Abstract
Coronary flow capacity (CFC) integrates quantitative assessment of hyperemic myocardial blood flow and coronary flow reserve. We aimed to evaluate the effect of elective percutaneous coronary revascularization (PCI) on CFC using serial stress transthoracic Doppler echocardiography (STDE). Overall, 148 stable patients underwent STDE of the left anterior descending arteries (LAD), before and after elective PCI. Coronary flow velocity reserve (CFVR) was measured using basal and hyperemic diastolic peak velocity (hDPV). Vessels were classified into four CFC categories: severely, moderately, or mildly reduced CFC, and normal flow. Changes in hDPV and CFC status post-PCI, as well as predictors of hDPV increase, were assessed. Despite improvements in fractional flow reserve (FFR) in all cases, 31 cases (20.9%) showed a decrease in hDPV following PCI. Vessels with ischemic CFC, defined as moderately or severely reduced CFC, decreased from 46.6% (69/148) to 19.6% (29/148) post-PCI. Conversely, CFC worsened in 15.5% of patients. Multivariable analysis showed lower pre-PCI hDPV and ischemic CFC were independently predictive of higher-level (> 50%) hDPV increase after PCI. Approximately 20% of FFR-guided LAD PCI resulted in decreased hDPV. CFC deterioration was not uncommon despite FFR improvement. Preprocedural non-invasive STDE may help identify lesions that benefit from revascularization.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-79896-w.
Subject terms: Cardiology, Interventional cardiology
Introduction
Coronary flow capacity (CFC) is a well investigated physiological index for myocardial ischemia severity that assesses flow limitation arising from combinations of epicardial coronary artery stenosis, diffuse disease, and microvascular dysfunction.1–6 Recent studies have highlighted the utility of CFC in identifying lesions amenable to revascularization that can lead to improved coronary flow7 and post-revascularization outcomes.8 Given the importance of myocardial ischemia severity as a prognostic factor in chronic coronary syndrome (CCS),9,10 revascularization procedures, such as percutaneous coronary intervention (PCI), should aim to increase vessel-specific coronary flow and improve coronary flow capacity, to effectively mitigate the potential risks associated with PCI and stent placement. Maximal myocardial blood flow as well as coronary flow reserve has been shown to be an independent prognostic factor.11 However, studies reporting the effect of PCI on coronary flow capacity or hyperemic coronary flow has been limited to modalities such as positron emission tomography (PET) scanning or an invasive intravascular velocity wire.7,12–14 Stress transthoracic doppler echocardiography (STDE) is a cost-effective, non-invasive, and widely available test that accurately evaluates diagnostic and quantitative data, such as coronary flow velocity and the coronary flow velocity reserve (CFVR). STDE can evaluate serial changes in coronary blood flow after revascularization, providing the ability to estimate its benefit with respect to coronary flow. The improvement of FFR may indicate the effectiveness of revascularization, whereas flow, not pressure index, has been reported to better indicate changes in coronary flow characteristics.7 CFVR and CFC measured by STDE can be a valuable tool to evaluate coronary physiology including coronary microcirculation. STDE provides prognostic information without the need for invasive ionizing radiation, radioactive tracers, gadolinium, or intravascular catheterisation.15 Therefore, the present study aimed to noninvasively obtain STDE-derived flow velocity as a surrogate of volumetric coronary flow for CFC classification. There are still few studies evaluating changes in CFC before and after PCI. Our study is the first to use STDE for the assessment of changes in coronary flow characteristics by CFC following PCI. Thereon, the impact of uncomplicated FFR-guided PCI on CFC status in the left anterior descending coronary artery (LAD) was investigated. Further, the pre-PCI STDE-derived physiological indices and CFC status were assessed to predict increased coronary flow after PCI in patients with CCS, in comparison or combination with FFR.
Materials and methods
Study design and patient population
We performed a retrospective analysis of pooled data from the institutional imaging and physiology registry at Tsuchiura Kyodo General Hospital by identifying patients with CCS who underwent elective FFR-guided PCI of de novo single LAD lesions with pre- and post-PCI STDE examinations between April 2019 and July 2023. All patients had symptoms suggestive of myocardial ischemia and functionally significant proximal LAD lesions with an FFR of ≤ 0.80. Those with acute coronary syndrome or angiographically visible collateral flow to LAD territory were excluded. Patients with contraindications to adenosine administration or who had an inability to provide consent were also not included. We further excluded patients with multivessel coronary disease, left main disease, in-stent restenosis, previous history of myocardial infarction in the LAD territory or coronary artery bypass graft surgery, occluded target vessels, severely reduced systolic function (left ventricular ejection fraction < 40%), atrial fibrillation at the time of STDE examination, and renal dysfunction. Optimal guideline-directed medical therapy was initiated immediately after diagnostic catheterization in all patients.16,17 As per the study protocol, no ad hoc PCI was performed in this study. In patients with functionally significant LAD lesions, pre- and post-PCI STDE were subsequently performed to coronary flow assessments. The present study was approved by an institutional ethics committee (#2022FY103/Tsuchiura Kyodo General Hospital) and was conducted in compliance with the tenets of the Declaration of Helsinki for human studies. All patients provided written informed consent for the institutional registry and future data utilization.
Invasive coronary angiography
Each patient initially underwent standard diagnostic coronary angiography via the radial artery using a 5 F system to assess epicardial coronary stenosis and functional severity. Quantitative coronary angiography analyses were performed using a CMS-MEDIS system (Medis Medical Imaging Systems, Leiden, Netherlands). All patients received a bolus injection of heparin (5,000 IU) prior to the procedure. An intracoronary bolus injection of nitroglycerin (0.2 mg) was administered at the start of the procedure and before functional assessments.
Physiological measurements
Physiological measurements were performed in the LAD using a Radi Analyzer Xpress instrument with a single 0.014-inch PressureWire™ (Abbott Vascular, St. Paul, MN, USA). The FFR value was calculated as the ratio of the mean distal coronary pressure (Pd) to the mean aortic pressure (Pa) during stable hyperemia, which was induced by the intravenous administration of adenosine (140 µg/kg/min through a central vein), as previously described.6,7 After pressure calibration, the wire was advanced as far distal as practical from the aortic ostium, and the intracoronary pressure distal to the coronary stenosis was measured. After FFR measurement, when the pressure sensor reached the tip of the guiding catheter during hyperemia via a pull-back maneuver, a mean Pd–Pa pressure drift of ≤ 2 mmHg was confirmed and documented. The institutional standard protocol mandated repeat assessment if the pressure drift was > 2 mmHg. All patients were instructed to strictly refrain from ingesting caffeinated beverages > 24 h before catheterization.
