Abstract
The EPIC III study showed that 52% of patients admitted to the intensive care unit (ICU) have infectious diseases and that the incidence of ICU-acquired infections is increasing, leading to longer ICU stays and higher mortality rates. Multiple-site decontamination, a type of selective decontamination program, has been associated with a reduction in the incidence of ICU-acquired infection and decreased mortality rates in some critically ill patients. However, the standardized implementation and actual effectiveness of multiple-site decontamination require further investigation.
1. Definition and development of multiple-site decontamination
The concept of decontamination originated in the 1960s, initially targeting patients with malignant hematological tumors who were immunosuppressed and susceptible to secondary infections, as well as those undergoing solid organ transplantation. By the 1980s, its application had expanded to critically ill patients in the intensive care unit (ICU). Selective digestive decontamination (SDD) is one of the most common methods currently used. It involves the application of nonabsorbable local antibacterial and antifungal drugs to the oral cavity and upper gastrointestinal tract of mechanically ventilated (MV) patients. While the specific implementation and drug protocols may vary, SDD typically includes oral administration of aminoglycosides (e.g., tobramycin, gentamicin), polymyxins (e.g., colistin, polymyxin B), and antifungal agents (e.g., amphotericin B, miconazole). Short-term intravenous administration of third-generation cephalosporins (e.g., cefotaxime) or fluoroquinolones (e.g., ciprofloxacin) can also be included [1], [2].
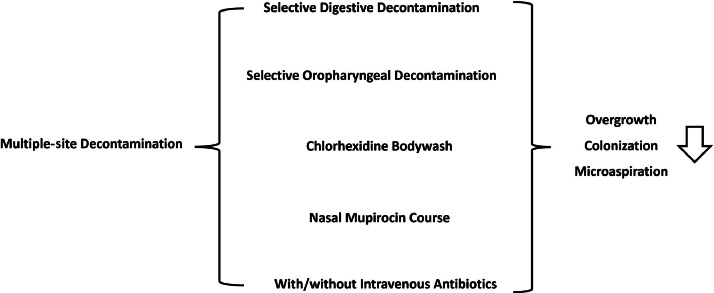
In recent years, the concept of multiple-site decontamination (MSD) has emerged, combining selective oropharyngeal decontamination with comprehensive body washing using chlorhexidine and nasal application of mupirocin, in addition to SDD. The primary aim of MSD is to prevent the excessive growth of antibiotic-resistant bacteria in the patient's oral cavity, skin, mucosa, and upper gastrointestinal tract. It seeks to prevent ventilator-associated pneumonia (VAP) by inhibiting the colonization of these microorganisms in the stomach and their subsequent microaspiration into the lungs. Additionally, it aims to reduce the translocation of intestinal microbiota and ICU-acquired bloodstream infections (BSIs) resulting from skin and mucosal damage (Fig. 1).
Fig. 1.
Multiple-site decontamination.
2. Clinical evidence of multiple-site decontamination
Table 1 summarizes key studies evaluating the efficacy of MSD in ICUs and includes some important studies related to SDD. The methods and main results of the MSD-related studies are as follows.
-
•
In 2023, Massart et al. [3] evaluated 295 immunocompromised hosts (ICHs) in French ICUs, dividing patients into an MSD group and a standard prevention group. The MSD protocol involved daily use of gentamicin (543 mg/day), colistin (400 mg/day), and amphotericin B (2000 mg/day) in the oropharynx and gastric tube; daily full-body bathing with 4% chlorhexidine; and a 5-day course of mupirocin applied to both nasal cavities without intravenous antibiotics. The study showed that MSD reduced the incidence of ICU-acquired infections (ICU-AI) (relative risk [RR], 0.39; 95% confidence interval [CI], 0.20–0.87) and lowered overall ICU mortality (hazard ratio [HR], 0.58; 95% CI, 0.34–0.95).
-
•
Massart et al. [4] conducted a retrospective observational study in 2022 involving 461 MV patients from 15 ICUs in France, with 89 patients receiving MSD. The study showed reductions in ICU-AI (RR, 0.56; 95% CI, 0.38–0.83), VAP (RR, 0.52; 95% CI, 0.33–0.89), and in-hospital mortality (16.9% vs. 30.1%, P = 0.017) in the MSD group [4].
