Abstract
Presynaptic inhibition of primary muscle spindle (group Ia) afferent terminals in motor nuclei of the spinal cord plays an important role in regulating motor output and is produced by a population of GABAergic axon terminals known as P boutons. Despite extensive investigation, the cells that mediate this control have not yet been identified. In this work, we use immunocytochemistry with confocal microscopy and EM to demonstrate that P boutons can be distinguished from other GABAergic terminals in the ventral horn of rat and mouse spinal cord by their high level of the glutamic acid decarboxylase (GAD) 65 isoform of GAD. By carrying out retrograde labeling from lamina IX in mice that express green fluorescent protein under the control of the GAD65 promoter, we provide evidence that the cells of origin of the P boutons are located in the medial part of laminae V and VI. Our results suggest that P boutons represent the major output of these cells within the ventral horn and are consistent with the view that presynaptic inhibition of proprioceptive afferents is mediated by specific populations of interneurons. They also provide a means of identifying P boutons that will be important in studies of the organization of presynaptic control of Ia afferents.
Keywords: GABA, Ia afferent, presynaptic inhibition
The inhibitory transmitter GABA is used by many neurons in the spinal cord and generates both postsynaptic inhibition at axo-dendritic and axo-somatic synapses and presynaptic inhibition at axo-axonic synapses (1). GABA is synthesized by the enzyme glutamic acid decarboxylase (GAD), and Abs against GAD have been used to identify GABAergic axonal boutons in the spinal cord (2, 3). More recently, two forms of the enzyme have been identified, and based on their molecular weights these forms have been named GAD65 and GAD67 (4). Both forms are present in the ventral horn of the rat spinal cord but have a very different distribution (5, 6). The majority of GABAergic boutons throughout the ventral horn show strong GAD67 immunoreactivity and a low level of GAD65. However, there are clusters of boutons in lamina IX that contain very high levels of GAD65. We have suggested (6) that these clusters may correspond to the P boutons that form axo-axonic synapses with terminals of group Ia afferents (7–10). In this work, we have used a variety of approaches to confirm this hypothesis and to identify the cells of origin of these boutons.
Methods
All experiments were approved by the Ethical Review Process Applications Panel of the University of Glasgow or the Animal Care and Protection Committee at the University of Debrecen and were performed in accordance with the U.K. Animals (Scientific Procedures) Act 1986 and the European Communities Council Directives. Immunocytochemical reactions were performed on free-floating 60-μm transverse Vibratome sections that had been treated with 50% ethanol for 30 min to enhance Ab penetration. Abs were diluted in PBS with 0.3% Triton X-100 (except on sections used for EM).
Primary Afferent Labeling. Transganglionic labeling of sciatic afferents with cholera toxin B subunit (CTb) was performed in three adult male Sprague–Dawley rats (300–350 g; Harlan UK, Bicester, U.K.). These rats were anesthetized with halothane and received injections of 4 μl of 1% CTb (Sigma) into the left sciatic nerve. After 3 days, they were deeply anesthetized with pentobarbitone and perfused with fixative containing 4% freshly depolymerized formaldehyde. Sections from the third to fifth lumbar (L3–L5) segments were incubated for 72 h in goat anti-CTb (List Biological Laboratories, Campbell, CA; 1:5,000), monoclonal mouse anti-GAD65 (GAD6, Developmental Studies Hybridoma Bank, University of Iowa; 1:100), and rabbit anti-vesicular glutamate transporter 1 (VGLUT1) (Synaptic Systems, Göttingen, Germany; 1:5,000). We have previously shown that VGLUT1 is present in all myelinated primary afferent terminals in lamina IX (11). They were then reacted with species-specific secondary Abs (Jackson ImmunoResearch; raised in donkey; 1:100) conjugated to FITC, rhodamine (Rhodamine Red-X), or cyanine 5.18 and mounted in glycerol-based medium (Vectashield, Vector Laboratories). Sections from the L4 segment of each rat were scanned with a confocal microscope (Bio-Rad MRC1024 or Radiance 2100) through an ×60 oilimmersion lens for quantitative analysis. Sixty primary afferent boutons in lamina IX (identified by the presence of both CTb and VGLUT1) were randomly selected from each rat, and the number of GAD65-immunoreactive profiles in contact with each one was determined. The selection of boutons was made before GAD65 immunoreactivity was viewed. In a separate analysis, 253, 359, and 397 boutons in lamina IX that showed strong GAD65 immunoreactivity were selected from the three animals and the proportion that were in contact with a VGLUT1-immunoreactive axon was determined. For this part of the analysis, we examined contacts with axons that contained VGLUT1, because not all primary afferents were labeled with CTb. To avoid bias toward GAD65-immunoreactive boutons that were in clusters, an arbitrary threshold value for pixel luminance was set for GAD65 immunofluorescence in each confocal image stack, and all boutons that contained at least one pixel brighter than this were included in the analysis.
