Abstract
Objective
The flow re-direction endoluminal device (FRED) is a safe and effective treatment option for intracranial aneurysms. The novel FRED X features an antithrombotic surface coating (“X Technology”) on an otherwise unmodified stent design. This two-center study evaluates the clinical safety and efficacy of FRED X and compares it to the literature.
Methods
Consecutive patients treated between 2020 and 2023 were retrospectively reviewed for aneurysm characteristics, procedural details and complications, and angiographic outcomes. A mini-review of the literature for FRED X clinical trials was performed and results were pooled using a random effects model.
Results
Thirty-four patients (mean age 56 years) were treated for 34 aneurysms. The mean aneurysm size was 7.7 ± 5.0 mm, 7 (21%) were ruptured, 6 (18%) were recurrent after previous treatment, 11 (32.3%) were located in the posterior circulation, and 4 (12.5%) had non-saccular morphology. All procedures were technically successful and no balloon angioplasty was required. There was 1 (2.9%) symptomatic complication (a transient ischemic attack) and no procedural morbidity or mortality. Technical asymptomatic events included 1 procedural stent occlusion that was reopened with thrombectomy and 3 cases of vasospasm. Complete and adequate occlusion rates were 68% (19/28) and 89% (25/28) at a mean follow-up time of 6 months, respectively. The results of this study are comparable to previous FRED X studies.
Conclusions
The results demonstrate a high feasibility and procedural safety of the FRED X with adequate mid-term occlusion rates. Long-term and comparative studies are needed to evaluate the full potential of the FRED X.
Keywords: aneurysm, angiography, device, flow diverter, intervention
Introduction
Flow diverters (FDs) have greatly expanded the treatment options for endovascular management of complex aneurysms, including wide-neck, large, and fusiform aneurysms.1–3 The mechanism of action is based on a tightly woven mesh of stent struts that redirects blood flow within the target vessel, ultimately leading to thrombosis of the aneurysm. The metal coverage of the vessel wall is higher than that of intracranial stents, which carries the risk of inducing thrombosis within the FD. This is a complication of concern, as it can lead to distal embolization and stent occlusion, which may contribute to ischemic stroke.
The introduction of antithrombotic coatings is an emerging trend in flow diversion technology, as they may reduce the risk of thrombosis-related complications. 4 The flow re-direction endoluminal device (FRED; MicroVention, Aliso Viejo, CA, USA) and its smaller-vessel variant, the FRED Jr., are well-established flow diverters that have recently received a surface coating (“X-technology”) and are now marketed as “FRED X”. 5 Other than the coating, the stent design was unchanged. Initial studies with this device have shown a favorable procedural safety profile and comparatively high mid-term occlusion rates.6,7
The objective of this two-center study was to evaluate our experience with the FRED X for the treatment of intracranial aneurysms and to summarize the results of the published clinical studies of this device.
Methods
Study design
All patients treated with the FRED X at two neurovascular centers between 2020 and 2023 were retrospectively reviewed. Data collection adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines and was approved by local ethics committees for retrospective data retrieval. Informed consent was obtained from all patients prior to the study.
FRED X
FRED X features a dual-layered design with a low-porosity inner mesh and a high-porosity outer stent, mirroring the structure of the classic “FRED” (for stent diameters ≥ 3.5 mm) and “FRED Jr.” (for stent diameters ≤ 3.0 mm). The technical novelty is the incorporation of an antithrombotic surface coating (“X-technology”) based on poly-2-methoxyethyl acrylate, which is commonly used in cardiovascular devices. This surface treatment is an amphiphilic polymer with a hydrophobic segment facing the device and a hydrophilic segment facing the vessel lumen (and blood) or vessel wall. This configuration creates a boundary layer adjacent to the stent struts that is designed to minimize protein denaturation and consequently reduce platelet adhesion. The FRED X is available in implant diameters from 2.5 to 5.5 mm and lengths from 13 to 36 mm. Deployment can be achieved with conventional 0.021” microcatheters for implant diameters ≤ 3.0 mm, while diameters ≥ 3.5 mm require a 0.027” microcatheter.
Procedure and anti-platelet regimen
Procedures were performed under general anesthesia with femoral access. Heparin was administered with an initial bolus of 5000 IU followed by subsequent aliquots of 1000 IU/h. A triaxial approach was used with an 8F guiding catheter for the internal carotid artery (ICA) or a 6F or 7F guiding catheter for the vertebral artery (VA), along with an appropriate intermediate catheter. FRED X was deployed using either a 0.021” microcatheter (Headway 21, Microvention, Tustin, CA, USA) or a 0.027” microcatheter (Headway 27) as deemed appropriate. In complex cases with a dysplastic parent vessel or incomplete wall apposition or neck coverage, multiple flow diverters were implanted. Balloon angioplasty was performed when the flow diverter showed inadequate wall apposition. Adjunctive coils were deployed according to operator preference using a microcatheter jailing technique.
