Abstract
Huajiao water (HW) has a wide range of applications in the new food service industry. However, few process and flavor studies have been conducted on HW. The aim of this study was to investigate the effect of boiling process on HW flavor as well as the mechanisms by which important aroma compounds affect the flavor. The results showed that HW-20 min had better flavor quality and typical flavor of Huajiao. Boiling time mainly affected the content of terpenoid, which subsequently affected the overall flavor of HW. Compared to dried Huajiao (DH), HW had significantly lower volatile compounds. Limonene and linalool were abundant volatile compounds in DH and HW. Finally, the potential degradation pathways of limonene and linalool were summarized based on model experiments and isotopic tracer techniques, respectively. This study provided a feasible solution for the investigation of the transformation pathway and the flavor regulation of HW in industry.
Keywords: Huajiao (Zanthoxylum bungeanum Maxim.) water, Boiling, Limonene, Linalool, Degradation pathway, Isotopic tracer
Highlights
-
•
HW-20 min had the best flavor, fully revealed the characteristic aroma of Huajiao.
-
•
Boiling time affected terpenoid content and therefore the flavor of HW.
-
•
Degradation products of linalool and limonene were determined by isotopic tracing.
-
•
Four degradation pathways were summarized for limonene and linalool, respectively.
1. Introduction
Huajiao (Zanthoxylum bungeanum Maxim.) were originally grown in China, and have been widely distributed in tropical and subtropical regions of Asia, America, Africa and Oceania, with more than 250 species found around the world (Epifano et al., 2011). There are five species of Zanthoxylum L. native to China, including pepper (Zanthoxylum bungeanum), wild pepper (Z. simulans), bamboo-leaf pepper (Z. armatum), Sichuan-Shaanxi pepper (Z. piasezkii), and green pepper (Z. schinifolium) (Chen, 2022). According to the color of the peel at maturity, it can be simply divided into green pepper and red pepper (Dong et al., 2023). In terms of origin, it has been developed into four major pepper producing areas in China, mainly in Hanyuan, Sichuan, Jiangjin, Chongqing, Hancheng, Shaanxi and Laiwu, Shandong. Among them, Hanyuan County, Ya'an City, Sichuan Province, is the famous origin of pepper, known as the “hometown of Chinese pepper”. Hanyuan pepper is known as the “Millennium Tribute pepper” with rich flesh and excellent aroma quality (Zhu et al., 2011). As a typical woody oilseed crop for the poverty alleviation and rural revitalization, Huajiao has been developing rapidly in China with its great economic value, medicinal value as well as ecological value.
Huajiao is rich in edible and medicinal values and is widely used in food, medicine and other industries. Huajiao can be used to treat fever, toothache, diarrhea, ascariasis, etc., and has been included in the Pharmacopoeia of the People's Republic of China (Phuyal et al., 2019; Zhao et al., 2023). As the primary source of numbness, Huajiao provide a unique flavor profile for the restaurant industry. In recent years, with the in-depth study of food flavor substances and innovation in cooking methods, the boundary of hemp flavor has gradually been broken, and new flavor combinations have been emerging, such as “hemp and sweet”, “hemp and salty” and other distinctive flavor types. In particular, new tea drinks, coffee, western fast food and other food and beverage categories have also tried to integrate Huajiao into their products, developing a series of pepper products. For example, Dahongpao Huajiao Lemon Tea, Huajiao Ice Americano, Huajiao Ice Cream and so on, provide consumers with a rich taste experience. It's showed that Huajiao Water (HW) has a wide range of applications in the new restaurant industry.
As a traditional Chinese spice, Huajiao have a series of applications in Chinese cooking, giving a unique flavor to food. At the same time, it can also be used as a food additive to inhibit the formation of heterocyclic amine (Zeng et al., 2018). In Chinese cooking, Huajiao are usually deep-fried at high temperatures to bring out their characteristic flavor. Sun et al. analyzed the aroma characteristics of fried red pepper oil from Hancheng and Hanyuan regions, and the results indicated that 1,8-cineole, (E)-2-heptenal, β-myrcene, β-ocimene, limonene and linalool were the essential aroma contributing components (Sun et al., 2020). Ni et al. studied the fried pepper oils of five types of Huajiao (red Huajiao from Hancheng and Hanyuan, green Huajiao from Shaanxi, Jiangjin and Pinchang) and showed that the red pepper oils exhibited strong herbal, spicy, fatty and rosin attributes, while the green pepper oils presented high levels of green and citrus-like notes, and linalool, linalyl acetate, and 1,8-cineol were identified as markers to distinguish fried red and green pepper oils (Ni et al., 2022). Besides fried pepper oil, HW is also a popular means of flavoring. In the study of the effect of Huajiao on the flavor of stewed sheep tail fat soup, it was found that the taste intensity of sheep tail fat soup stewed with 0.2 % Huajiao was significantly increased (Huang et al., 2022). Unprocessed meat often carries a strong smell of blood, adding Huajiao to raw meat after slaughter for wet curing treatment can eliminate the blood and other unpleasant odors. For example, by adding Huajiao and other spices during the wet-submerged fermentation process of mandarin fish, the aroma compounds increased significantly compared to the simple wet-submerged fermentation, and the flavor of the fish was significantly enhanced (Zhou et al., 2019). In summary, Huajiao often play a flavor-modifying role in water-soluble systems including cooking and pickling. However, little research has been done on the aroma characteristics of boiled Huajiao. During the boiling process, Huajiao underwent a series of complex physicochemical changes, such as thermal oxidation and hydrolysis reactions, which have far-reaching effects on the composition of volatile compounds, thus altering their flavor characteristics. For instance, linalool and limonene can be converted to α-terpineol by double bond hydration, dehydration, cyclization and ester hydrolysis (Pérez-López et al., 2006; Rodríguez et al., 2017). At present, no researcher has analyzed the aroma compound compositions of dried Huajiao (DH) and Huajiao Water (HW) comparatively to explore the mechanism of aroma compound changes during the cooking process of Huajiao.
In order to achieve the identification of volatile flavor components within Huajiao samples, the first task is to effectively carry out the isolation and extraction work of Huajiao ‘s aroma compounds. Solvent-assisted flavor evaporation (SAFE) is a sample pretreatment method that separates non-volatile components under high vacuum (103–105 Pa) and ultra-low temperature freezing conditions to efficiently catch volatile aroma compounds. In this way, the loss of heat-sensitive volatile components is effectively reduced, so that the natural characteristics of the food product can be preserved. As a consequence of this, SAFE is rapidly becoming the standard method for solvent extraction of volatile compounds to avoid by-products in academic and food industry research. It has been shown that SAFE was suitable for the extraction of volatile compounds from Huajiao products (Sun et al., 2020). Food flavors are formed by the interaction of abundant volatile compounds. To effectively separate and analyze these volatile compounds, gas chromatography techniques have been widely used. Gas chromatography-mass spectrometry (GC–MS) is one of the most common equipment for the characterization and quantification of volatile compounds. Due to the complexity of food flavor components, it is crucial to explore as much valuable information as possible from large and complex data. Therefore, the use of chemometrics in food science and technology is becoming more and more widespread, especially in the identification of food authenticity and traceability (González-Domínguez et al., 2022). Among them, principal component analysis (PCA) and partial least squares regression (PLSR) have been widely used in the research field of food flavor chemistry. Liu et al. established correlation analysis (CRA), PCA and cluster analysis (CA) models for the comprehensive assessment of quality characteristics of green pepper in Southwest China (Liu et al., 2023). Tian et al. used PLSR to correlate the volatile compounds of four different fried shallot-flavored oil with the corresponding sensory descriptors, and found that 2-ethyl-3,5-dimethyl-pyrazine, 2,3-dihydro-benzofuran, and benzaldehyde contributed to “grease” flavor (Tian et al., 2020). Currently, modeling reactions are the main method to explore the metabolic pathways of chemical components in complex food systems. Model reaction experiments by Cao et al. determined that the stale odor components of Longjing tea originated mainly from the oxidative degradation of linoleic acid (Cao et al., 2024). As is known to us all, isotope tracer technique is the most accurate and direct way to verify the mechanism of chemical reactions. Luo et al. determined the sources of dimethyl sulfide and 3-(methylthio)propanal in melon juice during thermal processing by isotope tracer experiments (Luo et al., 2018).
In this study, red Huajiao were used as the research object to investigate the effect of boiling process on the aroma components of Huajiao. Firstly, the volatile components of the HW with different boiling times were analyzed by SAFE and GC–MS, and the differences in volatile components were compared. Secondly, PCA and PLSR were applied to construct a correlation model between samples, volatile compounds and sensory attributes to further investigate the effect of boiling time on the aroma compounds in HW. After that, a simulated reaction system was constructed to identify the volatile compounds by headspace solid-phase microextraction-gas chromatography–mass spectrometry (HS-SPME-GC–MS) to preliminarily investigate the degradation pathways of key flavor compounds in HW. Finally, the transformation pathways of the major aroma compounds were verified by isotopic tracer techniques.
