Abstract
Introduction
Primary dedifferentiated liposarcomas of the spine mark a rare tumor entity.
Research question and case description
We present a rare case of a primary dedifferentiated liposarcoma of the thoracic spine. A 36-year-old previously completely healthy woman presented with a sudden ascending paresthesia of both legs, persistently increasing over the course of two days before initial presentation.
Case report
Computed tomography and magnetic resonance imaging revealed an expansively growing tumor mass extending from T5 to T6 and absolutely compressing the dural sac and spinal cord. The patient's neurological function completely recovered after emergency posterior decompression via laminectomy with intralesional tumor debulking. The tumor was histologically classified as primary grade 2 dedifferentiated liposarcoma (DDLPS) of the spine and after referral to a sarcoma center, the patient was treated with three courses of polychemotherapy (doxorubicin plus ifosfamide). Chemotherapy was followed by aggressive resection by en-bloc spondylectomy in cooperation with a spine tumor center. Subsequently, the patient also underwent radiation therapy.
Results
The patient still undergoes structured tumor aftercare and is tumor- and metastasis-free 53 months after tumor resection.
Discussion and conclusion
DDLPS rarely occur in the spine, with definitive resection of the tumor being the treatment of choice. Surgery should be accompanied by other (radio-) oncological treatment options in cases where only subtotal resection is possible. Also, referral of patients with primary sarcomas of the spine to specialized sarcoma centers is essential, so they can be provided with individual treatment options and structured interdisciplinary aftercare, that ensure the best possible outcome.
Keywords: Liposarcoma, Dedifferentiated liposarcoma, Primary spinal tumor, Thoracic spine, En-bloc spondylectomy
Highlights
-
•
Primary sarcoma of the spine marks a very rare tumor entity with only a few cases mentioned in literature.
-
•
Surgery in accordance to the Enneking recommendations is the treatment of choice for sarcomas of the spine.
-
•
Patients should be treated in specialized sarcoma centers that also have advanced spinal surgical expertise.
-
•
After definitive treatment of the tumor, structured multidisciplinary aftercare is vital for patients with spine sarcomas.
1. Introduction
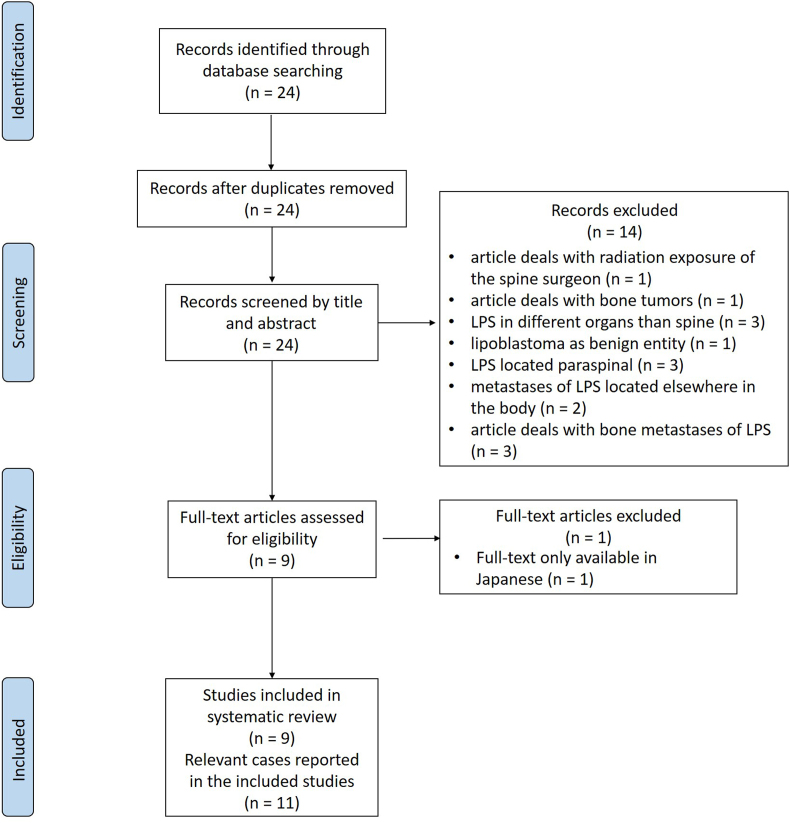
Liposarcomas are rare malignant tumors of adipocytic differentiation that account for approximately 15–20% of all soft tissue sarcomas in adults (Ducimetière et al., 2011). According to the 5th edition of the World Health Organization Classification of Soft Tissue and Bone Tumors, issued in 2020, they can be further classified into five distinct subtypes: well-differentiated LPS (synonym: atypical lipomatous tumor), dedifferentiated LPS, myxoid LPS, pleomorphic LPS and the new entity of myxoid pleomorphic LPS, which has been added to the classification with its last revision (Choi and Ro, 2021; Sbaraglia et al., 2021). While MPLPS typically affect children and adolescents (Alaggio et al., 2009), the other subtypes have their peak incidence at an age of around 50 years (Lee et al., 2018). Amongst the subtypes typically affecting adults, DDLPS are the third most common, making up a proportion of around 15–20% (Lee et al., 2018). DDLPS typically present as a painless mass and therefore are mostly found by chance (Dei Tos et al., 2020). They are associated with high rates of local and metastatic recurrence (Dei Tos et al., 2020). Typical sites of origin are the retroperitoneum, the spermatic cord and more rarely the mediastinum, head, neck and the extremities (Dei Tos et al., 2020; Lee et al., 2018). Primary occurrence in the trunk and especially in the spine is rare, a feature DDLPS share with WDLPS, MLPS and PLPS. We conducted a systematic search in Pubmed and Embase using the search terms “primary AND liposarcoma AND spine” as well as “primary AND liposarcoma AND epidural AND space” to find all previously described cases of primary LPS of the spine (Fig. 1). This search retrieved only nine reports, presenting a total of eleven cases in which LPS primarily arose from the spine - all of them being either MLPS or PLPS (de Moraes et al., 2012; Halevi et al., 2015; Hamlat et al., 2005; Kaneuchi et al., 2016; Lmejjati et al., 2008; Morales-Codina et al., 2016; Rovlias et al., 2017; Turanli et al., 2000; Zhao et al., 2016). To the best of our knowledge, this current study reports the first case of a primary DDLPS of the thoracic spine up to date, including descriptions of its clinical, histological and treatment characteristics. Written informed consent was obtained from the patient prior to publication of this case report.
