Abstract
Background and Aims
The clinical characteristics and risk factors involved in the development of liver fibrosis in the subtypes of steatotic liver disease (SLD) remain unknown. We examined the clinical characteristics of SLD subtypes using a large Japanese cohort.
Methods
We performed a cross-sectional analysis (total n = 108,446). In this cohort, SLD was diagnosed by ultrasonography. Individuals with none of the cardiometabolic risk factors were excluded.
Results
According to their nonalcoholic fatty liver disease (NAFLD) and metabolic dysfunction-associated steatotic liver disease (MASLD) status based on the database, participants with cardiometabolic criteria were allocated to the MASLD, MASLD with increased alcohol intake (MetALD), and alcohol-associated liver disease (ALD) with metabolic dysfunction groups. Of 30,857 subjects with SLD, 21,488 (69.6%) had NAFLD, and 20,922 (67.8%) had MASLD. There were few differences in the clinical characteristics between NAFLD and MASLD. After adjustment for clinical variables, we found that male patients with MetALD [odds ratio (OR) 2.26; 95% confidence interval (CI) 1.87–2.84] and ALD with metabolic dysfunction (OR 3.92; 95% CI 2.85–5.39) had a significantly higher risk for advanced liver fibrosis (diagnosed by Fibrosis-4 (FIB-4) index >2.67) compared to those with MASLD. In female patients with ALD, metabolic dysfunction (OR 5.80; 95% CI 2.51–13.4) and systemic blood pressure of ≥130 mmHg were significant risk factors for high FIB-4 (males: OR 3.38, 95% CI 2.51–4.55; females: OR 4.34, 95% CI 2.66–7.07, P < .001).
Conclusion
Alcohol intake and systolic blood pressure are independent contributors to liver fibrosis progression assessed by FIB-4 in SLD.
Keywords: Metabolic Dysfunction, Alcohol Intake, Systolic Blood Pressure, Steatotic Liver Disease, FIB-4 Index
Globally, nonalcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease.1 Experts from multiple hepatology societies have recently suggested using the term “metabolic dysfunction-associated steatotic liver disease (MASLD)” instead of NAFLD.2 The new nomenclature will also include a new category called “MetALD” to describe patients with MASLD who consume greater amounts of alcohol per week (alcohol intake: 140–350 g/wk for females and 210–420 g/wk for males).
One recent report revealed that the discrepancy between MASLD and NAFLD is minimal; thus, is reasonable to consider that findings from older NAFLD studies will remain valid under the new MASLD definition.3 Li et al found from the Third National Health and Nutrition Examination Survey (NHANES III) that compared to those without hepatic lipidosis, pure MASLD (hazard ratio [HR] 1.234; 95% confidence interval [CI] 1.118–1.362), MetALD (HR 1.690; 95% CI 1.213–2.355), and alcohol-associated liver disease (ALD) with metabolic dysfunction (HR 1.995; 95% CI 1.275–3.121) were associated with significantly higher all-cause mortality after adjustments for age, sex, and ethnicity.4
Given the definition of MetALD, there is now a growing clinical demand to investigate how moderate alcohol consumption, which has been relatively overlooked in the past, influences metabolic factors and the progression of liver fibrosis. In this study, we used a large health examination cohort collected in a multicenter study to identify 1) whether there are clinical differences between NAFLD and MASLD and 2) variables independently involved in advanced liver fibrosis assessed by FIB-4 >2.67 in each steatotic liver disease (SLD) subtype.
Experimental Procedures
This study, based on a registry, was conducted across multiple centers and involved a historical cohort. The study received approval from the Institutional Review Board of Osaka Metropolitan University in a batch review (approval no. 2022-031, received on September 14, 2022). The study was also registered in the University Hospital Medical Information Network (UMIN) Clinical Trials Registry (UMIN-CTR no. UMIN000049419). Informed consent requirements were waived because the study was retrospective and relied solely on existing information. Instead, we offered an opt-out choice, which was explained in the instructions available on each hospital's website. The study adhered to the Strengthening the Reporting of Observational Studies in Epidemiology reporting guidelines.5
Data Sources
We utilized data from the Japan Study Group of NAFLD, specifically the Medical Health Checkup Registry for Metabolic Syndrome, Chronic Kidney Disease, and Fatty Liver in Japan (MIRACLE-J) database, to gather information about individuals who underwent health checkups.6 This database includes information from 13 health checkup centers in Japan, which are: JA Yamanashi Koseiren Health Care Center, MedCity 21, Asahikawa-Kosei General Hospital, KKR Takamatsu Hospital, Heart Life Hospital, Shimane Institute of Health Science, Saga Health and Clinical Examination Center, Nara Medical University, Japanese Red Cross Asahikawa Hospital, Kawasaki Medical Center, Kanagawa Dental University Yokohama Clinic, Loco Medical Eguchi Hospital, and Northern OKINAWA Medical Center. All study data were collected and managed through REDCap electronic data capture tools, hosted at Osaka Metropolitan University.7,8
