Abstract
Tofacitinib is a targeted JAK inhibitor used to treat rheumatoid arthritis. Despite some recent safety concerns, it is considered effective and safe with appropriate patient selection. Between May 2015 and May 2024, data were retrospectively analyzed from 112 patients with a diagnosis of RA in a tertiary care hospital who had received tofacitinib for at least 1 month, with or without prior biologic DMARDs. The mean disease duration was 12 years, and the median duration of tofacitinib use was 32.5 months. The p‐value for all disease activity parameters evaluated for effectiveness between the 1st‐ and 3rd‐month visits was <0.001, except CRP (p = 0.097). Adverse events occurred in 15 (13.4%) patients, with an incidence rate of 4.54 per 100 patient‐years. Observed were one myocardial infarction (0.3/100 patient‐years), two pulmonary embolisms (0.6/100 patient‐years), three herpes zoster (HZ) (0.9/100 patient‐years), and one basal cell carcinoma (BCC) (0.3/100 patient‐years). Median drug‐free survival was 68 (95% CI: 54.8–81.2) months. The drug was discontinued in 28 (25%) patients due to ineffectiveness and in 13 (11.6%) due to side effects. A significant difference in drug survival rates was observed between patients who had not previously used bDMARDs and those who had received at least one bDMARD before tofacitinib (p < 0.001). Drug survival was 46.35 months in the prior bDMARD group and 71.09 months in the bDMARD‐naive group. This study found significant reductions in disease activity indices at 3 and 6 months after starting tofacitinib, with sustained effectiveness. Although adverse event rates were somewhat higher than reported in the literature, tofacitinib can be used effectively and safely in appropriate patient populations for RA treatment.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by inflammatory synovitis and joint degeneration. Tofacitinib, a targeted synthetic Disease‐Modifying Antirheumatic Drug (tsDMARD), is used to treat RA patients with moderate to severe activity, showing efficacy in clinical trials. Real‐world data on the long‐term effectiveness and safety of tofacitinib is essential for understanding its broader clinical impact.
WHAT QUESTION DID THIS STUDY ADDRESS?
This study aimed to evaluate the effectiveness, safety, and drug survival of tofacitinib in RA patients treated at a tertiary center over approximately 9 years.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
The study confirmed significant reductions in disease activity indices at the 3rd and 6th months of tofacitinib treatment, with no loss of effectiveness by the 6th month. The most common adverse events were herpes zoster (HZ) and pulmonary embolism (PE), with an overall adverse event rate of 4.54 per 100 patient‐years. Drug survival analysis indicated that tofacitinib was more effective in patients who had not previously received biologic Disease‐Modifying Antirheumatic Drugs (bDMARDs), with a median survival time of 68 months.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
This study underscores the importance of real‐world data in assessing the long‐term safety and efficacy of tofacitinib. The findings suggest that tofacitinib is a viable treatment option for RA, especially in patients who are naïve to biologic Disease‐Modifying Antirheumatic Drugs (bDMARDs), providing clinicians with valuable insights into its use in diverse populations. The study highlights the need for continuous monitoring and evaluation of tofacitinib's safety profile, particularly concerning cardiovascular events and infections.