Fig. 1.
PRISMA flowchart showing the selection process for articles included and excluded in this case report.
2. Case report
2.1. History and examination
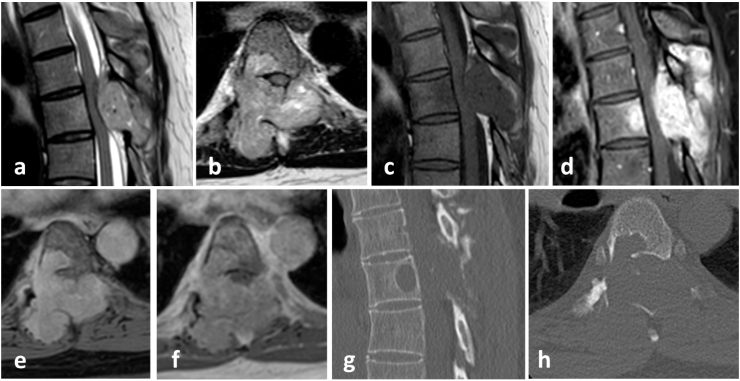
A previously healthy 36-year-old woman presented at our institution in November 2019 without peripheral motor dysfunction, but ascending paresthesia of both legs, persistently increasing during the last two days before ambulatory presentation. The patient did not suffer from any neurological symptoms before. Native CT and MR with contrast medium showed a tumor mass extending in the vertebral arch, spinous process of T5 and partially T6, expansively growing into the spinal canal and absolutely compressing the dural sac/spinal cord (grade 3 according to Bilski et al., 2010) with high CM uptake (Fig. 2). Emergency posterior decompression via laminectomy at T6 with intralesional tumor debulking and bisegmental posterior instrumentation from T5 to 7 was performed. (Fig. 3). After surgery, the patient's neurological function completely recovered.
Fig. 2.
Initial MRI without contrast medium: a) sagittal T2W_TSE, b) axial T2W_TSE, c) sagittal T1W_TSE; and MRI with contrast medium: d) sagittal s3D_T1W-mDixon_W TSE W KM, e) axial s3D_T1W-mDixon IP KM and f) axial s3D_T1W-mDixon W KM and native CT: g) sagittal and h) axial, showing a lytic lesion in T6 with spinal cord compression grade 3 according to Bilsky by CM enhancing tumor masses.
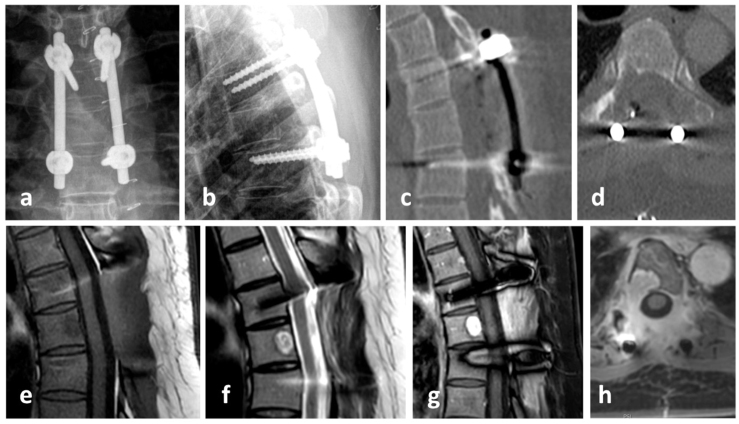
Fig. 3.
X-Ray one week post-surgery: a) anterior-posterior and b) lateral; computed tomography one day post-surgery: c) sagittal and d) axial; magnetic resonance tomography 4 weeks post-surgery: e) sagittal T1w TSE, f) sagittal T2w TSE, g) sagittal T1 DIXON water-only with CM and h) axial T1-DIXON water-only with CM, showing a short level posterior fixation T5 to T7 with sufficient decompression of the spinal cord compression (grade 0 according to Bilsky) and CM enhancing tumor masses in T6 as well scar tissue in the approach and former posterior tumor region.
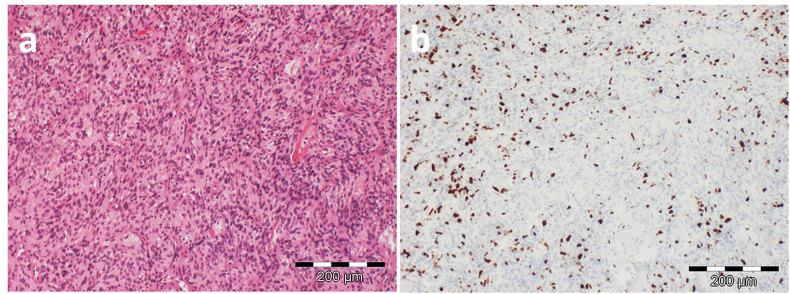
After almost one month during which histological, immunohistochemical and additional molecular pathological examinations were performed, the final diagnosis of dedifferentiated liposarcoma was given. Showing poor differentiation, moderate mitotic activity and also an absence of necrosis, the tumor was classified as grade 2, with a total score of 4 according to the FNCLCC grading of soft tissue tumors (Fig. 4).