Percutaneous coronary intervention
PCI was performed according to the latest guidelines.18 All patients underwent second- or third-generation coronary drug-eluting stent implantation with pre-dilatation. Successful and uncomplicated PCI was defined as residual stenosis of < 20%, along with Thrombolysis in Myocardial Infarction flow grade 3 with no side branch occlusion or distal embolization, and no PCI-related myocardial infarction according to the fourth universal definition of myocardial infarction.19 According to the study protocol, all eligible patients underwent STDE examination before elective PCI for LAD lesions.
Coronary flow velocity assessment by STDE
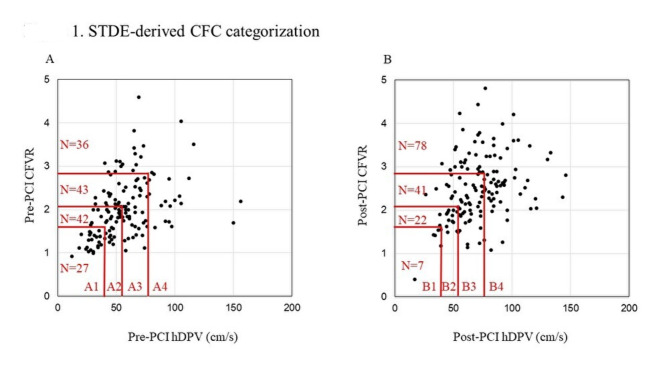
Eligible patients underwent pre- (1 day before) and post-PCI (average: 3 [range: 2–5] days after PCI) coronary flow assessments of LAD by STDE, as previously described.20,21 Echocardiographic studies were performed according to the American Society of Echocardiography guidelines22 using an ultrasound system (GE Vivid E95; GE Vingmed Ultrasound, Horten, Norway) with a multifrequency transducer and second-harmonic technology, as previously reported.20 Briefly, after the standard examination, coronary flow in the mid-distal portion of the LAD was visualized in a modified three-chamber view. The velocity range was set as 16–24 cm/s. A sample volume was selected at the mid-to-distal LAD, to measure blood flow velocity. The peak diastolic coronary flow velocity was measured at basal conditions (bDPV) and during maximal hyperemia (hDPV) by the induction using intravenous adenosine (140 µg/kg per min through the central vein). All data were digitally stored for data analyses. Three optimal flow signal profiles at rest and during hyperemia were obtained offline from the recorded data. CFVR was calculated as the ratio of the hyperemic peak diastolic flow velocity to the basal peak diastolic flow velocity. Coronary flow increase was evaluated by the metric of % hDPV-increase, defined as (post-PCI hDPV minus pre-PCI hDPV)/pre-PCI hDPV × 100. Two TDE experts who were blinded to the clinical data independently analysed all stored data at a 1-week interval and performed the analyses twice to evaluate the reproducibility of the STDE-derived data. Figure 1 shows a map of STDE-CFC categorization in the present cohort.
Fig. 1.
STDE-derived CFC categorization. The distribution of 148 LAD vessels across the two-dimensional map of hyperemic DPV (x-axis) and CFVR (y-axis) with four categories. (A) Pre-PCI CFC categorization. A total of 27 (18.2%, A1), 42 (28.4%, A2), 43 (29.1%, A3), and 36 (24.3%, A4) vessels were categorized as severely reduced, moderately reduced, mildly reduced, and normal STDE-derived CFC, respectively. (B) Post-PCI CFC categorization. A total of 7 (4.7%, B1), 22 (14.9%, B2), 41 (27.7%, B3), and 78 (52.7%, B4) vessels were categorized as severely reduced, moderately reduced, mildly reduced, and normal STDE-derived CFC, respectively. CFC, coronary flow capacity, CFVR, coronary flow velocity reserve; LAD, left anterior descending artery; STDE, stress transthoracic Doppler echocardiography; PCI, percutaneous coronary flow intervention; DPV, diastolic peak velocity.
CFC definition by STDE
The definition and categorization of CFC using PET-derived and invasive velocity wire-derived data have been previously described.1,23 According to the concept of CFC, categorizations were performed using CFVR and hDPV in this study. Since the cut-off threshold of CFVR and hDPV based on STDE measurements have not been established for CFC categorizations, we applied the following values, as previously described 1,4,6,24: normal STDE-CFC was defined as CFVR ≥ 2.8 with its corresponding hDPV ≥ 78 cm/s (14 percentiles); mildly reduced STDE-CFC as 2.1 < CFVR < 2.8 and the corresponding hDPV as 60 cm/s ≤ hDPV < 78 cm/s (14–37 percentiles); moderately reduced STDE-CFC as CFVR ≤ 2.1 and CFVR > 1.7 and the corresponding hDPV as 44 cm/s ≤ hDPV < 60 cm/s (37–66 percentiles); and severely reduced STDE-CFC was defined as CFVR ≤ 1.7 with corresponding hDPV < 44 cm/s (over 66 percentiles). According to these categories, delta STDE-CFC was defined as a numeric improvement in categories between pre- and post-PCI STDE-CFC (post-PCI minus pre-PCI: +3, indicated improvement from severely reduced to normal; STDE-CFC, + 2 indicated severely reduced to mildly reduced STDE-CFC or moderately reduced to normal STDE-CFC). Furthermore, as reported in previous CFC grading,1 we defined severely or moderately reduced STDE-CFC as ischemic STDE-CFC, and mildly reduced or normal STDE-CFC as non-ischemic STDE-CFC, in the present study. The same criteria for CFC categorizations were applied for the pre- and post-PCI physiological assessment. As for flow velocity improvement, an average increase in absolute coronary flow after PCI was reportedly 46% 25. We defined a > 50% increase in hDPV as an arbitrary surrogate metric for absolute higher-level coronary flow increase, while this cut-off value corresponded to the highest tertile of hDPV increase. In the present study, the average peak velocity (APV) of the whole cardiac cycle was also measured at basal conditions (bAPV) and during maximal hyperemia (hAPV) in 113/148 patients, while the APV was not available due to the difficulty to trace the flow waves in 35 patients. In the 113 patients, significant linear relationships were observed between “bDPV and bAPV”, and “hDPV and hAPV” (Supplemental Fig. 1). CFVR calculated by DPV and APV also showed significant linear relationship (Supplemental Fig. 1). As for another factor used in the CFC definition, not like strictly defined CFC thresholds for myocardial flow impairment introduced using PET11 and the intracoronary invasive CFC definition,1,26 as no such thresholds exist for hyperemic average maximum flow velocity, we matched maximum flow thresholds to well-defined CFR cut-offs according to the corresponding percentiles of hAPV. Thus, it is plausible to use hDPV for the CFC definition and classification in the present study, considering technical difficulties to trace flow waves of the whole cycle using STDE. The recent recommendation that STDE-derived CFVR should be expressed as the ratio of hyperemic to rest peak diastolic flow velocity has been also issued by the clinical consensus statement.27
Statistical analysis
Statistical analyses were performed using R version 4.3.0 (R Foundation for Statistical Computing, Vienna, Austria). Categorical data are expressed as numbers and percentages and were compared using the chi-square or Fisher’s exact tests, as appropriate. The normality of the distributed values was assessed using Shapiro–Wilk statistics. Continuous variables are presented as median (25th−75th percentile) since all the variables showed non-normal distributions and were compared by the Mann-Whitney U test. Associations were evaluated by analyzing Spearman’s correlation for non-normally distributed data. Differences in clinical, physiological, and angiographical variables according to CFC categories were compared by Kruskal-Wallis or chi-squared, or Fisher’s exact test. Receiver-operating characteristic (ROC) curve analysis was performed to compare variables to predict lesions with higher-level increase (> 50%) in coronary flow. Univariable and multivariable logistic regression analyses were performed to predict a higher-level coronary flow increase (> 50% increase). The associated variables with a P-value of < 0.10 in univariate analyses were entered in the multivariable model, and a forward stepwise regression method was used to fit the multivariable model. The Akaike information criterion was applied to test the model fitness to avoid over-fitting.
