Abstract
Granule cells in the olfactory bulb (OB) are continually produced and added into the neuronal circuit in the adult brain. Sensory input to the OB plays a key role in the survival of newly generated granule cells. Here, we examined in the adult mice whether there is a time window after the generation of new granule cells when their survival is strongly influenced by sensory input. New granule cells were labeled by BrdUrd injection, and the mice were deprived of sensory input unilaterally by naris cauterization. During the initial 14 days after BrdUrd labeling, the number of BrdUrd-positive granule cells was similar for deprived and undeprived OBs. At 28 days or later, the BrdUrd-positive cell number remarkably decreased in the deprived OB. Cauterization at days 14–28 effectively reduced the number of BrdUrd-positive granule cells, whereas 2-week cauterization before or after this period produced little effect. Administration of diazepam, a GABAA receptor modulator, decreased the number of BrdUrd-positive granule cells. The diazepam administration was most effective at days 14–28. Histochemical examination showed that activation of caspase-3 was accompanied by apoptotic cell death of granule cells that was induced by sensory deprivation or diazepam administration. Double labeling with activated caspase-3 and BrdUrd indicated that granule cells at days 14–20 were most susceptible to cell death. These results indicate that there is a critical period when the survival of new granule cells is determined in a sensory experience-dependent manner and that the pharmacological manipulation can mimic the effect of sensory deprivation.
Keywords: neurogenesis, sensory deprivation, apoptosis
Even in adult mammals the central nervous system retains the potential to generate new neurons. In the hippocampal dentate gyrus and olfactory bulb (OB), large numbers of new neurons are generated (1–3). Proliferating cells in the periventricular region migrate along the rostral migratory stream into the OB. In the OB, new neurons differentiate into local interneurons, granule cells or periglomerular cells, and are functionally integrated into preexisting neuronal circuits (4, 5).
In the OB, a subset of newly generated granule cells is selected to survive, while the others are eliminated. BrdUrd and 3H-thymidine labeling experiments in rodent OB showed that nearly half of new granule cells are eliminated within several months (6, 7). Olfactory input plays a key role in the survival of new granule cells. In the OB of adult mice, the survival rate of new granule cells increases when the mice are raised in an odor-enriched environment (8). Conversely, in mice that are deficient in odor signal transduction in sensory neurons, the survival rate of new granule cells decreases (6). However, we still lack the cellular and molecular mechanisms that determine the survival or death of newly generated neurons and the integration of the neurons into a preexisting neuronal circuit.
Sensory experience-dependent neuronal plasticity is a crucial mechanism for establishing a finely tuned neuronal circuit in the development of the brain. For example, deprivation of visual input from one eye shifts the response property of binocular zone neurons in the visual cortex preferentially toward the undeprived eye input (9, 10). An interesting feature of ocular dominance plasticity is the existence of a time window after the birth of animals, called the critical period, when monocular deprivation effectively shifts the ocular dominance. The critical period related to sensory experience is also shown in the barrel fields in the somatosensory cortex (11). The purpose of this study is to examine whether new granule cells in the adult have a critical period when olfactory sensory experience influences their survival. Here, we have applied a naris cauterization method that has been used to reveal sensory experience-dependent changes in the OB (12–16).
In the OB of rodents, the number of apoptotic granule cells increases after deprivation of olfactory sensory input (13–17). In many brain regions, apoptotic neuronal death is mediated by the activation of caspases (18). We thus examined the activation of caspase-3 in granule cells after sensory deprivation and determined the age of apoptotic cells. We show a specific time window for new granule cells when their fate between survival and death is crucially regulated by olfactory sensory experience.
Materials and Methods
Animals and BrdUrd Labeling. C57BL/6 male mice at 8 weeks of age were used throughout this study. Mice were injected i.p. with BrdUrd (300 mg/kg) (19). For the determination of the age of caspase-3-activated cells, BrdUrd was injected once per day for 7 consecutive days. All experiments were performed by procedures approved by the Experimental Animal Research Committee at the University of Tokyo.
Naris Cauterization. Mice were deeply anesthetized with diethyl ether, and one nostril of each mouse was cauterized unilaterally. Cauterization was performed at 8–12 h after the BrdUrd injection to avoid its possible influence on the proliferation of progenitors.
Diazepam Administration. Diazepam (Wako Biochemicals, Osaka) was dissolved in a solution containing 50% distilled water, 40% propylene glycol, and 10% dimethyl sulfoxide with a final concentration of 6 mg/ml. The solution was injected i.p. (30 mg/kg). To define the critical period, diazepam was injected once per day for 14 consecutive days. Diazepam was administered at least 8 h after the BrdUrd injection to avoid its possible influence on the proliferation of progenitors.