INTRODUCTION
Rheumatoid arthritis (RA) is a chronic, progressive autoimmune disease characterized by inflammatory synovitis and joint degeneration. The cornerstone of treatment RA, which often affects women, is to prevent destruction by controlling inflammation in the joint. To this end, conventional synthetic Disease‐Modifying Antirheumatic Drugs (csDMARDs) [methotrexate (MTX), leflunomide, sulfasalazine, hydroxychloroquine (HCQ)], biologic Disease‐Modifying Antirheumatic Drugs (bDMARDs) (tumor necrosis factor‐alpha inhibitors (anti‐TNF‐alpha), tosilizumab, abatasept, rituximab), and targeted synthetic Disease‐Modifying Antirheumatic Drugs (tsDMARDs) (tofacitinib, baricitinib, upadatisinib) are used in RA treatment. 1 , 2 , 3
The Janus Kinase (JAK) family, which includes JAK‐1, JAK‐2, and JAK‐3, as well as tyrosine kinase 2, is required for the signaling pathways of various cytokines and growth factors implicated in the pathogenesis of RA. These cytokines and growth factors are essential to lymphocyte function, and inhibiting their signaling modulates multiple aspects of the immune response (e.g., IL‐2, IL‐6, IL‐7, IL‐12, IL‐15, IL‐17, IL‐23, granulocyte‐macrophage colony‐stimulating factor, and interferon [IFN]). Tofacitinib, a tsDMARD, preferentially inhibits signaling by receptors associated with JAK3 as the primary target, with JAK1 and JAK2 also affected at higher concentrations due to a debilitating effect on signaling pathways with nanomolar potency, resulting in the inhibition of Signal Transducer and Activator of Transcription (STAT) molecules' transmigration to the nucleus. 4 , 5 It is used in patients with moderately to severely active RA at a dose of 5 mg twice daily or 11 mg as a single daily dose. 6 Efficacy and safety evaluations have been conducted for tofacitinib in the ORAL Start, ORAL Solo, ORAL Strategy, ORAL Scan, ORAL Standard, ORAL Sync, and ORAL Step trials in patients with methotrexate naïve, inadequate response to methotrexate or biologic therapies. 7 Treatment with tofacitinib was approved after phase trials were shown to be effective and safe. However, as with any approved and marketed medical treatment, data on long‐term effectiveness and safety should have been analyzed in post‐marketing studies. For this reason, detailed assessments were conducted with scientific studies based on real‐life experience, particularly safety data. The first major studies to present real‐world experience came from the United States of America (USA), where tofacitinib has been used since 2012, but there are also studies from Japan, Switzerland, Latin America, Argentina, Russia, Taiwan, China, Colombia, Canada, Israel, Turkey, and Australia. 8 , 9 Tofacitinib for the treatment of RA was approved by the FDA (United States Food and Drug Administration) in November 2012 and by the EMA (European Medicines Agency) in March 2017. 10 In Turkey, it has been approved for the treatment of RA since May 2015.
Although there are numerous studies on the use of tofacitinib, the value of real‐world data remains critical for a comprehensive understanding of its long‐term effects. In this study, we present 9 years of real‐world data from RA patients treated with tofacitinib in a tertiary care center, evaluating the drug's effectiveness, safety, and survival. This analysis provides meaningful insights into the management of rheumatoid arthritis and contributes to the growing body of evidence supporting tofacitinib's use in clinical practice.
MATERIALS AND METHODS
Study design
This was a retrospective, single‐center observational study. Data were analyzed from patients aged >18 years who were followed up in a tertiary care hospital between May 2015 and May 2024 with a diagnosis of RA, who had or had not previously received bDMARDs, and who had received tofacitinib 5 mg twice daily or tofacitinib 11 mg once daily for at least 1 month. The parameters analyzed were age, sex, age at diagnosis, disease duration, time from symptom onset to diagnosis, body mass index, smoking status, serologic tests [Rheumatoid Factor (RF) and Anti‐Cyclic Citrullinated Peptide Antibody (ACPA) positivity], organ and system involvement, comorbidities [diabetes mellitus (DM), hypertension (HT), chronic kidney disease (CKD), coronary artery disease (CAD), heart failure (HF), hyperlipidemia, cerebrovascular accident, cardiac arrhythmias, pulmonary embolism (PE), pulmonary arterial hypertension (PAH), asthma, chronic obstructive pulmonary disease (COPD), hypothyroidism, psoriasis, etc.)], previously used bDMARDs and csDMARDs, duration of tofacitinib use, type of tofacitinib use (as monotherapy or together with csDMARDs such as MTX), preference for tofacitinib among DMARDs other than csDMARDs, reasons for tofacitinib discontinuation, side effects of tofacitinib, and steroid use during the switch to tofacitinib were retrospectively evaluated. Patient Global Assessment (PtGA), Physician Global Assessment (PGA), Visual Analog Scale (VAS), VAS Pain, VAS Fatigue, Simplified Disease Activity Index (SDAI), Clinical Disease Activity Index (CDAI), Health Assessment Questionnare (HAQ), Disease Activity Score‐28‐C reactive protein (DAS‐28‐CRP), DAS28‐ ESR (Erythrocyte Sedimentation Rate), Swollen Joint Count (SJC), Tender Joint Count (TJC), C reactive protein (CRP), and Erythrocyte Sedimentation Rate (ESR) data were analyzed. These data were obtained from the TURKBIO and TReasure registries.
