Abstract
Background
Thyroid hormone resistance syndrome (RTH) is a rare hereditary endocrine disease that can manifest as hyperthyroidism, hypothyroidism, or remain asymptomatic. It can easily be confused with other types of thyroid diseases. The diagnosis of the disease depends on genetic testing.
Case Report
We report a 19-year-old male patient with elevation of thyroid hormones. Serological examination showed elevated thyroid hormone levels, and thyroid-stimulating hormone levels within the reference interval. The patient was finally diagnosed with RTH after genetic testing that identified a gene mutation inherited from his mother. Due to timely diagnosis, the patient’s condition has been well controlled, and his prognosis is good.
Conclusion
The clinical manifestations of RTH lack specificity, and serological examination typically shows elevated thyroid hormone levels and unsuppressed thyrotropin levels. Differential diagnosis requires a combination of serological examination, imaging studies, and functional tests to distinguish RTH from other conditions. The purpose of this treatment is to improve symptoms and should not involve the blind administration of antithyroid drugs, thyroid surgery, or radiotherapy.
Keywords: Inappropriate secretion of thyroid-stimulating hormone, resistance to thyroid hormone, thyroid hormone receptor β, THRB gene
Introduction
Thyroid hormone resistance syndrome (RTH), also known as thyroid hormone insensitivity syndrome (THIS), is mainly caused by mutations in the gene encoding the thyroid hormone receptor β (TRβ). These mutations result in the resistance of target organs or tissues to thyroid hormone (TH), characterized by increased serum TH levels and a lack of thyroid-stimulating hormone (TSH) inhibition.1 The diversity in gene mutations and TH resistance severity lead to a range of clinical manifestations in RTH patients; some are asymptomatic, some have hyperthyroidism, and some have hypothyroidism.2 The clinical manifestations are more complicated in patients with RTH comorbid with other thyroid diseases. The substantial heterogeneity of RTH results in missed diagnoses, misdiagnoses, and mistreatment. Here, we describe the diagnosis and treatment of a patient with RTH at our hospital, review the related literature, and introduce the relevant knowledge of the disease to provide a reference for clinicians in diagnosing and treating RTH.
Case Report
Clinical Data
A 19-year-old male presented at the endocrinology department of our hospital on January 11, 2023. Two months prior, the patient developed emotional agitation without obvious inducement, accompanied by chest tightness, hand-shaking, and diarrhea. Thyroid function was examined at another hospital on November 8, 2022, and revealed the following: triiodothyronine (T3), 2.85 (normal range, 1.01–2.95) nmol/L; thyroxine (T4), 196.00 (normal range, 55.34–160.88) nmol/L; free triiodothyronine (FT3), 8.69 (normal range, 2.77–6.50) pmol/L; free thyroxine (FT4), 37.50 (normal range, 10.43–24.32) pmol/L; and TSH, 3.48 (normal range, 0.40–4.34) mIU/L. At that time, the patient was unable to receive further diagnosis and treatment for personal reasons. One week before presenting at our hospital, the patient developed palpitations, and the reported symptoms were slightly worse than before. The outpatient clinic examined his thyroid function and revealed the following: T3, 2.20 (normal range, 1.01–2.95) nmol/L; T4, 209.90 (normal range, 55.34–160.88) nmol/L; FT3, 8.63 (normal range, 2.77–6.50) pmol/L; FT4, 34.06 (normal range, 10.43–24.32) pmol/L; TSH, 4.13 (normal range, 0.40–4.34) mIU/L; thyroid peroxidase antibody (TPOAb), 43.80 (normal range, <60) U/mL; and thyroglobulin antibody (TGAb), <15 (normal range, <60) U/mL. The patient was admitted to the hospital from the outpatient clinic with suspected syndrome of inappropriate secretion of TSH (SITSH). During the course of the disease, the patient did not experience fever, chills, or sweating. The patient’s appetite was unaffected, and he did not experience weight loss, fatigue, constipation, or other discomfort. The patient was in good health and had no history of chronic disease or radiation exposure to the neck. His parents were not consanguineous, and no genetic history in the family.
