Abstract
Background
Therapeutic hypothermia has shown potential in cardiac intervention for years; however, its adoption into the neurovascular space has been limited. Studies have pointed to slow cooling and limited depth of hypothermia yielding negative outcomes. Here we present an insulated catheter that allows for consistent infusion of chilled saline directly to the brain. Direct delivery of cold saline allows a faster depth of hypothermia, which could have a benefit to the growth of ischemic lesions.
Methods
Ten canines were randomized to either receive selective brain cooling or no additional therapy. Eight animals were successfully enrolled (n = 4 per group). Each animal underwent a temporary middle cerebral artery occlusion (MCAO) for a total of 45 min. Five minutes prior to flow restoration, chilled saline was injected through the ipsilateral internal carotid artery using an insulated catheter to ensure delivery temperature. The treatment continued for 20 min, after which the animal was transferred to an MRI scanner for imaging.
Results
Of the 8 animals that were successfully enrolled in the study, 3 were able to survive to the 30-day endpoint with no differences between the cooled and control animals. There was no difference in the initial mean infarct size between the groups; however, animals that did not receive cooling had infarcts continuing to progress more rapidly after the MCAO was removed (13.8% vs 161.3%, p = 0.016, cooled vs control).
Conclusions
Selective hypothermia was able to reduce the post-MCAO infarct progression in a canine model of temporary MCAO.
Keywords: Acute ischemic stroke, animal model, neurointerventional surgery, magnetic resonance imaging
Introduction
Over the past decade mechanical thrombectomy (MT) has become the clinical standard of care for patients presenting with large vessel occlusion (LVO) stroke. The most recent studies have shown rates of thrombolysis in cerebral infarction (TICI) 2b/3 of over 80%,1,2 and rates of first pass success increasing from around 25% to over 50%.3–5 Despite this dramatic increase in the recanalization success rate, the number of patients that achieve good clinical outcome, modified Rankin scale (mRS) of 0–2, is at most 49% even among those with successful first pass recanalization,6–8 and rates are lower in those requiring multiple passes. 9 To further improve patient outcomes, other approaches such as adjunct neuroprotection, are of great interest. 10
Therapeutic hypothermia (TH) is arguably the most potent neuroprotective strategy supported by rigorous scientific evidence from pre-clinical models.11–13 However, clinical translation remains elusive. Whole-body or systemic TH does not fit well into the Acute Ischemic Stroke (AIS) care path due to associated negative side effects and its sluggish temperature reduction capabilities, often on the scale of tens of minutes to hours.14–16 To address these limitations, selective delivery of cold fluids through standard catheters are being explored.17–19 A recently published clinical work showed encouraging results in the potential for infarct reduction that motivated a larger randomized controlled trial to further evaluate efficacy. 19
The canine model of ischemic damage has been used to test preclinical methods of stroke treatment. 20 There are two primary endovascular methods to cause a large vessel occlusion in the canine; either an embolus can be injected resulting in a permanent occlusion of the middle cerebral artery (MCA) 21 or a detachable coil can be deployed into the MCA, without detachment, and then removed at a specified time for a transient occlusion. 22 Herein, we have selected the transient occlusion model to simulate mechanical thrombectomy. This model has two major advantages for determining the effect of local hypothermia: 1) the duration of the ischemia can be controlled, and 2) a craniotomy is not required to effect the ischemic region, 23 which has been shown to reduce post-infarct mortality. 24 We hypothesized that localized TH would reduce infarct growth as compared to non-treated animals.
Methods
Twelve purpose bread hound cross canines (sex: male, weight: 23.5 ± 1.5 kg) were used in this experiment, after ethical approval from the Institutional Animal Care and Use Committee (IACUC). On the day of surgery all procedures were performed under general anesthesia using strict aseptic technique. Prior to all surgical or imaging procedures, the animals were pre-anesthetized by an intramuscular injection of glycopyrrolate (0.01 mg/kg), acepromazine (0.06 mg/kg) and for surgical procedures, buprenorphine (0.02 mg/kg). Anesthesia was induced by an intravenous injection of propofol (3–4 mg/kg) and maintained with mechanical ventilation of 1–3% isoflurane. The physiologic status of the animal was assessed using continuous monitoring of respiration rate, heart rate, oxygen saturation level, end tidal CO2 level, and temperature.
