Abstract
Importance
Efficient approaches to prevent postoperative atrial fibrillation (POAF) after coronary artery bypass grafting (CABG) are still needed.
Objective
To investigate whether partial cardiac denervation, achieved by cutting off the ligament of Marshall (LOM) and resecting the fat pad along the Waterston groove, can reduce the risk of POAF following CABG.
Design, Setting and Participants
This single-center, randomized clinical trial enrolled adult patients scheduled for isolated CABG in China. Enrollment was from August 15, 2022, to December 13, 2023; follow-up visits were 30 days after discharge.
Interventions
Participants were randomized into the intervention group (CABG plus partial cardiac denervation) and the control group (CABG only) in a 1:1 pattern. All participants were continuously monitored for the incidence of POAF until day 6 after the operation.
Main outcome and Measures
The primary end point was the incidence of POAF in 6 days, defined as a supraventricular arrhythmia lasting for more than 30 seconds.
Results
The trial enrolled 430 patients (79 [18.4%] female; mean [SD] age, 61.9 [7.8] years). Compared with the control group, the 6-day incidence of POAF was significantly lower in the intervention group (18.1% vs 31.6%; P = .001; risk ratio, 0.57 [95% CI, 0.41-0.81]). To further support these results, a sensitivity analysis performed with Kaplan-Meier survival curves also showed a significant reduction in the occurrence of POAF in the intervention group (hazard ratio, 0.53 [95% CI, 0.36-0.79]; P = .002). Safety assessments showed no difference between the 2 groups, while postoperative medical cost was reduced in the intervention group.
Conclusions and Relevance
This randomized clinical trial found that partial cardiac denervation was an effective procedure to reduce the occurrence of POAF after isolated CABG without additional postoperative complications. These results suggest that partial cardiac denervation may be a good option for cardiac surgeons to consider for preventing POAF after CABG.
Trial Registration
ClinicalTrials.gov Identifier: NCT05009914
This randomized clinical trial examines whether a partial cardiac denervation procedure reduces the incidence of postoperative atrial fibrillation among patients undergoing isolated coronary artery bypass grafting (CABG) in China.
Key Points
Question
Among patients undergoing isolated coronary artery bypass grafting (CABG), does a partial cardiac denervation procedure reduce the incidence of postoperative atrial fibrillation (POAF)?
Findings
In this randomized clinical trial of 430 patients undergoing isolated CABG, partial cardiac denervation, achieved by cutting off the ligament of Marshall and resecting the fat pad along the Waterston groove, reduced the incidence of POAF without an increase in complications
Meaning
This study’s results suggest that partial cardiac denervation may be an effective approach for cardiac surgeons to prevent POAF after CABG .
Introduction
Postoperative atrial fibrillation (POAF) is a common complication after coronary artery bypass grafting (CABG), which occurs most commonly within 1 week after surgery, with an incidence ranging from 5% to 40%. POAF may prolong the length of hospitalization and elevate the risk of stroke and mortality, thereby increasing medical costs. Furthermore, POAF might also increase the long-term risk of cumulative cerebrovascular accidents.
The cardiac autonomic nerve system (CANS) plays a vital role in the development of POAF. Nevertheless, the prevalence of POAF following CABG still stands at 21.1% despite the widespread use of β-blockers, which inhibit cardiac sympathetic excitability and are the sole medication recommended as a class I treatment by the current guidelines to prevent POAF. The intrinsic CANS mainly consists of ganglia and relative nerves found in the epicardial adipose tissue (EAT) surrounding the cardiac major arteries and veins, including the ligament of Marshall (LOM) and the fat pad along the Waterston groove. The activation of CANS embedded in the EAT is considered to take part in the development of atrial fibrillation (AF), which is the main theory for cutting off the LOM and resecting the fat pad along the Waterston groove during Maze procedure for the treatment of AF. Therefore, we hypothesized that partial cardiac denervation through these 2 sites might be useful for reducing the risk of POAF after CABG. By cutting off the LOM and resecting the fat pad along the Waterston groove, this trial aimed to test the hypothesis that partial cardiac denervation could reduce POAF after isolated CABG.
Methods
Study Design and Patient Eligibility Criteria
This single-center, prospective, randomized clinical trial was conducted according to the Declaration of Helsinki, approved by the institutional review board of Fuwai Hospital, and registered at ClinicalTrials.gov in June 2021 (protocol record: NCRC2020003; and identifier: NCT05009914). Reporting of this trial followed the Consolidated Standards of Reporting Trials (CONSORT) guideline. The study was overseen by both the institutional review board and the data monitoring committee for safety and efficacy concerns during the entire process. The study protocol was previously published.
Adult patients scheduled for isolated CABG were screened for eligibility. Exclusion criteria were as follows: (1) age younger than 18 years; (2) urgent CABG; (3) prior cardiac surgery history; (4) concurrent cardiac surgery (such as repair of congenital heart disease, left ventricular reconstruction, valvular surgery, or surgery for aortic diseases); (5) critical condition requiring mechanical or pharmaceutical sustainment before CABG; (6) AF history (defined as supraventricular heart rhythm disorder characterized by rapid and irregular electrical activity in the atrium); and (7) taking antiarrhythmic medications other than β-blockers in 2 weeks before the surgery.