Patient population
We retrospectively analyzed the records of a total of 132 patients whose diagnosis was confirmed by the 2010 American College of Rheumatology (ACR)/European Alliance of Associations for Rheumatology (EULAR) RA classification criteria. Six patients diagnosed with seronegative RA and taking tofacitinib for a period of time were diagnosed with psoriatic arthritis (PsA) during follow‐up. Among the remaining 126 patients, one additional patient with seronegative RA was diagnosed with inflammatory bowel disease associated with peripheral spondyloarthritis. Thirteen patients were excluded from the study because of missing data. The remaining 112 patients were enrolled in the study. Patients taking the drug for at least 6 months were included in the effectiveness evaluation. Since 22 of the 112 patients discontinued tofacitinib treatment before 6 months and/or missing data were available for effectiveness evaluation, 90 patients could be used for effectiveness evaluation (Figure 1).
FIGURE 1.
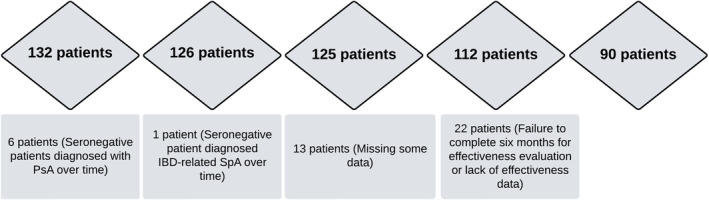
Flow chart for study design.
Statistical analysis
Descriptive statistics were used for the sociodemographic, clinical, and laboratory parameters of the patients. After the normal distribution of the quantitative data was determined by Shapiro–Wilk and Kolmogorov–Smirnov tests, the quantitative data that did not conform to the normal distribution were reported as median and Interquartile range (IQR) values, whereas the data that conformed to the normal distribution were reported as mean ± standard deviation, minimum, maximum. For patient effectiveness assessment, Friedman test was used to determine whether there was a relationship between the data PtGA, PGA, VAS Pain, VAS Fatigue, SDAI, CDAI, HAQ, DAS28‐CRP, DAS28‐ESR, SJC, TJC, CRP, and ESR at 1st, 3rd, and 6th months. Then 0th–3rd month visit, 0th–6th month visit, and 3rd–6th month visit, the Wilcoxon signed‐rank test was used. Bonferroni correction was performed for three separate comparisons of paired groups, and a new significant p‐value <0.0167 was accepted. The factors associated with the development of adverse events were initially evaluated using univariate logistic regression analysis to identify potential correlations between individual variables and adverse outcomes. Variables that reached statistical significance in the univariate analysis were subsequently included in a multivariate logistic regression model. This multivariate approach was employed to control for confounding variables and to determine independent predictors of adverse events. Given that multivariate logistic regression provides a more comprehensive and accurate assessment of independent risk factors, the emphasis in the results has been placed on the findings from this analysis to enhance the clarity and interpretability of the prognostic factors. Drug survival analysis was performed using the Kaplan–Meier method. Both the difference between anti‐TNFα‐naive patients and patients who had received anti‐TNFα at least once previously and the relationship between the groups receiving tofacitinib as monotherapy and the groups receiving tofacitinib in combination with leflunomide or methotrexate were compared using Cox regression analysis. p‐Value <0.05 was accepted as the limit of statistical significance. IBM SPSS Statistical software (version 28.0.0.0, IBM Corp., Armonk, NY) was used for all statistical analyses.
Ethics statement
The study protocol was approved by the Clinical Research Ethics Committee of Uludağ University Faculty of Medicine (Decision number: 2022‐3/30).