For elective procedures, patients received dual antiplatelet therapy (DAPT) with 100 mg acetylsalicylic acid (ASA) and 75 mg clopidogrel or 2 × 90 mg ticagrelor. In both regimens, antiplatelet therapy was started 5–7 days before the procedure and continued for at least 4 months, followed by continuous ASA monotherapy. In emergency situations, intravenous tirofiban was administered during the procedure, followed by loading doses of ASA (250 mg) and clopidogrel (300 mg) or ticagrelor (180 mg). Maintenance antiplatelet therapy was consistent with elective procedures.
Data collection
Clinical and demographic information, as well as details on aneurysm size, morphology, and location, the number and size of implants, and the use of adjunctive devices and balloon angioplasty, were retrospectively collected. Technical success was defined as complete coverage of the targeted aneurysm neck with the studied device. All symptomatic complications with neurological deficits (e.g., ischemic stroke, hemorrhage, contrast-induced encephalopathy, cranial nerve palsy) and symptomatic complications without neurological deficits (e.g., groin hematoma) were collected. Moreover, technical asymptomatic complications (e.g., intraprocedural thromboembolism, arterial wall dissection, vessel perforation, and severe vasospasm requiring medical therapy) were recorded. Procedural morbidity was defined as an increase of at least one point on the modified Rankin Scale (mRS) at discharge compared to baseline that could be attributed to a procedure-related complication. Immediate contrast retention within the aneurysm sac was assessed as present or absent, and follow-up occlusion status was classified as complete occlusion, neck remnant, or aneurysm remnant. Complete occlusion and neck remnants were considered as adequate occlusion.
Literature review
A mini literature review was performed using PubMed/MEDLINE, EMBASE, and Cochrane databases to identify clinical trials with at least 10 patients treated with FRED X. Given the nature of this mini-review, the search process did not strictly adhere to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Information on study design, patient and aneurysm characteristics, procedural details, and antiplatelet regimen were extracted. Treatment outcomes were determined using the same definitions as described above.
Statistical analysis with meta-analysis
Qualitative variables are presented as numbers and percentages and compared using the chi-squared test, and Fisher's exact test. Quantitative variables are presented as means with standard deviations and compared using Student's t-test (normally distributed data) and the Wilcoxon-Mann-Whitney test (non-normally distributed data).
Statistical analyses were performed using SPSS software (IBM SPSS Statistics for Windows, version 25.0, Armonk, NY, USA), and a significance level of p < 0.05 was considered statistically significant.
For the meta-analysis, the incidence rates of ischemic complications within 7 days of treatment and complete occlusion at ≤1 year were aggregated was performed using a random effects model. Results were expressed as event rate per procedure with 95% confidence intervals. The I2 statistic was used to assess statistical heterogeneity between studies, with a value of >50% indicating significant heterogeneity. Forest plots were generated to illustrate proportions and estimated overall rates. The meta-analysis was performed using OpenMeta[Analyst], an open-source statistical software available at http://www.cebm.brown.edu/openmeta/.
Results
Patient and aneurysm characteristics
Thirty-four patients (mean age: 55.7 ± 9.7 years; 23 [67.6%] female) were treated with FRED X for 34 aneurysms. Six (17.6%) aneurysms were recurrent, previously treated (4 coiling, 1 stent, 1 clipping) and 7 (20.6%) were ruptured. The aneurysms were located at the paraophthalmic internal carotid artery (ICA) in 20 (58.8%), at the ICA terminus in 1 (2.9%), at the posterior communicating artery (Pcom) in 2 (5.9%), at the basilar artery (BA) trunk in 2 (5.9%), at the vertebral artery (VA) in 5 (14.7%), and at the posterior inferior cerebellar artery in 4 (11.8%). Thirty (88.2%) aneurysms were saccular, 2 (5.9%) fusiform, 1 (2.9%) dissecting, and 1 (2.9%) blister-like. Three (8.8%) aneurysms were partially thrombosed and 3 (8.8%) had a vessel arising from the aneurysm sac. The mean aneurysm size was 7.7 ± 5.0 mm, including 9 (26.5%) large aneurysms > 10 mm. The mean neck width was 5.0 ± 3.3 mm with a mean dome-to-neck ratio of 1.4 ± 0.7. The proximal and distal diameters of the parent artery were 3.7 ± 0.5 mm and 3.5 ± 0.7 mm, respectively. Baseline patient and aneurysm characteristics are summarized in Table 1.
Table 1.
Baseline patient and aneurysm characteristics for the overall cohort and the FRED X with adjunctive coiling and FRED X only subgroups.
