Summary
Background
Advances in neonatal care have increased survival rates for premature or low birth weight (LBW) infants but raised concerns about long-term neurosensory and psychomotor challenges. Objective: to investigate perinatal factors linked to visual and auditory problems in ex-preterm or LBW young adults, assessing their long-term quality of life.
Methods
Participants from a 20-year-old randomised controlled trial comparing Kangaroo-Mother Care (KMC) to conventional care were re-enrolled. A group of 50 at term individuals without risk factors was assessed as a reference group.
Findings
5.9% of participants had functional visual issues and 8.1% experienced hearing problems. Those with hearing or visual impairments had longer hospital stays and more neonatal complications. Correlations were found between Griffiths auditory sub-scale results at 6 months and long-term auditory outcomes. Only 27.5% of those with deafness had access to cochlear implants or hearing aids, resulting in lower IQ scores, learning difficulties, and increased risk of depression and self-harm. Participants with visual impairments exhibited lower IQ scores, self-esteem, and HOME test acceptance. However, they did not differ from the group with normal vision in terms of quality of life, depression, or attachment scores. All participants, whether they had issues or not, rated their quality of life higher than their parents did.
Interpretation
Preterm or LBW infants with visual and hearing deficits are more likely to face cognitive and emotional challenges in adulthood. This study underscores the importance of a multidisciplinary approach to promptly address these vulnerabilities, reducing the risk of long-term neurodevelopmental and functional issues.
Funding
The Grand Challenge Canada, Fulbright Colciencias and Colombia Cientifica – Alianza, The World Bank, managed by the Colombian Administrative Department of Science, Technology and Innovation (COLCIENCIAS).
Keywords: Infant, Preterm, Low birth weight, Sequelae, Audition, Vision, Cohort study, Kangaroo-mother care method
Research in context.
Evidence before this study
According to a 2023 UN report, the global average prematurity rate has remained constant at 10% for more than a decade. These fragile children are responsible for 60–70% of neonatal and infant mortality worldwide, and probably more than 50% of cognitive and behavioural problems in early childhood. Over the last decade, developing countries have implemented high-tech neonatal units that have increased survival rates for premature or low birth weight (LBW) infants but raised concerns about long-term neurosensory and neuro-psychomotor challenges. Preterm or LBW babies face a higher risk of visual problems, hearing loss and impaired auditory processing, which can affect their overall quality of life, academic performance, language skills, and intelligence quotient (IQ) at adulthood and these sensorial issues are often diagnosed late.
In Latin America, little is known about the long-term follow-up of infants born prematurely or with LBW. Since 1978, the Kangaroo-Mother Care (KMC) method has been practiced in Colombia as an alternative for the management of these vulnerable infants, and since 1993 the Kangaroo Foundation has been working on the improvement and dissemination of this method based on the kangaroo position, exclusive breastfeeding as far as possible and early discharge home with strict outpatient follow-up.
Between 1994–1996, the Kangaroo Foundation research group carried out the first Randomized Controlled Trial (RCT) on the KMC method. The short- and medium-term results on mortality, morbidity, mother-child relationship, and somatic and neuro-psychomotor development were published in 1997–2001 and used in all the systematic reviews that now show the multiple benefits of this intervention.
In 2012 our research group aimed at evaluating the long-term results, by re-enrolling 20 years later the same cohort than in the 1994–1996 RCT and carrying out a complete cross-sectional study that looked not only at the physical condition of the patients, but also at their social integration, quality of life, psychological, neurological, mental health and sensory status. We have been analyzing and publishing the results of this large project and dataset over the past few years.
The main goal of our study is to assess long term hearing and visual impairment in preterm and LBW population, associated risk factors and consequences, as well as the probable impact of these issues in the quality of life of this population.
Added value of this study
In this paper we focus on the results of the sub-sample of young adults ex-preterm/LBW with hearing or visual problems from our original RCT (14%). We analyzed the impact of perinatal factors and follow-up variables in the first year of life on the onset of visual and hearing problems at age 20, and looked at quality of life, IQ and physical and mental health at age 20 in the disabled group. The results of this study stress the need for rigorous follow-up for all preterm/LBW infants to minimize neurosensory, cognitive, and motor sequelae affecting quality of life and developmental potential of this population. The availability of technology and its possible misuse have long-term implications that cannot be recovered. The state and resources in use 20 years ago in neonatology in Colombia are like the ones found in many low-income countries that are beginning to implement modern neonatology technologies. The lessons learned from this study, and over 20 years of KMC research and practice provide a clear path to not repeat historical errors, and to introduce modern technology, hand-in-hand with the humanization of the care, the non-separation from the mother and continuous KMC from birth whenever possible with strict monitoring for at least the first 2 years of life. This while also being cognizant that for the highest risk group detected by the KMC follow up should receive further follow-up and support should be provided at least until adolescence.
Implications of all the available evidence
The results of the 20-year follow up of young adults ex-preterm/LBW with hearing or visual problems from our original RCT (14%) reveals many critical useful lessons. For the group where hearing problems were detected 20 years ago; the associated factors were linked to their stay in the neonatal care unit, having received aminoglycosides, oxygen, phototherapy, parenteral nutrition and having been ventilated aggressively. On the other hand, for the group where visual problems were detected, the main factors associated with the appearance of visual problems at 20 years were immaturity and having received phototherapy.
Despite the 12-month follow-up to detect hearing and visual problems, quality of life in adulthood is not up to standard, there are cognitive problems, and as a consequence learning problems and difficulties in school that reduce the potential in adulthood life. Our results support that strict multidisciplinary follow-up is essential to early detection of development problems with the aim to enhance brain plasticity and ensure a better quality of life. Only 27% of the young adults with deafness had access to cochlear implants and often no had rehabilitation. Half of young adults with visual problems didn’t wear their glasses. This can be linked to essentially non-existent governmental support. This study, presenting the first long-term outcomes in our country at 20 years of age, underscores the importance of shifting from ensuring survival to ensuring a better quality of life for this vulnerable population.
This is a message for all organizations that support development. We know that technology alone, focused only on survival, will not have the desired impact, quality of this survival must be equally the challenge. The rights of premature/LBW infants are the same, whatever their race or origin or place of birth. We must do better, and we hope our results can contribute to knowledge about the follow-up of premature/LBW children and inform available current and future preventive interventions.
Introduction
Preterm birth occurs when a baby is born <37 weeks of gestational age (GA), and low birth weight (LBW) refers to babies born weighing <2500 g. Globally 15 million preterm and LBW babies are born each year, constituting around 11% of all births, and one million of these infants will not survive beyond their fifth birthday, making it the leading cause of mortality under 5.1,2 Up to three-quarters of these deaths could be prevented through cost-effective interventions.3
Determining the global prevalence of preterm births is complex. In Colombia, a 2021 report from the Health Ministry estimated that around 10.85% of births were preterm, and 4% full-term with LBW.4
In recent decades, substantial progress has been made in neonatal care.5,6 Improved survival rates for premature and LBW infants, however, might come with an increase in long-term health issues, including sensory and developmental problems, which can lead to chronic challenges like hearing and vision deficits.5,7 Notably, there is limited data on these issues in adulthood, despite significant research into childhood neurosensory problems.8 For example, a study in Japan involving 40,728 very LBW patients found that 7.1% had cerebral palsy, 1.8% experienced blindness, and 0.9% had hearing impairment.6
Preterm or LBW babies face a higher risk of hearing loss and impaired auditory processing, which can affect their overall quality of life, academic performance, language skills, and intelligence quotient (IQ).9 These hearing issues are often diagnosed late, typically after the age of 2.10 The estimated incidence of sensorineural deafness ranges from 1.2 to 5.7 cases per 1000 live births, emphasizing the urgent necessity for universal and early screening, as recommended by the American Academy of Pediatrics.11 Deafness is 10–20 times more prevalent in infants in Neonatal Intensive Care Units (NICUs) than in the general population.9 The global population with hearing disabilities has grown substantially, with 60% of deafness cases being preventable. Birth complications, prematurity, and LBW contribute to 17% of these cases.11 In Colombia, despite mandatory neonatal hearing screening programs, those are not widely available.12
Visual impairments are also common among preterm and LBW infants, often linked to a condition called retinopathy of prematurity (ROP). In 2019 ROP caused 101.6 thousand cases of vision impairment CI 95% (77.5–128.2). In Latin America published prevalence of severe ROP, enough to require treatment ranged from 1.2 to 23.8%13, 14, 15, 16, 17
This study aimed to investigate visual and auditory impairment in a group of former preterm and LBW infants born in 1994. They were closely monitored throughout their first year in a high-risk follow-up program and assessed again at ages 18–20 through comprehensive clinical and psychological evaluations. Looking at this high-risk group recovered in adulthood we intend to understand whether different grades of deafness and blindness or refractive impairments associated with perinatal noxae can be identified early through high-risk outpatient follow-up, ultimately trying to attenuate sequelae that may occur in adulthood.