Physical examination revealed the following: body temperature, 36.5°C; pulse, 97 bpm; breathing, 19 bpm; blood pressure, 120/78 mmHg; height, 172 cm; weight, 70 kg; BMI, 23.66 kg/m2; clear mind; normal development, intelligence, vision, and hearing; no exophthalmos; free eye movements; mild thyroid enlargement and softness, with no tenderness and with swallowing up and down; no vascular murmur; no positive signs in the heart, lung or abdomen; normal muscle strength and tension of the extremities; no mucinous edema of lower limbs.
Supplementary Examination
Re-examination of thyroid function on January 12, 2023, revealed the following results: T3, 1.99 (normal range, 1.01–2.95) nmol/L; T4, 184.30 (normal range, 55.34–160.88) nmol/L; FT3, 8.17 (normal range, 2.77–6.50) pmol/L; FT4, 32.74 (normal range, 10.43–24.32) pmol/L; TSH, 2.30 (normal range, 0.40–4.34) mIU/L; TPOAb, 52.00 (normal range, <60) U/mL; TGAb, <15 (normal range, <60) U/mL; thyroid stimulating receptor antibody (TRAb), <0.10 (normal range, <1.00) U/L; and reverse triiodothyronine (rT3), 66.13 (normal range, 35.00–95.00) ng/dl. There were no obvious abnormalities in the blood routine, biochemistry, cortisol rhythm, adrenocorticotropin, growth hormone, or sex hormone test results. The 2-hour thyroid iodine uptake rate was lower than the normal value, and the 6-hour thyroid iodine uptake rate was normal. Thyroid color Doppler ultrasound revealed a right thyroid follicular cyst (TI-RADS-II). Thyroid single photon emission computed tomography revealed an enlarged thyroid volume. Bone age: adult bone age. No obvious abnormalities were found on routine or enhanced magnetic resonance imaging (MRI) of the pituitary gland. The patient’s electrocardiogram results were normal.
Genetic Testing
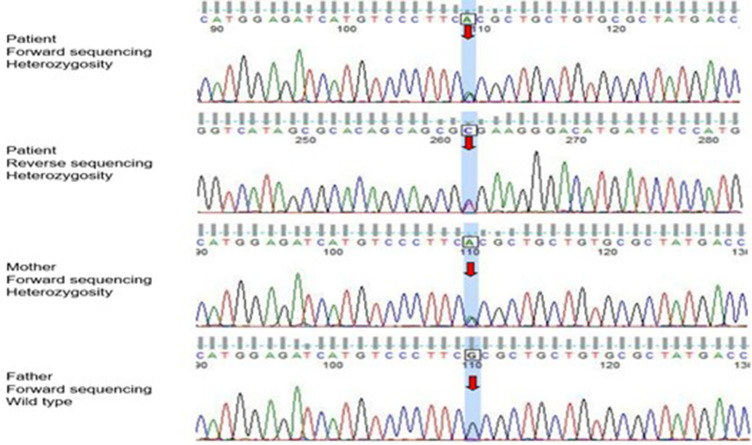
The patient showed elevated FT3, FT4, and T4 levels and some manifestations of thyrotoxicosis; however, his TSH and thyroid autoantibody levels were normal, and the thyroid iodine uptake rate decreased, which did not support the diagnosis of primary hyperthyroidism. The physical and other auxiliary examinations also ruled out thyroiditis, TSH secreting pituitary adenoma (TSHoma), and other central hyperthyroidism. The patient was highly suspected to have RTH. Genetic analyses were performed on the patient and his family after obtaining informed consent. The full exome of genomic DNA was captured and sequenced by KingMed Diagnostics Co., Ltd. The result revealed a heterozygous mutation in the THRB gene encoding thyroid hormone receptor β (TRβ) on chromosome 3. The 947 base G in the gene’s coding region was converted to A, and the nucleotide codon changed from CGC to CAC, converting amino acid 316 from arginine to histidine (R316H). Missense mutations can lead to RTH. The mother and the patient had a heterozygous mutation at the same site; the father had no mutation (Figure 1).
