Oligomerization of G protein-coupled receptors (GPCRs) has received much attention over the past several years (1, 2). The consequences arising from oligomeric arrangements of GPCRs are proposed to play a central role in signal transduction (3). Except for a few cases (e.g., refs. 4–6), many of the recent studies investigating GPCR oligomerization have involved heterologous expression systems of native and modified receptors (7). The studies by Waldhoer et al. in this issue of PNAS (8) broaden the previous work by placing it in a physiologically relevant context.
The opioid receptors studied by Waldhoer et al. (8) belong to the GPCR superfamily and exist as three types: δ, κ, and μ. All three types have been shown previously to form homooligomers individually and heterooligomers with each other (5, 9–12). Waldhoer et al. demonstrate the existence of a functional heteromer of δ and κ opioid receptors in vivo that localizes specifically in the spinal cord. This study gives credence to the wealth of evidence for GPCR oligomerization in vitro. The δ–κ heteromer has a novel pharmacology and is activated by the heteromer-specific agonist 6′-guanidinonaltrindole (6′-GNTI) (Fig. 1), which illustrates the added diversity offered by heterooligomerization (Fig. 2). The novel pharmacological profile of the δ–κ heteromer and the availability of ligands that are specific to this heterooligomer (13, 14) open the door to more selectively targeted therapeutics with reduced side effects.
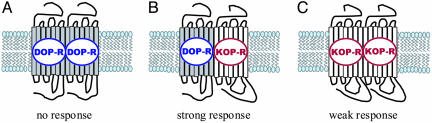
Fig. 1.
Novel pharmacology produced through the heterooligomerization of the δ opioid receptor (DOP-R) and κ opioid receptor (KOP-R). The functional response to the binding of 6′-GNTI is different for the three forms of the opioid receptor presented: δ (A), κ (C), and δ–κ heteromer (B). Only the heteromeric complex (B) is capable of producing a robust response to the analgesic.
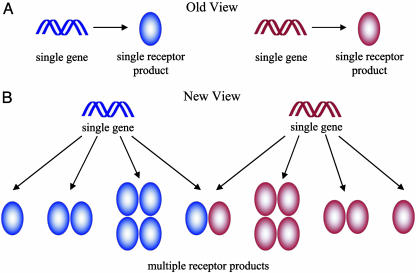
Fig. 2.
Diversity through oligomerization. (A) The classical view of GPCR systems presumes that the functional receptor unit is the product of a single gene. The study by Waldhoer et al. (8) demonstrates that GPCRs are not restricted to this linear process. (B) The repertoire of functional receptor units may also include homooligomers of a single gene product and heterooligomers of multiple gene products.
The concept of what constitutes a receptor continues to evolve as more is learned about these systems (1). Traditional wisdom has depicted GPCRs as a linear system wherein the receptor is the product of a single gene and is activated by an agonist in a 1:1 stoichiometry (Fig. 2). The knowledge that GPCRs can exist and function as oligomers prompts an alternate conceptualization of the system. The ability to study GPCR oligomerization under in vivo or close to in vivo conditions will greatly facilitate our understanding of how these systems function.
The studies of Waldhoer et al. (8) demonstrate in vivo the nonlinearity of GPCR systems. The agonist 6′-GNTI has been shown to produce a robust response specific to the δ–κ heteromer. The response elicited by 6′-GNTI from the δ–κ heteromer is different from that observed with either of the opioid receptor types expressed alone. 6′-GNTI is antagonistic with pure δ opioid receptors, whereas the response is severely attenuated with pure κ opioid receptors (Fig. 1). This finding demonstrates the pharmacological uniqueness of the δ–κ heteromer compared with the two parent molecules.
Positive cooperativity has been observed previously in the binding of the δ-selective agonist [d-Pen2,d-Pen5]-enkephalin and the κ-selective agonist U69593 to the δ–κ heteromer from a heterologous expression system coexpressing the two types of opioid receptors (10). Although neither ligand alone was able to bind the δ–κ heteromer with an appreciable affinity, the apparent affinity of both ligands increased by at least 50-fold when used in combination. Receptor “cross-talk” has also been detected in heteromeric complexes of the μ and δ opioid receptors (5, 11).
The oligomeric potential of G protein-coupled receptors allows for more complex ligand–receptor relationships.
The conceptualization of GPCR systems is becoming increasingly complex. The product of a single gene is no longer necessarily the functional unit. For instance, the product of a single gene may exist as a monomer, dimer, or larger oligomer, all of which may have unique signaling properties or roles in the signaling process (Fig. 2). The oligomeric potential of GPCRs allows for more complex ligand–receptor relationships than those predicted by the law of mass action and expands the repertoire of unique signaling units. Add to this complexity the ability of two different types of receptor to heterooligomerize, and the repertoire is expanded even further. The diversity created by GPCR oligomerization provides the opportunity to design novel pharmaceutical agents, such as bivalent ligands specific to heterodimers (13, 14).