-
•
In 2023, Massart et al. [5] examined 241 patients who underwent venovenous extracorporeal membrane oxygenation (VV-ECMO) for acute respiratory distress syndrome in 3 ICUs in France, with 69 patients in the MSD group and 172 patients in the standard treatment group. The study showed a decrease in ECMO-related infections (RR, 0.42; 95% CI, 0.23–0.60) and multidrug-resistant organism (MDRO) infections (RR, 0.13; 95% CI, 0.03–0.56) in the MSD group, but there was no significant difference in the mortality rate (45% vs. 43%, P = 0.90) [5].
-
•
Also in 2023, Massart et al. [6] analyzed 346 MV patients from 5 ICUs in western France, including 334 in the MSD group and 1012 in the standard care group. A previous before-and-after study showed that MSD reduced the incidence of ICU-AI (RR, 0.33; 95% CI, 0.18–0.60), VAP (3.6% vs. 16.2%), and BSI (3.0% vs. 7.2%) (P < 0.05) [6]. By contrast, the four SDD studies listed in Table 1 remain controversial in terms of their impact on the incidence of ICU-AI, mortality, length of ICU stay, and other outcomes.
Table 1.
Important studies evaluating the impact of decontamination in the ICU.
| Study/Year | Study Type | Intervention Group | Control Group | Outcomes |
|---|---|---|---|---|
| Recent studies focused on MSD | ||||
| Nicolas Massart et al. (2023) [3] | Before after study | MSD | Standard care | MSD reduces the incidence of ICU-AI and all-cause mortality |
| Nicolas Massart et al. (2022) [4] | Retrospective observational study | MSD | Standard care | MSD reduces the incidence, VAP and all-cause mortality, but not the incidence of BSI |
| Nicolas Massart et al. (2023) [5] | Retrospective observational study | MSD | Standard care | MSD decreases the incidence of ECMO-AI and MDRO infection, but not the all-cause mortality |
| Nicolas Massart et al. (2023) [6] | Retrospective observational study | MSD | Standard care | MSD decreases the incidence of ICU-AI, VAP, BSI, but not the incidence of MDRO infection |
| Nicolas Massart et al. (2023) [7] | Before after study | MSD | Standard care | MSD decreases the incidence of IFI |
| Recent studies focused on SDD | ||||
| Plantinga et al. (2018) [8] | Meta-analysis of six RCTs | SOD or SDD | Standard care | SDD improves ICU and hospital survival |
| Wittekamp et al. (2018) [9] | Three-arm cluster crossover trial | CHX 2%, SOD or SDD | Standard care | SDD does not decrease the incidence of ICU-BSI |
| Myburgh et al. (2022) [10] | Cluster crossover trial | SDD | Standard care | No difference can be found in mortality, MV time and long of ICU stay |
| Hammond et al. (2022) [11] | Meta-analysis of 32 RCTs | SDD | Standard care | SDD reduces the incidence of VAP and ICU-BSI |
Abbreviations: CHX, chlorhexidine; IFI, invasive fungal infection; SOD, selective oral decontamination; RCT, randomized controlled trial.
Recent analyses indicate that high-risk populations for ICU-acquired MDRO infections include ICHs, patients with severe COVID-19, patients undergoing VV-ECMO, and patients with ARDS. Some studies have revealed ICU-AI rates as high as 56% [12]. Adopting selective decontamination strategies with or without systemic antibiotics may reduce the occurrence of VAP and BSI in critically ill patients, decrease MDRO infections, and potentially reduce broad-spectrum antibiotic use. In the current era of global antibiotic resistance, these findings are encouraging. However, most studies were conducted in ICUs with low MDRO detection rates, such as in France and the Netherlands, while ICUs with higher MDRO rates have reported opposite outcomes.
As part of MSD, the role of chlorhexidine gluconate bathing in reducing hospital-acquired MDRO infections remains unclear. In areas with a high MDRO prevalence, such bathing significantly reduced the risk of MDRO and hospital-acquired BSIs. However, a randomized controlled trial in France showed no reduction in ICU-AI compared with placebo. Later studies revealed that combining mupirocin and chlorhexidine gluconate with oral-pharyngeal and digestive tract decontamination had a synergistic preventive effect, with encouraging results across AI, VAP, BSI, and MDRO [13]. Moreover, several studies showed lower overall mortality rates in the MSD group than in controls. The attribution of VAP-related mortality remains controversial. Smet et al. [14] indicated that MV patients with moderate to severe conditions have higher VAP-related mortality, but many confounding factors affect these rates. Determining the true efficacy of MSD will require further adjustment or subgroup analysis.