Intra-axonal labeling of Ia afferents was carried out in three adult male Sprague–Dawley rats (320–380 g), which were anesthetized with halothane and subsequently maintained by doses of sodium pentobarbitone (Rhône-Mérieux Laboratory, Harlow, Essex, U.K.; 10 mg/kg i.v.), given as required. The depth of anesthesia was assessed by monitoring pedal withdrawal reflexes, the corneal reflex, and blood pressure. During recording, animals were paralyzed with Pancuronium bromide (Pavulon; Faulding Pharmaceuticals, Leamington Spa, U.K.; 0.3 mg/kg i.v. every 40 min) and artificially ventilated. Anesthetic was then administered at a frequency commensurate with that required before paralysis, and adequacy of anesthesia was checked by monitoring blood pressure and the absence of changes in response to noxious stimuli. Core temperature was maintained at ≈38°C, mean blood pressure at >80 mm Hg, and pCO2 at 4–4.5%. The left sciatic nerve was exposed and placed on bipolar stimulating electrodes, and a laminectomy was performed to expose the spinal cord. Single primary afferent fibers in the dorsal columns that were driven orthodromically from the sciatic nerve were impaled with Neurobiotin-filled glass microelectrodes as described in ref. 12. Ia afferents were identified by their regular firing rate, which was altered by manipulation of ipsilateral hindlimb muscles, and by their conduction velocity. After successful impalement, Neurobiotin was injected by ionophoresis (5–10 nA continuous positive current for 4–30 min). Animals were maintained under anesthesia for 1 h and then perfused with 4% formaldehyde. Conduction distances between stimulating electrodes on the sciatic nerve and the recording sites were measured and used to calculate conduction velocities (12). Sections from the region of the injection site were incubated for 48 h in mouse anti-GAD65 (1:100) and streptavidin conjugated to Rhodamine Red (Jackson ImmunoResearch, 1:1,000) and then in donkey anti-mouse IgG conjugated to fluorescein (Jackson ImmunoResearch; 1:100). They were scanned with the confocal microscope to examine the relationship between GAD65-immunoreactive boutons and labeled Ia terminals in lamina IX.
GAD65, GAD6, and VGLUT1 in Mouse and Rat Spinal Cord. To compare expression of GAD isoforms in putative P boutons in rat and mouse, sections of formaldehyde-fixed lumbar spinal cord from three adult male C57BL/6 mice and three adult male Sprague–Dawley rats were incubated in the following primary Ab cocktails: (i) mouse anti-GAD65 (1:100), rabbit anti-GAD67 (K2 Ab, Chemicon; 1:5,000), and guinea-pig anti-VGLUT1 (Chemicon; 1:20,000); and (ii) rabbit anti-GAD65 (Sigma-Aldrich, catalog no. G4913; 1:5,000), mouse anti-GAD67 (Chemicon; 1:5,000 or 10,000), and guinea-pig anti-VGLUT1 (1:20,000). These Abs were revealed with species-specific donkey secondary Abs conjugated to Alexa Fluor 488 (Molecular Probes), Rhodamine Red, or cyanine 5.18. To test the specificity of the GAD65 Abs, sections from three adult male GAD65 knock-out mice (NOD.GAD65–/–) (13) were reacted with both of these Ab combinations.
EM. Two adult male Wistar rats (240–270 g) were deeply anesthetized with pentobarbitone and perfused with fixative containing 1% glutaraldehyde and 1% formaldehyde. Sections of midlumbar spinal cord were treated with 1% sodium borohydride for 30 min and incubated for 72 h in rabbit anti-GAD65 (1:50,000 or 1:100,000), followed by 24 h each in biotinylated donkey anti-rabbit IgG (Jackson ImmunoResearch; 1:500) and extravidin-peroxidase (Sigma–Aldrich; 1:1,000). Peroxidase activity was revealed with 3,3′-diaminobenzidine in the presence of H2O2, and sections were osmicated, dehydrated, block-stained with uranyl acetate, and flat-embedded in Durcupan. One section from each rat was selected, photographed through a light microscope to allow identification of clusters of boutons in lamina IX with strong GAD65 immunoreactivity, and then mounted onto a stub of cured resin. Series of ultrathin sections were cut from each block and stained with lead citrate. Immunoreactive boutons in the clusters identified with light microscopy were followed through serial ultrathin sections with a Philips CM100 electron microscope to determine whether they formed axo-axonic synapses.
