Abstract
Study Design
Retrospective comparative cohort.
Objective
(1) Describe the prevalence of the basivertebral vessel (BVV) in a cohort of spinal epidural abscesses (SEA) at lumbar or thoracic (2) correlate the presence of BVV to the risk of conservative treatment failure (CTF).
Methods
Twenty-six patients successfully managed without surgery were compared to 26 who required surgical management due to failed conservative management (lumbar and thoracic). Two observers sought the BVV on the sagittal T1 with contrast sequences of the initial MRI in a blinded fashion for Kappa score calculation. BVV-/BVV+: absence/presence. Demographic, radiological, and laboratory parameters, as well as functional scores, were recorded.
Results
For both observers, 29/52 patients had a BVV+ (55.7%); the agreement was 84% (Kappa: 0.80 CI 95% [0.70-0.90]). 5/23 (21.7%) BVV- patients had a successful medical treatment, while the proportion was 21/29 (72%) for BVV+ (P = .0003). The positive predictive value for BVV+, predicting successful conservative treatment, was 81%. The negative predictive value for BVV- predicting CTF was 69%. BVV- was predictive of CTF in multivariable logistic regression: OR = 40, CI 95% [5-880], P = .02, for agreed observations between observers. For both observers, the proportion of dorsal abscess was the highest for BVV+ (P = .01).
Conclusion
The BVV is part of the epidural network. The absence of BVV was strongly correlated with an increased risk of CTF, leading to the need for subsequent surgical treatment. SEA’s location pattern varied according to BVV detection. Although the spinal vascular anatomy has been well-known for over 100 years, there are still very few studies on its pathophysiological implications.
Keywords: spinal epidural abscess, vascularity, prognostic score, treatment failure
Introduction
Spinal epidural abscess (SEA) presents a significant medical challenge associated with neurological deficits in up to 50% and carries a significant 90-day mortality risk of approximately 15%.1,2 Furthermore, SEA may be responsible for a long-term quality of life decline. 3 The global incidence of SEA has increased due to factors such as increasing comorbid conditions, increased life expectancy, and improved diagnostic capabilities. 2 Medical treatment is commonly attempted first, but this approach faces a substantial risk of failure, reaching up to 40%.1,2
Current prognostic scores, including the modified Frailty Index (mFI-11),4,5 Mortality in Spinal Infection (MSI), 6 Charlson Comorbidity Index (CCI),5,7 SORG, 8 or SITE, 9 primarily rely on demographic data, comorbidities, severity indicators, and specific radiological information, mostly related to stability. While these existing scores effectively predict mortality, they exhibit poorer performances when predicting the failure of medical treatment.5,10 Similarly, single parameters such as the CRP 7 or existing mellitus diabetes 11 have been identified as risk factors for treatment failure or neurological compromise. However, they are not effective enough to be used alone. Given the limitations of these current tools, there is an imperative to explore new avenues and develop innovative prognostic tools that can more accurately anticipate the complexities of SEA and enhance clinical decision-making.
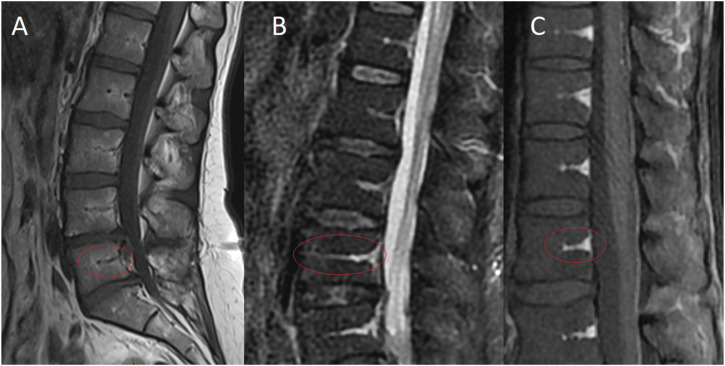
The concept of ‘seeding’ refers to the spread of infection through the bloodstream, which is related to the hematogenous nature of SEA, and was described around 1940. 12 However, despite its crucial role in the pathophysiology of SEA, epidural vascularization remains largely understudied. As of the 1870 s, several authors extensively described the epidural vascular anatomy12-18; the intra-vertebral body vascular system is a branch of the epidural network. The basivertebral vessel (BVV), is the main artery originating from the posterior part of the vertebral body, directly from the intra-spinal canal epidural vessels.14,19 Normal basivertebral arteries penetrate the body centrally, maintaining its caliber and giving coil ramifications. 14 The intravertebral venous and lymphatic plexus are analogous to the arterial network, with drainage primarily occurring in the posterior region of the vertebral body. 20 Epidural veins may be valveless,19,21 making venous bidirectional, which can favor retrograde seeding. The BVV is visible in imaging, particularly magnetic resonance imaging (MRI), so it may reflect the epidural vascular network (Figure 1). Delivery of antibiotics to the infective focus is a key parameter for infection control, and a healthy vascular network will facilitate this. The spine contains numerous avascular structures, for instance, the intervertebral discs, whose vascularization depends on the quality of surrounding vascularized tissues. 22 Additionally, pharmacological studies have shown poor penetration of antibiotics into the spine.23-25 These two last parameters make SEA management even more complex.
Figure 1.
The basivertebral vessel (BVV) is the main artery originating from the posterior part of the vertebral body directly from the intra-spinal canal epidural vessels. Normal basivertebral arteries penetrate the body centrally, maintaining its caliber and giving coil ramifications. The BVV is visible in imaging, particularly magnetic resonance imaging so that it may reflect the epidural vascular network. It may appear hypointense with T1 without contrast (A), hyperintense with T2 sequences (especially T2 STIR and Water, (B), and T1 with contrast (C).