Fig. 4.
Representative histological pictures of the tumor: a) H&E section (magnification: x100) showing a cellular neoplasm with spindle-shaped nuclei and b) Ki67 (magnification: x100) labeling demonstratings the high proliferation activity of the tumor.
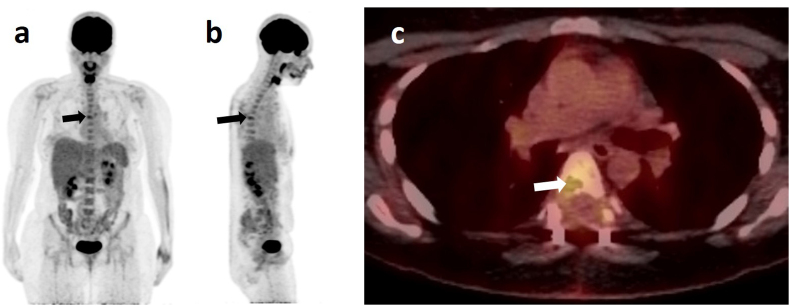
Whole body 18F-fluorodeoxyglucose positron emission tomography/computed tomography was performed, revealing markedly elevated FDG-uptake in the region of the osteolysis at T6 without any further FDG-avid foci, indicating presently a solitary lesion with no metastases (Fig. 5). Therefore, the DDLPS of the thoracic spine was diagnosed as primary. Still the patient did not suffer from any neurological deficits. The patient was presented to the interdisciplinary sarcoma board and it was decided to make a curative approach with three courses of neoadjuvant polychemotherapy using doxorubicin (adriamycin) and ifosfamide, followed by restaging imaging and - depending on whether the lesion remained solitary - aggressive resection by extralesional en-bloc excision. After completion of the well-tolerated cytostatic therapy, the patient again underwent an MRI scan of the thoracic spine in preparation for the upcoming surgery. The scan showed a largely unaltered homogenous contrast-enhancing process in the right dorsal part of the T6 vertebral body, extending into the pedicle. Also, a displacement of the epidural fatty tissue could be seen after dorsal stabilization from T5 to T7 without any signs of a loosening of the implant. This could be attributed to the fact that unlike carcinomas, sarcomas do rarely infiltrate the surrounding tissue, but rather grow expansively and thereby compress adjacent structures. An intrathecal spread of the tumor could not be delineated. Therefore, progression of the remaining tumor tissue during systemic therapy could not be confirmed. Furthermore, there were no signs of spinal canal compression or myelopathy. An additional angiographic CT of the chest, abdomen and pelvis using contrast agent, was performed. The scan showed the residual tumor at the dorsal margin of the vertebral body of T6, reaching into the right pedicle. It had a size of 17 × 9mm, being constant in size compared to previous scans. An arterial supply of the process was no longer distinguishable, and evidence of metastasis was also absent.
Fig. 5.
PET- (a) sagittal view and b) coronal view) and PET-CT- (c) axial view at the height of T6) scans taken ten weeks after emergency decompression. PET-scans reveal no other tumor in the patient's body besides the one found in T6 (arrow). This primary tumor is also clearly visible in the PET-CT-scan, where the site of the osteolysis in the vertebral body shows an increased glucose metabolism (arrow).
2.2. Surgery
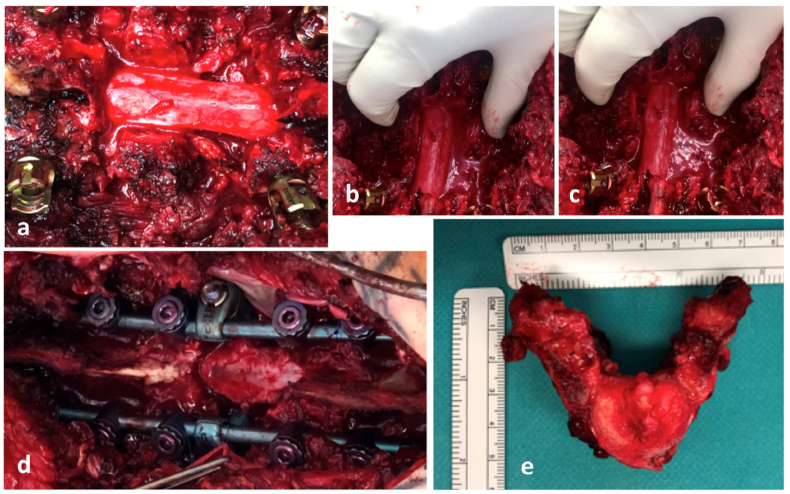
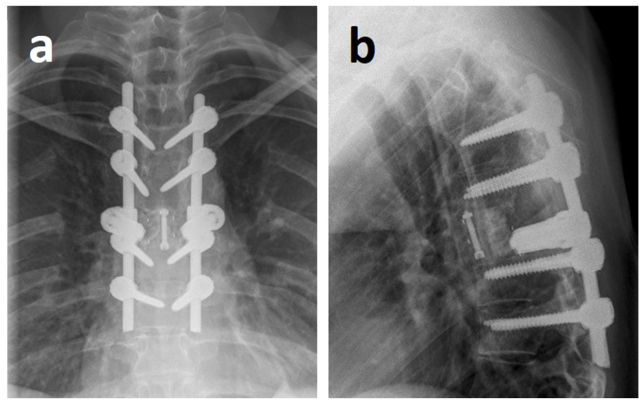
According to the interdisciplinary sarcoma board decision, the patient was scheduled for aggressive resection by en-bloc spondylectomy using the technique originally described by Tomita et al. (1994). Due to limited experience in that surgical technique within the sarcoma center, the patient was referred to a spine tumor center. There tumor resection was performed in cooperation using a posterior costotransversal approach on both sides. Extralesional resection of the tumor was performed according to the preoperative planning. As T6 lamina had already been resected in the initial emergency surgery, en-bloc laminectomy was no longer possible. Instead, scar tissue along the spinal canal had to be removed. Histopathological specimens were also sent to pathology for further work-up. Following costotransversectomy on both sides, bi-manual detection of the anterior larger vessels was performed. After the anterior corridor was established, S-shaped hooks were inserted to separate the spinal column from the anterior structures. With the hooks in place, secure dissection of the intervertebral discs adjacent to the tumor-involved vertebra was possible. Using a rotatory turning-out maneuver, the vertebral body was resected in total. Reconstruction was performed using a posterior pedicle-screw construct from T4/5 to T7/8, which was interconnected to a modular carbon-composite cage (Coligne, Zürich, Switzerland), fixed to the posterior pedicle-screw-rod system by metal-sleeved screws (“neopedicles”) in sense of a 360° stabilization (Fig. 6, Fig. 7) following the technique described by Druschel et al. (2012).