Immunohistochemistry. At various periods after BrdUrd labeling, mice were deeply anesthetized with diethyl ether and perfused with 4% paraformaldehyde. Cryosections of the OB were prepared serially to 20-μm thickness. Primary antibodies used were: rat anti-BrdUrd antibody (Oxford Biotechnology, Oxfordshire, U.K.; 1/100), mouse anti-NeuN antibody (Chemicon; 1/200), and rabbit anti-cleaved caspase-3 antibody (Cell Signaling Technology, Beverly, MA; 1/100). Secondary antibodies were Alexa Fluor 488- or 546-conjugated antibodies (Molecular Probes; 1/300). Sections were incubated with appropriate antibodies and then stained with DAPI and/or Alexa Fluor 647 NeuroTrace (Molecular Probes). For BrdUrd detection, sections were incubated with 0.025 M HCl for 30 min at 65°C, rinsed with 0.1 M boric acid (pH 8.5), and analyzed immunohistochemically as reported (20). For the morphological analysis of caspase-3-activated cells, paraformaldehyde-fixed OBs were sliced to 50-μm thickness with a microslicer (DTK-3000, Dosaka, Kyoto, Japan). The samples were immunostained and analyzed with a confocal laser scanning microscope (Bio-Rad).
TUNEL Assay. Paraformaldehyde-fixed OBs were cryosectioned to 12-μm thickness. The samples were postfixed with ethanol/acetate (2:1) for 5 min at -20°C and subjected to TUNEL assay (MEBSTAIN apoptosis kit II, MBL, Nagoya, Japan) according to the manufacturer's instructions.
Quantitative Analysis. Coronal sections of the OB from the rostral tip to the caudal end were selected at the rate of one per 10 serial sections and labeled with antibodies or subjected to TUNEL assay. Labeled cells in the granule cell layer (GCL), internal plexiform layer (IPL), and mitral cell layer (MCL) were counted under a fluorescent microscope. The counted numbers were added and then multiplied by 10 to estimate the total number of labeled cells per whole OB. Statistical analysis was made by unpaired or paired Student's t test.
Results
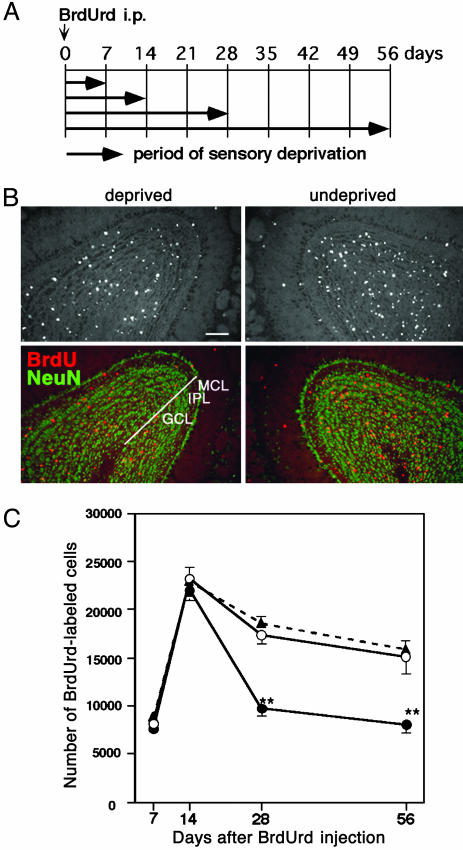
Decreased Number of New Granule Cells in the Sensory-Deprived OB. Among two types of OB interneurons (granule cell and periglomerular cell), we concentrated on the analysis of granule cells that are the predominant population generated in the adult (1, 2, 4–6). Newly generated cells in 8-week-old mice were labeled by i.p. BrdUrd injection. At 8–12 h after the injection, one nostril was cauterized to deprive olfactory sensory input. Fig. 1A shows the timetable for the experiment in four groups of mice. The day of BrdUrd injection was defined as day 0, and the OBs were processed at days 7, 14, 28, and 56.
Fig. 1.
Olfactory sensory deprivation reduces the number of BrdUrd-labeled cells. (A) Shown is the timetable for sensory deprivation. Newly generated cells were labeled by i.p. BrdUrd injection at day 0. On the same day, one nostril was cauterized. At days 7–56 after the injection, mice were perfusion-fixed and analyzed. (B) Coronal sections through the OBs were examined at day 28. (Upper) BrdUrd-labeled cells in the OB. (Lower) The layer organization using anti-NeuN antibody staining (green). BrdUrd-labeled cells (red) in the GCL, IPL, and MCL were counted. (Scale bar: 100 μm.) (C) The number of BrdUrd-labeled cells in the OB at various periods is shown. ▴, control OB; ○, undeprived OB of animals that received unilateral sensory deprivation; •, sensory-deprived OB. Values indicate mean ± SD from six OBs for control, three OBs for undeprived side, and three OBs for deprived side. Statistical analysis was conducted by paired or unpaired Student's t test (**, P < 0.01).
Fig. 1B shows coronal sections of the OBs at day 28. We counted the number of BrdUrd-positive cells within the GCL, IPL, and MCL that were determined by anti-NeuN antibody staining. Because NeuN is a classical neuronal marker that labels interneurons but not mitral cells in the OB (21), NeuN-positive cells in these layers are considered to be granule cells. The number of BrdUrd-positive cells decreased in the sensory-deprived OB compared with the undeprived OB (Fig. 1B).
