week in differentiation medium, 25 clones developed cells with phenotype of cardiomyocytes, SMCs, ECs, and fibroblasts. Additionally, c-kitPOS, MDR1POS, and Sca-1-likePOS cells, which expressed transcription factors of cardiac cell lineages (GATA-4, MEF2C, GATA-6, and Ets1), were detected in the different preparations (Fig. 10, which is published as supporting information on the PNAS web site). Five subclones were obtained and showed properties essentially identical to those of the primary clones. Similar protocols were used to collect progenitor cells that had only one surface antigen. This collection was accomplished by (i) using immunobeads coated with the antibody of interest and (ii) depleting the collected pool of cells positive for the other two antigens; the depletion was accomplished through negative selection with immunobeads. Finally, single cells were plated in individual wells by FACS sorting and automated deposition. Forty-one clones were developed (11 c-kitPOS, 16 MDR1POS, and 14 Sca-1-likePOS), and five to seven clones obtained from each cell class were cultured in differentiation medium, and their progeny were characterized. The expanded clones behaved similarly, and each of them gave rise to all cardiac cell lineages (Fig. 1C), mimicking the growth of the clones derived from CSCs with multiple antigens.
CSC Antigens and Growth and Differentiation Properties. To determine whether the expression of distinct antigens on CSCs influenced quantitatively the differentiated progeny, the relative proportion of cardiomyocytes, SMCs, and ECs formed from each clone was measured. We found no statistical difference in the percentage of each cardiac cell type generated from founder cells that exhibited the three antigens or were only c-kitPOS, MDR1POS, or Sca-1-likePOS. In all cases, the generation of myocytes (≈50%) and SMCs (≈40%) was higher than ECs (≈10%) (Fig. 11, which is published as supporting information on the PNAS web site). However, when the same number of cells from each distinct clone was placed in differentiating medium, the total number of committed cells differed, whether they were generated from c-kitPOS, MDR1POS, or Sca-1-likePOS clonogenic cells or from c-kit-MDR1-Sca-1-likePOS cells. Over a period of 4 weeks, 10.5 × 105, 3.9 × 105, and 2.4 × 105 cells were formed from c-kitPOS, MDR1POS, and Sca-1-likePOS cells, respectively. c-kit-MDR1-Sca-1-likePOS clonogenic cells created 1.6 × 105 cells. The ability of clonogenic c-kitPOS cells to create myocardial cells was 2.3-, 5.9-, and 7.6-fold greater than MDR1POS, Sca-1-likePOS, and c-kit-MDR1-Sca-1-likePOS clonogenic cells, respectively (Fig. 12, which is published as supporting information on the PNAS web site). Clonogenic MDR1POS cells produced 2.6- and 3.4-fold more myocardial cells than did Sca-1-likePOS and c-kit-MDR1-Sca-1-likePOS cells. In all cases, little difference was found between the growth of Sca-1-likePOS and c-kit-MDR1-Sca-1-likePOS clonogenic cells.
CSCs, HGF, and IGF-1. CSCs of the mouse and rat heart possess the HGF–c-Met receptor and the IGF-1–IGF-1 receptor systems. The first is implicated in cell migration and, to a lesser extent, in cell growth and survival, whereas the second has a predominant effect on cell multiplication and viability without affecting cell motility (19). To test whether identical systems were present in the canine CSC compartment, these growth factors (GFs) and receptor proteins were identified in CSCs and ECCs by Western blot (Fig. 1D), and in vitro studies of cell motility were performed.