| Overall (n = 34) | FRED X only (n = 21) | FRED X + coiling (n = 13) | P | |
|---|---|---|---|---|
| Patient age (years) | 55.7 ± 9.7 | 51.5 ± 7.6 | 62.5 ± 8.9 | 0.01 |
| Female sex | 23 (67.6%) | 14 (66.7%) | 9 (69.2%) | 0.88 |
| Aneurysm location | 0.14 | |||
| ICA-paraopthalmic | 20 (58.8%) | 11 (52.4%) | 9 (69.2%) | |
| ICA-posterior communicating | 2 (5.9%) | 1 (4.8%) | 1 (7.7%) | |
| ICA-terminus | 1 (2.9%) | 0 (0%) | 1 (7.7%) | |
| Basilar artery | 2 (5.9%) | 1 (4.8%) | 1 (7.7%) | |
| Vertebral artery | 5 (14.7%) | 5 (23.8%) | 0 (0%) | |
| Posterior inferior cerebellar artery | 4 (11.8%) | 3 (14.3%) | 1 (7.7%) | |
| Aneurysm morphology | 1.0 | |||
| Saccular | 30 (88.2%) | 18 (85.7%) | 12 (92.3%) | |
| Fusiform | 2 (5.9%) | 1 (4.8%) | 1 (7.7%) | |
| Dissecting | 1 (2.9%) | 1 (4.8%) | 0 (0%) | |
| Blister-like | 1 (2.9%) | 1 (4.8%) | 0 (0%) | |
| Recurrent aneurysms | 6 (17.6%) | 4 (19.0%) | 2 (15.4%) | 0.79 |
| Ruptured aneurysms | 7 (20.6%) | 5 (23.8%) | 2 (15.4%) | 0.68 |
| Partial thrombosed aneurysms | 3 (8.8%) | 1 (4.8%) | 2 (15.4%) | 0.54 |
| Vessel arising from aneurysm sac | 4 (11.8%) | 3 (14.3%) | 1 (7.7%) | 0.58 |
| Aneurysm size (mm) | 7.7 ± 5.0 | 5.9 ± 4.2 | 10.6 ± 4.9 | <0.01 |
| Neck width (mm) | 5.0 ± 3.3 | 4.5 ± 3.3 | 5.8 ± 3.2 | 0.26 |
| Dome-to-neck ratio | 1.4 ± 0.7 | 1.3 ± 0.6 | 1.7 ± 0.7 | 0.06 |
| Proximal diameter of parent artery | 3.7 ± 0.5 | 3.5 ± 0.4 | 3.9 ± 0.7 | 0.06 |
| Distal diameter of parent artery | 3.5 ± 0.7 | 3.5 ± 0.5 | 3.5 ± 1.0 | 0.83 |
Data are presented either as means with standard deviation or as numbers with percentages, as appropriate. ICA: internal carotid artery.
Treatment
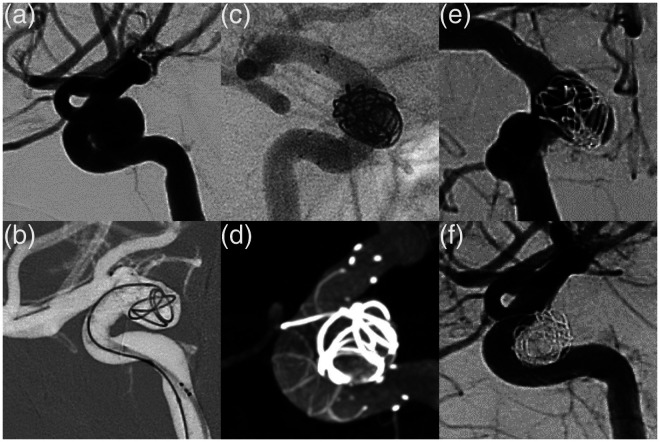
Procedural characteristics are summarized in Table 2. A total of 36 FDs were successfully implanted in all procedures, resulting in a median of 1 FD per procedure (range: 1–2 FDs per procedure). Two FDs each were used in a fusiform dissecting VA aneurysm and another VA aneurysm with a dysplastic parent artery to increase wall anchorage. The 3.5 mm diameter FRED X was used in 8 (23.5%) procedures, the 4.0 mm diameter in 13 (38.2%), the 4.5 mm diameter in 9 (26.5%), the 5.0 diameter in 2 (5.9%), and the 5.5 diameter in 2 (5.9%). Adjunctive coiling was performed in 13 patients (38.2%). No additional balloon angioplasty was required. The mean fluoroscopy time was 22 ± 12 min and the procedure time was 95 ± 41 min with a mean dose area product of 95 ± 52 Gy cm2. At the end of the procedure, contrast retention was observed in 28 (82.4%) aneurysms. An illustrative case of FRED X treatment is shown in Figure 1.
Table 2.
Procedural characteristics and outcomes for the overall cohort and the FRED X with adjunctive coiling and FRED X only subgroups.