2. Materials and methods
2.1. Materials
Red Huajiao samples (from Hanyuan County, Ya'an City, Sichuan Province) were purchased from You Jia Co., Ltd. (Chengdu City, Sichuan Province, China). Pure water was purchased from Wahaha (Hangzhou City, Zhejiang Province, China).
2.2. Chemicals
Dichloromethane and sodium sulfate were purchased from Mreda (Beijing, China). 1,2-Dichlorobenzene was purchased from Aladdin (Shanghai, China). Acetic acid was purchased from Anpel (Shanghai, China). n-Alkanes (C6–C30) were purchased from Sigma-Aldrich (Shanghai, China). Acetonitrile (HPLC) was purchased from Fisher Scientific (Germany). Standards (≥95 %): α-pinene, β-pinene, sabinene, myrcene, limonene, o-cymene, δ-cadinene, 2-methyl-3-buten-2-ol, 2-heptanol, 1-hexanol, leaf alcohol, (E)-2-hexen-1-ol, 1-octen-3-ol, 1-heptanol, linalool, terpinen-4-ol, α-terpineol, L-carveol, 2-(4-methylphenyl)propan-2-ol, geraniol, benzyl alcohol, phenethyl alcohol, dihydro cuminyl alcohol, 4-(1-methylethyl) benzenemethanol, α-bisabolol, cis,trans-farnesol, crotonaldehyde, hexanal, heptaldehyde, (E,E)-2,4-hexadienal, benzaldehyde, citral, perillaldehyde, 2-pentanone, 3-penten-2-one, 4-methyl-3-penten-2-one, 2-heptanone, acetoin, hydroxyacetone, 6-methyl-5-hepten-2-one, α-thujone, 2,5-hexanedione, 3-methyl-2-cyclopenten-1-one, 6-methyl-3,5-heptadien-2-one, piperitone, carvone, 4-methylacetophenone, isobutyl acetate, heptyl acetate, linalyl acetate, 4-hexanolide, terpinyl acetate, neryl acetate, geranyl acetate, phenylethyl acetate, methyl 2-methoxybenzoate, methyl vanillate, 1,8-cineole, limonene oxide, 2-acetylfuran, 5-methyl-2(5H)-furanone and caryophyllene oxide were purchased from Sigma-Aldrich (Shanghai, China). Linalool oxide, perillen, hydroxy-α-sanshool, hydroxy-β-Sanshool, hydroxy-γ-Sanshool, were purchased from Yuanye (Shanghai, China). [13C3]- linalool and [13C, 2H2]- limonene were purchased from IsoReag (Shanghai, China).
2.3. HW sample preparation
Dried Huajiao granules (25 g) were added to purified water (200 mL) and boiled in an oil bath at 100 °C for 10 min, 20 min, 30 min, and 40 min, respectively. The samples were cooled and filtered to obtain HW. HW preparation experiments were repeated 3 times, sealed and refrigerated at −18 °C for backup.
2.4. Quantitative descriptive analysis (QDA)
The sensory evaluation panel consisted of 12 judges (6 males and 6 females, aged 20–30 years), all of them without any olfactory impairment or related medical history. The panel was trained for a week to familiarize themselves with the aroma characteristics of Huajiao. This part included the training of sensory description of Huajiao, recognition of Huajiao's aroma attributes, and evaluation of aroma intensity. Standards of major aroma active compounds in Huajiao were used as reference in training. Prior to the start of the formal experiment, sensory panelists were asked not to consume flavor-intense foods, such as coffee and candy in order to avoid experimental errors.
HW (5 g) was poured into covered odorless, transparent glass vials (volume = 20 mL). The evaluation was performed in a sensory laboratory with controlled temperature (25 °C). First, the sensory panel provided sensory words describing the aroma of the samples based on sniffing, summarized and counted all the descriptors, and screened the seven descriptors with the highest frequency of occurrence as the basis for subsequent sensory descriptions. Based on the results of the sensory descriptions, seven sensory attributes were determined to be pepper, spice, citrus-like, herbal, floral, piney, and grassy. The panelists scored the intensity of each aroma for the four samples using a ten-point linear scale from 0 to 9 (Table S1).
This sensory evaluation experiment has been approved and licensed by the Scientific Research Ethics Committee of Beijing Technology and Business University (Reference number: No. 205 of 2024 year). All panels were informed about the aim of the sensory evaluation and detailed information about their privacy and right to withdraw from the study at any time. They all signed a consent for the sensory study and the use of their information.
2.5. Isolation of volatiles by SAFE
HW (100 mL) was taken in a separating funnel (500 mL), followed by CH2Cl2 solvent (200 mL) and of internal standard (1, 2-dichlorobenzene, 0.210 g/mL, 20 μL) for extraction. Liquid-liquid extraction was carried out using a GGC-C separating funnel vertical shaker (Beijing Guohuan High tech Automation Technology Research Institute, Beijing, China) at 300 r/min for 20 min, and the lower clear liquid was collected by static stratification. The sample was transferred to a SAFE dropping funnel for high-vacuum distillation. When the pressure of the whole device dropped to 1 × 10−5 mbar, slowly rotate the piston of the dropping funnel to start distillation. After that, HW volatile component extracts were obtained. After the temperature of the extract was stabilized to room temperature, the appropriate amount of anhydrous Na2SO4 was added to remove the water. The final distillate was concentrated to 1 mL using Vigreux column (50 cm × 1 cm) (Beijing Jingxing Glassware Co., Ltd., China) and BF-2000 nitrogen drying instrument (Beijing Bafang Century Technology Co., Ltd., China).
For DW, dried Huajiao granules (25 g) were taken in dichloromethane (200 mL) and solid-liquid extraction was carried out using a split funnel, and other operations were performed as above.
2.6. GC–MS analysis
GC–MS analysis was performed by a Thermo Fisher Trace 1310 gas chromatograph (Thermo Fisher Scientific, Waltham, MA, USA) combined with a Thermo Fisher mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). Separation was performed using TG-Wax column (30 m × 0.25 mm i.d., 0.25 μm, Thermo Fisher Scientific, Waltham, MA, USA).
The paper referred to the method of Sun et al. (2020), with slight modifications. Helium was used as the carrier gas and delivered at a fixed flow rate of 1.2 mL/min to the column. For the TG-Wax column, the oven temperature was initially 40 °C, followed by a 1 min hold; increased to 140 °C at a rate of 2 °C/min, followed by a 1 min hold; and finally increased to 220 °C at a rate of 6 °C/min, followed by a 1 min hold; injection volume 1 μL, split ratio 100:1. The mass detector condition: ionization energy, 70 eV; MS transfer line temperature, 240 °C, ion source temperature, 250 °C; scanning mode, full scan; mass range, m/z 40–350; and solvent delay, 5 min.
2.7. Qualitative and quantitative analysis
The volatile compounds in the samples were identified using the NIST14 library, retention index (RI) combined with standards (Std). The formula for calculating RI is as follows:
where n and n + 1 represented the number of carbon atoms of the n-alkane before and after the flow out to be measured; t’(i) was the adjusted retention time of the component to be measured, min; t’(n) and t’(n + 1) were the adjusted retention time of the n-alkane with n and n + 1 carbon atoms, respectively, min.
The quantitative analysis was performed by the internal standard method, i.e. the relative content of each volatile compound in HW was quantified by the ratio of the peak area of the component to be measured in the sample to the peak area of the internal standard.
2.8. Model reaction experiment of limonene and linalool
2.8.1. Construction of transformation pathways for characteristic aroma compounds
According to the content of limonene (3 g) and linalool (1.4 g) in dried Huajiao, limonene and linalool were added separately to purified water (60 mL). Cooking method was the same as 2.3 HW sample preparation. Finally, limonene solution (LMS) and linalool solution (LNS) were obtained respectively.
Extraction of volatile compounds from linalool and limonene reaction models using HS-SPME-GC–MS. LMS/LNS (1 g) was weighed and added to a 20 mL glass vial with a silicon septum, and 1, 2-dichlorobenzene (20 μL, 0.210 g/mL for LMS, 10 μL, 0.021 g/mL for LNS) was added as an internal standard. For extraction by HS-SPME, a 75 μm Carboxen™/ polydimethylsiloxane (CAR-PDMS) fiber (Supelco, Inc., Bellefonte, PA) (Yang et al., 2022; Dong et al., 2024) was used to isolate the volatiles after conditioning at 250 °C for 20 min. At the same time, the glass vial was heated in the water bath at 60 °C. After that, the fiber was exposed to the sample headspace for 30 min at 60 °C in order to extract the volatiles. At last, the fiber was transferred to the injector port and desorbed for 5 min at 250 °C for the GC–MS analysis. GC–MS method was the same as 2.6 GC–MS analysis.