Study Cohort
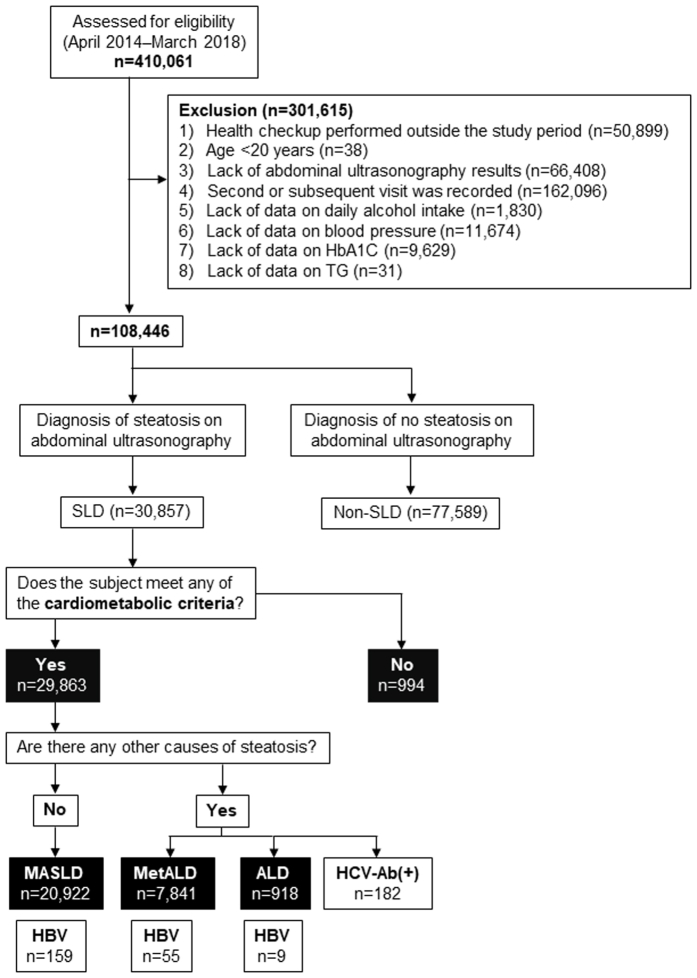
In this cross-sectional multicenter study, we retrospectively included individuals who underwent a health checkup between April 1, 2014, and March 31, 2018. Initially, the study encompassed 410,061 participants who underwent medical examinations (Figure A1). The exclusion criteria were as follows: 1) the health checkup was performed outside the study time period (n = 50,899), 2) age <20 years (n = 38), 3) lack of abdominal ultrasonography results (n = 66,408), 4) a second or subsequent visit was recorded (n = 162,096), 5) lack of data on daily alcohol intake (n = 1830), 6) data on blood pressure (BP) (n = 11,674), 7) lack of data on hemoglobin A1C (HbA1C) (n = 9629), and 8) lack of data on triglycerides (TG) (n = 31). After the exclusion criteria were applied, a total of 108,446 participants were analyzed (Figure A1). We excluded diseases affecting platelet counts (eg, idiopathic thrombocytopenic purpura and essential thrombocythemia) from this study whenever possible. Considering the positivity rate in Japanese health checkups, we judged the missing data in HBsAg and hepatitis C virus (HCV) antibodies as negative. Cases with missing data other than HBsAg and HCV-Ab were excluded from the analysis. Before statistical analysis, the medical records were reviewed for data inconsistencies regarding the use of medications for diabetes mellitus, hypertension, and dyslipidemia, as well as inconsistencies regarding smoking status (ie, between smoking history and the number of cigarettes smoked per day).
Statistical Analysis
Descriptive statistics (mean [standard deviation (SD)] or number [%]) were calculated for all variables. For continuous variables, Dunnett’s analysis was used to test for multiple comparisons of MetALD and ALD against the MASLD group (control). Categorical variables were compared using Fisher’s exact test. Logistic regression models were adjusted for cardiometabolic criteria, alcoholic intake, and smoking status (never vs experienced (ex) and current smoker). Because alcohol consumption has different effects based on sex and also based on systolic and diastolic BP,9 both systolic and diastolic BP values were used as factors in this analysis. Values of P < .05 were considered indicative of statistical significance. Statistical analyses were conducted using JMP 17.2.0 (SAS Institute Inc, Cary, NC, USA).
Results
Subject Characteristics and the Prevalence of SLD
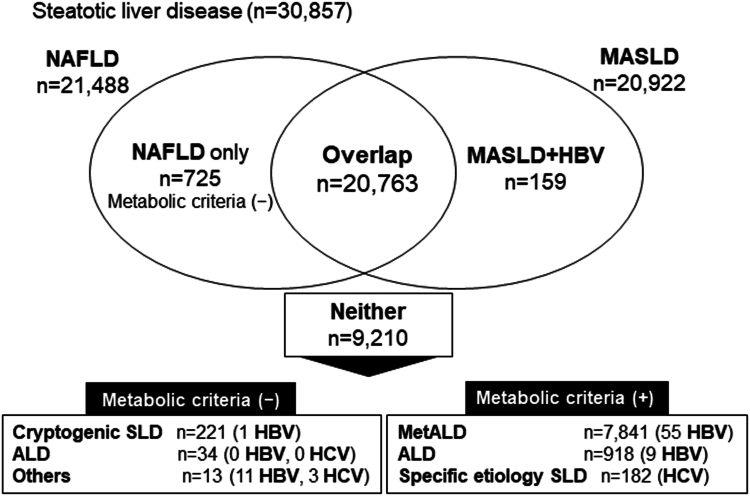
Of the 108,446 participants, 30,857 (28.4%) were diagnosed with SLD on abdominal ultrasound (Figure A1). Furthermore, of the 30,857 subjects with SLD, 29,863 (96.8%) met the cardiometabolic criteria. The percentages matching each of the cardiometabolic criteria in subjects with SLD are shown in Table A1). Of the 29,863 subjects, 20,922 had MASLD, 725 had NAFLD only (ie, those not meeting the cardiometabolic criteria), 20,763 had overlapping NAFLD/MASLD, 7841 had MetALD, 918 had ALD with metabolic dysfunction, and 182 had specific-etiology SLD (ie, HCV) (Table A2, Figures A1 and 1).
Figure 1.
Prevalence of NAFLD and MASLD in our study cohort. Among 30,857 subjects, the number of NAFLD-only subjects was 21,488, and the number of MASLD-only subjects was 20,922. The number of overlapping subjects (ie, those with NAFLD and MASLD) was 20,763. Approximately 97% of subjects with NAFLD and 99% of subjects with MASLD were overlapping subjects in our cohort.
The MetALD group was significantly younger than the MASLD group (MetALD vs MASLD; 53.4 vs 54.1 years, P < .001), had a significantly lower proportion of females (11% vs 38%), and had a higher proportion of patients with hypertension (41% vs 33%, P < .001, respectively) (Table 1). In addition, the alanine aminotransferase (ALT, 28.5 vs 25.4 U/L), gamma glutamyl transpeptidase (GGT, 75.9 vs 42.1 U/L), and UA (6.43 vs 5.96 mg/dL) levels, the aspartate aminotransferase (AST)/ALT ratio (0.94 vs 0.90), and the FIB-4 (1.27 vs 1.14) were significantly higher in the MetALD group than in the MASLD group (all P < .001). Moreover, the ALD group was characterized by a significantly lower age (ALD vs MASLD; 50.8 vs 54.1 years, P < .001), a significantly lower proportion of females (13% vs 38%), and a significantly higher rate of hypertension (42% vs 33%) (all P < .001) (Table 1). ALT (35.4 vs 25.4 U/L), GGT (123 vs 42.1 U/L), UA (6.72 vs 5.96 mg/dL), AST/ALT ratio (1.01 vs 0.90), and FIB-4 (1.38 vs 1.14) (ALD vs MASLD, P < .001 for all). FIB-4 >2.67 (7.2% vs 1.6%) in ALD group were significantly higher than in the MASLD group (P < .001).
Table 1.