RESULTS
Basic characteristics of patients
The demographic characteristics of a total of 112 patients with RA, who received tofacitinib 2 × 5 mg/day or 11 mg/day for at least 1 month, are shown in Table 1. When comorbidity data were analyzed, a total of 78 (69.6%) patients had at least one comorbidity. The most common comorbidity was HT at 51.8%. Other comorbidities included hyperlipidemia (27.7%), DM (25.9%), cardiovascular disease (25%), asthma (25%), COPD (10.7%), cardiac arrhythmias (8.9%), HF (6.3%), peripheral arterial disease (2.7%), cerebrovascular disease (1.8%), CKD (1.8%), and lung disease HT (0.9%). To diagnose hyperlipidemia, patients were considered to have received anti‐hyperlipidemic treatment at least once (Table 1).
TABLE 1.
Baseline characteristics of patients using tofacitinib (n = 112).
| Age (years), Mean ± SD (Min., Max.) | 58.01 ± 12.50 (30, 84) |
| Gender, Female/Male (%) | 95/17 (84.8/15.2) |
| Age at diagnosis (years), Mean ± SD (Min., Max.) | 43.81 ± 14.14 (12, 79) |
| Disease duration (years), Median (IQR) | 12 (13) |
| Duration from symptom onset to diagnosis, Median (IQR) | 12 (4.75) |
| BMI (kg/m2), Median (IQR) | 28.06 (8.36) |
| Obesity, n (%) (BMI ≥ 30) | 44 (39.3) |
| Smoking status | |
| Never smoked, n (%) | 70 (62.5) |
| Ex‐smoker, n (%) | 17 (15.2) |
| Active smoker, n (%) | 25 (22.3) |
| Seropositivity, n (%) | 84 (75.0) |
| RF positivity, n (%) | 68 (60.7) |
| ACPA positivity, n (%) | 74 (66.1) |
| Both RF and ACPA positivity, n (%) | 58 (51.8) |
| History of malignancy before tofacitinib, n (%) | 2 (1.8) |
| Colon cancer, n (%) | 1 (0.9) |
| Breast cancer, n (%) | 1 (0.9) |
| Secondary Sjögren, n (%) | 8 (7.1) |
| System and/or organ involvement, n (%) | 6 (5.4) |
| Lung involvement, n (%) | 6 (5.4) |
| Interstitial lung disease, n (%) | 5 (4.5) |
| Rheumatoid nodule, n (%) | 1 (0.9) |
Abbreviations: ACPA, anti‐cyclic citrullinated peptide antibodies; BMI, body mass index; IQR, interquartile range; Max., maximum; Min., minimum; RF, rheumatoid factor; SD, standart deviation.
Data on drug use
Among non‐csDMARDs, tofacitinib was the first choice in 61 (54.5%) patients, second choice in 29 (25.9%), third choice in 12 (10.7%), and fourth choice or later in 9 (8%). The median duration of tofacitinib use was 32.5 months. Forty‐seven (42%) patients received tofacitinib as monotherapy, 30 (27.0%) patients MTX + tofacitinib, and 35 (31.0%) patients tofacitinib + leflunomide. The median number of csDMARDs taken prior to tofacitinib treatment was three. MTX and leflunomide were used before tofacitinib treatment in 106 (94.6%) and 89 (79.5%) patients, respectively. When analyzing the use of bDMARDs prior to treatment with tofacitinib, it was found that 48 (42.8%) patients were taking at least one bDMARD, with anti‐TNFs being the most commonly used bDMARDs in 50 (44.64%) patients. Besides anti‐TNF agents, Tocilizumab (15.2%) was the most frequently used bDMARD. In terms of usage frequency among anti‐TNF agents, it was as follows: Adalimumab 15 (13.4%) patients, etanercept 13 (11.6%) patients, sertolizumab pegol 9 (8%) patients, golimumab 9 (8%) patients, infliximab 4 (3.6%) patients. Other bDMARDs used prior to treatment with tofacitinib were rituximab 13 (11.6%) patients, and abatasept 8 (7.1%) patients. At the time of switch to tofacitinib, 80 (71.4%) patients were taking steroids, and the median prednisolone dose was 5 mg.