Randomization
After giving written informed consent, a computer-generated minimized random allocation approach was used to randomly assign individuals. As POAF is strongly associated with various factors, including age, left ventricular systolic function, a history of AF and left atrial enlargement, to ensure a proper baseline balance after randomization, randomization was stratified by age, left ventricular ejection fraction (LVEF), and history of myocardial infarction (eMethods 1 in Supplement 1). All surgeons were informed of the patient’s specific group assignment once their patients were under general anesthesia in the operating room.
Interventions
Before scheduled CABG, the intervention group underwent a partial cardiac denervation procedure achieved by cutting off the LOM and resecting the fat pad along the Waterston groove. If the patient’s hemodynamics was unstable by the judgement of the surgeon during off-pump CABG, the partial cardiac denervation procedure was performed after anastomosis of grafts. The specific procedure was described previously and detailed in eMethods 2 in Supplement 1. The anatomic sites of the LOM and the fat pad along the Waterston groove were shown in eFigures 1 and 2 in Supplement 1. Thorough cardiac denervation to the surface of myocardium was necessary. In the control group, patients underwent isolated CABG without an additional surgical procedure.
At the beginning, all surgeons taking part in this trial observed how the intervention procedure was performed by 2 experienced chief surgeons. After completing no more than 3 procedures with their guidance, all of the surgeons could solely and proficiently master this technique.
To confirm the existence of intrinsic CANS in the resected EAT and that partial cardiac denervation was achieved, 10 pairs of Waterston fat pad samples from participants (1 with and 1 without POAF in each pair) were selected to perform histologic analysis. All samples were fixed in formalin and cut (from myocardial to epicardial side) into 2 or 3 segments according to their size. Each segment was embedded in paraffin, sliced into sections and stained by hematoxylin-eosin. The example of the fat pad along the Waterston groove and the example of histologic results are presented separately in eFigures 3 and 4 in Supplement 1. Ganglia and/or nerve fibers could be seen in all samples (eTables 1 and 2 in Supplement 1).
Outcomes
By wearing the NS-SP-B-01 Attached Dynamic Electrocardiogram (ECG) Recording System (Ensense, Shanghai, China), all participants were continuously monitored from within 1 hour after the surgery to postoperative day 6. Additional 12-lead ECGs would be conducted if necessary. After the surgery, all of the patients were prescribed β-blockers unless there were complications with contradictions such as bradycardia or atrioventricular block. Participants were assessed by 12-lead standard ECG and echocardiogram on the day of discharge. The 30-day follow-up after discharge was completed by outpatient visit and/or phone calls.
The primary outcome was the occurrence of POAF in 6 days, defined as a supraventricular arrhythmia lasting for more than 30 seconds. Additionally, the number of AF episodes and AF burden (defined as the ratio of total AF time to recording time) were compared between the 2 groups.
The secondary outcomes included both perioperative and follow-up events. These were: (1) safety assessments including the incidence of transferring to on-pump CABG, secondary operation for bleeding, requirement for blood transfusions, delayed pericardial effusion, and critical arrhythmias except AF within 30 days after discharge; (2) economic assessments of the postoperative length of hospitalization and postoperative medical cost; and (3) major adverse cardiovascular and cerebrovascular events (MACCE), which were defined as the composite of stroke, myocardial infarction, repeat coronary revascularization and all-cause mortality during the 30-day follow-up. Delayed pericardial effusion was defined as new-onset pericardial effusion (moderate or more) within 30 days after discharge. Critical arrhythmias other than AF were defined as arrhythmias requiring immediate clinical intervention, such as complete atrioventricular block.
Statistical Analysis
We assumed a rate of POAF of 23% after cardiac surgery according to previous studies and that cardiac denervation would reduce the occurrence by 50%. An estimation of 408 participants were required to provide 80% power and .05 α (2-sided). Considering a certain rate of protocol violation, participant withdrawal and loss to follow-up, a total of 430 participants (215 in each group) was sufficient for this trial.
All analyses were conducted according to the intention-to-treat principle. Continuous variables were expressed as mean (SD) or median (IQR), and tested by t test or Mann-Whitney U test according to their distributions. Categorical variables were presented as numbers (%) and tested by χ2 test or by Fisher exact test, as appropriate. To further confirm the results, a sensitivity analysis regarding the primary outcome was carried out by the time-to-event analysis performed with Kaplan-Meier survival curves and compared by the log-rank test.
Subgroup analysis was conducted based on the risk factors of POAF reported by previous studies, including on/off-pump, sex, age (≥65 years vs <65 years), LVEF (>55% vs ≤55%), body mass index (calculated as weight in kilograms divided by height in meters squared; ≥25 vs <25), left atrium size (≥40 mm vs <40 mm), and history of myocardial infarction, diabetes, and hypertension. Two-tailed P < .05 was considered statistically significant. Statistical analyses were performed using R 4.0.2 (R Core Team) and Stata 15.0 (StataCorp).