For endovascular MCA occlusion, bi-lateral femoral access with 8F hemostatic introducers were placed and a guide catheter (AXS Catalyst 5, Stryker Neurovascular, Freemont CA) was navigated under fluoroscopic guidance into the vertebral artery and a microcatheter (Headway Duo, Microvention) was navigated into the MCA through the ipsilateral posterior communicating artery (Pcomm) where a coil was deployed as previously described. 25 Once the coil was deployed into the MCA, digital subtraction angiography (DSA) was performed to ensure adequate occlusion (Figure 1). The coil was left in place for a total of 45 min and then removed to restore flow. Fourty-five minutes of occlusion was chosen to ensure the presence of a treatable penumbra at flow restoration. 26 Contemporaneously, the insulated Khione catheter (FocalCool, Mullica Hill, New Jersey, USA) was placed in the ipsilateral common carotid artery (CCA) just proximal to the internal carotid artery (ICA) origin. This catheter was designed to insulate a microcatheter from body heat, allowing cold fluid delivery. 27 A microcatheter (XT-27, Stryker Neurovascular, Freemont CA) was navigated through the Khione into the ICA to allow for delivery of chilled saline. By placing the Khione catheter at the origin of the ICA, heat transfer was insulated between the microcatheter and blood. Prior to randomization, if there were any procedure complications the animal was excluded from the study. The animals were pairwise randomized 1:1 to receive either cooled saline or no adjunctive therapy to serve as a control. Delivery of chilled saline began 5 min prior to reperfusion, and continued for 20 min post-reperfusion, for a total of 25 min of cooling time.
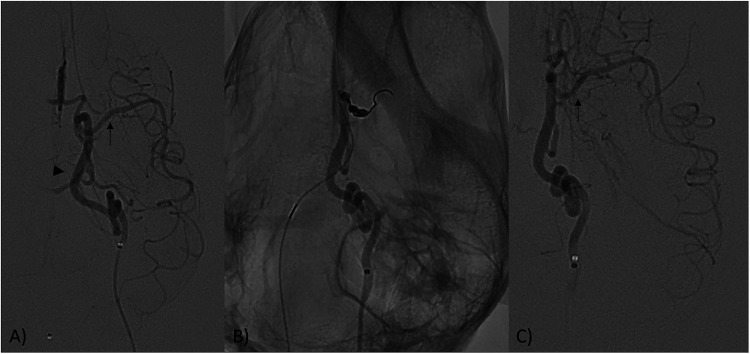
Figure 1.
(A) Digital subtraction angiography (DSA) for the internal carotid artery (ICA, arrowhead) and middle cerebral artery (MCA, arrow) of the canine before occlusion. (B) Aneurysm coil is deployed into the MCA to mimic an M1 occlusion. (C) After 45 min the coil is removed, and flow is restored to the MCA (arrow).
Immediately after cooling, all devices were removed, the femoral arteries ligated, and the incisions closed with suture in layers. The animal was transferred to the 3T MRI scanner (Philips Ingenia, Best, the Netherlands). All MRI examinations followed the same standard protocol using a 16-channel knee coil. The MR protocol included time-of-flight (TOF) angiographic imaging (TR/TE 20/4 ms, FA = 20°, matrix = 332 × 212), diffusion weighted imaging (DWI) (TR/TE 2000/76 ms, FA = 90°, b-values = 0, 1000 s/mm2, directions = 3, NSA = 6, matrix = 144 × 144), intravoxel incoherent motion (IVIM) diffusion weighted MRI (TR/TE 2000/76 ms, FA = 90°, 10 b-values = [0, 50, 100, 150, 200, 250, 300, 500, 700, 900] s/mm2, directions = 3, NSA = 4, matrix = 128 × 128), susceptibility weighted imaging (SWI) (TR/TE 32/7.2 ms, FA = 17°, NSA = 2, matrix = 268 × 268, slice thickness = 0.5 mm), T2-wighted fluid attenuated inversion recovery (T2-FLAIR) (TR/TE = 11,000/125 ms, TI = 2800 ms, FA = 90°, NSA = 2, matrix = 160 × 156, slice thickness 1.5 mm), T1-weighted magnetization prepared rapid acquisition of a gradient echo (T1w-MPRAGE) (TR/TE = 10/4.75 ms, TI = 900 ms, acceleration = 161, FA = 8°, NSA = 2, matrix = 172 × 169, slice thickness = 0.75 mm), and finally a repeat of the DWI imaging 1 h post-reperfusion to assess infarct growth. After the TOF imaging, if the MCA was found to be occluded, the animal was excluded from the study even though it was post randomization as the intention of the study was a reperfusion model, and such a finding would show lack of reperfusion. Following the completion of the MRI examination, if the infarct was less than 1/3 of the MCA territory, the animal was recovered. If the infarct was above this pre-defined threshold, the infarct was deemed to be too severe for recovery and the animal was euthanized at that time. The animals that recovered from the procedure were re-imaged at 48 h and 30 days following the procedure with the same protocol described above. All surviving animals underwent weekly neurological testing as previously described. 25 Briefly, animals were tested for gaze deficit, loss of visual field, front or hind limb gate changes, and inability to balance when on 3 limbs.