For all these reasons, we hypothesize that poor epidural vascularization is associated with inadequate antibiotic penetration and, therefore, an increased risk of medical treatment failure. This study examines the BVV, a branch of the epidural network, in the thoracic and lumbar spine with contrast-enhanced MRI. The first aim is to study inter-observer agreement for BVV detection; the second is to investigate the association between the presence or absence of BVV and the risk of medical treatment failure. We hypothesized that poorer vascularity would be associated with a greater risk of medical treatment failure.
Material and Methods
Ethics
This analysis received the Hospital Review Board approval (Waikato Hospital Clinical Audit Support Unit; registration Number 4341P). As data were analyzed in retrospect, patient consent was not required.
Setting and Participants
This study was conducted at a tertiary referral spine center servicing over 900 000 people. The hospital coding provided a list of patients over 18 years old diagnosed with spinal epidural abscesses at the thoracic or lumbar from January 2010 to November 2022. Patients included in the study had their electronic medical and digital radiology records checked to confirm a radiological diagnosis of SEA. A total of 124 patients were pre-selected, and only the patients with an early MRI with contrast were included (MRI within the first week of symptoms onset). Twenty-six patients with conservative treatment failure were matched with 26 patients successfully managed with antibiotics alone. The matching was done manually by similar ages.
Variables
The demographic data collected were the age at admission, gender, ethnicity, comorbidities, the SORG, mFI-11, and CCI.4,5,8 The clinical information was the neurological status (using the Frankel classification: from E, no neurological deficit, to A, complete motor and sensitive deficit) 26 and the presence or absence of multifocal infection. The laboratory values were the C-reactive protein (CRP, mg/L), Haemoglobin (g/L), White Blood Cell Count (WBC, g/L), Platelets, and renal profile. The radiological information obtained from MRI was the presence of osteomyelitis or discitis and the location of the SEA (ventral or dorsal).
Outcomes
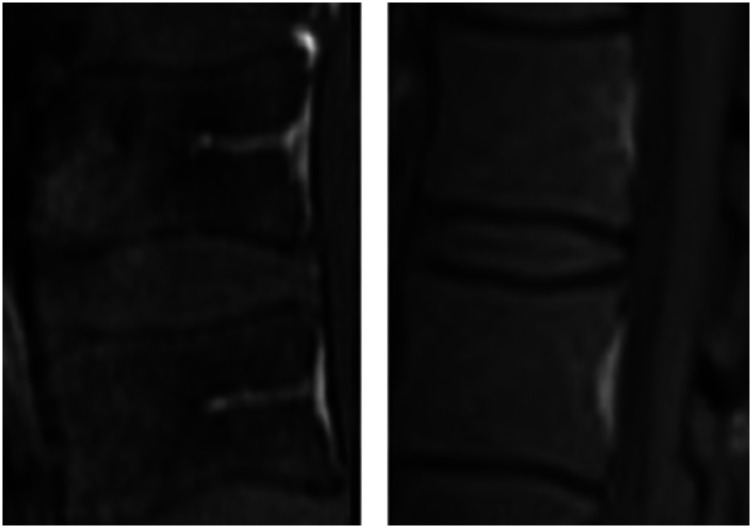
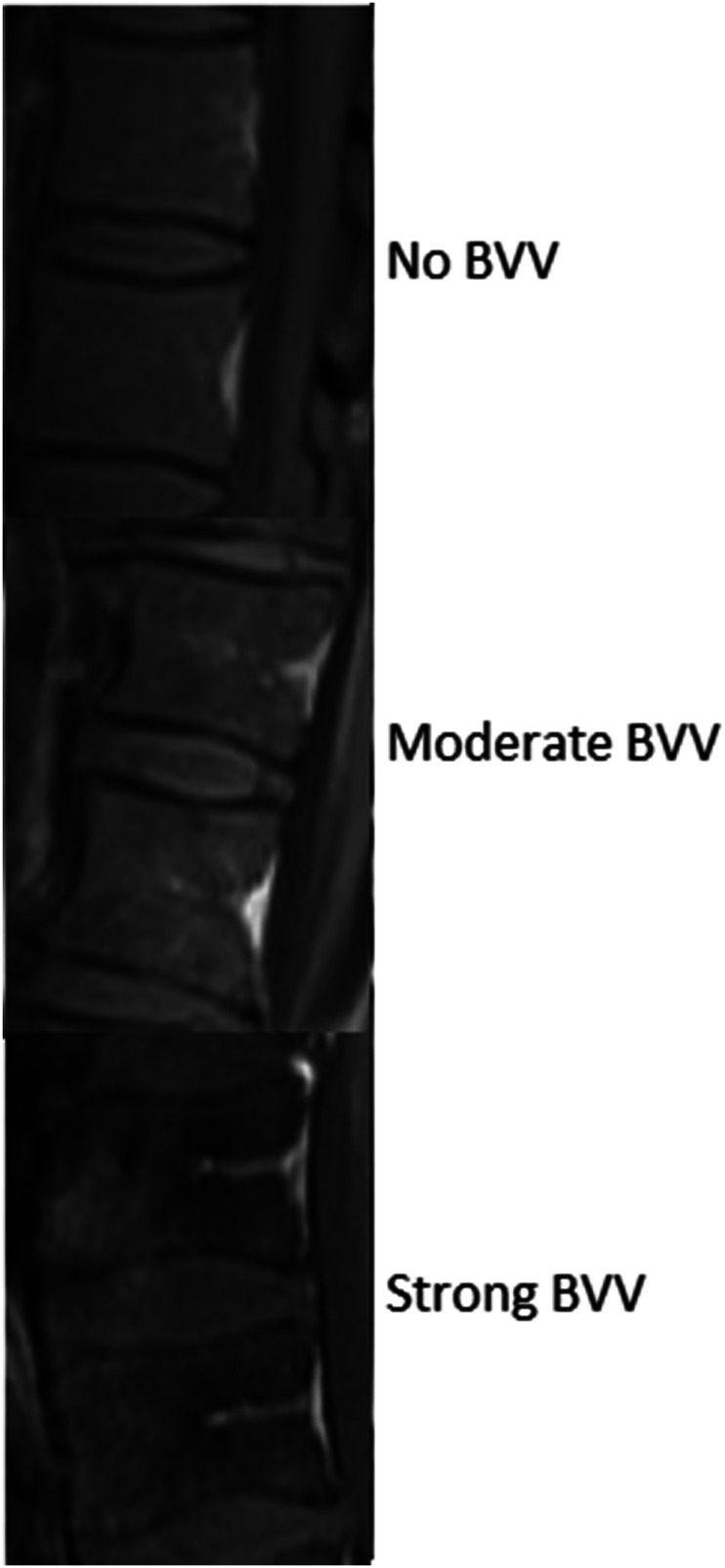
The main outcome was the presence of the Basi-Vertebral Vessel. BVV was searched around the SEA (two levels above and below) and at the SEA level when feasible (edema and pus sometimes made the detection difficult at the SEA level). The best possible score was accepted – if clearly present at one level but not others, then the BVV was considered present. The sagittal T1 sequences with contrast were used; the BVV is hyperintense with contrast (Figures 2 and 3), and hypointense with T1 without contrast 27 (Figure 1). Two scales were used; the simplest scale was BVV + for detection and BVV- for no detection (Figure 2). The more refined scale was no BVV detection, moderate BVV detection, and strong BVV detection (Figure 3). Search was made by two senior spine surgeons, in a blinded fashion regarding the treatment outcome.