Fig. 6.
En-bloc spondylectomy using the technique originally described by Tomita et al. (Tomita et al., 1994) via posterior costotransversal approach on both sides: a) posterior approach and removal of scar tissue and “cleared” dura, b) and c) mobilization of T6 vertebra, d) posterior long level instrumentation T4/5 to T7/8 with “neopedicles” connection to anterior carbon fibre cage and e) resected vertebra T6.
Fig. 7.
X-ray after tumor resection via a posterior costotransversal approach on both sides with posterior instrumentation from T4/5 to T7/8 as well as anterior reconstruction using a modular carbon cage with neopedicles connected to the dorsal rod system in sense of a 360° fusion: a) anterior-posterior view, b) lateral view.
Although an aggressive resection was planned, clean surgical margins could neither be entirely expected nor reached due to the initial intralesionally performed emergency decompression (R1-resection). Histopathological findings of the retrieved tumor subtype were consistent with the ones from the initial decompression procedure. Nevertheless, the rationale to perform an aggressive resection despite intralesional previous surgery, was to attain a better resection status than R2. Furthermore, aggressive resection was favored in order to minimize the tumor-derived blood loss with inevitable dissemination of tumor/sarcoma cells during intralesional piece-meal resection, thereby decreasing the risk of a local recurrence very difficult to manage.
2.3. Postoperative course
After surgery, the patient underwent radiation using 6 MeV photons with an overall dose of 50,4 Gy to the extended area of the primary tumor including the scar as well as a sequential boost to the tumor area (60,4 Gy in total). The patient again was assessed one month after the end of the radiation therapy. Here, clinical and laboratory examination, MRI of the thoracic spine and CT of the thorax showed no signs of local tumor recurrence as well as metastases. The patient still undergoes structured tumor aftercare and up to date (53 months after tumor resection) has remained tumor- and metastasis-free.
3. Discussion
It is assumed that DDLPS are WDLPS showing progression to usually non-lipogenic sarcomas of variable histological grade, as around 90% of all DDLPS are found within a primary WDLPS and ca. 10% within areas of locally recurrent WDLPS (Singer et al., 2003). DDLPS are characterized by highly cellular areas of high-grade undifferentiated sarcomas that typically transition abruptly within an already existing WDLPS (Ducimetière et al., 2011). This thesis is also supported by the fact that DDLPS and WDLPS share some important molecular characteristics. In both entities supernumerary ring or giant rod chromosomes are found, consisting of amplified segments of 12q13-15, including MDM2 and CDK4 cell cycle oncogenes important for tumorigenesis (Forus et al., 1995; Nishio, 2011). This noticeable increase in MDM2 and CDK4-expression was also visible in our case. For DDLPS, also a rare histologic pattern of dedifferentiation has been described on several occasions, that is characterized by formation of neural-like or meningothelial-like whorls or spherical bodies found in clusters and scattered throughout the tumor. This pattern has first been described independently in 1998 (Fanburg-Smith and Miettinnen, 1998; Nascimento et al., 1998) and has also been present in our case.
Definitive diagnosis of DDLPS is made histopathologically and cytogenetically. Usually DDLPS show an abrupt transition between well-differentiated and dedifferentiated areas, with the former consisting of mature fat cells significantly varying in size and of atypical, hyperchromatic stromal spindle cells (Nishio and Nakayama, 2023). Also, a varying number of mono- or multi-vacuolated lipoblasts can be visible. Dedifferentiated areas can show a wide spectrum of morphologies, but mostly they resemble undifferentiated PLPS or intermediate- to high-grade myxofibrosarcomas (Dei Tos et al., 2020; Nishio and Nakayama, 2023). DDLPS show a mitotic activity that is variable and usually lower than in other high-grade sarcomas (Nishio and Nakayama, 2023). On some occasions, as in the present study, the presence of peculiar neural- or meningothelial-like whorling patterns has been described (Fanburg-Smith and Miettinnen, 1998; Nascimento et al., 1998). The main molecular characteristics of DDLPS are genetic abnormalities (ring or giant chromosomes and double minutes) which lead to amplification of genes located on chromosome 12q13-15, amongst them MDM2 and CDK4 (Casadei et al., 2022; Chamberlain et al., 2021; Shon and Billings, 2020). The amplification of MDM2, the consequent MDM2 protein overexpression and the so-induced inhibition of p53 and its tumor-suppressing function mark the main driver of DDLPS growth and progression (Casadei et al., 2022). Consistent nuclear reactivity of MDM2 and CDK4, which both was given in the case presented here, is the only reliable immunohistochemical marker for DDLPS, allowing separation of homologous DDLPS from PLPS (Nishio and Nakayama, 2023; Sirvent et al., 2007).