3. Controversies regarding widespread settlement in multiple regions
Despite studies showing that SDD can reduce ICU-AI rates and its recommendation in some guidelines as a standard strategy for preventing VAP, the implementation of SDD in ICUs remains low. International guideline groups have consistently not recommended routine use of this intervention, mainly because of the lack of standardized methods and concerns about increased antibiotic resistance. Excessive antibiotic use, particularly local application, is associated with higher detection rates of MDRO, especially methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus, which are prevalent in ICU-AI. SDD may also be linked to increased MDR bacteria in patients with inflammatory bowel disease, likely because of disruptions in the intestinal microbiota balance from local antibiotics. However, elucidating the true impact of SDD on host microbiota still requires long-term follow-up with large sample sizes.
Most research on the impact of SDD on antibiotic resistance patterns comes from France and the Netherlands, countries with a lower prevalence of MDRO. A recent study included patients from five Dutch ICUs with an average follow-up of 7 years. The study showed that after implementing a decolonization strategy, the detection rate of tobramycin-resistant pathogens decreased over time, while resistance to colistin remained unchanged (0.5%) [15]. Further analysis is needed to determine whether data from countries with lower antibiotic resistance apply to those with higher resistance rates.
A systematic review of antibiotic-resistant bacteria emergence after SDD showed no increase in colonization or infection rates of methicillin-resistant Staphylococcus aureus (odds ratio [OR], 1.46; 95% CI, 0.90–1.68) or vancomycin-resistant Enterococcus (OR, 0.63; 95% CI, 0.39–1.02). Resistance to aminoglycosides (OR, 0.73; 95% CI, 0.51–1.05) or quinolones (OR, 0.52; 95% CI, 0.16–1.68) did not increase, while resistance to glycopeptides (OR, 0.58; 95% CI, 0.46–0.72) and third-generation cephalosporins (OR, 0.33; 95% CI, 0.20–0.52) significantly decreased [16]. Although concerns about increased resistance rates exist, published clinical trials do not support this claim. In fact, SDD is associated with reduced overall antibiotic use and decreased prescription of “wild-type” antibiotics. The amount of antibiotics used in SDD is negligible relative to total hospital usage.
Another barrier to SDD implementation is the lack of standardized, commercially prepared, and tested drug formulations. Most studies rely on formulations prepared in hospital pharmacies or at the bedside, complicating consistent implementation.
Patients with traumatic brain injury are particularly prone to VAP because of their impaired consciousness and decreased immune function. Long-term antimicrobial use for infection prevention or treatment in these patients can lead to drug-resistant bacteria. Young et al. [17] performed a post hoc analysis of nearly 6000 patients with traumatic brain injury and found lower mortality rates in the SDD group than in the conventional treatment group (32.3% vs. 38.0%, P = 0.004). However, further analysis of factors such as pathogen resistance and changes in intestinal microbiota was not conducted. The generalizability and long-term effects of SDD and MSD in these patients require further clarification through large-sample randomized controlled trials [17].
4. Limitations and prospects of multisite extensive decontamination
The primary goal of implementing MSD is to reduce the emergence and spread of MDR pathogens. However, some studies suggest that MSD may alter patients’ internal microecological balance, potentially inducing MDR pathogen production. Wand et al. [18] found that exposure of Klebsiella pneumoniae to chlorhexidine may induce mutation of the smvR gene, enhancing the efflux pump mechanism and further mutating the PhoPQ gene, ultimately leading to colistin resistance in Klebsiella pneumoniae [18].
Some clinical studies have analyzed the impact of MSD on the minimum inhibitory concentration of pathogens, antibiotic resistance, and patient microflora. Although some studies show that MSD can reduce all-cause mortality, it also increases the detection of antibiotic-resistant bacteria. Machuca et al. [19] evaluated the use of oral aminoglycoside antibiotics for patients colonized with Klebsiella pneumoniae carbapenemase (KPC)-producing Klebsiella pneumoniae and found a secondary gentamicin resistance rate of 13.6% in the decolonization group, higher than in the control group (3.0%, P = 0.008). Another single-center study implementing SDD for patients at risk of resistant bacterial infection detected two colistin-resistant strains and five gentamicin-resistant strains in the SDD group, while none were found in the control group [20]. Later research by Oren et al. [21] reached similar conclusions.
