Abstract
Cortico-basal ganglia circuits are key parts of the brain's habit system, but little is yet known about how these forebrain pathways function as ingrained habits are performed. We simultaneously recorded spike and local field potential (LFP) activity from regions of the frontal cortex and basal ganglia implicated in visuo-oculomotor control as highly trained macaque monkeys performed sequences of visually guided saccades. The tasks were repetitive, required no new learning, and could be performed nearly automatically. Our findings demonstrate striking differences between the relative timing of striatal and cortical activity during performance of the tasks. At the onset of the visual cues, LFPs in the prefrontal cortex and the oculomotor zone of the striatum showed near-synchronous activation. During the period of sequential-saccade performance, however, peak LFP activity occurred 100–300 msec later in the striatum than in the prefrontal cortex. Peak prefrontal activity tended to be peri-saccadic, whereas peak striatal activity tended to be post-saccadic. This temporal offset was also apparent in pairs of simultaneously recorded prefrontal and striatal neurons. In triple-site recordings, the LFP activity recorded in the supplementary eye field shared temporal characteristics of both the prefrontal and the striatal patterns. The near simultaneity of prefrontal and striatal peak responses at cue onsets, but temporal lag of striatal activity in the movement periods, suggests that the striatum may integrate corollary discharge or confirmatory response signals during sequential task performance. These timing relationships may be signatures of the normal functioning of striatal and frontal cortex during repetitive performance of learned behaviors.
Keywords: basal ganglia, local field potential, prefrontal cortex, synchrony
The basal ganglia and neocortex are strongly interconnected by pathways that lead from widespread areas of the neocortex to the striatum and from the striatum through multisynaptic pathways back to the neocortex, mainly the frontal cortex (1). These cortico-basal ganglia loops are thought to release cortical activity phasically from tonic inhibitory control by the basal ganglia, thereby modulating cortical function (2, 3). Neuroimaging studies have demonstrated that cortical and striatal components of these forebrain circuits can be coactivated during voluntary behavior, and that such coactivation patterns are regionally selective (4, 5). Very little evidence is available, however, about how the temporal dynamics of ongoing activity states in the basal ganglia and neocortex are interrelated as animals perform the habitual, semi-automatic behaviors that cortico-basal ganglia pathways are thought to enable.
Resolving this issue is at the limits of current technology, because both population-level analysis across multiple brain regions and high spatiotemporal resolution at a local level are required, together with large-scale identification of anatomically connected pairs of single neurons (6–9). As a step toward analyzing corticostriatal dynamics, we identified striatal and cortical components of the oculomotor cortico-basal ganglia circuit in macaque monkeys and simultaneously recorded neural activity in these regions with multiple chronically implanted electrodes as the monkeys viewed a sequence of visual targets and made saccades to each in order to receive reward. We recorded from the oculomotor region of the caudate nucleus, the dorsolateral prefrontal cortex anterior to the frontal eye fields, in which we have found saccade-related activity (10) and, in some experiments, the supplementary eye field (SEF) as well. To achieve a population-level analysis, we recorded local field potential (LFP) activity (11–15) in addition to the spike activity of individual neurons. Our goal in these experiments was to determine the patterns of activity that occur in these compartments of oculomotor corticobasal ganglia circuits as the monkeys performed the sequential tasks in a stereotyped, habitual fashion. We therefore made the recordings after the monkeys had been extensively trained on the saccade tasks, so that no new learning would be required of them.
Our findings demonstrate that neural activity in each of the striatal and cortical regions was differentially modulated during the course of task performance, but that, remarkably, the relative timing of peak neural activities in these regions varied dynamically during the task. Cortical and striatal components of cortico-basal ganglia loops thus do not have a single timing relationship during repetitive performance of familiar sequential tasks. Instead, both striatal and cortical levels appear to register sensory inputs at nearly the same time but have activities that diverge in time during the execution phase of task performance.
Materials and Methods
Two adult female monkeys (Maccaca mulatta), M6 and M7, were trained over a period of 3 years to perform visuomotor tasks (10). For the main sequential-saccade task, the monkeys were required to fixate for 1 sec on a red 0.8°-diameter target presented at the center of a 9 × 9 array of gray potential target dots on a computer screen 30 cm in front of the monkey. The fixation point was then extinguished, and the monkeys were required to make saccades within 400 msec to one to six targets successively illuminated in red (Go signals; estimated onset jitter, ≈14 msec). The lengths of the saccade sequences and target presentation intervals were varied in blocks of 30–40 trials. The monkeys received water reward 400–800 ms after the last target offset if they successfully completed a given saccade sequence in a single trial. A 1.5-sec inter-trial interval followed. Target sequences for each trial were chosen pseudorandomly from 64 sequences for the standard four-target task, eliciting saccades in combinations of up, down, right, and left. Thus, in activity averaged over successive trials, the effects of saccade direction were cancelled out. The monkeys were also trained on fixed-sequence blocks of trials not reported here (10). The standard number of saccades per trial was four, and the standard interval between the visual Go signals was 400 msec. In a variant of the standard task, on which monkeys were trained for >1.5 months before recording, the color of the last target was yellow instead of red. A simple fixation task was also given, requiring 1-sec fixation followed by reward 400–800 msec later. Trials with reward only, without reward but with reward-associated click, or with clicks only were also given in blocks of 30 trials.