Primary ineffectiveness refers to the lack of a therapeutic response from the outset of treatment, meaning the drug did not achieve the desired clinical outcomes in the patient. Secondary ineffectiveness indicates that the drug initially worked but lost its effectiveness over time, leading to a recurrence or worsening of symptoms. The drug was discontinued in 6 (5.4%) patients because of primary ineffectiveness, in 22 (19.6%) because of secondary ineffectiveness, and in 13 (11.6%) because of adverse events. In addition to primary ineffectiveness, secondary ineffectiveness, and the development of some adverse events, other reasons for discontinuing tofacitinib included two patients who discontinued treatment due to pneumonia caused by COVID‐19 infection and acute coronary syndrome, respectively. One patient with pulmonary involvement had tofacitinib discontinued and was switched to rituximab. Furthermore, two patients (1.8%) voluntarily discontinued the treatment, and one patient discontinued due to pregnancy. Additionally, one patient discontinued tofacitinib following a diagnosis of basal cell carcinoma (BCC) during follow‐up (Table 2).
TABLE 2.
Usage characteristics of tofacitinib (n = 112).
| Duration of tofacitinib use (months), Median (IQR) | 32.5 (43.5) |
| Order of preference for tofacitinib among DMARDs other than csDMARD, n (%) | |
| First line | 61 (54.5) |
| Second line | 29 (25.9) |
| Others | 22 (19.6) |
| Tofacitinib usage, n (%) | 112 (100) |
| Monotherapy, n (%) | 47 (42.0) |
| With MTX, n (%) | 30 (26.8) |
| With leflunomide, n (%) | 35 (31.2) |
| Reason for tofacitinib discontinuation | |
| Primary ineffectiveness, n (%) | 6 (5.4) |
| Secondary ineffectiveness, n (%) | 22 (19.6) |
| Development of side effects, n (%) | 13 (11.6) |
| Others, n (%) | 7 (6.3) |
| Number of csDMARDs used before, median (IQR) | 3 (1) |
| Number of bDMARDs used before, median (IQR) | 0 (1) |
| Previous bDMARDs usage, n (%) | |
| Anti‐TNFs | 50 (44.64) |
| Adalimumab | 15 (13.4) |
| Golimumab | 9 (8.0) |
| İnfliksimab | 4 (3.6) |
| Certolizumab pegol | 9 (8.0) |
| Etanercept | 13 (11.6) |
| Tocilizumab | 17 (15.2) |
| Abatacept | 8 (7.1) |
| Rituximab | 13 (11.6) |
| Steroid usage during transition to tofacitinib, n (%) | 80 (71.4) |
| Prednisolone dose used to switch to tofacitinib, median (IQR) | 5 (5) |
Abbreviations: bDMARDs, biologic disease‐modifying antirheumatic drugs; csDMARDs, conventional disease‐modifying antirheumatic drugs; IQR, interquartile range; MTX, methotrexate.
Safety data
Adverse events occurred in 15 patients (13.4%), and the rate of adverse events was 4.54 per 100 patient‐years. One patient experienced a myocardial infarction (MI) as a major cardiovascular event (MACE) (0.3/100 patient‐years). Two patients developed PE due to thrombotic processes (0.6/100 patient‐years). A total of 11 patients (3.33/100 patient‐years) developed drug‐related infections. The most common infections were herpes zoster (HZ) (0.9/100 patient‐years) and urinary tract infections (0.9/100 patient‐years), each occurring in three patients. In addition, pneumonia developed in two patients (0.6/100 patient‐years), cellulitis in two patients (0.6/100 patient‐years), and tuberculous meningitis in one patient (0.3/100 patient‐years). In addition, one patient (0.3/100 patient‐years) developed gastrointestinal symptoms (GI) such as nausea and dyspepsia, while another patient had elevated liver enzymes.