The P-value for linear trend was estimated by the association of STDE-CFC in ranks (severely, moderately, mildly reduced, and normal STDE-CFC) or delta STDE-CFC (ranging from − 3 to + 3) with flow increase in velocity or % increase. The discrimination ability of STDE-CFC was assessed by various nested logistic regression models: Model 1 included age, sex, hypertension, diabetes mellitus, hyperlipidemia, current smoking, angiographic minimum lumen diameter, and diameter stenosis; Model 2 included Model 1 plus pre-PCI FFR; Model 3 was Model 2 plus pre-PCI hDPV, Model 4 was Model 3 plus pre-PCI ischemic STDE-CFC. The discriminating improvements were evaluated by net reclassification improvement (NRI) and integrated discrimination improvement (IDI). Two expert investigators, blinded to the clinical and guidewire-based physiological data, assessed all STDE flow waves. Inter- and intraobserver variability of hDPV was assessed using intraclass correlation coefficients. A level of P < 0.05 was considered significant.
Results
Baseline patient characteristics and physiological findings
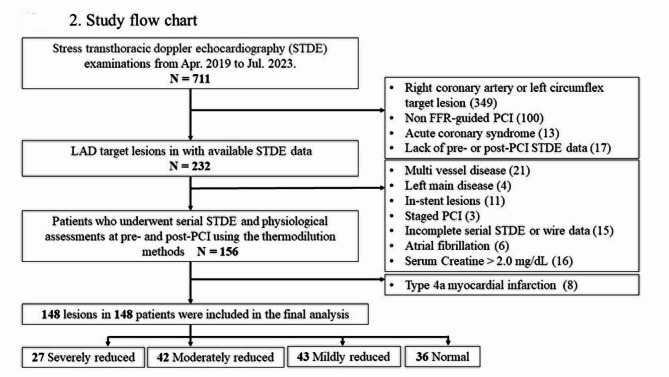
From the initially enrolled cohort of patients (N = 232), 93 individuals were excluded from the final analysis, due to multivessel disease (n = 21), left main lesion (n = 4), in-stent lesion (n = 11), staged PCI (n = 3), insufficient guidewire-based physiological data (n = 11), insufficient STDE data acquisition (n = 4), atrial fibrillation at the time of STDE examination (n = 6), and baseline serum creatinine levels exceeding 2.0 mg/dl (n = 16). Additionally, 8 patients who experienced type 4 A myocardial infarction were excluded. Consequently, the final analysis comprised of a total of 148 vessels (Fig. 2).
Fig. 2.
Study flow chart. CCS, chronic coronary syndrome; FFR, fractional flow reserve; LAD, left anterior descending artery; STDE, stress transthoracic Doppler echocardiography; PCI, percutaneous coronary intervention.
Supplemental Table 1 presents the patient characteristics and physiological parameters before and after PCI, stratified across STDE-CFC categories and based on ischemic and non-ischemic STDE-CFC. The pre-PCI FFR and S-TDE-derived CFVR were 0.66 ± 0.10 and 2.00 ± 0.67, respectively. Pre-PCI bDPV and hDPV were 29.0 ± 12.0 cm/s and 56.1 ± 23.7 cm/s, respectively. No significant difference in blood pressure and heart rate were observed pre- and post-PCI STDE examinations.
Before PCI, 27 (18.2%), 42 (28.4%), 43 (29.1%), and 36 (24.3%) patients were categorized as having severely reduced, moderately reduced, mildly reduced, and normal STDE-CFC status before PCI, respectively (Fig. 2). Thus, ischemic and non-ischemic STDE-CFC comprised 69 (46.6%) and 79 (53.4%) patients, respectively. As depicted in Supplemental Table 1, a worse STDE-CFC status generally correlated with a more adverse physiologic profile.
Coronary flow capacity and flow velocity changes following PCI
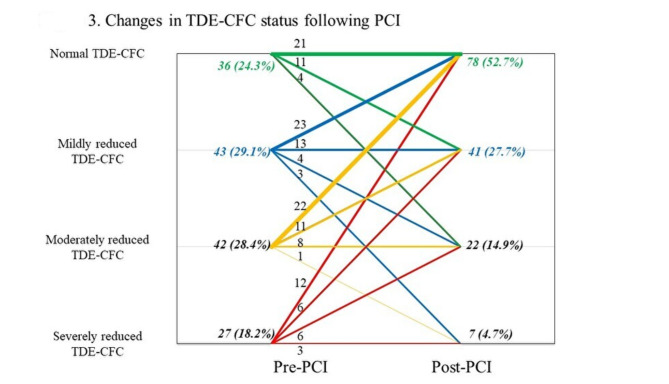
Post-PCI, the FFR and STDE-derived CFVR were 0.84 [0.80, 0.87] and 2.44 [1.97, 2.94], respectively. Improvement in FFR was observed in all cases, whereas 24.3% (36/148) of the total cohort exhibited a decrease in CFVR. Figure 3 shows the changes in STDE-CFC status following PCI.
Fig. 3.