2.8.2. Identification of linalool and limonene transformation pathways in HW
To further simulated the HW system, red Huajiao (7.5 g) and limonene (3 g)/ linalool (1.4 g) were taken and boiled in purified water (60 mL), obtained the limonene-HW solution (LM-HW) and linalool-HW solution (LN-HW). Other Cooking conditions was the same as 2.3 HW sample preparation. In addition, HW was used as a blank control group. The volatile compounds were qualified and quantified by HS-SPME-GC–MS, and the internal standard addition of HW was 10 μL, 2.10 g/L, other conditions were the same as the method in 2.9.1.
2.9. Validation of transformation pathways by isotopic tracer techniques
Purified water (8 mL), Huajiao (1 g) and isotope standards (10 mg) were added to a round-bottomed flask and boiled under the same conditions as in method 2.9.1. Subsequently, HS-SPME-GC–MS analysis was performed to verify the degradation products of linalool and limonene in the HW system by isotopic labeling. In other words, the generation of degradation products was finally determined by verifying the presence of isotopically labeled products (M + 3) in the mass spectra of the suspected degradation products.
2.10. Statistical analysis
Experimental data were collected and organized using Excel (Microsoft Office 2016, Redmond, WA). The results of the experiments were expressed as the mean of three experiments ± standard deviation and analyzed by one-way analysis of variance using IBM SPSS version 27 (SPSS Inc., Chicago, IL). Heat map was created by TBtools software (https://github.com/CJ-Chen/TBtools). Aroma profiles and bar chart were plotted using Origin version 2023b (OriginLab Corporation, Northampton, MA.). PLSR analysis was conducted using XLSTAT 2019. PLSR analysis was conducted using XLSTAT 2019 (Addinsoft, New York, NY). Degradation pathways were drawn via ChemDraw 15 (CambridgeSoft, USA).
3. Results and discussion
3.1. Sensory profiles of HWs and DH
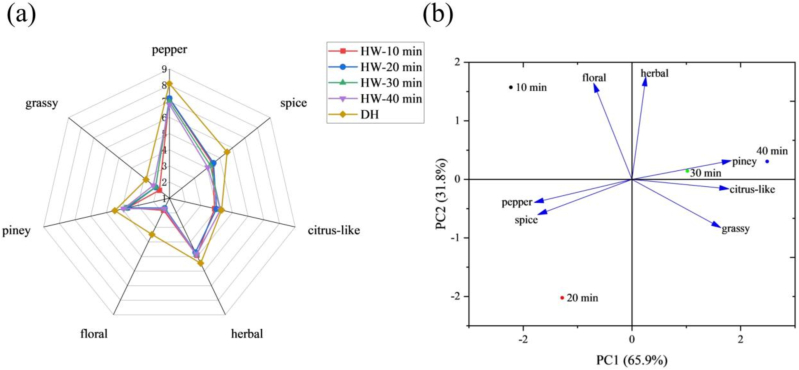
QDA was used to evaluate and compare the 7 aroma attributes of pepper, spice, citrus-like, herbal, floral, piney, and grassy aroma of the 5 samples. From Fig. 1a, the overall aroma profile strength of DH was obviously stronger than that of HWs. The aroma profile of DH indicated that DH has the strongest pepper aroma, followed by herbal and spice notes. In addition, it had a light piney, floral, grassy, citrus-like aroma. The floral attributes of DH weakened the most after boiling, with little change in the intensity of the citrus-like aroma. It suggested that the boiling process reduced pungent odors (spice and pepper), contributing to the overall acceptability of Huajiao product. The flavor profiles of different HWs were similar, with pepper, spice, citrus-like, herbal, and piney playing an important role in the four HWs. However, the rest of the attributes had little effect on the aroma characteristics of the HWs, especially the 10 min (grassy 1.79) and 20 min (floral 1.67) samples. In the meanwhile, the seven aroma attributes showed different degrees of differences among the four HWs, among which three aroma attributes (piney, floral, and herbal) did not show significant differences. HW-20 min was superior to the other samples in terms of pepper and spicy, while HW-10 min showed similar sensory attributes to HW-20 min, but the intensity of both pepper and spicy aroma was lower than that of HW-20 min. HW-40 min had a strong citrus-like and grassy aroma, followed by HW-30 min.
Fig. 1.
Sensory evaluation of samples. (a) Aroma profiles of DH and HW with different boiling times. (b) PCA charts for sensory evaluation of different HWs.
In order to further explore the differences in aroma characteristics between different HW samples, the sensory evaluation data of different HWs were analyzed by PCA. As shown in Fig. 1b, the contribution of the first principal component (PC1) is 65.9 % and that of the second principal component (PC2) is 31.8 %, with a total contribution of 97.7 %, which meant that the two principal components explain most of the odor information of all samples. From the perspective of PC1, the 30 min and 40 min samples were in the positive half-axis, while the 10 min and 20 min samples were in the negative half-axis. From the perspective of PC2, the 10 min, 30 min, and 40 min samples were in the positive half-axis, while the 20 min sample was in the negative half-axis. The above analysis showed that PCA could effectively distinguish the four HWs, with more prominent floral aroma in HW-10 min, more pronounced herbal and piney aroma in HW-30 min and HW-40 min, and strong pepper and spicy aroma in HW-20 min, which was in agreement with the results of the above QDA analysis. In summary, the quality of HW-20 min was the best, which fully expressed the unique pepper aroma and spicy flavor of Huajiao.
3.2. Analysis of volatile compounds in HW with different boiling times
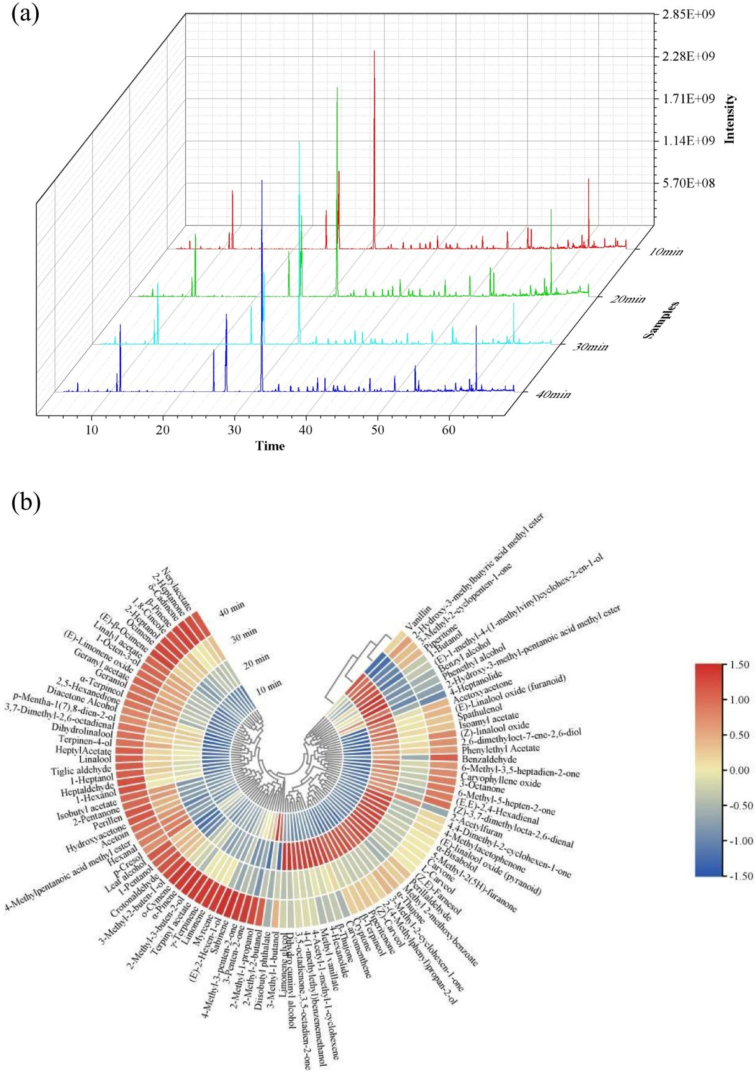
The total ion chromatograms of the 4 HWs were shown in Fig. 2a. A total of 104 volatiles, including 11 terpenes, 33 alcohols, 10 aldehydes, 23 ketones, 17 esters, 1 phenol and 9 heterocycles, were detected from the 4 HWs (Table S2). Boiling time affected the content of compounds, especially terpenoids, which in turn influenced the overall flavor of HW. Among them, alcohols (4866.77–5927.05 μg/g), heterocycles (3274.83–3940.94 μg/g) and esters (1709.32–2320.20 μg/g) were the most abundant and might play an important role in the aroma presentation of HWs. The highest content among the alcohols was linalool (2147–2628.81 μg/g). As an important volatile compound in Huajiao, linalool provided HWs with floral characteristic aromas (Sun et al., 2020). The highest content among the esters was linalyl acetate (1436.95–1945.39 μg/g). Linalyl acetate similarly acted as a key aroma-active compound for Huajiao, providing HWs with floral and fruity odor (Dong et al., 2023). The content of 1,8-cineole (1309.74–1633.43 μg/g) was the highest among the heterocyclic group. 1,8-Cineole also contributed prominently to the herb aroma, providing the grassy and woody characteristic aroma of HWs (Sun et al., 2020). Although the total amount of terpenes was relatively poor, limonene (358.96–700.45 μg/g) was particularly rich in them. Limonene was also one of the key flavor compounds of Huajiao (Dong et al., 2023). From the perspective of compound type and composition, there were 102 shared components in different HWs, indicating that the volatiles of the HWs did not differ significantly.
