Abstract
Background
Radiographic visualization of liquid embolic agents (LEAs) during embolization procedures in neurovascular territory represents a crucial feature to ensure efficacy and safety for the patients during endovascular treatment of arteriovenous shunting lesions. Radiopacity of available LEAs varies significantly and limited methods are currently available for comparison. The purpose of this study was to compare the contrast resolution (CR) during injection under blank roadmap of various LEAs, as well as standard contrast material.
Methods
An injectable angiographic phantom was designed consisting of parallel tubings between 313 and 1000 micron. Under roadmap, eight radiopaque liquid agents were injected and analyzed: Onyx18®, 34® Squid®12, 18, PHIL®25% (PHIL®25), PHIL®30% (PHIL®30).
TrufillTM (NBCA), 30% dilution and Omnipaque®300. CR was evaluated as a contrast to noise ratio (CNR) and calculated as mean peak signal (Sa) minus mean background signal (Sb) divided by the standard deviation of the background signal (Std) .
Results
Omnipaque 300 and NBCA were found to have the highest CR. PHIL®25 demonstrated the lowest CNR (45% of Omnipaque CNR). Onyx 18 and 34 (Both around 82% of Omnipaque CNR) demonstrated higher CNR compared to Squid®12 and 18 (52–55% of Omnipaque CNR). On average, at 500 micron there is a >70% reduction in CNR, and at 313 micron there is a 90% reduction in CNR compared to 1000 micron.
Conclusions
Significantly different CNR between most LEAs and iodinated contrast media was evident under roadmap conditions and should be considered prior to injection.
Keywords: Neurovascular embolization, liquid embolics, contrast resolution
Introduction
Endovascular treatment (EVT) has become an integral part of the clinical management of arteriovenous vascular malformations and fistulas of the brain and spinal cord such as arteriovenous malformations (AVMs) and arteriovenous fistulas (AVFs) of the brain and spinal cord. The increasing technical success rate and improved clinical outcome of EVT is to a large degree based on significant technological advancements over the past 20 years. Catheter and guidewire materials, properties of liquid embolic agents (LEAs) and image quality for better visual control during EVT procedures have markedly improved over the last 20 years. 1 Transarterial, and more recently, transvenous embolization techniques play an increasing role in the clinical management of these diseases. In addition, LEAs are used for tumor embolization and increasingly for MMA embolization in the management of chronic subdural hematomas (cSDHs).2,3
Besides high-resolution DSA and roadmap capabilities, sufficient radiopacity of embolic materials that allows for a maximum of visual control is considered crucial during embolization procedures. For decades, operators relied solely on the use of a nonradiopaque acrylic glue (NBCA), mixed with the oily contrast medium lipiodol to document nidal penetration and occlusion of AV shunts.
For some time, DMSO based LEAs (Onyx®, Squid®) that are mixed with metallic powder (tantalum) to ensure fluoroscopic visibility during manual injections, have been introduced. One disadvantage of these tantalum based embolics is the measurable loss in radiopacity which steadily increases over time as tantalum undergoes sedimentation after preparation. 4 To resolve this issue, a novel DMSO based LEA covalently bonded with iodine has been developed, PHIL®, which allows for constant radiopacity even over long injections. 5 Although the industry has undertaken attempts to improve angiographic image quality data to allow more effective and safer embolization procedures, objective and accurate assessment of differences in radiographic visibility between various embolic products has been until recently, completely lacking. Further, no comparison among roadmap or DSA quality of various vendors exists.
We present a study in which a novel, recently patented injectable angiographic phantom for testing and comparing the contrast resolution (CR) of currently available LEAs used for neurovascular embolization procedures.