Migration and invasion assays showed that HGF exerted a powerful chemoattractive and translocation effect that was not detected with IGF-1. IGF-1 in combination with HGF did not increase the degree of cell migration obtained by HGF alone. Modest motogenic effects were seen with basic FGF, granulocyte colony-stimulating factor (CSF), granulocyte–macrophage-CSF, EGF, stromal cell-derived factor 1, and VEGF, whereas an intermediate response was observed with stem cell factor (SCF). The inhibition of matrix metalloproteinase (MMP)-2/MMP-9 significantly abrogated the invasive properties of cardiac progenitor cells stimulated by HGF or SCF (Fig. 13, which is published as supporting information on the PNAS web site). To characterize further the role of HGF and IGF-1 in cell growth and cell death, CSCs-ECCs were cultured in serum-free medium in the presence of HGF and/or IGF-1. Both GFs significantly increased cell survival, but the antiapoptotic effect of IGF-1 was ≈2-fold greater than that of HGF. Together, HGF and IGF-1 did not improve the level of cell viability reached by IGF-1 alone. Similarly, IGF-1 resulted in a degree of cell proliferation that was ≈2-fold higher than that induced by HGF. There was no synergy between the two GFs in the potentiation of cell division (Fig. 14, which is published as supporting information on the PNAS web site). The stimulation of CSCs-ECCs by HGF or IGF-1 led to the synthesis and secretion of the corresponding GF (Fig. 15, which is published as supporting information on the PNAS web site). Thus, HGF and IGF-1 may protect in vivo CSCs-ECCs from death signals present during the translocation of these cells to the damaged myocardium.
Activation of CSCs After Infarction. Chronically instrumented dogs were used. In each dog, one or two sets of sonomicrometer crystals were implanted in the myocardial territory supplied by the left anterior descending coronary artery (LAD) 2 weeks before the occlusion of the vessel. After coronary occlusion, the functional evolution of the infarcted heart was measured weekly over a period of 28 days. The structural properties of the damaged myocardium were analyzed at 28 days. Dogs were infarcted by inflating a hydraulic occluder around the LAD. Within minutes, the myocardium comprised within the crystals and supplied by the occluded artery ceased contracting. Four hours later, when paradoxical motion of the infarcted region was observed, HGF and IGF-1 were injected in the border zone to mobilize and activate the CSCs-ECCs distributed in the surrounding viable myocardium. This interval was selected because 4 h after coronary occlusion, there is little or no recovery of contractile function, and all myocytes within the infarct are dead, mostly by apoptosis in the absence of inflammation (20). The lack of inflammation may improve the viability and growth of progenitor cells.
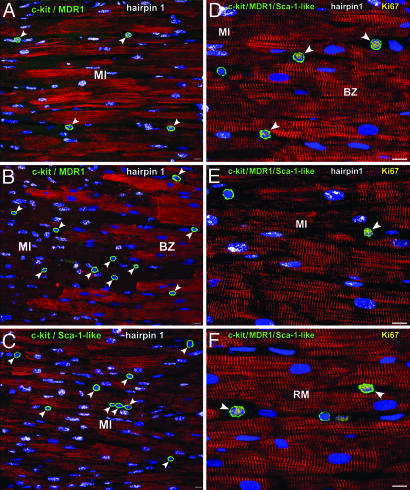
The localization, activation, and lineage commitment of c-kit, MDR1, and Sca-1-positive progenitor cells 8 h after coronary artery occlusion and 4 h after the administration of HGF and IGF-1 are discussed first. In nontreated infarcted dogs (n = 3), the number of progenitor cells in the myocardium adjacent to and distant from the infarct was similar to that found in corresponding areas of sham-operated controls. Progenitor cells within the infarct of dogs not injected with GFs were all dead by apoptosis. Conversely, in GF-treated infarcted dogs (n = 4), the number of progenitors per cm3 of myocardium increased ≈11-, 16-, and 7-fold in the infarct, border zone, and remote area, respectively (Fig. 2 A–C; see also Fig. 16, which is published as supporting information on the PNAS web site).
Fig. 2.