Figure 2.
The simplest scale was BVV+ (left) for detection and BVV- (right) for no detection; sagittal T1 sequences with contrast.
Figure 3.
The more refined scale was no BVV detection, moderate BVV detection, and strong BVV detection; sagittal T1 sequences with contrast.
The second key result was the treatment outcome. The medical treatment failure was defined as the progression of the SEA despite adequate initial antibiotic treatment, leading to a neurological compromise or failure of biological improvement as evidenced by persistently elevated CRP 7 or increasing pain or spine instability and requiring surgical management.
Bias
The main bias considered was the comparability between the two groups. As specified above, the patients were matched according to their age. Due to a power constraint, no further matching was possible.
Statistics
When describing the data, we report the median, first, and third quartiles for quantitative variables. We report counts for categorical variables. The associations between all the variables and the outcome were tested using a Generalized Linear Regression (univariable tests).
The first outcome was the inter-observer agreement, which was the number and proportion of agreed observations for BVV detection. Then, a Cohen’s Kappa score was calculated 28 with its 95% confidence interval (95% CI), and we used Landis and Koch’s table to interpret the coefficient 28 : between 0.41 and 0.60, moderate agreement; between 0.61 and 0.80, strong agreement; and between 0.81 and 1, almost perfect agreement. Calculations were made for the two scales.
The second objective was to assess the association between BVV and the risk of medical treatment failure. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated. Then, every variable amongst the BVV detection was tested for association with the treatment outcome, using invariable tests (Generalized Linear Regression). Those with some degree of association with the treatment outcome (P-value <.2) were selected for a multivariable logistic regression. Before entering these explicative variables in the multivariable regression, the absence of collinearity between every variable was tested (because collinearity between explicative variables impairs the estimates 29 ).
Analyses were performed on R software (R 3.6.0, R Foundation for Statistical Computing). The alpha risk was set at 5% (threshold for significance: P-value <.05).
Results
Descriptive Analysis and Agreement
The full description of the cohort is given in Table 1.
Table 1.
Characteristics of the entire cohort and by BVV subgroup.