Methods
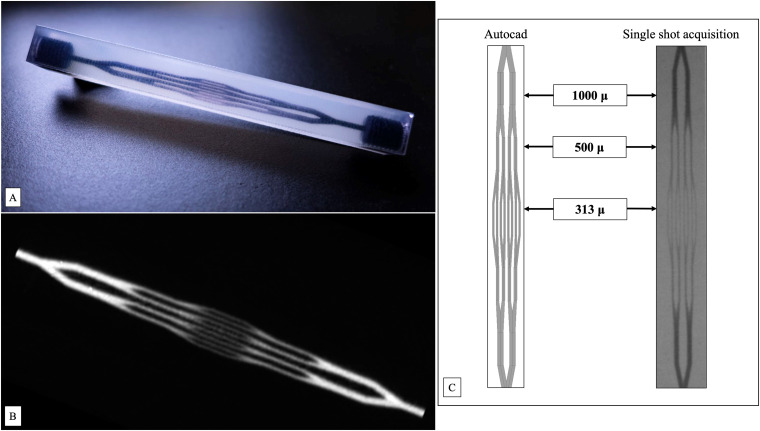
An injectable angiographic polymethyl methacrylate phantom with a branching pattern was designed consisting of parallel tubings between 313 and 1000 micron 6 (Figure 1(A), (B) and (C)). Multiple identical copies of the phantom were printed with a ProJet®3500 HDPlus with VisiJet® EX 200 resin (3D Systems, Rock Hill, South Carolina, USA). Under roadmap, eight radiopaque liquid agents were injected: Onyx®18, 34 (Medtronic, Dublin, Ireland), Squid®12, 18 (Balt, Montmorency, France), PHIL®25% (PHIL®25), PHIL®30% (PHIL®30) (MicroVention, Tustin, CA, USA), TrufillTM (NBCA) (Cerenovus, Fremont, Ca, USA) 30% dilution mixed with Lipiodol® (Guerbet, France) and Omnipaque®300 (GE Healthcare, Chicago, IL, USA). A separate phantom was used for each injection. For each LEA, one injection was performed, a total of eight injections. Each injection was performed using a new, previously unused phantom by the same operator with over 30 years of experience with LEAs using a consistent force during each injection.
Figure 1.
(A) Photo of the three-printed PMMA phantom. A branching pattern was designed consisting of parallel tubings between 313 and 1000 micron, here injected with an ink dye to illustrate inner design. (B) Maximum intensity projection (MIP) reconstruction of a 20 s cone beam scan (Siemens Zeego) of the phantom after injection of Omnipque 300. (C) Autocad design file and single shot X-ray acquisition. The size of the tubings is indicated by the following labels: 313 micron, 500 micron, and 1000 micron.
PMMA: polymethyl methacrylate.
Image data was obtained from a monoplane angiographic system: Artis Zeego (Siemens Healthineers, Erlangen, Germany) with the following settings: detector size 16 cm, table height −15 cm, SID 90 cm, RM Glue RM K40 EA3, 15 p/s, KV 68, mA 17, and dose rate 0.5 mGy/min. Image analysis was performed with ImageJ (NIH, Bethesda, Maryland) and Matlab (MathWorks, Inc., Natick, MA). The find peak function in Matlab was used to automatically detect signal from each different tube size (313-, 500-, and 1000-micron diameter tubes).
CR was evaluated as a contrast to noise ratio (CNR) and calculated as mean peak signal (Sa) minus mean background signal (Sb), divided by the standard deviation of the background signal (Std) . For each injection, the entire fluoro cine run was saved in form of single fluoro images using the “store monitor function” for subsequent analysis. Thus for each injection, a total of 25–30 frames captured the injections from beginning to end. From this, the highest peak signal from 10 different frames was selected automatically from the findpeaks function. These 10 separate measurements were averaged to obtain the peak signal (Sa) value.