Methods
Study design
Retrospective cohort study involved young adults born with LBW (≤2000 g, 87.2% < 37 weeks of GA). Participants were part of a randomized controlled trial (RCT) conducted between 1994 and 1996.18 The RCT compared two methods of care: Kangaroo-Mother Care (KMC) and conventional incubator care. Additionally, a reference group not randomized of 150 individuals born at term in the same hospital and appropriate for their GA was included in the study. All participants were followed up until they reached one year of age and a recovered cohort was assessed again at the age of 18–20 years.
Exclusion criteria
Newborns with genetic syndromes, hypoxic-ischemic encephalopathy, major congenital malformations, and severe perinatal clinical conditions like pulmonary hypertension and intraventricular hemorrhage were excluded. The study also excluded patients who were referred to other healthcare institutions, faced difficulties in follow-up due to relocation, or were abandoned infants given up for adoption.
Procedures
In the original study, 746 preterm or LBW newborns were randomly assigned to receive either KMC or conventional incubator care based on weight categories. These infants were closely monitored in high-risk KMC ambulatory programs until they reached one year of corrected age (CA). Clinical status, anthropometric measures, nutrition, neuromotor and neurosensorial strict follow-up was performed. Optometric evaluation was done at 3 months CA. Auditory screening was performed at 3 months of CA and general development quotient with Griffiths test at 6 and 12 months. During the first year of follow up 30 participants passed away.18,19
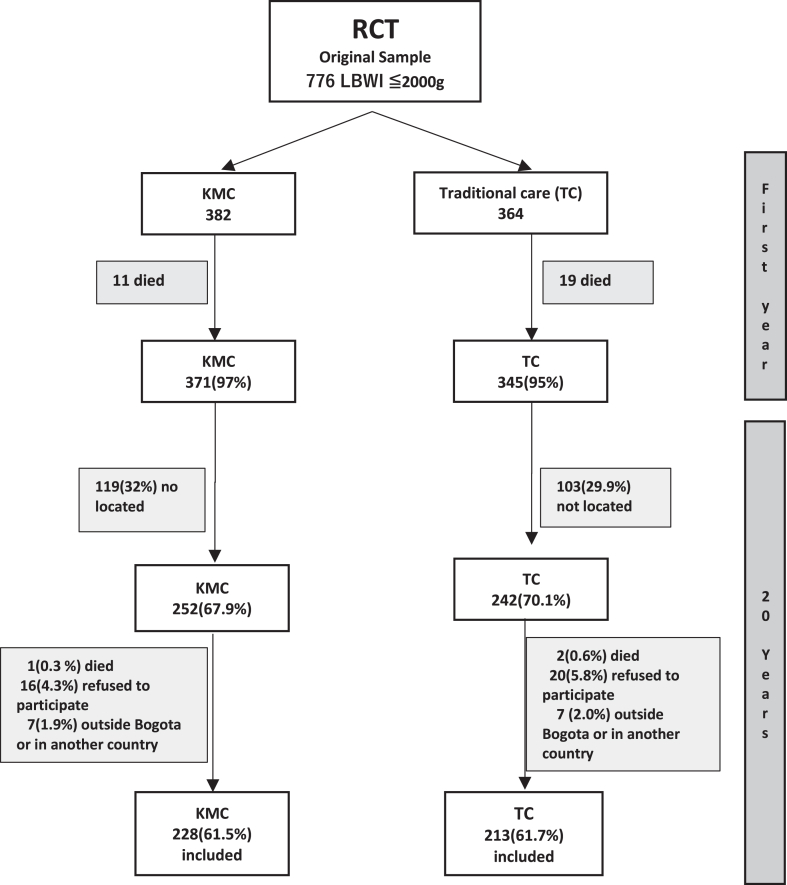
Between 2013 and 2014, 69% (494/716) of the original RCT participants were successfully located through various means, including direct telephone contact, domiciliary visits and mass media (radio, television, newspapers and socialmedia like facebook). Furthermore, 50 of 150 (33%) participants from the original at-term- without risk factors reference group were recruited (Fig. 1). Before measurements were made, all the participants were referred for complete optometry and audiology tests to ensure that they could participate in all the tests; glasses or hearing aids were provided or adjusted, as needed and when feasible. Recovered participants we classified according to their auditory or visual performance as impaired vision if (i) both sides abnormal vision, (ii) one side abnormal vision and the other side moderate vision with or without correction, according to the Snellen’s chart and WHO classification (https://www.paho.org/en/topics/visual-health), and impaired hearing if they had (i) uni or (ii) bilateral hearing loss according to audiometry.
Fig. 1.
Cohort recovery flowchart. ∗Percentages on the number of patients at the end of the first year of life (716), when RCT outcomes were evaluated. ∗∗The sample size calculated for the original RCT was 776 LBW infants (preterm and at term with IUGR). Recovered cohort N = 441 (62% of the original sample).
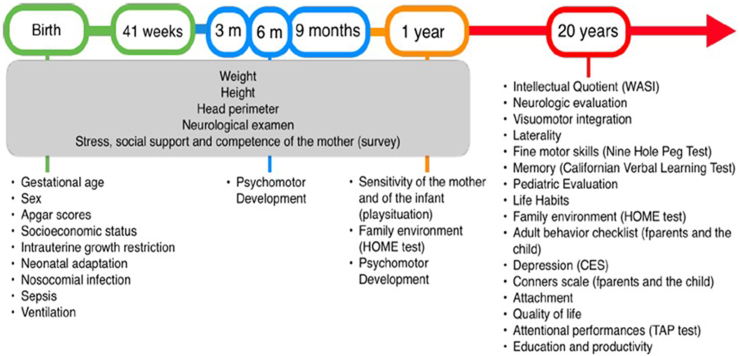
The 491 re-enrolled young adults (441 from the RCT and 50 from the reference group) underwent a comprehensive series of evaluations, with prior informed consent obtained. The evaluations encompassed a general health assessment, complete hearing and vision assessments, and a battery of neuropsychological tests, including IQ evaluation, memory, attention, and fine motor skills assessments. The mother-child relationship was assessed using the Inventory of Parent and Peer Attachment (IPPA), depression was measured using the Center for Epidemiological Studies–Depression scale (CESd), and social behaviour was reported by parents using the Adult Behaviour Checklist (ABCL) and the CONNERS test. Participants also provided self-assessments of their social behaviour using the Adult Self Report (ASR) and the CONNERS test. A family assessment was conducted using the HOME test20, 21, 22, 23, 24, 25, 26 (Fig. 2). Additionally, validated questionnaires were used to inquire about social risk, physical violence, and the presence of feelings of distress or anxiety (LIFE –H 3.0 General, adapted to spanish).27
Fig. 2.
Summary of the Battery of tests performed between birth up to 20 years of age.
For sensory data, a clean and verified database previously used in one general study conducted at 20 years of age with the same patients was employed.
To address the research objectives, the study evaluated a range of sociodemographic and perinatal variables, including sex, APGAR score, GA, birthweight, Intrauterine Growth Restriction (IUGR), hospital stay, and neonatal history (including Neonatal Intensive Care Unit (NICU) stay, invasive ventilation, need for phototherapy, use of aminoglycosides, sepsis, intraventricular hemorrhage, ROP, total days on oxygen, total days of hospitalization, and type of feeding at discharge). The study also considered the follow-up history during the first year of life, including assessments by ophthalmology and phonoaudiology in the KMC follow-up program, as well as neuromotor and psychomotor development at 3, 6, 9, and 12 months of CA. Evaluations were conducted at 18–20 years of age, including clinical assessments, neurosensory evaluations, and neuropsychological tests (Fig. 2).
Statistical analysis
To assess whether the recruitment process introduced selection bias, distributions of main variables of interest (exposure, main outcomes at 41 weeks of GA and at 1 year of CA and potential confounders and effect modifiers) were compared between the original recruited sample (776 subjects) and the recovered sample (441) as a whole and also according to experimental allocation (KMC or conventional incubator care). Given that only survivors up to 1 year of CA were available for re-enrolment, a survival cohort effect was anticipated. In addition, during the first year of follow up more control than KMC infants died, therefore an imbalance in mortality risk factors and other potential confounders could also be anticipated.