Clinical Characteristics of MASLD, MetALD, and ALD With Metabolic Dysfunction
| Characteristics | MASLD | MetALD | ALD with |
|---|---|---|---|
| Metabolic dysfunction | |||
| Number | 20,922 | 7841 | 918 |
| Age (y) | 54.1 (11.0) | 53.4 (10.3) | 50.8 (9.5) |
| Sex (female) | 7915 (38%) | 836 (11%) | 116 (13%) |
| BMI (kg/m2) | 26.1 (3.7) | 26.0 (3.4) | 26.1 (3.8) |
| Waist circumference (cm) | 90.2 (9.1) | 91.0 (8.6) | 92.0 (9.1) |
| Systolic blood pressure (mmHg) | 123 (15.0) | 126 (14.8) | 127 (15.8) |
| Diastolic blood pressure (mmHg) | 76.6 (10.7) | 79.7 (10.6) | 81.0 (11.1) |
| Type 2 diabetes mellitus | 3107 (15%) | 1139 (15%) | 142 (16%) |
| Hypertension | 6835 (33%) | 3155 (41%) | 365 (42%) |
| Dyslipidemia | 10,791 (52%) | 3546 (46%) | 408 (47%) |
| Alcohol intake (g/day) | 4.74 (6.6) | 37.4 (10.0) | 71.7 (6.5) |
| Smoking (never vs ex + current) (%) | 54.6/45.4 | 26.5/73.5 | 21.2/78.8 |
| Biochemistry | |||
| Platelet count (×109/L) | 238 (55.5) | 229 (53.4) | 232 (54.4) |
| AST (U/L) | 25.4 (12.0) | 28.5 (16.7) | 35.4 (35.3) |
| ALT (U/L) | 32.6 (22.4) | 34.4 (25.1) | 38.2 (31.2) |
| ALP (U/L) | 220 (63.7) | 206 (58.4) | 206 (61.5) |
| GGT (U/L) | 42.1 (39.6) | 75.9 (84.7) | 123 (163) |
| Cholinesterase (U/L) | 375 (65.7) | 367 (65.6) | 362 (68.5) |
| Albumin (g/dL) | 4.40 (0.27) | 4.42 (0.28) | 4.39 (0.29) |
| Total bilirubin (mg/dL) | 1.04 (1.19) | 1.12 (1.28) | 1.03 (1.04) |
| Triglycerides (mg/dL) | 140 (89.0) | 164 (124) | 191 (146) |
| HDL-cholesterol (mg/dL) | 52.4 (12.4) | 55.0 (13.9) | 57.4 (15.8) |
| LDL-cholesterol (mg/dL) | 131 (31.0) | 126 (30.9) | 120 (33.0) |
| Non-HDL-cholesterol (mg/dL) | 154 (34.2) | 152 (33.8) | 151 (35.3) |
| Fasting blood glucose (mg/dL) | 106 (22.5) | 109 (22.7) | 111 (26.9) |
| HbA1c (%) | 5.99 (0.79) | 5.90 (0.75) | 5.86 (0.83) |
| BUN (mg/dL) | 13.9 (3.5) | 13.7 (3.4) | 13.1 (3.4) |
| Creatinine (mg/dL) | 0.78 (0.21) | 0.83 (0.16) | 0.79 (0.15) |
| eGFR (mL/min/1.73 m2) | 75.7 (14.9) | 76.9 (14.7) | 81.1 (15.4) |
| CRP (mg/dL) | 0.15 (0.34) | 0.15 (0.36) | 0.17 (0.34) |
| Uric acid (mg/dL) | 5.96 (1.31) | 6.43 (1.30) | 6.72 (1.34) |
| Noninvasive tests | |||
| AST/ALT ratio | 0.90 (0.30) | 0.94 (0.32) | 1.01 (0.40) |
| FIB-4 index | 1.14 (0.60) | 1.27 (0.70) | 1.38 (1.01) |
| FIB-4 >2.67 | 343 (1.6%) | 300 (3.8%) | 67 (7.2%) |
ALP, alkaline phosphatase; BUN, blood urea nitrogen; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c.
Daily Alcohol Intake by Age and Sex
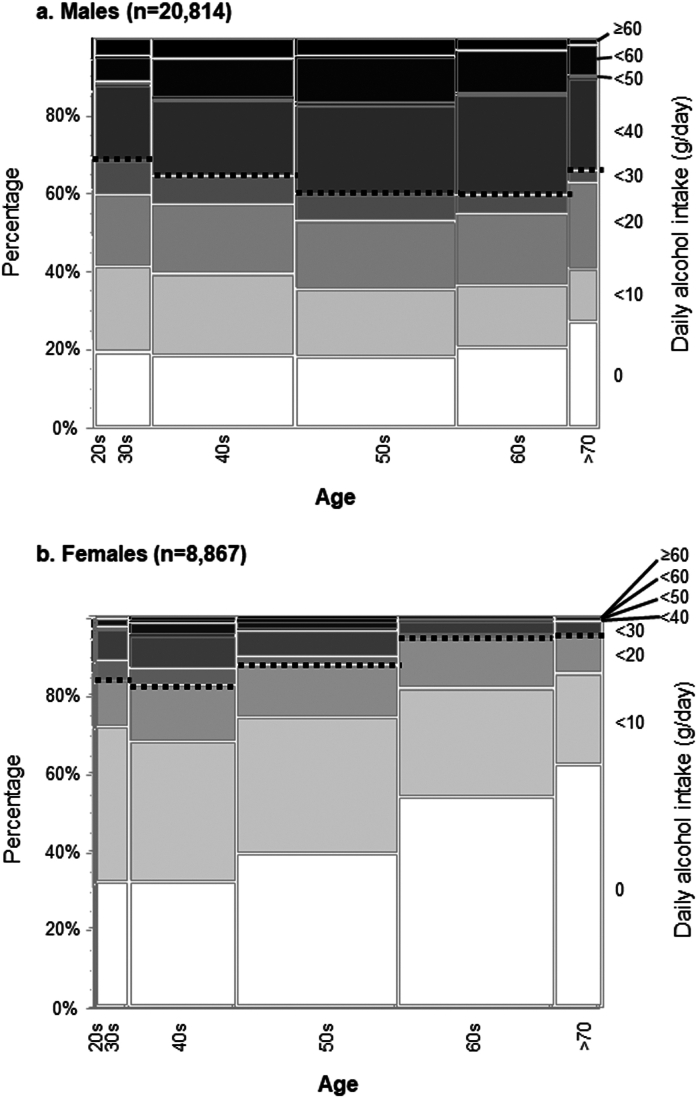
We compared daily alcohol intake stratified by age groups and sex in Figure 2, with the dotted lines indicating the threshold values for drinkers (males >30 g/day, females >20 g/day). More than 30% of males fell into the category of drinkers in all generations. In contrast, the percentage of females who were drinkers declined as they aged, with only 4.9% of those in their 70s meeting the criteria for drinkers. Our data indicated that young females (<60 years) had higher levels of alcohol consumption than older females (≥60 years) (P < .001).