The phantom was mounted and secured to the angiographic table. Prior to each injection with a liquid embolic, the phantom was flushed with normal saline followed by injection of Omnipaque®300 (GE Healthcare, Chicago, IL, USA) and imaged under the settings described above. After the injection, the phantom was flushed again with normal saline. For the tantalum based embolics, Onyx® and Squid®, the liquids were prepared according the instructions for use of the manufacturers with the Vortex Genie shaker for 20 min. The shaker was kept next to the angiotable, and identical steps for all tantalum based LEAs were used in order to minimize variations. Beginning the moment the vial was removed from the shaker, the LEA was withdrawn with the syringe and connected to the phantom via tubing. Except for NBCA, the phantom was filled with DMSO prior to the injection with LEA followed by the embolization exactly at 3 min after removal from the shaker. For NBCA, the phantom was filled with 5% dextrose prior to embolization.
Results
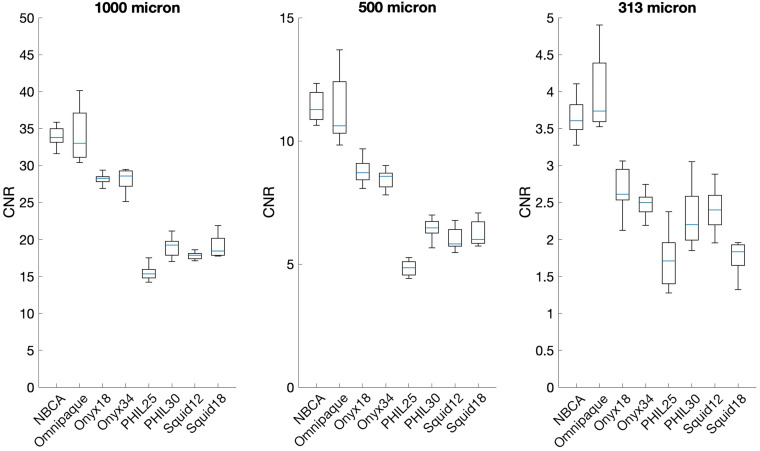
We have demonstrated the feasibility of using an injectable angiographic phantom with a branching pattern to calculate and compare CR of various LEAs by measuring the CNR in pixel data extracted from DICOM images (Figure 2(A), (B) and (C)). We also showed under our experimental conditions, confirmed by ANOVA test (p < .01) the following (Table 1 and Figures 3, 4(A), (B), (C)): Omnipaque™ 300 and NBCA mixed with Lipidol® (30%) have the highest, PHIL®25 has the lowest CNR. Onyx®18 and 34 have higher CNR compared to Squid®12 and 18, Onyx®18 and 34 have marginal differences. Squid®12 and 18 have marginal differences, and overall poor CNR at 313 micron during roadmap injections.
Figure 2.
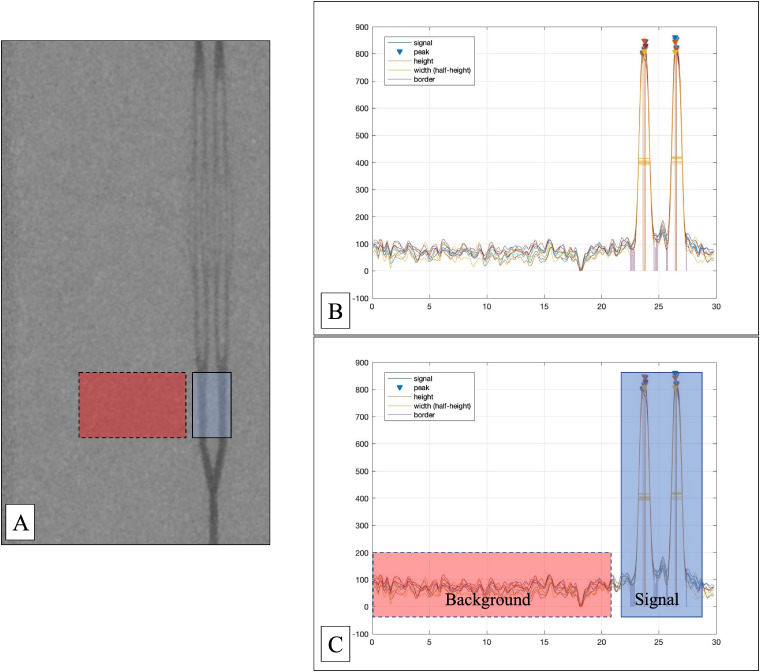
(A) Still image from the injection under road map. A still image from the injection under road map shows the model with the adjacent background. The blue region indicates where the signal CNR was obtained for the 1000 micron tubes, whereas the red region indicates where the background noise measures were obtained. (B) Pixel data analysis. This graph demonstrates the Matlab findpeak function used to analyze the cine run from an injection. It shows the multiple different pixel data extracted from the same cine run. Two separate peaks are shown, in this case, for the two separate 1000 micron tubes. (C) Regions of signal and background noise extraction. The blue region with solid outline here indicates the region used to extract the signal. The red region with dashed outline was used to extract the background noise. This corresponds to the same regions seen on (A).