Fig. 2.
GC–MS analysis results of HW samples with different boiling times. (a) Total ion chromatograms of HW with different boiling times. (b) Clustering heat map of volatile compounds with different HWs.
In order to compare the differences between different HWs more clearly, the content of volatile compounds was visualized using hierarchical cluster analysis (HCA). The gradation of colors from red to blue in the heat map (Fig. 2b) reflects high to low volatiles content, where the maximum value of color intensity is 1.5 (red) and the minimum value is −1.5 (blue). The results of HCA showed that HWs could be clearly classified into three groups. HW-10 min and HW-20 min were grouped into their respective groups, while HW-30 min and HW-40 min were categorized into another group. The above results agreed with the results of PCA analysis for sensory evaluation. Based on the color of the clustered heat map, volatile compounds could be roughly classified into 4 parts. First, diisobutyl phthalate and 3-methyl-1-butanol, which were higher in HW-10 min, were classified as part 1. The content of 3-methyl-1-butanol, which presented the floral aroma, was relatively high in the first 10 min of boiling. Since the volatile compounds in huajiao have not been completely dissolved, it might lead to the relative prominence of the floral aroma in HW-10 min and the weakness of the characteristic pepper aroma and spicy flavor of the huajiao. The second part included 26 compounds which were found in higher levels in HW-20 min. In particular, 4-methylacetophenone, (E)-linalool oxide (pyranoid) and carvone, which were associated with pepper and spicy aroma attributes, contributed significantly to the aroma profile of the 20-min sample. The third part, consisting of 25 compounds, showed high levels in both HW-20 min and HW-40 min. In particular, piperitone and phenethyl alcohol gave HW herbal and floral aroma in HWs. The remaining 51 compounds were categorized as part 4, with higher levels in HW-40 min. When the cooking time exceeded 30 min, the content of most of the compounds increased, and p-cresol and 4-methylpentanoic acid methyl ester appeared only in HW-30 min and HW-40 min. The above two compounds were not detected in the HW-10 min and HW-20 min, which might be due to the insufficiently long boiling time resulting in low levels that did not reach the detection limit of GC–MS. In this case, p-cresol exhibited an irritating odor similar to that of smoke and herb and had a low odor threshold (0.0039 mg/kg) (Gemert, 2011). The presence of p-cresol in small quantities generated a pleasing herbal and piney aroma. However, large amounts of p-cresol may impart a pungent odor to HWs. It has been shown that p-cresol was formed from tyrosine (Watkins et al., 2014) and the fecal off-flavor in white pepper rose in intensity because of its presence (Jagella & Grosch, 1999). In summary, HW with different boiling times did not differ much in terms of compound species, but there were significant differences in terms of compound content. The effect of compound content on the overall aroma profile of HW requires further exploration.
3.3. Correlation analysis of aroma attributes and aroma compounds
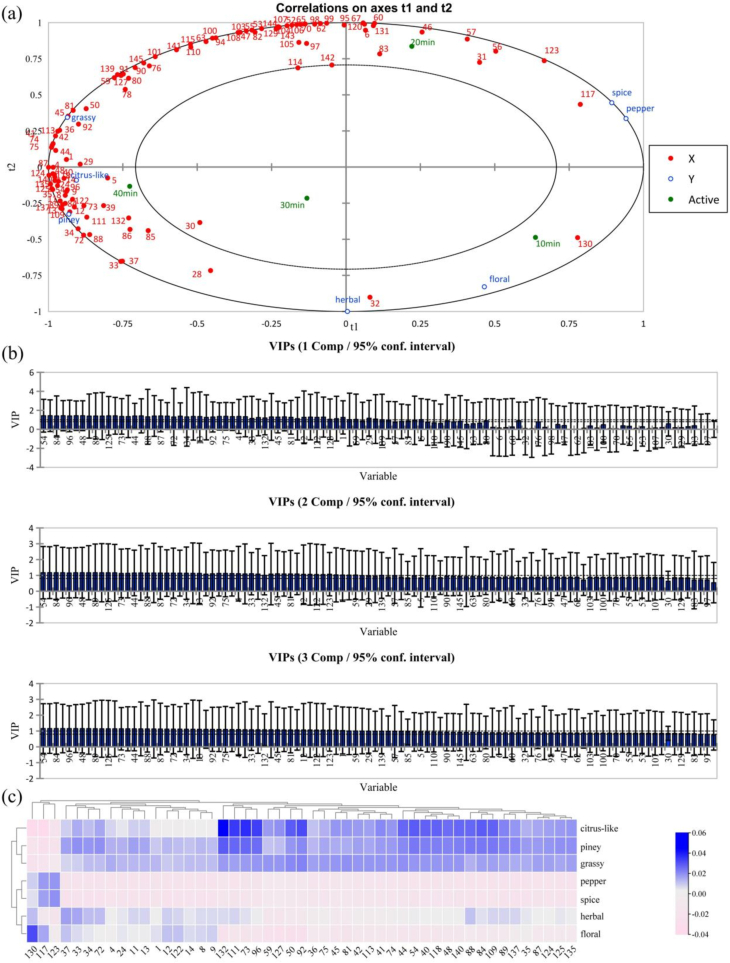
In order to explore the correlation between sensory attributes and flavor compounds of HWs, PLSR was used to construct correlation analysis model. In this case, 104 volatiles were used as the X variable, and 7 sensory attributes were used as the Y variable. The majority of the X and Y variables were located in 0.5 < r2 < 1, indicating that this model could explain the above variables well (Gao et al., 2024). The PLSR loading map was shown in Fig. 3a, and the 4 HW samples were well distinguished from each other. HW- 10 min was located in the fourth quadrant, HW-20 min was located in the first quadrant, and HW-30 min & HW-40 min were located in the third quadrant, suggesting that the different boiling times resulted in significant differences between samples. In particular, distinguishing it from the other 3 samples, HW-20 min was located in the positive semiaxis of the t1 axis, indicating that it was significantly different from the other 3 samples. In terms of distance between the two points, HW-40 min had strong citrus-like and piney characteristics, HW-10 min had more prominent floral notes, while HW-20 min had a stronger correlation with pepper and spice. As the consequence of that, HW-20 min was further identified as the sample with the most characteristic flavor of Huajiao. The results of the above analysis were in agreement with the results of the sensory evaluation.
Fig. 3.
Correlation analysis between volatile compounds and aroma attributes in HWs. (a)Correlation loadings of the PLSR model between sensory attributes and volatile compounds in HWs. (b)Variable importance for the projection (VIP) scores and 95 % confidence interval (bootstrap method) of active compounds for the first three components in the PLSR model. (The full figure is shown in the supplementary material) (c)Heat map of correlation between sensory attributes and important aroma active compounds (VIP ≥ 1).
Variable importance in projection (VIP) is one of the important variables in the PLSR model that reflects the strength of the correlation. As shown in Fig. 3b, a total of 49 compounds with VIP > 1 for the first three principal components had a significant effect on the aroma of HWs. In order to observe the relationship between aroma compounds and sensory attributes more intuitively, a heat map analysis of 49 aroma compounds was carried out based on the standardized coefficients (Fig. 3c). 2-Hydroxy-3-methyl-pentanoic acid methyl ester (117) and 4-heptanolide (123) were positively correlated with pepper aroma and spice. p-Cresol (132), hexanal (73), 2,5-hexanedione (96) and 4-methylpentanoic acid methyl ester (111) were significantly and positively correlated with citrus-like. 1-Pentanol (33) and leaf alcohol (37) were positively correlated with herbal. Diisobutyl phthalate (130) was significantly positively correlated with floral. 1-Octen-3-ol (40), terpinen-4-ol (44), α-terpineol (48), geraniol (54), crotonaldehyde (72), hexanal (73), 2-pentanone (84), acetoin (88), hydroxyacetone (89), 2,5-hexanedione (96), isobutyl acetate (109), 4-methylpentanoic acid methyl ester (111), linalyl acetate (118), p-cresol (132), perillen (137) and (E)-limonene oxide (140) were positively correlated with pine aroma. Therefore, the above compounds might be main aroma compounds that affect the aroma of HW.