A headpost, a recording chamber, and an eye coil (16) were surgically implanted in sterile surgeries performed by methods in accordance with National Institutes of Health guidelines and approved by the Massachusetts Institute of Technology Committee on Animal Care. Spike and LFP activities were recorded simultaneously from the caudate nucleus bilaterally and from the dorsolateral prefrontal cortex in the left hemisphere of each monkey with 24–64 Epoxylite-coated tungsten microelectrodes (FHC, Bowdoinham, ME; 0.2–1.5 mohm) advanced through supradural guide tubes in a grid. Up to 12 microelectrodes were also placed in the left SEF in monkey M7. Electrode placement was guided by pre-recording MRI images and by stimulation and recording mapping (10, 17). Electrodes were initially placed at sites with well-isolated unit activity, regardless of whether task-related activity was recorded. Electrodes were moved in daily sessions only if signal-to-noise ratios were poor, and they were not moved during recording periods. One guide tube served as a reference channel for both spike and LFP recordings.
Spike, LFP, eye position, and behavioral task-event data were collected by a Cheetah recording system (Neuralynx, Tucson, AZ) and were time-stamped and analyzed with in-house software. Spike activity was sampled at 32 kHz, with LFP activity (filtered between 1 and 300 Hz at input) at 1 kHz and eye position at 1 kHz. For LFP recordings, 16 channels were selected daily from the channels used for spike recording, with a fraction of them overlapping those of the previous recording session.
Units were accepted for analysis based on manually guided cluster cutting and autocorrelogram analyses. The activity of each accepted unit during each peri-event window was compared with the activity of that unit during a 500-msec baseline period set at the center of the 1.5-sec-long inter-trial interval. If peri-event activity summed over all trials of a block in peri-event time histograms was significantly greater than that recorded during the control period (Mann–Whitney, P < 0.05), the unit was classified as a task-related neuron.
LFPs were analyzed by in-house software by use of matlab and python scripts. Single LFP traces were aligned by specific task events for all trials of each trial block for each task condition, and these were averaged to generate peri-event-triggered LFPs. To avoid possible duplicative recording and oversampling of same-site activity from day to day, only LFPs recorded from different sites, after electrode movement, were included in the population analyses. To compare LFP activity across conditions, population LFPs were normalized by using the formula: normalized averaged LFP of condition x = {(averaged LFP of condition x) – (minimum value of averaged LFP of standard four-saccade condition)}/(range of averaged LFP of standard four-saccade condition). Normalized LFPs were used to identify peaks in the field activity by moving a 150-msec time window in 1-msec steps across the entire trial time and comparing the LFP activity in the first and second 75-msec-long halves of each window. A peak rise was identified if 10 successive windows exhibited increases in LFP signal at the P < 0.01 level (Wilcoxin test). The decline of the peak was identified when 10 successive windows showed significant decreases in LFP signal. The maximum value of LFP activity within the defined peak was taken as the time of peak occurrence for subsequent analyses.
Cross-covariance analysis was applied to averaged normalized LFPs recorded simultaneously in the caudate nucleus (n = 213), prefrontal cortex (n = 52), and SEF (n = 25) of the left hemisphere. We applied a moving-window cross-covariance analysis (matlab) to all pairs of simultaneously recorded LFPs to determine the temporal comodulation of LFP activity in the three structures. Two-hundred-msec-wide time windows were moved in steps of 100 msec across each pair of normalized prefrontal-striatal, prefrontal-SEF, and SEF-striatal LFPs recorded over 60-trial blocks of the default four-saccade task. Cross-correlograms were normalized by using the mean and standard deviation. We also applied the peak detection algorithm used above to the cross-correlation for each individual time window to derive average peak and valley timing distribution plots.
Results
Task-Related Single-Unit Activity Recorded Simultaneously in Striatum and Prefrontal Cortex. We first analyzed neuronal activity in paired simultaneous recordings of units at up to 21 sites in the dorsolateral prefrontal cortex and up to 20 sites in the caudate nucleus (Fig. 8, which is published as supporting information on the PNAS web site). The resulting sample of 750 well isolated units (n = 156 in PFC, n = 594 in caudate nucleus) included 90 task-related neurons, of which 54 were in the caudate nucleus, and 36 were in the dorsolateral PFC. Altogether, 26 striatal and prefrontal units had task-related activity simultaneously on the same recording day.