CNR: contrast to noise ratio.
Table 1.
Raw CNR data with associated standard deviations (std) for various LEAs.
| 1000 micron | CNR ± std | 500 micron | CNR ± std | 313 micron | CNR ± std |
|---|---|---|---|---|---|
| NBCA | 34.1 ± 1.7 | NBCA | 11.4 ± 0.6 | NBCA | 3.7 ± 0,3 |
| Omni 300 | 34.2 ± 3.6 | Omni 300 | 11.3 ± 1.3 | Omni 300 | 4 ± 0.5 |
| Onyx 18 | 28.2 ± 1.1 | Onyx 18 | 8.8 ± 0.5 | Onyx 18 | 2.7 ± 0.3 |
| Onyx 34 | 28.1 ± 1.4 | Onyx 34 | 8.5 ± 0.4 | Onyx 34 | 2.5 ± 0.2 |
| PHIL25 | 15.4 ± 1 | PHIL25 | 4.8 ± 0.3 | PHIL25 | 1.8 ± 0.4 |
| PHIL30 | 18.9 ± 1.3 | PHIL30 | 6.4 ± 0.4 | PHIL30 | 2.3 ± 0.4 |
| Squid 12 | 17.8 ± 0.5 | Squid 12 | 6 ± 0.4 | Squid 12 | 2.4 ± 0.3 |
| Squid 18 | 19 ± 1.4 | Squid 18 | 6.2 ± 0.5 | Squid 18 | 1.8 ± 0.2 |
NBCA: nonradiopaque acrylic glue; CNR: contrast to noise ratio; LEA: liquid embolic agent
Figure 3.
CNR comparison of LEAs and contrast. CNR measures for 1000, 500, and 313 micron tubes for the various LEAs and iodine contrast. We also showed under our experimental conditions, the following confirmed by ANOVA test; Omnipaque 300 and NBCA have the highest contrast resolution, PHIL 25 has the lowest contrast resolution, Onyx 18 and 34 have higher contrast resolution compared to Squid 12 and 18, Onyx 18 and 34 have marginal differences in contrast resolution, Squid 12 and 18 have marginal differences in contrast resolution, and, overall poor contrast resolution at 313 micron during roadmap injections.
NBCA: nonradiopaque acrylic glue; CNR: contrast to noise ratio; LEA: liquid embolic agent; ANOVA: analysis of variance.
Figure 4.