MSD has gradually become a standard practice for preventing ICU-AI in European countries such as France and the Netherlands, where MDRO detection rates are lower. It remains uncertain whether findings from studies by Massart et al. apply to regions with higher MDRO detection rates, such as the United States or Asia. The true impact of MSD on the ICU environment and host microbiota also requires long-term follow-up studies with large sample sizes. Additionally, the heterogeneity in defining ICHs may necessitate subgroup analyses of the effectiveness of preventive strategies across different settings. With the increasing use of novel biological agents and immunosuppressive therapies in clinical practice, there is also a need for standardized definitions and classifications of ICHs.
Finally, all completed MSD research, primarily observational studies by Massart et al., lack blinded or randomized controlled trial designs, affecting the reliability and generalizability of the results. Large-sample randomized controlled trials are necessary to evaluate the real effects of MSD, and further analysis is required to determine the contribution of each component of MSD.
Funding
This work was supported by the Project of the Key Laboratory of Multiple Organ Failure, Ministry of Education (2023KF07), the Key Laboratory of Intelligent Pharmacy and Individualized Treatment in Huzhou City (HZKF-20240101), and the Guangxi Key Laboratory for Diagnosis and Treatment of Acute Respiratory Distress Syndrome (ZZH2020013-3), China.
CRediT authorship contribution statement
Yuetian Yu: Conceptualization, Data curation. Bin Lin: Data curation, Writing – original draft. Lihui Wang: Validation, Writing – review & editing. Chunhui Xu: Data curation, Software. Cheng Zhu: Methodology, Writing – original draft. Yuan Gao: Writing – review & editing.
Acknowledgments
Acknowledgments
None.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data available statement
Not applicable.
Ethics statement
Ethical approval was not required.
Informed consent
Not applicable.
Contributor Information
Cheng Zhu, Email: zhucheng1203@163.com.
Yuan Gao, Email: 17811@renji.com.
References
- 1.Vincent J.L., Sakr Y., Singer M., et al. Prevalence and outcomes of infection among patients in intensive care units in 2017. JAMA. 2020;323(15):1478–1487. doi: 10.1001/jama.2020.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Houston M., Hendrickson R.G. Decontamination. Crit. Care. Clin. 2005;21(4):653–672. doi: 10.1016/j.ccc.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Massart N., Dupin C., Legris E., et al. Prevention of ICU-acquired infection with decontamination regimen in immunocompromised patients: a pre/post observational study. Eur. J. Clin. Microbiol. Infect. Dis. 2023;42(10):1163–1172. doi: 10.1007/s10096-023-04650-5. [DOI] [PubMed] [Google Scholar]
- 4.Massart N., Reizine F., Fillatre P., et al. Multiple-site decontamination regimen decreases acquired infection incidence in mechanically ventilated COVID-19 patients. Ann. Intensive Care. 2022;12(1):84. doi: 10.1186/s13613-022-01057-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Massart N., Camus C., Nesseler N., et al. Multiple-site decontamination to prevent acquired infection in patients with veno-venous ECMO support. Ann. Intensive Care. 2023;13(1):27. doi: 10.1186/s13613-023-01120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Massart N., Dupin C., Legris E., et al. Multiple-site decontamination in mechanically ventilated ICU patients: a real-life study. Infect. Dis. Now. 2023;53(3) doi: 10.1016/j.idnow.2023.104666. [DOI] [PubMed] [Google Scholar]
- 7.Massart N., Reizine F., Dupin C., et al. Prevention of acquired invasive fungal infection with decontamination regimen in mechanically ventilated ICU patients: a pre/post observational study. Infect. Dis. (Lond) 2023;55(4):263–271. doi: 10.1080/23744235.2023.2170460. [DOI] [PubMed] [Google Scholar]
- 8.Plantinga N.L., de Smet A.M.G.A., Oostdijk E.A.N., et al. Selective digestive and oropharyngeal decontamination in medical and surgical ICU patients: individual patient data meta-analysis. Clin. Microbiol. Infect. 2018;24(5):505–513. doi: 10.1016/j.cmi.2017.08.019. [DOI] [PubMed] [Google Scholar]