The different pharmacologies arising from the oligomerization of GPCRs may have several different mechanistic origins. For instance, oligomerization may generate a novel binding pocket, an alternate structural response to ligands, or a combination of the two, as suggested by Waldhoer et al. (8) in their studies of the δ–κ heteromer. A complex of receptor protomers also allows for cooperative effects wherein the binding of a ligand to one protomer can potentially affect the binding of ligands to other protomers in the complex (e.g., refs. 1 and 15). Each member of the oligomeric complex may play a different role in the function of the receptor. For instance, one protomer may be responsible for binding the ligand while another is responsible for coupling to the G protein (e.g., ref. 16). In such a case, the requirement for full function of the receptor may require the activation of only a single protomer (e.g., ref. 17), and the activation of a single protomer may promote the activation of others within the same complex. Oligomerization may also change the nature of the interaction between the receptor and its associated heterotrimeric G protein (e.g., refs. 11 and 18). A unique binding surface may be produced upon oligomerization, altering the specificity for G proteins and other interacting proteins.
The detection of a δ–κ heteromer of the opioid receptor in the spinal cord demonstrates that functional oligomers of GPCRs are relevant to signal transduction in vivo (8). This observation, however, does not exclude the possible presence and function of monomeric GPCRs nor does it describe the complement of functional receptor units in any given cell. Both monomers and oligomers have been detected in vitro (e.g., refs. 19 and 20), and various nonclassical effects have been attributed to the latter species (1). Model systems that will allow testing of these different effects, particularly in vivo, will greatly enhance our ability to determine the phenomena that are relevant to signaling.
See companion article on page 9050.
References
- 1.Park, P. S.-H., Filipek, S., Wells, J. W. & Palczewski, K. (2004) Biochemistry 43, 15643–15656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Terrillon, S. & Bouvier, M. (2004) EMBO Rep. 5, 30–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chidiac, P., Green, M. A., Pawagi, A. B. & Wells, J. W. (1997) Biochemistry 36, 7361–7379. [DOI] [PubMed] [Google Scholar]
- 4.Fotiadis, D., Liang, Y., Filipek, S., Saperstein, D. A., Engel, A. & Palczewski, K. (2003) Nature 421, 127–128. [DOI] [PubMed] [Google Scholar]
- 5.Gomes, I., Gupta, A., Filipovska, J., Szeto, H. H., Pintar, J. E. & Devi, L. A. (2004) Proc. Natl. Acad. Sci. USA 101, 5135–5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chinault, S. L., Overton, M. C. & Blumer, K. J. (2004) J. Biol. Chem. 279, 16091–16100. [DOI] [PubMed] [Google Scholar]
- 7.Milligan, G. (2001) J. Cell Sci. 114, 1265–1271. [DOI] [PubMed] [Google Scholar]
- 8.Waldhoer, M., Fong, J., Jones, R. M., Lunzer, M. M., Sharma, S. K., Kostenis, E., Portoghese, P. S. & Whistler, J. L. (2005) Proc. Natl. Acad. Sci. USA 102, 9050–9055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cvejic, S. & Devi, L. A. (1997) J. Biol. Chem. 272, 26959–26964. [DOI] [PubMed] [Google Scholar]
- 10.Jordan, B. A. & Devi, L. A. (1999) Nature 399, 697–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.George, S. R., Fan, T., Xie, Z., Tse, R., Tam, V., Varghese, G. & O'Dowd, B. F. (2000) J. Biol. Chem. 275, 26128–26135. [DOI] [PubMed] [Google Scholar]
- 12.Wang, D., Sun, X., Bohn, L. M. & Sadee, W. (2005) Mol. Pharmacol. 67, 2173–2184. [DOI] [PubMed] [Google Scholar]
- 13.Bhushan, R. G., Sharma, S. K., Xie, Z., Daniels, D. J. & Portoghese, P. S. (2004) J. Med. Chem. 47, 2969–2972. [DOI] [PubMed] [Google Scholar]
- 14.Daniels, D. J., Kulkarni, A., Xie, Z., Bhushan, R. G. & Portoghese, P. S. (2005) J. Med. Chem. 48, 1713–1716. [DOI] [PubMed] [Google Scholar]
- 15.Agnati, L. F., Fuxe, K. & Ferre, S. (2005) Trends Biochem. Sci. 30, 188–193. [DOI] [PubMed] [Google Scholar]
- 16.Pin, J. P., Kniazeff, J., Binet, V., Liu, J., Maurel, D., Galvez, T., Duthey, B., Havlickova, M., Blahos, J., Prezeau, L. & Rondard, P. (2004) Biochem. Pharmacol. 68, 1565–1572. [DOI] [PubMed] [Google Scholar]
- 17.Hlavackova, V., Goudet, C., Kniazeff, J., Zikova, A., Maurel, D., Vol, C., Trojanova, J., Prezeau, L., Pin, J. P. & Blahos, J. (2005) EMBO J. 24, 499–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Filipek, S., Krzysko, K. A., Fotiadis, D., Liang, Y., Saperstein, D. A., Engel, A. & Palczewski, K. (2004) Photochem. Photobiol. Sci. 3, 628–638. [DOI] [PubMed] [Google Scholar]
- 19.Park, P. S.-H. & Wells, J. W. (2003) Biochemistry 42, 12960–12971. [DOI] [PubMed] [Google Scholar]
- 20.Jastrzebska, B., Maeda, T., Zhu, L., Fotiadis, D., Filipek, S., Engel, A., Stenkamp, R. E. & Palczewski, K. (2004) J. Biol. Chem. 279, 54663–54675. [DOI] [PMC free article] [PubMed] [Google Scholar]