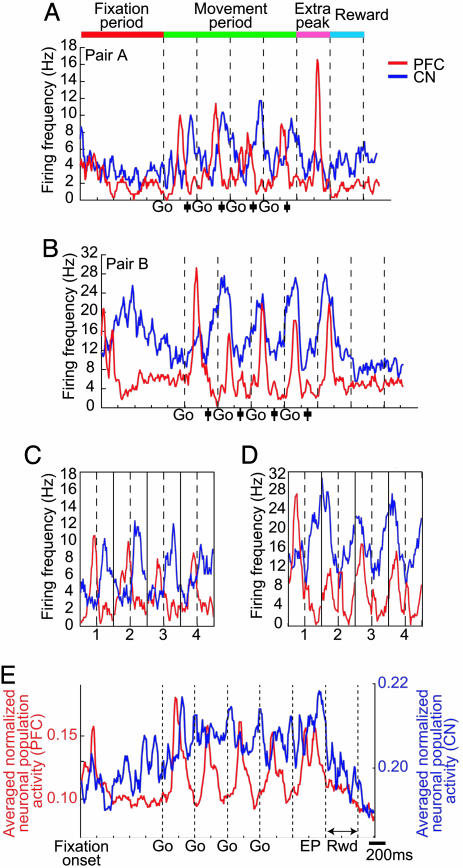
Fig. 1 illustrates the spike activity of two of these pairs, recorded as monkey M7 performed the standard four-target sequential-saccade task on different days (2 weeks apart) within a single 10-week period of chronic recording. The prefrontal neurons generated phasic peri-saccadic responses for each of the four saccades, together with a phasic “extra” peak (see ref. 10) that occurred ≈270 msec after the last target off. The striatal neurons of each pair also had phasic activity related to each of the four saccade events during the movement period but not sharp extra peaks. The striatal neuron shown in Fig. 1B increased its firing rate during the fixation period, and there was some modulation of tonic firing during the task.
Fig. 1.
Spike activity of neurons recorded simultaneously in the striatum and prefrontal cortex during performance of the standard four-saccade task. Prefrontal activity is shown in red, striatal activity in blue. Task events shown at top. (A and B) Peri-event time histograms of the activity of two pairs of neurons active during 60 trials of task performance by monkey M7. Average times of saccade onset are shown by black vertical lines and their standard deviations by black boxes. (C and D) Movement-period activity of the two pairs of neurons illustrated as in A and B but realigned on saccade onset. (E) Population histogram of simultaneously recorded prefrontal (n = 36) and striatal (n = 54) neurons from monkeys M7 and M6 normalized for each unit and then averaged.
A striking feature of both of these simultaneously recorded unit-recordings is that the peaks of prefrontal spike activity during the movement period preceded those of the striatal neurons. The temporal lead was ≈130 msec for the prefrontal neuron in Fig. 1 A and ≈350 msec for the prefrontal neuron in Fig. 1B. For both pairs, however, the initial increases in striatal and prefrontal spike discharge after the first target onset had nearly the same latency. The peaks of prefrontal spike activity were peri-saccadic, whereas the striatal spike activity peaks for each saccade period were post-saccadic, lagging the prefrontal peaks by ≈100–320 msec (Fig. 1 C and D). This pattern was consistent for the other pairs of simultaneously recorded pairs of units when both exhibited phasic target/saccade responses to the same targets/saccades of the sequences.
The population spike histograms for all simultaneously recorded task-related units, however, did not give such a clear view of the relative time of spike activity in the two structures and caudate nucleus (Fig. 1E). Sharp phasic peaks in the averaged activity of the prefrontal neurons did occur after each target onset (together with a doublet extra peak), but a tonic rise in activity during the movement period dominated the striatal population response, obscuring discrete, phasic single-neuron responses such as those shown in Fig. 1 A and B. To investigate the relative timing of the striatal and prefrontal responses, we therefore turned to LFP recordings.
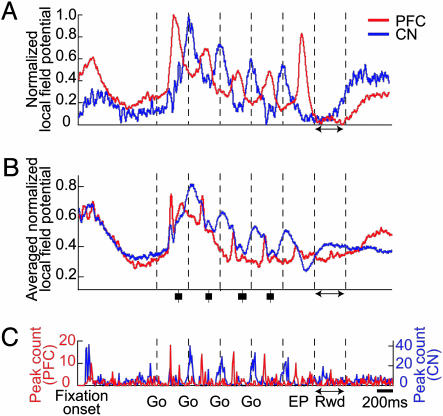
LFP Activity Recorded Simultaneously in Striatum and Prefrontal Cortex. In sharp contrast to the weak phasic patterning in the population spike responses of striatal neurons, there was clear phasic patterning in the LFPs recorded in the striatum at the same time (Fig. 2A). Peaks in LFP activity occurred in both the striatum and the prefrontal cortex for each of the four target/saccade events in the sequence. The relative timing of these peaks, however, varied markedly during different phases of the task. During the fixation period, the population LFPs in the two recording samples were nearly identical (Fig. 2B), and when the first target was presented, LFP activity in each structure rose abruptly and nearly synchronously with a ≈140-msec latency. However, the prefrontal LFP activity then continued to rise rapidly, whereas the striatal LFP activity rose and reached its peak ≈200 ms later than the prefrontal LFP response.