Cardiac progenitor cells in the acutely infarcted heart. Sections of infarcted myocardium 8 h after coronary occlusion in a nontreated (A) and a GF-treated (B–F) heart. (A) c-kit-MDR1-positive cardiac progenitor cells (green, arrowheads) and myocytes are dead by apoptosis (hairpin 1, white). (B) The viable myocardium of the border zone (BZ) and the dead myocardium of the infarcted region (MI) are shown. c-kit-MDR1-positive cells (green, arrowheads) are viable as shown by the absence of hairpin 1 and the presence of propidium iodide staining of their nuclei. In contrast, myocytes are all apoptotic (hairpin 1, white). (C) Similarly, in the center of the infarcted segment, c-kit-Sca-1-like-positive cells (green, arrowheads) are viable and dispersed among dead myocytes. (D and E) Similarly, c-kit-MDR1-Sca-1-like-positive progenitor cells (green) in the border (D), infarct (E), and remote myocardium (RM) (F) of GF-treated hearts express Ki67 (yellow, arrowheads). In D and E, the replicating progenitor cells are located between dead myocytes (hairpin 1, white). (Scale bars, 10 μm.)
The nuclear protein Ki67 was used to measure the number of progenitors within the cell cycle. The number of Ki67-labeled CSCs-ECCs per cm3 of tissue was ≈44-, 81-, and 39-fold higher in the infarct, border zone, and remote myocardium of GF-treated infarcted dogs than in the equivalent anatomical regions of nontreated infarcted and sham-operated animals. Moreover, in GF-treated animals, the value of Ki67-positive progenitors in the border of the infarct was 2-fold greater than in the other two regions of the ventricle (Fig. 2 D–F; see also Fig. 17, which is published as supporting information on the PNAS web site). The analysis of apoptosis by hairpin 1 and necrosis by hairpin 2 in situ ligation showed that the death of progenitor cells increased in both groups of infarcted hearts. But the GFs markedly attenuated cell death in treated infarcted hearts (Fig. 18, which is published as supporting information on the PNAS web site). HGF and IGF-1 had positive effects on the mobilization, proliferation, and survival of cardiac progenitor cells. However, these acute cellular responses did not ameliorate the contractile behavior of the infarcted segments at this early time (data not shown).
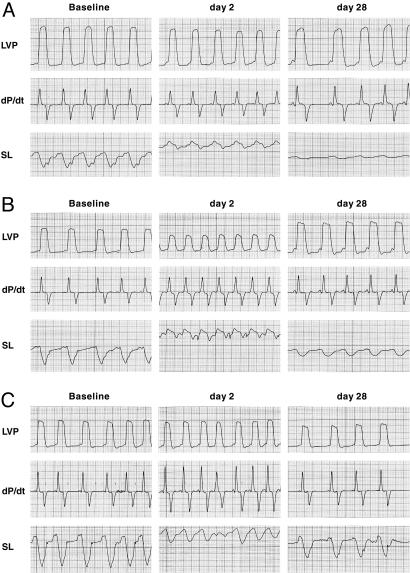
Recovery of Function in the Infarcted Myocardium. To determine whether activation of resident progenitor cells led, with time, to the reinstitution of contractile activity within the dead myocardium, the changes in the dyskinetic and hypokinetic regions of the infarcted segments included within the sonomicrometers were examined over a period of 28 days. In GF-treated dogs (n = 8), segments that had paradoxical motion (holosystolic bulging) at 2 days (n = 6) showed a significant degree of recovery of contraction at 1 week. Fractional shortening continued to improve at 2 and 3 weeks, reaching 50% control value at 4 weeks (Fig. 3). Stroke work reversed from a negative [–18 ± 11 mm × mmHg (1 mmHg = 133 Pa)] to a positive (53 ± 10 mm × mmHg) value. In contrast, paradoxical motion of the infarcted segments (n = 4) in nontreated dogs (n = 6) never recovered contractile activity, and dyskinesis persisted throughout (Fig. 19, which is published as supporting information on the PNAS web site).
Fig. 3.
GFs improve regional cardiac performance after infarction. Myocardial contraction measured by sonomicrometer crystals is shown. (Left) Baseline conditions before coronary artery occlusion. (Center) Recordings at 2 days after infarction. (Right) Recordings at 28 days after infarction. (A) Data from an infarcted segment from a nontreated heart. (B and C) Data from infarcted segments from GF-treated hearts. All panels show data from infarcted segments with holosystolic bulging. In A, loss of function and paradoxical motion at 2 days (Bottom Center) is still present at 28 days (Bottom Right). Conversely, in B and C, the loss of function and paradoxical motion at 2 days (Bottom Center in each) is followed by significant recovery of contraction at 28 days (Bottom Right in each). LVP, left ventricular pressure; SL, segment length.