Results
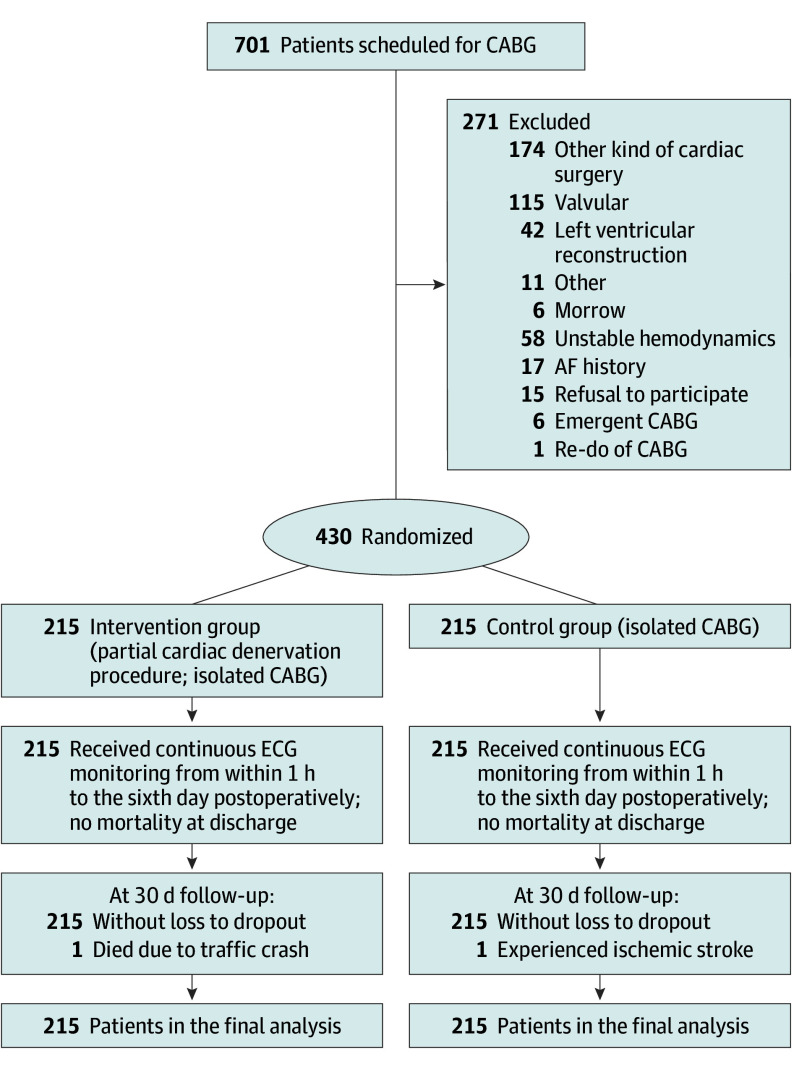
From August 15, 2022, to December 13, 2023, a total of 701 patients were screened for eligibility, and 430 patients were enrolled in this study (Figure 1). The mean (SD) age was 61.9 (7.8) years, and 79 participants (18.4%) were female. Baseline characteristics were well balanced after randomization between the intervention group (n = 215) and the control group (n = 215) (Table 1).
Figure 1. Flowchart of the Study.
AF indicates atrial fibrillation; CABG, coronary artery bypass grafting; ECG, electrocardiogram.
Table 1. Baseline and Intraoperative Characteristics.
| Variables | Control (n = 215) | Intervention (n = 215) |
|---|---|---|
| Baseline | ||
| Age, mean (SD), y | 61.9 (7.6) | 61.9 (7.9) |
| Sex, No. (%) | ||
| Female | 40 (18.6) | 39 (18.1) |
| Male | 175 (81.4) | 176 (81.9) |
| BMI, median (IQR) | 26.0 (23.8-28.0) | 25.9 (24.1-27.8) |
| NYHA III or IV, No. (%) | 35 (16.3) | 37 (17.2) |
| Hypertension, No. (%) | 165 (76.7) | 152 (70.7) |
| Diabetes, No. (%) | 93 (43.3) | 108 (50.2) |
| Insulin users, No. (%) | 31 (14.4) | 40 (18.6) |
| Dyslipidemia, No. (%) | 178 (82.8) | 173 (80.5) |
| Chronic kidney dysfunction, No. (%) | 2 (0.9) | 3 (1.4) |
| Left main disease, No. (%) | 42 (19.5) | 36 (16.7) |
| Triple vessel diseases, No. (%) | 188 (87.4) | 183 (85.1) |
| Myocardial infarction, No. (%) | 85 (39.5) | 85 (39.5) |
| Prior PCI, No. (%) | 41 (19.0) | 49 (22.8) |
| Stroke, No. (%) | 39 (18.1) | 42 (19.5) |
| Peripheral arterial disease, No. (%) | 40 (18.6) | 32 (14.9) |
| Chronic lung disease, No. (%) | 1 (0.5) | 3 (1.4) |
| Smoking, No. (%) | 107 (49.8) | 110 (51.2) |
| NT-pro BNP, median (IQR), pg/mL | 121.0 (59.9-302.0) | 115.0 (51.9-239.0) |
| T3, median (IQR), ng/mL | 1.1 (1.0-1.2) | 1.1 (1.0-1.2) |
| hsCRP, median (IQR), mg/L | 1.0 (0.5-2.7) | 1.0 (0.4-3.2) |
| ALT, median (IQR), IU/L | 21.0 (16.0-29.0) | 22.0 (14.0-35.0) |
| AST, median (IQR), IU/L | 22.0 (17.0-28.0) | 23.0 (17.0-30.0) |
| Creatinine, median (IQR), μmol/L | 68.4 (59.5-77.9) | 67.3 (58.8-78.1) |
| LVEF, median (IQR) | 63.0 (60.0-66.0) | 63.0 (60.0-67.0) |
| Left atrium, median (IQR), mm | 36.0 (34.0-40.0) | 36.0 (34.0-38.0) |
| LVEDD, median (IQR), mm | 48.0 (45.0-52.0) | 48.0 (45.0-51.0) |
| Mitral regurgitation, No. (%) | ||
| No/trivial | 159 (74.0) | 173 (80.5) |
| Mild | 56 (26.1) | 42 (19.5) |
| β-Blocker, No. (%) | 188 (87.4) | 188 (87.4) |
| Intraoperative | ||
| On-pump, No. (%) | 91 (42.3) | 86 (40) |
| CPB duration, median (IQR), min | 107.0 (82.0-133.0) | 102.0 (80.0-123.0) |
| Cross clamp time, median (IQR), min | 73.0 (57.0-95.0) | 72.5 (55.0-89.0) |
| Operation time, median (IQR), min | 234.0 (200.0-260.0) | 228.0 (203.0-255.0) |
| No. of grafts, mean (SD) | 3.8 (0.8) | 3.7 (0.8) |
| Arterial grafts, mean (SD) | 1.1 (0.4) | 1.0 (0.3) |
| Venous grafts, mean (SD) | 2.7 (0.8) | 2.7 (0.9) |
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CPB, cardiopulmonary bypass; hs-CRP, high-sensitivity C-reactive protein; LVEDD, left ventricular end diastolic diameter; LVEF, left ventricular ejection fraction; NT-pro BNP, N-terminal B-type natriuretic peptide; NYHA, New York Heart Association; PCI, percutaneous coronary intervention.