Treatment was discontinued in two patients who developed PE and one patient who experienced MI thrombotic processes. With the exception of one patient who had frequent acute cystitis infections but benefited from tofacitinib treatment and was symptom‐free during the past year on prophylactic antibiotherapy, treatment was discontinued in all other patients with infection‐related adverse events (tuberculous meningitis, cellulitis, HZ, resistant urinary tract infection requiring intravenous antibiotic treatment, and pneumonia). Another patient with GI symptoms and intolerance to tofacitinib was also discontinued. In one patient with elevated liver enzyme levels, aspartate aminotransferase (AST) and alanine aminotransferase(ALT) were less than three times the upper normal limit, and treatment was not discontinued. The tofacitinib dose was halved (1 × 5 mg/day), and the levels of AST and ALT were normalized after 2 weeks. When the tofacitinib treatment dose was increased to 2 × 5 mg/day in patients, no liver function test increase was observed. To develop a model that could predict the occurrence of adverse events, factors that were clinically associated with adverse events and could be included in the model were first identified using univariate logistic regression analysis. When factors influencing the development of side effects were evaluated by univariate logistic regression analysis, gender (p = 0.044, OR: 3.542, 95% CI: 1.033–12.141), duration of tofacitinib use (p = 0.011, OR: 0.961, 95% CI: 0.932–0.991), steroid use during the transition to tofacitinib (p = 0.028, OR: 0.288, 95% CI: 0.094–0.877) and comorbidities HT (p = 0.028, OR: 4.435, 95% CI: 1.177–16.708), CAD (p = 0.044, OR: 3.167, 95% CI: 1.030–9.740), HF (p = 0.032, OR: 5.812, 95% CI: 1.158–29. 171), cardiac arrhythmias (atrial fibrillation) (p = 0.018, OR: 5.515, 95% CI: 1.345–22.621) had a statistically significant impact on the development of adverse events. In a multivariate logistic regression analysis with these factors, gender (p = 0.012, OR: 8.889, 95% CI: 1.623–48.691), duration of tofacitinib use (p = 0.002, OR: 0.943, 95% CI: 0.908–0.979), HT (p = 0. 009, OR: 8.541, 95% CI: 1.712–42.603) and steroid use during the switch to tofacitinib (p = 0.015, OR: 0.164, 95% CI: 0.038–0.703) had a significant and independent impact on the development of adverse events (Table 3).
TABLE 3.
Factors affecting infection‐related side effects in RA patients receiving tofacitinib (n = 112).
| Univariate logistic regression | Multivariate logistic regression | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p‐Value | OR | 95% CI | p‐Value | |
| Age (years), Mean ± SD | 1.005 | 0.962–1.051 | 0.809 | |||
| Gender, Female/Male (%) | 3.542 | 1.033–12.141 | 0.044 | 8.889 | 1.623–48.691 | 0.012 |
| Disease duration (years), Median | 1.009 | 0.941–1.083 | 0.799 | |||
| Duration from symptom onset to diagnosis, Median | 1.014 | 0.996–1.033 | 0.120 | |||
| BMI (kg/m2), Median | 1.060 | 0.971–1.167 | 0.167 | |||
| Obesity, n (%) (BMI ≥ 30) | 1.937 | 0.648–5.788 | 0.237 | |||
| Smoking status, n (%) | 1.316 | 0.380–4.558 | 0.665 | |||
| Count of csDMARDs use before tofacitinib, Median | 0.842 | 0.430–1.650 | 0.617 | |||
| Seropositivity, n (%) | 0.622 | 0.193–2.005 | 0.426 | |||