The collateral circulation of the canine heart partially protects the myocardium from ischemic events so that coronary occlusion may result in hypokinesis instead of dyskinesis of the affected segments. In these cases, partial restoration of contraction was observed from 1 to 4 weeks in both groups of infarcted dogs. However, fractional shortening in hypokinetic segments (n = 5) of GF-treated dogs returned to normal values; a 50% defect persisted in hypokinetic segments (n = 6) of nontreated dogs (Figs. 19 and 20, which are published as supporting information on the PNAS web site). Stroke volume more than doubled, and ejection fraction increased 61% in infarcted treated dogs from 2 days to 4 weeks (n = 6 in this case). Conversely, no significant changes occurred in untreated infarcted dogs during the same interval (Fig. 19). Infarct size measured by the fraction of myocytes lost by the left ventricle was 30% larger in treated (26 ± 9%) than in untreated (20 ± 7%) dogs. These values corresponded to a loss of 600 × 106 and 460 × 106 myocytes, respectively.
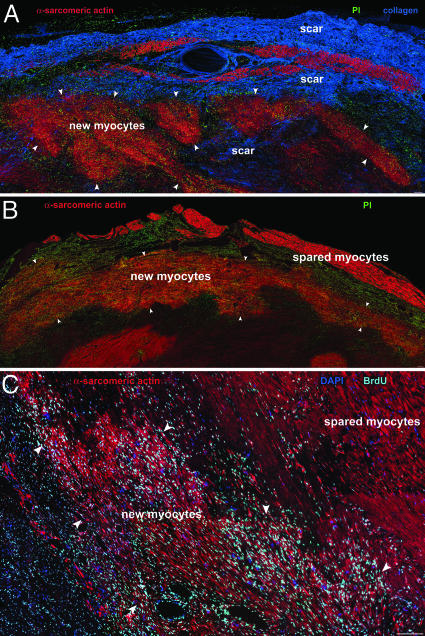
Regeneration of Infarcted Myocardium. A possible mechanism for the improvement in myocardial performance of the 11 infarcted segments from treated dogs was the regeneration of myocytes and coronary vessels mediated by the administration of GFs. In contrast, the absence or modest amelioration of myocardial contraction in the 10 infarcted segments from nontreated dogs was consistent with the lack or, at most, limited formation of parenchymal cells and coronary vasculature. To facilitate the recognition of newly formed myocytes, arterioles, and capillaries, animals were injected daily with BrdUrd for the entire 28-day period. Extensive foci of myocardial regeneration were found in each of the 11 samples of infarcted myocardium obtained from GF-treated hearts (Fig. 4). However, myocardial regeneration was absent in the 10 specimens from untreated hearts. The de novo formation of myocardium consisted of myocytes and dispersed coronary arterioles and capillary profiles surrounded by connective tissue (Fig. 5A).
Fig. 4.
Myocardial regeneration after infarction in GF-treated hearts. Newly formed myocytes are clustered together (α-sarcomeric actin, red, arrowheads). Areas of scarring are visible in A (collagen I-III, blue). (C) Bright fluorescence in nuclei corresponds to BrdUrd labeling of accumulated newly formed myocytes. PI, propidium iodide (green). (Scale bar, 100 μm.)
Fig. 5.