SI conversion factors: To convert ALT to μkat/L, multiply 0.0167; AST to μkat/L, multiply 0.0167; creatinine to mg/dL, divide by 88.4; hs-CRP to mg/dL, divide by 10; NT-pro BNP to ng/L, multiply by 1.
All participants underwent scheduled CABG successfully. The partial cardiac denervation procedure did not significantly increase total operative time or bypass time (Table 1) and was successfully performed on each patient in the intervention group. Nineteen patients who underwent off-pump CABG emerged with a transient increase in heart rate when resecting the fat pad along the Waterston groove. No other complications or adverse events occurred during the partial cardiac denervation procedure.
There were no in-hospital deaths in either group. One patient in the intervention group and 3 in the control group developed new-onset stroke, respectively. For blood inflammatory biomarkers, the level of postoperative interleukin (IL)-4, IL-6, IL-8, tumor necrosis factor α and high-sensitivity C-reactive protein of the patients were comparable between the intervention and control group (eTable 3 in Supplement 1).
Primary Outcome
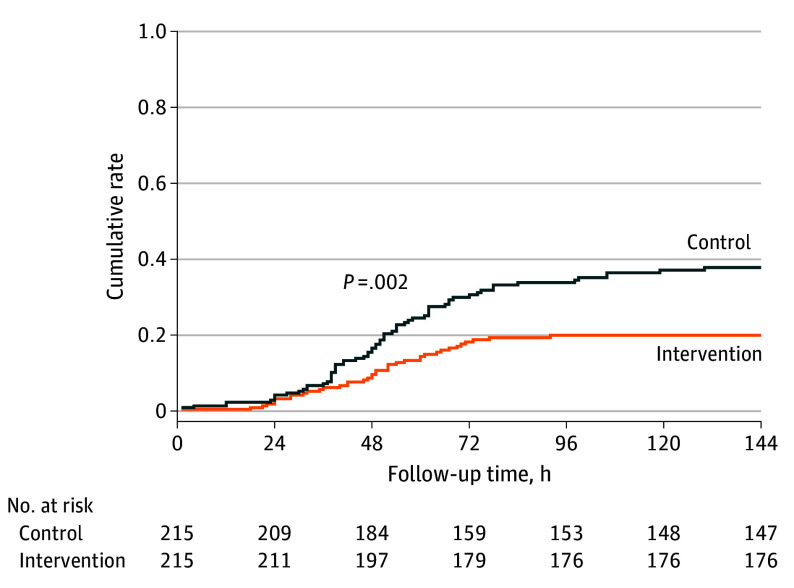
All patients were included in the final analysis. The postoperative use of β-blockers did not differ in each group (206 [95.8%] vs 200 [93.0%]; P = .21). Thirty-nine patients in the intervention group and 68 patients in the control group developed POAF, respectively. As compared with the control group, partial cardiac denervation reduced the risk of POAF (18.1% vs 31.6%; P = .001; risk ratio: 0.57 [95% CI, 0.41-0.81]). In the sensitivity analysis, the Kaplan-Meier analysis also showed a significant reduction in the occurrence of POAF in the intervention group (hazard ratio, 0.53 [95% CI, 0.36-0.79]; P = .002) (Figure 2).
Figure 2. Kaplan-Meier Curves of the Primary Outcome.
Subgroup analysis for the primary outcome was conducted in terms of potential risk factors. The results showed that partial cardiac denervation had similar treatment effect in each subgroup (eFigure 5 in Supplement 1). The incidence of POAF was similar between on-pump (30.8%) and off-pump CABG (31.5%) in the control group.
POAF-Related Measurements
Median (IQR) AF episodes were significantly reduced in the intervention group (0 [0-0] vs 0 [0-3]; P = .002), with a maximum of 223 in the intervention group and 158 in the control group, respectively. AF burden was lower in the intervention group (AF burden of 0%: 147 [68.4%] vs 176 [81.9]; >0% to ≤10%: 58 [27.0%] vs 31 [14.4%]; >10%: 10 [4.7%] vs 8 [3.7%]; P = .004) (Table2), while 2 patients in the control group were diagnosed with POAF that lasted more than half of the monitoring time, detailed as 99.97% and 100%. These were the only 2 patients with persistent AF on the day of discharge.