GAD65 in GFP-Expressing Mice. Spinal cords from mice expressing GFP under the control of the GAD65 promoter (GAD65-GFP mice) (14) were used to investigate the cells of origin of the P boutons. Sections from formaldehyde-fixed adult animals were incubated in rabbit anti-GAD65 (1:5,000), guinea-pig anti-VGLUT1 (1:10,000), and sheep anti-GFP (Biogenesis, Bournemouth, U.K.; 1:1,000), which were revealed with species-specific secondary Abs labeled with Alexa Fluor 546/555, 647, and 488, respectively. They were mounted in Vectashield and scanned with a Zeiss LSM 510 confocal microscope to examine the relationship between GAD65-, VGLUT1-, and GFP-immunoreactive axonal varicosities.
Three adult mice of this strain were terminally anesthetized with pentobarbitone (30 mg/kg). The vertebral column was quickly cut out and immersed in ice-cold oxygenated artificial cerebrospinal fluid. The lumbosacral spinal cord was dissected and placed in a chamber superfused with oxygenated artificial cerebrospinal fluid at room temperature. Glass micropipettes (tip diameter 10–20 μm) were filled with 1% rhodamine-biotinylated dextran amine (R-BDA; Mr 3,000; Molecular Probes), which was injected into lamina IX at the level of L4 segment on one side by iontophoresis with positive current (10 μA for 10 min). After the tracer injection, the spinal cord was kept in the superfusion chamber for 4–6 h and then transferred to fixative containing 4% formaldehyde. Sections through the L3–L5 segments were processed to reveal GFP, and the distribution of GFP-expressing neurons that were also R-BDA-labeled was plotted onto an outline of the spinal cord.
Results
Primary Afferent Labeling. As described in ref. 6, many boutons with strong GAD65 immunoreactivity were present in lamina IX of the rat spinal cord, and these frequently formed clusters. Much lower levels of GAD65 were seen in many other boutons throughout the ventral horn; however, these could easily be distinguished from the strongly immunoreactive boutons, which for convenience are referred to as GAD65-intense. After injection of CTb into the sciatic nerve, the clusters of GAD65-intense boutons were invariably centered around VGLUT1-immunoreactive axon terminals, many of which contained CTb (Fig. 1). Most of the GAD65-intense boutons in lamina IX that were not in clusters were also adjacent to VGLUT1-immunoreactive axon terminals. GAD65-intense boutons were also associated with CTb-labeled terminals in lamina VII. Primary afferent terminals in lamina IX (identified by the presence of VGLUT1 and CTb) were associated with 0–11 GAD65-intense boutons, with the mean number per terminal ranging from 2.8 to 3.2 in the three rats (mean 3.1). Between 92% and 95% (mean 93%) of the primary afferent terminals were in contact with at least one GAD65-intense bouton. Quantitative analysis revealed that 88–89% (mean 89%, n = 3) of GAD65-intense boutons in lamina IX were in contact with a VGLUT1-immunoreactive terminal. Although the quantitative data were obtained from the lateral motoneuronal cell groups in L4, clusters of GAD65-intense boutons were observed in all motor nuclei in the L3–L5 segments.
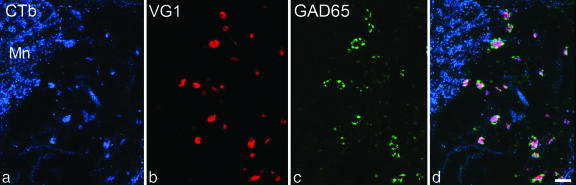
Fig. 1.
Immunostaining for CTb, VGLUT1 (VG1), and GAD65 in lamina IX of a rat in which CTb had been injected into the ipsilateral sciatic nerve. CTb labeling is present in a motoneuron (Mn) cell body and several proprioceptive afferent terminals. The latter also contain VGLUT1 and appear purple in the merged image. Clusters of boutons with strong GAD65 immunoreactivity are associated with the afferent terminals, which they often surround. The images are projections from nine optical sections at 0.5-μm z-separation. (Bar: 5 μm.)
A single intraaxonally labeled Ia afferent was recovered in each of three experiments. Conduction velocities ranged from 34 to 77 ms–1, and the morphology of their central arbors resembled that described for Ia afferents in the rat (10). Between 19 and 108 labeled axon terminals in lamina IX were analyzed, and most of these (74% of 19, 90% of 42, and 96% of 108) were associated with GAD65-immunoreactive boutons (Fig. 2). The mean numbers of GAD65-intense boutons that contacted labeled terminals belonging to each of the three Ia afferents were 3.1 (±2.9 SD, range 0–9), 3.9 (±2.5, 0–10), and 3.6 (±2.0, 0–11), respectively. GAD65-intense boutons were also associated with Neurobiotin-labeled Ia terminals in lamina VII.