Calculation of infarct volume was done by an automated validated MATLAB (MathWorks, Natick, MA) function. 26 Briefly, apparent diffusion coefficient (ADC) maps were generated from the average of the 3 different DWI directions, and then regionally thresholded to 533*10−6 mm2/s. The ADC-derived infarct map was manually adjusted if there were infarct areas detected outside the MCA distribution as areas outside the MCA territory could only be caused by the procedure and not the occlusion. Due to the known variability in infarct size the percent change in infarct size after reperfusion has been shown to be a more consistent measurement, 26 thus this was taken as the primary outcome of the study. IVIM analysis was performed with a two-step fitting approach to calculate the IVIM parameters (D, D*, f and fD*) using in-house code written in MATLAB, based on previously published methodology. 28 The parameters were compared between the cooled and control animals within the infarct territory, within the contralateral matched territory, and within each animal between the infarct territory and a contralateral matched territory.
All statistical calculations were done in R4.2.0 (the R foundation), with a p-value of less than 0.05 considered to be significant, and a Shapiro-Wilk normality test was applied. For normally distributed continuous data, an analysis of variance (ANOVA) was used, while for non-normally distributed data a Mann-Whitney-U test was used. In the case of categorical or discrete data, a Fisher's exact t-test was used.
Results
Before randomization, two animals were excluded due to basilar artery perforator injury leading to subarachnoid hemorrhage. Overall, 10 of the 12 animals were randomized (83%) to receive either the cooling or control treatment, resulting in n = 5 for each group. Of the 10 dogs that were randomized, 8 (67% of total), comparable with published success rates, 25 were included in the analysis (n = 4 for each group). After randomization, we excluded two animals due to: 1.) Lack of MCA recanalization after the coil was retrieved (cooled animal), and 2.) evidence of global brain infarct (ADC < 533*10−6 mm2/s for the entire brain) on the first DWI MR post-operatively (control animal). After the post-operative MRI, 4 animals (n = 3 cooled and n = 1 control) met euthanasia criteria (infarct > 1/3 of the MCA territory), and 1 animal (control) was euthanized because it did not recover from the MRI even though it had a small area of infarct (0.68 mm3), although these 5 animals were euthanized after the post treatment MRI they were considered in the intention to treat analysis. This resulted in 3 animals continuing to the 30-day designated endpoint (n = 1 cooled and n = 2 control), with no differences in the neurological outcome or rate of survival.
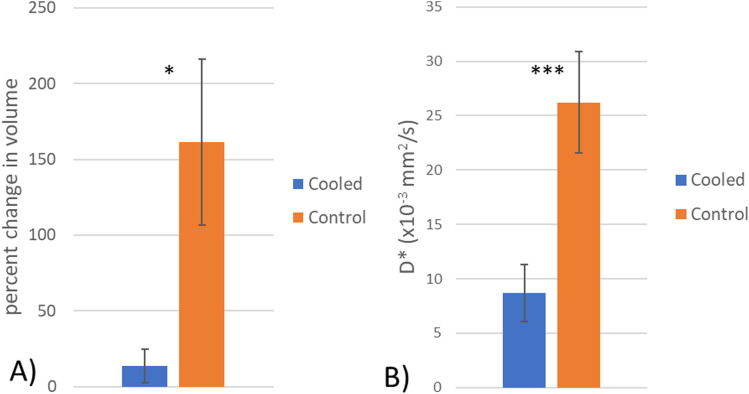
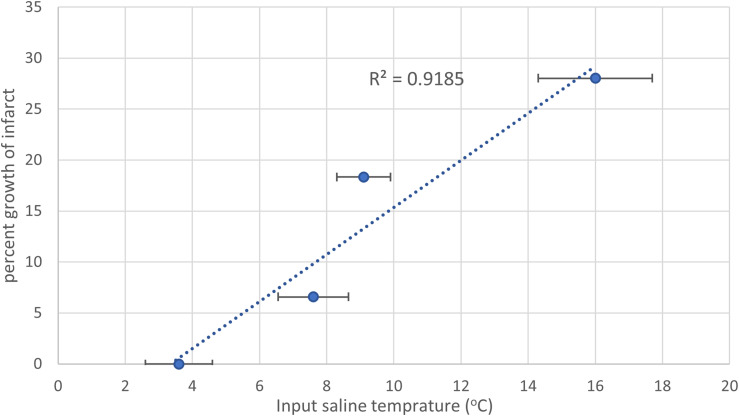
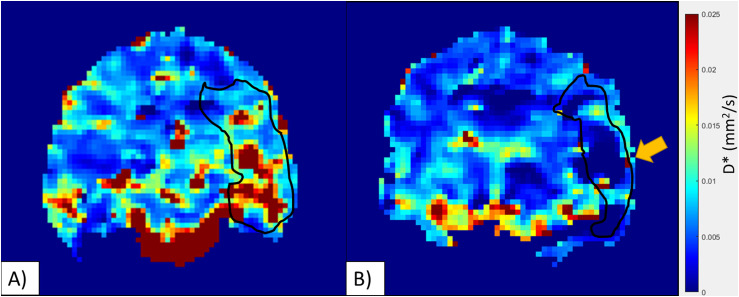