To assess whether the resulting expected unbalance in the set of potential confounders between experimental groups could bias the comparisons in the re-enrolled cohort, a Rasch model was fitted to estimate an overall degree of vulnerability (fragility index) due to factors present prior to random allocation to the interventions. A set of 22 binary indicators was selected to represent injuries that might have occurred during pregnancy, birth or the neonatal period before randomisation. The set includes retrospective measures at 1 year (indicators 16, 19, 20 and 22) and measures at 20 years (indicators 15 and 18) of detectable consequences of a pre-allocation probable injury. This fragility index is based on individual factorial scores, on the assumption that a common latent variable measures the non-specific personal fragility of an infant. These indicators were published in pediatrics in a previous study and added as Supplementary Table S1.28
In this statistical perspective, the probability of observing an indicator is a logit function of the level of fragility in an equation with two parameters: difficulty and discrimination. An easy indicator (negative value) is any observed fragility; a difficult indicator (positive value) is seen only at the most severe levels. When the probability increases with fragility, the discrimination parameter is low; however, when the probability passes from 0 to 1, with a small change in fragility, the discrimination parameter is high. In the classical Rasch model, discrimination is presumed to be equal for all indicators. This assumption is unrealistic, as the indicators reflect a wide variety of situations, from pregnancy to neonatal events. Therefore, we used a two-parameter logistic model. The analysis was conducted with the LTM package available with R statistical software version 3.0.2 (www.r-project.org).
After assessing the potential effect of imbalances of fragility indicators, a comparison of the distribution of variables of interest between the set of survivors at 1 year of CA (716 subjects) and the re-enrolled sample at 20 years was made. Bivariate analysis compared baseline characteristics, antenatal, perinatal and follow-up outcomes up to one year of CA between survivors at one year and the actual recovered sample, and also according to experimental groups. Categorical variables were compared by Chi-square test or Fisher’s exact test, as appropriate. Numerical discrete and continuous variables were compared using appropriate parametric or non-parametric tests. An alpha p-value of less than 0.05 was considered significant. We concluded that those who were followed up were similar to those who were not.
The analysis of this paper was done using SPSS 28 and STATA 14. In the initial phase, an exploratory analysis aimed to identify perinatal factors associated with sensory problems in the 491 patients. Sociodemographic and clinical characteristics were compared in patients from the recovered cohort with or without impairments. Subsequently, clinical characteristics and outcomes were compared during follow-up up to 20 years of age, according to the auditory and visual diagnoses at that age.
Quantitative variables were reported with averages and standard deviations or medians and minimum and maximum values, depending on their distribution. Qualitative variables were presented with absolute frequencies and percentages. For the bivariate analysis, a Chi-square test or Fisher’s exact test was used for qualitative variables, and the Student’s or Mann Whitney U test was used for quantitative variables, depending on their distribution. Adjusted p-values were obtained using Family-Wise Error Rate (FWER) correction.
In the second phase, multivariate logistic regression analysis was conducted to assess vision and hearing outcomes at 12 months and 20 years, adjusting for variables that showed significant differences in the bivariate analysis (adjusted p-value < 0.05) and considered confounding variables. The analysis included an evaluation of the goodness of fit of the models and the percentage of correct classification.
Ethics approval
This study was conducted in agreement with the principles established in the Declaration of Helsinki and with the standards of Good Clinical Practice in the country. The ethics and research committee of the Kangaroo Foundation approved the protocol.
Role of the funding source
Study funders did not have any role in study design, data collection, data analysis, interpretation, writing of the report or decision to submit.
Results
Comparison between recovered RCT cohort (preterm, LBW) and reference population
When analyzing sociodemographic characteristics, we found that the median per capita income of the families in the recovered RCT cohort was slightly lower than the reference group (70,000 vs. 90,000 COP) and mothers’ median age was 4 years older, (27.5 vs. 23 years).
Regarding birth characteristics, 18% (79/441) of the RCT cohort had birth weights <1500 g and 48% (212/441) between 1500 and 1800 g. Thirty-two (141/441) percent had GA ≤ 32 weeks and 29% (129/441) between 32 and <35 weeks.
In the RCT recovered cohort 19.9% (66/332) had some type of neonatal adaptation maneuver, and 8.5% (29/341) presented Apgar <5 at 5 min; 61% (269/441) were hospitalized in the NCU for a median of 15 days, min–max (1–64) and 15% (66/441) of them were in the NICU. Regarding antibiotic use, 55%147/269 received aminoglycosides. Hospital discharge was at a median of 35 weeks and 1700 g and 75% (300/399) were breastfed for more than 40 weeks GA.
During the first 12 months follow-up, more neuromotor alterations were found in the INFANIB test at 3 months in the RCT recovered cohort (20% (76/398) vs. 2% (1/47)). At 12 months 4.7% (16/402) continued with motor problems vs. none in the reference group and 2% (8/403) were diagnosed with CP. In developmental assessments at 6 and 12 months using the Griffiths test,29 we found disparities at 6 months, particularly in hearing and language domains. However, by 12 months, no score differences were observed.
In terms of sensory problems, 2.6% (10/390) of the RCT-recovered cohort had regressive Retinopathy of Prematurity (ROP) and 1% (4/390) had ROP requiring treatment. Unilateral hearing deficit was detected in 2.3% (9/396) of participants and bilateral deficits were found in 1% (4/396).
At 20 years old, the schooling was similar between groups. We didn’t find differences in cognitive performance (Wasi test) or Visual-Motor Integration (VMI). However, the recovered RCT cohort had a higher prevalence of learning disabilities (21% (91/440) vs. 6% (3/50)).
Socially, reference and RCT groups showed similar quality of life based on the Kidscreen questionnaire, self-esteem, stress, social risk, and depression scores. There were no differences in employment status, relationships, suicidal behaviours or substance abuse. In terms of the family environment, living arrangements and family structures were similar; however, the recovered RCT cohort exhibited higher attachment scores (median IPPA 63 vs. 56), and lower regulatory activity scores in the HOME test (median 6 vs. 8). Interestingly, the self-perceived quality of life scores reported by the young individuals were higher than those reported by their parents.
In the general cohort, 5.9% (29/491) had functional visual impairment, and 8.1% (40/491) experienced hearing problems at the age of 20. Table 1 describes auditory and visual evaluation at 20 years in the RCT recovered cohort and the reference group.
Table 1.
Auditory and visual evaluation at 20 years in the RCT recovered cohort and in the reference group.
| Variables N (%) | RCT recovered cohort ≤ 2000 g N = 441 | Reference group N = 50 | pa |
|---|---|---|---|
| Visual functional evaluation at 20 years (2 categories) N (%) | |||
| Normal bilateral or unilateral vision | 405/434 (93.3) | 49/49 (100) | 0.06 |
| One eye abnormal and the other eye abnormal or moderately abnormal with or without correction | 29/434 (6.7) | 0 | |
| Myopia or amblyopia at 20 years N (%) | |||
| Normal vision or other refractive defects | 232/441 (52.6) | 26/50 (52.0) | 0.94 |
| Uni- or bilateral myopia or amblyopia | 209/441 (47.4) | 24/50 (48.0) | |
| Requires lens correction, as assessed by optometrist N (%) | 232/437 (53.1) | 23/49 (46.9) | 0.41 |
| Functional hearing evaluation at 20 years N (%) | |||
| Normal hearing | 405/441 (91.8) | 46/50 (92.0) | 0.26 |
| Uni or bilateral deafness -general | 36/441 (8.2) | 4/50 (8.0) | |
| Uni or bilateral neurosensorial deafness | 30/441 (6.8) | 2/50 (4.0) | |
| Uni or bilateral conductive deafness | 6/441 (1.4) | 2/50 (4.0) |
Tables were based on retrospective data. There are different denominators according to data available during follow-up. Sometimes patients didn’t attend follow up.
Chi2 or Fisher’s exact test was used for qualitative variables and Mann Whitney or Student’s t-test for quantitative variables, according to the distribution of each variable in the comparison groups.