Fig. 2.
LFP activity recorded simultaneously in the prefrontal cortex (red) and caudate nucleus (blue) during performance of the standard four-saccade task. (A) Examples of LFPs recorded from a single pair of prefrontal and striatal electrodes, averaged over 60 trials and normalized. (B) Averaged normalized LFPs recorded simultaneously over 60 trials at separate recording sites in the prefrontal cortex (n = 52 sites) and caudate nucleus (n = 213). Saccade onsets shown as in Fig. 1. (C) Plot of number of peaks detected in the LFP data shown in B by peak-detection algorithm applied to sliding 150-msec window moved in 100-msec steps across task time.
During the movement period, phasic peaks in prefrontal activity occurred after each Go signal, but the tonic level of the prefrontal response declined sharply during the movement period. The tonic level of the striatal LFP response also declined but did so more slowly. The striatal LFP peaks were longer in duration than those in the prefrontal cortex and occurred ≈250–300 msec after the prefrontal LFP peaks. The average rise time for the prefrontal peaks was 126 msec (n = 1,105), significantly shorter than the 200-ms average rise time for the striatal peaks (n = 3,596, P < 0.01, two-tailed t test). The main LFP activity recorded in the prefrontal cortex and caudate nucleus thus alternated between peaks of peri-saccadic prefrontal activity and strong peaks of later, post-saccadic striatal activity (Fig. 2C).
Small prefrontal and striatal responses to the onset of the fixation point occurred nearly simultaneously, however, and small striatal peaks also occurred nearly simultaneously with the larger prefrontal peaks after each subsequent target onset. Thus, the predominant timing relations between peak LFP activity in the prefrontal cortex and caudate nucleus varied from being nearly in-phase during the initial and subsequent target-onset periods to being out-phase, with a large prefrontal lead, during the remainder of the movement sequence. These modulations of LFP activity occurred in a context of stable baseline activity: in control trial blocks in which the monkey simply rested, no phasic LFP activity was detected (Fig. 9A, which is published as supporting information on the PNAS web site).
To investigate the consistency of these timing relationships, we applied variations in the task conditions and compared the LFPs recorded from the same set of prefrontal and striatal sites. We reasoned that if these relative timing relations between prefrontal and striatal field activity were tied to the particular saccade timing imposed by the standard four-saccade task, they should change when such task timing parameters were altered. They were not. During blocks of trials in which the four-saccade task was performed with inter-target intervals of 600 or 800 ms (Fig. 9 B and C), the relative timing of the peak prefrontal and striatal activity was maintained, as was the timing of the peaks relative to target presentations. What did change were the fall times of the striatal peaks and the late near-plateau periods of the prefrontal peaks (as judged within the limits of the 1-Hz low-pass filter) and, because of the longer intertarget intervals, the late striatal peaks did not overlap the next target onsets as they did in the default task. In the single-saccade task (Fig. 9 D and E), striatal and prefrontal responses were again nearly simultaneous at target onset, and the peak prefrontal response led by ≈125–135 ms. In fixation-task trials, in which there was no requirement for the monkey to make a saccade in response to the offset of the fixation target (Fig. 9F), the population LFPs in the striatum and prefrontal cortex also showed similar initial phasic increases, at fixation point offset, and then late peak with prefrontal cortex (peak 330 msec) leading striatum (peak 450 msec) by >100 msec. Similar patterns were present in trial blocks in which free reward (accompanied by a click) was given every 5 sec (Fig. 9G) or in which only the clicks were delivered every 5 sec (Fig. 9H).