Table 2. POAF-Related Measurements.
| Variables | Control (n = 215) | Intervention (n = 215) | P value |
|---|---|---|---|
| AF burden, No. (%) | |||
| 0 | 147 (68.4) | 176 (81.9) | .004 |
| >0 and ≤10% | 58 (27.0) | 31 (14.4) | |
| >10% | 10 (4.7) | 8 (3.7) | |
| POAF lasting time, No. (%) | |||
| 0 | 146 (67.9) | 175 (81.4) | .01 |
| >0 and ≤6 min | 5 (2.3) | 2 (0.9) | |
| >6 min and ≤6 h | 38 (17.7) | 22 (10.2) | |
| >6 h and ≤24 h | 23 (10.7) | 11 (5.1) | |
| >24 h | 3 (1.4) | 5 (2.4) | |
| AF episodes, No. (%) | 0 (0 to 3) | 0 (0 to 0) | .002 |
| Antiarrhythmic therapy | |||
| β-Blocker, No. (%) | 200 (93.0) | 206 (95.8) | .21 |
| Cardioversion, No. (%) | 0 | 0 | NA |
| Amiodarone, No. (%) | 57 (26.5) | 31 (14.4) | .002 |
| % of Premature atrial contractions, median (IQR) | 0.044 (0.007 to 0.489) | 0.026 (0.007 to 0.233) | .47 |
| % of Single premature atrial contractions, median (IQR) | 0.041(0.005 to 0.397) | 0.024 (0.007 to 0.200) | .42 |
| % of Couplets, median (IQR) | 0.001 (0 to 0.019) | 0.001 (0 to 0.005) | .42 |
| % of Nonsustained atrial tachyarrhythmias, median (IQR) | 0.002 (0 to 0.023) | 0.001 (0 to 0.008) | .31 |
| Highest HR, median (IQR), beats/min | 146 (125 to 182) | 133 (119 to 166) | .002 |
| Lowest HR, median (IQR), beats/min | 56 (49 to 61) | 59 (52 to 65) | .001 |
| Mean HR, median (IQR), beats/min | 81 (77 to 87) | 83 (78 to 88) | .24 |
| Acceleration capacity, median (IQR), ms | −3.1 (−2.7 to −3.8) | −2.8 (−2.4 to −3.4) | <.001 |
| Deceleration capacity, median (IQR), ms | 2.8 (2.3 to 3.4) | 2.6 (2.2 to 3.1) | .008 |
Abbreviations: AF, atrial fibrillation; HR, heart rate; NA, not applicable; POAF, postoperative atrial fibrillation.
There was no difference regarding the percentage of single premature atrial contractions, couplets, nonsustained atrial tachyarrhythmias and mean heart rate between the 2 groups postoperatively. However, the acceleration capacity and the deceleration capacity were different between the 2 groups, presenting a potential autonomic modulation (Table 2).
Secondary Outcomes
For the safety assessments, the incidence of perioperative blood transfusion (33 [15.4%] vs 44 [20.5%]; P = .17), reoperation for postoperative bleeding (0 [0%] vs 2 [0.9%]; P = .50), and delayed pericardial effusion (1 [0.5%] vs 2[0.9%]; P > .99) was similar between groups (Table 3). No patient transferred to on-pump CABG intraoperatively or developing critical arrhythmias within the 30-day follow-up. However, 1 patient in each group developed AF after discharge.
Table 3. Secondary Outcomes.
| Variables | Control (n = 215) | Intervention (n = 215) | P value |
|---|---|---|---|
| Perioperative | |||
| Blood transfusion, No. (%) | 44 (20.5) | 33 (15.4) | .17 |
| Reoperation for bleeding, No. (%) | 2 (0.9) | 0 | .50 |
| Postoperative length of hospitalization, mean (SD), days | 7.3 (3.3) | 6.8 (1.8) | .09 |
| Medical cost, median (IQR), US $ | 4052.9 (3338.2-5278.8) | 3909.2 (3226.5-4917.9) | .05 |
| Follow-up | |||
| Delayed epicardial effusion, No. (%) | 2 (0.9) | 1 (0.5) | >.99 |
| MACCE within 30 d, No. (%) | 1 (0.5) | 1 (0.5) | >.99 |
Abbreviation: MACCE, major adverse cardiovascular and cerebrovascular events.
For the economic assessments, the postoperative length of hospitalization was a mean (SD) of 6.8 (1.8) days in the intervention group vs 7.3 (3.3) days in the control group (P = .09) (Table 3). Patients in the intervention group had shorter median (IQR) intensive care unit stay times than the control group (21.0 [17.0-42.0] vs 22.0 [17.0-51.0] hours; P = .05) (eTable 3 in Supplement 1). The median (IQR) cost was also reduced in the intervention group (US $3909.2 [US $3226.5-4917.9] vs US $4052.9 [US $3338.2-5278.8]; P = .05).
Within the 30-day follow-up, 1 patient in the intervention group died of traffic accident and 1 patient in the control group suffered from ischemic stroke. There was no difference of MACCE between each group (1 [0.5%] vs 1 [0.5%]; P > .99) (Table 3).