DDLPS mostly occur in the retroperitoneum, but they have also been reported to develop in the extremities, the mediastinum, the head and neck region as well as paratesticular (Dei Tos et al., 2020; Lee et al., 2018). Primary occurrence in the trunk and especially in the spine is rare, as in all subtypes of LPS found in adults. WDLPS are reported to occur in the extremities, retroperitoneal, and rarely in the mediastinum and paratesticular (Lee et al., 2018). MLPS typically present within deep soft tissues of the extremities, most often the thighs (Kilpatrick et al., 1996) while PLPS most commonly occur on the extremities and on rare occasion also in the trunk wall, the retroperitoneum and the spermatic cord (Pedeutour and Montgomery, 2020). Primary occurrence of LPS in the spine is rare. When reviewing the available literature, we found only eleven reported instances of LPS of the spine, amongst them no case of a DDLPS (Table 1) (de Moraes et al., 2012; Halevi et al., 2015; Hamlat et al., 2005; Kaneuchi et al., 2016; Lmejjati et al., 2008; Morales-Codina et al., 2016; Rovlias et al., 2017; Turanli et al., 2000; Zhao et al., 2016).
Table 1.
Overview of the clinical and demographic characteristics of the present case as well as of the only eigth cases of liposarcoma already documented in the literature, and comparison of treatments applied in each case and their results.
| Study | Sarcoma subtype | Age (years) | Sex | Localization | Clinical Data | Initial Treatment | Result | Follow-up |
|---|---|---|---|---|---|---|---|---|
| presentcase | DDLPD | 36 | f | thoracic(T5/T6) | Paresisofbothlegs.Paresthesiaofbothlegs,ascendingintosupraum-bilicalregion.2-dayterm | LaminectomyofT6andinternalstabilizationT5toT7.Chemotherapy(doxorubicin+ifosfamide,3courses).ResectionofT6withdorsalinstrumentationfromT5toT7andventralre-constructionplusresectionofcostae5to7.60,4Gy radiationtherapy. | Neurologicalrecovery.Stiffnessinthethoracicregionofthespine.Deafnessalongintercostalnerves. | 53 months.Nolocalrecurrenceormetastasis. |
| Turanli et al. (2000) | MLPS | 65 | f | lumbar | Low back pain. Weakening of left ante-rior tibial muscle (Grade 4/5). Hyperactivity in left L4 dermatoma. Positive Lasègue test at 60° on left side. 6-week term. | Hemilaminectomy of L3. Marginal resection of the tumor. Posterior pedicle screws from L2 to L4. | No problems in performing daily activities. | 13 months. No local recurrence or metastasis. |
| Kaneuchi et al. (2016) | MLPS | 22 | f | thoracic | Back pain. Hypalgesia and hypesthesia below level of the xiphoid cartilage. Numbness of the lower extremities. | Laminectomy of T4 – T6. Intralesional resection. Chemotherapy (doxorubicin + ifosfamide, 3 courses). | Rapid recovery of paralysis without any disturbance. | 18 months. No local recurrence or metastasis. |
| Rovlias et al. (2017) | MLPS | 79 | m | thoracic | Aggravating weakness of lower limbs. Posterior pain. Spastic paraparesis with a fifth thoracic sensory level. 2-month term. | Laminectomy of T4 and T5. Piece-meal subtotal excision of tumor. T3 to T7 posterior ins-trumentation. 45Gy palliative radiotherapy. | No functional neurological improvement. Significant dorsal pain relief. | 7 months. Gradual physical deterioration. Death. |
| Zhao et al., (2016) (3 cases) | MLPS |
44 |
m |
sacral |
Low back pain. Radicular pain, numbness and weakness of the left leg. Weight decrease. |
Piece-meal resection. Lumbar-iliac fusion. |
No details given. |
18 months. Local recurrence and metastasis. Death. |
| MLPS | 37 | m | lumbar (L4-ilium) | Low back pain. Radicular pain, numbness and weakness of the lower extremities. | Piece-meal resection. Lumbar-iliac fusion. | No details given. | 20 months. No evidence of disease. | |
| MLPS | 32 | m | cervical (C1-4) | Neck pain and limitation of neck motion. Palpable mass. | Piece-meal resection. Occipital fusion. | No details given. | 20 months. Local recurrence and metastasis. Alive. | |
| Hamlat et al. (2005) | PLPS | 45 | f | thoracic (T7/T8) | Backache. Paraplegia. Sensory level at T4. Clonus and bilateral extensor plantar reflex. | Laminectomy + instrumentation +45 Gy radiation therapy. | No neurological improvement (paraplegia). Lung and costal area were irradiated. | 13 months. Physical deterioration. Alive. |
| Lmejjati et al. (2008) | PLPS | 35 | m | lumbar (L4/L5) | Bilateral lumbosciatica. Gait abnormality. Cauda equina syndrome. No alteration in the sphincters. 2-month term. | Surgical decompression + 45Gy radiation therapy. | Neurological recovery. Death at 3 months. | 3 months. Death. |
| de Moraes et al. (2012) | PLPS | 60 | f | lumbar (L4) | Lumbosciatica on the left side. No motor or sensory deficit. 6-month term. | Resection of L4, L3 to L5 arthrodesis. Radiation therapy, chemotherapy. | At 18 months, neither pain, nor neurologic deficit, recurrence, or metastasis. | 36 months. Lung metastasis. |
| Halevi et al. (2015) | PLPS | 70 | m | thoracic (T5) | Sudden onset lower extremity weakness. Constipation. Back pain. | Emergency decompressive laminectomy of T5 and T6. Radiation therapy. | Tumor recurrence after 3 months with repeat surgery, radiation and chemotherapy. | 12 months. Widespread metastasis. Death. |
| Morales-Codina et al. (2016) | PLPS | 61 | m | lumbar (L1-L3) | Bilateral lumbosciatica. Motor deficit in the lower limbs. Hypoesthesia in L1, L2, and L3. No alteration in the sphincters. Change in gait. 4-month term. | En-bloc resection in L1, L2 and L3 + instrumentation. | Dehiscence and a deep wound infection. Inflamma-tory polymyopathy. | 2 months. Local recurrence. Hepatic metastasis. Extensive thrombosis. Type I hepato-renal syndrome. Death. |
DDLPS show local recurrence in at least 40% of all cases (Dei Tos et al., 2020; Lee et al., 2018). Distant metastases can be found in 15–20% of all patients with an overall mortality rate of around 30% at 5-year- follow-up and the lung being the preferred site of metastasis (Henricks et al., 1997). In our case, the patient underwent structured aftercare and restaging every six months. The most recent examination was performed 53 months after resection of the tumor, which marks the longest follow-up period of all cases reported in literature until now. The examination showed no signs of either local tumor recurrence or metastasis. The patient also completely recovered from her initial neurological symptoms.