The number of BrdUrd-positive cells per bulb was calculated and plotted (Fig. 1C). From day 7 to day 14, BrdUrd-positive cells increased in number, which presumably reflected the entry of new granule cells into the OB through the rostral migratory stream (1, 2, 4–7). The number of BrdUrd-positive cells at days 7 and 14 did not show a significant difference between deprived and undeprived OBs. At day 14, BrdUrd-positive cells remaining in the core of the OB (NeuN-negative region) were small both in undeprived and deprived OBs (1.7 ± 0.2% and 2.0 ± 0.2%, respectively, compared with those in GCL, IPL, and MCL). These results indicate that radial migration of BrdUrd-positive cells into these layers has been essentially completed by day 14, and the migration is not significantly affected by sensory deprivation. At days 28 and 56, the number of BrdUrd-positive cells decreased from that at day 14. The decline was most prominent in the deprived OB. At day 28, the number of BrdUrd-positive cells in the deprived OB decreased by 56% compared with that at day 14, whereas that in the undeprived OB decreased by 25%. At day 56, the number of BrdUrd-positive cells decreased slightly both in deprived and undeprived OBs compared with that at day 28. The BrdUrd-positive cell numbers in the undeprived OB were similar to those in the OBs of animals without naris cauterization (Fig. 1C). The results indicate that sensory deprivation reduces the survival rate of new granule cells after day 14.
A majority of the BrdUrd-positive cells were NeuN-expressing neurons at days 14, 28, and 56 (Table 1), indicating that most of the BrdUrd-positive cells were granule cells. In both deprived and undeprived OBs, nearly four-fifths of BrdUrd-positive cells were NeuN-positive. This result indicates that sensory deprivation did not affect the differentiation of newly generated cells into NeuN-expressing granule cells.
Table 1. Percentages of NeuN-positive cells among BrdUrd-labeled cells.
| % of NeuN-labeled cells
|
||
|---|---|---|
| Days after BrdUrd injection | Undeprived | Deprived |
| 7 | 12.0 ± 1.0 | 8.7 ± 3.5 |
| 14 | 80.7 ± 3.2 | 79.3 ± 6.1 |
| 28 | 87.3 ± 3.8 | 87.0 ± 3.5 |
| 56 | 90.7 ± 1.2 | 90.3 ± 2.1 |
All data are the average ± SD of three bulbs.
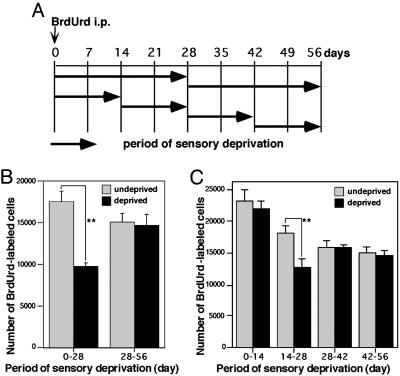
Sensory Deprivation During Days 14–28 Was Most Effective in Reducing the Survival of New Granule Cells. To examine whether there is a specific time window when sensory deprivation affects the survival of new granule cells, the starting date and duration of naris cauterization were changed (Fig. 2A). Deprivation for 4 weeks from days 0 to 28 decreased the number of surviving new granule cells by 44% compared with the undeprived side (Fig. 2B). In contrast, deprivation for 4 weeks from days 28 to 56 produced no significant effect on survival. Next, the length of deprivation period was narrowed to 2 weeks (Fig. 2 A). Two weeks of deprivation during days 14–28 decreased survival by 30% (Fig. 2C). However, the 2-week deprivation before or after this period had no significant effect. These data indicate that for new granule cells days 14–28 are the critical period when survival is strongly affected by sensory deprivation.
Fig. 2.
A critical period for the sensory experience-dependent survival of new granule cells. (A) A diagram for the protocol of sensory deprivation. Newly generated cells were labeled by BrdUrd injection at day 0. One nostril was cauterized at various periods after the BrdUrd labeling. (B and C) The effect of sensory deprivation for 28 (B) and 14 (C) days. Hatched columns show the number of labeled cells in the undeprived OB, and filled columns show the number of labeled cells in the deprived OB. Values indicate mean ± SD from three OBs. Statistical analysis was conducted by paired Student's t test (**, P < 0.01).