Discussion
In this randomized clinical study, partial cardiac denervation reduced the occurrence of POAF by 47%, as well as the AF burden and number of AF episodes after isolated CABG. This procedure was cost-efficient, because patients in the intervention group had shorter intensive care unit stays and less postoperative medical costs without additional complications.
The mechanisms of POAF after cardiac surgery are extremely complicated and remain unclear. Generally, the development of POAF is based on 3 aspects, including (1) the atrial remodeling substrate, such as left atrial enlargement and fibrosis, which is associated with aging, hypertension, and genetics; (2) surgery-induced substrate resulting from cardiopulmonary bypass or atriotomy; and (3) transient postoperative factors such as CANS, inflammation, or oxidative stress.
Numerous research studies have investigated the effects of autonomic neuromodulation therapies and surgical means to avert POAF. Several studies attempted to prevent POAF by excising the fat pads surrounding the heart’s major vessels and injecting botulinum toxin into the epicardial fat pads. The results were inconsistent due to limited sample size and nonoptimal trial design. Wang et al tried to suppress the function of 4 major ganglionated plexi by injecting calcium chloride and reported a reduction of POAF. However, this study only enrolled patients undergoing off-pump CABG and the injection procedure required a certain learning curve. The NOVA study also showed similar outcomes, though the sample size was limited. As the largest study on this clinical field, the current pCAD-POAF trial enrolled either on-pump or off-pump patients. In this study, partial cardiac denervation was achieved by surgical resection of LOM and fat pad along the Waterston groove. The results showed that the incidence of POAF, AF episodes, and AF burden were reduced after the partial cardiac denervation procedure, which meanwhile did not significantly increase the operation time, cause bleeding or critical arrhythmias. Although the postoperative length of hospital stay was not statistically different, partial cardiac denervation still brought economic benefit and decreased the intensive care unit stay.
One major concern may be the safety of the procedure. In our study, there was no difference between the 2 groups regarding the percentage of premature atrial contractions (single/couplets/nonsustained atrial tachyarrhythmias), mean heart beats, echocardiogram parameters at discharge and the 30 days follow-up visit (eTable 3 in Supplement 1). Even though no postoperative complications were observed, long-term follow-up to assess patients’ performance such as exercise tolerance and functional capacity, is still needed to fully evaluate the safety and long-term outcomes of this procedure.
It is believed that the stimulation of sympathetic tone may lead to POAF, because higher levels of norepinephrine and more administration of inotropic agents were found in patients with POAF. Nevertheless, both higher and lower heart rate variability could be seen before POAF, suggesting that dysfunction of not only sympathetic but also vagal tone may participate in the process. With the histologic results in this trial, we confirm the existence of ganglia and nerve fibers in the EAT we resected and the preventive role of partial cardiac denervation. However, the exact interactions between CANS and myocardium, as well as how the balance of sympathetic and vagal tone changes remain uncertain.
There is scarce evidence directly derived from human samples to provide information of the postoperative change in patients with POAF. Most studies tried to associate blood inflammatory biomarkers such as IL-4 and IL-6 with POAF, but the results were inconsistent. In our study, these results were comparable between the 2 groups. Under this circumstance, the local ganglia regulation and inflammatory effect of EAT may play a vital role in the development of POAF.
Cutting off the LOM and resecting the fat pad along the Waterston groove was not a highly-demanding technique and could be mastered easily after a short-term training. Therefore, surgical partial cardiac denervation might be an effective and convenient approach for cardiac surgeons to prevent POAF after CABG.
Limitations
Our study has limitations. First, due to the impact of COVID-19 pandemic, we encountered challenges in conducting a multicenter trial, thus heterogeneity across cardiac surgery centers in implementing the intervention has not been examined. Second, given that POAF predominantly manifests within the first week after surgery, our study primarily focused on outcomes during hospitalization and short-term follow-up. However, it remains imperative to conduct long-term follow-ups to thoroughly investigate the safety and efficacy of this partial cardiac denervation technique. We intend to invite all patients to participate in a long-term assessment after 1 year or more in the future.