In summary, the results of the correlation analysis showed that different cooking time had significant effects on the flavor of HWs. Among them, HW-20 min had the most characteristic Huajiao aroma. 25 volatile compounds have been hypothesized to be important aroma-active compounds affecting HW aroma profiles.
3.4. Comparative analysis of DH and HW volatile compounds
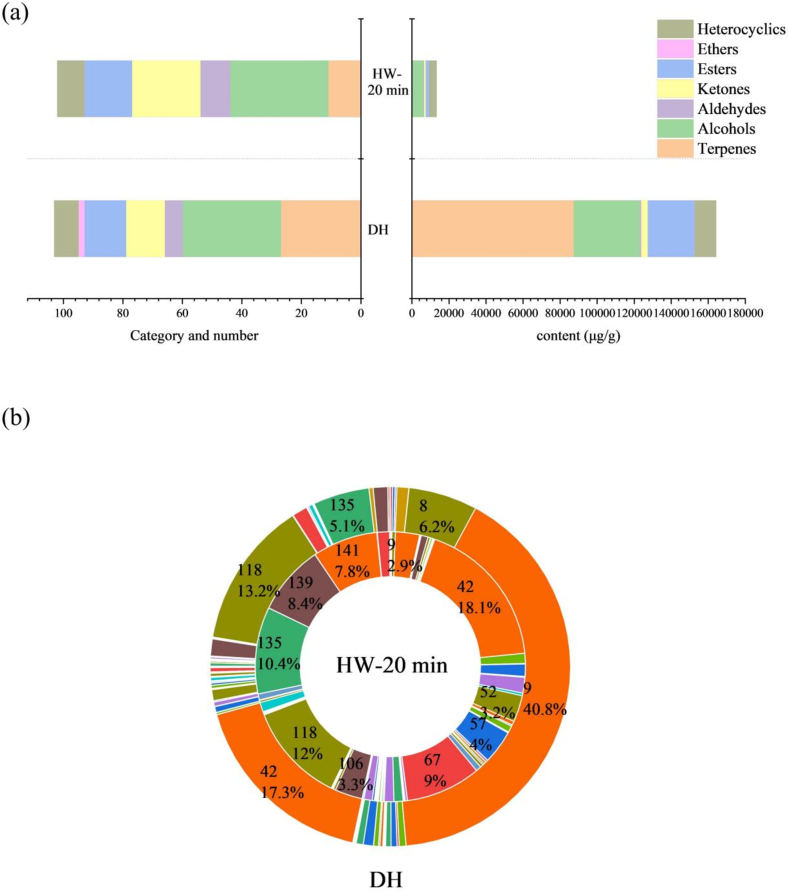
To further explore the mechanism of HW aroma presentation, DH was analyzed by SAFE and GC–MS. Combining these results with the GC–MS results of HW, a total of 145 volatiles were identified, including 27 terpenes, 43 alcohols, 13 aldehydes, 25 ketones, 23 esters, 1 phenol, 2 ethers and 11 heterocyclics (Table S2). A plot of the compound species of DH vs. HW was shown in Fig. 4a. There were 103 volatile compounds in DH and 104 compounds in HWs. DH contained all the aroma compounds in HW except p-cresol and 4-methylpentanoic acid methyl ester. It is noteworthy that the composition of the aroma compounds of Huajiao changed significantly after being boiled in water. In terms of number of compounds, terpenes and alcohols were the most abundant in DH. And from the perspective of the content of various compounds, terpenes were most abundant in DH. The volatile compounds of DH were primarily terpenes and alcohols, with alcohols being the most abundant, but terpenes dominate in relative content. However, the composition of volatile compounds in HW was dominated by alcohols, followed by ketones. There was a significant increase in aldehydes, ketones, esters and heterocyclic aroma compounds in HW, except for a decrease in ether and terpene species. This might be due to the fact that some aroma compounds undergo decomposition during heating or react with other compounds to produce new compounds (Zhang, Song, et al., 2017). The above changes have made the HW and DH aroma profiles significantly different.
Fig. 4.
Comparison of volatile compounds in DH and HW-20 min. (a) Number and content of each type of compounds in DH and HW-20 min. (b) Percentage of each compound in DH and HW-20 min.
After boiling, the total content of volatile compounds in Huajiao decreased, with the most pronounced decrease in terpene content (Fig. 4a). Terpenoids are the most abundant and structurally diverse secondary metabolites in plants and play an important role in plant biology (Huang et al., 2018). The species and content of terpenes varied greatly before and after boiling, and their concentration decreased significantly from 87,469.05 μg/g to 483.49 μg/g. Terpene content accounted for 53.26 % of total volatiles in DH, while terpene content accounted for 3.66 % of total volatiles in HW. It is noteworthy that the content of each compound of HW decreased after boiling, but the percentage of alcohols, aldehydes, ketones and heterocycles increased, especially alcohols (44.61 %) and heterocycles (28.24 %). Thereafter, the relative content of each compound in DH and HW was analyzed. As shown in Fig. 4b, the two compounds with the highest percentage of content in DH were limonene (40.8 %) and linalool (17.3 %). After 20 min of boiling, the content of limonene and linalool changed significantly. The percentage of the above 2 compounds in HW was 2.9 % for limonene and 18.1 % for linalool, respectively. In the meantime, limonene and linalool had been reported as key aroma compounds in DH (Dong et al., 2023). As the consequence of that, in order to investigate the mechanism of aroma formation in HW, limonene and linalool are two important research subjects. The chemical reactions and metabolites of linalool and limonene during the boiling process deserved further investigation.
3.5. Preliminary study on the transformation pathway of characteristic aroma compounds
3.5.1. Basic modeling of the transformation pathways of limonene and linalool
In order to deeply investigate the effect of the boiling process on the aroma of HW, the boiling reaction conditions were simulated, and the reactions of LMS and LNS were modeled to explore the degradation products, respectively. The degradation products of limonene and linalool under boiling for 20 min were shown in Table S3a & S3b. The analysis led to the initial identification of 46 suspected degradation products in LMS and 12 suspected degradation products in LNS. A total of 27 suspected degradation products in LMS were also present in HW-20 min. Particular compounds were listed in Table S3a. A total of 11 suspected degradation products in LNS were also present in HW-20 min. In particular, although nerol was not detected in LNS, its esterification product neryl acetate was present in HW-20 min. Considering the differences between the LMS/LNS and HW system, a more realistic simulation of the system was required to determine the degradants.
3.5.2. Transformation pathways of limonene and linalool in the HW model
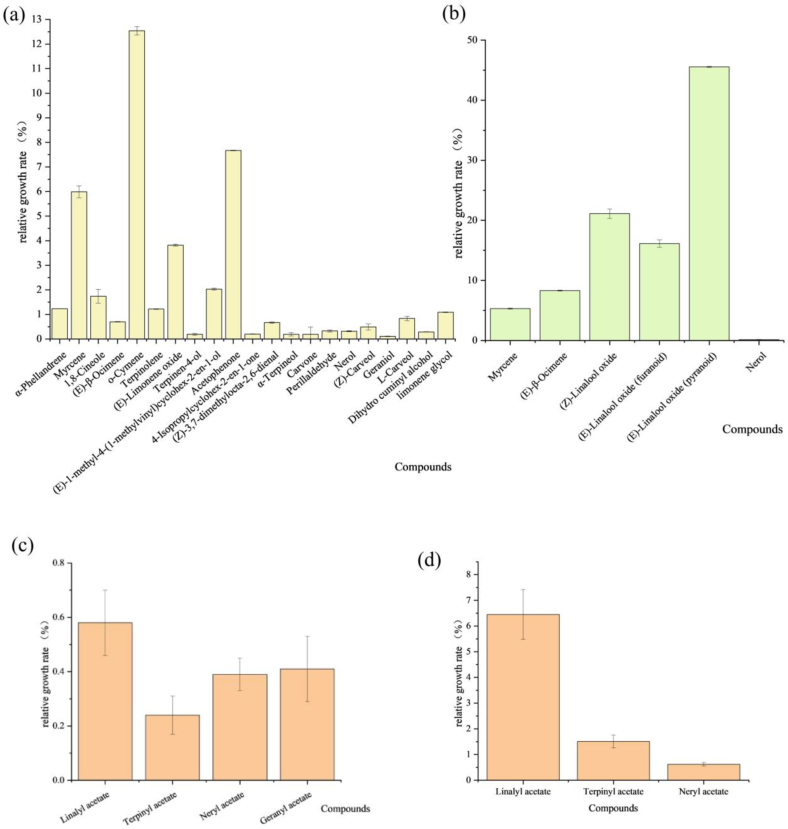
In order to deeply determine the degradation pathways of limonene and linalool in the HW system, limonene (LM-HW) and linalool (LN-HW) were added to the HW system for boiling, respectively. The growth rate of the suspected degradation products obtained from 3.5.1 was calculated by comparing with the blank group (HW). The relative growth rates of limonene and linalool degradation products were shown in Fig. 5a & b. Compared to the sample without limonene addition (HW), 21 compounds were increased in the LM-HW. Six compounds were increased in LN-HW compared to the samples without linalool addition (HW). According to the growth rate, o-cymene, acetophenone and myrcene were the main degradation products of limonene. (Z)-linalool oxide, (E)-linalool oxide (furanoid) and (E)-linalool oxide (pyranoid) were the main degradation products of linalool. In addition, esters corresponding to linalool, pinacol, nerol and geraniol may also be secondary degradation products of limonene and linalool (Fig. 5c & d). The above compounds were identified as possible degradation products based on growth rates.