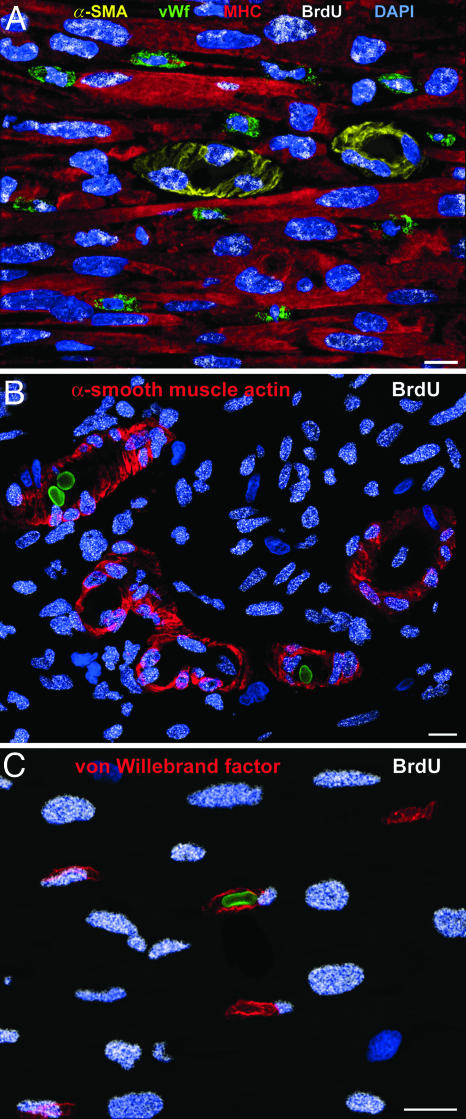
Formation of coronary vessels after infarction in GF-treated hearts. (A) Higher magnification of regenerated myocytes (cardiac myosin heavy chain, red) and coronary arterioles in which SMCs are positive for α-smooth muscle actin (yellow) and ECs are positive for von Willebrand factor (green). (B and C) Arterioles (B) and capillaries (C) contain red blood cells (green). BrdUrd appears as white fluorescence dots in nuclei. (Scale bars, 10 μm.)
The new myocytes were small and oriented in parallel and were labeled by BrdUrd. They expressed cardiomyocyte nuclear and cytoplasmic proteins. MEF2C was clearly detected in the nucleus, whereas α-sarcomeric actin, cardiac myosin heavy chain, troponin I, and α-actinin were identified in the cytoplasm (Fig. 21, which is published as supporting information on the PNAS web site). The junctional proteins, connexin 43, and N-cadherin were seen at the surface of these developing myocytes (Fig. 21). The new arterioles and capillaries were also labeled by BrdUrd. The presence of red blood cells within the lumen (Fig. 5 B and C) suggested that these vessels were functional and connected with the primary coronary circulation.
The volume of regenerated myocytes varied from 400 to 17,000 μm3, with an average volume of 2,300 ± 600 μm3. Only a few of these myocytes had a volume of 10,000 μm3 or larger (Fig. 21), indicating that the majority of cells resembled fetal-neonatal myocytes (2); adult canine myocytes have a volume of 25,000 μm3 (21). Together, 1.3 × 109 myocytes were formed by the administration of GFs, markedly exceeding the 600 × 106 cells lost by the infarcted ventricle. However, because of the small size of the new myocytes, only 17% of the dead myocyte mass was reconstituted. Moreover, there were 95 ± 33 BrdUrd-positive resistance arterioles and 920 ± 220 BrdUrd-positive capillaries per mm2 of tissue: significantly less than the ≈4,000 capillaries per mm2 present in the adult dog heart.
The regeneration of myocytes and vascular structures was not detected in the infarcted segments of nontreated dogs. However, a relevant degree of myocyte proliferation was observed in the surviving myocardium bordering the infarcted segments in both groups of dogs (Fig. 22, which is published as supporting information on the PNAS web site). Over a period of 28 days of labeling, 15% BrdUrd-positive myocytes were found in the border zone of GF-treated infarcted hearts, whereas only 7% BrdUrd-positive cells were noted in the corresponding region of nontreated infarcted hearts. BrdUrd-labeled myocytes were less frequent and similar in magnitude in the remote myocardium of both groups of dogs (Fig. 23, which is published as supporting information on the PNAS web site). Myocardial regeneration in treated animals was accompanied by attenuation of reactive hypertrophy in the spared myocytes (Fig. 24, which is published as supporting information on the PNAS web site).