Conclusions
This RCT found that the occurrence of POAF after isolated CABG could be efficiently reduced by partial cardiac denervation through cutting off the LOM and resecting the fat pad along the Waterston groove. These results suggest that partial cardiac denervation may be viable option for cardiac surgeons to consider for reducing the risk of POAF after CABG
eMethods 1. Details of the Randomization Scheme
eMethods 2. Partial Cardiac Denervation Procedure
eTable 1. Histologic Results of the Waterston fat Pad Resected From Patients Who Developed POAF
eTable 2. Histologic Results of the Waterston Fat Pad Resected From Patients Without POAF
eTable 3. Other Postoperative and Follow-Up Results
eFigure 1. Anatomic Site of the LOM
eFigure 2. Anatomic Site of the Fat Pad Along the Waterston Groove
eFigure 3. Example of the Fat Pad Along the Waterston Groove
eFigure 4. Examples of the Histologic Results Performed on the Fat Pad Along the Waterston Groove
eFigure 5. Subgroup Analysis for Primary Outcome
Trial Protocol and Statistical Analysis Plan
Data Sharing Statement
References
- 1.Villareal RP, Hariharan R, Liu BC, et al. Postoperative atrial fibrillation and mortality after coronary artery bypass surgery. J Am Coll Cardiol. 2004;43(5):742-748. doi: 10.1016/j.jacc.2003.11.023 [DOI] [PubMed] [Google Scholar]
- 2.Katritsis DG, Pokushalov E, Romanov A, et al. Autonomic denervation added to pulmonary vein isolation for paroxysmal atrial fibrillation: a randomized clinical trial. J Am Coll Cardiol. 2013;62(24):2318-2325. doi: 10.1016/j.jacc.2013.06.053 [DOI] [PubMed] [Google Scholar]
- 3.Eikelboom R, Sanjanwala R, Le ML, Yamashita MH, Arora RC. Postoperative atrial fibrillation after cardiac surgery: a systematic review and meta-analysis. Ann Thorac Surg. 2021;111(2):544-554. doi: 10.1016/j.athoracsur.2020.05.104 [DOI] [PubMed] [Google Scholar]
- 4.Maesen B, Nijs J, Maessen J, Allessie M, Schotten U. Post-operative atrial fibrillation: a maze of mechanisms. Europace. 2012;14(2):159-174. doi: 10.1093/europace/eur208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benedetto U, Gaudino MF, Dimagli A, et al. ; ART Investigators* . Postoperative atrial fibrillation and long-term risk of stroke after isolated coronary artery bypass graft surgery. Circulation. 2020;142(14):1320-1329. doi: 10.1161/CIRCULATIONAHA.120.046940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dobrev D, Aguilar M, Heijman J, Guichard JB, Nattel S. Postoperative atrial fibrillation: mechanisms, manifestations and management. Nat Rev Cardiol. 2019;16(7):417-436. doi: 10.1038/s41569-019-0166-5 [DOI] [PubMed] [Google Scholar]
- 7.Frendl G, Sodickson AC, Chung MK, et al. ; American Association for Thoracic Surgery . 2014 AATS guidelines for the prevention and management of perioperative atrial fibrillation and flutter for thoracic surgical procedures. J Thorac Cardiovasc Surg. 2014;148(3):e153-e193. doi: 10.1016/j.jtcvs.2014.06.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng Z, Jayaram R, Jiang L, et al. Perioperative rosuvastatin in cardiac surgery. N Engl J Med. 2016;374(18):1744-1753. doi: 10.1056/NEJMoa1507750 [DOI] [PubMed] [Google Scholar]
- 9.Mehall JR, Kohut RM Jr, Schneeberger EW, Taketani T, Merrill WH, Wolf RK. Intraoperative epicardial electrophysiologic mapping and isolation of autonomic ganglionic plexi. Ann Thorac Surg. 2007;83(2):538-541. doi: 10.1016/j.athoracsur.2006.09.022 [DOI] [PubMed] [Google Scholar]
- 10.Kim DT, Lai AC, Hwang C, et al. The ligament of Marshall: a structural analysis in human hearts with implications for atrial arrhythmias. J Am Coll Cardiol. 2000;36(4):1324-1327. doi: 10.1016/S0735-1097(00)00819-6 [DOI] [PubMed] [Google Scholar]
- 11.Tan AY, Li H, Wachsmann-Hogiu S, Chen LS, Chen PS, Fishbein MC. Autonomic innervation and segmental muscular disconnections at the human pulmonary vein-atrial junction: implications for catheter ablation of atrial-pulmonary vein junction. J Am Coll Cardiol. 2006;48(1):132-143. doi: 10.1016/j.jacc.2006.02.054 [DOI] [PubMed] [Google Scholar]
- 12.Onorati F, Curcio A, Santarpino G, et al. Routine ganglionic plexi ablation during Maze procedure improves hospital and early follow-up results of mitral surgery. J Thorac Cardiovasc Surg. 2008;136(2):408-418. doi: 10.1016/j.jtcvs.2008.03.022 [DOI] [PubMed] [Google Scholar]
- 13.Yang Z, Tiemuerniyazi X, Huang S, Song Y, Xu F, Feng W. Partial cardiac denervation to prevent postoperative atrial fibrillation after coronary artery bypass grafting (pCAD-POAF): study protocol for a randomized controlled trial. Am J Cardiol. 2024;221:120-125. doi: 10.1016/j.amjcard.2024.04.018 [DOI] [PubMed] [Google Scholar]
- 14.Abouarab AA, Leonard JR, Ohmes LB, et al. Posterior left pericardiotomy for the prevention of postoperative atrial fibrillation after cardiac surgery (PALACS): study protocol for a randomized controlled trial. Trials. 2017;18(1):593. doi: 10.1186/s13063-017-2334-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang H, Zhang Y, Xin F, et al. Calcium-Induced Autonomic denervation in patients with post-operative atrial fibrillation. J Am Coll Cardiol. 2021;77(1):57-67. doi: 10.1016/j.jacc.2020.10.049 [DOI] [PubMed] [Google Scholar]