According to the Enneking recommendations, aggressive tumor resection with wide local excision and tumor-free surgical margins (Enneking-adequate) is the treatment of choice for all forms of localized LPS (Gamboa et al., 2020; Shon and Billings, 2020). In our case, resection margins were limited due to the previously performed intralesional emergency decompression, inevitably resulting in a R1-resection at the intraspinal epidural interface. But even with LPS situated in the spine, extralesional en-bloc resection should be attempted in order to minimize piece-meal resection induced sarcoma cell dissemination, to prevent tumor-derived blood loss (again contributing to seeding of tumor cells) and reduce the risk of local recurrence. In addition, as the surgical technique of monosegmental total en-bloc spondylectomy (Tomita et al., 1994) for tumors without massive extracompartmental growth or vascular involvement allows en-bloc excision via a single posterior surgery only, a second anterior approach (thoracotomy) typically needed for additional intralesional corpectomy, could be circumvented.
While surgical resection is the mainstay of treatment for primary DDLPS, it is considered useful to combine it with polychemotherapy. Currently anthracycline-based therapy regimens, typically using doxorubicin, are the first line of treatment (Chamberlain et al., 2021; Gamboa et al., 2020; Shon and Billings, 2020), as the EORTC 62012 trial has demonstrated that liposarcomas respond better to polychemotherapy than other sarcoma subtypes (Young et al., 2017). The question whether DDLPS should be treated with adjuvant radiation therapy is still controversially discussed. While local radiotherapy is generally assumed to reduce the risk of local recurrence of lesions in the extremities, there is no data that undermines that it improves survival or that justifies the use of this treatment in other localizations (Morales-Codina et al., 2016).
4. Conclusion
DDLPS in the spine are a very rare tumor entity. To the best of our knowledge, this study marks the first case of a primary DDLPS occurring in the spine presented in literature. Surgery is the definitive treatment of choice and in this case, initial intralesional emergency decompression was necessary to avoid detrimental neurological outcome for the patient. Nevertheless, when a primary tumor of the spine is suspected, the treatment ideally should begin with referring the patient to a specialized center with advanced spinal surgical expertise, as the complex spinal anatomy with immediate proximity to essential and vital structures like spinal cord, large vessels and visceral organs complicates aggressive resection and attainment of clean surgical margins (Schaser et al., 2009). This center should also closely collaborate with an oncological department and/or a specialized sarcoma center (Dandurand et al., 2021; Disch et al., 2020). Only in such specialized centers interdisciplinary diagnostic and therapeutic concepts - applied by multidisciplinary teams experienced in diagnosing as well as treating this rare tumor types - can secure the best possible outcome for the individual patient. As first step, a biopsy of the tumor should be taken to confirm the diagnosis, and further therapy should be adapted to its outcome. This is especially important in sarcomas, where neoadjuvant chemotherapy is a vital part of the treatment algorithm. After an eventual neoajuvant chemotherapy, definitive resection in primary tumors should be performed in accordance to the Enneking recommendations (Disch et al., 2023) with the goal to achieve marginal or wide resection margins. Consequently, intraoperative tumor dissemination and inherent risk for local recurrence can be minimized. Nevertheless, in such very rare cases when extralesional resection has to be expected to be marginal and only subtotal resection is possible, other oncological/radiooncological treatment options should be added. Finally, following interdisciplinary multimodal treatment of the primary tumor, the patient should undergo structured interdisciplinary aftercare, including restaging imaging at short time intervals.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Structured review
The search was performed in Pubmed an Embase using the search terms “primary AND liposarcoma AND spine” as well as “primary AND liposarcoma AND epidural AND space”, to find all the relevant articles to be included in this case report. The search retrieved 24 articles in total, none of them were duplicates. After screening the articles by title and abstract, 14 of them had to be excluded from the review process, the reasons for their exclusion can be seen in Fig. 1. Of the nine full-text articles that were assessed for eligibility, one had to be excluded as it was only available in Japanese. Nine studies were finally included in this systematic review, comprising a total of eleven individual cases.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Handling Editor: F Kandziora
References
- Alaggio R., Coffin C.M., Weiss S.W., Bridge J.A., Issakov J., Oliveira A.M., Folpe A.L. Liposarcomas in young patients: a study of 82 cases occurring in patients younger than 22 years of age. Am. J. Surg. Pathol. 2009;33:645–658. doi: 10.1097/PAS.0b013e3181963c9c. [DOI] [PubMed] [Google Scholar]