Fig. 5.
Analysis of degradation products of limonene and linalool in HW systems. (a) Relative growth rates of limonene degradation products. (b) Relative growth rates of linalool degradation products. (c) Relative growth rates of suspected secondary degradation products of limonene. (d) Relative growth rates of suspected secondary degradation products of linalool.
3.5.3. Isotope tracing to verify transformation pathways
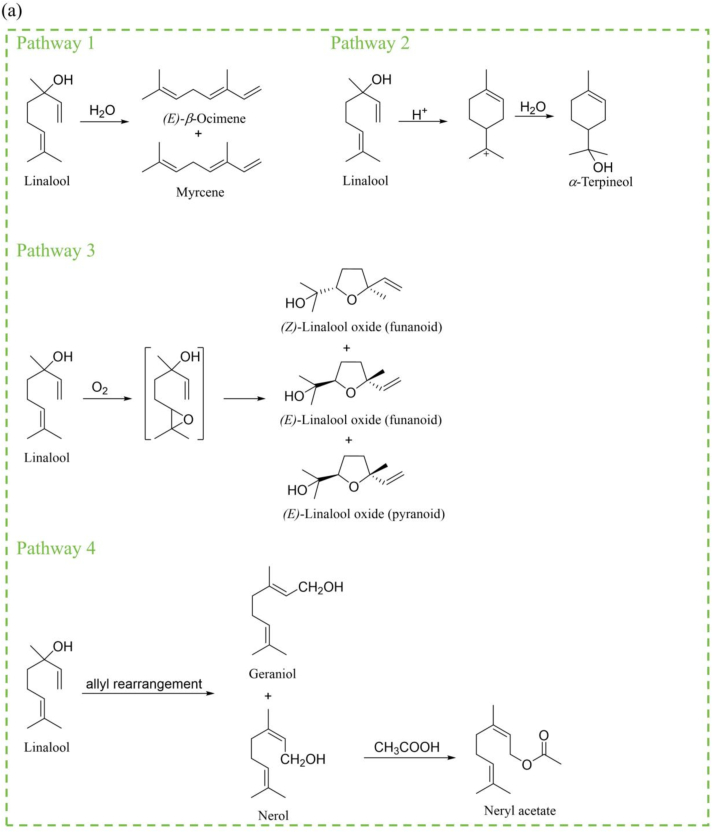
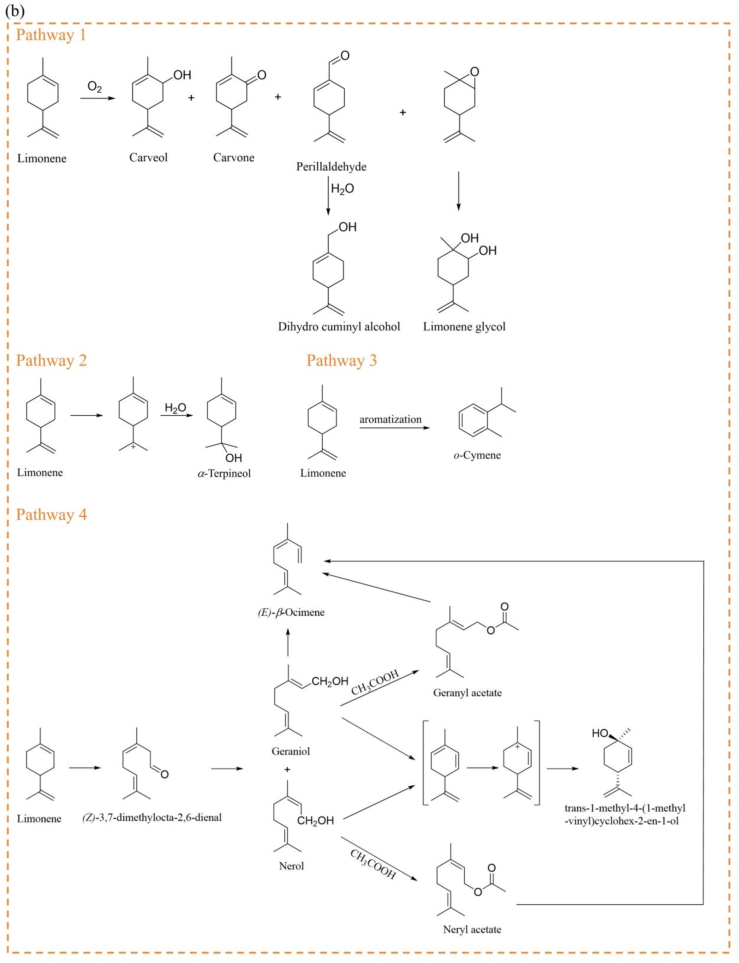
To further validate the linalool and limonene degradants, isotope tracer experiments were conducted. During HW preparation, [13C3]- linalool was added to detect the conversion pathways by isotope labeling during the boiling process. The results showed that 9 compounds were isotopically labeled during the boiling process (Fig. 6a). Linalool showed mainly floral aroma and might have contributed to the floral, pepper aroma of HW (Dong et al., 2024; Sun et al., 2020). Its degradation products included myrcene (herb), (E)-β-ocimene (sweet, leaf), linalool oxide (floral), α-terpineol (pine), geraniol (rose, fruit), nerol (floral, fruit) and neryl acetate (floral, fruit). A total of 15 degradation products were identified with the addition of [13C, 2H2]- limonene during the boiling process (Fig. 6b). Limonene provides mainly lemon and citrus-like aromas (Dong et al., 2023; Dong et al., 2024). Its corresponding degradation products were (E)-β-ocimene (sweet, leaf), o-cymene, trans-1-methyl-4-(1-methylvinyl)cyclohex-2-en-1-ol (mint), (Z)-3,7-dimethylocta-2,6-dienal (lemon), α-terpineol (pine), carvone (mint), Perillaldehyde (mint), nerol (floral, fruit), geraniol (rose, fruit), carveol (fat, fresh, spearmint), dihydro cuminyl alcohol (fat, green, pungent), limonene glycol (mint), neryl acetate (floral, fruit) and geranyl acetate (lavender, rose). The above odor descriptions were found in https://www.femaflavor.org/flavor-library and https://www.chemicalbook.com. These aroma active compounds worked together with other composition to influence the overall flavor of HW. Next, specific degradation pathways were analyzed and discussed.
Fig. 6.
Possible degradation pathways of linalool and limonene in HW. (a) Main degradation pathways of linalool.
Possible degradation pathways of linalool and limonene in HW. (b) Main degradation pathways of limonene.
3.5.4. Degradation pathways of limonene and linalool in HW systems
Degradation pathways were predicted based on the degradation products and literature. The generation of the above products of linalool could be divided into 4 main routes (Fig. 6a). Firstly, linalool can dehydrate to form (E)-β-ocimene (Chen et al., 2021) and myrcene (Brodkorb et al., 2010). Secondly, linalool underwent a cyclization reaction under acidic conditions and formed intermediate with carbocation (Zhang, Catti, et al., 2017). Thereafter, the intermediate combined with H2O of HW to obtain α-terpineol. Third, linalool is oxidized to form linalool oxide intermediates, which were converted into different configurations of linalool oxide (Moreno Rueda et al., 2013; Vilanculo et al., 2020). Lastly, linalool could be converted to nerol and geraniol by alkyl rearrangement (Semikolenov et al., 2003). In HW systems, nerol could also undergo esterification to form neryl acetate.