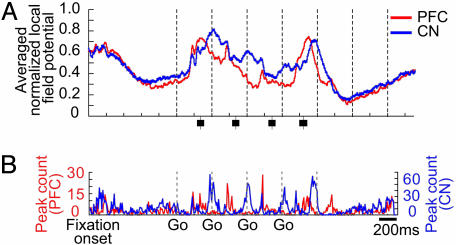
In previous work, we developed a modified sequential-saccade task in which we used a yellow last target condition to cue explicitly the end of the task (10). This changed the cognitive conditions of the task but left the motor demands of the task the same. We found that single-unit activity in the prefrontal cortex was strongly affected by the introduction of the task-end cue (10). We used this condition while recording LFP activity simultaneously in prefrontal cortex and striatum. We found, as shown in Fig. 3, that the LFPs recorded in the prefrontal cortex and caudate nucleus in this condition (Fig. 3A) were dramatically different from those recorded in the standard task (Fig. 2B). Peak timing was disrupted during the movement period, and large peaks occurred in response to the yellow target both in the prefrontal cortex and in the striatum, with the prefrontal peak leading by ≈70 msec (Fig. 3B). The percent correct performance of the monkeys was as high (>90%) in this task as in the standard task. This result suggests that the LFP activity recorded in the caudate nucleus and prefrontal cortex was not exclusively tied to the movement parameters of the sequential-saccade task, which could be changed without disrupting striatal-prefrontal LFP timing relationships, but that the relative timing of LFP activity in the prefrontal cortex and striatum could be altered by introduction of an explicit end signal.
Fig. 3.
LFP activity recorded simultaneously in prefrontal cortex (red) and caudate nucleus (blue) during performance in 30 trials of the yellow last target version of the four-saccade task. (A) Averaged normalized LFPs recorded at same sites from which data in Fig. 2B were recorded (52 prefrontal and 213 striatal sites). Conventions as in Fig. 2. (B) LFP peak counts for data shown in A, obtained as for Fig. 2C.
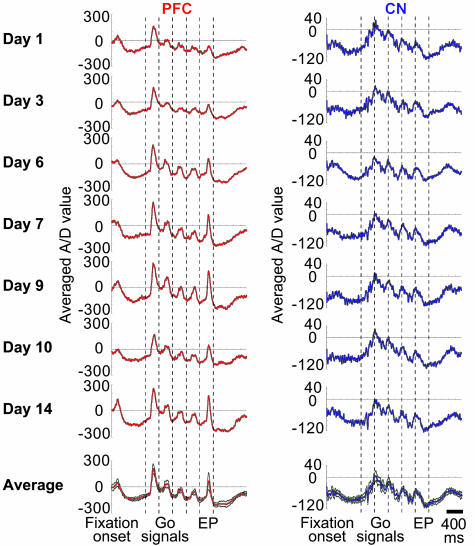
If such LFP activity were used as a signal for building neural representations, it should occur reliably when recorded under similar conditions. It was. As an example, the LFP activity illustrated in Fig. 4 was recorded simultaneously from the same single prefrontal and striatal sites for >14 daily sessions as monkey M7 performed the default four-saccade task. LFP peaks occurred at almost identical task times from day to day, and the amplitudes of the responses, although varying slightly from day to day, were also similar.
Fig. 4.
Chronic recordings of LFP activity in prefrontal cortex (red) and caudate nucleus (blue) as monkey M7 performed the standard four-saccade task in 60 trial sessions over a 2-week period. Results are shown for seven selected individual sessions (top) and for the average of all 14 sessions (bottom). Plots show raw analogue/digital (A/D) values. Green lines in averaged plots indicate ± 2 standard deviations around the mean.
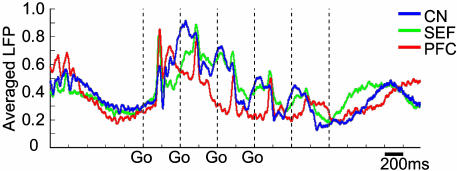
LFP Activity Recorded Simultaneously in the Prefrontal Cortex, SEF, and Striatum. In monkey M7, we recorded LFP activity from electrodes implanted in the SEF (n = 25) as well as in the caudate nucleus (n = 111) and the prefrontal cortex (n = 36). LFP activity in the SEF underwent clear temporal modulation during the saccade task, and this modulation differed from that of both the prefrontal cortex and the caudate nucleus (Fig. 5). The population LFPs in the SEF had sharp peri-saccadic peaks, as did the population LFP activity in the prefrontal cortex, but the SEF also had late LFP peaks that overlapped the late peaks in the striatal LFPs. Recordings of population LFP activity in each of the task variants confirmed that the SEF, prefrontal cortex, and striatal sites exhibited distinct, although temporally coordinated, activity (Fig. 10, which is published as supporting information on the PNAS web site).
Fig. 5.
Averaged normalized LFPs recorded from 36 prefrontal (red), 35 SEF (green), and 111 striatal (blue) sites, recorded in 60 trials during performance of the standard four-saccade task.