Changes in STDE-CFC status following PCI. Changes in STDE-CFC following PCI in the current population. The plot visualizes the number of vessels regarding pre-PCI STDE-CFC and post-PCI STDE-CFC. Numbers indicate the number of vessels corresponding to the STDE-CFC changes, illustrated as the lines. Thickness of the lines reflects the number of vessels in each STDE-CFC change. The majority of the vessels improved their STDE-CFC status, while 15.5% (23/148) of patients showed worsened status. CFC, coronary flow capacity; STDE, stress transthoracic Doppler echocardiography; PCI, percutaneous coronary intervention.
In comparison to the pre-PCI STDE-CFC status, 54.1% (80/148) of patients demonstrated an improvement in STDE-CFC status, resulting in 52.7% (78/148) of patients attaining a post-PCI normal STDE-CFC status. The prevalence of vessels with ischemic CFC decreased from 46.6% (69/148) to 19.6% (29/148), after PCI. Generally, worse pre-PCI STDE-CFC status was associated with a more unfavorable post-PCI STDE-CFC status and a higher possibility of experiencing a substantial improvement in STDE-CFC status.
Supplemental Table 2 outlines the characteristics of patients with each delta STDE-CFC status. The degree of the changes in STDE-CFC status ranged from worsening to no change, + 1, +2, and + 3 category improvement. The number (%) of patients for changes in STDE-CFC status from worsening, no change, + 1, +2, + 3, respectively ranged from, 23 (15.5%), 45 (30.4%), 40 (27.0%), 28 (18.9%), and 12 (8.1%). No significant differences in clinical demographics were detected between these groups, while lower pre-PCI FFR, worse STDE-CFC, CFVR, and lower hDPV were associated with greater improvement in STDE-CFC status. Patients with worsening STDE-CFC were characterized as relatively higher pre-PCI FFR, CFVR and hDPV. (Supplemental Table 2)
The median pre-PCI hDPV was 52.5 [41.0, 67.3] cm/s and increased to 70.0 [55.0, 84.0] cm/s following PCI (P < 0.001). In total, 33.8% of patients (50/148) exhibited an hDPV increase of more than 50% after PCI, while 20.9% of patients (31/148) experienced a decrease in hDPV despite FFR improvement. No significant difference in post-PCI peak cardiac troponin levels was detected in patients with, versus, without higher-level hDPV increase (> 50%) after PCI (358 [114, 1138] vs. 373 [174, 1275] pg/mL, P = 0.423).
When patients were stratified into pre-PCI ischemic and non-ischemic STDE-CFC groups, 63.8% (44/69) from the ischemic and 7.6% (6/79) from the non-ischemic STDE-CFC group demonstrated a higher-level coronary flow increase after PCI (P < 0.001, Supplemental Table 1). Patients in the pre-PCI ischemic STDE-CFC group exhibited greater angiographic stenosis, lower FFR, CFR, and STDE-derived CFVR compared with non-ischemic group (Supplemental Table 1). No significant differences in post-PCI physiological parameters were observed between pre-PCI ischemic versus non-ischemic STDE-CFC groups (Supplemental Table 1).
Supplemental Table 3 shows clinical, angiographic, and physiological factors according to the post-PCI STDE-CFC categorization. Clinical demographics, angiographic findings and pre-PCI physiological parameters showed no significant differences between post-PCI STDE-CFC status, except that pre-PCI hDPV was slower in lesions with ischemic CFC. Post-PCI FFR showed no significant differences between post-PCI STDE-CFC status, post-PCI basal and hyperemic mean transit time tended to be longer, and post-PCI index of microcirculatory resistance tended to be higher in lesions with post-PCI ischemic STDE-CFC, compared with lesions with post-PCI non-ischemic STDE-CFC. Regarding post-PCI STDE-derived parameters, bDPV was not associated with post-PCI CFC status.
Baseline patient characteristics showed no significant differences between patients with versus without higher-level hDPV increase (> 50%) (Supplemental Table 4). As for pre-PCI physiological parameters, FFR was low, hyperemic mean transit time was longer, CFR tended to be lower, the index of microcirculatory resistance tended to be higher, hDPV and CFVR was lower in patients with higher-level hDPV increase (Supplemental Table 4).
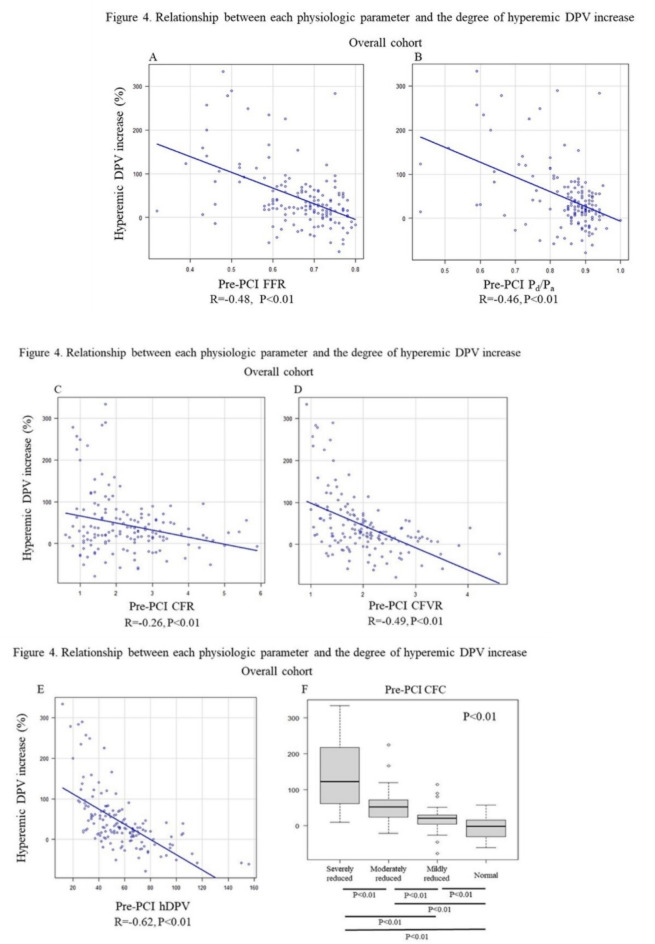
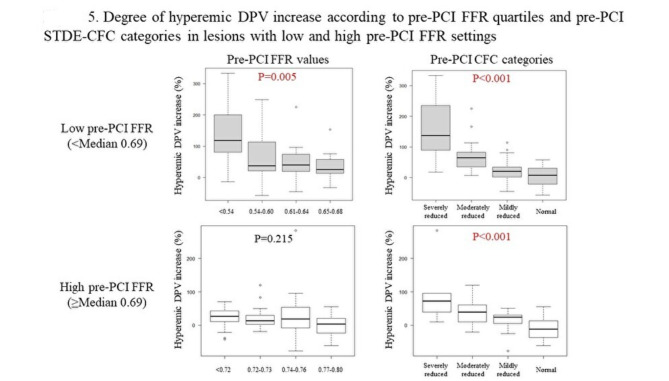
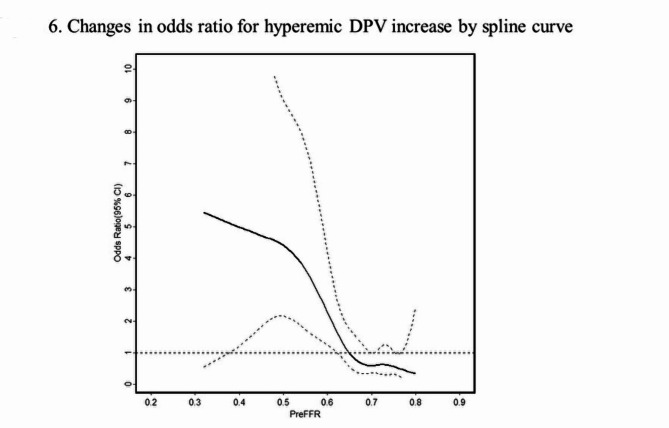