The degradation of limonene has also been separated into 4 main pathways (Fig. 6b). First, limonene was oxidized during heat processing to produce a series of aldehydes and alcohols. These included carveol, carvone (Oszajca et al., 2023), perillaldehyde (Naróg et al., 2008), dihydro cuminyl alcohol (Wróblewska et al., 2018) and limonene glycol (Gomes et al., 2023; Wróblewska et al., 2018). In addition, limonene may form carbocation intermediate during the heating process, further producing α-terpineol (Zítová et al., 2021). In the tracer modeling experiment with linalool, there was similar reaction. During the processing, limonene also reacted by aromatization to form o-cymene (Albeck & Tamary, 1991). Notably, based on the experimental results, it was hypothesized that limonene may suffer a ring -opening reaction and oxidized to (Z)-3,7-dimethylocta-2,6-dienal (cis-citral) under heating conditions. This reaction has not been reported in the literature. Thereafter, cis-citral was reduced to give geraniol and nerol (Shabade et al., 2022). Geraniol and nerol were esterified to give the corresponding esters, respectively. Nerol and geraniol underwent cyclization, deprotonation and dehydrogenation to obtain carbocation intermediate, which combined with H2O to form trans-1-methyl-4-(1-methylvinyl)cyclohex-2-en-1-ol (Wang & Liu, 1998). Additionally, geraniol (da Silva & Chaves, 2018) neryl acetate and geranyl acetate (Friesen, 2001) suffered an elimination reaction to produce (E)-β-ocimene. These pathways are mainly predicted based on the literature and could be further verified by experiments.
4. Conclusions
In this study, sensory data showed that HW-20 min had the best aroma quality, fully revealed the characteristic aroma of Huajiao. A total of 104 compounds were detected by SAFE-GC–MS from 4 HW samples with different boiling times. To further determine the relationship between compounds and aroma attributes, PLSR analysis was performed. A total of 49 aroma-active compounds were found to contribute significantly to HW aroma. When DH and HW samples were analyzed in comparison, the content of compounds was more abundant in DH. Among them, the most obvious changes were in terpenes. The two of the most abundant compounds in DH were limonene and linalool. In modeling experiments, the degradation products of limonene and linalool in the HW system were determined individually. 9 degradation products of linalool and 15 degradation products of limonene were identified by isotopic tracer techniques. Finally, 4 potential degradation pathways were hypothesized for each of the two compounds in relation to the literature. In conclusion, this experiment determined the preferred boiling time for the preparation of HW and the degradation products of linalool and limonene during thermal processing, and the degradation pathways were predicted and summarized. In the future, flavor regulation of HW might be carried out based on the above findings to enhance the aroma quality of HW.
Informed consent
Not applicable.
CRediT authorship contribution statement
Tianyu Dong: Writing – original draft, Methodology, Data curation. Shuwei Wang: Supervision, Methodology, Investigation. Nan Qi: Software, Data curation, Conceptualization. Jie Sun: Writing – review & editing, Resources, Methodology, Conceptualization. Haitao Chen: Writing – review & editing, Resources, Conceptualization. Shuqi Wang: Writing – review & editing, Formal analysis. Baoguo Sun: Validation, Supervision, Resources.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The present work was supported by the National Natural Science Foundation of P.R. China (No. 32202215).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2024.101939.
Contributor Information
Jie Sun, Email: sunjie@btbu.edu.cn.
Haitao Chen, Email: chenht@th.btbu.edu.cn.
Shuqi Wang, Email: wangshuqi@btbu.edu.cn.
Baoguo Sun, Email: sunbg@btbu.edu.cn.
Appendix A. Supplementary data
Supplementary material
Data availability
Data will be made available on request.
References
- Albeck M., Tamary T. TeCl4 in organic synthesis. Elimination reactions. Journal of Organometallic Chemistry. 1991;420(1):35–42. doi: 10.1016/0022-328X(91)86442-S. [DOI] [Google Scholar]
- Brodkorb D., Gottschall M., Marmulla R., Lüddeke F., Harder J. Linalool dehydratase-isomerase, a bifunctional enzyme in the anaerobic degradation of monoterpenes*. Journal of Biological Chemistry. 2010;285(40):30436–30442. doi: 10.1074/jbc.M109.084244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Huang C., Guo Y., Xu Y., Gong S., Chu Q., Chen P. Unraveling the contributing factors of stale odor in Longjing tea through a sensomics approach. Food Chemistry. 2024;441 doi: 10.1016/j.foodchem.2023.138301. [DOI] [PubMed] [Google Scholar]
- Chen K.Q., Shen J., Wang Z.X., Chen X.Y. A donor-acceptor complex enables the synthesis of E-olefins from alcohols, amines and carboxylic acids. Chemical Science. 2021;12(19):6684–6690. doi: 10.1039/D1SC01024G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z.H. Chinese pepper industry development status and future development prospects. Contemporary Horticulture. 2022;45(08):16–18. doi: 10.14051/j.cnki.xdyy.2022.08.056. [DOI] [Google Scholar]
- Dong T., Tian Z., Wang S., Sun J., Chen H., Wang S., Sun B. Identification of key off-flavor compounds during storage of fried pepper (Zanthoxylum bungeanum Maxim.) oils by sensory-directed flavor analysis and partial least squares regression (PLSR) Journal of Food Composition and Analysis. 2024;131:106268. doi: 10.1016/j.jfca.2024.106268. [DOI] [Google Scholar]
- Dong T.Y., Qi N., Liu R.J., Sun J., Zhang N., Chen H.T. Comparative analysis of key flavor substances of green and red pepper. Fine Chemicals. 2023;40(04):869–877. doi: 10.13550/j.jxhg.20220957. [DOI] [Google Scholar]
- Epifano F., Curini M., Marcotullio M.C., Genovese S. Searching for novel cancer chemopreventive plants and their products: The genus Zanthoxylum. Current Drug Targets. 2011;12(13):1895–1902. doi: 10.2174/138945011798184128. [DOI] [PubMed] [Google Scholar]
- Friesen R.W. Georg Thieme Verlag KG; Stuttgart: 2001. Product subclass 2: Palladium-allyl complexes. Category 1, Organometallics (Vol. volume 1) [Google Scholar]
- Gao L., Shi B., Zhao L., Wang H., Xiang Y., Zhong K. Aroma characteristics of green Huajiao in Sichuan and Chongqing area using sensory analysis combined with GC-MS. Foods. 2024;13(6):836. doi: 10.3390/foods13060836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemert L.J.V. Oliemans Punter & Partners BV; Netherlands: 2011. Odour thresholds. [Google Scholar]
- Gomes D.M., Silva A.F., Gomes A.C., Neves P., Valente A.A., Gonçalves I.S., Pillinger M. Pyrazine-bridged molybdenum(0) carbonyl and molybdenum(VI) oxide network solids as catalysts for epoxidation and sulfoxidation. Catalysis Today. 2023;418 doi: 10.1016/j.cattod.2023.114050. [DOI] [Google Scholar]
- González-Domínguez R., Sayago A., Fernández-Recamales Á. An overview on the application of chemometrics tools in food authenticity and traceability. Foods. 2022;11(23):3940. doi: 10.3390/foods11233940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Liu Y., Yang W., Liu Y., Zhang Y., Huang M., Sun B. Characterization of the potent odorants contributing to the characteristic aroma of Beijing douzhi by gas chromatography-Olfactometry, quantitative qnalysis, and odor activity value. Journal of Agricultural and Food Chemistry. 2018;66(3):689–694. doi: 10.1021/acs.jafc.7b04839. [DOI] [PubMed] [Google Scholar]
- Huang Y., Pu D., Hao Z., Liang L., Zhao J., Tang Y., Zhang Y. Characterization of taste compounds and sensory evaluation of soup cooked with sheep tail fat and prickly ash. Foods. 2022;11(7):896. doi: 10.3390/foods11070896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagella T., Grosch W. Flavour and off-flavour compounds of black and white pepper (Piper nigrum L.)III. Desirable and undesirable odorants of white pepper. European Food Research and Technology. 1999;209(1):27–31. doi: 10.1007/s002170050451. [DOI] [Google Scholar]