| Variable | Agreed Observations | ||||
|---|---|---|---|---|---|
| All Cohort | No BVV | Moderate BVV | Strong BVV | P | |
| n = 52 | n = 18 | n = 11 | n = 10 | ||
| Age | 66.5 (56.0-73.2) | 63.5 (60.5-74.2) | 70.0 (62.0-79.0) | 59.5 (47.8-73.0) | 0.44 |
| SORG | 10.6 (4.2-19.4) | 11.2 (7.8-18.0) | 17.6 (9.0-26.3) | 9.2 (4.0-21.5) | 0.72 |
| MFI | 1.0 (0.0-2.0) | 2.0 (1.0-3.0) | 1.0 (0.0-2.0) | 0.5 (0.0-1.0) | 0.19 |
| CCI | 2.0 (1.0-4.0) | 3.0 (1.5-3.0) | 3.0 (2.0-5.8) | 3.0 (0.8-6.0) | 0.24 |
| Group | |||||
| Surgical | 26 (50.0) | 16 (89) | 4 (36) | 1 (10) | 0.0001 |
| Conservative | 26 (50.0) | 2 (11) | 7 (64) | 9 (90) | |
| Gender | |||||
| Male | 36 (69.2) | 11 (61) | 7 (64) | 8 (80) | 0.58 |
| Female | 16 (30.8) | 7 (39) | 4 (36) | 2 (20) | |
| Ethnicity | |||||
| European | 24 (46.2) | 9 (50) | 7 (64) | 4 (40) | 0.06 |
| Maori | 18 (34.6) | 8 (44) | 0 (0) | 3 (30) | |
| Other | 10 (19.2) | 1 (6) | 4 (36) | 3 (30) | |
| Dorsal SE | |||||
| Yes | 28 (58.3) | 9 (50) | 5 (45) | 10 (100) | 0.015 |
| Ventral SEA | |||||
| Yes | 24 (50.0) | 11 (61) | 7 (64) | 0 (0) | 0.003 |
| Discitis | |||||
| Yes | 30 (58.8) | 12 (67) | 6 (60) | 5 (50) | 0.69 |
| Osteomyelitis | |||||
| Yes | 26 (51.0) | 11 (61) | 7 (70) | 2 (20) | 0.06 |
| Initial Frankel score | |||||
| C | 2 (4.3) | 0 (0) | 1 (10) | 1 (10) | 0.59 |
| D | 14 (30.4) | 4 (27) | 3 (30) | 1 (10) | |
| E | 30 (65.2) | 11 (73) | 6 (60) | 8 (80) | |
| Multifocal infection | |||||
| Yes | 28 (60.9) | 12 (80) | 5 (50) | 5 (50) | 0.19 |
| CRP | 173 (124-260) | 180 (144-243) | 165 (113-254) | 218 (132-285) | 0.96 |
| WBC | 12.4 (8.9-14.8) | 13.6 (12.4-16.2) | 9.8 (8.2-11.5) | 11.2 (7.2-13.5) | 0.07 |
| Haemoglobin | 12.4 (10-13) | 12 (10-13) | 11.6 (10-12) | 13.4 (12.3-14.0) | 0.35 |
| Albumin | 28 (23-33) | 28.0 (22-31) | 30.0 (25-33) | 30.0 (24.8-36.5) | 0.75 |
Regarding BVV-/BVV + only, 29/52 patients had a BVV+ (55.7%), with both observers. The observers found similar results in 42/50 cases (84%), and the Kappa score was 0.80 CI 95% [0.70-0.90].
When considering the more refined scale, the only types of disagreement between the two observers were between no BVV/moderate BVV or moderate BVV/strong BVV. There was no disagreement type, no BVV/strong BVV. The observers found similar results in 39/50 cases (75%), and the kappa score was 0.61 CI 95% [0.39-0.83].
For both observers, the proportion of dorsal abscess was the highest for strong BVV: 12/13 (92%) for observer 1, P = .015, 10/10 (100%) for observer 2, P = .01. Conversely, for both observers, the proportion of ventral abscess was the lowest for strong BVV, 2/13 (15%) for observer 1, P = 0.01, and 0/10 for observer 2, P = .001. For observer 1, the proportion of Māori was more prevalent for no BVV, (57% vs 19%, P = .007), but this difference did not reach significance for agreed observations (P = .06, Table 1). There was no significant difference in the disease severity parameters (biological and radiological parameters).
Association with the Risk of Surgical Treatment
For both observers, 23 patients were BVV-, and 5/23 had a successful conservative treatment (21.7%, considering agreed observations). Considering agreed observations, twenty-nine patients were BVV+, and 21/29 had a successful conservative treatment P = .0003 (72%). The PPV was 81%, which means the likelihood of successful conservative treatment amongst patients with BVV+. The NPP was 69%, which means the likelihood of unsuccessful conservative treatment amongst patients with BVV-. The specificity and sensibility of BVV- as a predictor of surgical treatment were 80% and 69%, respectively.
When considering the more refined scale, for observer 1, 18/23 (78%) of the patients without BVV had surgical treatment, 6/16 (37%) for moderate BVV, and 2/13 (15%) for strong BVV; the NPV without BVV was 90%, and the PPV in case of strong BVV was 69%. For observer 2, 18/23 (78%) of the patients without BVV had surgical treatment, 7/19 (37%) for moderate BVV, and 1/10 (10%) for strong BVV; the NPV without BVV was 95%, and the PPV in case of strong BVV was 64%.
The presence or absence of BVV was predictive of surgical treatment: OR = 8.5 CI 95% [2.5-33], P = .001, for both observers, in univariable tests (Table 2). CRP was significantly associated with surgical treatment: OR = 1.007 for each unit increase in CRP, CI 95% [1.0006-1.01], P = .04. Females also had some association with surgery (OR = 3.06 CI 95% [0.91-0.11], P = 0.08).
Table 2.
Characteristics of patients manged with either surgery or conservative therapy.