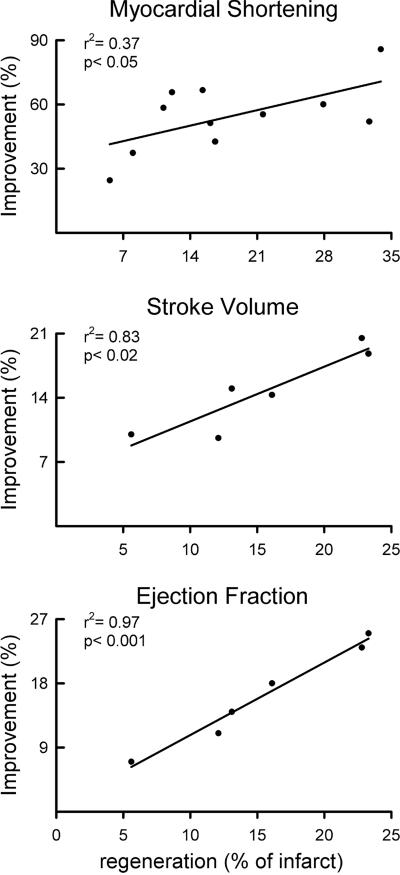
Myocardial Regeneration and Functional Recovery. To test whether the reconstitution of the dead tissue and myocyte proliferation and hypertrophy in the surviving myocardium were the mechanisms of functional recovery of the injured heart, these processes were correlated with segmental shortening in the infarcted regions and stroke volume and ejection fraction of the ventricle. The extent of tissue regeneration in each of the 11 hypokinetic and dyskinetic segments of the infarcted myocardium was linearly related to the improvement in local contractility and to the increases in stroke volume and ejection fraction of GF-treated infarcted hearts (Fig. 6). Conversely, there was a negative correlation between infarct size and segmental myocardial performance and ventricular stroke volume and ejection fraction in the nontreated infarcted hearts. Importantly, rhythm disturbances in the form of monomorphic and polymorphic ventricular tachycardia and ventricular fibrillation were rare in GF-treated infarcted dogs but were rather common in nontreated infarcted dogs (Fig. 25, which is published as supporting information on the PNAS web site).
Fig. 6.
GFs improve cardiac performance after infarction. Shown are linear correlations between the magnitude of myocardial regeneration and indices of regional and global ventricular function.
Discussion
The results of the current study indicate that the dog heart has a stem cell compartment and that the canine CSCs are self-renewing, clonogenic, and multipotent. Additionally, this population of resident CSCs-ECCs can be recruited and activated by GFs after infarction to invade the damaged tissue and promote the formation of new myocardium. The differentiation of these primitive cells into myocytes and coronary vessels repairs the infarcted heart, restores local wall motion, improves ventricular hemodynamics, and positively interferes with pathologic ventricular remodeling. Although myocardial regeneration after infarction has been shown in rodents by the local delivery of clonogenic CSCs (6), the clinical implications of these studies have been viewed with skepticism. This early work left unanswered two critical questions: (i) whether cardiac repair would occur in large mammals, suggesting that a similar growth adaptation may be induced in humans and (ii) whether the partial restoration of dead myocardium results in recovery of contraction in the infarcted segment of the wall. Our findings provide evidence in favor of the notion that the adult heart is a self-renewing organ with a powerful growth reserve that can be coaxed to reconstitute lost, damaged myocardium.
Until recently, repair of the infarcted heart was considered an impossible task. After ischemic injury in humans and animals, myocyte regeneration occurs but is restricted to the surviving portion of the ventricle. Replicating myocytes are unable to migrate to the area of damage, multiply, and reconstitute the infarct (22). Healing is activated and collagen accumulates, leading to the formation of a thick, scarred ventricular wall. In the last few years, embryonic cells, bone marrow-derived adult progenitor cells, or more primitive cells have been mobilized into the circulation of infarcted animals or injected directly in the proximity of the necrotic heart tissue (1). With two exceptions (3, 4), these attempts have successfully replaced dead myocardium with contracting parenchymal cells and functional coronary vessels. The recognition that resident stem cells are present in the rat and mouse heart (6–8) and, when properly administrated, reconstitute infarcts with new myocytes, arterioles, and capillaries has raised the possibility of unique approaches for the treatment of ischemic heart disease (2). In this regard, the G-actin sequestering peptide thymosin β4 can promote the migration of cardiac progenitor cells in vitro (23).