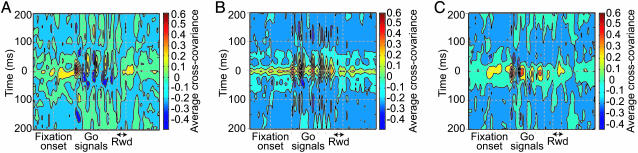
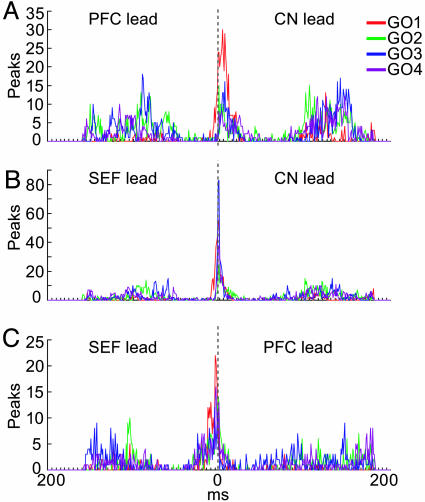
A cross-covariance analysis of these LFP activities is shown in Fig. 6. The striatal-prefrontal cross-covariance (Fig. 6A) suggests close synchrony in the prefrontal and striatal population LFPs during the post-fixation period after the target onsets, particularly for the first target and again after reward delivery. Anti-phase activity occurred in relation to each saccade. Referred to the first target-onset time, these alternations showed clear prefrontal leads for each successive target/saccade phase, producing the striatal lag pattern in the cross-covariogram. The cross-covariance between the striatal and SEF population LFPs (Fig. 6B) also demonstrated near-synchrony at the first target onset and longer periods of near-synchronous activity alternating during the task with shorter periods of anti-phase activity. Prefrontal and SEF LFPs (Fig. 6C) exhibited prominent periods of synchronous activity throughout the task, with weaker outphase or unrelated activity at other times. Very little temporal covariation was evident in the simultaneously recorded LFPs during the intertrial intervals, when the monkey was not engaged in the task. The standard deviations of the cross-covariance averages were generally low at times of high cross-covariance in-phase and anti-phase and high in between (Fig. 11, which is published as supporting information on the PNAS web site). Analysis of the cross-covariance peak distributions showed that the prefrontal and striatal population activity peaks were most commonly nearly synchronous at first target onset, with the striatal peaks leading by ≈10 msec (Fig. 7A). The SEF and striatal LFP responses to the target onsets were synchronous (Fig. 7B). The SEF responses to the target onsets led the prefrontal LFP peaks slightly (Fig. 6C). The cross-covariance peak distributions within each structure were highly consistent (Fig. 12, which is published as supporting information on the PNAS web site).
Fig. 6.
Sliding-window cross-covariance plots of the data for total of 739 simultaneous pairs of recording sites. (A) Three hundred fifteen striatal–prefrontal pairs; (B) 316 striatal–SEF pairs; and (C) 108 prefrontal–SEF pairs. A 200-msec-wide window was moved in 100-msec steps across trial time. Pseudocolor plot indicates strength of cross-covariance (+1.0, same shape waveform; –1.0, 180° inverted waveform).
Fig. 7.
Distribution of in-phase activity peaks in cross-covariance plots of LFP activity illustrated in Fig. 6, shown for 400-msec time windows centered around each target onset (Go signal).
Discussion
Our findings demonstrate that functionally related parts of the macaque monkey striatum and frontal cortex, identified by their task-related single-unit spike responses during repetitive performance of sequential-saccade tasks, have individually specific patterns of LFP activity that bring them into and out of synchronous activation during task performance. When monkeys performed sequential saccades cued by visual targets, sharp peri-saccadic peaks in LFP activity occurred in the prefrontal cortex and SEF. Small peri-saccadic peaks also occurred in the striatal LFPs, but the largest peaks in striatal LFP activity occurred as much as 100–300 msec later. Such large post-saccadic peaks were absent from prefrontal LFPs but did occur in the field activity recorded in the SEF, which thus shared during the movement period characteristics of both the prefrontal and the striatal LFP patterns. All three regions exhibited similar modulation during fixation, and they exhibited nearly synchronous activation at the onset of the visual target presentations. Striatal activity thus did not always lag or lead cortical activity, as judged by the LFP responses. Instead, lead-lag relationships for the neocortex and striatum varied during the performance of the task.
Temporal Structure of Simultaneously Recorded LFPs and Single-Unit Spike Activity. We found patterns of spike activity very similar to the LFP activity patterns in some simultaneously recorded pairs of single units in the striatum and prefrontal cortex, but the relative timing of peak striatal and prefrontal activity during the movement period varied pair by pair. Moreover, the averaged spike-activity patterns resembled the averaged LFP patterns for the prefrontal cortex but were different for the striatum. Tonic firing levels were so high in the averaged striatal spike activity that it was impossible to analyze adequately phasic activity patterns. Yet the LFPs recorded from subsets of the same electrodes had clear phasic activity. The fact that the prefrontal recordings were concentrated in a restricted part of the dorsolateral prefrontal cortex in and anterior to area 8A may have led to the consistency in prefrontal spike and LFP pattern. We have found many neurons in this region to respond phasically in sequential-saccade tasks (10). In the oculomotor zone of the caudate nucleus targeted in our experiments, we found more varied phasic activity of single units and a greater tendency for tonic responses. This variability could reflect the modular arrangement of inputs to this region from oculomotor and other areas of the cortex (18). It is remarkable that, despite this variability, the striatal LFPs were distinguished by clear phasic patterning.