Comparisons according to hearing problems
Table 2, Table 3 and Supplementary Tables S2 and S3 outline the results of follow-up assessments up to age 20. The groups with and without hearing issues showed no differences in sociodemographic or gestational characteristics, except for slightly lower birth weight in the hearing-impaired group. Those with hearing problems also had longer stays in the newborn unit, more days on oxygen, invasive ventilation, aminoglycoside use, parenteral nutrition, and phototherapy requirements (Supplementary Table S2). Developmental assessments at 6 months revealed lower scores in hearing and language sub-scale for the hearing-impaired group. Audiometry tests at 12 months confirmed hearing deficits (Supplementary Table S3). At the 20-year mark, only 30.5% (11/36) of those with deafness had access to cochlear implants or hearing aids and exhibited more significant cognitive deficits, with lower scores in various tests and a higher prevalence of learning disorders (Table 2). Quality of life, attachment, self-esteem, stress, anxiety, social risk, and HOME test scores showed no significant differences between participants with hearing sequelae and those with normal hearing. However, participants with hearing impairment reported a tendency of higher self-perceived depression levels. Despite this, there were no distinctions in the frequency of suicidal ideation or suicide attempts. Regarding family dynamics, no notable differences were identified in living arrangements with parents or the preservation of original family structures (Table 3).
Table 2.
Cognitive outcomes according to functional deafness diagnosis at 20 years.
| Variables | Normal audition N = 451 | Uni/bilateral deafness N = 40 | pa | Adjusted pb |
|---|---|---|---|---|
| Wasi total score-4 components Mean (SD) | 88.2 (13.6) | 80.0 (14.8) | 0.0005c | <0.01c |
| Wasi- verbal comprehension composite score Mean (SD) | 89.5 (14.0) | 79.6 (18.6) | 0.0001c | <0.01c |
| Wasi- perceptual reasoning composite score mean (SD) | 89.3 (14.1) | 82.8 (14.2) | 0.005c | 0.06 |
| Wasi-categories by score n/N (%) | ||||
| Borderline and very low (<80) | 116/448 (25.9) | 19/36 (52.8) | 0.004c | 0.02c |
| Low average (80–89) | 126/448 (28.1) | 5/36 (13.9) | ||
| Normal average (90–110) | 183/448 (40.9) | 12/36 (33.3) | ||
| Above average > 110 | 23/448 (5.1) | 0 | ||
| VMI -Total score 4 standardized domains P50 (min–max) | 92 (45–107) | 89.5 (45–107) | 0.33 | |
| VMI- visual P50 (min–max) | 92 (45–107) | 86 (45–107) | 0.08 | |
| VMI- motor P50 (min–max) | 92 (47–107) | 86 (47–97) | 0.025c | 0.28 |
| Schooling 20 years n/N (%) | ||||
| Secondary | 101/437 (23.1) | 21/40 (52.5) | 0.001c | <0.01c |
| Technique | 155/437 (35.5) | 8/40 (20.0) | ||
| University | 163/437 (37.3) | 11/40 (27.5) | ||
| Others | 18/437 (4.1) | 0 | ||
| ICFES n/N (%) | 344/451 (76.3) | 21/40 (52.5) | 0.001c | <0.01c |
| Passed ICFES in 2014 or 2018 n/N (%) | 378/451 (83.8) | 27/40 (67.5) | 0.009c | 0.04c |
| ICFES- Standardized score in language zscore mean (SD) | 0.01 (0.89) | −0.40 (0.87) | 0.024c | 0.27 |
| ICFES- Standardized score in mathematics zscore mean (SD) | 0.00 (1.00) | −0.45 (0.86) | 0.023c | 0.26 |
| ICFES- Standardized score in social sciences zscore mean (SD) | 0.03 (0.93) | −0.10 (0.99) | 0.50 | |
| ICFES- Standardized score in philosophy zscore mean (SD) | 0.06 (0.99) | −0.16 (0.94) | 0.25 | |
| ICFES- Standardized score in chemistry zscore mean (SD) | 0.01 (0.95) | −0.24 (0.85) | 0.20 | |
| ICFES- Standardized score in physics zscore P50 (min, max) | −0.09 (−4.6, 2.7) | 0.25 (−3.77, 1.62) | 0.35 | |
| ICFES- Standardized score in biology zscore mean (SD) | −0.02 (0.95) | −0.24 (1.08) | 0.25 | |
| Learning disorders n/N (%) | 78/450 (17.3) | 16/40 (40.0) | <0.0001c | <0.01c |
Tables were based on retrospective data. There are different denominators according to data available during follow-up. Sometimes patients didn’t attend follow up.
SD, Standard Deviation. Wasi, Wechsler Abbreviated Scale of Intelligence. VMI, test of Visual Motor Integration. P50, percentile 50 (median). ICFES test, Instituto Colombiano de Fomento para la Educación Superior (state exams for university entrance in Colombia).
Chi2 or Fisher’s exact test was used for qualitative variables and Mann Whitney or Student’s t-test for quantitative variables, according to the distribution of each variable in the comparison groups.
Family-Wise Error Rate (FWER) correction for unadjusted significant values of p.
p < 0.05.
Table 3.
Quality of life and social performance outcomes at 20 years according to auditory functional diagnosis at 20 years.
| Variables | Normal audition N = 451 | Uni/bilateral deafness N = 40 | pa | Adjusted pb |
|---|---|---|---|---|
| Active worker n/N (%) | 325/442 (73.5) | 25/40 (62.5) | 0.13 | |
| Studying and working n/N (%) | 99/442 (22.4) | 6/40 (15.0) | 0.28 | |
| Attachment score (IPPA) P50 (min- max) | 63.0 (9–87) | 59.50 (31.0–81.0) | 0.16 | |
| Self-esteem score P50 (min- max) | 32 (14–40) | 31.5 (24–38) | 0.23 | |
| Percentile self-perceived depression score (DSM) P50 (min- max) | 62.0 (50.0–99.0) | 76.0 (50.0–99.0) | 0.019c | 0.11 |
| Percentile score depression perception parents (ABCL) P50 (min- max) | 76.0 (50.0–99.0) | 79.0 (50.0–98.0) | 0.74 | |
| Percentile best friend depression score (ABCL) P50 (min- max) | 58.0 (50.0–100.0) | 67.0 (50.0–99.0) | 0.08 | |
| Victim of school violence n/N (%) | 132/448 (29.5) | 11/40 (27.5) | 0.79 | |
| Smoker n/N (%) | 98/449 (21.8) | 12/40 (30) | 0.041c | 0.28 |
| Psychoactive drug use n/N (%) | 46/451 (10.2) | 0 | 0.024c | 0.18 |
| Alcohol consumption n/N (%) | ||||
| ≤ 1 time per week | 349/364 (95.9) | 29/33 (87.9) | 0.06 | |
| >1 time per week | 15/364 (4.1) | 4/33 (12.1) | ||
| Has considered suicide in the last 12 months n/N (%) | 39/449 (8.7) | 7/40 (17.5) | 0.07 | |
| Attempted suicide n/N (%) | 24/37 (64.9) | 4/7 (57.1) | 0.69 | |
| Social risk in the near environment p50 (min, max) | 11 (7–12) | 11 (8–12) | 0.76 |
Tables were based on retrospective data. There are different denominators according to data available during follow-up. Sometimes patients didn’t attend follow up.
IPPA, Inventory of Parental-Peer Attachment. P50, percentile 50(median). ABCL, Adult Behaviour Checklist.
Chi2 or Fisher’s exact test was used for qualitative variables and Mann Whitney or Student’s t-test for quantitative variables, according to the distribution of each variable in the comparison groups.
Family-Wise Error Rate (FWER) correction for unadjusted significant values of p.
p < 0.05.
Comparisons according to visual sensory problems
Table 4, Table 5, Table 6 and Supplementary Tables S4 and S5 report visual function comparisons. While no significant sociodemographic or gestational differences were noted, individuals with visual deficits exhibited lower GA and birth weight. They also have a tendency to longer hospital stays, higher frequency of necrotising enterocolitis (NEC), phototherapy, aminoglycosides treatment, extended parenteral nutrition, and oxygen therapy (Supplementary Table S4).
Table 4.