The current study advances the field of regenerative cardiology through identification and characterization of a multipotent CSC in the dog heart. Moreover, the demonstration that GFs induce translocation of resident progenitor cells to the infarct, rebuilding the lost myocardium in larger mammals, closes the gap between rodents and humans and strengthens the feasibility and applicability of local stem cell activation in the patient population. This strategy is minimally invasive and avoids the complication of rejection and the effects of time on the onset of therapy linked to the acquisition and in vitro expansion of the patient's own progenitor cells (24, 25). Additionally, the risks inherent in the systemic mobilization of a large number of hematopoietic and other stem cells to nontarget organs are prevented. Cellular therapy becomes organ-specific.
This notion is advanced because three objectives have been accomplished here. The first involved the unequivocal documentation that a segment of myocardium was irreversibly damaged and that the administration of GFs resulted in the translocation of progenitor cells to the infarcted area; the second included the demonstration that homed cells proliferate and differentiate in the various cardiac lineages, initiating myocardial repair; and the third comprised proof that the regenerated myocardium reached functional competence, ameliorating chronically segmental contraction, global ventricular performance, and ejection fraction.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants HL-38132, AG-15756, HL-65577, HL-66923, HL-65573, AG-17042, AG-023071, HL-43023, HL-50142, and HL-081737.
Author contributions: A. Linke, P.M., A. Leri, T.H.H., J.K., and P.A. designed research; A. Linke, P.M., D.N., C. Casarsa, D.T., A.N., C. Castaldo, S.C., M.B., F.Q., K.U., A. Leri, T.H.H., and J.K. performed research; A. Linke, P.M., D.N., C. Casarsa, D.T., A.N., C. Castaldo, S.C., M.B., F.Q., K.U., A. Leri, T.H.H., J.K., and P.A. analyzed data; and A. Leri, T.H.H., J.K., and P.A. wrote the paper.
Abbreviations: CSC, cardiac stem cell; HGF, hepatocyte growth factor; IGF-1, insulin-like growth factor 1; GF, growth factor; SMC, smooth muscle cell; EC, endothelial cell; ECC, early committed cell.
References
- 1.Rosenthal, N. (2003) N. Engl. J. Med. 349, 267–274. [DOI] [PubMed] [Google Scholar]
- 2.Anversa, P., Sussman, M. A. & Bolli, R. (2004) Circulation 109, 2832–2838. [DOI] [PubMed] [Google Scholar]
- 3.Balsam, L. B., Wagers, A. J., Christensen, J. L., Kofidis, T., Weissman, I. L. & Robbins, R. (2004) Nature 428, 668–673. [DOI] [PubMed] [Google Scholar]
- 4.Murry, C. E., Soonpaa, M. H., Reinecke, H., Nakajima, H., Nakajima, H. O., Rubart, M., Pasumarthi, K. B., Virag, J. I., Bartelmez, S. H., Poppa, V., et al. (2004) Nature 428, 664–668. [DOI] [PubMed] [Google Scholar]
- 5.Kajstura, J., Rota, M., Whang, B., Cascapera, S., Hosoda, T., Bearzi, C., Nurzynska, D., Kasahara, H., Zias H., Bonafe, M., et al. (2005) Circ. Res. 96, 127–137. [DOI] [PubMed] [Google Scholar]
- 6.Beltrami, A. P., Barlucchi, L., Torella, D., Baker, M., Limana, F., Chimenti, S., Kasahara, H., Rota, M., Musso, E., Urbanek, K., et al. (2003) Cell 114, 763–776. [DOI] [PubMed] [Google Scholar]