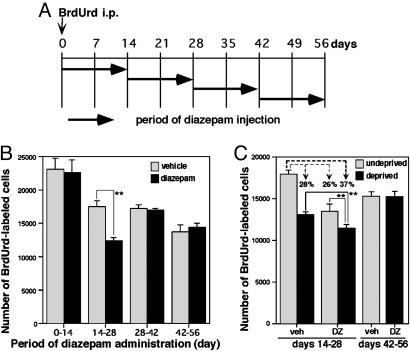
Diazepam Mimicked the Effect of Sensory Deprivation on Granule Cell Survival. We next examined whether pharmacological manipulation can mimic the effect of sensory deprivation. We chose diazepam, a GABAA receptor allosteric modulator (22). Systemic administration of diazepam reduces the overall neuronal activity within the brain (23). Diazepam was injected i.p. once per day for consecutive 14 days, and the starting date of the administration was shifted (Fig. 3A). Diazepam administration during days 14–28 decreased the survival of new granule cells by 30% compared with the vehicle injection (Fig. 3B). However, diazepam administration before or after this period had no obvious effect on the BrdUrd-positive granule cells. Diazepam administration during days 0–14 did not affect the migration of granule cells (only 2.0 ± 0.4% of BrdUrd-positive cells remained in the OB core at day 14) or the differentiation into NeuN-positive neurons (81.7 ± 4.2% of BrdUrd-positive cells were NeuN-positive at day 14; compare Table 1).
Fig. 3.
Diazepam administration mimics the effect of sensory deprivation on the survival of new granule cells. (A) A diagram for the protocol of diazepam administration. Newly generated cells were labeled by BrdUrd at day 0. Diazepam was i.p. administered once per day for 14 consecutive days at various periods after the BrdUrd labeling. (B) The effect of diazepam on the survival of new granule cells. Hatched columns indicate the number of BrdUrd-labeled cells of vehicle-administered animals. Filled columns show the number of BrdUrd-labeled cells of diazepam-administered animals. Values indicate mean ± SD from six OBs. (C) Simultaneous manipulation of sensory deprivation and diazepam administration during days 14–28 or days 42–56. Hatched and filled columns represent undeprived and deprived OBs, respectively. Veh, vehicle-injected animals; DZ, diazepam-injected animals. Values indicate mean ± SD from four OBs. Statistical analysis was conducted by paired or unpaired Student's t test (**, P < 0.01).
To examine how mechanisms leading to reduced cell survival with sensory deprivation and those with diazepam administration are related, animals were simultaneously subjected to the two manipulations (Fig. 3C). Sensory deprivation or diazepam administration during days 14–28 reduced the number of BrdUrd-positive cells by 28% or 26%, respectively (Fig. 3C). In the OBs receiving both manipulations simultaneously, the reduction was more prominent (37%). The extent of the reduction rate (37%) was less than the sum of the reduction rate with either of the two manipulations (28% + 26% = 54%). These results suggest that mechanisms of the two manipulations lead to reduced cell survival overlap with each other rather than competition or segregation. Simultaneous manipulation during days 42–56 showed no significant effect on cell survival (Fig. 3C), confirming the existence of the critical time window.
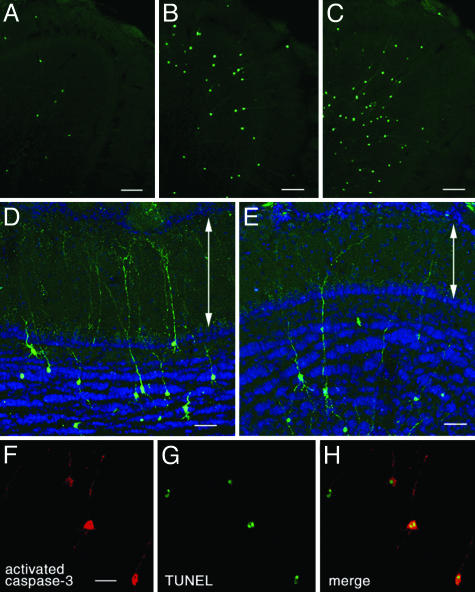
Caspase Activation by Sensory Deprivation and Diazepam Administration. We hypothesized that during days 14–28 after the generation new granule cells become susceptible to apoptotic cell death with sensory deprivation and diazepam administration. We examined the activation of caspase-3, which functions at the most downstream of the caspase activation cascade (18), by using an antibody specific for the active (cleaved) form of caspase-3 (24). In the OB of control mice, we found only a few cells with activated caspase-3 immunoreactivity in the GCL (Fig. 4A). After 28 days of sensory deprivation, the number of caspase-3-activated cells increased in the GCL (Fig. 4B). Similarly, at 8 h after diazepam administration, caspase-3-activated cells increased in number (Fig. 4C), whereas vehicle administration showed no significant change (data not shown). Most of the caspase-3-activated cells in the GCL had radially extending dendrite-like processes (Fig. 4 B and C), indicating they were granule cells. Magnified views showed that some dendrites of caspase-3-activated granule cells extended into the external plexiform layer (EPL) and made branches there, both in the sensory-deprived OB (Fig. 4D) and the OB of diazepam-treated animals (Fig. 4E). EPL is the layer in which granule cells make dendrodendritic synapses with mitral/tufted cells (25). We could not find any caspase-3-activated mitral/tufted cells (Fig. 4 A–E).
Fig. 4.