In image analysis, 2 (20%) cases required manual refinement of the ADC maps due to detection of infarct areas in the contralateral anterior cerebral artery (ACA) (cooled animal, n = 1) and posterior cerebral artery (PCA) distribution (control animal, n = 1). Immediately post-reperfusion no differences were observed between the infarct size in the cooled and control animals (5.58 ± 4.06 cm3 vs 1.55 ± 1.04 cm3, p = 0.34, Mann-Whitney-U, Table 1). This was most likely due to both groups having a mix of slow and fast evolvers, 26 and thus the standard deviation of both groups was over 70% of the mean. At one-hour post-reperfusion the infarct volume showed no difference between the groups (6.68 ± 4.97 cm3 vs 4.56 ± 3.73 cm3, p = 0.68, Mann-Whitney-U cooled vs control, Table 1). To account for bio-variability associated with canine slow and fast evolvers, 26 similar to humans, the percent change in the infarct volume from post-reperfusion to 1-h post-reperfusion was calculated (Figure 2). There was a significant difference between the cooled and control animals (13.8% vs 161.3%, p = 0.016, ANOVA, Figure 3(a)), with all 4 of the cooled animals showing reduced infarct growth compared to any of the control animals, indicating that the localized hypothermia was able to reduce the growth of post-reperfusion infarct compared to the control group. There was some variation in the input temperature for the cooled group (range: 2.6–9°C), which resulted in a linear increase in the infarct growth with the higher input temperatures (R2 = 0.93, Figure 4). From the IVIM analysis, the microvascular perfusion (D*, Figure 5) within the infarct was found to be significantly lower in the cooled group compared to the control group (0.0086 vs 0.026, p < 0.001, cooled vs control, Figure 3(b)) showing that the cooling reduced the microvascular perfusion in the infarct territory. Furthermore, comparing the microvascular perfusion in the contralateral side between the cooled and control animals showed no difference (0.017 vs 0.019, p = 0.1, cooled vs control) showing the cooling was able to achieve the desired effect only within the planned territory. Finally, although not significant, there was an elevation in the microvascular perfusion in the infarct territory compared to the contralateral matching territory in the control animal (0.026 vs 0.020, p = 0.06, ipsilateral vs contralateral).
Table 1.
Group wise infarct progression for the selective hypothermia and control groups. All data is presented as mean ± standard deviation.
| Group | Post reperfusion infarct size (cc) | 1 h post reperfusion infarct size (cc) | Percent change in infarct size (%) |
|---|---|---|---|
| Local Hypothermia (n = 4) | 5.6 ± 4.1 | 6.7 ± 5.0 | 13.8 ± 11.1 |
| Control (n = 4) | 1.6 ± 1.1 | 4.56 ± 3.7 | 161.3 ± 54.6 |
| P-value | 0.34 | 0.68 | 0.016 |
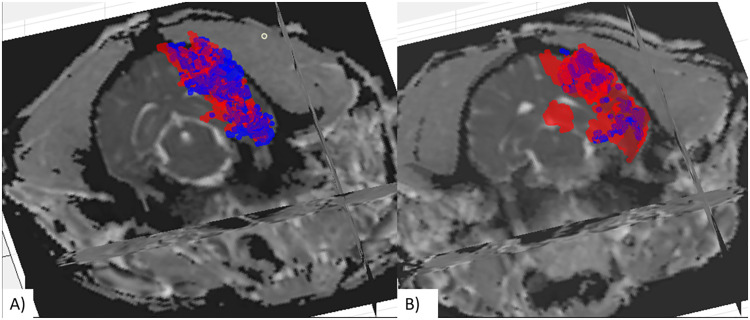
Figure 2.
Post-procedure infarct growth three-dimensional reconstruction of the infarct size immediately following reperfusion (blue) and then 1 h following reperfusion (red). Panel A shows a representative canine that underwent localized hypothermia and had little increase in the infarct size (6.6%). Panel B shows a representative control dog with a much larger infarct at the 1 h follow up than post-reperfusion (230%).
Figure 3.
(A) Mean change in the infarct size between the selective hypothermia group (blue) and the control group (orange). There was a significantly larger increase in the infarct size in the control group (p = 0.016) (B) Mean difference in D* between the infarct territory in the cooled and control groups there was a significant difference between the two groups (p < 0.001). *-p < 0.05, ***-p < 0.001.
Figure 4.