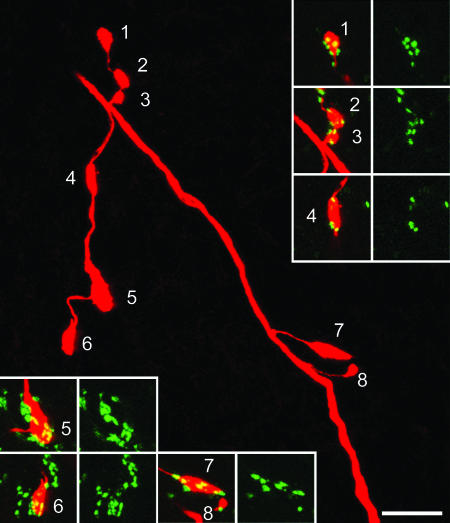
Fig. 2.
Association of GAD65 terminals with a Neurobiotin-injected Ia afferent in the rat. The main image is a projection of confocal scans through part of a collateral with eight varicosities in lamina IX (Neurobiotin appears red). The remaining pairs of images show the association of GAD65 immunostaining (green) with each varicosity. Note that all of the Ia varicosities are contacted by at least one GAD65-immunoreactive bouton. The main image is a projection of 37 optical sections at 0.5-μm z-spacing, whereas those in the Insets are projected from 5, 13, 6, 5, 5, and 9 optical sections, respectively. (Bar: 10 μm.)
GAD65, GAD67, and VGLUT1 in Mouse and Rat Spinal Cord. We reported (6) that the GAD65-intense boutons in lamina IX of the rat spinal cord contained low levels of GAD67 immunoreactivity. However, the rabbit Ab against GAD67 used in that study is known to cross-react weakly with GAD65 (15). In sections of rat spinal cord reacted with mAb against GAD67, the great majority of GAD65-intense boutons that surrounded VGLUT1-immunoreactive axon terminals had no detectable GAD67 immunoreactivity (Fig. 3 a–c) whereas a few showed very weak labeling. Although it is possible that our failure to detect GAD67 immunoreactivity in most P boutons in the rat is due to lack of sensitivity of the GAD67 mAb, a more likely explanation is that in our previous study the labeling seen in these boutons with rabbit GAD67 Ab represented cross-reactivity with GAD65.
Fig. 3.
Expression of GAD isoforms in P boutons in rat and mouse. Sections were stained with rabbit anti-GAD65 (green), mouse mAb against GAD67 (red), and guinea-pig anti-VGLUT1 (VG1, blue). (a–c) GAD65-positive boutons surrounding a VGLUT1-labeled terminal in the rat. These boutons are not GAD67 immunoreactive. (d–f) In the mouse, boutons with strong GAD65 immunoreactivity are also associated with VGLUT1-containing terminals, but these boutons show moderate levels of GAD67. (Bar: 2 μm.)
Sections of mouse spinal cord that had been reacted with either combination of GAD Abs showed numerous boutons that were strongly GAD65-immunoreactive in lamina IX. These boutons had a similar distribution to those in the rat spinal cord and were also generally associated with VGLUT1-immunoreactive terminals. Unlike the situation in the rat, these boutons invariably showed a low to moderate level of immunoreactivity with the mAb against GAD67 (Fig. 3 d–f). As in the rat, the GAD65-intense boutons could easily be distinguished from other GABAergic boutons in the ventral horn that contained much lower levels of GAD65 immunoreactivity. Staining with both GAD65 Abs was completely absent from the spinal cords of GAD65–/– mice.
EM. Thirty-eight boutons from lamina IX that showed strong GAD65 immunoreactivity were identified with EM (26 and 12 from the two rats). When followed through serial sections, these boutons were found to be arranged in clusters that surrounded large nonimmunoreactive terminals that resembled those belonging to Ia afferents (9, 10). All of these GAD65-immunoreactive boutons were presynaptic to the unlabeled central terminals at axo-axonic synapses (Fig. 4). These were identified by the clustering of vesicles adjacent to small presynaptic densities. These features are characteristic of axo-axonic synapses formed by P boutons, which lack a pronounced synaptic specialization (16).