| RF positivity, n (%) | 0.705 | 0.236–2.105 | 0.531 | |||
| ACPA positivity, n (%) | 0.527 | 0.179–1.613 | 0.268 | |||
| Both RF and ACPA positivity, n (%) | 0.577 | 0.191–1.746 | 0.330 | |||
| Duration of tofacitinib use (months), Median | 0.961 | 0.932–0.991 | 0.011 | 0.943 | 0.908–0.979 | 0.002 |
| Presence of comorbid diseases, n (%) | 3.200 | 0.681–15.042 | 0.141 | |||
| HT, n (%) | 4.435 | 1.177–16.708 | 0.028 | 8.541 | 1.712–42.603 | 0.009 |
| DM, n (%) | 2.145 | 0.690–6.667 | 0.187 | |||
| CAD, n (%) | 3.167 | 1.030–9.740 | 0.044 | |||
| HF, n (%) | 5.812 | 1.158–29.171 | 0.032 | |||
| HL, n (%) | 1.648 | 0.446–6.090 | 0.454 | |||
| CRD, n (%) | 6.857 | 0.405–115.960 | 0.182 | |||
| COPD, n (%) | 2.444 | 0.580–10.308 | 0.223 | |||
| Asthma, n (%) | 1.106 | 0.322–3.799 | 0.873 | |||
| Peripheral arterial disease, n (%) | 3.393 | 0.288–39.918 | 0.331 | |||
| Pulmonary embolism, n (%) | 10.000 | 0.580–172.362 | 0.113 | |||
| Cardiac arrhythmia, n (%) | 5.515 | 1.345–22.621 | 0.018 | |||
| Lung involvement, n (%) | 1.314 | 0.143–12.094 | 0.809 | |||
| Count of bDMARDs use before tofacitinib, median | 0.845 | 0.496–1.440 | 0.535 | |||
| Steroid usage during transition to tofacitinib, n (%) | 0.288 | 0.094–0.877 | 0.028 | 0.164 | 0.038–0.703 | 0.015 |
| Prednisolone dose used to switch to tofacitinib, median | 0.944 | 0.805–1.106 | 0.473 | |||
Note: p < 0.05 was accepted as the limit of statistical significance, it has been italicized.
Abbreviations: ACPA, anti‐cyclic citrullinated peptide antibodies; bDMARDs, biologic disease‐modifying antirheumatic drugs; BMI, body mass index; CAD, coronary artery disease; CI, confidence interval; COPD, chronic obstructive pulmonary disease; CRD, chronic renal disease; csDMARDs, conventional synthetic disease‐modifying antirheumatic drugs; DM, diabetes mellitus; HF, heart failure; HL, hyperlipidaemia; HT, hypertension; OR, odds ratio; RF, rheumatoid factor; RTX, rituximab; SD, standart deviation.
Drug survival
In the Kaplan–Meier analysis performed to represent drug survival, the median drug survival time was 68 months (95% CI: 54.8–81.2) (Figure 2). When analyzing the drug survival of patients who did not receive bDMARDs and patients who had previously received at least one bDMARD, the drug survival of patients who did not receive bDMARDs was 71.09 ± 4.49 (95% CI: 62.289–79.894) months, while the drug survival of patients who had previously received at least one bDMARD was 46.35 ± 4.71 (95% CI: 37.117–55.590) months. The Log‐rank analysis revealed a statistically significant difference between the two groups (p < 0.001) (Figure 3). When comparing the drug survival times of patients receiving tofacitinib as monotherapy with those of patients receiving tofacitinib plus MTX or leflunomide, the drug survival times of patients receiving tofacitinib as monotherapy was 50.33 ± 5.57 (95% CI: 39.414–61.238) months, while the drug survival of patients receiving tofacitinib plus methotrexate or leflunomide was 58.82 ± 4.41 (95% CI: 50.183–67.465) months, and log‐rank analysis showed no statistically significant difference between the two groups (p = 0.835) (Figure 4).
FIGURE 2.
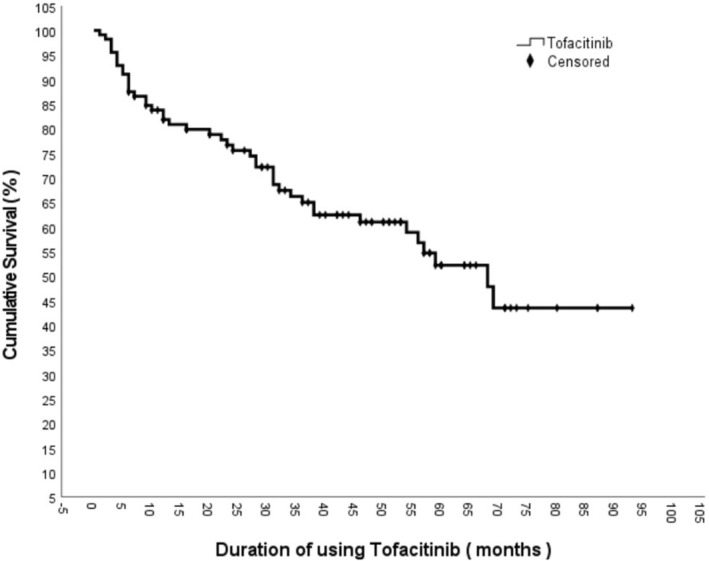
Tofacitinib survival curve.