Figure 1.
Genetic test results of the patient and his parents (red arrow indicates mutation location).
Discussion
Most cases of RTH, a family hereditary disease, exhibit autosomal dominant inheritance, and some cases exhibit autosomal recessive inheritance.1,3 Sporadic cases are rare. The main cause of RTH is a mutation in the gene encoding TR, divided into TRα and TRβ.4 Mutations in the THRB gene, encoding TRβ, are the most common cause of RTH.5 A small number of RTH patients do not have THRB gene mutations and instead have mutations in the genes encoding TRα,6,7 TH cell membrane transporters,8 TH intracellular metabolic factors,9,10 or other coregulatory factors.11 THRB gene mutations can also be selectively expressed in some tissues, that is, somatic mutations,12 and cannot be detected in peripheral blood.
According to the degree of resistance of target organs or tissues to TH, RTH can be divided into generalized resistance to thyroid hormone (GRTH), pituitary resistance to thyroid hormone (PRTH), and peripheral tissue resistance to thyroid hormone (PTRTH). GRTH is the most common presentation, in which pituitary and peripheral tissues are resistant to TH, TH compensatory increase occurs, and TSH is normal or increased. Most patients can be compensated by increasing TH and TSH, generally without obvious symptoms, while decompensated patients show hypothyroidism. Only the pituitary gland is resistant to TH in PRTH; the response of peripheral tissue to TH is normal, TSH is oversecreted, and TH synthesis is increased. The clinical manifestation is mild to moderate hyperthyroidism with no exophthalmos or pretibial mucinous edema. PTRTH is extremely rare; only peripheral tissue is resistant to TH, the pituitary response to TH is normal, TH is elevated, and TSH is normal or elevated. The clinical manifestation is hypothyroidism.1 The clinical manifestations of patients with RTH vary greatly. According to existing reports, the most common presentation is goiter, and other symptoms include tachycardia, short stature, growth retardation, intellectual disability, attention deficit, abnormal hearing, and visual impairment.13,14 RTH can also be comorbid with other thyroid diseases, complicating the clinical manifestations. The level of TSH in most patients with RTH increases, and as the TSH level increases, the risk of thyroid nodules increases, potentially leading to cancer.15–17 Moreover, long-term stimulation with TSH can cause chronic lymphocyte infiltration, leading to thyroid damage and autoimmune thyroid disease (AITD), including Graves’ disease and Hashimoto’s thyroiditis.18 However, some studies have shown that the prevalence of AITD with age is not affected by the THRB gene.19 In addition, cardiovascular diseases, such as arrhythmia and heart valvular disease, are more common in RTH patients, and the condition is milder.20 Decreased TH sensitivity is associated with diabetes, hypertension, metabolic syndrome, osteoporosis, fracture, and decreased renal function.21–24
When diagnosing RTH, general thyroid diseases, such as Graves’ disease and primary hypothyroidism, should first be ruled out by serological examination. For patients suspected to have RTH, thyroid function, sex hormone binding globulin (SHBG), and serum ferritin require multiple repeat tests to rule out assay interference. SHBG and serum ferritin are effector proteins of TH, which are mainly produced by the liver and generally increased in patients with hyperthyroidism; patients with RTH do not demonstrate increased SHBG and serum ferritin due to the resistance of the liver to TH. Second, disorders leading to abnormal binding of TH in the serum, including familial dysalbuminemic hyperthyroxinemia, abnormal thyroxine-binding globulin, or transthyretin, should be excluded. The clinical manifestation of TSHoma is hyperthyroidism, and the serological manifestation is elevated TH and a lack of TSH inhibition, similar to the manifestations of RTH.25 Occupying lesions can usually be found on pituitary MRI in patients with TSHoma; however, there are clinical reports of RTH complicated with pituitary nonfunctional microadenoma or TSHoma.26–29 Thus, it is particularly important to determine whether pituitary tumors secrete TSH. In the thyrotropin-releasing hormone (TRH) stimulation