| Overall (n = 34) | FRED X only (n = 21) | FRED X + coiling (n = 13) | P | |
|---|---|---|---|---|
| Mutliple FRED X implanted | 2 (5.9%) | 1 (4.8%) | 1 (7.7%) | 1.0 |
| FRED X diameter | 0.44 | |||
| 3.5 mm | 8 (23.5%) | 6 (28.6%) | 2 (15.4%) | |
| 4.0 mm | 13 (38.2%) | 10 (47.6%) | 3 (23.1%) | |
| 4.5 mm | 9 (26.5%) | 3 (14.3%) | 6 (46.2%) | |
| 5.0 mm | 2 (5.9%) | 2 (9.5%) | 0 (0%) | |
| 5.5 mm | 2 (5.9%) | 0 (0%) | 2 (15.4%) | |
| Fluoroscopy time (min) | 22 ± 12 | 26 ± 19 | 35 ± 18 | 0.28 |
| Procedure time (min) | 95 ± 41 | 89 ± 36 | 103 ± 49 | 0.44 |
| Dose area product (Gy cm2) | 95 ± 52 | 92 ± 57 | 99 ± 48 | 0.79 |
| Immediate contrast retention | 28 (82.4%) | 16 (76.2%) | 12 (92.3%) | 0.37 |
| Complications | ||||
| Overall | 4 (11.8%) | 1 (4.8%) | 3 (23.1%) | 0.11 |
| Symptomatic | 1 (2.9%) | 0 (0%) | 1 (7.7%) | 0.38 |
| Asymptomatic | 3 (8.8%) | 1 (4.8%) | 2 (15.4%) | 0.54 |
| Complete occlusion at follow-up | 19/28 (67.9%) | 13/18 (72.2%) | 6/10 (60.0%) | 0.51 |
Data are presented either as means with standard deviation or as numbers with percentages, as appropriate.
Figure 1.
Digital subtraction angiography shows a 9 mm wide neck aneurysm in the paraophthalmic segment of the right internal carotid artery (a). The aneurysm was treated with a FRED X 4 × 23 mm and coils in the Jailing technique. (b) shows the deployment of the FRED X. Unsubtracted angiography after device deployment clearly shows the 4 radiopaque markers at both ends of the FRED X (c). An additional Vaso-CT also shows the radiopaque spiral filaments in the FRED X contour, indicating complete vessel wall apposition (d). Immediate control DSA shows immediate contrast retention within the aneurysm sac (OKM A3). Six-month angiographic follow-up shows complete aneurysm occlusion (OKM D) and no intimal hyperplasia (e). There were no procedural complications or treatment-related morbidity.
Complications and clinical outcome
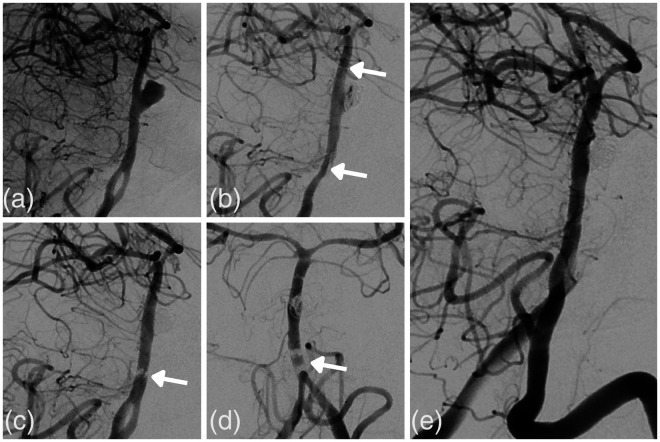
There was 1 (2.9%) neurological adverse event after treatment: A patient treated with a single FRED X for a ruptured Pcom aneurysm developed hemihypesthesia after the procedure, which was interpreted as a transient ischemic attack because there was no cerebral ischemia on magnetic resonance imaging. The symptoms resolved completely within a few days. There were no hemorrhagic events. Technical asymptomatic events included apposition thrombus formation within the proximal end of an implanted FRED X 3.5 × 17 mm with high grade stenosis during treatment of a mycotic BA trunk aneurysm. The patient had a history of oropharyngeal carcinoma and responded well to ASA and clopidogrel. The thrombosis was successfully treated by aspiration thrombectomy and intravenous tirofiban infusion and remained asymptomatic. The case is shown in Figure 2. Further technical complications included severe vasospasm in 3 cases (2 para-ophthalmic ICA, 1 BA) using FRED X 3.5 mm and 4 mm diameters. There was no procedure-related morbidity. There were two in-hospital deaths, both unrelated to the disease or treatment: The patient with the mycotic BA aneurysm died of organ failure, although he had no neurological symptoms after the complicated FD treatment. The second patient had a ruptured ICA aneurysm and died of a vasospastic infarction.
Figure 2.
Digital subtraction angiography in working projection shows a mycotic basilar trunk aneurysm (6 mm) in a patient with oropharyngeal carcinoma (a). Antiplatelet testing showed an adequate response to acetylsalicylic acid and clopidogrel. The aneurysm was treated with a FRED X 3.5 × 17 mm and coils using the Jailing technique. The arrows indicate the proximal and distal markers of the deployed FRED X (b). Two consecutive control series show progressive apposition thrombus formation at the proximal flow diverter with high grade stenosis (c + d). The thrombus was successfully dissolved with aspiration thrombectomy and i.a. tirofiban, resulting in complete patency (e).
Angiographic and clinical follow-up
A total of 28 (82.4%) patients were available for angiographic follow-up (FU) with a mean FU time of 5.6 ± 3.4 months. Complete occlusion was achieved in 19 aneurysms (67.9%), 6 (21.4%) had neck remnants, and 3 (10.7%) had aneurysm remnants. The rate of adequate occlusion was 89.3% (25/28). One patient (3.6%) had <50% in-stent stenosis and was treated with continuous dual antiplatelet therapy. Clinical follow-up was available for 31 patients with a mean FU time of 6.0 ± 3.6 months. One patient died of a hemorrhagic stroke unrelated to the treated aneurysm. The other patients had no late complications or new morbidity.