Figure 4 illustrates the relationship between pre-PCI FFR, basal Pd/Pa, CFR, CFVR, hyperemic DPV, STDE-CFC categorization and relative %hDPV increase, respectively. Notably, when vessels are divided into two groups by the median value of pre-PCI FFR (0.69), pre-PCI STDE-CFC categorization maintained the ability to discriminate lesions which achieved higher-level hyperemic DPV increase (> 50%) in both groups, while FFR lost discriminating ability in higher FFR vessels (Fig. 5). Pre-PCI FFR value was inversely associated with the degree of hDPV increase in vessels with FFR < 0.69 (median), while no significant association was observed in vessels with FFR ≥ 0.69 (Supplemental Fig. 2). An ROC curve analysis showed that the best cut-off value of pre-PCI FFR to predict higher-level hDPV increase was 0.67 with c-statistics (95% confidence interval [CI]) of 0.71 (0.61–0.80). The spline curve analysis showed possible hDPV increase (> 50%) for the vessels of pre-PCI FFR lower than about 0.65 (Fig. 6).
Fig. 4.
Relationship between each physiologic parameter and the degree of hyperemic DPV increase. Scatterplots show inverse relationships between pre-PCI FFR (A), basal Pd/Pa (B), CFR (C), CFVR (D), hyperemic DPV (E). Boxplots show a graded degree of hyperemic DPV increase among STDE-derived CFC categories. CFC, coronary flow capacity; CFR, coronary flow reserve; CFVR, coronary flow velocity reserve; FFR, fractional flow reserve; DPV, diastolic peak velocity; STDE, stress transthoracic Doppler echocardiography; Pa, aortic pressure; PCI, percutaneous coronary intervention; Pd, distal coronary pressure.
Fig. 5.
Degree of hyperemic DPV increase according to pre-PCI FFR quartiles and pre-PCI STDE-CFC categories in lesions with low and high pre-PCI FFR settings. Vessels are divided into two groups by the median value of pre-PCI FFR (0.69). Pre-PCI STDE-CFC categorization maintained the ability to discriminate lesions, which potentially achieve higher-level hyperemic DPV increase (> 50%) in both groups, while FFR lost discriminating ability in higher FFR vessels. CFC, coronary flow capacity; DPV, diastolic peak velocity; FFR, fractional flow reserve; STDE, stress transthoracic Doppler echocardiography; PCI, percutaneous coronary intervention.
Fig. 6.
Changes in odds ratio for hyperemic DPV increase by spline curve. The spline curve analysis showed a possible hyperemic DPV increase (> 50%) for the vessels of pre-PCI FFR lower than 0.65. DPV, diastolic peak velocity; FFR, fractional flow reserve; PCI, percutaneous coronary intervention.
Predictors of higher-level coronary flow increase and determinants of hDPV change after PCI
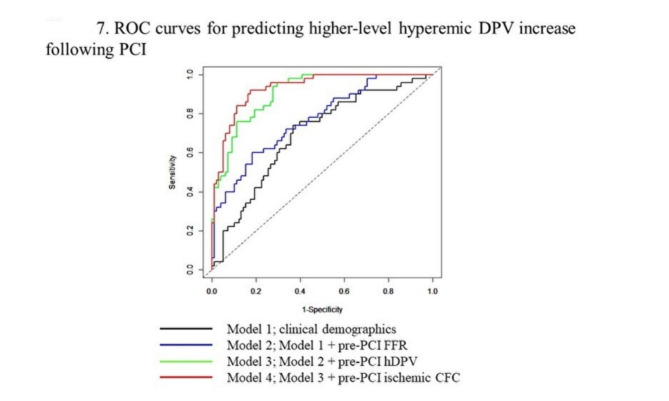
Predictors of a higher-level coronary flow increase (> 50% hDPV increase) were assessed using uni- and multivariable logistic regression analyses (Table 1). Multivariable logistic regression analysis showed that pre-PCI hDPV (odds ratio: 0.92, 95% CI: 0.88–0.97. P < 0.01) and ischemic CFC status (odds ratio: 7.85, 95% CI: 2.19–28.2. P < 0.01) were independently predictive of a higher-level hyperemic DPV increase. Supplemental Tables 5 and Fig. 7 show the prediction of higher-level hDPV increase (> 50%) by various nested models for improvement in the discrimination. The reference Model 1 consisted of basic clinical demographics and angiographic findings. It showed a c-statistics (95% CI) of 0.69 (0.60–0.78) and the addition of pre-PCI FFR and pre-PCI hDPV improved the discrimination step-by-step. Model 4, additionally including pre-PCI ischemic STDE-CFC, showed better discrimination ability compared with the model without ischemic CFC (Supplemental Table 5, Fig. 7).
Table 1.
Predictors of higher-level coronary hyperemic DPV increase after PCI (> 50% increase).
| Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Pre-PCI FFR, per 0.01 | 0.92 | 0.89, 0.96 | < 0.01 | 0.95 | 0.90, 0.97 | 0.08 |
| Pre-PCI hyperemic Tmn, s | 4.84 | 1.69, 13.9 | < 0.01 | -− | − | − |
| Pre-PCI CFR | 0.71 | 0.50, 1.00 | 0.048 | − | − | − |
| Pre-PCI corrected IMR | 1.03 | 1.00, 1.05 | 0.02 | − | − | − |
| Pre-PCI hyperemic DPV, cm/s | 0.89 | 0.86, 0.93 | < 0.01 | 0.92 | 0.88, 0.97 | < 0.01 |
| Pre-PCI CFVR | 0.11 | 0.04, 0.26 | < 0.01 | − | − | − |
| Pre-PCI HMR | 10.7 | 3.84, 29.8 | < 0.01 | − | − | − |
| Pre-PCI ischemic CFC | 21.4 | 8.15, 56.3 | < 0.01 | 7.85 | 2.19–28.2 | < 0.01 |
DPV, diastolic peak velocity; PCI, percutaneous coronary intervention; OR, odds ratio; CI, confidence interval; FFR, fractional flow reserve; Tmn, transit time; CFR, coronary flow reserve; IMR, index of microcirculatory resistance; CFVR, coronary flow velocity reserve; HMR, hyperemic microvascular resistance; CFC, coronary flow capacity.