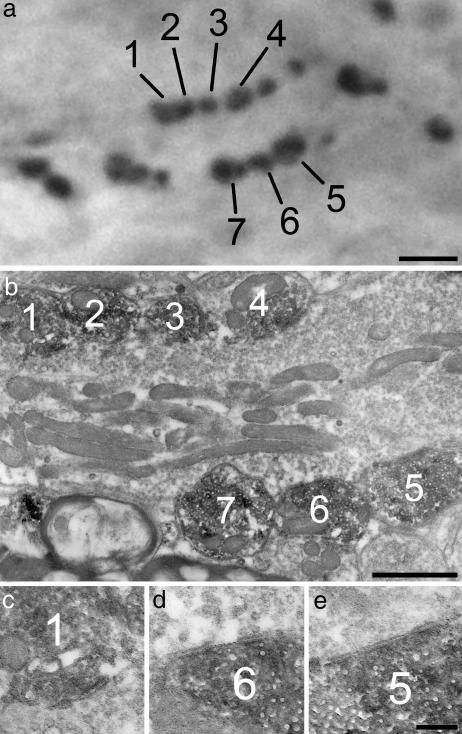
Fig. 4.
GAD65-intense boutons form axo-axonic synapses. (a) A photomicrograph showing a cluster of boutons with strong GAD65 immunoreactivity. (b) A low-magnification electron micrograph reveals seven of these boutons (corresponding to those numbered in a) surrounding a large unlabeled axon terminal, which presumably belongs to a Ia afferent. When followed through serial sections, all of the labeled boutons were seen to form axo-axonic synapses with the central axon. Three of these are shown at higher magnification in c–e. [Bars: 2 μm(a), 1 μm(b), and 0.2 μm(c–e).]
GAD65 in GFP-Expressing Mice. GFP-labeled cell bodies were mostly confined to the dorsal horn in the GAD65-GFP mice. They were particularly numerous in laminae I–III, present at lower density in laminae IV–VI, and only occasionally observed in laminae VII–VIII. Labeled processes were present throughout the gray matter of both dorsal and ventral horns. GFP labeling also was seen in axons in the deepest part of the dorsal columns; however, these axons lacked GAD65 and GAD67 immunoreactivity and probably reflect ectopic expression of GFP in corticospinal fibers. The distribution of GAD65-immunoreactive boutons in the ventral horn of GAD65-GFP mice was identical to that observed in nontransgenic animals (Fig. 5b). GAD65-intense boutons in these mice were adjacent to, and often formed clusters around, VGLUT1-containing terminals in lamina IX (Fig. 5 b–d). Most of the GAD65-intense boutons that contacted VGLUT1-immunoreactive axon terminals also expressed GFP, although some did not (Fig. 5 a and d). In the majority of cases, all of the GAD65-intense boutons in contact with a single VGLUT1-containing terminal either expressed GFP or were GFP-negative (Fig. 5). Some of the GFP-labeled varicosities in lamina IX did not correspond to the GAD65-intense boutons.
Fig. 5.
Confocal images from lamina IX of a GAD65-GFP mouse. Numerous boutons with strong GAD65 immunoreactivity can be seen (b), and most are associated with VGLUT1-containing terminals (c and d). Some, but not all, of these GAD65 boutons are labeled with GFP (a and d). Arrows point to four clusters of GAD65/GFP boutons. Note that some GAD65-intense boutons in contact with VGLUT1 terminals are not GFP labeled, and a cluster of these is indicated with arrowheads. The images were projected from eight optical sections at 1-μm z-spacing. (Bar: 10 μm.)
After injections of R-BDA (17, 18) that covered an area with a diameter of 150–200 μm in the lateral motor column of the GAD-GFP mice, large numbers of retrogradely labeled neurons were recovered in the spinal gray matter (Fig. 6a). With a few exceptions, the R-BDA-labeled neurons were confined to lamina V–VII along a three-segment length of the spinal cord ipsilateral to the injection site, with a similar distribution to that reported after injection of BDA into lamina IX in the rat in vivo (18). Despite the extensive retrograde labeling, cells that contained both R-BDA and GFP were present in limited numbers and were restricted to the medial aspect of laminae V–VI on the ipsilateral side (Fig. 6 b and c). These double-labeled neurons were only seen within an 800- to 900-μm length of spinal cord centered on the rostrocaudal level of the injection site.
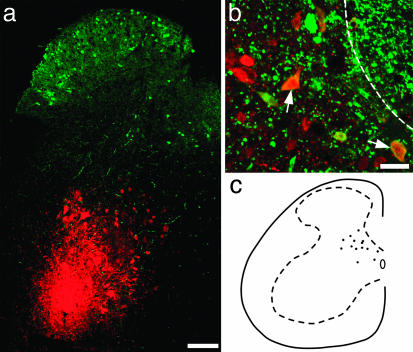
Fig. 6.