Subanalysis FRED X with coils versus FRED X only
A subgroup analysis was performed comparing aneurysms treated with FRED X and adjunctive coiling to cases treated with FRED X alone. The baseline characteristics and outcomes of the two groups are detailed in Tables 1 and 2. Cases treated with adjunctive coiling were characterized by significantly higher patient age and aneurysm size. The dome-to-neck ratio and proximal artery diameter tended to be significantly higher. Procedural characteristics and complication rates were comparable between the two groups and a similar proportion of aneurysms achieved complete occlusion (FRED X + coils: 6/10 [60.0%], FRED X only: 13/18 [72.2%], p = 0.51).
Literature review and meta-analysis
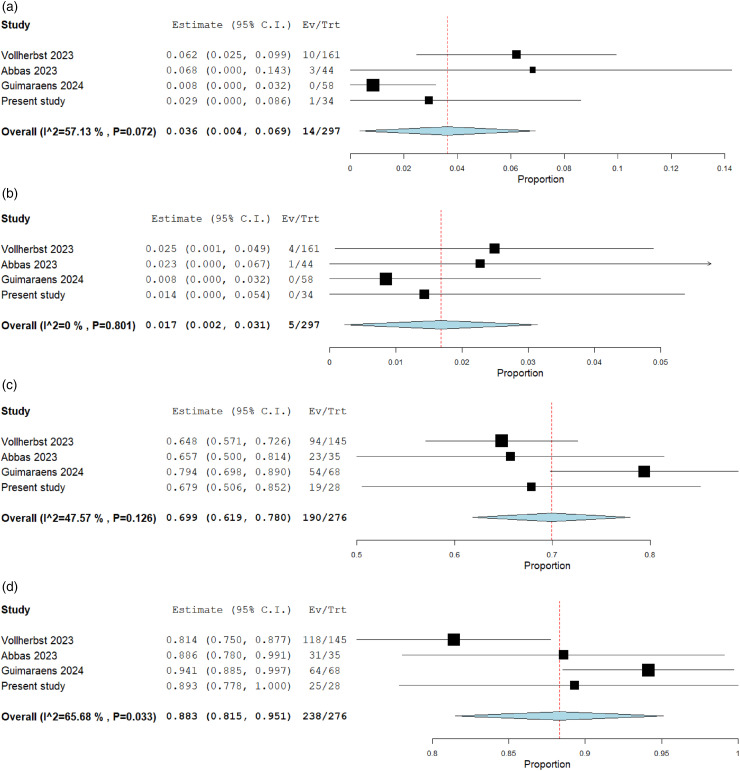
Three FRED X studies with a cumulative population of 263 patients and 314 aneurysms were identified (2 retrospective, 1 prospective).6–8 The characteristics and results of each study are summarized in Tables 3 and 4. The technical success rate was 100%. Balloon angioplasty was performed in 2.5% in the study by Vollherbst et al. and in 0% in the study by Abbas et al. The percentage of adjunctive coiling ranged from 0% to 29.8%. All authors reported FRED X implantation under DAPT, with the majority using a combination of ASA and clopidogrel. The pooled estimate for ischemic complications was 3.6% (95%CI, 0.4%–6.9%) (Figure 3). Vollherbst et al. reported 3 major strokes (1 procedural territory infarction, 1 early regrowth of a giant basilar artery aneurysm with brainstem infarction, and 1 in-stent thrombosis at 2 weeks) and 7 minor strokes. Abbas et al. reported 1 major territory stroke and 1 transient ischemic attack. There were no ischemic complications in the study by Guimaraens et al. There was one symptomatic hemorrhagic complication in the study by Vollherbst et al., which was a rebleeding after placement of an external ventricular drain under DAPT in a patient with subarachnoid hemorrhage. The pooled estimate for procedural morbidity was 1.7% (95%CI, 0.2%–3.1%). Mid-term occlusion rates were available for 276 aneurysms with a pooled complete occlusion rate of 69.9% (95%CI, 61.9%–78.0%) and an adequate occlusion rate of 88.3% (95%CI, 81.5%–95.1%) (Figure 3).
Table 3.