Auditory and visual evaluation in all the sample at 20 years according to functional vision diagnosis.
| Sensory performance evaluation | Functional vision normal N = 454 | Functional vision impairment N = 29 | pa |
|---|---|---|---|
| Refractive problems n/N (%) | |||
| Uni- or bilateral myopia or amblyopia | 210/454 (46.3) | 21/29 (72.4) | <0.01b |
| Normal vision or other refractive defects | 244/454 (53.7) | 8/29 (27.6) | |
| Required lens correction, as assessed by optometrist n/N (%) | 226/454 (49.8) | 26/29 (89.7) | <0.01b |
| Functional hearing evaluation 20 years n/N (%) | |||
| Normal hearing | 418/454 (92.1) | 25/29 (86.2) | 0.29 |
| Uni or bilateral hearing loss | 36/454 (7.9) | 4/29 (13.8) |
Chi2 or Fisher’s exact test was used for qualitative variables and Mann Whitney or Student’s t-test for quantitative variables, according to the distribution of each variable in the comparison groups.
p < 0.05.
Table 5.
Cognitive Performance outcomes according to functional vision diagnosis at 20 years.
| Sensory and cognitive performance evaluation | Functional vision normal N = 454 | Functional vision impairment N = 29 | pa | Adjusted pb |
|---|---|---|---|---|
| Wasi total score- 4 components Mean (SD) |
88.1 (13.4) | 82.2 (17.5) | 0.026c | 0.29 |
| Wasi- verbal comprehension composite score P50 (min–max) | 90 (45–123) | 79 (45–118) | 0.032c | 0.35 |
| Wasi- mean perceptual reasoning composite score (SD) | 89.1 (13.9) | 84.8 (17.4) | 0.11 | |
| Wasi-categories by score N (%) | ||||
| Borderline and very low (<80) | 119/449 (26.5) | 15/29 (51.7) | 0.007c | 0.03c |
| Low average (80–89) | 123/449 (27.4) | 5/29 (17.2) | ||
| Normal average (90–110) | 187/449 (41.7) | 6/29 (20.7) | ||
| Above average > 110 | 20/449 (4.5) | 3/29 (10.3) | ||
| VMI -Total score 4 standardized domains P50 (min–max) | 92 (45–107) | 85 (45–107) | 0.25 | |
| VMI- visual P50 (min–max) | 92 (45–107) | 89 (45–101) | 0.28 | |
| VMI- motor P50 (min–max) | 92 (47–107) | 86 (64–102) | 0.13 | |
| Schooling 20 years n/N (%) | ||||
| Secondary | 110/443 (24.8) | 10/28 (35.7) | 0.10 | |
| Technique | 151/443 (34.1) | 10/28 (35.7) | ||
| University | 165/443 (37.3) | 7/28 (25.0) | ||
| Others | 17/443 (3.8) | 1/28 (3.6) | ||
| Passed ICFES N (%) | 379/454 (83.5) | 22/29 (75.9) | 0.29 | |
| ICFES- Standardized score in language zscore mean (SD) | −0.00 (0.90) | −0.36 (0.82) | 0.07 | |
| ICFES- Standardized score in mathematics zscore mean (SD) | −0.00 (1.01) | −0.44 (0.82) | 0.05 | |
| ICFES- Standardized score in social sciences zscore mean (SD) | 0.03 (0.92) | 0.02 (0.92) | 0.98 | |
| ICFES- Standardized score in philosophy zscore mean (SD) | 0.07 (0.97) | −0.35 (1.14) | 0.05 | |
| ICFES- Standardized score in chemistry zscore mean (SD) | 0.01 (0.95) | −0.33 (0.96) | 0.10 | |
| ICFES- Standardized score in physics zscore P50 (min, max) | −0.03 (−4.6, 2.7) | −0.11 (−1.54, 1.62) | 0.78 | |
| ICFES- Standardized score in biology zscore mean (SD) | −0.01 (0.94) | −0.44 (1.19) | 0.039c | 0.41 |
| Learning disorders n/N (%) | 78/453 (17.2) | 12/29 (41.4) | 0.001c | <0.01c |
Tables were based on retrospective data. There are different denominators according to data available during follow-up.
Wasi, Wechsler Abbreviated Scale of Intelligence. SD, Standard Deviation. VMI, test of Visual Motor Integration. P50, percentile 50 (median). ICFES test, Instituto Colombiano de Fomento para la Educación Superior (state exams for university entrance in Colombia).
Chi2 or Fisher’s exact test was used for qualitative variables and Mann Whitney or Student’s t-test for quantitative variables, according to the distribution of each variable in the comparison groups.
Family-Wise Error Rate (FWER) correction for unadjusted significant values of p.
p < 0.05.
Table 6.
Quality of life and social performance outcomes according to functional vision diagnosis at 20 years.
| Variables | Functional vision Normal N = 454 | Functional vision impairment N = 29 | pa | Adjusted pb |
|---|---|---|---|---|
| Active worker n/N (%) | 324/447 (72.5) | 22/29 (75.9) | 0.69 | |
| Studying and working n/N (%) | 98/447 (21.9) | 4/29 (13.8) | 0.36 | |
| Quality of life score according to parents (Kid screen) mean (SD) | 40.5 (3.8) | 40.6 (3.7) | 0.68 | |
| Quality of life score according to patients (Kid screen) p50 (min–max) | 46.9 (32.4–62.8) | 48.0 (33.4–60.8) | 0.30 | |
| Attachment score (IPPA) P50 (min- max) | 62.0 (9.0–87.0) | 66.0 (23.0–85.0) | 0.46 | |
| Self-esteem score P50 (min- max) | 32.0 (14.0–40.0) | 30.0 (16.0–38.0) | 0.041c | 0.40 |
| Percentile score depression parental perception (ABCL) P50 (min- max) | 76.0 (50.0–99.0) | 89.0 (50.0–99.0) | 0.18 | |
| Victim of school violence n/N (%) | 127/452 (28.1) | 13/29 (44.8) | 0.06 | |
| Has a partner n/N (%) | 203/454 (44.7) | 10/29 (34.5) | 0.28 | |
| Smoker n/N (%) | 99/453 (21.9) | 8/29 (27.6) | 0.75 | |
| Still lives with parents n/N (%) | 412/453 (91.0) | 26/29 (89.7) | 0.74 | |
| Alcohol consumption n/N (%) | ||||
| ≤ 1 time per week | 352/370 (95.1) | 22/22 (100) | 0.61 | |
| >1 time per week | 18/370 (4.9) | 0/22 | ||
| Home Total p50 (min, max) | 21.0 (−23.0, 53.0) | 18.0 (−15.0, 47.0) | 0.34 | |
| Home-physical environment p50 (min, max) | 5.0 (−7.0, 7.0) | 5.0 (−1.0, 7.0) | 0.09 | |
| Home-material learning mean (DS) | 0.6 (4.2) | 0.3 (5.3) | 0.83 | |
| Home-autosufficiency p50 (min, max) | 2.0 (−4.0, 4.0) | 2.0 (−2.0, 4.0) | 0.65 | |
| Home-regulatory activities p50 (min, max) | 6.0 (−6.0, 10.0) | 6.0 (−2.0, 10.0) | 0.97 | |
| Home-accompanying family mean (SD) | 0.10 (3.7) | 0.0 (3.1) | 0.87 | |
| Home-acceptance p50 (min–max) | 8.0 (−10.0, 10.0) | 4.0 (−6.0, 10.0) | 0.022c | 0.23 |
Tables were based on retrospective data. There are different denominators according to data available during follow-up.
P50, percentile 50 (median). IPPA, Inventory of Parental-Peer Attachment. ABCL, Adult Behaviour Checklist. Home, Home observation measurement of the environment.
Chi2 or Fisher’s exact test was used for qualitative variables and Mann Whitney or Student’s t-test for quantitative variables, according to the distribution of each variable in the comparison groups.
Family-Wise Error Rate (FWER) correction for unadjusted significant values of p.
p < 0.05.
Follow-up assessments up to 12 months of CA revealed an association between ROP screening and later visual issues. Anthropometric measurements and dietary patterns did not vary significantly between the groups. The 12-month ophthalmology and optometry evaluations were also linked to visual deficits at 20 years of age, however, there were no differences in the risk of cerebral palsy at 12 months between the groups (Supplementary Table S5).
At the 20-year evaluation, 72% of patients with functional visual impairment had uni or bilateral myopia or amblyopia and 86% had normal hearing (Table 4). Those with functional visual impairment scored lower in cognitive tests (Table 5). While no significant differences were observed in terms of quality of life, depression, or attachment scores, those with visual deficits had a tendency to lower self-esteem and acceptance scores on the HOME test. A higher proportion of them experienced school violence. No differences were found in the frequencies of participants residing with parents or belonging to preserved original families (Table 6).