The LFP patterns that we recorded were region-specific, consistent for a given task performed in a given context, stable over weeks of recording, and highly responsive in relation to task events. In each region, LFP activity was modulated during each of the visuomotor tasks performed, from simple fixation to single- and sequential-saccade tasks, and was also modulated in relation to reward delivery and the auditory stimuli associated with the rewards, even when no prior behavioral response was required. Thus the LFP modulations were sensitive indicators of a broad spectrum of task events. By contrast, single units varied cell by cell in their response preferences, so that it was necessary to identify particular subsets of units in order to predict, from the neural activity, the occurrence of particular task events.
Although the LFP responses were time-locked to signal specific sensory or motor events, their general form, for each region, was impervious to changes in the numbers of targets and saccades per trial or the number of inter-target intervals in these trials. Yet they could be altogether reconfigured when the significance of a particular task event was altered, as in the yellow last target condition. Performance in this task condition was similar to that in the standard task up until the yellow target was illuminated. LFP activity was strikingly different from that characteristic of the standard condition, as we have found also for single-unit activity in the prefrontal cortex (10). This result suggests that the LFP responses were affected by multiple cognitively integrated aspects of the tasks performed with a sensitivity equivalent to the spike activity of single units. Notably, the patterns of task-related temporal crosscovariance that we observed across striatal and cortical sites were visible without reference to oscillatory activity within particular frequency bands (19–22).
Timing of Neural Activity at Cortical and Striatal Levels of Cortico-Basal Ganglia Circuits. We found that, across recording sites within any given region, task-dependent patterns of LFP activity were consistent. The timing of peak LFP activity relative to task events, however, was different for each cortical area and for the striatum. As a result, their LFP activities were continuously brought into different temporal relationships as the monkeys performed the sequential-saccade tasks. LFP activity reflects the total electrical current near each recording site. LFPs likely strongly reflect synaptic currents and therefore may mainly reflect the activity of incoming afferents and local circuit activity (11, 13, 14). According to this interpretation, our results suggest that, at the population level, input and local activity in the oculomotor zone of the caudate nucleus, the dorsolateral prefrontal cortex, and the SEF respond with similar timing to sensory triggers for saccades but with different timing in relation to the sequential saccades themselves. Our findings thus argue for highly dynamic relationships between cortical and striatal activity during habitual task performance.
It is possible that these relative timing relationships characterized populations of cortical and striatal neurons directly connected by corticostriatal or corticocortical pathways, but there is no guarantee of this based on our results. Our findings do emphasize that some striatal-prefrontal pairs of units have relative temporal activity closely matching that of the LFPs. In these pairs, unit responses to the target onsets and offsets occurred almost simultaneously in prefrontal cortex and striatum but lagged in the striatum during the saccade period.
The very large post-saccadic delays in peak LFP activity in the striatum and SEF were particularly notable. This late striatal activity could, in part, consist of feedback signals consequent upon the eye movements. If so, it could represent signals for performance monitoring, confirmatory signaling, or visuooculomotor coordinate map resetting (10, 23–25). In particular, corollary discharge (efference copy) function could reasonably account for some of this activity, but the several hundred-millisecond delay in peak striatal activity, together with the broadness of the striatal peaks, suggests they might represent the integration of confirmatory responses signaling the consequences of the motor commands.
After the first saccade of the saccade sequences, LFP activity declined differentially in the prefrontal cortex, so that the late postsaccadic activity in the striatum (and in the SEF) appeared to dominate more and more as the over-learned sequences were performed. This differential decline did not occur in the yellow last target condition. It may, therefore, have represented a feature of the nearly automatic behavioral performance of the monkeys in the default task. This interpretation is in accord with the general conclusion suggested by our findings, that functional engagement of cortical and basal ganglia circuits is dynamic, varying, and task- and region-specific as habitual behaviors are repetitively performed. These patterns of relative aggregate response in the basal ganglia and frontal cortex may represent timing templates for normal behavioral control. Disruptions of these temporal relationships could in turn contribute to cortico-basal disorders.
Supplementary Material
Acknowledgments
We thank Dr. Peter Schiller for his valuable comments. This work was funded by National Institutes of Health Grant EY12848.
Author contributions: A.M.G. designed research; N.F. and A.M.G. performed research; N.F. and A.M.G. analyzed data; A.M.G. wrote the paper; and N.F. helped with the paper.