- 7.Oh, H., Bradfute, S. B., Gallardo, T. D., Nakamura, T., Gaussin, V., Mishina, Y., Pocius, J., Michael, L. H., Behringer, R. R., Garry, D. J., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 12313–12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsuura, K., Nagai, T., Nishigaki, N., Oyama, T., Nishi, J., Wada, H., Sano, M., Toko, H., Akazawa, H., Sato, T., et al. (2004) J. Biol. Chem. 279, 11384–11391. [DOI] [PubMed] [Google Scholar]
- 9.Sun, L., Lee, J. & Fine, H. A. (2004) J. Clin. Invest. 113, 1364–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bunting, K. D. (2002) Stem Cells (Dayton) 20, 11–20. [DOI] [PubMed] [Google Scholar]
- 11.Asakura, A. (2003) Trends Cardiovasc. Med. 13, 123–128. [DOI] [PubMed] [Google Scholar]
- 12.Urbanek, K., Quaini, F., Tasca, G., Torella, D., Castaldo, C., Nadal-Ginard, B., Leri, A., Kajstura, J., Quaini, E. & Anversa, P. (2003) Proc. Natl. Acad. Sci. USA 100, 10440–10445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Messina, E., De Angelis, L., Frati, G., Morrone, S., Chimenti, S., Fiordaliso, F., Salio, M., Battaglia, M., Latronico, M. V., Coletta, M., et al. (2004) Circ. Res. 95, 911–921. [DOI] [PubMed] [Google Scholar]
- 14.Roger, V. L., Weston, S. A., Redfield, M. M., Hellermann-Homan, J. P., Killian, J., Yawn, B. P. & Jacobsen, S. J. (2004) J. Am. Med. Assoc. 292, 344–350. [DOI] [PubMed] [Google Scholar]
- 15.Hamasuna, R., Kataoka, H., Moriyama, T., Itoh, H., Seiki, M. & Koono, M. (1999) Int. J. Cancer 82, 274–281. [DOI] [PubMed] [Google Scholar]
- 16.Powell, E. M., Mars, W. M. & Levitt, P. (2001) Neuron 30, 79–89. [DOI] [PubMed] [Google Scholar]
- 17.Arsenijevic, Y., Weiss, S., Schneider, B. & Aebischer, P. (2001) J. Neurosci. 21, 7194–7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reiss, K., Cheng, W., Ferber, A., Kajstura, J., Li, P., Li, B., Olivetti, G., Homcy, C. J., Baserga, R. & Anversa, P. (1996) Proc. Natl. Acad. Sci. USA 93, 8630–8635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Torella, D., Urbanek, K., Rota, M., Whang, B., Nurzynska, D., Baker, M., Hosoda, T., Cascapera, S., Bearzi, C., Musso, E., et al. (2004) Circulation 110, III-170. [Google Scholar]
- 20.Anversa, P. & Olivetti, G. (2002) in Handbook of Physiology, eds. Page, E., Fozzard, H.-A. & Solaro, R.-J. (Oxford Univ. Press, New York), Section 2, Vol. 1, pp. 75–144. [Google Scholar]
- 21.Kajstura, J., Zhang, X., Liu, Y., Szoke, E., Cheng, W., Olivetti, G., Hintze, T. H. & Anversa, P. (1995) Circulation 92, 2306–2317. [DOI] [PubMed] [Google Scholar]
- 22.Anversa, P. & Nadal-Ginard, B. (2002) Nature 415, 240–243. [DOI] [PubMed] [Google Scholar]
- 23.Bock-Marquette, I., Saxena, A., White, M. D., Dimaio, J. M. & Srivastava, D. (2004) Nature 432, 466–472. [DOI] [PubMed] [Google Scholar]
- 24.Britten, M. B., Abolmaali, N. D., Assmus, B., Lehmann, R., Honold, J., Schmitt, J., Vogl, T. J., Martin, H., Schachinger, V., Dimmeler, S., et al. (2003) Circulation 108, 2212–2218. [DOI] [PubMed] [Google Scholar]
- 25.Wollert, K. C. & Drexler, H. (2005) Circ. Res. 96, 151–163. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.