- Bilski M.H., Laufer I., Fourney D.R., Groff M., Schmidt M.H., Varga P.P., Vrionis F.D., Yamada Y., Gerszten P.C., Kuklo T.R. Reliability analysis of the epidural spinal cord compression scale. J. Neurosurg. Spine. 2010;13(3):324–328. doi: 10.3171/2010.3.SPINE09459. [DOI] [PubMed] [Google Scholar]
- Casadei L., de Faria F.C.C., Lopez-Aguiar A., Pollock R.E., Grignol V. Targetable pathways in the treatment of retroperitoneal liposarcoma. Cancers. 2022;14(6) doi: 10.3390/cancers14061362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain F., Benson C., Thway K., Huang P., Jones R.L., Gennatas S. Pharmacotherapy for liposarcoma: current and emerging synthetic treatments. Future Oncol. 2021;17(20):2659–2670. doi: 10.2217/fon-2020-1092. [DOI] [PubMed] [Google Scholar]
- Choi J.H., Ro J.Y. The 2020 WHO classification of tumors of soft tissue: selected changes and new entities. Adv. Anat. Pathol. 2021;28(1):44–58. doi: 10.1097/PAP.0000000000000284. [DOI] [PubMed] [Google Scholar]
- Dandurand C., Fisher C.G., Rhines L.D., Boriani S., Charest-Morin R., Gasbarrini A. Feasibility of achieving planned surgical margins in primary spine tumor: a PTRON study. Neurosurg. Focus. 2021;50(5):E16. doi: 10.3171/2021.2.FOCUS201091. [DOI] [PubMed] [Google Scholar]
- de Moraes F.B., Cardoso A.L.P., Tristão N.A., Pimenta W.E., Daher S., de Souza Carneiro S., Barbosa N.P.M., de Lima Malta N., Ribeiro N.B. Primary liposarcoma of the lumbar spine: case report. Revista Brasileira de Ortopedia (English Edition) 2012;47(1):124–129. doi: 10.1016/s2255-4971(15)30356-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dei Tos A.P., Marino-Enriquez A., Pedeutour F. The WHO Classification of Tumours Editorial Board (Ed.), WHO Classification of Tumours Soft Tissue and Bone Tumours. fifth ed. International Agency for Research on Cancer; 2020. Dedifferentiated liposarcoma; pp. 39–41. [Google Scholar]
- Disch A.C., Boriani S., Luzzati A., Rhines L.D., Fisher C.G., Lazary A., Gokaslan Z.L., Chou D., Clarke M.J., Fehlings M.G., Schaser K.D., Germscheid N.M., Reynolds J.J. Extradural primary malignant spinal tumors in a population younger than 25 Years: an ambispective international multicenter study on onco-surgical outcomes. Cancers. 2023;15(3) doi: 10.3390/cancers15030845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disch A.C., Kleber C., Redemann D., Druschel C., Liljenqvist U., Schaser K.D. Current surgical strategies for treating spinal tumors: results of a questionnaire survey among members of the German Spine Society (DWG) Eur. J. Surg. Oncol. 2020;46(1):89–94. doi: 10.1016/j.ejso.2019.08.019. [DOI] [PubMed] [Google Scholar]
- Druschel C., Disch A.C., Melcher I., Luzzati A., Haas N.P., Schaser K.D. Multisegmentale En-bloc-Spondylektomie: Indikation, Staging und chirurgische Technik. Operat. Orthop. Traumatol. 2012;24(3):272–283. doi: 10.1007/s00064-011-0070-6. [DOI] [PubMed] [Google Scholar]
- Ducimetière F., Lurkin A., Ranchère-Vince D., Decouvelaere A.V., Péoc’h M., Istier L., Chalabreysse P., Muller C., Alberti L., Bringuier P.P., Scoazec J.Y., Schott A.M., Bergeron C., Cellier D., Blay J.Y., Ray-Coquard I. Incidence of sarcoma histotypes and molecular subtypes in a prospective epidemiological study with central pathology review and molecular testing. PLoS One. 2011;6(8) doi: 10.1371/journal.pone.0020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanburg-Smith J.C., Miettinnen M. Liposarcoma with meningothelial-like whorls: a study of 17 cases of a distinctive histological pattern associated with dedifferentiated liposarcoma. Histopathology. 1998;33(5):414–424. doi: 10.1046/j.1365-2559.1998.00536.x. [DOI] [PubMed] [Google Scholar]
- Forus A., Weghuis D.O., Smeets D., Fodstad O., Myklebost O., van Kessel A.G. Comparative genomic hybridization analysis of human sarcomas: I. Occurrence of genomic imbalances and identification of a novel major amplicon at 1q21-q22 in soft tissue sarcomas. Genes Chromosomes Cancer. 1995;14(1):8–14. doi: 10.1002/gcc.2870140103. [DOI] [PubMed] [Google Scholar]
- Gamboa A.C., Gronchi A., Cardona K. Soft-tissue sarcoma in adults: an update on the current state of histiotype-specific management in an era of personalized medicine. CA Cancer J Clin. 2020;70(3):200–229. doi: 10.3322/caac.21605. [DOI] [PubMed] [Google Scholar]
- Halevi P.D., Ramirez-de-Noriega F., Fellig Y., Gomori J.M., Cohen J.E., Itshayek E. Primary pleomorphic liposarcoma of the thoracic epidural space: case report. Spine J. 2015;15(12):e71–e75. doi: 10.1016/j.spinee.2015.08.059. [DOI] [PubMed] [Google Scholar]
- Hamlat A., Saikali S., Gueye E.M., Le Strat A., Carsin-Nicol B., Brassier G. Primary liposarcoma of the thoracic spine: case report. Eur. Spine J. 2005;14(6):613–618. doi: 10.1007/s00586-004-0866-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henricks W.H., Chu Y.C., Goldblum J.R., Weiss S.W. Dedifferentiated liposarcoma: a clinicopathological analysis of 155 cases with a proposal for an expanded definition of dedifferentiation. Am. J. Surg. Pathol. 1997;21(3):271–281. doi: 10.1097/00000478-199703000-00002. [DOI] [PubMed] [Google Scholar]