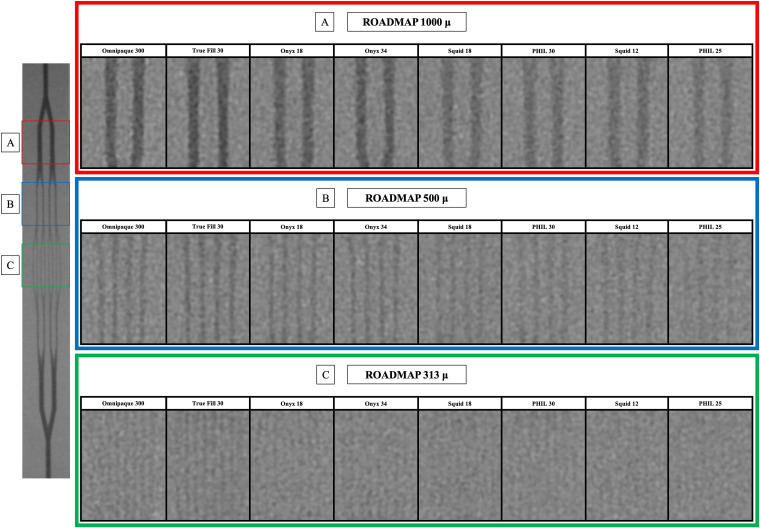
Comparison of LEAs and standard contrast medium. Still images from each injection under road map ordered from highest to lowest CNR. On the left side a single shot acquisition (nonsubtracted) of the entire phantom during injection of Omnipaque is displayed for reference. (A) 1000 micron. (B) 500 micron. (C) 313 micron.
CNR: contrast to noise ratio; LEA: liquid embolic agent.
Using the CNR of Omnipaque™ 300 as a reference, we can consider the relative CNR of the LEAs as a percentage of the CNR of Omnipaque™ 300 (Table 2). Most notably, at 1000 micron, PHIL®25, PHIL®30, Squid®12, and Squid®18 demonstrated 55% or less the CNR of Omnipaque™, with PHIL®25 demonstrating 45% the CNR of Omnipaque™. On average, when comparing Omnipaque™ and the various LEAs, at 500 micron there is a >70% reduction in CNR, and at 313 micron there is on average almost 90% reduction in CNR compared to 1000 micron. The CNR detected at 313 microns was demonstrably the lowest CNR measured for all LEAs. Additional single shot (unsubtracted) images demonstrated that all LAEs and Omnipaque™ are filling the 313 micron channels of the phantom, despite being difficult to appreciate on the roadmap images.
Table 2.
Relative CNR compared to omnipaque: at 1000 micron, CNR are displayed as a percentage of omnipaque CNR.
| Embolic | CNR | Percentage of Omnipaque CNR |
|---|---|---|
| NBCA | 34.1 | 99.6% |
| Onyx 18 | 28.2 | 82.4% |
| Onyx 34 | 28.1 | 82.2% |
| PHIL25 | 15.4 | 45% |
| PHIL30 | 18.9 | 55.4% |
| Squid 12 | 17.8 | 52% |
| Squid 18 | 18.9 | 55.4% |
NBCA: nonradiopaque acrylic glue; CNR: contrast to noise ratio
Discussion
Our study demonstrates measurable differences of CNRs across the tested LEAs with significantly lower CNRs for some when compared to standard angiographic contrast medium. This can be translated into better or poorer radiographic visibility of the various LEAs currently used for EVT of neurovascular lesions such AVMs, AVFs, tumors or cSDHs. These differences are mainly due to the type of radiopaque agent added to the radiolucent embolic liquid and its concentration in the suspension. The longest available embolic materials, NBCA showed in a standard concentration of 30% (70% Lipiodol) the highest CNR, and thus best radiographic visibility. On the other hand, one of the newest materials PHIL® shows the lowest CNR, which translates into poorest radiographic visibility among the tested substances.
Our study measures and compares CNR as an indicator for radiopacity or radiographic visibility of multiple LEAs in comparison to standard angiographic contrast medium during realistic manual injections under identical roadmap settings. Typical radiographic and angiographic phantoms are static models with a fine grid of high-density materials such as lead that allow for characterization of spatial and CR of angiographic machines. Digital subtraction angiography techniques such as road-mapping, regularly used for real time monitoring during endovascular procedures, present unique challenges when attempting to measure these variables objectively and reproducibly.