Infarct growth as a function of the input temperature. The temperature shown is the average input saline temperature in the cooled animals. There is a linear increase in the infarct growth for the cooled group (blue) based on the temperature input. Error bars are the standard deviation of input temperature per animal.
Figure 5.
(A) Control animal, there is an elevation in D* within the area of infarct (black outline). (B) Cooled animal, there is a reduction in D* in the area of infarct (black outline). This was significant between the groups (P < 0.001). The black outline is the diffusion weighted infarct area.
Of the 3 (30% of the randomized) animals that recovered from the infarct, none of them had new infarcts at either the 48-h or the 30-day follow-up timepoints. All 3 animals showed the same progression of the FLAIR lesion volume, maximum size at 48-h follow up that then regressed to only slightly larger than the 1-h post-reperfusion ADC-derived infarct volume. The control dogs showed larger FLAIR lesion volume at 48-h and 30-day follow ups; however, since only 1 cooled and 2 control animals survived, no statistical comparisons are made. The neurological assessment showed no change in status for two of the animals, while one (control) showed signs of blindness after the initial infarct, with improvement of symptoms and resolution by one week.
Discussion
We have shown that localized hypothermia in a large animal ischemic reperfusion model reduces infarct growth post reperfusion. The majority of studies on infarct size perform the measurement at least one day post-reperfusion, 29 whereas we assessed infarct size post-reperfusion, and 1 h later. There was a statistically significant reduction in the growth of the infarct region between the two assessment points (p = 0.016), and at the 48-h follow-up, the surviving dogs in the control group exhibited a larger T2-FLAIR hyperintensity. However, due to the post-infarct mortality rate, no statistical significance can be drawn. Although the input temperature in the cooled group had some variation, a strong linear trend between input saline temperature and infarct growth was observed where lower input temperatures were correlated with smaller post-reperfusion infarct growth.
A number of recent animal studies have looked into the effect of cooling, both systemically and selectively. 29 Omileke et al. 30 found that a 4.3°C drop in systemic body temperature resulted in a significant reduction in both post-reperfusion intracranial pressure and final infarct volume in rats after a temporary middle cerebral artery occlusion (MCAO). Two different hypothermia treatments were tested, either cooling only contemporaneously with the MCAO or continued hypothermia after reperfusion. The results showed no differences between the two methods. This mechanism likely resulted in the continuation of growth in the control dogs in our study, while those that underwent localized hypothermia had little to no increase in the infarct size post-reperfusion. Sun et al. 31 compared the use of localized hypothermia to normothermia to decouple the effects of hypothermia and saline perfusion, thought to remove harmful metabolic products.32,33 They showed that compared to both normothermia and no saline infusion, the hypothermia was able to reduce both neurological deficit and infarct volume. Mattingly et al. 23 caused a temporary MCAO by placing a clip on the MCA in pigs for 3 h. Following reperfusion, those animals in the hypothermia group had cold saline flushed into the CCA via a heat exchanger catheter for 3 h. They found on postmortem MRI that the percent of hemisphere infarction was significantly smaller in the hypothermia group. However, similar to our findings, due to the variance in the infarct volume, there was no statistically significant reduction in infarct volume (0.99 ± 1.00 cm3 vs 0.57 ± 0.76 cm3, control vs Neuroprotection). 23 Taken as a whole, our results of a reduction in the reperfusion ischemic injury after localized hypothermia are consistent with other studies. Furthermore, our results show that the effect of hypothermia is most likely acting primarily on the reperfusion injury, and not on the acute damage during the ischemic event.
The blood brain barrier (BBB) protects the brain from infiltration by immune cells, thus reducing the level of inflammation after injury. It has been established that after an ischemic event, the BBB is often compromised and no longer viable in performing its vital function, potentially causing post-reperfusion injury. 34 The canine model used in this study has shown a similar pattern of greatly increased BBB penetration in infarcted tissue, and moderate increase in the region surrounding the infarct. 35 Zhao et al. 36 showed in a mouse model that selective brain hypothermia maintained the integrity of the BBB and dramatically reduced the pro-inflammatory sequelae. By reducing both the inflammation and intracranial pressure, the overall infarct size can be reduced. Post-MT hyperperfusion, 37 causing a heightened stress on the blood vessels, has been shown to be an independent predictor of poor outcome. One of the side effects of hypothermia is vasoconstriction and reduced perfusion. 29 By both reducing the level of BBB breakdown and the hyper-perfusion following recanalization, hypothermia is able to halt infarct progression, as we have shown here.