Figure 2.
Prevalence rates of alcohol consumption in males (A) and females (B) stratified by age daily alcohol intake is illustrated by age group and sex. Dotted lines indicate the threshold values for drinkers (males >30 g/day, females >20 g/day).
Association Between Daily Alcohol Intake and Smoking Status by Sex
The association between daily alcohol intake and smoking status was stratified by sex (Figure A2). Of all males, 28.5% were current smokers, and the percentage remained generally constant regardless of the increase in alcohol consumption. In contrast, only 8.4% of all females were current smokers, but the percentage increased with increasing alcohol consumption, with 31.4% of females being smokers in the ≥60 g/day group compared to 6.4% of females in the nondrinkers group.
Percentages of FIB-4 Index Scores (<1.3, 1.3–2.67, >2.67) according to Daily Alcohol Intake in Young (<60 years) and Older (>60 years) Individuals Stratified by Sex
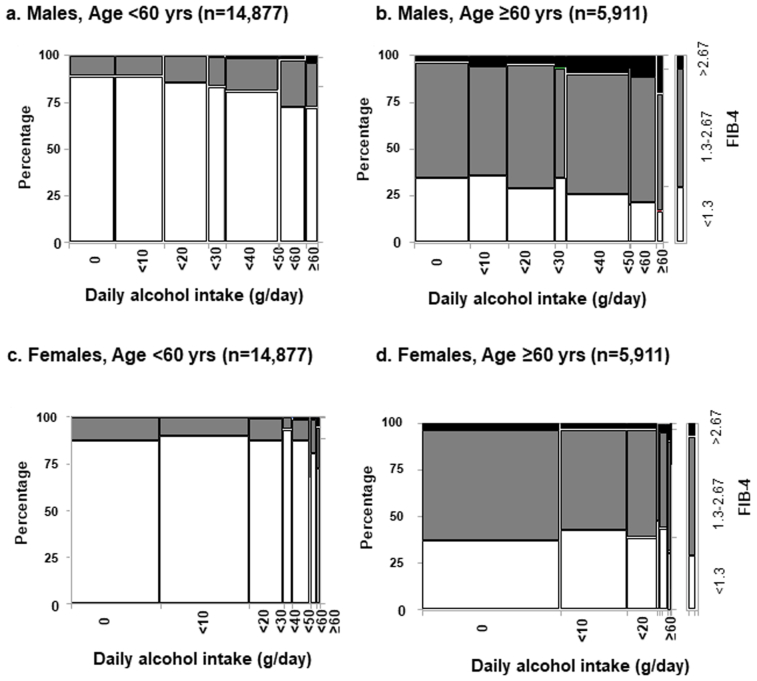
We investigated the changes in fibrosis-4 index (FIB-4 index) scores (<1.3, 1.3–2.67, >2.67) according to age group, sex, and alcohol consumption in SLD. The percentages of FIB-4 index scores stratified by sex and alcohol consumption are shown in Figure A3. The percentages of FIB-4 >2.67 were 0.9% in the younger (<60 years) male group and 7.0% in the older (≥60 years) male group. The percentage of males with FIB-4>2.67 increased with increasing alcohol consumption in both the younger and older groups. Among males, 4.2% of the young and 19.8% of the older individuals with daily alcohol intake levels ≥60 g/day had FIB-4 >2.67 (Figure A3A and B). In contrast, the percentages of FIB-4 >2.67 were 0.4% among younger females (<60 years) and 3.5% among older females (≥60 years). In the older female group, only 46 people in total drank 40 g or more per day. The percentage of females with FIB-4 >2.67 did not increase with increasing alcohol intake in either the young or the older group (Figure A3C and D).
Relationships Between Cardiometabolic Factors, Alcohol Consumption, Smoking Status, and Advanced Liver Fibrosis
We next examined the relationships between cardiometabolic factors, daily alcohol intake, smoking, and advanced liver fibrosis defined by FIB-4>2.67 and stratified by sex. Tables 2 and 3 show the results of the univariate and multivariate analyses. Although many variables were significant in the univariate analysis, the multivariate analysis revealed that among males (n = 20,814), MetALD [odds ratio (OR) 2.26, P < .001], ALD (OR 3.92, P < .001), body mass index (BMI) criteria (OR 0.76, P = .020), systolic BP ≥ 130 mmHg (OR 3.38, P < .001), FBS criteria (OR 1.78, P < .001), and TG criteria (OR 0.80, P = .025) were identified as independent factors. In contrast, among females (n = 8867), ALD (OR 5.80, P < .001), smoking status (OR 0.41, P = .003), BMI criteria (OR 0.57, P = .002), systolic BP ≥ 130 mmHg (OR 4.34, P < .001), and HDL criteria (OR 1.83, P < .001) were identified as independent factors.
Table 2.
Univariate and Multivariate Odds Ratios for FIB-4 >2.67 by Sex (Males; n = 20,814)
| Variable | Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P value | Odds ratio | 95% CI | P value | |
| Alcohol intake | ||||||
| MASLD | 1 [reference] | 1 [reference] | ||||
| MetALD | 2.68 | 2.23–3.21 | <.001 | 2.26 | 1.87–2.84 | <.001 |
| ALD | 4.92 | 3.64–6.65 | <.001 | 3.92 | 2.85–5.39 | <.001 |
| Smoking status | ||||||
| Ex or current smoker | 1.45 | 1.19–1.76 | <.001 | 1.22 | 0.99–1.49 | .060 |
| BMI criteria | 0.77 | 0.62–0.96 | .019 | 0.76 | 0.61–0.96 | .020 |
| Sys BP ≥ 130 mmHg | 4.11 | 3.33–5.08 | <.001 | 3.38 | 2.51–4.55 | <.001 |
| Dia BP ≥ 85 mmHg | 2.91 | 2.41–3.52 | <.001 | 1.07 | 0.82–1.39 | .64 |
| FBS criteria | 2.19 | 1.71–2.82 | <.001 | 1.78 | 1.38–2.31 | <.001 |
| TG criteria | 0.88 | 0.73–1.04 | .133 | 0.80 | 0.67–0.97 | .025 |
| HDL criteria | 0.64 | 0.54–0.75 | <.001 | 0.84 | 0.70–1.03 | .069 |
FBS, fasting blood sugar; HDL, high density lipoprotein; TG, triglycerides.