- Kaneuchi Y., Hakozaki M., Yamada H., Tajino T., Watanabe K., Otani K., Hojo H., Hasegawa T., Konno S. Primary dumbbell-shaped epidural myxoid liposarcoma of the thoracic spine: a case report and review of the literature. Oncol. Lett. 2016;11(2):1421–1424. doi: 10.3892/ol.2016.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick S.E., Doyon J., Choong P.F., Sim F.H., Nascimento A.G. The clinicopathologic spectrum of myxoid and round cell liposarcoma. A study of 95 cases. Cancer. 1996;77(8):1450–1458. doi: 10.1002/(SICI)1097-0142(19960415)77:8<1450::AID-CNCR5>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Lee A.T.J., Thway K., Huang P.H., Jones R.L. Clinical and molecular spectrum of liposarcoma. J. Clin. Oncol. 2018;36(2):151–159. doi: 10.1200/JCO.2017.74.9598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lmejjati M., Loqa C., Haddi M., Hakkou M., BenAli S.A. Primary liposarcoma of the lumbar spine. Joint Bone Spine. 2008;75(4):482–485. doi: 10.1016/j.jbspin.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Morales-Codina A.M., Martín-Benlloch J.A., Corbellas Aparicio M. Primary pleomorphic liposarcoma of the spine. Case report and review of the literature. International Journal of Surgery Case Reports. 2016;25:114–119. doi: 10.1016/j.ijscr.2016.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento A.G., Kurtin P.J., Guillou L., Fletcher C.D. Dedifferentiated liposarcoma: a report of nine cases with a peculiar neurallike whorling pattern associated with metaplastic bone formation. Am. J. Surg. Pathol. 1998;22(8):945–955. doi: 10.1097/00000478-199808000-00004. [DOI] [PubMed] [Google Scholar]
- Nishio J. Contributions of cytogenetics and molecular cytogenetics to the diagnosis of adipocytic tumors. J. Biomed. Biotechnol. 2011;2011 doi: 10.1155/2011/524067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio J., Nakayama S. Biology and management of high-grade myxofibrosarcoma: state of the art and future perspectives. Diagnostics. 2023;13(19) doi: 10.3390/diagnostics13193022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedeutour F., Montgomery E.A. The WHO Classification of Tumours Editorial Board (Ed.), WHO Classification of Tumours Soft Tissue and Bone Tumours. fifth ed. International Agency for Research on Cancer; 2020. Pleomorphic liposarcoma; pp. 45–46. [Google Scholar]
- Rovlias A., Balanika A., Nomikos A., Melissaris S. Primary myxoid liposarcoma of the upper thoracic spine in an elderly patient. J. Neurosci. Rural Pract. 2017;8(Suppl. 1):120–122. doi: 10.4103/jnrp.jnrp_99_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbaraglia M., Bellan E., Dei Tos A.P. The 2020 WHO classification of soft tissue tumours: news and perspectives. Pathologica. 2021;113(2):70–84. doi: 10.32074/1591-951X-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaser K.D., Melcher I., Luzzati A., Disch A.C. Bone sarcoma of the spine. Recent Results Cancer Res. 2009;179:141–167. doi: 10.1007/978-3-540-77960-5_10. [DOI] [PubMed] [Google Scholar]
- Shon W., Billings S.D. Soft tissue special issue: selected topics in the pathology of adipocytic tumors. Head and Neck Pathology. 2020;14(1):1–11. doi: 10.1007/s12105-019-01112-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S., Antonescu C.R., Riedel E., Brennan M.F., Pollock R.E. Histologic subtype and margin of resection predict pattern of recurrence and survival for retroperitoneal liposarcoma. Ann. Surg. 2003;238(3):358–371. doi: 10.1097/01.sla.0000086542.11899.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirvent N., Coindre J.-M., Maire G., Hostein I., Keslair F., Guillou L., Ranchère-Vince D., Terrier P., Pedeutour F. Detection of MDM2-CDK4 amplification by fluorescence in situ hybridization in 200 paraffin-embedded tumor samples: utility in diagnosing adipocytic lesions and comparison with immunohistochemistry and real-time PCR. Am. J. Surg. Pathol. 2007;31(10):1476–1489. doi: 10.1097/PAS.0b013e3180581fff. [DOI] [PubMed] [Google Scholar]
- Tomita K., Kawahara N., Baba H., Tsuchiya H., Nagata S., Toribatake Y. Total en bloc spondylectomy for solitary spinal metastases. Int. Orthop. 1994;18(5):291–298. doi: 10.1007/BF00180229. [DOI] [PubMed] [Google Scholar]
- Turanli S., Özer H., Özyürekoglu T., Cakiroglu E. Liposarcoma in the epidural space. Spine. 2000;25(13):1733–1735. doi: 10.1097/00007632-200007010-00021. [DOI] [PubMed] [Google Scholar]
- Young R.J., Litière S., Lia M., Hogendoorn P.C.W., Fisher C., Mechtersheimer G., Daugaard S., Sciot R., Collin F., Messiou C., Grünwald V., Gronchi A., van der Graaf W., Wardelmann E., Judson I. Predictive and prognostic factors associated with soft tissue sarcoma response to chemotherapy: a subgroup analysis of the European Organisation for Research and Treatment of Cancer 62012 study. Acta Oncol. 2017;56(7):1013–1020. doi: 10.1080/0284186X.2017.1315173. [DOI] [PubMed] [Google Scholar]
- Zhao C., Han Z., Xiao H., Yang C., Zhao Y., Fan T., Sun Z., Liu T., Xiao J. Surgical management of spinal liposarcoma: a case series of 7 patients and literature review. Eur. Spine J. 2016;25(12):4088–4093. doi: 10.1007/s00586-015-4374-3. [DOI] [PubMed] [Google Scholar]