Identification of GFP-labeled cells that project to lamina IX. (a) Transverse section of spinal cord scanned to reveal R-BDA (red) and GFP (green). The injection site is located within lamina IX. Note that the R-BDA has been scanned with low laser power to demonstrate the distribution of tracer at the injection site, and therefore only neurons with intense R-BDA labeling are visible in this image. (b) A high-magnification image from a different section showing cells labeled with R-BDA and containing GFP (two of which are marked with arrows) in the deep medial part of the dorsal horn. The dashed line represents the edge of the dorsal column. (c) A drawing showing the distribution of all double-labeled cells in seven randomly selected Vibratome sections from the L4 segment. [Bars: 100 μm(a) and 20 μm(b).]
Discussion
GAD65 in P Boutons. Axo-axonic synapses in the motor nuclei of the spinal cord were identified by Conradi (19), who defined the presynaptic axons as P boutons. He also demonstrated that the postsynaptic elements were of primary afferent origin because they degenerated after dorsal rhizotomy (20). It was subsequently shown that P boutons are presynaptic to terminals of Ia afferents (7–10) and that they are immunoreactive with Abs against GAD (2) and GABA (10, 16, 21, 22). These findings are consistent with the suggestion that GABAergic axo-axonic synapses are the anatomical basis for the presynaptic inhibition of Ia afferents and the accompanying primary afferent depolarization (23).
We previously identified clusters of axon terminals that were intensely stained with GAD65 Ab in lamina IX of the rat spinal cord and suggested that these might be the P boutons (6). The results of the present study confirm this suggestion by showing that GAD65-intense boutons are associated with (and often surround) primary afferent terminals and that they form axoaxonic synapses. There are two lines of evidence to suggest that all P boutons belong to the GAD65-intense population. Because P boutons are strongly GABA-immunoreactive (10, 16, 22), they must contain at least one GAD isoform. Although we saw many axons with significant levels of GAD67 in lamina IX, these were very seldom adjacent to VGLUT1-labeled boutons, which include all proprioceptive afferents (11). In addition, we found that the mean number of GAD65-intense boutons contacting each Ia afferent terminal was 3.6 (data pooled from three experiments), whereas quantitative electron microscopic studies in cat (9) and rat (10) have reported that the mean number of P boutons presynaptic to each Ia terminal in lamina IX is 2.6–2.7. The slightly higher figure that we obtained could be accounted for by GABAergic boutons that were in contact with the Ia terminal but did not form an axo-axonic synapse. However, a more likely explanation is that it arises from the difficulty of identifying P boutons at either end of the Ia terminals in the section series in the EM studies. Axo-axonic synapses formed by these boutons would have been nearly parallel to the plane of section, and thus impossible to distinguish with EM. In contrast, P boutons in the equivalent locations could be readily identified with the confocal microscope. Our results also suggest that the great majority of GAD65-intense axon terminals in lamina IX are P boutons because 89% of them were in contact with a VGLUT1-containing varicosity. GAD65-intense axons are greatly outnumbered by boutons that are GAD67-immunoreactive in lamina IX (6). Most of the latter express the glycine transporter GLYT2 and therefore presumably use both GABA and glycine as transmitters, whereas the GAD65-intense boutons lack GLYT2 (6). Consistent with this observation, it has been reported that P boutons in lamina IX are GABA-immunoreactive, but not glycine-immunoreactive, whereas many other GABAergic boutons in this lamina are enriched with glycine (10, 22). The presence of clusters of GAD65-intense boutons in all motor nuclei in the L3–L5 segments implies that Ia afferent input from all hindlimb muscles is under presynaptic control by P boutons.
One possible explanation for the difference in distribution of GAD isoforms between P boutons and other GABAergic axons in the ventral horn is that individual neurons can give rise to boutons that have widely varying concentrations of the two GADs, with the ratio being determined by the postsynaptic target. However, a more likely explanation is that the GAD65-intense axons are derived from a discrete population of GABAergic neurons that have undetectable levels of GAD67 in their axons. Because the GAD65-intense boutons are predominantly associated with primary afferent terminals in the ventral horn, it is likely that they originate from a population of neurons that are involved principally in mediating presynaptic inhibition in this region. The dorsal horn contains many axons with high levels of GAD65, and it is not known whether any of these are axon collaterals from these neurons.