Bold values demonstrate statistically significant values.
Table 3.
Univariate and Multivariate Odds Ratios for FIB-4 >2.67 by Sex (Females; n = 8867)
| Variable | Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P value | Odds ratio | 95% CI | P value | |
| Alcohol intake | ||||||
| MASLD | 1 [reference] | 1 [reference] | ||||
| MetALD | 0.93 | 0.53–1.66 | .81 | 1.19 | 0.65–2.19 | .57 |
| ALD | 3.78 | 1.73–8.27 | <.001 | 5.80 | 2.51–13.4 | <.001 |
| Smoking status | ||||||
| Ex or current smoker | 0.44 | 0.26–0.75 | .003 | 0.41 | 0.23–0.74 | .003 |
| BMI criteria | 0.65 | 0.46–0.92 | .016 | 0.57 | 0.40–0.82 | .002 |
| Sys BP ≥ 130 mmHg | 3.64 | 2.49–5.33 | <.001 | 4.34 | 2.66–7.07 | <.001 |
| Dia BP ≥ 85 mmHg | 2.08 | 1.50–2.89 | <.001 | 0.75 | 0.49–1.15 | .19 |
| FBS criteria | 1.68 | 1.05–2.69 | .032 | 1.48 | 0.90–2.43 | .009 |
| TG criteria | 0.88 | 0.60–1.30 | .53 | 0.68 | 0.45–1.04 | .073 |
| HDL criteria | 1.54 | 1.10–2.14 | .01 | 1.83 | 1.29–2.61 | <.001 |
FBS, fasting blood sugar; HDL, high density lipoprotein; TG, triglycerides.
Bold values demonstrate statistically significant values.
FIB-4 may have a lower rate of positive diagnosis, especially among older individuals, due to the inclusion of age in the formula.10,11 Therefore, Tables 2 and 3 were divided into subanalyses for the younger group (<60 years; n = 20,092) and the older group (≥60 years; n = 9589). Tables 4 and 5 show the results of the univariate and multivariate analyses stratified by sex with FIB-4 >2.67 as the outcome in the younger age group. Multivariate analysis revealed that among males (n = 14,899), MetALD (OR 2.84, P < .001), ALD (OR 7.54, P < .001), BMI criteria (OR 0.45, P < .001), and systolic BP ≥ 130 mmHg (OR 2.79, P < .001) were identified as independent factors. Among females (n = 5193), MetALD (OR 3.44, P = .026), ALD (OR 16.6, P < .001), BMI criteria (OR 0.20, P < .001), and systolic BP ≥ 130 mmHg (OR 3.18, P = .047) were identified as independent factors. Tables 6 and 7 show the results of the univariate and multivariate analyses by sex with FIB-4 >2.67 as the outcome in the group of older individuals. Multivariate analysis revealed that among males (n = 5915), MetALD (OR 2.23, P < .001), ALD (OR 4.93, P < .001), systolic BP ≥ 130 mmHg (OR 1.91, P < .001), and FBS criteria (OR 1.61, P = .008) were identified as independent factors. Among females (n = 3674), ALD (OR 6.12, P = .006), systolic BP ≥ 130 mmHg (OR 3.29, P < .001), TG criteria (OR 0.54, P = .012), and HDL criteria (OR 2.09, P < .001) were identified as independent factors.
Table 4.
Univariate and Multivariate Odds Ratios for FIB-4 >2.67 by Sex. Age <60 (Males; n = 14,899)
| Variable | Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | p value | Odds ratio | 95% CI | P value | |
| Alcohol intake | ||||||
| MASLD | 1[reference] | 1[reference] | ||||
| MetALD | 3.58 | 2.40–5.36 | <.001 | 2.84 | 1.87–4.32 | <.001 |
| ALD | 10.5 | 6.34–17.5 | <.001 | 7.54 | 4.41–12.9 | <.001 |
| Smoking status | ||||||
| Ex or current smoker | 1.73 | 1.15–2.61 | .009 | 1.27 | 0.83–1.94 | .26 |
| BMI criteria | 0.46 | 0.31–0.69 | <.001 | 0.45 | 0.29–0.68 | <.001 |
| Sys BP ≥ 130 mmHg | 4.46 | 2.97–6.68 | <.001 | 2.79 | 1.56–4.99 | <.001 |
| Dia BP ≥ 85 mmHg | 3.89 | 2.64–5.75 | <.001 | 1.66 | 0.95–2.90 | .077 |
| FBS criteria | 1.24 | 0.83–1.86 | .28 | 0.99 | 0.66–1.50 | .98 |
| TG criteria | 1.22 | 0.86–1.71 | .27 | 0.99 | 0.69–1.44 | .99 |
| HDL criteria | 0.69 | 0.49–0.98 | .038 | 0.97 | 0.67–1.41 | .88 |
FBS, fasting blood sugar; HDL, high density lipoprotein; TG, triglycerides.
Bold values demonstrate statistically significant values.
Table 5.
Univariate and Multivariate Odds Ratios for FIB-4 >2.67 by Sex. Age <60 (Females; n = 5193)
| Variable | Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P value | Odds ratio | 95% CI | P value | |
| Alcohol intake | ||||||
| MASLD | 1 [reference] | 1 [reference] | ||||
| MetALD | 2.63 | 0.93–7.40 | .067 | 3.44 | 1.16–10.2 | .026 |
| ALD | 14.9 | 4.8–46.7 | <.001 | 16.6 | 4.53–61.0 | <.001 |
| Smoking status | ||||||
| Ex or current smoker | 0.89 | 0.33–2.42 | .82 | 0.52 | 0.17–1.61 | .26 |
| BMI criteria | 0.22 | 0.09–0.50 | <.001 | 0.20 | 0.08–0.49 | <.001 |
| Sys BP ≥ 130 mmHg | 2.34 | 0.99–5.48 | .051 | 3.18 | 1.01–10.01 | .047 |
| Dia BP ≥ 85 mmHg | 1.30 | 0.54–3.15 | .56 | 0.60 | 0.19–1.93 | .40 |
| FBS criteria | 1.66 | 0.56–4.90 | .36 | 2.29 | 0.66–7.99 | .192 |
| TG criteria | 2.14 | 0.91–5.01 | .081 | 1.63 | 0.63–4.19 | .31 |
| HDL criteria | 1.30 | 0.54–3.10 | .56 | 1.49 | 0.55–4.03 | .43 |
FBS, fasting blood sugar; HDL, high density lipoprotein; TG, triglycerides.