Our model was designed with a branching pattern consisting of tubes with the size of 313, 500, and 1000 microns. The sizes were selected to simulate average sizes, including small perforating cerebral arteries, which range on average 330 to 500 microns 7 or less. The choice of 313 as the smallest diameter tube was related to the manufacturer's technological capabilities.
A recent study using similar methods but a linear, non-branching, injectable phantom8,9 with the smallest diameter being 500 micron, provides a foundation for comparison revealing both similarity and differences. One notable difference in our current study is that we used smaller diameter tubing, 313 micron versus 500 micron in the prior study. We consider this critical as the radiographic visibility in smaller vessels decreases and is limited also by the spatial resolution of the imaging system which is relevant for clinical practice. That limit is currently in the range of 150 micron based on the size of the detector element size of 154 micron.
While the linear model, also in our experience, may serve as a valuable initial tool, its simplistic design introduces significant limitations, particularly in terms of fluid dynamics, as flow resistance increases with a decrease in tube diameter. This potentially affects the visibility of tantalum-based LEAs due to pressure-induced variations. Our prior work using such linear model navigated these concerns with a step-wise diameter reduction akin to the model employed in a recently published study.6,10 To enhance the realism and accuracy 10 of our measurements, we revised and advanced this simplistic design to feature a branching pattern, 8 offering a more realistic representation of the human blood circulation. 11 Another important difference is the measurement of the ROIs. In the prior study 9 manual drawings of ROI measurements were made on a single frame, which may introduce undesirable measurement variations; whereas, in the current study, the entire fluoro cine run of the injection was analyzed automatically—the highest 10 frames were isolated using the findpeaks Matlab function. The advantage of an automated method is that it helps to eliminate human error that may be introduced by even meticulous manual measurements. Most importantly, in our current study's methods, measurements were made on the roadmap-based images, in comparison to fluoroscopic (unsubtracted) images which are typically not used during embolization with LEAs. Road-mapping is generally used during the critical injections, and most difficult to analyze from an image quality standpoint given the lack of phantoms and methods for this purpose. In addition to the aforementioned differences, scanner type and variations in settings, table positions, etc., all may play a role in the variation in results of CNR for some LEAs.
In contrast to the prior study 9 that reported “acceptable visibility” and observed no major differences between LEAs such as Onyx® and Squid®—notable differences were found in the present study as variations in CNR between both Onyx®, Squid®, and PHIL®. This is also consistent with clinical experience and prior research. 12 Interestingly, the prior study noted no difference between PHIL® and other tantalum-based LEAs, something inconsistent with prior research 4 as well as with clinical experience. The discrepancy between our results and Schmitt et al. could stem from various differences in methodology, or perhaps even an error introduced by their use of manual measurements, but at a minimum it highlights the need for further work to refine a standardized method and reference for visualization of LEAs.
Intravascular use of LEAs in the cerebral circulation has well-known risks including untoward nontarget embolization with occlusion of normal neurovascular territory and subsequent neurological deficits. To keep this risk at a minimum is paramount and largely based on operator's skill and experience, the use of neuroanatomic knowledge, proper positioning of the microcatheter and meticulous injection technique. One of the most critical parts of a safe embolization procedure is continuous and attentive visual monitoring of the flow of the liquid into the target territory. This is enabled by high-resolution monitors and the blank roadmap option commonly used on modern angiographic systems that allows monitoring the flow and penetration of the radiopaque liquid only. The quality of this visualization is mainly determined by two factors: (1) level of image quality determined by the technical characteristics of the angiographic machine including size of focal spot, size of detector elements, dynamic range of the flat panel detector (e.g., 14 vs 16 bit), binning mode (2 × 2 or 1 × 1), the X-ray dose (kV, mAs), and (2) the radiopacity of the liquid itself determined by its absorption of X-rays.