| Variables | Surgical | Conservative | P |
|---|---|---|---|
| n = 26 | n = 26 | ||
| Age | 64.50 (59.25-70.75) | 71.00 (51.00-77.75) | .59 |
| SORG | 11.20 (8.70-18.20) | 9.20 (4.00-21.15) | .53 |
| MFI | 1.00 (0.25-2.75) | 1.00 (0.00-2.00) | .59 |
| CCI | 3.00 (2.00-4.00) | 2.00 (1.00-5.00) | .57 |
| Diabetes | |||
| Yes | 5 (19) | 3 (11) | .17 |
| Immunocompromised | |||
| Yes | 2 (8) | 1 (4) | .51 |
| Observer 1 | |||
| BVV- | 18 (69) | 5 (19) | .0003 |
| BVV+ | 8 (31) | 21 (81) | |
| Observer 2 | |||
| BVV- | 18 (69) | 5 (19) | .0003 |
| BVV+ | 8 (31) | 21 (81) | |
| Agreed observations (n = 42) | |||
| BVV- | 18 (90) | 6 (27) | <.0001 |
| BVV+ | 2 (10) | 16 (73) | |
| Gender | |||
| Male | 15 (58) | 21 (81) | .071 |
| Female | 11 (42) | 5 (19) | |
| Ethnicity | |||
| European | 12 (46) | 12 (46) | .73 |
| Maori | 10 (38) | 8 (31) | |
| Other | 4 (15) | 6 (23) | |
| Dorsal SEA | |||
| Yes | 15 (58) | 16 (62) | .78 |
| Ventral SEA | |||
| Yes | 15 (58) | 12 (46) | .41 |
| Discitis | |||
| Yes | 16 (64) | 15 (58) | .64 |
| Osteomyelitis | |||
| Yes | 13 (52) | 14 (54) | .89 |
| Initial Frankel score | |||
| C | 1 (5) | 1 (4) | .32 |
| D | 9 (41) | 5 (21) | |
| E | 12 (55) | 18 (75) | |
| Multifocal infection | |||
| Yes | 16 (73) | 12 (50) | .11 |
| CRP | 185 (165-262) | 123.50 (79-221) | .022 |
| WBC | 13.00 (9.96-15.85) | 10.35 (8.00-13.85) | .13 |
| Haemoglobin | 12.0 (10.2-13.4) | 13.0 (11.4-14.1) | .44 |
| Albumin | 27 (21-31) | 30 (26-36) | .61 |
There was no association between CRP, gender, and BVV (P > .2), so there was no risk of collinearity, and these three variables could be entered together in the multivariable model. In the multivariable model, BVV- was still associated with the risk of surgical treatment: OR = 18 CI 95% [4-132], P = .001 for observer1, OR = 11 CI 95% [2.7-62], P = .001 for observers 2 and OR = 40, CI 95% [5-880], P = .02 for agreed observations. For agreed observations, CRP and Gender no longer have a significant association with surgical treatment: OR = 1.005 CI 95% [0.99-1.02], P = .33 and OR = 4.3 CI 95% [0.63-35], P = .15, respectively.
Discussion
There was at least a substantial agreement between the two observers to detect BVV with T1 sequence and contrast. The agreement was almost perfect when considering the simplest gradation (BVV+/BVV-). The only source of disagreement was for the patient with moderate BVV, and there was no disagreement with no BVV/strong BVV. The absence of BVV was strongly associated with the risk of surgical treatment. BVV was the only factor that remained significantly associated with the risk of surgery in the multivariable model. The use of the simplest scale was the most reproducible and provided a good PPV, meaning the likelihood of successful conservative treatment in case of BVV+. The use of the more refined scale increased the NPV for patients without BVV, meaning the likelihood, but was less reproducible.
Apart from BVV, the CRP was the only other parameter significantly associated with the risk of medical treatment failure, which is a well-known information.30,31 However, there is little data in the literature to compare the results regarding BVV with. It is well known that SAE or from a hematogenous spreading, and the notion of “seeding” of the infection is usually employed.12,32 As of 1940, Batson 12 identified that the vertebral venous plexus could be the entry point for prostatic metastasis cells. More recently, with the improvement of the imaging and the development of the MRI, several authors had a closer look at the epidural vessels’ physiopathology. For infections, the seeding occurs more in high blood flow areas,13,33 which explains why roughly 60% of the infections occur at the lumbar spine. The seeding could be from the arterial network but also the venous network. Some epidural veins are valveless,19,21 meaning that the blood flow is bidirectional, which could lead to retrograde seeding.34,35 Morikawa et al found that dilated spinal epidural plexus were more frequently associated with SAE. 36 In the context of degenerative disease, Ju et al identified several patterns of intra-canal epidural vein varicosities, some of which could compress the nerve roots, contributing to pain. 37 Slin’ko et al reviewed these compressive varicosities’ surgical options. 38 Vernon et al found similar results in dogs. 39 However, as Ju et al mentioned, vascularity is difficult to visualize and is often overlooked in daily practice. 37
The description of the absence of epidural vessels and its potential consequences is barely studied, and we can only formulate hypotheses based on available knowledge. The strong correlation between poor vascularity and conservative treatment failure may be explained by a poor diffusion of antibiotics and a poorer capacity to fight against infection. From an anatomical point of view, the spine contains avascular structures, such as the endplates and discs, fed by capillarity. 22 The disappearance of the BVV might contribute to a less good distribution of the antibiotics around the nutrient pedicle. Also, the lymphatic vessels go alongside the venous plexus, 20 and it is plausible that no BVV was associated with no lymphatic drainage. Almost two-thirds of the patients had associated discitis; however, it remains unclear whether the SEA originated from the discitis or if the discitis was a consequence of the SEA. Although the result did not reach statistical significance (P = .06), patients with strong BVV had less osteomyelitis. These findings support the theory that infection seeding occurs in areas with poor vascularization and that reduced vascularity limits the ability to fight infection locally. From a pharmacological standpoint, several authors have demonstrated that antibiotics have poor diffusion into the spine, with limited penetration and washout.23-25 Reduced vascularity may exacerbate these features.