Caspase-3 activation and apoptotic cell death in granule cells induced by sensory deprivation and diazepam administration. Immunoreactivity for activated caspase-3 is shown in green (A–E) or red (F and H). (A–C) Coronal section of the control OB (A), OB after 28 days of sensory deprivation (B), and OB after 8 h of diazepam administration (C). (D and E) Morphology of dendrites of caspase-3-activated granule cells in the OB after 28 days of sensory deprivation (D) and after 8 h of diazepam administration (E). Some radially elongating dendrites extended into EPL and made branches in the layer. The layer structure was shown by fluorescent Nissl staining (blue). Arrows indicate EPL. The pictures are the stack of confocal images within 15-μm thickness. (F and G) Confocal images of double labeling for activated caspase-3 (F) and TUNEL (G) after 28 days of sensory deprivation. (H) Merged view of F and G. (Scale bars: 100 μm, A–C; 50 μm, D and E; and 20 μm, F.)
Fig. 4 F–H shows the double detection for active caspase-3 and DNA fragmentation after 28 days of sensory deprivation. Many of the caspase-3-activated cells were colabeled with TUNEL. Double detection was also performed for the OBs of control animals and diazepam-treated animals (Table 2). Sensory deprivation and diazepam administration increased the number of caspase-3-activated cells and TUNEL-positive cells nearly four times. In both conditions, about two-thirds of activated caspase-3-immunoreactive cells were TUNEL-positive, and about four-fifths of TUNEL-positive cells were caspase-3-activated cells. The high rates of co-occurrence indicate that a majority of caspase-3-activated granule cells undergo apoptotic cell death and that most apoptotic granule cells are accompanied by the activation of caspase-3.
Table 2. Number of caspase-3-activated cells and TUNEL-positive cells in the GCL, IPL, and MCL of the OB.
| Treatment | Caspase-3 | TUNEL | Double§ | Double/caspase-3, % | Double/TUNEL, % |
|---|---|---|---|---|---|
| Control* | 770 ± 64 | 628 ± 93 | 493 ± 102 | 63.6 ± 8.3 | 78.2 ± 8.4 |
| Odor deprivation† | 3,173 ± 567 | 2,763 ± 472 | 2,506 ± 509 | 78.7 ± 2.2 | 90.3 ± 4.2 |
| Diazepam‡ | 3,605 ± 173 | 2,905 ± 240 | 2,400 ± 207 | 66.5 ± 4.2 | 82.6 ± 2.4 |
OBs of control mice. No. of bulbs analyzed; n = 6
OBs after 28 days of sensory deprivation (n = 3)
OBs after 8 h of diazepam administration (n = 6)
No. of double-positive cells for activated caspase-3 and TUNEL
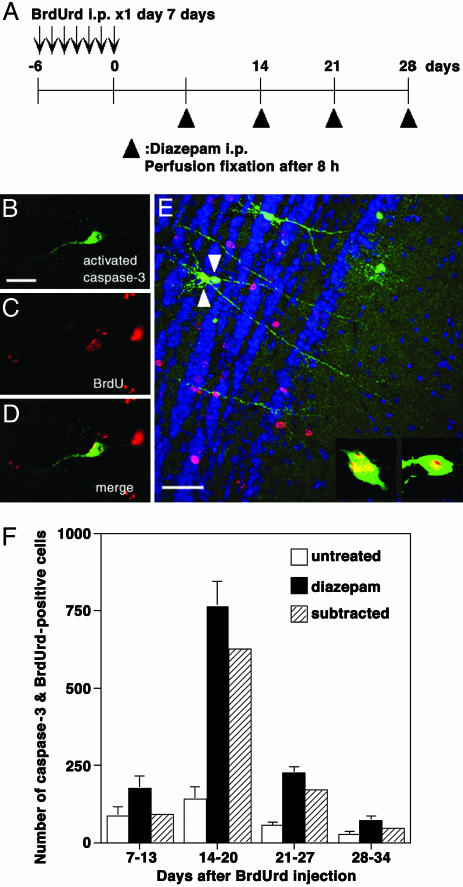
A Majority of the Caspase-3-Activated Cells Were at the Age of Days 14–20. Lastly, we examined the age of apoptotic granule cells. BrdUrd was injected for 7 consecutive days, and the last day of BrdUrd injection was defined as day 0 (Fig. 5A). After various intervals, diazepam was administered and the OBs were processed after 8 h of the drug administration for BrdUrd and active caspase-3 detection. Fig. 5 B–D shows an example of the double-labeled granule cell in the animal that received diazepam administration at day 14. In this case, the age of the BrdUrd-labeled cell should be at days 14–20 after its generation.
Fig. 5.