Multivariate analysis with logistic regression for sensory outcomes
Hearing
To assess factors during the first year of life associated with hearing loss, a multivariate analysis using logistic regression was conducted. The dependent variables were the diagnosis of deafness at 12 months and uni- or bilateral hearing loss at 20 years of age. The independent variables were those that showed significant differences in the bivariate analyses of neonatal characteristics and the first year of follow-up, according to the auditory functional diagnosis of deafness or normal hearing at 20 years (Supplementary Tables S2 and S3). The most concise models revealed that, for the auditory diagnosis at 12 months, the associated variables were the number of days on mechanical ventilation and the auditory development score on the Griffiths scale at 6 months (R2 = 0.51; 371 observations; Hosmer–Lemeshow chi2 (4) = 0.43, p = 0.98; correctly classified 98.9%). For the auditory diagnosis at 20 years, the associated variables were the number of days of stay in the Neonatal Intensive Care Unit (NICU) and, once again, the auditory development score on the Griffiths scale at 6 months (R2 = 0.09; 438 observations; Hosmer–Lemeshow chi2 (4) = 6.38, p = 0.17; correctly classified 91.6%) (Table 7).
Table 7.
Multivariate analyses-auditory evaluation- 12 months and at 20 years.
| Multivariate analysis-auditory evaluation 12 months Remarks = 371 LR Chi2 (2) = 17.87 Pseudo R2 = 0.51 p > chi2 <0.01 | |||
|---|---|---|---|
| Outcome variable: Uni- or bilateral hearing loss at 12 months of age | Raw OR 95% CI | Adjusted OR 95% CI | p |
| Days in invasive mechanical ventilation | 1.22 [0.97, 1.54] | 1.36 [0.98, 1.87] | <0.01 |
| Auditory and language development score at 6 months (Griffiths’ test) | 0.92 [0.88, 0.96] | 0.91 [0.87, 0.96] | 0.06 |
| Multivariate analysis-auditory evaluation 20 years Remarks = 438 LR Chi2 (1) = 15.75 Pseudo R2 = 0.06 p > chi2 < 0.01 | |||
|---|---|---|---|
| Outcome variable: Uni- or bilateral hearing loss at 20 years. | Raw OR 95% CI | Adjusted OR 95% CI | p |
| Days in NICU | 1.07 [1.03, 1.11] | 1.06 [1.02, 1.10] | <0.01 |
| Auditory and language development score at 6 months (Griffiths’ Test) | 0.96 [0.94, 0.98] | 0.96 [0.94, 0.98] | <0.01 |
LR, Likelihood ratio. Chi2, Chi2 test. OR, Odds ratio. CI, confidence Interval.
Vision
Multivariate analysis using logistic regression was performed with dependent variables being the diagnosis of refractive errors or ROP at 12 months, and functional visual compromise at 20 years of age. Independent variables included those that displayed significant differences in the bivariate analyses of neonatal characteristics and the first year of follow-up, according to visual functional diagnosis (Supplementary Tables S4 and S5). Table 8 shows that for visual outcomes at 12 months and 20 years, the most influential variable is GA at birth.
Table 8.
Multivariate analyses–visual assessment at 12 months and at 20 years.
| Multivariate analysis-visual assessment at 12 months Remarks = 491 LR Chi2 (2) = 19.07 Pseudo R2 = 0.08 p > chi2 <0.01 | ||
|---|---|---|
| Outcome variable: refractive error or ROP at 12 months vs. normal vision. | raw OR 95% CI | p |
| Ballard at birth | 0.82 [0.71, 0.94] | <0.01 |
| Visual assessment at 20 years | ||
|---|---|---|
| Outcome variable: Uni or bilateral normal vision vs. abnormal bilateral vision or abnormal unilateral vision and moderate refractive error in the other eye (with or without correction). | raw OR 95% CI | p |
| Ballard at birth (483 obs; R2 0.05) | 0.79 [0.69, 0.91] | <0.01 |
| Total days with oxygen in the neonatal period (265 obs; R2 0.04) | 1.06 [1.01, 1.10] | <0.01 |
LR, Likelihood ratio. Chi2, Chi2 test. ROP, Retinopathy of prematurity. GA, GA. OR, Odds ratio. CI, confidence Interval. Obs, observations.
Discussion
In 1994–1996, the Kangaroo Foundation research group in Colombia carried out the first RCT on the KMC method, an intervention directed at the care of premature or LBW children. The short- and medium-term results on mortality, morbidity, mother-child relationship, and somatic and neuro-psychomotor development were published in 1997–2001 and used in all the systematic reviews that now show the multiple benefits of this method. As an example, the World Health Organization (WHO) recently published a position paper in the Lancet in support of KMC.30
In 2012 our research group aimed at evaluating the long-term results, by re-enrolling 20 years later the same cohort than in the 1994–1996 RCT and carrying out a complete cross-sectional study that looked not only at the physical condition of the patients but also at their social integration, quality of life, psychological, neurological, mental health and sensory status. Neuroimages were performed in a subsample of participants. We have been analyzing and publishing the results of this large project and dataset over the past few years.
In this study, we used a cohort of participants recovered from the original RCT conducted between 1994 and 1996 in Colombia. After 20 years, 62% of the initial population were successfully tracked for long-term evaluation. This comprehensive assessment had not been previously undertaken in our country; the recovered cohort allowed us to analyze both the short and long-term outcomes that the young ex-preterm or with LBW presented. We were able to compare outcomes with a reference group of full-term infants without risk factors, born at the same institution where the RCT participants were born (50 recovered from the original reference group). The present study aimed to investigate perinatal factors linked to visual and auditory problems in ex-preterm or LBW young adults, assessing their long-term quality of life.
Preterm or LBW infants are part of the small vulnerable population responsible not only for a great part of morbimortality in infancy but health in adulthood and neurodevelopment. According to Barker’s hypothesis, this concept is not only relevant for metabolic programming, but it also suggests that stimuli applied during early development cause permanent changes that persist throughout the lifespan. Therefore, programming is not just limited to the in-utero environment but extends into childhood, where different organs and systems continue to adapt to various cues.31 The target population of this study, comprehends all LBW newborn with birth weight less than 2000 g. In our recovered cohort, 87.5% were premature infants and around 12.5% were at-term infants small for GA. LBW infants with damage associated with placental hypoperfusion, are at increased risk for cognitive, sensory and motor problems due to the immaturity of their brains and possibly the adverse intrauterine environment to which they were exposed.32 These facts highlight the need for strict follow-up for these fragile and vulnerable small babies. Therefore, we are examining the associated perinatal risk factors to enable timely diagnosis and intervention, taking advantage of early brain plasticity.
The study revealed more pronounced motor and neurosensorial issues in the general recovered cohort at 12 months, including a 2% risk of cerebral palsy, 2.6% regressive ROP and 1% non-regressive ROP. Additionally, 2.3% had unilateral hearing loss, and 1% bilateral hearing loss. Evidence shows that LBW infants face a higher risk of motor, cognitive, and sensory problems due to their immature brains, particularly affecting areas like white matter, germinal matrix, and cerebellum.33 Numerous studies highlight the elevated risk of hearing and vision issues in preterm or LBW infants.7
In this study it is striking that we found hearing loss rates of 8.2% in the recovered cohort as well as 8.0% in the reference group (at-term with no risk factors); probably this is due to different causes of hearing loss not identified in the reference group such as type STORCH infections or conductive deafness. In the reference group, half had conductive deafness. In participants born premature or LBW, the highest percentage was sensorineural deafness. This fact emphasizes the importance of universal hearing screening for all newborns, with known risk factors or not.
Our study noted a higher proportion of visual deficits among young adults born preterm or with LBW. Although not statistically significant, some had suboptimal vision, abnormal bilateral vision, and refractive defects.
In terms of quality of life and social functioning, the recovered cohort displayed a higher attachment score, potentially due to perceived vulnerability and increased dependence on parents. The exposure to kangaroo follow-up, available for both the intervention and control groups, may have contributed to this finding. The study observed a lower median score for the regulatory activities subitem in the family environment, indicating potential parental overprotection of premature or LBW children.34
Assessment of auditory functioning at 20 years of age
When comparing all participants, including the reference group, based on the diagnosis of unilateral or bilateral hearing deficits, we did not find significant demographic or gestational differences. On the other hand, our analyses found that factors influencing hearing functionality were primarily originated during the neonatal period.