test, patients with TSHoma do not demonstrate TSH being excited by TRH, while patients with RTH show normal or overreactive TSH. In the L-triiodothyronine inhibition test, TSH is not inhibited in patients with TSHoma but is significantly inhibited in patients with RTH. In the somatostatin test, TSH in patients with TSHoma can usually be inhibited by somatostatin, which can decrease the size of the tumor. The response to somatostatin is low in patients with RTH. However, in clinical practice, the criteria for the somatostatin test have not been unified, and the rates of TSH inhibition in patients with different diseases overlap; therefore, this test has not been popularized at present. The determination of serum glycoprotein hormone alpha-subunit (α-GSU) is also helpful for distinguishing between the two. The α-GSU or α-GSU/TSH ratio is increased in patients with TSHoma but is normal in patients with RTH.30,31 The diagnosis of RTH depends on genetic analysis, but a small number of patients do not have THRB gene mutations. If RTH is highly suspected in the clinic, we can use related functional tests to assist in diagnosis and further sequence other related genes. In the patient described in this paper, a missense mutation in the THRB gene was found by genetic detection, in addition serological examination and clinical manifestations were consistent with RTH. The diagnosis of RTHβ was clear.
The treatment of RTH is mainly aimed at improving symptoms rather than returning the TH levels to normal. In most patients, elevated TH is sufficient to compensate for the resistance of the target organs to TH, and the symptoms typically resolve without treatment. Patients with hypothyroidism, especially infants, should receive timely supplementation with exogenous TH, and levothyroxine is the first-line treatment.32 Patients with hyperthyroidism are treated with β-blockers or anti-anxiety drugs according to their main symptoms. For patients with high TSH levels, triiodothyroacetic acid (TRIAC), a T3 analog, should be selected to control TSH levels and reduce the incidence of thyroid nodules, thyroid cancer, and AITD. TRIAC can inhibit TSH, relieve goiter, reduce the level of TH, and improve the symptoms of hyperthyroidism.33,34 Other drugs, such as bromocriptine, octreotide, or glucocorticoids, can also effectively reduce the levels of TSH and TH; however, these drugs are associated with serious adverse reactions, and the long-term effects are insufficient.1 Antithyroid drugs should be avoided but can be used in small doses when RTH is complicated by Graves’ disease. Thyroid surgery or radiotherapy is prohibited to prevent refractory hypothyroidism, pituitary cell proliferation, and other consequences. Highly selective TRβ agonists, which can target mutant TRβ, are important for the treatment of RTHβ and help to explore the molecular structure of TRβ.35,36 A review of the patient in this paper revealed that he had symptoms of hyperthyroidism, such as chest tightness, palpitations, and emotional agitation and that his TSH level was normal. Metoprolol (47.5 mg) was given once a day for treatment. The palpitations improved, and there was no other obvious discomfort.
Conclusion
The clinical manifestations of RTH are not characteristic; thus, it is easy to misdiagnose and administer inappropriate treatment that aggravates the disease. In neonatal RTH screening, the levels of serum TSH and TH should be assessed at the same time.37,38 Children with a family history of RTH should be examined comprehensively, especially those with mental retardation, deafness, and growth retardation. The possibility of RTH should also be considered for other patients whose thyroid function is inconsistent with clinical manifestations. Once RTH is diagnosed, we should immediately administer corresponding treatment according to the patient’s symptoms, review thyroid function every three months and thyroid color Doppler ultrasound every six months, and pay close attention to the progression of the disease. In addition, attention should be paid to monitoring blood pressure, blood sugar, blood lipids, cardiac function, and renal function. If hypertension, diabetes, hyperlipidemia, and other concomitant diseases occur, comprehensive management should be conducted to improve patient prognosis.