Determination of the age of caspase-3-activated cells. (A) A diagram showing the experimental procedure. Newly generated cells were labeled by BrdUrd injection for 7 consecutive days. The last day of BrdUrd injection was defined as day 0. At either day 7, 14, 21, or 28, diazepam was administered, and the animals were perfusion-fixed 8 h after the diazepam administration. (B and C) Confocal images of a cell that was positive for both activated caspase-3 (B) and BrdUrd (C) in the animal that received diazepam administration at day 14. (D) Merged view of B and C. (E) Morphology of caspase-3-activated cells at days 14–20. An OB slice was obtained from an animal that received diazepam administration at day 14. Activated caspase-3 is shown in green, BrdUrd in red, and fluorescent Nissl staining in blue. Cells indicated by arrowheads are positive for both activated caspase-3 and BrdUrd. The picture is the stack of confocal images within 15-μm thickness. The magnified views of single confocal images of cell somata are indicated. (Scale bars: 20 μm, B;50 μm, E.) (F) The age of caspase-3-activated cells determined by the codetection of BrdUrd. Each column indicates the number of double-positive cells for activated caspase-3 and BrdUrd in the GCL, IPL, and MCL of the OB. Open columns, untreated OBs; filled columns, OBs 8 h after diazepam administration; hatched columns, subtracted values between filled columns and open columns showing the net increase caused by diazepam administration. Values indicate mean ± SD from six OBs.
The number of double-positive cells at different ages is shown in Fig. 5F. A majority of the double-positive cells after diazepam administration was at the age of days 14–20. The number of younger or older cells was smaller. Although the number of double-positive cells in the control OB was small, cells at days 14–20 were the largest in number. Subtraction of this value from that of diazepam administration shows the net increase induced by diazepam, which again peaked at days 14–20. These results indicated that granule cells at days 14–20 were most susceptible to apoptotic cell death for both normal condition and diazepam administration. This period roughly matched the period when BrdUrd-positive cell numbers decreased with sensory deprivation and diazepam administration.
Fig. 5E shows the morphology of caspase-3-activated cells at days 14–20. The cells indicated by arrowheads in Fig. 5E extended dendrites into EPL and made branches there. These cells thus represent the new granule cells at days 14–20 after the generation that have extended dendrites into EPL and are undergoing apoptosis.
Discussion
We have identified the critical time window for new granule cells in the adult mouse OB when their survival was strongly influenced by sensory experience. This period corresponds to days 14–28 after the birth of cells. Petreanu and Alvarez-Buylla (6) examined the morphological maturation of new granule cells by retrovirus labeling. A majority of granule cells at days 14–30 after the labeling become class 4 or class 5 cells, which elongate dendrites into EPL and make dendrodendritic synapses with mitral/tufted cells (25). In accordance with this study, we observed that many of the caspase-3-activated cells have extended dendrites into EPL (Fig. 4). Furthermore, we observed that at least some of the caspase-3-activated granule cells that elongated dendrites into EPL were at days 14–20 (Fig. 5). These results suggest that new granule cells at the period of synapse formation with mitral/tufted cells in EPL are susceptible to cell death in a sensory experience-dependent manner. This notion is consistent with the observation that class 4 and 5 granule cells were present in cyclic nucleotide-gated channel knockout mice at 14 days after retrovirus labeling, but decreased in number at day 45 (6).
In neonatal CNS, chemicals such as ethanol, NMDA receptor antagonist, and GABA mimetics induce neuronal death (26, 27). Importantly, the susceptible period of neurons to drug-induced cell death corresponds to the period of their synaptogenesis. Therefore, whatever the age of animals, the choice of neurons between survival and death might be executed when neurons start to synaptically interact with target neurons.
Possible Mechanism for Diazepam to Reduce Survival of New Granule Cells. This study indicates that the effect of diazepam on the survival of new granule cells resembled that of sensory deprivation in the following aspects: (i) Diazepam reduced the survival of new granule cells. (ii) The time window for the effect of diazepam was similar to that of sensory deprivation. (iii) Diazepam administration and sensory deprivation both induced caspase-3 activation and apoptosis in granule cells.
GABA generally inhibits the firing of neurons either by hyperpolarizing the membrane potential or shunting the excitatory postsynaptic potentials (22, 28, 29). Diazepam potentiates the GABA action and reduces the overall neuronal activity within the brain (23). We suppose that reduced survival of new granule cells by diazepam is related to reduced neuronal activity. Diazepam administration may potentiate the inhibitory input to mitral/tufted cells and reduce their activity. Consequently, new granule cells may be under the influence of reduced activity of mitral/tufted cells, to which new granule cells make synapses. Glutamatergic input from mitral/tufted cells might serve as a surviving signal for granule cells. Systemic administration of NMDA receptor antagonist, MK-801, increases apoptotic granule cells in the OB (15). Alternatively, because granule cells express GABAA receptor from the early stage of their development (5, 30), diazepam may directly reduce the activity of new granule cells. The activity of neurons regulates their own survival in developing brain and culture cells (31). Other mechanisms such as reduced activity of centrifugal fibers may be crucial. The augmented effect of the simultaneous manipulation of sensory deprivation and diazepam administration (Fig. 3C) is consistent with the idea that each manipulation reduces but does not completely silence neuronal activity in the OB, and that simultaneous manipulation augments the reduction in neuronal activity and thus in cell survival. Further pharmacological studies with various agonists and antagonists would reveal the possible role of neuronal activity during the critical period.