Abbreviations: SEF, supplementary eye field; LFP, local field potential.
References
- 1.Middleton, F. A. & Strick, P. L. (2002) Cereb. Cortex 12, 926–935. [DOI] [PubMed] [Google Scholar]
- 2.Saka, E. & Graybiel, A. (2004) Brain Dev. 25 Suppl. 1, S15–S19. [DOI] [PubMed] [Google Scholar]
- 3.Graybiel, A. M. & Saka, E. (2004) in The New Cognitive Neurosciences, ed. Gazzaniga, M. S. (MIT Press, Cambridge, MA), 3rd Ed., pp. 495–510.
- 4.Saint-Cyr, J. A. (2003) J. Int. Neuropsychol. Soc. 9, 103–127. [DOI] [PubMed] [Google Scholar]
- 5.Willingham, D. B., Salidis, J. & Gabrieli, J. D. (2002) J. Neurophysiol. 88, 1451–1460. [DOI] [PubMed] [Google Scholar]
- 6.Dicarlo, J. J. & Maunsell, J. H. (2005) J. Neurophysiol. 93, 2974–2986. [DOI] [PubMed] [Google Scholar]
- 7.Schmolesky, M. T., Wang, Y., Hanes, D. P., Thompson, K. G., Leutgeb, S., Schall, J. D. & Leventhal, A. G. (1998) J. Neurophysiol. 79, 3272–3278. [DOI] [PubMed] [Google Scholar]
- 8.Holdefer, R. N., Miller, L. E., Chen, L. L. & Houk, J. C. (2000) J. Neurophysiol. 84, 585–590. [DOI] [PubMed] [Google Scholar]
- 9.Medvedev, A. V. & Kanwal, J. S. (2004) J. Neurophysiol. 92, 52–65. [DOI] [PubMed] [Google Scholar]
- 10.Fujii, N. & Graybiel, A. (2003) Science 301, 1246–1249. [DOI] [PubMed] [Google Scholar]
- 11.Logothetis, N. K., Pauls, J., Augath, M., Trinath, T. & Oeltermann, A. (2001) Nature 412, 150–157. [DOI] [PubMed] [Google Scholar]
- 12.Pesaran, B., Pezaris, J. S., Sahani, M., Mitra, P. P. & Andersen, R. A. (2002) Nat. Neurosci. 5, 805–811. [DOI] [PubMed] [Google Scholar]
- 13.Andersen, R. A., Musallam, S. & Pesaran, B. (2004) Curr. Opin. Neurobiol. 14, 720–726. [DOI] [PubMed] [Google Scholar]
- 14.Csicsvari, J., Henze, D. A., Jamieson, B., Harris, K. D., Sirota, A., Bartho, P., Wise, K. D. & Buzsaki, G. (2003) J. Neurophysiol. 90, 1314–1323. [DOI] [PubMed] [Google Scholar]
- 15.Mehring, C., Rickert, J., Vaadia, E., Cardosa de Oliveira, S., Aertsen, A. & Rotter, S. (2003) Nat. Neurosci. 6, 1253–1254. [DOI] [PubMed] [Google Scholar]
- 16.Fuchs, A. F. & Robinson, D. A. (1966) J. Appl. Physiol. 21, 1068–1070. [DOI] [PubMed] [Google Scholar]
- 17.Blazquez, P., Fujii, N., Kojima, J. & Graybiel, A. M. (2002) Neuron 33, 973–982. [DOI] [PubMed] [Google Scholar]
- 18.Parthasarathy, H. B., Schall, J. D. & Graybiel, A. M. (1992) J. Neurosci. 12, 4468–4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Engel, A. K., Fries, P. & Singer, W. (2001) Nat. Rev. Neurosci. 2, 704–716. [DOI] [PubMed] [Google Scholar]
- 20.Courtemanche, R., Fujii, N. & Graybiel, A. (2003) J. Neurosci. 23, 11741–11752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baker, S. N., Kilner, J. M., Pinches, E. M. & Lemon, R. N. (1999) Exp. Brain Res. 128, 109–117. [DOI] [PubMed] [Google Scholar]
- 22.Murthy, V. N. & Fetz, E. E. (1996) J. Neurophysiol. 76, 3968–3982. [DOI] [PubMed] [Google Scholar]
- 23.Schall, J. D., Stuphorn, V. & Brown, J. W. (2002) Neuron 36, 309–322. [DOI] [PubMed] [Google Scholar]
- 24.Carpenter, R. H. (2004) Curr. Biol. 14, R416–R418. [DOI] [PubMed] [Google Scholar]
- 25.Nakamura, K., Roesch, M. R. & Olson, C. R. (2004) J. Neurophysiol. 93, 884–908. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.