Baseline characteristics and technical experience reported in the included studies.
| Study, year | Design/ country/ no. of centers | Age (mean years)/ sex (%F) | Total no. of unruptured/ruptured aneurysms | Aneurysm sizes/neck widths (mean mm) | Fusiform, dissecting, or blister morphology (%) | Locations (%) | Balloon angioplasty/coiling (%) |
|---|---|---|---|---|---|---|---|
| Vollherbst, 2023 | Retro/Germany/9 | 55/77.6 | 166/18 | 7.8/4.7 | 18.6 | 72.7 ICA 6.2 MCA 8.1 BA 5.6 VA 7.5 Other |
2.5/29.8 |
| Abbas, 2023 | Retro/USA/4 | 68/84.1 | 40/5 | 5.6/1.6 | 8.9 | 75.6 ICA 4.4 MCA 13.3 ACA 4.4 PCA 2.2 AICA |
0/4.4 |
| Guimaraens, 2024 | Pro/Spain/2 | 55/82.8 | 70/15 | * | 11.8 | 58.8 ICA 15.3 MCA 5.9 ACA 19.0 Posterior |
*/0 |
| Present study | Retro/Germany/2 | 56/67.6 | 27/7 | 7.7/5.0 | 11.8 | 67.6 ICA 5.9 BA 14.7 VA 11.8 PICA |
0/38.2 |
Retro: retrospective; Pro: prospective; ICA: internal carotid artery; MCA: middle cerebral artery; ACA: anterior cerebral artery; BA: basilar artery; VA: vertebral artery; PCA: posterior cerebral artery; PICA: posterior inferior cerebellar artery; AICA: anterior inferior cerebellar artery. *Not specified
Table 4.
Individual outcome measures reported in the included studies.
| Study, year | Technical success (%) | Symptomatic Ischemic/hemorrhagic complications (%) | Major adverse events/Mortality (%) | Complete/Adequate occlusion (%) | In-stent stenosis/occlusion (%) | Length of follow-up (months) |
|---|---|---|---|---|---|---|
| Vollherbst, 2023 | 100 | 6.2/0.6 | 2.5/0.6 | 66.2/83.1 | 10.6/0.7 | 7 |
| Abbas, 2023 | 100 | 6.8/0 | 2.3/0 | 65.7/88.6 | 11.4/0 | 6 |
| Guimaraens, 2024 | 100 | 0/0 | 0/0 | 79.4/94.1 | */* | * |
| Present study | 100 | 1/0 | 0/0 | 67.9/89.3 | 3.6/0 | 6 |
FD: flow diverter. *Not specified
Figure 3.
Forest plots with random effects models showing the overall rate of ischemic complications (a), the morbidity rate (b), and the mid-term complete (c) and adequate (d) occlusion rate. Ev/Trt indicates event (complication/occlusion) per treated patient/aneurysm.
Discussion
The current study retrospectively analyzed consecutive patients treated with FRED X, reflecting a real-world scenario. The results of this study suggest a favorable safety profile for FRED X procedures, with a 2.9% incidence of ischemic events and no procedural morbidity or mortality. In addition, the study demonstrated a high technical success rate of 100%, with a single FD used in 89% of cases. The overall rates of complete and adequate occlusion at 6 months were 68% and 89%, respectively. These results are consistent with previous research on the FRED X, which was included in this study.
Morbidity associated with flow diversion is primarily due to ischemic stroke caused by intraprocedural thromboembolic events, perforator occlusion, or delayed in-stent stenosis. Using an in vitro blood loop model, Yoshizawa et al. demonstrated reduced platelet adhesion to the FRED X surface compared to the uncoated FRED version. 9 In the present study, we encountered only 1 (2.9%) ischemic event, a transient ischemic attack. In addition, there was one intraprocedural thrombus formation within the FD. This raises questions about the efficacy of the antithrombotic coating, especially since the patient was an ASA and clopidogrel responder. However, the patient had a history of cancer, which can confer a prothrombotic or hypercoagulable state. 10
However, pooling the ischemic event rates of the available studies for FRED X results in a low rate of 3.6% with a morbidity rate of 1.7%. The complication rate of the FRED X appears to be lower than that reported for the uncoated FRED in multicenter trials: In the SAFE study, the rate of thromboembolic events was 4.9% and the rate of morbidity was 3.0%. 11 In the FRED Italian Registry, the morbidity rate was 7.3% and the mortality rate was 4.3%. 12 Khorasanizadeh et al. reported thromboembolic complications in 6.9% and morbidity in 8.6% and McDougall et al. reported a major stroke rate of 6.2%.13,14
These findings are supported by the comparative registry study by Guimaraens et al., which reported fewer complications for FRED X (2.4%) than for FRED (8.3%), however, without reaching statistical significance. 7 The safety profile of FRED X also appears to be at least equivalent to other coated FD types: The major event rates were 3.0% for the Pipeline Vantage Embolization Device with Shield Technology (Medtronic, Dublin, Ireland) in the study by de Villiers et al., 5.5% for the Derivo embolization device (Acandis, Pforzheim, Germany) in the study by Trivelato et al., and 7% for the p64 HPC in the study by Ernst et al.15–17
Compared to the FRED, the FRED X has only added the surface coating, while the stent design is otherwise unchanged. This is an ideal setting for a prospective comparative study to evaluate whether the coating has a real clinical benefit in terms of thromboembolic events. Such a study should rigorously evaluate thromboembolic lesions, e.g., by diffusion-weighted imaging (DWI) or transcranial Doppler ultrasound. Cortez et al. retrospectively analyzed DWI lesions for the Pipeline Shield compared to the uncoated Pipeline Flex and found no significant difference. 18 However, the study was limited by the small number of patients enrolled and the retrospective design, and further studies are needed in this area.