Patients with hearing deficits displayed several distinctive characteristics. They had a median birth weight 200 g lower than those with normal hearing and experienced a higher incidence of neonatal clinical issues. Significantly, there were more than twice as many patients with hearing deficits who had been hospitalized in the Neonatal Intensive Care Unit (NICU) compared to those with normal hearing (27.5% vs. 12.2%). This group also spent over double the median number of days in the NICU, and they faced a higher incidence of neonatal sepsis, greater use of aminoglycosides, and a longer duration of aminoglycoside treatment. Furthermore, patients with hearing deficits had a higher frequency of invasive ventilation, phototherapy, longer durations of oxygen therapy, and more days of parenteral nutrition. These findings underscore the need for hearing assessments in NICU graduates.
In the first 12 months of CA, participants with hearing deficits exhibited similar anthropometric measures compared to those with normal hearing. Their developmental assessments at 6 months indicated more substantial deficits, particularly in the hearing and language sub-item (92 vs. 100). By 20 years of age, participants with hearing impairment continued to show cognitive deficits, with lower IQ scores. Their scores on the WASI were notably lower than those with normal hearing, with significant differences in verbal comprehension sub-item. Furthermore, they exhibited a higher prevalence of learning disorders. In terms of education, fewer participants with hearing impairment passed the Colombian State Tests for University Admission (ICFES).
Regarding the quality of life, participants with hearing impairment reported a tendency to higher levels of self-perceived depression (76 vs. 62; p = 0.02), a higher incidence of suicidal ideation (17.5% vs. 8.7%), and a higher percentage of smoking (30% vs. 22%).
Our multivariate analysis revealed that two significant factors correlated with hearing loss at 12 months: the duration of invasive mechanical ventilation and the hearing and language score at 6 months. This could suggest a link between hearing loss and the noise associated with mechanical ventilation.35,36
The 6-month auditory development score also appears crucial for early intervention in hearing loss. Delays in hearing assessments can lead to developmental issues.37 Timely hearing assessments are crucial to avoid developmental issues. It’s concerning that only 27.5% (11/40) of individuals with hearing deficits had access to hearing aids or cochlear implants, highlighting the need for more accessible diagnostic and treatment options to enhance their development and quality of life.38
The established connection between hearing issues and global neurodevelopmental delay underscores the significance of addressing hearing impairments. These impairments can disrupt a child’s brain maturation and overall development. Comprehensive rehabilitation is often necessary, and in some cases, correcting hearing impairments can lead to improved developmental outcomes.39 Children with severe hearing loss face an increased risk of behavioural disorders, impacting their academic performance and overall quality of life. This issue is a major contributor to years lived with disability in this population and is a significant public health concern. The prevalence of hearing loss varies globally, emphasizing the importance of early hearing assessments and interventions to support well-rounded development and mitigate the potential long-term consequences of hearing impairments.12
Assessment of visual functioning at 20 years of age
Analyzing participants based on their visual diagnosis at 20 years, including the reference group, revealed no family or gestational history differences. However, visually impaired individuals had lower GA (32.6 vs. 34.4 weeks), and a tendency to higher frequency of neonatal hospitalizations (76% vs. 54%), and NEC (18% vs. 9%). They also had a higher significative prevalence of phototherapy (62% vs. 34%) and although not significative difference, spent more days on oxygen (median 9 vs. 5 days).
Surprisingly, there were no significant differences in nutritional, anthropometric, and neuromotor assessments during the first 12 months of CA between participants with visual deficits and those with normal vision. This suggests that visual deficits might be more associated with immaturity and inflammation, potentially aggravated by oxygen exposure.
At the 20-year evaluation, cognitive deficits became apparent, with lower total IQ scores on the WASI test. No significant differences were observed in VMI scores, educational attainment, or the proportion of individuals who took and passed state tests. Learning disorders were more prevalent in those with visual deficits.
Regarding quality of life, there was a trend towards lower self-esteem scores in the group with visual deficits. Additionally, a lower acceptance sub-item score was observed for the group with visual deficits when evaluating the family environment with the HOME test.
Limitations of the study
This study has several limitations derived from the fact that it is a cohort recovered 20 years after the performance of an RCT in LBW newborns who were followed up to one year of life with a reference group of at-term, non-randomized newborns. In order to minimize the probable selection bias, the recovered patients were compared with those of the original cohort by means of a fragility test, finding similar characteristics. On the other hand, there are multiple unknown covariates during the life course of these vulnerable youngsters that could have influenced the outcomes found and that is why the results must be evaluated with a margin of uncertainty.
As can be seen in the tables, we have information collected retrospectively, with incomplete follow-up data for some patients. On the other hand, the effect of time means that the findings related to management and the level of health care 30 years ago may not be extrapolated to the current condition of our newborns. However, similar practices still persist in low- and middle-income countries and these findings can be an input for high-risk follow-up policies in this population of vulnerable infants.
It is important to take into account that all associations suggested in the results should be interpreted with caution given the usual caveats of association studies—i.e., unmeasured and unpredicted confounders. Also, the study design precludes any causal inferences and further longitudinal RCTs should be able to confirm or expand the findings discussed here.
Conclusions
Our study offers valuable insights into the long-term outcomes of preterm and LBW individuals. These findings pertain to patients born three decades ago. While neonatal care has improved in urban Colombian areas, similar practices persist in many regions of the country and in other low to middle-income countries. Our results emphasize the importance of enhancing neonatal care practices, including non-invasive ventilation and oxygen management, prudent aminoglycoside usage, and consistent at least first-year follow-up. Early and timely detection of neonatal issues affecting cognitive, motor, sensory functions, and emotional well-being, with implications for adult social functioning, is paramount. Implementing comprehensive follow-up programs, including systematic hearing and ROP screenings, as well as optometric assessments, can facilitate early identification of neurosensory impairment and effective intervention, thereby promoting optimal cognitive development and an improved quality of life.
Contributors
-
•
NC was responsible for conceptualisation, access and validated raw data, data curation, formal analysis, funding acquisition, investigation, methodology, supervision, validation, visualisation, writing original draft, and writing– review & editing.
-
•
AM was responsible for access and validated raw data, data curation, formal analysis, investigation, methodology, validation, visualisation, writing—original draft, and writing—review & editing.
-
•
LR was responsible for conceptualisation, investigation, validation, visualization and writing– review & editing.
-
•
CL was responsible for investigation, validation, visualization and writing– review & editing.
-
•
DC was responsible for conceptualisation, access and validated raw data, data curation, formal analysis, funding acquisition, investigation, validation, visualisation, writing original draft, and writing– review & editing.
All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
Data sharing statement
Data collected for this study will be made available by the authors, without undue reservation.
Declaration of interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgements
The Grand Challenge Canada (GCC grant# 0059-03) financed the recuperation of the original RCT population, and the cross-sectional study carried out on the reenrolled cohort.
The authors thank financial support from Fulbright Colciencias and Colombia Cientifica – Alianza EFI #60185 contract #FP44842- 220-2018, funded by The World Bank through the Scientific Ecosystems, managed by the Colombian Administrative Department of Science, Technology and Innovation (COLCIENCIAS) for these new analyses.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lana.2024.100921.