Cells of Origin of the P Boutons. A similar arrangement of GAD65-intense boutons associated with VGLUT1-containing terminals was seen in the mouse, indicating that GAD65 immunoreactivity can also be used to distinguish P boutons in this species. The expression of GFP in P boutons in the GAD65-GFP mice allowed us to investigate their cells of origin by retrogradely labeling GFP-expressing neurons with R-BDA from lamina IX. The lack of double-labeled cells dorsal to lamina V is unlikely to be due to limited transport of the tracer, because neurons with strong R-BDA labeling were found up to 3 mm rostral and caudal to the injection site, whereas the distance from the injection site to medial lamina IV is ≈800 μm. Because not all GFP-containing axons in lamina IX correspond to the P boutons and because there may have been some uptake of R-BDA by fibers of passage (see ref. 18 for discussion), some neurons other than those giving rise to the P boutons also will have been double-labeled. In addition, the cell bodies of those P boutons that were not GFP-labeled will not have been detected. However, the majority of neurons that give rise to the P boutons within the region of the injection site are presumably included among the double-labeled cells. Our results therefore suggest that P boutons are derived from neurons in the medial parts of laminae V and VI on the ipsilateral side at a similar rostrocaudal level to the Ia terminals that they innervate. This interpretation is consistent with a report that electrical microstimulation of this area results in primary afferent depolarization in Ia afferents (24). Primary afferent depolarization in these afferents can be evoked by stimulation of Ia and Ib fibers (23), and it has been proposed on the basis of latency measurements that this effect involves a trisynaptic pathway (24). Because both Ia and Ib afferents terminate in the medial part of laminae V and VI (25), our finding that cells of origin of the P boutons are located in this region raises the possibility that there may be a disynaptic circuit, with P bouton cells being activated directly by group I proprioceptive afferents.
Although most P boutons contained GFP in the GAD-GFP mice, some did not, and this finding is likely to reflect an unpredictable variability in the expression of GFP within the GABAergic neuronal population. Interestingly, the P boutons associated with individual primary afferents were usually consistently positive or negative for GFP, which suggests that the P boutons in a cluster around an afferent terminal are derived from a single neuron.
It has been reported that in addition to forming axo-axonic synapses with Ia terminals, P boutons can also form axo-dendritic synapses in triadic arrangements and are therefore not solely involved in generating presynaptic inhibition (9). Although we did not study this relationship systematically, we also saw examples of triads involving P boutons. There is evidence that primary afferent depolarization-generating neurons can also produce postsynaptic inhibition of motoneurons, which is picrotoxin (but not strychnine) sensitive (23). This observation is compatible with a triadic arrangement involving P boutons because these are GABAergic but not glycinergic. Despite their role in producing postsynaptic inhibition of motoneurons at synaptic triads, the P boutons clearly form a different functional population from the majority of GABAergic boutons in the ventral horn, which form axo-somatic and axodendritic synapses, and which include some of the F boutons that are presynaptic to motoneurons (18, 21, 22). Recent studies have identified four classes of ventral interneurons in the developing neural tube (26). Of these, the V1 class (characterized by transient expression of the En1 transcription factor) appears to give rise to various types of inhibitory interneuron including some that are premotor, such as Renshaw cells (27) and Ia-inhibitory interneurons (F. J. Alvarez, personal communication). We have recently examined mice in which synthesis of GFP is driven by transient expression of En1 and found that although many GAD67-immunoreactive axons in the ventral horn were GFP labeled, the P boutons were not (R.H., A.J.T., and F.J.A., unpublished observations). This finding provides further evidence that P boutons are derived from a different population of neurons than those that give rise to other GABAergic axons in the ventral horn. From their location, it is likely that these arise from one of the dorsal progenitor domains (28).
Acknowledgments
We thank Drs. R. Tisch and J. Valtschanoff (both of University of North Carolina, Chapel Hill, NC) for providing GAD65 knock-out tissue; Dr. D. T. Scott for assisting in some of the experiments; and Mr. R. Kerr, Mrs. C. Watt, and Mrs. M. M. McGill for expert technical assistance. The work was supported by the Wellcome Trust, the Biotechnology and Biological Sciences Research Council, the Human Frontiers Science Program Organization, the Hungarian National Scientific Research Fund, and the Hungarian Academy of Sciences.
Abbreviations: CTb, cholera toxin B subunit; GAD, glutamic acid decarboxylase; R-BDA, rhodamine-biotinylated dextran amine; VGLUT1, vesicular glutamate transporter 1.
References
- 1.Todd, A. J. & Maxwell, D. J. (2000) in GABA in the Nervous System: The View at Fifty Years, eds. Martin, D. L. & Olsen, R. W. (Lippincott, Philadelphia), pp. 439–457.