Vessel perforation by the FD and delivery system is a rare complication, but can lead to significant morbidity. Notably, no hemorrhagic events were observed in this study, and there was only one hemorrhagic complication in the FRED X study by Vollherbst et al., was due to antiplatelet therapy and not to the procedure. 6 In contrast, the rate of bleeding for the Pipeline was 2.4% in the IntrePED study and 3.7% in the ASPIRe study.1,19 Although the small sample size of the present study precludes a systematic comparison, we believe that the flexible structure of the FRED X and its delivery systems may be advantageous in preventing device-related aneurysm and vessel perforation.
FRED X deployment was straightforward and successful in all cases, with a median of 1 device implanted per aneurysm. Similarly, a 100% technical success rate was reported in all three FRED X studies included in this analysis, with the majority of aneurysms treated by a single FD. Of note, FRED X achieved excellent wall apposition, and balloon angioplasty was not required in the present study. Similarly, Abbas et al. did not require balloon angioplasty in any case, while Vollherbst et al. reported balloon angioplasty in 2.5%.6,8 In the present study, adjunctive coiling was performed in 38%, mainly for larger aneurysms. Additional coiling did not impact occlusion rates. In the other three FRED X studies, the rate of additional coiling varied from 0% in the study by Guimaraens to 30% in the study by Vollherbst, which may be related to different care practices.6,7 As Guimaraens et al. reported the highest occlusion rates, it might be speculated that additional coiling is not necessary for aneurysm thrombosis, however, it might be used in specific cases such as ruptured and large aneurysms to provide immediate aneurysm occlusion.
The in vitro study by Yoshizawa et al. showed comparable endothelial cell adhesion between FRED X and uncoated FRED, which is important for neoendothelialization and progressive aneurysm occlusion. 9 The pooled mid-term complete occlusion rate of the FRED X was 68%, which appears to be comparable to the uncoated FRED. For the latter, complete occlusion rates were 73% at 1 year in the SAFE trial, 64% at 3–6 months in the Italian FRED registry, 55% at 7 months in the study by Khorasanizadeh et al., and 58% at 1 year in the study by McDougall et al.12–14,20 Guimaraens reported a higher complete occlusion rate for the FRED X (79%) than for the FRED (59%), but it must be acknowledged that the complete occlusion rate for the FRED X was higher than in the other FRED X studies, which ranged from 65% in the Vollherbst et al. study to 68% in the present study.6,7
For competing FD types, complete occlusion rates at 6 months were 82% for the Pipeline Vantage in the de Villiers et al. study, 81% for the Derivo in the Trivelato et al. study, and 87% for the p48 HPC in the Pierot et al. study.16,17,21 These mid-term occlusion rates appear to be slightly higher than the pooled FRED X occlusion rate (68%), but occlusion rates can vary depending on aneurysm location and geometry, and whether they are core lab or self-reported. 22 Because FDs provide progressive aneurysm occlusion, long-term results are usually superior to mid-term results. In this context, Hohenstatt et al. recently reported complete occlusion rates for the uncoated FRED of 77% (with 98% adequate occlusion) at 2 years and 85% (100% adequate occlusion) at 5 years. 23
Although preliminary safety and efficacy results for FRED X appear promising, further studies with long-term follow-up are needed, particularly comparative studies of coated and uncoated flow diverters.
Limitations
The primary limitations of this study are related to its retrospective nature and limited patient cohort. The lack of core laboratory evaluation increases the potential for confounding. The study group was heterogeneous with respect to baseline aneurysm characteristics and treatment approaches. In addition, follow-up data were available for only 82% of the patients, and long-term follow-up is still pending. Another factor contributing to heterogeneity was the use of different imaging modalities for angiographic control.
Conclusions
This study demonstrates a high level of feasibility and procedural safety for the FRED X, which appears to exceed that of first generation devices. Mid-term occlusion rates are adequate, but long-term and comparative studies are needed to evaluate the full potential of the FRED X and other coated FDs.
Footnotes
Data statement: All data will be made available upon request in an anonymized manner.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: CK serves as consultant for Acandis GmbH (Pforzheim, Germany) and as proctor for MicroVention Inc./Sequent Medical (Aliso Viejo, CA, USA).