The reasons why the BVV was not visualized in some patients remain unclear, and several hypotheses can be proposed, including degenerative processes. Basivertebral branches are short, fragile vessels within the intravertebral plexus and may be particularly sensitive to aging and degeneration.13,19 Basiverebral vessels also transit through the posterior longitudinal ligament, 13 which can be ossified in generative disease. 40 Modic bone marrow remodeling is a well-known phenomenon in disk disease, with the sequence inflammation/fatty dehiscence/ sclerosis. 41 As BVV is inside the trabecular bone, any structure modification may alter the vascularity. Laffosse et al showed that abnormal mechanical load can decrease the bone permeability and capillary network around the endplates. 42 Derivative processes may exist; for instance, Capossela et al found that severe disc degeneration is associated with increased cellular signals for alternative vascularisation. 43 The second hypothesis is related to pressure. The intravertebral epidural network is dynamic and can regulate its pressure. In particular, the longitudinal veins of the internal venous plexus (to which the basivertebral pedicle is connected) have walls containing smooth muscles. Stringer et al 19 reviewed several flow control or redirection mechanisms. 19 Degenerative conditions can also alter the pressure; for instance, Jayson et al described the disappearance of the venous flow due to disc prolapse. 44 The absence of BVV may be linked to differences in blood flow direction, as well as the varying proportions of dorsal and ventral abscesses between BVV+ and BVV- cases.
This study has several limitations. It is unclear what MRI sequence is the best to detect the BVV. We used T1 sequences with contrast, but the BVV was also seen with STIR or T245,46 sequences or even T1 without contrast. CT scanner is another alternative option to detect BVV pedicle. 47 The use of contrast was not possible for several patients with renal failure, leading to their exclusion from the study. The nature of the vessel seen is not clear either: it could be the vein or the artery, as these vessels are very small and close to each other. The detection of the vessel also depends on the quality of the MRI, the artifact movements, and the thickness of the slices. Osteomyelitis and edema might also have disrupted the detection of BVV. Physiological and pathological situations change the aspect of the bone marrow,48,49 which may modify the detection of the BVV. As described above, the intravertebral plexus is dynamic, so detection could be inconstant. From a methodological point of view, observer 1 was not blinded to the treatment outcome because he selected eligible patients with adequate MRI, which could have introduced bias. There was a probable lack of power, especially when analyzing the more refined scale with no/moderate/strong BVV. The lack of power may also explain why some usual risk factors did not reach significance, such as diabetes.1,31 For instance, the optimal total number of patients to adequately explore a difference between 11% and 19% for diabetes, with an alpha risk of 5% and a power of 80%, is roughly 130. Finally, the outcome definition (conservative or surgical treatment) was not perfectly controlled. All the patients received medical treatment first, so surgery was automatically considered a failure. However, we did not accurately describe the medical treatment, for instance, the time between the first symptoms and the first antibiotic therapy or between the start of the antibiotic therapy and surgery. Some patients might have been restricted from surgery and considered palliative because they were too fragile.
Conclusion
We evaluated epidural vascularity through the basivertebral vessels. The BVV was a radiological sign with a good inter-rater agreement, and its presence was significantly associated with a lower rate of medical treatment failure. Dorsal abscesses were more frequently observed in patients with strong BVV. The use of the simplest scale, BVV+/BVV + provided a good combination with a good agreement and a good positive predictive value for BVV + predicting successful conservative treatment. Although the spinal vascular anatomy has been well-known for over 100 years, there are still very few studies on its pathophysiological implications. This study confirms the need for further investigations into spinal vascularization and its relationship with treatment response in pyogenic spinal infection. We suspect that poorer spinal vascularity was associated with poorer antibiotic penetrance and, hence, poorer infection control. This hypothesis remains to be confirmed and could lead to new diagnostic and therapeutic options.
ORCID iDs
Baptiste Boukebous https://orcid.org/0000-0002-7361-8392
Joseph F. Baker https://orcid.org/0000-0002-8518-8780
References
- 1.Tetsuka S, Suzuki T, Ogawa T, Hashimoto R, Kato H. Spinal epidural abscess: a review highlighting early diagnosis and management. JMA J. 2020;3(1):29-40. doi: 10.31662/jmaj.2019-0038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwab JH, Shah AA. Spinal epidural abscess: diagnosis, management, and outcomes. J Am Acad Orthop Surg. 2020;28(21):e929-e938. doi: 10.5435/JAAOS-D-19-00685 [DOI] [PubMed] [Google Scholar]