- 2.McLaughlin, B. J., Barber, R., Saito, K., Roberts, E. & Wu, J. Y. (1975) J. Comp. Neurol. 164, 305–322. [DOI] [PubMed] [Google Scholar]
- 3.Hunt, S. P., Kelly, J. S., Emson, P. C., Kimmel, J. R., Miller, R. J. & Wu, J. Y. (1981) Neuroscience 6, 1883–1898. [DOI] [PubMed] [Google Scholar]
- 4.Erlander, M. G. & Tobin, A. J. (1991) Neurochem. Res. 16, 215–226. [DOI] [PubMed] [Google Scholar]
- 5.Feldblum, S., Dumoulin, A., Anoal, M., Sandillon, F. & Privat, A. (1995) J. Neurosci. Res. 42, 742–757. [DOI] [PubMed] [Google Scholar]
- 6.Mackie, M., Hughes, D. I., Maxwell, D. J., Tillakaratne, N. J. K. & Todd, A. J. (2003) Neuroscience 119, 461–472. [DOI] [PubMed] [Google Scholar]
- 7.Conradi, S., Cullheim, S., Gollvik, L. & Kellerth, J.-O. (1983) Brain Res. 265, 31–39. [DOI] [PubMed] [Google Scholar]
- 8.Fyffe, R. E. W. & Light, A. R. (1984) Brain Res. 300, 201–209. [DOI] [PubMed] [Google Scholar]
- 9.Pierce, J. P. & Mendell, L. M. (1993) J. Neurosci. 13, 4748–4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watson, A. H. & Bazzaz, A. A. (2001) J. Comp. Neurol. 433, 335–348. [DOI] [PubMed] [Google Scholar]
- 11.Todd, A. J., Hughes, D. I., Polgár, E., Nagy, G. G., Mackie, M., Ottersen, O. P. & Maxwell, D. J. (2003) Eur. J. Neurosci. 17, 13–27. [DOI] [PubMed] [Google Scholar]
- 12.Hughes, D. I., Scott, D. T., Todd, A. J. & Riddell, J. S. (2003) J. Neurosci. 23, 9491–9499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kash, S. K., Johnson, R. S., Tecott, L. H., Noebels, J. L., Mayfield, R. D., Hanahan, D. & Baekkeskov, S. (1997) Proc. Natl. Acad. Sci. USA 94, 14060–14065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Marchis, S., Temoney, S., Erdelyi, F., Bovetti, S., Bovolin, P., Szabo, G. & Puche, A. C. (2004) Eur. J. Neurosci. 20, 1307–1317. [DOI] [PubMed] [Google Scholar]
- 15.Esclapez, M., Tillakaratne, N. J. K., Kaufman, D. L., Tobin, A. J. & Houser, C. R. (1994) J. Neurosci. 14, 1834–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holstege, J. C. & Calkoen, F. (1990) Brain Res. 530, 130–137. [DOI] [PubMed] [Google Scholar]
- 17.Rajakumar, N., Elisevich, K. & Flumerfelt, B. A. (1993) Brain Res. 607, 47–53. [DOI] [PubMed] [Google Scholar]
- 18.Puskár, Z. & Antal, M. (1997) J. Comp. Neurol. 389, 377–389. [DOI] [PubMed] [Google Scholar]
- 19.Conradi, S. (1969) Acta Physiol. Scand. 332, Suppl., 54–58. [PubMed] [Google Scholar]
- 20.Conradi, S. (1969) Acta Physiol. Scand. 332, Suppl., 85–115. [PubMed] [Google Scholar]
- 21.Destombes, J., Horcholle-Bossavit, G., Simon, M. & Thiesson, D. (1996) Neurosci. Res. 24, 123–130. 22. [DOI] [PubMed] [Google Scholar]
- 22.Örnung, G., Shupliakov, O., Lindå, H., Ottersen, O. P., Storm-Mathisen, J., Ulfhake, B. & Cullheim, S. (1996) J. Comp. Neurol. 365, 413–426. [DOI] [PubMed] [Google Scholar]
- 23.Rudomin, P. & Schmidt, R. F. (1999) Exp. Brain Res. 129, 1–37. [DOI] [PubMed] [Google Scholar]
- 24.Jankowska, E., McCrea, D., Rudomin, P. & Sykova, E. (1981) J. Neurophysiol. 46, 506–516. [DOI] [PubMed] [Google Scholar]
- 25.Brown, A.G. (1981) Organization of the Spinal Cord: The Anatomy and Physiology of Identified Neurones (Springer, Berlin).
- 26.Briscoe, J., Pierani, A., Jessell, T. M. & Ericson, J. (2000) Cell 101, 435–445. [DOI] [PubMed] [Google Scholar]
- 27.Sapir, T., Geiman, E. J., Wang, Z., Velasquez, T., Mitsui, S., Yoshihara, Y., Frank, E., Alvarez, F. J. & Goulding, M. (2004) J. Neurosci. 24, 1255–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Helms, A. & Johnson, J. E. (2003) Curr. Opin. Neurobiol. 13, 42–49. [DOI] [PubMed] [Google Scholar]