- 9.Wittekamp B.H., Plantinga N.L., Cooper B.S., et al. Decontamination strategies and bloodstream infections with antibiotic-resistant microorganisms in ventilated patients: a randomized clinical trial. JAMA. 2018;320(20):2087–2098. doi: 10.1001/jama.2018.13765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.SuDDICU Investigators for the Australian and New Zealand Intensive Care Society Clinical Trials Group. Myburgh J.A., Seppelt I.M., et al. Effect of selective decontamination of the digestive tract on hospital mortality in critically ill patients receiving mechanical ventilation: a randomized clinical trial. JAMA. 2022;328(19):1911–1921. doi: 10.1001/jama.2022.17927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hammond N.E., Myburgh J., Seppelt I., et al. Association between selective decontamination of the digestive tract and In-hospital mortality in intensive care unit patients receiving mechanical ventilation: a systematic review and meta-analysis. JAMA. 2022;328(19):1922–1934. doi: 10.1001/jama.2022.19709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daneman N., Sarwar S., Fowler R.A., et al. Effect of selective decontamination on antimicrobial resistance in intensive care units: a systematic review and meta-analysis. Lancet Infect. Dis. 2013;13(4):328–341. doi: 10.1016/S1473-3099(12)70322-5. [DOI] [PubMed] [Google Scholar]
- 13.Wittekamp B.H., Oostdijk E.A., de Smet A.M., et al. Colistin and tobramycin resistance during long- term use of selective decontamination strategies in the intensive care unit: a post hoc analysis. Crit. Care (Lond. Engl.) 2015;19(1):113. doi: 10.1186/s13054-015-0838-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Smet A.M., Kluytmans J.A., Cooper B.S., et al. Decontamination of the digestive tract and oropharynx in ICU patients. N. Engl. J. Med. 2009;360(1):20–31. doi: 10.1056/NEJMoa0800394. [DOI] [PubMed] [Google Scholar]
- 15.Cuthbertson B.H., Campbell M.K., MacLennan G., et al. Clinical stakeholders’ opinions on the use of selective decontamination of the digestive tract in critically ill patients in intensive care units: an international Delphi study. Crit. Care (Lond. Engl.) 2013;17(6):R266. doi: 10.1186/cc13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plantinga N.L., de Smet A.M.G.A., Oostdijk E.A.N., et al. Selective digestive and oropharyngeal decontamination in medical and surgical ICU patients: individual patient data meta-analysis. Clin. Microbiol. Infect. 2018;24(5):505–513. doi: 10.1016/j.cmi.2017.08.019. [DOI] [PubMed] [Google Scholar]
- 17.Young P.J., Devaux A., Li Q., et al. Selective digestive tract decontamination in critically ill adults with acute brain injuries: a post hoc analysis of a randomized clinical trial. Intensive Care Med. 2024;50(1):56–67. doi: 10.1007/s00134-023-07261-y. [DOI] [PubMed] [Google Scholar]
- 18.Wand M.E., Bock L.J., Bonney L.C., et al. Mechanisms of increased resistance to chlorhexidine and cross-resistance to colistin following exposure of klebsiella pneumoniae clinical isolates to chlorhexidine. Antimicrob. Agents Chemother. 2016;61(1) doi: 10.1128/AAC.01162-16. e01162–e01116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Machuca I., Gutiérrez-Gutiérrez B., Pérez Cortés S., et al. Oral decontamination with aminoglycosides is associated with lower risk of mortality and infections in high-risk patients colonized with colistin-resistant, KPC-producing Klebsiella pneumoniae. J. Antimicrob. Chemother. 2016;71(11):3242–3249. doi: 10.1093/jac/dkw272. [DOI] [PubMed] [Google Scholar]
- 20.Wand M.E., Bock L.J., Bonney L.C., et al. Mechanisms of increased resistance to chlorhexidine and cross-resistance to colistin following exposure of klebsiella pneumoniae clinical isolates to chlorhexidine. Antimicrob. Agents Chemother. 2017;61(1) doi: 10.1128/AAC.01162-16. e01162–e01116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oren I., Sprecher H., Finkelstein R., et al. Eradication of carbapenem-resistant Enterobacteriaceae gastrointestinal colonization with nonabsorbable oral antibiotic treatment: a prospective controlled trial. Am. J. Infect. Control. 2013;41(12):1167–1172. doi: 10.1016/j.ajic.2013.04.018. [DOI] [PubMed] [Google Scholar]