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article
ORCID iDs: Lukas Goertz https://orcid.org/0000-0002-2620-7611
Cornelius Deuschl https://orcid.org/0000-0001-9262-7289
References
- 1.Kallmes DF, Hanel R, Lopes D, et al. International retrospective study of the pipeline embolization device: a multicenter aneurysm treatment study. Am J Neuroradiol 2015; 36: 108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brinjikji W, Murad MH, Lanzino Get al. et al. Endovascular treatment of intracranial aneurysms with flow diverters: a meta-analysis. Stroke 2013; 44: 442–447. [DOI] [PubMed] [Google Scholar]
- 3.Chiu A, Cheung A, Wenderoth J, et al. Long-term follow-up results following elective treatment of unruptured intracranial aneurysms with the pipeline embolization device. Am J Neuroradiol 2015; 36: 1728–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White TG, Santhumayor BA, Turpin J, et al. Flow diverter surface modifications for aneurysm treatment: a review of the mechanisms and data behind existing technologies. Interv Neuroradiol 2023: 15910199231207550. [DOI] [PubMed] [Google Scholar]
- 5.Waqas M, Dossani RH, Alkhaldi M, et al. Flow redirection endoluminal device (FRED) for treatment of intracranial aneurysms: a systematic review. Interv Neuroradiol 2022; 28: 347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vollherbst D, Lücking H, DuPlessis J, et al. The FRESH study: treatment of intracranial aneurysms with the new FRED X flow diverter with antithrombotic surface treatment technology-first multicenter experience in 161 patients. Am J Neuroradiol 2023; 44: 474–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guimaraens L, Saldaña J, Vivas E, et al. Flow diverter stents for endovascular treatment of aneurysms: a comparative study of efficacy and safety between FREDX and FRED. J Neurointerv Surg 2024. [DOI] [PubMed] [Google Scholar]
- 8.Abbas R, Lan M, El Naamani K, et al. First United States multicenter experience with the new-generation FRED X surface-modified flow diversion stent: feasibility, safety, and short-term efficacy. J Neurosurg 2023; 1: 1–10. [DOI] [PubMed] [Google Scholar]
- 9.Yoshizawa K, Kobayashi H, Kaneki A, et al. Poly (2-methoxyethyl acrylate)(PMEA) improves the thromboresistance of FRED flow diverters: a thrombogenic evaluation of flow diverters with human blood under flow conditions. J Neurointerv Surg 2023; 15: 1001–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nasser NJ, Fox J, Agbarya A. Potential mechanisms of cancer-related hypercoagulability. Cancers (Basel) 2020; 12: 566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pierot L, Spelle L, Berge J, et al. Feasibility, complications, morbidity, and mortality results at 6 months for aneurysm treatment with the flow re-direction endoluminal device: report of SAFE study. J Neurointerv Surg 2018; 10: 765–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piano M, Valvassori L, Lozupone Eet al. et al. FRED Italian registry: a multicenter experience with the flow re-direction endoluminal device for intracranial aneurysms. J Neurosurg 2019; 133: 174–181. [DOI] [PubMed] [Google Scholar]
- 13.Khorasanizadeh M, Shutran M, Schirmer CM, et al. North American multicenter experience with the flow redirection endoluminal device in the treatment of intracranial aneurysms. J Neurosurg 2022; 138: 933–943. [DOI] [PubMed] [Google Scholar]
- 14.McDougall CG, Diaz O, Boulos A, et al. Safety and efficacy results of the flow redirection endoluminal device (FRED) stent system in the treatment of intracranial aneurysms: uS pivotal trial. J Neurointerv Surg 2022; 14: 577–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ernst M, Jamous A, Bartl M, et al. Multicenter study of the safety and effectiveness of intracranial aneurysm treatment with the p64MW-HPC flow modulation device. Interv Neuroradiol 2023: 15910199231220964. [DOI] [PubMed] [Google Scholar]
- 16.de Villiers L, do Nascimento VC, Domitrovic Let al. et al. Vanguard Study: Initial experience with the new fourth generation Pipeline Vantage Flow Diverter (PVFD): 6-month results, technical and clinical considerations. J Neurointerv Surg 2024. [DOI] [PubMed] [Google Scholar]
- 17.Trivelato FP, Abud DG, Ulhôa AC, et al. Derivo embolization device for the treatment of intracranial aneurysms: a multicenter study of 183 aneurysms. Stroke 2019; 50: 2351–2358. [DOI] [PubMed] [Google Scholar]
- 18.Cortez GM, Benalia VH, Sauvageau Eet al. et al. Diffusion-weighted imaging lesions after intracranial aneurysm treatment with Pipeline Flex and Pipeline Flex with Shield technology: a retrospective cohort analysis. J Neurointerv Surg 2023; 16(4): 385–391. [DOI] [PubMed] [Google Scholar]
- 19.Kallmes DF, Brinjikji W, Boccardi E, et al. Aneurysm study of pipeline in an observational registry (ASPIRe). Interv Neurol 2016; 5: 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pierot L, Spelle L, Berge J, et al. SAFE Study (safety and efficacy analysis of FRED embolic device in aneurysm treatment): 1-year clinical and anatomical results. J Neurointerv Surg 2019; 11: 184–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pierot L, Soize S, Cappucci Met al. et al. Surface-modified flow diverter p48-MW-HPC: preliminary clinical experience in 28 patients treated in two centers. J Neuroradiol 2021; 48: 195–199. [DOI] [PubMed] [Google Scholar]
- 22.Rezek I, Lingineni R, Sneade Met al. et al. Differences in the angiographic evaluation of coiled cerebral aneurysms between a core laboratory reader and operators: results of the cerecyte coil trial. Am J Neuroradiol 2014; 35: 124–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hohenstatt S, Ulfert C, Herweh C, et al. Long-term follow-up after aneurysm treatment with the flow redirection endoluminal device (FRED) flow diverter. Clin Neuroradiol 2023; 34(1): 181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]