Acknowledgments
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Funding Statement
This work was supported by the Key Program of Nature Science Foundation of Anhui Education Committee [grant numbers 2022AH051524]; the Key Project of Natural Science Foundation of Bengbu Medical College [grant numbers 2021byzd043]; the Postgraduate Scientific Research and Innovation Program of Bengbu Medical College in 2023 [grant numbers Byycx23102].
Consent for Publication
The informed consent for publication was obtained from the patient, along with the case details, the patient also consented to have any accompanying images published. The hospital did not require ethical clearance for the case report.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Refetoff S, Weiss RE, Usala SJ. The syndromes of resistance to thyroid hormone. Endocr Rev. 1993;14(3):348–399. doi: 10.1210/edrv-14-3-348 [DOI] [PubMed] [Google Scholar]
- 2.Pappa T, Refetoff S. Resistance to Thyroid Hormone Beta: a Focused Review. Front Endocrinol. 2021;12:656551. doi: 10.3389/fendo.2021.656551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Refetoff S, DeWind LT, DeGroot LJ. Familial syndrome combining deaf-mutism, stuppled epiphyses, goiter and abnormally high PBI: possible target organ refractoriness to thyroid hormone. J Clin Endocrinol Metab. 1967;27(2):279–294. doi: 10.1210/jcem-27-2-279 [DOI] [PubMed] [Google Scholar]
- 4.Ortiga-Carvalho TM, Sidhaye AR, Wondisford FE. Thyroid hormone receptors and resistance to thyroid hormone disorders. Nat Rev Endocrinol. 2014;10(10):582–591. doi: 10.1210/edrv-14-3-348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Refetoff S, Bassett JH, Beck-Peccoz P, et al. Classification and proposed nomenclature for inherited defects of thyroid hormone action, cell transport, and metabolism. J Clin Endocrinol Metab. 2014;99(3):768–770. doi: 10.1210/jcem-27-2-279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moran C, Agostini M, Visser WE, et al. Resistance to thyroid hormone caused by a mutation in thyroid hormone receptor (TR)α1 and TRα2: clinical, biochemical, and genetic analyses of three related patients. Lancet Diabetes Endocrinol. 2014;2(8):619–626. doi: 10.1038/nrendo.2014.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Gucht ALM, Moran C, Meima ME, et al. Resistance to Thyroid Hormone due to Heterozygous Mutations in Thyroid Hormone Receptor Alpha. Curr Top Dev Biol. 2017;125:337–355. doi: 10.1016/bs.ctdb.2017.02.001 [DOI] [PubMed] [Google Scholar]
- 8.Dumitrescu AM, Liao XH, Best TB, Brockmann K, Refetoff S. A novel syndrome combining thyroid and neurological abnormalities is associated with mutations in a monocarboxylate transporter gene. Am J Hum Genet. 2004;74(1):168–175. doi: 10.1086/380999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dumitrescu AM, Liao XH, Abdullah MS, et al. Mutations in SECISBP2 result in abnormal thyroid hormone metabolism. Nat Genet. 2005;37(11):1247–1252. doi: 10.1038/ng1654 [DOI] [PubMed] [Google Scholar]
- 10.Lee KW, Shin Y, Lee S, Lee S. Inherited Disorders of Thyroid Hormone Metabolism Defect Caused by the Dysregulation of Selenoprotein Expression. Front Endocrinol. 2022;12:803024. doi: 10.3389/fendo.2021.803024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reutrakul S, Sadow PM, Pannain S, et al. Search for abnormalities of nuclear corepressors, coactivators, and a coregulator in families with resistance to thyroid hormone without mutations in thyroid hormone receptor beta or alpha genes. J Clin Endocrinol Metab. 2000;85(10):3609–3617. doi: 10.1210/jcem.85.10.6873 [DOI] [PubMed] [Google Scholar]