- 16.Gaudino M, Sanna T, Ballman KV, et al. ; PALACS Investigators . Posterior left pericardiotomy for the prevention of atrial fibrillation after cardiac surgery: an adaptive, single-centre, single-blind, randomised, controlled trial. Lancet. 2021;398(10316):2075-2083. doi: 10.1016/S0140-6736(21)02490-9 [DOI] [PubMed] [Google Scholar]
- 17.Biancari F, Mahar MA. Meta-analysis of randomized trials on the efficacy of posterior pericardiotomy in preventing atrial fibrillation after coronary artery bypass surgery. J Thorac Cardiovasc Surg. 2010;139(5):1158-1161. doi: 10.1016/j.jtcvs.2009.07.012 [DOI] [PubMed] [Google Scholar]
- 18.Yadava M, Hughey AB, Crawford TC. Postoperative atrial fibrillation: incidence, mechanisms, and clinical correlates. Heart Fail Clin. 2016;12(2):299-308. doi: 10.1016/j.hfc.2015.08.023 [DOI] [PubMed] [Google Scholar]
- 19.Waldron NH, Cooter M, Haney JC, et al. Temporary autonomic modulation with botulinum toxin type A to reduce atrial fibrillation after cardiac surgery. Heart Rhythm. 2019;16(2):178-184. doi: 10.1016/j.hrthm.2018.08.021 [DOI] [PubMed] [Google Scholar]
- 20.Romanov A, Pokushalov E, Ponomarev D, et al. Long-term suppression of atrial fibrillation by botulinum toxin injection into epicardial fat pads in patients undergoing cardiac surgery: three-year follow-up of a randomized study. Heart Rhythm. 2019;16(2):172-177. doi: 10.1016/j.hrthm.2018.08.019 [DOI] [PubMed] [Google Scholar]
- 21.Melo J, Voigt P, Sonmez B, et al. Ventral cardiac denervation reduces the incidence of atrial fibrillation after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2004;127(2):511-516. doi: 10.1016/S0022-5223(03)01283-2 [DOI] [PubMed] [Google Scholar]
- 22.Alex J, Guvendik L. Evaluation of ventral cardiac denervation as a prophylaxis against atrial fibrillation after coronary artery bypass grafting. Ann Thorac Surg. 2005;79(2):517-520. doi: 10.1016/j.athoracsur.2004.08.011 [DOI] [PubMed] [Google Scholar]
- 23.Omran AS, Karimi A, Ahmadi H, Yazdanifard P, Sheikh Fahtollahi M, Tazik M. Prophylactic ventral cardiac denervation: does it reduce incidence of atrial fibrillation after coronary artery bypass grafting? J Thorac Cardiovasc Surg. 2010;140(5):1036-1039. doi: 10.1016/j.jtcvs.2009.12.024 [DOI] [PubMed] [Google Scholar]
- 24.Piccini JP, Ahlsson A, Dorian P, et al. ; NOVA-AF Investigators . Efficacy and safety of botulinum toxin type A for the prevention of postoperative atrial fibrillation. JACC Clin Electrophysiol. 2024;10(5):930-940. doi: 10.1016/j.jacep.2024.01.020 [DOI] [PubMed] [Google Scholar]
- 25.Kalman JM, Munawar M, Howes LG, et al. Atrial fibrillation after coronary artery bypass grafting is associated with sympathetic activation. Ann Thorac Surg. 1995;60(6):1709-1715. doi: 10.1016/0003-4975(95)00718-0 [DOI] [PubMed] [Google Scholar]
- 26.Argalious M, Motta P, Khandwala F, et al. “Renal dose” dopamine is associated with the risk of new-onset atrial fibrillation after cardiac surgery. Crit Care Med. 2005;33(6):1327-1332. doi: 10.1097/01.CCM.0000166876.41694.CA [DOI] [PubMed] [Google Scholar]
- 27.Hogue CW Jr, Domitrovich PP, Stein PK, et al. RR interval dynamics before atrial fibrillation in patients after coronary artery bypass graft surgery. Circulation. 1998;98(5):429-434. doi: 10.1161/01.CIR.98.5.429 [DOI] [PubMed] [Google Scholar]
- 28.Amar D, Zhang H, Miodownik S, Kadish AH. Competing autonomic mechanisms precede the onset of postoperative atrial fibrillation. J Am Coll Cardiol. 2003;42(7):1262-1268. doi: 10.1016/S0735-1097(03)00955-0 [DOI] [PubMed] [Google Scholar]
- 29.Kaireviciute D, Blann AD, Balakrishnan B, et al. Characterisation and validity of inflammatory biomarkers in the prediction of post-operative atrial fibrillation in coronary artery disease patients. Thromb Haemost. 2010;104(1):122-127. doi: 10.1160/TH09-12-0837 [DOI] [PubMed] [Google Scholar]
- 30.Kourliouros A, Yin X, Didangelos A, et al. Substrate modifications precede the development of atrial fibrillation after cardiac surgery: a proteomic study. Ann Thorac Surg. 2011;92(1):104-110. doi: 10.1016/j.athoracsur.2011.03.071 [DOI] [PubMed] [Google Scholar]
- 31.Del Campo A, Roldán J, Verdejo HE, et al. Increased C-reactive protein plasma levels are not involved in the onset of post-operative atrial fibrillation. J Cardiol. 2017;70(6):578-583. doi: 10.1016/j.jjcc.2017.03.011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. Details of the Randomization Scheme
eMethods 2. Partial Cardiac Denervation Procedure
eTable 1. Histologic Results of the Waterston fat Pad Resected From Patients Who Developed POAF
eTable 2. Histologic Results of the Waterston Fat Pad Resected From Patients Without POAF
eTable 3. Other Postoperative and Follow-Up Results
eFigure 1. Anatomic Site of the LOM
eFigure 2. Anatomic Site of the Fat Pad Along the Waterston Groove
eFigure 3. Example of the Fat Pad Along the Waterston Groove
eFigure 4. Examples of the Histologic Results Performed on the Fat Pad Along the Waterston Groove
eFigure 5. Subgroup Analysis for Primary Outcome
Trial Protocol and Statistical Analysis Plan
Data Sharing Statement