NBCA is nonradiopaque and made visible only by adding Lipiodol® in various concentrations. The most common DMSO-based LEAs have added materials with high radiopacity, for example, tantalum. Tantalum powder is very radiopaque and allows good visual control during injections but it tends to sediment out which decreases its radiopacity especially during longer injections. 4 Tantalum can also be added to NBCA to further increase radiopacity during glue injections. 13 Different from the previous study we found a higher CNR for NBCA which can be attributed to the higher percentage of Lipiodol® in a 30/70 mixture versus 50/50 which is less frequently used in clinical practice except for high-flow fistulas. Thus, we believe the current study's use of 30% (or even lower) is more realistic as it is more widely used and relevant to point out that none of the modern LEAs has such high radiopacity.
The introduction of both, Squid®and PHIL® is an attempt to overcome these known limitations. 14 Controversy exists whether micronized tantalum powder as is used in Squid®would reduce this sedimentation sufficiently enough to increase radiographic visualization during long embolization procedures. To overcome the problem of sedimentation and to prevent decreased visualization and loss of visual control during longer EVT, the newest embolic liquid included in the test, uses covalently bonded Iodine (PHIL®). Unlike tantalum based LEAs it maintains a constant radiopacity overtime. 4
The systematic study of radiographic visualization characteristics of LEAs has been challenging due to a lack of suitable phantoms and methods for their use. Our work demonstrates the feasibility of a novel phantom that allows to assess and compare one of the most crucial properties of current LEAs, their radiographic visibility. It is interesting to note that normal undiluted contrast medium containing 300 mg/ml Iodine had a significantly higher CNR than nearly all embolic liquids used in this study. This is an important aspect to consider when performing an embolization in close proximity to a normal territory as the operator cannot fully rely on an identical vascular visualization during the embolization, especially with vessels around 300 micron or less. The latter is the average size of cerebral perforators 7 which may not be reliably visualized using super-selective DSA runs prior to the actual embolization which can result in additional nontarget embolization unnoticed by the operator.
Optimal visualization is paramount to ensure operator's visual control for both, sufficient penetration and avoiding potential complications when using LEAs. 11 Machine settings can play a crucial role in optimizing LEA visualization. Some modern angiographic systems feature specialized settings for PHIL® and Onyx®, enhancing visibility and increasing CNR. Future studies should investigate radiopacity and visualization using these specific settings for different embolic materials, such as Obtura® which offers a decreasing radiopacity over time that would eliminate CT-associated artifacts and allow better assessment of residual AV shunting compartments in brain AVMs. 10 While our study did not aim to determine the LEA providing the best visualization for specific clinical scenarios, this remains an ongoing research topic.
Limitations
Several limitations of this study need to be considered.
First of all, only one injection per embolic agent was performed related to the relatively high costs of the materials. Secondly, some of the newer angiographic systems offer modified program settings in order to optimize visualization of LEAs with different radiopacity. We did not evaluate radiopacity of the LEAs under these settings. In addition, systems using newer generation of flat detectors with a higher dynamic range may provide overall higher CNRs which, however, would not change the differences between various LEAs. Finally, the manual injection being used here may somewhat contribute to a slightly less consistent injection pressure, and therefore could introduce variabilities. For this reason, future studies may consider the use of an automated pump or power injector which, on the other hand again, would also increase the overall costs as a higher amount of liquid material will be needed.
Conclusion
There is significant variability in CNR for the various LEAs agents when injected under blank roadmap conditions as used in this study. Onyx® and TrufillTM demonstrated high CNR relative to other LEAs. These differences should be considered when LEAs are selected for embolization procedures in patients with neurovascular AV shunts, vascular tumors or even cSDHs. Knowledge and understanding of the different visualization performance among various LEAs may be important to minimize and avoid non-target vessel embolization. Caution is advised when using LEAs with a lower CNR in areas with high bone density. Subsequent studies should leverage the methodology and phantom outlined in our study to advance the validation of this technique, thereby contributing to the ongoing enhancement of radiographic image quality and visual monitoring. Such improvements are crucial for augmenting patient safety during EVT in neurovascular territories.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics approval disclosure statement: This research was conducted under the approval of an institutional review board at Baylor College of Medicine, 51999-I.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Carter Technology Fund, Balt Extrusion (Grant No. 000, 0000).