Fig. 7.
ROC curves for predicting higher-level hyperemic DPV increase following PCI. The ROC curves showing the prediction of hyperemic DPV increase by clinical demographics (model 1, black), model 1 plus pre-PCI FFR (model 2, blue), model 2 plus pre-PCI hyperemic DPV (model 3, green), and model 3 plus pre-PCI ischemic STDE-CFC (model 4, red). CFC, coronary flow capacity, FFR, fractional flow reserve; LAD, left anterior descending artery; STDE, stress transthoracic Doppler echocardiography; PCI, percutaneous coronary intervention; ROC, receiver operating characteristic; DPV, diastolic peak velocity.
STDE measurement reproducibility
The reproducibility of hDPV measurements was satisfactory in the interobserver (intraclass correlation: 0.80) and intraobserver (intraclass correlation: 0.78) reproducibility analysis.
Discussion
This study is the first to categorize vessels into CFC classification before and after elective PCI using STDE, and to evaluate the impact of PCI on changes in CFC status and coronary flow velocity. We further assessed the effectiveness of pre-PCI STDE-CFC to predict coronary flow increase associated with PCI in comparison with FFR. In patients undergoing elective FFR-guided PCI, the essential findings are as follows: (1) the prevalence of the severely reduced, moderately reduced, mildly reduced, and normal pre-PCI CFC categories using STDE were 18%, 28%, 29% and 24%, respectively. After PCI, severely reduced, moderately reduced, mildly reduced, and normal STDE-CFC values were 5%, 15%, 28%, and 53%, respectively. Ischemic CFC, defined as a composite of moderately and severely reduced CFC, comprised of 46.6% (69/148) cases before PCI, while the frequency reduced to 19.6% (29/148) of cases after PCI; (2) PCI provided an increased hyperemic coronary flow in 79% patients documented by STDE-derived flow velocity, while FFR improvement was achieved in all patients, indicating that FFR improvement was not associated with the coronary flow increase of 21% in the patients.
Furthermore, (3) the mean hDPV increase after PCI was 45 ± 72% and 34% of the total study patients, showed a higher-level (> 50%, highest tertile) increase after PCI. Ischemic pre-PCI STDE-CFC territories showed higher-level coronary flow increase in 64% of the target territories, while only 8% in non-ischemic pre-PCI STDE-CFC territories exhibited flow increase after PCI; (4) changes in STDE-CFC category status were 5% of −2, 11% of −1, 30% of 0, 27% of + 1, 19% of + 2, and 8% of + 3; (5) reduced pre-PCI STDE-CFC tended to show a greater improvement in the STDE-CFC category, and had a greater increase in coronary flow; and (6) pre-PCI ischemic STDE-CFC provided incremental value, adding to baseline clinical demographics, the pre-PCI FFR, and pre-PCI hDPV for the prediction of higher-level coronary flow increase (> 50%) after PCI; (7) ischemic post-PCI STDE-CFC was associated with lower post-PCI STDE-derived hyperemic coronary flow and higher microvascular resistance in LAD territory, while post-PCI FFR was non-different between ischemic and non-ischemic post-PCI STDE-CFC status.
To the best of our knowledge, the present study is the first to demonstrate that non-invasive pre-PCI STDE examination can categorize CFC status of LAD territories undergoing elective PCI, and its predictability for coronary flow increase after PCI. We also assessed the changes in STDE-CFC status after PCI and observed that pre-PCI STDE-CFC categorization could discriminate coronary flow increase after PCI, more precisely, compared with pre-PCI FFR. Serial STDE can noninvasively evaluate a change in coronary flow in LAD following PCI at low cost and low risk. Our results are merely hypothesis generating and prospective large-scale study is needed to test our results. STDE may be an ideal tool to noninvasively measure serial changes in coronary flow after PCI without an effect of acute flow turbulence associated with PCI. Comprehensive assessment using STDE and FFR may further optimize PCI for the benefit obtained by an increase in coronary flow.
Rationale of revascularization and clinical implications of STDE CFC
Revascularization is most fundamentally expected to improve coronary flow limitation in an ischemic myocardium by relief or bypass of epicardial coronary artery stenosis. Coronary intervention without this rationale would not improve outcomes in CCS patients and rather potentially be harmful by the exposure of patients to procedure-related, stent-related, and bleeding risks due to dual antiplatelet therapy or a combination of anticoagulation therapies in patients with atrial fibrillation. A recent study using STDE showed a decrease in the peak flow in 20% of vessels treated with successful and uncomplicated PCI for LAD lesions.20 In the present study, 20.9% of patients showed a decrease in hyperemic peak diastolic flow in LAD after successful PCI, while FFR improved in all cases, which was in line with the previously reported results.20 In the territories showing a lower pre-PCI myocardial resistance (MR) under the presence of epicardial stenosis, the microvasculature might already be fully dilated; thus, coronary flow may not increase after PCI, despite the anatomic reduction of epicardial stenosis. In these territories, pre-PCI hyperemic coronary flow is already high, and a potential coronary flow increase after PCI might be limited by a responsive increase in MR with no capability for further dilation. These territories may be described as nonflow limiting territories despite their pre-PCI FFR values, and PCI might decrease coronary flow because of increased hyperemic MR. In addition, considering that the extent and severity of induced myocardial ischemia have been proposed to be the most important contributing factors for worse prognosis10,28, coronary intervention without an expected higher-level increase in coronary flow or reduced ischemia may not be expected to improve patient outcomes. Considering the recent clinical trials including ISCHEMIA29 and REVIVED-BCIS230, demonstrating that revascularization did not provide further benefit compared with guideline directed medical therapy, current PCI indication in patients with CCS could be further optimized. In this regard, PCI indication strongly requires the physiological basis of coronary flow increase as our results and previously reported data consistently