Bold values demonstrate statistically significant values.
Table 6.
Univariate and Multivariate Odds Ratios for FIB-4 >2.67 by Sex. Age ≥60 (Males; n = 5915)
| Variable | Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P value | Odds ratio | 95% CI | P value | |
| Alcohol intake | ||||||
| MASLD | 1 [reference] | 1 [reference] | ||||
| MetALD | 2.35 | 1.90–2.89 | <.001 | 2.23 | 1.79–2.78 | <.001 |
| ALD | 5.59 | 3.67–8.52 | <.001 | 4.93 | 3.17–7.67 | <.001 |
| Smoking status | ||||||
| Ex or current smoker | 1.06 | 0.84–1.34 | .59 | 0.96 | 0.76–1.23 | .77 |
| BMI criteria | 1.15 | 0.88–1.51 | .30 | 1.19 | 0.90–1.58 | .22 |
| Sys BP ≥ 130 mmHg | 2.03 | 1.58–2.61 | <.001 | 1.91 | 2.51–4.55 | <.001 |
| Dia BP ≥ 85 mmHg | 1.58 | 1.27–1.97 | <.001 | 0.89 | 0.65–1.22 | .47 |
| FBS criteria | 1.56 | 1.11–2.19 | .010 | 1.61 | 1.14–2.28 | .008 |
| TG criteria | 0.95 | 0.77–1.17 | .65 | 0.92 | 0.73–1.15 | .46 |
| HDL criteria | 0.71 | 0.58–0.87 | .001 | 0.86 | 0.69–1.07 | .168 |
FBS, fasting blood sugar; HDL, high density lipoprotein; TG, triglycerides.
Bold values demonstrate statistically significant values.
Table 7.
Univariate and Multivariate Odds Ratios for FIB-4 >2.67 by Sex. Age ≥60 (Females; n = 3674)
| Variable | Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P value | Odds ratio | 95% CI | P value | |
| Alcohol intake | ||||||
| MASLD | 1 [reference] | 1 [reference] | ||||
| MetALD | 1.29 | 0.62–2.69 | .49 | 1.39 | 0.63–3.09 | .42 |
| ALD | 4.69 | 1.36–16.1 | .014 | 6.12 | 1.67–22.3 | .006 |
| Smoking status | ||||||
| Ex or current smoker | 0.57 | 0.29–1.10 | .092 | 0.49 | 0.24–1.00 | .052 |
| BMI criteria | 1.02 | 0.69–1.52 | .92 | 0.90 | 0.60–1.35 | .60 |
| Sys BP ≥ 130 mmHg | 2.53 | 1.64–3.91 | <.001 | 3.29 | 1.88–5.75 | <.001 |
| Dia BP ≥ 85 mmHg | 1.54 | 1.07–2.20 | .020 | 0.69 | 0.44–1.11 | .125 |
| FBS criteria | 0.85 | 0.49–1.44 | .54 | 0.86 | 0.50–1.49 | .60 |
| TG criteria | 0.72 | 0.46–1.13 | .152 | 0.54 | 0.34–0.88 | .012 |
| HDL criteria | 1.67 | 1.17–2.39 | .005 | 2.09 | 1.42–3.06 | <.001 |
FBS, fasting blood sugar; HDL, high density lipoprotein; TG, triglycerides.
Bold values demonstrate statistically significant values.
Discussion
Upon evaluating a cohort of Japanese health examination subjects, we found that 1) NAFLD and MASLD were clinically identical, and 2) daily alcohol consumption (MetALD and ALD with metabolic dysfunction) and systolic BP were independent factors among both young and older subjects for the FIB-4 high category in SLD.
Alcohol is a major risk factor for liver cirrhosis, with the risk increasing exponentially as intake increases.12 Alcohol consumption triggers hepatocyte damage (ballooning), which releases hedgehog ligands. These ligands induce hedgehog-responsive genes, which activate hepatic stellate cells in a paracrine fashion and promote extracellular matrix deposition.13 While it is clear that prolonged heavy drinking poses a risk for liver fibrosis and hepatocellular carcinoma, debates regarding the specific threshold and duration of alcohol consumption required to trigger fibrosis are ongoing.12,13 Chang et al found that, over a median follow-up period of 4.9 years, light (1–9.9 g/day) to moderate (10–19.9 d/day) drinking independently increased the degree of fibrosis assessed by FIB-4 by HR 1.08 (0.98–1.16) and HR 1.29 (1.18–1.40), respectively.14 Recently, Islaelsen et al investigated the prognosis of MASLD, MetALD, and ALD in a prospective cohort study.15 As alcohol intake increased, the rate of decompensated liver cirrhosis transition increased, and the overall survival rate decreased. The authors demonstrated that increased alcohol consumption was a prognostic factor independent of age, sex, and liver stiffness.
In Japan, alcohol consumption among young females has been increasing (Ministry of Health, Labor, and Welfare of Japan published guidelines regarding drinking (available in Japanese only; https://www.mhlw.go.jp/content/12200000/001211974.pdf)), with the issue becoming a social problem. Females have less fluid in their bodies and lower liver alcohol dehydrogenase activity levels than males; therefore, alcohol consumption among females poses more medical risks than among males.16,17 In addition, estrogen may be a cofactor in alcohol-associated liver damage.18 In our study, we found that younger females consumed higher amounts of alcohol than older females. Due to this increased consumption, there are concerns that the number of cases of liver fibrosis among females will increase in the future. Very recently, the Ministry of Health, Labor, and Welfare of Japan published guidelines regarding drinking. Such educational resources regarding limiting the amount of alcohol consumed will become increasingly important in the future.
Recently, there has been increasing evidence connecting MASLD and hypertension. A systematic review including 25,260 participants in a total of 11 studies demonstrated that MASLD and hypertension were risk factors independent of each other.19 Federico et al showed that alcohol consumption had significant linear effects on systolic BP, and diastolic BP was modified by sex in a systematic analysis.9 Our study results also demonstrated that systolic BP, but not diastolic BP, was an independent risk factor for a high FIB-4 score, and the FIB-4 score increased as alcohol consumption increased. These results indicate that both systolic BP and alcohol consumption are independent risk factors for the rising FIB-4 index value.