Caspase Activation in Granule Cells by Sensory Deprivation and Diazepam Administration. We showed that apoptosis of OB granule cells was accompanied by caspase-3 activation in both normal and experimental conditions. Labeling with antiactivated caspase-3 antibody revealed the morphology of apoptotic granule cells. Because activated caspase-3 might not spread widely within the cell and cells with caspase-3 activation might already start changing their morphology, it was difficult to morphologically classify all of the caspase-3-activated cells as in previous studies (5, 6). Nevertheless, the present analysis showed that many apoptotic granule cells extended dendrites into EPL, where granule cells make synapses with mitral/tufted cells.
Distribution of the activated caspase-3 immunoreactivity raises a possibility that the activation might occur locally in dendrites. In cultured hippocampal neurons, focal application of glutamate induces caspase-3 activation in the dendrites (32). At the earliest time examined in our study (2 h) after diazepam administration, activated caspase-3 was observed both in the dendrites in EPL and soma (data not shown). Further temporal and spatial dissection of caspase activation might reveal how the apoptotic signals initiate in the new granule cells.
The co-occurrence of caspase-3 activation and DNA fragmentation also suggests that apoptosis of granule cells is mediated mainly by the caspase activation. We have observed the activation of caspase-9 in TUNEL-positive granule cells (unpublished observation). Caspases might serve as key molecules to reveal the molecular basis for how the sensory experience is converted into survival signals for new granule cells. Analysis using caspase knockout mice and caspase inhibitors might be a promising strategy (33).
Significance of the Critical Period Identification. This study indicates that the survival of new granule cells is under the strong influence of olfactory sensory input when the cells are at their critical period. Each odorant activates OB regions with distinct but stereotypical spatial patterns, generating an activity map in the OB (34–36). Because odorants that animals perceive may change in different environments and situations, the OB regions effectively integrating new granule cells may vary in the same animals. In this way OB neuronal circuit may be remodeled temporally and spatially depending on the sensory experience.
The existence of the critical period for new granule cells indicates that survival of granule cells at a younger or older age is less influenced by sensory experience. Mechanisms that open and then close the critical period should be the focus of additional studies. In the high vocal center of song birds, BDNF application during days 14–20 after the generation of new neurons, but not during other periods, enhances cell survival (37). Function of tropic factors like BDNF in the mammalian OB needs to be clarified. Our results showed that granule cells at days 14–20 are most susceptible to caspase-3 activation. It is tempting to speculate that the critical period is defined by mechanisms that make caspase activation permissive. Detailed analysis of positive and negative controls of the apoptotic pathways might provide a clue toward understanding the mechanisms for the critical period for sensory input-dependent cell survival and the integration of new granule cells into the preexisting neuronal circuit.
Neuronal transplantation is a hopeful therapy for neurodegenerative diseases and traumatic injuries of CNS. Transplanted neurons may have a time window for activity-dependent survival, although the onset and duration should vary among different types of neurons. Understanding the nature of the critical period should facilitate the effective utilization of sensory input for urging transplanted neurons to generate appropriate neuronal circuits and recover CNS functions.
Acknowledgments
We thank our colleagues in the Department of Physiology, University of Tokyo for advice and valuable discussion. This work was supported by Grants-in-Aid from Creative Scientific Research and Basic Scientific Research from the Japan Society for the Promotion of Science.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: OB, olfactory bulb; IPL, internal plexiform layer; EPL, external plexiform layer; GCL, granule cell layer; MCL, mitral cell layer.