indicate that FFR-guided PCI does not necessarily increase coronary flow in a non-negligible portion of patients.20,21,31 The present study indicated that the contemporary FFR-guided PCI provided worsened STDE-CFC and non-improved STDE-CFC in 15.5% (23/148) and 45.9% (68/148) of patients, respectively. Our results also demonstrated that conducting pre-PCI CFC assessments help identify lesions as candidates for revascularization, which most likely associates beneficial impact by providing higher-level coronary flow increase. In this context, although FFR also captures the epicardial stenosis severity, it may not directly indicate the status of coronary flow impairment, including microvascular function. In this study, pre-PCI FFR showed no predicting power when pre-PCI FFR was larger than 0.65. Therefore, reliable and widely available methods to identify lesions or guide decision-making to optimize PCI are necessary, preferably by non-invasive pre-PCI testing. Our results suggested that STDE-derived CFC when combined with FFR, which can also be obtained non-invasively using FFR-CT, could integrate the information of hyperemic coronary flow impairment and myocardial ischemia. Furthermore, this would help identify patients who could potentially benefit from coronary flow increase through PCI. Further multicenter large sample size studies are required to test our hypothesis, and to assess the association between coronary flow increase and better prognosis. STDE can also measure microvascular function and cardiac diastolic function. Coronary flow is theoretically directly linked with microvascular resistance. Therefore, noninvasive assessment using STDE may elucidate not only the relationship between coronary flow and prognosis but the relationship between flow, microvascular resistance, and heart failure associated with myocardial ischemia in future studies.32
Limitations
This study has several important limitations, which should be taken into consideration. First, the present study evaluated a small number of patients, and carried the inherent limitations due to its small sample size, single-center study, and observational nature. Our small sample size precluded extensive subgroups analyses and a selection bias cannot be canceled. Second, in the STDE examination, pre- and post-PCI LAD flow data were comparable only when measured at identical positions and constant vessel diameters under similar hemodynamics. These issues were carefully addressed by ensuring the exact echo-probe positioning and maintaining the specified timeframe for examination (around 10 am for both pre- and post-PCI STDE). Nevertheless, we cannot exclude the possibility of a change in the diameter of the LAD or a difference in the echo probe positioning during flow measurement. Third, the absolute coronary flow volume was not assessed in this study. The limited coronary flow volume in the ischemic region may also depend on the microvascular dysfunction. Further studies using absolute flow and microvascular resistance measurements are needed to test our results. However, the main strength of the present study was the paired comparison performed for serial STDE examinations of the proximal LAD lesions for each patient, which might at least partially alleviate this limitation. Fourth, coronary flow may serially change after PCI, and our results are based on a one-time window of a median of 3 days after PCI. Further studies are required to serially quantify the changes in coronary flow and/or microvascular resistance at different time windows. Fifth, inter-rater variability in TTE evaluation cannot be completely eliminated. However, the depiction of the LAD by TTE generally exceeds 80%, and in our laboratory, it reaches nearly 95%. Furthermore, examiners acquire a certain level of skill with a 3 to 6 month training period, achieving inter-rater and intra-rater reliability of 0.92 and 0.94, respectively. A previous studies have reported an interobserver agreement for CFVR evaluation of 90%, suggesting that a similar level of quality can be expected in other facilities.33 Sixth, no prognostic data was provided in the present study. Seventh, approximately 8% of patients were not adequately evaluated by echocardiography, which may have influenced the results. Finally, and most importantly, future studies should test whether changes in coronary flow in ischemic regions after PCI could provide prognostic information.
Conclusions
STDE-CFC provided pre- and post-PCI CFC categorization in more than 90% of patients undergoing de novo functionally significant LAD PCI. Ischemic CFC (composite of severely reduced and moderately reduced CFC) decreased to 19.6% from 46.6% after uncomplicated successful PCI. The contemporary FFR-guided PCI provided coronary flow increase in about 79% of patients after successful LAD PCI, while coronary flow decrease was observed in 21% of patients despite of FFR improvement in all cases. The noninvasively obtained pre-PCI STDE-derived CFC categorization yielded incremental predictive information over pre-PCI FFR for a higher-level increase (> 50%, the highest tertile) in coronary flow after PCI, which might support our decision to optimize revascularization. We could further optimize beneficial PCI by integrating FFR and STDE-derive data. Post-PCI ischemic STDE status may provide unfavorable coronary flow status after PCI and may help identify patients at high risk for worse outcomes after PCI, although this hypothesis needs to be tested in future studies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank all the clinical technicians in the Department of Clinical Laboratory at Tsuchiura Kyodo General Hospital for their assistance in obtaining the echocardiographic data of the patients involved in the study.
Author contributions
H.U, E.U, and T.K contributed to the conception and design of this study.M.H, Y.K, T.S, M.H, T.N, Y.H, K.N, M.S, K.S, T.T, K.M, R.S, T.S, H.S, T.W, T.M, and T.Y made effort to enroll the patients and analyzed. H.U, E.U, and T.K conducted the analysis and interpretation of the data.H.U, E.U, and T.K drafted the manuscript.All authors approved this manuscript prior to submission.
Data availability
The datasets used and analysed during the current study available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hiroki Ueno and Eisuke Usui contributed equally to this work.