- 12.Yen PM. Molecular basis of resistance to thyroid hormone. Trends Endocrinol Metab. 2003;14(7):327–333. doi: 10.1016/s1043-2760(03)00114-0 [DOI] [PubMed] [Google Scholar]
- 13.Weiss RE, Dumitrescu A, Refetoff S. Approach to the patient with resistance to thyroid hormone and pregnancy. J Clin Endocrinol Metab. 2010;95(7):3094–3102. doi: 10.1210/jc.2010-0409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campi I, Cammarata G, Bianchi Marzoli S, et al. Retinal Photoreceptor Functions Are Compromised in Patients With Resistance to Thyroid Hormone Syndrome (RTHβ). J Clin Endocrinol Metab. 2017;102(7):2620–2627. doi: 10.1210/jc.2016-3671 [DOI] [PubMed] [Google Scholar]
- 15.Karakose M, Caliskan M, Arslan MS, Cakal E, Yesilyurt A, Delibasi T. Thyroid hormone resistance in two patients with papillary thyroid microcarcinoma and their BRAFV600E mutation status. Arch Endocrinol Metab. 2015;59(4):364–366. doi: 10.1590/2359-3997000000091 [DOI] [PubMed] [Google Scholar]
- 16.Vinagre J, Borges F, Costa A, et al. Differentiated thyroid cancer in patients with resistance to thyroid hormone syndrome. A novel case and a review of the literature. Front Mol Biosci. 2014;1:10. doi: 10.3389/fmolb.2014.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fiore E, Vitti P. Serum TSH and risk of papillary thyroid cancer in nodular thyroid disease. J Clin Endocrinol Metab. 2012;97(4):1134–1145. doi: 10.1210/jc.2011-2735 [DOI] [PubMed] [Google Scholar]
- 18.Gavin C, Meggison H, Ooi TC. Proposing a causal link between thyroid hormone resistance and primary autoimmune hypothyroidism. Med Hypotheses. 2008;70(5):1024–1028. doi: 10.1016/j.mehy.2007.08.015 [DOI] [PubMed] [Google Scholar]
- 19.Barkoff MS, Kocherginsky M, Anselmo J, Weiss RE, Refetoff S. Autoimmunity in patients with resistance to thyroid hormone. J Clin Endocrinol Metab. 2010;95(7):3189–3193. doi: 10.1210/jc.2009-2179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Illouz F, Briet C, Mirebeau-Prunier D, et al. Cardiac complications of thyroid hormone resistance syndromes. Ann Endocrinol. 2021;82(3–4):167–169. doi: 10.1016/j.ando.2020.03.008 [DOI] [PubMed] [Google Scholar]
- 21.Mehran L, Delbari N, Amouzegar A, Hasheminia M, Tohidi M, Azizi F. Reduced Sensitivity to Thyroid Hormone Is Associated with Diabetes and Hypertension. J Clin Endocrinol Metab. 2022;107(1):167–176. doi: 10.1210/clinem/dgab646 [DOI] [PubMed] [Google Scholar]
- 22.Laclaustra M, Moreno-Franco B, Lou-Bonafonte JM, et al. Impaired Sensitivity to Thyroid Hormones Is Associated With Diabetes and Metabolic Syndrome. Diabetes Care. 2019;42(2):303–310. doi: 10.2337/dc18-1410 [DOI] [PubMed] [Google Scholar]
- 23.Liu C, Hua L, Liu K, Xin Z. Impaired sensitivity to thyroid hormone correlates to osteoporosis and fractures in euthyroid individuals. J Endocrinol Invest. 2023;46(10):2017–2029. doi: 10.1007/s40618-023-02035-1 [DOI] [PubMed] [Google Scholar]
- 24.Yang S, Lai S, Wang Z, Liu A, Wang W, Guan H. Thyroid Feedback Quantile-based Index correlates strongly to renal function in euthyroid individuals. Ann Med. 2021;53(1):1945–1955. doi: 10.1080/07853890.2021.1993324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Concolino P, Costella A, Paragliola RM. Mutational Landscape of Resistance to Thyroid Hormone Beta (RTHβ). Mol Diagn Ther. 2019;23(3):353–368. doi: 10.1007/s40291-019-00399-w [DOI] [PubMed] [Google Scholar]