References
- 1.Luskin, M. B. (1993) Neuron 11, 173-189. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez-Buylla, A. & Garcia-Verdugo, J. M. (2002) J. Neurosci. 22, 629-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kempermann, G., Wiskott, L. & Gage, F. H. (2004) Curr. Opin. Neurobiol. 14, 186-191. [DOI] [PubMed] [Google Scholar]
- 4.Belluzzi, O., Benedusi, M., Ackman, J. & LoTurco, J. J. (2003) J. Neurosci. 23, 10411-10418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carleton, A., Petreanu, L. T., Lansford, R., Alvarez-Buylla, A. & Lledo, P. M. (2003) Nat. Neurosci. 6, 507-518. [DOI] [PubMed] [Google Scholar]
- 6.Petreanu, L. & Alvarez-Buylla, A. (2002) J. Neurosci. 22, 6106-6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winner, B., Cooper-Kuhn, C. M., Aigner, R., Winkler, J. & Kuhn, H. G. (2002) Eur. J. Neurosci. 16, 1681-1689. [DOI] [PubMed] [Google Scholar]
- 8.Rochefort, C., Gheusi, G., Vincent, J. D. & Lledo, P. M. (2002) J. Neurosci. 22, 2679-2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gordon, J. A. & Stryker, M. P. (1996) J. Neurosci. 16, 3274-3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hensch, T. (2003) Neurosci. Res. 47, 17-22. [DOI] [PubMed] [Google Scholar]
- 11.Stern, E. A., Maravall, M. & Svoboda, K. (2001) Neuron 31, 305-315. [DOI] [PubMed] [Google Scholar]
- 12.Brunjes, P. C. (1994) Brain Res. Rev. 19, 146-160. [DOI] [PubMed] [Google Scholar]
- 13.Corotto, F. S., Henegar, J. R. & Maruniak, J. A. (1994) Neuroscience 61, 739-744. [DOI] [PubMed] [Google Scholar]
- 14.Najbauer, J. & Leon, M. (1995) Brain Res. 674, 245-251. [DOI] [PubMed] [Google Scholar]
- 15.Fiske, B. K. & Brunjes, P. C. (2001) J. Neurobiol. 47, 223-232. [DOI] [PubMed] [Google Scholar]
- 16.Fiske, B. K. & Brunjes, P. C. (2001) J. Comp. Neurol. 431, 311-319. [PubMed] [Google Scholar]
- 17.Biebl, M., Cooper, C. M., Winkler, J. & Kuhn, H. G. (2000) Neurosci. Lett. 291, 17-20. [DOI] [PubMed] [Google Scholar]
- 18.Yuan, J., Lipinski, M. & Degterev, A. (2003) Neuron 40, 401-413. [DOI] [PubMed] [Google Scholar]
- 19.Cameron, H. A. & McKay, R. D. G. (2001) J. Comp. Neurol. 435, 406-417. [DOI] [PubMed] [Google Scholar]
- 20.Yamaguchi, M., Saito, H., Suzuki, M. & Mori, K. (2000) NeuroReport 11, 1991-1996. [DOI] [PubMed] [Google Scholar]
- 21.Mullen, R. J., Buck, C. R. & Smith, A. M. (1992) Development (Cambridge, U.K.) 116, 201-211. [DOI] [PubMed] [Google Scholar]
- 22.Mehta, A. K. & Ticku, M. K. (1999) Brain Res. Rev. 29, 196-217. [DOI] [PubMed] [Google Scholar]
- 23.Eintrei, C., Sokoloff, L. & Smith, C. B. (1999) Br. J. Anaesth. 82, 596-602. [DOI] [PubMed] [Google Scholar]
- 24.Olney, J. W., Tenkova, T., Dikranian, K., Muglia, L. J., Jermakowicz, W. J., D'Sa, C. & Roth, K. A.(2002) Neurobiol. Dis. 9, 205-219. [DOI] [PubMed] [Google Scholar]
- 25.Shepherd, G. M., Chen, W. R. & Greer, C. A. (2004) in The Synaptic Organization of the Brain, ed. Shepherd, G. M. (Oxford Univ. Press, New York), pp. 165-216.
- 26.Ikonomidou, C., Bittigau, P., Ishimaru, M. J., Wozniak, D. F., Koch, C., Genz, K., Price, M. T., Stefovska, V., Horster, F., Tenkova, T., et al. (2000) Science 287, 1056-1060. [DOI] [PubMed] [Google Scholar]
- 27.Olney, J. W. (2002) Neurotoxicology 23, 659-668. [DOI] [PubMed] [Google Scholar]
- 28.Ben-Ari, Y. (2002) Nat. Rev. Neurosci. 3, 728-739. [DOI] [PubMed] [Google Scholar]
- 29.Owens, D. F. & Kriegstein, A. R. (2002) Nat. Rev. Neurosci. 3, 715-727. [DOI] [PubMed] [Google Scholar]
- 30.Stewart, R. R., Hoge, G. J., Zigova, T. & Luskin, M. B. (2002) J. Neurobiol. 50, 305-322. [DOI] [PubMed] [Google Scholar]
- 31.Mennerick, S. & Zorumski, C. F. (2001) Mol. Neurobiol. 22, 41-54. [DOI] [PubMed] [Google Scholar]
- 32.Mattson, M. P., Keller, J. N. & Begley, J. G. (1998) Exp. Neurol. 153, 35-48. [DOI] [PubMed] [Google Scholar]
- 33.Ranger, A. M., Malynn, B. A. & Korsmeyer, S. J. (2001) Nat. Genet. 28, 113-118. [DOI] [PubMed] [Google Scholar]
- 34.Mori, K., Nagao, H. & Yoshihara, Y. (1999) Science 286, 711-715. [DOI] [PubMed] [Google Scholar]
- 35.Uchida, N., Takahashi, Y. K., Tanifuji, M. & Mori, K. (2000) Nat. Neurosci. 3, 1035-1043. [DOI] [PubMed] [Google Scholar]
- 36.Nagao, H., Yamaguchi, M., Takahash, Y. K. & Mori, K. (2002) Microsc. Res. Tech. 58, 168-175. [DOI] [PubMed] [Google Scholar]
- 37.Alvarez-Borda, B., Haripal, B. & Nottebohm, F. (2004) Proc. Natl. Acad. Sci. USA 101, 3957-3961. [DOI] [PMC free article] [PubMed] [Google Scholar]