Although this study focuses on the use of catheter-based intra-arterial delivery of chilled saline, other methods can be used to cool ischemic tissue. Carlstrom et al. 38 demonstrated that cold infusion into the esophagus and nasal cavity and return through an oropharyngeal catheter could cool the brain without a large drop in the core body temperature. However, this approach is limited by a slow rate of cooling (approximately 0.15°C/minute) compared to the much more rapid results seen with the Khione catheter system (2.2°C/minute). 39 Furthermore, without a control group, it could not be determined if there was any benefit in infarct reduction. Moomiaie et al. 40 proposed a cranial burr hole with an insertion of a small catheter into the ventricles. This method allowed them to directly cool the CSF that surrounds the brain allowing for the brain parenchyma to be cooled to 34.5°C within 5 min of starting the flow. This depth of hypothermia was on average not as deep as that achieved with the Khione catheter, which was used in this study. 39 Also, the cranial burr hole method causes systemic brain cooling that cannot be targeted to the hemisphere where the infarct is located.
Limitations
A major limitation of this study is the variation in the post-reperfusion infarct size, since there was no way to a priori determine if the dog was a fast or slow evolver in this model. This could be the reason we were not able to determine if there was a localized cooling effect on the post-reperfusion infarct size. Another limitation of this study was the combination of the short follow-up on the animals that did not survive, only 1 h, and the low number of animals that survived the infarct. This model has been described several times, and the survival rate was not unexpected. Allowing for a longer follow-up period acutely could have demonstrated continued infarct growth in the control group, while the hypothermia group remained the same. Neither of these limitations impact the major finding of this study that localized hypothermia using an insulated catheter following successful revascularization reduces infarct growth.
Conclusions
Localized hypothermia in a large animal model of transient MCA occlusion reduces infarct progression following successful restoration of blood flow, and therefore TH may act as a neuroprotectant targeting reperfusion injury after mechanical thrombectomy.
Contributorship
RMK: responsible for data acquisition, data analysis and statistical analysis. Drafted the manuscript. MSS, ME, VA, SH, JM: responsible for data acquisition, data analysis and statistical analysis. Provided critical editing of the manuscript. NH: Responsible for data analysis, statistical analysis, and critical editing of the manuscript. CR: Responsle for data acquisition and editing of the manuscript. MJG, AJP, TM: Responsible for planning, conception and design of the study, acquisition of data, analysis, interpretation of data, and editing the manuscript. All authors approved the final version of this manuscript to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Data sharing: Data are available by contacting the corresponding author.
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: RMK, ME, NH, and CR declare no competing interest. VA: Consultant on a fee-per-hour basis for Stryker Neurovascular. MSS: 1. Consultant on a fee-per-hour basis for Sanofi; 2. Research support from the Breast Cancer Research Foundation. TLM, SH, and JM: employees of FocalCool. ASP: consultant for Medtronic Neurovascular, Stryker Neurovascular, Balt, Q’Apel Medical, Cerenovus, Microvention, Imperative Care, Agile, Merit, CereVasc, and Arsenal Medical; research grants from NIH, Microvention, Cerenovus, Medtronic Neurovascular, and Stryker Neurovascular; holds stocks in InNeuroCo, Agile, Perfuze, Galaxy and NTI. MJG: 1. Consultant on a fee-per-hour basis for Alembic LLC, Astrocyte Pharmaceuticals, BendIt Technologies, Cerenovus, Imperative Care, Jacob's Institute, Medtronic Neurovascular, Mivi Neurosciences, phenox GMbH, Q’Apel, Route 92 Medical, Scientia, Simcerre, Stryker Neurovascular, Stryker Sustainability Solutions, Wallaby Medical; holds stock in Imperative Care, InNeuroCo, Galaxy Therapeutics, Neurogami and Synchron; 2. Research support from the NIH, the United States – Israel Binational Science Foundation, Anaconda, ApicBio, Arsenal Medical, Axovant, Balt, Cerenovus, Ceretrieve, CereVasc LLC, Cook Medical, Galaxy Therapeutics, Gentuity, Gilbert Foundation, Imperative Care, InNeuroCo, Insera, Jacob's Institute, Magneto, MicroBot, Microvention, Medtronic Neurovascular, MIVI Neurosciences, Naglreiter MDDO, Neurogami, Q’Apel, Philips Healthcare, Progressive Medical, Pulse Medical, Rapid Medical, Route 92 Medical, Scientia, Stryker Neurovascular, Syntheon, ThrombX Medical, Wallaby Medical, the Wyss Institute and Xtract Medical; 3.
Ethics approval: All laboratory animal research activities were approved by the University of Massachusetts Chan Medical School's Institutional Animal Care and Use Committee under protocol #PROTO201900269.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was sponsored by FocalCool, LLC. Research reported in this publication was supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under award number R5R44NS095573-04. The content is solely the responsibility of the authors and does not necessarily represent the official views of the sponsor.