- 3.Xiong GX, Nguyen A, Hering K, Schoenfeld AJ. Long-term quality of life and functional outcomes after management of spinal epidural abscess. Spine J. 2023;24(23):759-767. doi: 10.1016/j.spinee.2023.11.019 [DOI] [PubMed] [Google Scholar]
- 4.Yagi M, Fujita N, Okada E, et al. Impact of frailty and comorbidities on surgical outcomes and complications in adult spinal disorders. Spine (Phila Pa 1976). 2018;43(18):1259-1267. doi: 10.1097/BRS.0000000000002596 [DOI] [PubMed] [Google Scholar]
- 5.Vettivel J, Bortz C, Passias PG, Baker JF. Pyogenic vertebral column osteomyelitis in adults: analysis of risk factors for 30-day and 1-year mortality in a single center cohort study. Asian Spine J. 2019;13(4):608-614. doi: 10.31616/asj.2018.0295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lener S, Wipplinger C, Lang A, Hartmann S, Abramovic A, Thomé C. A scoring system for the preoperative evaluation of prognosis in spinal infection: the MSI-20 score. Spine J. 2022;22(5):827-834. doi: 10.1016/j.spinee.2021.12.015 [DOI] [PubMed] [Google Scholar]
- 7.Hunter S, Cussen R, Baker JF. Predictors of failure for nonoperative management of spinal epidural abscess. Global Spine J. 2021;11(1):6-12. doi: 10.1177/2192568219887915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karhade AV, Shah AA, Bono CM, et al. Development of machine learning algorithms for prediction of mortality in spinal epidural abscess. Spine J. 2019;19(12):1950-1959. doi: 10.1016/j.spinee.2019.06.024 [DOI] [PubMed] [Google Scholar]
- 9.Pluemer J, Freyvert Y, Pratt N, et al. A novel scoring system concept for de novo spinal infection treatment, the Spinal Infection Treatment Evaluation Score (SITE Score): a proof-of-concept study. J Neurosurg Spine. 2022;38:396-404. doi: 10.3171/2022.11.SPINE22719 [DOI] [PubMed] [Google Scholar]
- 10.Boukebous B, Petrie L, Baker JF. Keeping it simple: developing a prognostic tool for spinal epidural abscess. Global Spine J. 2023;17:21925682231221497. doi: 10.1177/21925682231221497 [DOI] [PubMed] [Google Scholar]
- 11.Sircar K, Jung N, Kernich N, et al. Risk factors for neurologic deficits in patients with spinal epidural abscess: an analysis of one-hundred-forty cases. Global Spine J. 2023. Online ahead of print. doi: 10.1177/21925682231194467 [DOI] [PubMed] [Google Scholar]
- 12.Batson O. The function of the vertebral veins and their rôle in the spread of metastases - pmc. Ann Surg. 1940;112(1):138-149. doi: 10.1097/00000658-194007000-00016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaynes P, Verdié JC, Moscovici J, Zadeh J, Vaysse P, Becue J. Microsurgical anatomy of the internal vertebral venous plexuses. Surg Radiol Anat. 1998;20(1):47-51. doi: 10.1007/bf01628115 [DOI] [PubMed] [Google Scholar]
- 14.Ratcliffe JF. The arterial anatomy of the adult human lumbar vertebral body: a microarteriographic study. J Anat. 1980. [PMC free article] [PubMed] [Google Scholar]
- 15.Hyrtl J. Lehrbuch der Anatomie des Menschen : mit Rücksicht auf physiologische Begründung und praktische Anwendung. Braumüller. https://wellcomecollection.org/works/krejv86u. Accessed 16 January 2024. [Google Scholar]
- 16.Wagoner G, Pendergrass EP. Intrinsic circulation of the vertebral body. With roentgenologic considerations. Am J Roentgenol. 1932;27:818-826. [Google Scholar]
- 17.Harris RS, Jones DM. The arterial supply to the adult cervical vertebral bodies. J Bone Joint Surg Br. 1956;38-B(4):922-927. doi: 10.1302/0301-620X.38B4.922 [DOI] [PubMed] [Google Scholar]
- 18.Markhashov AM. Variations in the arterial blood supply of the spine. Vestn Khir Im I I Grek. 1965;94(1):64-74. [PubMed] [Google Scholar]
- 19.Stringer MD, Restieaux M, Fisher AL, Crosado B. The vertebral venous plexuses: the internal veins are muscular and external veins have valves. Clin Anat. 2012;25(5):609-618. doi: 10.1002/ca.21281 [DOI] [PubMed] [Google Scholar]
- 20.Green K, Reddy V, Hogg JP. Neuroanatomy, spinal cord veins. In: StatPearls [Internet]. StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK542182/ [PubMed] [Google Scholar]
- 21.van der Kuip M, Hoogland PV, Groen RJ. Human radicular veins: regulation of venous reflux in the absence of valves. Anat Rec. 1999;254(2):173-180. doi: 10.1002/(SICI)1097-0185 [DOI] [PubMed] [Google Scholar]
- 22.Rannou F, Mayoux-Benhamou MA, Poiraudeau S, Revel M. Disque intervertébral et structures voisines de la colonne lombaire : anatomie, biologie, physiologie et biomécanique. EMC Rhumatol-Orthopédie. 2004;1(6):487-507. doi: 10.1016/j.emcrho.2003.11.007 [DOI] [Google Scholar]
- 23.Jackson AR, Eismont A, Yu L, et al. Diffusion of antibiotics in intervertebral disc. J Biomech. 2018;76:259-262. doi: 10.1016/j.jbiomech.2018.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thabit AK, Fatani DF, Bamakhrama MS, Barnawi OA, Basudan LO, Alhejaili SF. Antibiotic penetration into bone and joints: an updated review. Int J Infect Dis. 2019;81:128-136. doi: 10.1016/j.ijid.2019.02.005 [DOI] [PubMed] [Google Scholar]
- 25.Hanberg P, Bue M, Birke Sørensen H, Søballe K, Tøttrup M. Pharmacokinetics of single-dose cefuroxime in porcine intervertebral disc and vertebral cancellous bone determined by microdialysis. Spine J. 2016;16(3):432-438. doi: 10.1016/j.spinee.2015.11.031 [DOI] [PubMed] [Google Scholar]