Because age is a component of the FIB-4 and an increase in the FIB-4 partly reflects aging itself, it must be carefully determined whether the worsening of the FIB-4 over time truly reflects a change in the fibrosis score over time. Recently, Blomdahl et al performed serial liver biopsies in 73 NAFLD patients during a median follow-up period of 17.2 years.20 Fibrosis progression was defined as the progression of ≥2 stages or the development of cirrhotic complications. Multivariate analysis revealed that fibrosis progression was generally associated with alcohol consumption levels of 10–20 g/day. Little is known about how the amount of alcohol consumption associated with MetALD (average 20–50 g/d for women and 30–60 g/d for men) affects the development of liver fibrosis. In our cross-sectional study, we examined the significance of the amount of alcohol consumption that falls under MetALD. We also performed a subanalysis in 2 groups: under 60 years of age and over 60 years of age. Our results demonstrated that alcohol consumption was an independent risk factor for high FIB-4 irrespective of age.
The FIB-4 is useful for evaluating the progression of liver fibrosis and predicting prognosis in chronic liver diseases.21 However, it should be noted that the FIB-4 may be overestimated in patients with alcoholic liver disease, as abnormalities in blood liver enzyme values are observed with a predominance of AST. Therefore, we examined each SLD category and FIB-4 score using the health checkup cohort. As expected, the FIB-4 score increased with increasing alcohol consumption, and alcohol consumption remained an independent factor for the high FIB4 category. At the same time, systolic BP remained an independent factor (it was a dominant independent factor in all groups, regardless of age or sex).
Interestingly, our data demonstrated that BMI criteria was an independent negative predictor for high FIB4 of young subjects in both male and female, while BMI criteria was not a significant predictor in older subjects (Table 2, Table 3, Table 4, Table 5, Table 6, Table 7). These findings would indicate that liver fibrosis might proceed in lean SLD than nonlean SLD in young subjects. In addition to alcohol intake and BP, lean SLD would be a significant risk factor for the liver fibrosis progression in Japanese young subjects. Further investigation would be needed to elucidate this issue.
Several limitations must be considered when interpreting our results. First, this was a retrospective cross-sectional study; therefore, causal relationships are unknown. Future longitudinal, prospective studies will be required to verify the accuracy of our conclusions. Second, selection bias is a major potential limitation. Most participants were healthy enough to be employed (in contrast to the general population) and were also sufficiently conscientious about their health to voluntarily undergo health checkups.22 The results of this study may not apply to individuals who are not generally healthy. Further studies in a heterogeneous population will be required to validate our findings. Third, we do not have data on how many of our subjects were of non-Japanese ethnicity. Fourth, we do not have data on the patatin-like phospholipase domain-containing 3 gene genotype.
Conclusion
In conclusion, although there are some limitations, our study using a Japanese cohort of individuals who received health checkups demonstrated that NAFLD and MASLD are clinically identical. In addition, daily alcohol consumption and systolic BP were independent factors among both young and older SLD subjects for advanced liver fibrosis, as assessed by FIB-4 >2.67.
Acknowledgments:
This research was financially supported by the Japan Strategic Medical Administration Research Center (J-SMARC). The authors are grateful to Ms. Keiko Ota and Ms. Masayo Kitano (Osaka Metropolitan University) for their technical assistance in creating this REDCap project. The authors are also grateful to Ms. Kozue Tashiro, Ms. Maki Miyahara (Saga University Hospital), and Ms. Miki Noguchi (Kawasaki Medical School) for their technical assistance in creating the virtual slides. The authors also thank BioScience Writers (https://www.biosciencewriters.com/) for providing English language editing services.
Authors’ Contributions:
Yoshihiro Kamada, Hideki Fujii, Masafumi Ono: Conceptualization, drafting of the manuscript; Yuichiro Suzuki, Koji Sawada, Miwa Tatsuta, Tatsuji Maeshiro, Hiroshi Tobita, Tsubasa Tsutsumi, Takemi Akahane, Chitomi Hasebe, Miwa Kawanaka, Takaomi Kessoku, Yuichiro Eguchi, Hayashi Syokita, Asahiro Morishita, Tsutomu Masaki, Takumi Ohmura, Toshio Watanabe, Yoshioki Yoda, Nobuyuki Enomoto: data collection, investigation; Kanako Fuyama, Kazufumi Okada, Naoki Nishimoto, Yoichi M. Ito: data analysis and review of the results; Yoichi M. Ito, Hirokazu Takahashi, Yoshio Sumida: review and editing the manuscript; Hirokazu Takahashi, Yoshio Sumida: supervision.
Footnotes
Conflicts of Interest: This author discloses the following: Hideki Fujii received an analysis stipend from the Japan Strategic Medical Administration Research Center (J-SMARC). The remaining authors disclose no conflicts.
Funding: The authors report no funding.
Ethical Statement: This study, based on a registry, was conducted across multiple centers and involved a historical cohort. The study received approval from the Institutional Review Board of Osaka Metropolitan University in a batch review (approval no. 2022-031, received on September 14, 2022). The study was also registered in the University Hospital Medical Information Network (UMIN) Clinical Trials Registry (UMIN-CTR no. UMIN000049419). Informed consent requirements were waived because the study was retrospective and relied solely on existing information. Instead, we offered an opt-out choice, which was explained in the instructions available on each hospital's website.
Data Transparency Statement: The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to their containing information that could compromise the privacy of research participants.
Reporting Guidelines: STROBE.
Material associated with this article can be found in the online version at https://doi.org/10.1016/j.gastha.2024.08.006.
Supplementary Materials
Figure A1.
Subject flowchart for this study.
Figure A2.
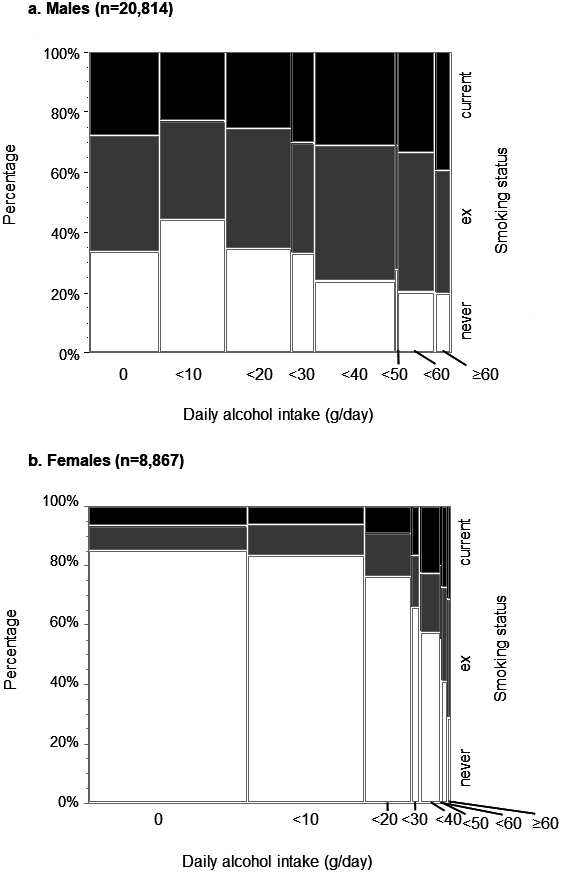
Prevalence rates of smoking status (never, ever, current) among males (A) and females. The association between daily alcohol intake and smoking status is illustrated by sex. White, grey, and black columns indicate never, experienced, and current smokers, respectively.