Appendix ASupplementary data
References
- 1.Walani S.R. Global burden of preterm birth. Int J Gynecol Obstet. 2020;150:31–33. doi: 10.1002/ijgo.13195. [DOI] [PubMed] [Google Scholar]
- 2.Perin J., Mulick A., Yeung D., et al. Global, regional, and national causes of under-5 mortality in 2000–19: an updated systematic analysis with implications for the sustainable development goals. Lancet Child Adolesc Health. 2022;6:106–115. doi: 10.1016/S2352-4642(21)00311-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization . 2023. Preterm birth.https://www.who.int/news-room/fact-sheets/detail/preterm-birth published online March 10. [Google Scholar]
- 4.MINSALUD . Ministerio de Salud y Protección Social; 2017. Actualización de los Lineamientos Técnicos para la implementación de Programas Madre Canguro en Colombia, con énfasis en la nutrición del neonato prematuro o de bajo peso al nacer. [Google Scholar]
- 5.Jesus LMR de, Basso C.S.D., Castiglioni L., Monserrat A.L., Arroyo MA. da S. Speech-language-hearing follow-up of preterm children: feeding and neuropsychomotor performance. Revista CEFAC. 2020;22 doi: 10.1590/1982-0216/202022415119. [DOI] [Google Scholar]
- 6.Kono Y. Neurodevelopmental outcomes of very low birth weight infants in the neonatal research network of Japan: importance of neonatal intensive care unit graduate follow-up. Clin Exp Pediatr. 2021;64:313–321. doi: 10.3345/cep.2020.01312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vollmer B., Stålnacke J. Young adult motor, sensory, and cognitive outcomes and longitudinal development after very and extremely preterm birth. Neuropediatrics. 2019;50:219–227. doi: 10.1055/s-0039-1688955. [DOI] [PubMed] [Google Scholar]
- 8.Purcell P.L., Cushing S.L., Papsin B.C., Gordon K.A. Unilateral hearing loss and single-sided deafness in children: an update on diagnosis and management. Curr Otorhinolaryngol Rep. 2020;8:259–266. [Google Scholar]
- 9.Prasad Sahu U., Kumar Singh B., Narayan Prasad K. Prospective observational assessment of hearing impairment in newborns admitted to A neonatal intensive care unit (NICU) study. Int J Pharmaceut Chem Res. 2022;14:695–702. [Google Scholar]
- 10.Mandal S., Banerjee M., Ghosh P., Mallick A.K., Kanjilal S. Early detection of hearing impairment in high-risk new-borns. Pediatr Oncall. 2019;16 doi: 10.7199/ped.oncall.2019.18. [DOI] [Google Scholar]
- 11.Neumann K., Chadha S., Tavartkiladze G., Bu X., White K.R. Newborn and infant hearing screening facing globally growing numbers of people suffering from disabling hearing loss. Int J Neonatal Screen. 2019;5:7. doi: 10.3390/ijns5010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olarte M., Bermúdez Rey M.C., Beltran A.P., et al. Detection of hearing loss in newborns: definition of a screening strategy in Bogotá, Colombia. Int J Pediatr Otorhinolaryngol. 2019;122:76–81. doi: 10.1016/j.ijporl.2019.03.016. [DOI] [PubMed] [Google Scholar]
- 13.Petriçli İ.S., Kara C., Arman A. Is being small for gestational age a risk factor for strabismus and refractive errors at 3 years of age? Turk J Pediatr. 2020;62:1049–1057. doi: 10.24953/turkjped.2020.06.017. [DOI] [PubMed] [Google Scholar]
- 14.Chaves-Samaniego M.J., Chaves-Samaniego M.C., Hoyos A.M., Serrano J.L.G. 2021. New evidence on the protector effect of weight gain in retinopathy of prematurity.www.analesdepediatria.org [DOI] [PubMed] [Google Scholar]
- 15.Hanif M., Ariff S., Ansar A., Ahmed K., Hussain A.S. 2020. Characteristics of preterm with sight treatening Rethinopathy of Prematurity.http://www.jamc.ayubmed.edu.pk [PubMed] [Google Scholar]
- 16.Zhang R.H., Liu Y.M., Dong L., et al. Prevalence, years lived with disability, and time trends for 16 causes of blindness and vision impairment: findings highlight retinopathy of prematurity. Front Pediatr. 2022;10:1–9. doi: 10.3389/fped.2022.735335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zimmermann Carrion J., Fortes Filho J.B., Tartarella M.B., Zin A., Dorneles Jornada I. Prevalence of retinopathy of prematurity in Latin America. Clin Ophthalmol. 2011;5:1687–1695. doi: 10.2147/OPTH.S25166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charpak N., Ruiz-Peláez J.G., Figueroa Z., et al. Kangaroo mother versus traditional care for newborn infants ≤2000 grams: a randomized, controlled trial. Pediatrics. 1997;100:682–688. doi: 10.1542/peds.100.4.682. [DOI] [PubMed] [Google Scholar]
- 19.Charpak N., Ruiz-Peláez J.G., Figueroade C.Z., Charpak Y. A randomized, controlled trial of kangaroo mother care: results of follow-up at 1 year of corrected age. Pediatrics. 2001;108:1072–1079. doi: 10.1542/peds.108.5.1072. [DOI] [PubMed] [Google Scholar]
- 20.Mathiowetz V., Weber K., Kashman N., Volland G. Adult norms for the nine hole peg test of finger dexterity. Occup Ther J Res. 1985;5:24–38. doi: 10.5014/ajot.39.6.386. [DOI] [PubMed] [Google Scholar]
- 21.Donders J. A confirmatory factor analysis of the California Verbal Learning Test-Second Edition (CVLT-II) in the standardization sample. Assessment. 2008;15:123–131. doi: 10.1177/1073191107310926. [DOI] [PubMed] [Google Scholar]
- 22.Irby S.M., Floyd R.G. Test review: wechsler abbreviated scale of intelligence, 2nd ed. Can J Sch Psychol. 2013;28:295–299. [Google Scholar]
- 23.Keller A.S., Jagadeesh A.V., Bugatus L., Williams L.M., Grill-Spector K. Attention enhances category representations across the brain with strengthened residual correlations to ventral temporal cortex. Neuroimage. 2022;249:1–35. doi: 10.1016/j.neuroimage.2022.118900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cismaru A.L., Gui L., Vasung L., Lejeune F., Hüppi P.S., Miller S. Altered amygdala development and fear processing in prematurely born infants. Front Neuroanat. 2016;10:1–10. doi: 10.3389/fnana.2016.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conners C.K. 1994. The Conners continuous performance test. Toronto, Canada. [Google Scholar]
- 26.Tessier R., Cristo M.B.M.B., Velez S., et al. Kangaroo Mother Care: a method for protecting high-risk low-birthweight and premature infants against developmental delay. Behav Dev. 2003;26:384–397. [Google Scholar]
- 27.Noreau L., Fougeyrollas P., Vincent C. The LIFE-H: assessment of the quality of social participation. Technol Disabil. 2002;14:113–118. [Google Scholar]
- 28.Charpak N., Tessier R., Ruiz J.G., et al. Twenty-year follow-up of kangaroo mother care versus traditional care. Pediatrics. 2017;139:1–10. doi: 10.1542/peds.2016-2063. [DOI] [PubMed] [Google Scholar]
- 29.Griffiths R. Manual the 1996 revision Huntley: association for research in infant and child development. 1996. The Griffiths mental development scales from birth to 2 years. [Google Scholar]
- 30.Darmstadt G.L., Kirkwood B., Gupta S., et al. WHO Global Position Paper and Implementation Strategy on kangaroo mother care call for fundamental reorganisation of maternal-infant care. Lancet. 2023 doi: 10.1016/S0140-6736(23)01000-0. [DOI] [PubMed] [Google Scholar]
- 31.Barker D.J.P. Adult consequences of fetal growth restriction. Clin Obstet Gynecol. 2006;49:270–283. doi: 10.1097/00003081-200606000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Parra-Saavedra M., Crovetto F., Triunfo S., et al. Neurodevelopmental outcomes of near-term small-for-gestational-age infants with and without signs of placental underperfusion. Placenta. 2014;35:269–274. doi: 10.1016/j.placenta.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 33.Inder T.E., Volpe J.J., Anderson P.J. Defining the neurologic consequences of preterm birth. N Engl J Med. 2023;389:441–453. doi: 10.1056/NEJMra2303347. [DOI] [PubMed] [Google Scholar]
- 34.Day K.L., Dobson K.G., Schmidt L.A., et al. Exposure to overprotective parenting and psychopathology in extremely low birth weight survivors. Child Care Health Dev. 2018;44:234–239. doi: 10.1111/cch.12498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kirchner L., Wald M., Jeitler V., Pollak A. In vitro comparison of noise levels produced by different CPAP generators. Neonatology. 2012;101:95–100. doi: 10.1159/000329558. [DOI] [PubMed] [Google Scholar]
- 36.Zimmerman E., Lahav A. Ototoxicity in preterm infants: effects of genetics, aminoglycosides, and loud environmental noise. J Perinatol. 2013;33:3–8. doi: 10.1038/jp.2012.105. [DOI] [PubMed] [Google Scholar]
- 37.Khurana P., Cushing S.L., Chakraborty P.K., et al. Early hearing detection and intervention in Canada. Paediatr Child Health. 2021;26:141–144. doi: 10.1093/pch/pxaa064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramesh B.Y., Kashyap H., Kini P., Shrikiran Characteristics of hearing impairment in children aged six months to two years with global developmental delay. J Nepal Paediatr Soc. 2021;41:140–146. [Google Scholar]
- 39.Le Clercq C.M.P., Labuschagne L.J.E., Franken M.C.J.P., et al. Association of slight to mild hearing loss with behavioral problems and school performance in children. JAMA Otolaryngol Head Neck Surg. 2020;146:113–120. doi: 10.1001/jamaoto.2019.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.