References
- 1. Van De Hoef, T. P. et al. Diagnostic and prognostic implications of coronary flow capacity: A comprehensive cross-modality physiological concept in ischemic heart disease. JACC Cardiovasc. Interv.8, 1670–1680 (2015). [DOI] [PubMed]
- 2. Hamaya, R. et al. Diagnostic and Prognostic Efficacy of Coronary Flow Capacity Obtained Using Pressure-Temperature Sensor–Tipped Wire–Derived Physiological Indices. JACC Cardiovasc. Interv.11, 728–737 (2018). [DOI] [PubMed]
- 3. Johnson, N. P. & Gould, K. L. Integrating noninvasive absolute flow, coronary flow reserve, and ischemic thresholds into a comprehensive map of physiological severity. JACC Cardiovasc. Imaging5, 430–440 (2012). [DOI] [PubMed]
- 4. Hamaya, R. et al. Clinical outcomes of fractional flow reserve-guided percutaneous coronary intervention by coronary flow capacity status in stable lesions. EuroIntervention17, 301–308 (2021). [DOI] [PMC free article] [PubMed]
- 5. Gould, K. L. & Johnson, N. P. Coronary Physiology Beyond Coronary Flow Reserve in Microvascular Angina: JACC State-of-the-Art Review. J. Am. Coll. Cardiol.72, 2642–2662 (2018). [DOI] [PubMed]
- 6. Hamaya, R. et al. Differential Impact of Coronary Revascularization on Long-Term Clinical Outcome According to Coronary Flow Characteristics: Analysis of the International ILIAS Registry. Circ. Cardiovasc. Interv.15, E011948 (2022). [DOI] [PubMed]
- 7. Murai, T. et al. Coronary flow capacity to identify stenosis associated with coronary flow improvement after revascularization: A combined analysis from define flow and ideal. J. Am. Heart Assoc.9, (2020). [DOI] [PMC free article] [PubMed]
- 8. De Winter, R. W. et al. The impact of coronary revascularization on vessel-specific coronary flow capacity and long-term outcomes: a serial [15O]H2O positron emission tomography perfusion imaging study. Eur. Heart J. Cardiovasc. Imaging23, 743–752 (2022). [DOI] [PMC free article] [PubMed]
- 9. Kimura, T. et al. Cost analysis of non-invasive fractional flow reserve derived from coronary computed tomographic angiography in Japan. Cardiovasc. Interv. Ther.30, 38–44 (2015). [DOI] [PMC free article] [PubMed]
- 10. Hachamovitch, R., Hayes, S. W., Friedman, J. D., Cohen, I. & Berman, D. S. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation107, 2900–2906 (2003). [DOI] [PubMed]
- 11. Ankur Gupta, MD, Viviany R. Taqueti, T. P. van de H. et al. Integrated Non-invasive Physiological Assessment of Coronary Circulatory Function and Impact on Cardiovascular Mortality in Patients with Stable Coronary Artery Disease. Circulation136, 2325–2336 (2017). [DOI] [PMC free article] [PubMed]
- 12. Aikawa, T. et al. Improved regional myocardial blood flow and flow reserve after coronary revascularization as assessed by serial 15O-water positron emission tomography/computed tomography. Eur. Heart J. Cardiovasc. Imaging21, 36–46 (2020). [DOI] [PubMed]
- 13. Bober, R. M. et al. The impact of revascularization on myocardial blood flow as assessed by positron emission tomography. Eur. J. Nucl. Med. Mol. Imaging46, 1226–1239 (2019). [DOI] [PMC free article] [PubMed]
- 14. Lance Gould, K. et al. Regional, artery-specific thresholds of quantitative myocardial perfusion by PET associated with reduced myocardial infarction and death after revascularization in stable coronary artery disease. J. Nucl. Med.60, 410–417 (2019). [DOI] [PMC free article] [PubMed]
- 15. Ciampi, Q. et al. Functional, Anatomical, and Prognostic Correlates of Coronary Flow Velocity Reserve During Stress Echocardiography. J. Am. Coll. Cardiol.74, 2278–2291 (2019). [DOI] [PubMed]
- 16. Ponikowski, P. et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J.37, 2129-2200m (2016).
- 17. Maddox, T. M. et al. 2021 Update to the 2017 ACC Expert Consensus Decision Pathway for Optimization of Heart Failure Treatment: Answers to 10 Pivotal Issues About Heart Failure With Reduced Ejection Fraction: A Report of the American College of Cardiology Solution Set Oversig. J. Am. Coll. Cardiol.77, 772–810 (2021). [DOI] [PubMed]
- 18. Levine, G. N. et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention. J. Am. Coll. Cardiol.58, e44–e122 (2011). [DOI] [PubMed]
- 19. Thygesen, K. et al. Fourth Universal Definition of Myocardial Infarction (2018). Circulation vol. 138 (2018). [DOI] [PubMed]
- 20. Yamaguchi, M. et al. Preprocedural transthoracic Doppler echocardiography to identify stenosis associated with increased coronary flow after revascularisation. Sci. Rep.12, 1–10 (2022). [DOI] [PMC free article] [PubMed]
- 21. Hada, M. et al. Early effect of percutaneous coronary intervention of non-left anterior descending artery on coronary flow velocity reserve of left anterior descending artery assessed by transthoracic Doppler echocardiography. PLoS One16, 1–12 (2021). [DOI] [PMC free article] [PubMed]
- 22. Lang, R. M. et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr.28, 1–39.e14 (2015). [DOI] [PubMed]
- 23. Johnson, N. P., Kirkeeide, R. L. & Gould, K. L. Is discordance of coronary flow reserve and fractional flow reserve due to methodology or clinically relevant coronary pathophysiology? JACC Cardiovasc. Imaging5, 193–202 (2012). [DOI] [PubMed]
- 24. Stegehuis, V. E. et al. Impact of clinical and haemodynamic factors on coronary flow reserve and invasive coronary flow capacity in non-obstructed coronary arteries: A patient-level pooled analysis of the DEBATE and ILIAS studies. EuroIntervention16, E1503–E1510 (2021). [DOI] [PMC free article] [PubMed]
- 25. Johnson, N. P. & Gould, K. L. Physiological basis for angina and ST-segment change: PET-verified thresholds of quantitative stress myocardial perfusion and coronary flow reserve. JACC Cardiovasc. Imaging4, 990–998 (2011). [DOI] [PubMed]
- 26. Ullrich, H. et al. Coronary Venous Pressure and Microvascular Hemodynamics in Patients with Microvascular Angina: A Randomized Clinical Trial. JAMA Cardiol.8, 979–983 (2023). [DOI] [PMC free article] [PubMed]
- 27. Picano, E. et al. The clinical use of stress echocardiography in chronic coronary syndromes and beyond coronary artery disease: A clinical consensus statement from the European Association of Cardiovascular Imaging of the ESC. Eur. Heart J. Cardiovasc. Imaging25, E65–E90 (2024). [DOI] [PubMed]
- 28. Tonino, P. A. L. et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N. Engl. J. Med.360, 213–24 (2009). [DOI] [PubMed]
- 29. Study, I., Effectiveness, C. H. & Approaches, I. Protocolo Ischemia. 124–135 (2019) doi:10.1016/j.ahj.2018.04.011.International.
- 30. Perera, D. et al. Percutaneous Revascularization for Ischemic Left Ventricular Dysfunction. N. Engl. J. Med.387, 1351–1360 (2022). [DOI] [PubMed]
- 31. Kanaji, Y. et al. Effect of Elective Percutaneous Coronary Intervention on Hyperemic Absolute Coronary Blood Flow Volume and Microvascular Resistance. Circ. Cardiovasc. Interv.10, 1–10 (2017). [DOI] [PubMed]
- 32. Shah, S. J. et al. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur. Heart J.39, 3439–3450 (2018). [DOI] [PMC free article] [PubMed]
- 33. Winter, R. et al. Feasibility of noninvasive transthoracic echocardiography/doppler measurement of coronary flow reserve in left anterior descending coronary artery in patients with acute coronary syndrome: a new technique tested in clinical practice. J Am Soc Echocardiogr.16, 464–468. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analysed during the current study available from the corresponding author on reasonable request.