- Liu J., Wan J., Zhang Y., Hou X., Shen G., Li S., Luo Q., Li Q., Zhou M., Liu X., Wen C., Zhu X., Zhang Z. The establishment of comprehensive quality evaluation model for flavor characteristics of green Sichuan pepper (Zanthoxylum armatum DC.) in Southwest China. Food Chemistry. 2023;X, 18 doi: 10.1016/j.fochx.2023.100721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo D., Pang X., Xu X., Bi S., Zhang W., Wu J. Identification of cooked off-flavor components and analysis of their formation mechanisms in melon juice during thermal processing. Journal of Agricultural and Food Chemistry. 2018;66(22):5612–5620. doi: 10.1021/acs.jafc.8b01019. [DOI] [PubMed] [Google Scholar]
- Moreno Rueda M.G., Guerrini A., Giovannini P.P., Medici A., Grandini A., Sacchetti G., Pedrini P. Biotransformations of terpenes by fungi from amazonian citrus plants. Chemistry & Biodiversity. 2013;10(10):1909–1919. doi: 10.1002/cbdv.201300112. [DOI] [PubMed] [Google Scholar]
- Naróg D., Szczepanik A., Sobkowiak A. Iron(II, III)-catalyzed oxidation of limonene by dioxygen. Catalysis Letters. 2008;120(3):320–325. doi: 10.1007/s10562-007-9290-7. [DOI] [Google Scholar]
- Ni R., Yan H., Tian H., Zhan P., Zhang Y. Characterization of key odorants in fried red and green huajiao (Zanthoxylum bungeanum maxim. and Zanthoxylum schinifolium sieb. et Zucc.) oils. Food Chemistry. 2022;377 doi: 10.1016/j.foodchem.2021.131984. [DOI] [PubMed] [Google Scholar]
- Oszajca M., Nitek W., Rafalska-Łasocha A., Pamin K., Połtowicz J., Łasocha W. Synthesis, crystal structure and selected properties of three new 4-propylanilinium polyoxomolybdates. Journal of Molecular Structure. 2023;1273 doi: 10.1016/j.molstruc.2022.134292. [DOI] [Google Scholar]
- Pérez-López A.J., Saura D., Lorente J., Carbonell-Barrachina A.A. Limonene, linalool, α-terpineol, and terpinen-4-ol as quality control parameters in mandarin juice processing. European Food Research and Technology. 2006;222(3–4):281–285. doi: 10.1007/s00217-005-0055-5. [DOI] [Google Scholar]
- Phuyal N., Jha P.K., Raturi P.P., Gurung S., Rajbhandary S. Essential oil composition of Zanthoxylum armatum leaves as a function of growing conditions. International Journal of Food Properties. 2019;22(1):1873–1885. doi: 10.1080/10942912.2019.1687517. [DOI] [Google Scholar]
- Rodríguez A., Peris J.E., Redondo A., Shimada T., Costell E., Carbonell I.…Peña L. Impact of D-limonene synthase up- or down-regulation on sweet orange fruit and juice odor perception. Food Chemistry. 2017;217:139–150. doi: 10.1016/j.foodchem.2016.08.076. [DOI] [PubMed] [Google Scholar]
- Semikolenov V.A., Ilyna I.I., Maksimovskaya R.I. Linalool to geraniol/nerol isomerization catalyzed by (RO)3VO complexes: Studies of kinetics and mechanism. Journal of Molecular Catalysis A: Chemical. 2003;204-205:201–210. doi: 10.1016/S1381-1169(03)00299-1. [DOI] [Google Scholar]
- Shabade A.B., Sharma D.M., Bajpai P., Gonnade R.G., Vanka K., Punji B. Room temperature chemoselective hydrogenation of C C, C O and C N bonds by using a well-defined mixed donor Mn(i) pincer catalyst. Chemical Science. 2022;13(46):13764–13773. doi: 10.1039/D2SC05274A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva M.J., Chaves D.M. SnCl2-catalyzed synthesis of carbamates from renewable origin alcohols. Chemical Papers. 2018;72(5):1169–1180. doi: 10.1007/s11696-017-0349-7. [DOI] [Google Scholar]
- Sun J., Sun B.G., Ren F.Z., Chen H.T., Zhang N., Zhang Y.Y. Characterization of key odorants in Hanyuan and Hancheng fried pepper (Zanthoxylum bungeanum) oil. Journal of Agricultural and Food Chemistry. 2020;68(23):6403–6411. doi: 10.1021/acs.jafc.0c02026. [DOI] [PubMed] [Google Scholar]
- Tian P., Zhan P., Tian H., Wang P., Lu C., Zhao Y. Effects of different vegetable oils on the aroma characteristics of deep-fried shallot flavoring evaluated by HS-SPME/GC-MS coupled with PLSR. Journal of Food Processing and Preservation. 2020;44(9) doi: 10.1111/jfpp.14698. [DOI] [Google Scholar]
- Vilanculo C.B., Da Silva M.J., Teixeira M.G., Villarreal J.A. One-pot synthesis at room temperature of epoxides and linalool derivative pyrans in monolacunary Na7PW11O39-catalyzed oxidation reactions by hydrogen peroxide. RSC Advances. 2020;10(13):7691–7697. doi: 10.1039/D0RA00047G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Liu Y.C. A novel cyclization of geraniol and nerol initiated by tris(p-bromophenyl)ammoniumyl radical cation. Journal of Chemical Research, Synopses. 1998;1:42–43. doi: 10.1039/A705326F. [DOI] [Google Scholar]
- Watkins P.J., Kearney G., Rose G., Allen D., Ball A.J., Pethick D.W., Warner R.D. Effect of branched-chain fatty acids, 3-methylindole and 4-methylphenol on consumer sensory scores of grilled lamb meat. Meat Science. 2014;96(2):1088–1094. doi: 10.1016/j.meatsci.2012.08.011. [DOI] [PubMed] [Google Scholar]
- Wróblewska A., Makuch E., Młodzik J., Koren Z.C., Michalkiewicz B. Oxidation of limonene over molybdenum dioxide-containing nanoporous carbon catalysts as a simple effective method for the utilization of waste orange peels. Reaction Kinetics, Mechanisms and Catalysis. 2018;125(2):843–858. doi: 10.1007/s11144-018-1468-z. [DOI] [Google Scholar]
- Yang Y., Yu P., Sun J., Jia Y., Wan C., Zhou Q., Huang F. Investigation of volatile thiol contributions to rapeseed oil by odor active value measurement and perceptual interactions. Food Chemistry. 2022;373 doi: 10.1016/j.foodchem.2021.131607. [DOI] [PubMed] [Google Scholar]
- Zeng M., Wang J., Zhang M., Chen J., He Z., Qin F.…Chen J. Inhibitory effects of Sichuan pepper (Zanthoxylum bungeanum) and sanshoamide extract on heterocyclic amine formation in grilled ground beef patties. Food Chemistry. 2018;239:111–118. doi: 10.1016/j.foodchem.2017.06.097. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Catti L., Pleiss J., Tiefenbacher K. Terpene cyclizations inside a supramolecular catalyst: Leaving-group-controlled product selectivity and mechanistic studies. Journal of the American Chemical Society. 2017;139(33):11482–11492. doi: 10.1021/jacs.7b04480. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Song H., Li P., Yao J., Xiong J. Determination of potential off-flavour in yeast extract. LWT - Food Science and Technology. 2017;82:184–191. doi: 10.1016/j.lwt.2017.04.030. [DOI] [Google Scholar]
- Zhao C., Han M., Tu T., Chen S., Hu W., Dong L., Zhang F., Zhao Y., Li Z. Comparative analysis of fatty acids, volatile and non-volatile components in red huajiao (Zanthoxylum bungeanum maxim.) and green huajiao (Zanthoxylum armatum DC.) using GC-MS, UPLC-LTQ-Orbitrap-MS/MS and HPLC-DAD. Industrial Crops and Products. 2023;204 doi: 10.1016/j.indcrop.2023.117371. [DOI] [Google Scholar]
- Zhou Y.X., Yan Y., Yin J.F., Huang J.J., Zhang F.S., Cui K.…Xie N.N. Effect of fermentation methods on microflora and volatile compounds in Huangshan smelly mandarin fish. Meat Research. 2019;33(10):36–43. doi: 10.7506/rlyj1001-8123-20190626-148. [DOI] [Google Scholar]
- Zhu R.X., Zeng W.C., Zhao Z.F., Gao H., Yan Z.N. Chemical components and antibacterial activity of Hanyuan Zanthoxylum bungeanum seed oil. Food Science. 2011;32(17):85–88. https://kns.cnki.net/kcms2/article/abstract?v=QuBpG80dbeAza3PWaBqhkryKWXbM97okBMc6QXZIfT0kq08_LQuOa_f41JO5mYcThmG25LW7DgFvEay36uNKQIK3WlyuN8XOKYVjnafWKUtYtGoI_SP_kB6gigPVBqZ4wOp0NC9XO4s=&uniplatform=NZKPT&language=CHS [Google Scholar]
- Zítová K., Vyskočilová E., Červený L. Preparation of α-terpineol and perillyl alcohol using zeolites beta. Research on Chemical Intermedlates. 2021;47(10):4297–4310. doi: 10.1007/s11164-021-04515-6. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Data will be made available on request.