ORCID iDs: J Ryan Mason https://orcid.org/0000-0002-6822-4460
Goetz Benndorf https://orcid.org/0000-0002-9824-6900
References
- 1.Vaidya S, Tozer K, Chen J. An overview of embolic agents. Semin Intervent Radiol 2008; 25: 204–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ku JC, Dmytriw AA, Essibayi MA, et al. Embolic agent choice in middle meningeal artery embolization as primary or adjunct treatment for chronic subdural hematoma: a systematic review and meta-analysis. Am J Neuroradiol 2023; 44: 297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pal A, Blanzy J, Gómez KJR, et al. Liquid embolic agents for endovascular embolization: a review. Gels 2023; 9: 378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mason JR, Dodge C, Benndorf G. Quantification of tantalum sedimentation rates in liquid embolic agents. Interv Neuroradiol 2018; 24: 574–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leyon JJ, Chavda S, Thomas Aet al. et al. Preliminary experience with the liquid embolic material agent PHIL (precipitating hydrophobic injectable liquid) in treating cranial and spinal dural arteriovenous fistulas: technical note. J Neurointerv Surg 2016; 8: 596–602. [DOI] [PubMed] [Google Scholar]
- 6.Benndorf G, Mason JR. Phantoms and methods and kits using the same. 2019; U.S. Paten. https://patents.google.com/patent/US10426418B2/en.
- 7.Lang J. Clinical anatomy of the head: neurocranium, orbit, craniocervical regions. Berlin, Germany: Springer-Verlag, 1983. [Google Scholar]
- 8.Mason J, Benndorf G. Comparison of image quality of liquid embolic agents [Conference Session]. In: Society of NeuroInterventional Surgery 16th Annual Meeting (Miami, Fl), 2019. [Google Scholar]
- 9.Schmitt N, Wucherpfennig L, Hohenstatt S, et al. Visibility of liquid embolic agents in fluoroscopy: a systematic in vitro study. J Neurointerv Surg 2023; 15: 594–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Llibre-Guerra JC, Guimaraens L, Kadziolka KB, et al. ihtObtura: A novel liquid embolic agent with post-embolization radiopacity loss, in endovascular treatment of brain arteriovenous malformations, dural arteriovenous fistulas, and tumors: CLARIDAD trial. J Neurointerv Surg 2024: jnis-2023-021442. doi: 10.1136/jnis-2023-021442 [DOI] [PubMed] [Google Scholar]
- 11.Zhang H, Liang S, Lv X. Proof-of-principle for AVM embolization complications caused by the proximal occlusion technique using onyx: a theoretical basis for ante-grade drifting technique. Neurol India 2022; 70: 1443–1447. [DOI] [PubMed] [Google Scholar]
- 12.Mason JR, Dodge C, Benndorf G. Quantification of tantalum sedimentation rates in liquid embolic agents. Interv Neuroradiol 2018; 24: 574–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samarage HM, Kim WJ, Zarrin D, et al. The “bright Falx” sign-midline embolic penetration is associated with faster resolution of chronic subdural hematoma after middle meningeal artery embolization: a case series. Neurosurgery 2022; 91: 389–398. [DOI] [PubMed] [Google Scholar]
- 14.Vollherbst DF, Chapot R, Bendszus Met al. et al. Glue, onyx, squid or PHIL? Liquid embolic agents for the embolization of cerebral arteriovenous malformations and dural arteriovenous fistulas. Clin Neuroradiol 2022; 32: 25–38. [DOI] [PMC free article] [PubMed] [Google Scholar]