- 26.Frankel HL, Hancock DO, Hyslop G, et al. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. I. Paraplegia. 1969;7(3):179-192. doi: 10.1038/sc.1969.30 [DOI] [PubMed] [Google Scholar]
- 27.Basivertebral veins - an overview | ScienceDirect Topics. Accessed November 16, 2022. https://www.sciencedirect.com/topics/neuroscience/basivertebral-veins [Google Scholar]
- 28.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159-174. [PubMed] [Google Scholar]
- 29.Harrell FE. Regression modeling strategies: with applications to linear models. In: Logistic and Ordinal Regression, and Survival Analysis. Springer International Publishing; 2015. doi: 10.1007/978-3-319-19425-7 [DOI] [Google Scholar]
- 30.Hunter S, Ou C, Baker JF. Early reduction in C-reactive protein following treatment for spinal epidural abscess: a potential treatment guide. Global Spine J. 2023;14:1296-1303. doi: 10.1177/21925682221139801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel AR, Alton TB, Bransford RJ, Lee MJ, Bellabarba CB, Chapman JR. Spinal epidural abscesses: risk factors, medical versus surgical management, a retrospective review of 128 cases. Spine J. 2014;14(2):326-330. doi: 10.1016/j.spinee.2013.10.046 [DOI] [PubMed] [Google Scholar]
- 32.Cornett CA, Vincent SA, Crow J, Hewlett A. Bacterial spine infections in adults: evaluation and management. J Am Acad Orthop Surg. 2016;24(1):11-18. doi: 10.5435/JAAOS-D-13-00102 [DOI] [PubMed] [Google Scholar]
- 33.Samy DA, Gandham S, DeMatas M. The diagnosis and management of discitis and spinal infection. Surgery. 2021;39(8):540-546. doi: 10.1016/j.mpsur.2021.07.001 [DOI] [Google Scholar]
- 34.Graeber A, Cecava ND. Vertebral osteomyelitis. In: StatPearls. StatPearls Publishing; 2023. Accessed 16 January 2024. [PubMed] [Google Scholar]
- 35.Mehkri Y, Felisma P, Panther E, Lucke-Wold B. Osteomyelitis of the spine: treatments and future directions. Infect Dis Res. 2022;3(1):3. doi: 10.53388/idr20220117003 [DOI] [Google Scholar]
- 36.Morikawa M, Sato S, Numaguchi Y, Mihara F, Rothman MI. Spinal epidural venous plexus: its MR enhancement patterns and their clinical significance. Radiat Med. 1996;14(5):221-227. [PubMed] [Google Scholar]
- 37.Ju JH, Ha HG, Jung CK, Kim HW, Lee CY, Kim JH. Patterns of epidural venous varicosity in lumbar stenosis. Korean J Spine. 2012;9(3):244-249. doi: 10.14245/kjs.2012.9.3.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slin’ko EI, Al-Qashqish II. Surgical treatment of lumbar epidural varices. J Neurosurg Spine. 2006;5(5):414-423. doi: 10.3171/spi.2006.5.5.414 [DOI] [PubMed] [Google Scholar]
- 39.Vernon JC, Durand A, Guevar J, et al. Vertebral venous system abnormalities identified with magnetic resonance imaging in sighthounds. Vet Radiol Ultrasound. 2017;58(4):399-410. doi: 10.1111/vru.12492 [DOI] [PubMed] [Google Scholar]
- 40.Mizuno J, Nakagawa H. Ossified posterior longitudinal ligament: management strategies and outcomes. Spine J. 2006;6(6 Suppl):282S-288S. doi: 10.1016/j.spinee.2006.05.009 [DOI] [PubMed] [Google Scholar]
- 41.Modic MT, Steinberg PM, Ross JS, Masaryk TJ, Carter JR. Degenerative disk disease: assessment of changes in vertebral body marrow with MR imaging. Radiology. 1988;166(1 Pt 1):193-199. doi: 10.1148/radiology.166.1.3336678 [DOI] [PubMed] [Google Scholar]
- 42.Laffosse JM, Accadbled F, Molinier F, Bonnevialle N, de Gauzy JS, Swider P. Correlations between effective permeability and marrow contact channels surface of vertebral endplates. J Orthop Res. 2010;28(9):1229-1234. doi: 10.1002/jor.21137 [DOI] [PubMed] [Google Scholar]
- 43.Capossela S, Bertolo A, Gunasekera K, Pötzel T, Baur M, Stoyanov JV. VEGF vascularization pathway in human intervertebral disc does not change during the disc degeneration process. BMC Res Notes. 2018;11(1):333. doi: 10.1186/s13104-018-3441-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoyland JA, Freemont AJ, Jayson MI. Intervertebral foramen venous obstruction. A cause of periradicular fibrosis? Spine (Phila Pa 1976). 1989;14(6):558-568. doi: 10.1097/00007632. [DOI] [PubMed] [Google Scholar]
- 45.Le Ster C, Gambarota G, Lasbleiz J, Guillin R, Decaux O, Saint-Jalmes H. Breath-hold MR measurements of fat fraction, T1 , and T2 * of water and fat in vertebral bone marrow. J Magn Reson Imaging. 2016;44(3):549-555. doi: 10.1002/jmri.25205 [DOI] [PubMed] [Google Scholar]
- 46.Maeder Y, Dunet V, Richard R, Becce F, Omoumi P. Bone marrow metastases: T2-weighted dixon spin-echo fat images can replace T1-weighted spin-echo images. Radiology. 2018;286(3):948-959. doi: 10.1148/radiol.2017170325 [DOI] [PubMed] [Google Scholar]
- 47.Spinal vascular anatomy. Accessed August 1, 2021. https://www.neurosurgicalatlas.com/volumes/neuroradiology/spinal-cord-disorders/spinal-vascular-anatomy
- 48.Nouh MR, Eid AF. Magnetic resonance imaging of the spinal marrow: basic understanding of the normal marrow pattern and its variant. World J Radiol. 2015;7(12):448-458. doi: 10.4329/wjr.v7.i12.448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Geith T, Stellwag AC, Baur-Melnyk A. [Posttherapeutic changes in bone marrow]. Radiologe. 2017;57(11):958-963. doi: 10.1007/s00117-017-0300-5 [DOI] [PubMed] [Google Scholar]