ORCID iDs: Robert M King https://orcid.org/0000-0002-5144-9110
Vania Anagnostakou https://orcid.org/0000-0001-5101-3192
Matthew J Gounis https://orcid.org/0000-0002-8034-2785
References
- 1.Flottmann F, Leischner H, Broocks G, et al. Recanalization rate per retrieval attempt in mechanical thrombectomy for acute ischemic stroke. Stroke 2018; 49: 2523–2525. [DOI] [PubMed] [Google Scholar]
- 2.Pereira VM, Gralla J, Davalos A, et al. Prospective, multicenter, single-arm study of mechanical thrombectomy using solitaire flow restoration in acute ischemic stroke. Stroke 2013; 44: 2802–2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bai X, Zhang X, Yang W, et al. Influence of first-pass effect on recanalization outcomes in the era of mechanical thrombectomy: a systemic review and meta-analysis. Neuroradiology 2021; 63: 795–807. [DOI] [PubMed] [Google Scholar]
- 4.Zaidat OO, Castonguay AC, Linfante I, et al. First pass effect: a new measure for stroke thrombectomy devices. Stroke 2018; 49: 660–666. [DOI] [PubMed] [Google Scholar]
- 5.Drouard-de Rousiers E, Lucas L, Richard S, et al. Impact of reperfusion for nonagenarians treated by mechanical thrombectomy: insights from the ETIS registry. Stroke 2019; 50: 3164–3169. [DOI] [PubMed] [Google Scholar]
- 6.Flottmann F, Brekenfeld C, Broocks G, et al. Good clinical outcome decreases with number of retrieval attempts in stroke thrombectomy: beyond the first-pass effect. Stroke 2021; 52: 482–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Requena M, Pinana C, Olive-Gadea M, et al. Combined technique as first approach in mechanical thrombectomy: efficacy and safety of REACT catheter combined with stent retriever. Interv Neuroradiol 2023; 29: 504–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ullberg T, von Euler M, Wasselius J, et al. Survival and functional outcome following endovascular thrombectomy for anterior circulation acute ischemic stroke caused by large vessel occlusion in Sweden 2017–2019-a nationwide, prospective, observational study. Interv Neuroradiol 2023; 29: 94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elfil M, Bahbah EI, Bayoumi A, et al. Repeated mechanical thrombectomy for recurrent large vessel occlusion: a systematic review and meta-analysis. Interv Neuroradiol 2022; 0. doi: 10.1177/15910199221134307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisher M, Saver JL. Future directions of acute ischaemic stroke therapy. Lancet Neurol 2015; 14: 758–767. [DOI] [PubMed] [Google Scholar]
- 11.Dumitrascu OM, Lamb J, Lyden PD. Still cooling after all these years: meta-analysis of pre-clinical trials of therapeutic hypothermia for acute ischemic stroke. J Cereb Blood Flow Metab 2016; 36: 1157–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rewell SS, Jeffreys AL, Sastra SA, et al. Hypothermia revisited: impact of ischaemic duration and between experiment variability. J Cereb Blood Flow Metab 2017; 37: 3380–3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Worp HB, Sena ES, Donnan GA, et al. Hypothermia in animal models of acute ischaemic stroke: a systematic review and meta-analysis. Brain 2007; 130(Pt 12): 3063–3074. [DOI] [PubMed] [Google Scholar]
- 14.Geurts M, Petersson J, Brizzi M, et al. COOLIST (Cooling for ischemic stroke trial): a multicenter, open, randomized, phase II, clinical trial. Stroke 2017; 48: 219–221. [DOI] [PubMed] [Google Scholar]
- 15.Kurisu K, Yenari MA. Therapeutic hypothermia for ischemic stroke; pathophysiology and future promise. Neuropharmacology 2018; 134(Pt B): 302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polderman KH, Herold I. Therapeutic hypothermia and controlled normothermia in the intensive care unit: practical considerations, side effects, and cooling methods. Crit Care Med 2009; 37: 1101–1120. [DOI] [PubMed] [Google Scholar]
- 17.Chen J, Liu L, Zhang H, et al. Endovascular hypothermia in acute ischemic stroke: pilot study of selective intra-arterial cold saline infusion. Stroke 2016; 47: 1933–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tokairin K, Osanai T, Abumiya T, et al. Regional transarterial hypothermic infusion in combination with endovascular thrombectomy in acute ischaemic stroke with cerebral main arterial occlusion: protocol to investigate safety of the clinical trial. BMJ Open 2017; 7: e016502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu C, Zhao W, An H, et al. Safety, feasibility, and potential efficacy of intraarterial selective cooling infusion for stroke patients treated with mechanical thrombectomy. J Cereb Blood Flow Metab 2018; 38: 2251–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taha A, Bobi J, Dammers R, et