- 26.Campi I, Covelli D, Moran C, et al. The Differential Diagnosis of Discrepant Thyroid Function Tests: insistent Pitfalls and Updated Flow-Chart Based on a Long-Standing Experience. Front Endocrinol. 2020;11:432. doi: 10.3389/fendo.2020.00432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sriphrapradang C, Srichomkwun P, Refetoff S, Mamanasiri S. A Novel Thyroid Hormone Receptor Beta Gene Mutation (G251V) in a Thai Patient with Resistance to Thyroid Hormone Coexisting with Pituitary Incidentaloma. Thyroid. 2016;26(12):1804–1806. doi: 10.1089/thy.2016.0450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teng X, Jin T, Brent GA, Wu A, Teng W, Shan Z. A Patient With a Thyrotropin-Secreting Microadenoma and Resistance to Thyroid Hormone (P453T). J Clin Endocrinol Metab. 2015;100(7):2511–2514. doi: 10.1210/jc.2014-3994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao J, Xu L, Li C, Wang F, Liao L, Dong J. The clinical characteristics and gene mutations associated with thyroid hormone resistance syndrome coexisting with pituitary tumors. Front Endocrinol. 2023;14:1131044. doi: 10.3389/fendo.2023.1131044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brucker-Davis F, Oldfield EH, Skarulis MC, Doppman JL, Weintraub BD. Thyrotropin-secreting pituitary tumors: diagnostic criteria, thyroid hormone sensitivity, and treatment outcome in 25 patients followed at the National Institutes of Health. J Clin Endocrinol Metab. 1999;84(2):476–486. doi: 10.1210/jcem.84.2.5505 [DOI] [PubMed] [Google Scholar]
- 31.Macchia E, Lombardi M, Raffaelli V, et al. Clinical and genetic characteristics of a large monocentric series of patients affected by thyroid hormone (Th) resistance and suggestions for differential diagnosis in patients without mutation of Th receptor β. Clin Endocrinol. 2014;81(6):921–928. doi: 10.1111/cen.12556 [DOI] [PubMed] [Google Scholar]
- 32.Yao B, Yang C, Pan C, Li Y. Thyroid hormone resistance: mechanisms and therapeutic development. Mol Cell Endocrinol. 2022;553:111679. doi: 10.1016/j.mce.2022.111679 [DOI] [PubMed] [Google Scholar]
- 33.Groeneweg S, Peeters RP, Visser TJ, Visser WE. Therapeutic applications of thyroid hormone analogues in resistance to thyroid hormone (RTH) syndromes. Mol Cell Endocrinol. 2017;458:82–90. doi: 10.1016/j.mce.2017.02.029 [DOI] [PubMed] [Google Scholar]
- 34.Visser WE. Therapeutic applications of thyroid hormone analogues. Ann Endocrinol. 2021;82(3–4):170–172. doi: 10.1016/j.ando.2020.03.004 [DOI] [PubMed] [Google Scholar]
- 35.Yao B, Wei Y, Zhang S, et al. Revealing a Mutant-Induced Receptor Allosteric Mechanism for the Thyroid Hormone Resistance. iScience. 2019;20:489–496. doi: 10.1016/j.isci.2019.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Q, Yao B, Zhao S, et al. Discovery of a Highly Selective and H435R-Sensitive Thyroid Hormone Receptor β Agonist. J Med Chem. 2022;65(10):7193–7211. doi: 10.1021/acs.jmedchem.2c00144 [DOI] [PubMed] [Google Scholar]
- 37.Cannarella R, Musmeci M, Garofalo V, et al. Resistance to Thyroid Hormones: a Case-Series Study. Int J Mol Sci. 2022;23(19):11268. doi: 10.3390/ijms231911268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vela A, Pérez-Nanclares G, Ríos I, et al. Thyroid hormone resistance from newborns to adults: a Spanish experience. J Endocrinol Invest. 2019;42(8):941–949. doi: 10.1007/s40618-019-1007-4 [DOI] [PubMed] [Google Scholar]