Figure A3.
Prevalence of FIB-4 index categories among males (A) and females (B) stratified by age; A, C) subjects <60 years, B, D) subjects ≥60 years. The percentages of FIB-4 scores by sex and alcohol consumption are shown. White, grey, and black columns indicate low (<1.3), intermediate (1.3–2.67), and high (>2.67) in FIB-4 scores, respectively.
References
- 1.Younossi Z.M., Wong G., Anstee Q.M., et al. The global burden of liver disease. Clin Gastroenterol Hepatol. 2023;21(8):1978–1991. doi: 10.1016/j.cgh.2023.04.015. [DOI] [PubMed] [Google Scholar]
- 2.Rinella M.E., Lazarus J.V., Ratziu V., et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. 2023;78(6):1966–1986. doi: 10.1097/HEP.0000000000000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song S.J., Che-To Lai J., Lai-Hung Wong G., et al. Can we use old NAFLD data under the new MASLD definition? J Hepatol. 2024;80(2):e54–e56. doi: 10.1016/j.jhep.2023.07.021. [DOI] [PubMed] [Google Scholar]
- 4.Li M., Xie W. Are there all-cause mortality differences between metabolic dysfunction-associated steatotic liver disease subtypes? J Hepatol. 2024;80(2):e53–e54. doi: 10.1016/j.jhep.2023.07.012. [DOI] [PubMed] [Google Scholar]
- 5.Von Elm E., Altman D.G., Egger M., et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495–1499. doi: 10.1016/j.ijsu.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 6.Fujii H., Suzuki Y., Sawada K., et al. Prevalence and associated metabolic factors of nonalcoholic fatty liver disease in the general population from 2014 to 2018 in Japan: a large-scale multicenter retrospective study. Hepatol Res. 2024;53(11):1059–1072. doi: 10.1111/hepr.13947. [DOI] [PubMed] [Google Scholar]
- 7.Harris P.A., Taylor R., Thielke R., et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris P.A., Taylor R., Minor B.L., et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95 doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Federico S., Filippini T., Whelton P.K., et al. Alcohol intake and blood pressure levels: a dose-response meta-analysis of nonexperimental cohort studies. Hypertension. 2023;80(10):1961–1969. doi: 10.1161/HYPERTENSIONAHA.123.21224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishiba H., Sumida Y., Tanaka S., et al. The novel cutoff points for the FIB4 index categorized by age increase the diagnostic accuracy in NAFLD: a multi-center study. J Gastroenterol. 2018;53(11):1216–1224. doi: 10.1007/s00535-018-1474-y. [DOI] [PubMed] [Google Scholar]
- 11.McPherson S., Hardy T., Dufour J.F., et al. Age as a Confounding factor for the accurate non-Invasive diagnosis of advanced NAFLD fibrosis. Am J Gastroenterol. 2017;112(5):740–751. doi: 10.1038/ajg.2016.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roerecke M., Vafaei A., Hasan O.S.M., et al. Alcohol consumption and risk of liver cirrhosis: a systematic review and meta-analysis. Am J Gastroenterol. 2019;114(10):1574–1586. doi: 10.14309/ajg.0000000000000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lackner C., Tiniakos D. Fibrosis and alcohol-related liver disease. J Hepatol. 2019;70(2):294–304. doi: 10.1016/j.jhep.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Chang Y., Cho Y.K., Kim Y., et al. Nonheavy drinking and worsening of noninvasive fibrosis markers in nonalcoholic fatty liver disease: a cohort study. Hepatology. 2019;69(1):64–75. doi: 10.1002/hep.30170. [DOI] [PubMed] [Google Scholar]
- 15.Israelsen M., Torp N., Johansen S., et al. Validation of the new nomenclature of steatotic liver disease in patients with a history of excessive alcohol intake: an analysis of data from a prospective cohort study. Lancet Gastroenterol Hepatol. 2024;9(3):218–228. doi: 10.1016/S2468-1253(23)00443-0. [DOI] [PubMed] [Google Scholar]
- 16.Bradley K.A., Badrinath S., Bush K., et al. Medical risks for women who drink alcohol. J Gen Intern Med. 1998;13(9):627–639. doi: 10.1046/j.1525-1497.1998.cr187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chrostek L., Jelski W., Szmitkowski M., et al. Gender-related differences in hepatic activity of alcohol dehydrogenase isoenzymes and aldehyde dehydrogenase in humans. J Clin Lab Anal. 2003;17(3):93–96. doi: 10.1002/jcla.10076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Osna N.A., Donohue T.M., Jr., Kharbanda K.K. Alcoholic liver disease: pathogenesis and current management. Alcohol Res. 2017;38(2):147–161. [PMC free article] [PubMed] [Google Scholar]
- 19.Li G., Peng Y., Chen Z., et al. Bidirectional association between hypertension and NAFLD: a systematic review and meta-analysis of observational studies. Int J Endocrinol. 2022;2022 doi: 10.1155/2022/8463640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blomdahl J., Nasr P., Ekstedt M., et al. Moderate alcohol consumption is associated with significant fibrosis progression in NAFLD. Hepatol Commun. 2023;7(1) doi: 10.1097/HC9.0000000000000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anstee Q.M., Berentzen T.L., Nitze L.M., et al. Prognostic utility of Fibrosis-4 index for risk of subsequent liver and cardiovascular events, and all-cause mortality in individuals with obesity and/or type 2 diabetes: a longitudinal cohort study. Lancet Reg Health Eur. 2024;36 doi: 10.1016/j.lanepe.2023.100780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moriya A., Iwasaki Y., Ohguchi S., et al. Roles of alcohol consumption in fatty liver: a longitudinal study. J Hepatol. 2015;62(4):921–927. doi: 10.1016/j.jhep.2014.11.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.