al. Comparison of large animal models for acute ischemic stroke: which model to use? Stroke 2022; 53: 1411–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shazeeb MS, King RM, Anagnostakou V, et al. Novel oxygen carrier slows infarct growth in large vessel occlusion dog model based on magnetic resonance imaging analysis. Stroke 2022; 53: 1363–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rink C, Christoforidis G, Abduljalil A, et al. Minimally invasive neuroradiologic model of preclinical transient middle cerebral artery occlusion in canines. Proc Natl Acad Sci U S A 2008; 105: 14100–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mattingly TK, Denning LM, Siroen KL, et al. Catheter based selective hypothermia reduces stroke volume during focal cerebral ischemia in swine. J Neurointerv Surg 2016; 8: 418–422. [DOI] [PubMed] [Google Scholar]
- 24.Back L, Nagaraja V, Kapur A, et al. Role of decompressive hemicraniectomy in extensive middle cerebral artery strokes: a meta-analysis of randomised trials. Intern Med J 2015; 45: 711–717. [DOI] [PubMed] [Google Scholar]
- 25.Christoforidis GA, Rink C, Kontzialis MS, et al. An endovascular canine middle cerebral artery occlusion model for the study of leptomeningeal collateral recruitment. Invest Radiol 2011; 46: 34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shazeeb MS, King RM, Brooks OW, et al. Infarct evolution in a large animal model of middle cerebral artery occlusion. Transl Stroke Res 2020; 11: 468–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merrill TL, Merrill DR, Nilsen TJ, et al. Design of a cooling guide catheter for rapid heart cooling. J Med Devices 2010; 4: 035001 (8 pages). [Google Scholar]
- 28.Federau C, O’Brien K, Meuli R, et al. Measuring brain perfusion with intravoxel incoherent motion (IVIM): initial clinical experience. J Magn Reson Imaging 2014; 39: 624–632. [DOI] [PubMed] [Google Scholar]
- 29.Liddle LJ, Kalisvaart ACJ, Abrahart AH, et al. Targeting focal ischemic and hemorrhagic stroke neuroprotection: current prospects for local hypothermia. J Neurochem 2022; 160: 128–144. [DOI] [PubMed] [Google Scholar]
- 30.Omileke D, Azarpeykan S, Bothwell SW, et al. Short-duration hypothermia completed prior to reperfusion prevents intracranial pressure elevation following ischaemic stroke in rats. Sci Rep 2021; 11: 22354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun G, Qin W, Wang Q, et al. Selective-cerebral-hypothermia-induced neuroprotection against-focal cerebral ischemia/reperfusion injury is associated with an increase in SUMO2/3 conjugation. Brain Res 2021; 1756: 147311. [DOI] [PubMed] [Google Scholar]
- 32.Ding Y, Li J, Rafols JA, et al. Prereperfusion saline infusion into ischemic territory reduces inflammatory injury after transient middle cerebral artery occlusion in rats. Stroke 2002; 33: 2492–2498. [DOI] [PubMed] [Google Scholar]
- 33.Duan H, Huber M, Ding JN, et al. Local endovascular infusion and hypothermia in stroke therapy: a systematic review. Brain Circ 2019; 5: 68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi Y, Zhang L, Pu H, et al. Rapid endothelial cytoskeletal reorganization enables early blood-brain barrier disruption and long-term ischaemic reperfusion brain injury. Nat Commun 2016; 7: 10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.King R, Caroff J, Gounis M. E-061 rapid blood brain barrier permeability imaging in a canine ischemic re-perfusion model. J Neurointerv Surg 2018; 10(Suppl 2): A78–AA9. [Google Scholar]
- 36.Zhao J, Mu H, Liu L, et al. Transient selective brain cooling confers neurovascular and functional protection from acute to chronic stages of ischemia/reperfusion brain injury. J Cereb Blood Flow Metab 2019; 39: 1215–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kneihsl M, Hinteregger N, Nistl O, et al. Post-reperfusion hyperperfusion after endovascular stroke treatment: a prospective comparative study of TCD versus MRI. J Neurointerv Surg 2022; 15: 983–988. [DOI] [PubMed] [Google Scholar]
- 38.Carlstrom LP, Perry A, Graffeo CS, et al. Novel focal therapeutic hypothermia device for treatment of acute neurologic injury: large animal safety and efficacy trial. J Neurol Surg B Skull Base 2022; 83: 203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caroff J, King RM, Mitchell JE, et al. Focal cooling of brain parenchyma in a transient large vessel occlusion model: proof-of-concept. J Neurointerv Surg 2020; 12: 209–213. [DOI] [PubMed] [Google Scholar]
- 40.Moomiaie RM, Gould G, Solomon D, et al. Novel intracranial brain cooling catheter to mitigate brain injuries. J Neurointerv Surg 2012; 4: 130–133. [DOI] [PubMed] [Google Scholar]