FIGURE 3.
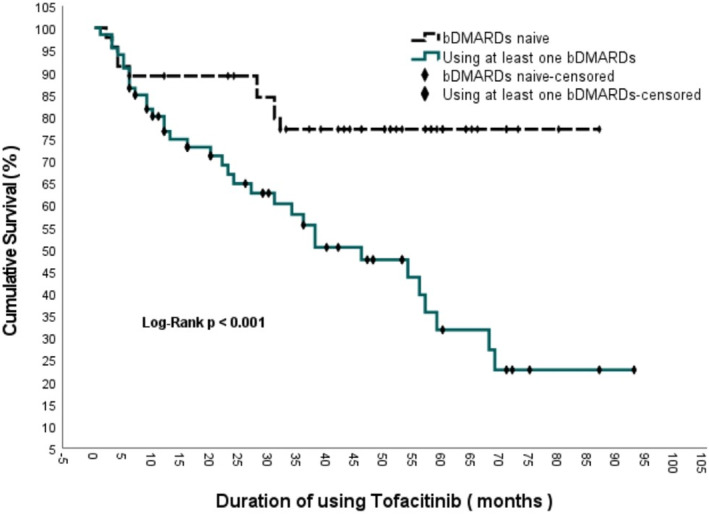
Comparison of survival with tofacitinib treatment in bDMARD‐naive patients and patients taking at least one bDMARD.
FIGURE 4.
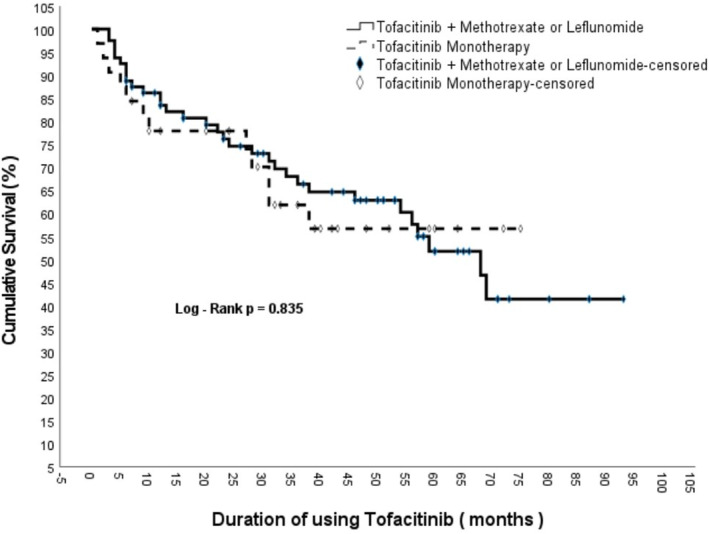
Comparison of drug survival rate between tofacitinib monotherapy and tofacitinib + methotrexate treatment.
Drug effectiveness
To evaluate the effectiveness of tofacitinib, parameters such as PtGA, PGA, VAS Pain, VAS Fatigue, SDAI, CDAI, HAQ, DAS28‐CRP, DAS28‐ESR, SJC, TJC, CRP, and ESR were assessed at the third and sixth month visits after the first visit. The Friedman test was used to examine whether there was a significant association between these parameters, which were examined at the 0, 3, and 6 month visits and were not normally distributed. Except for CRP (p = 0.284) and ESR (p = 0.060), the p‐value of all other parameters <was 0.001 and statistically significant. Next, the relationship between 1st month and 3rd month, 3rd month and 6th month, and 1st month and 6th month was analyzed in paired groups. A statistically significant p‐value of 0.0167 was considered for the three separate comparisons with Bonferroni correction. The p‐value of all parameters analyzed for effectiveness evaluation between the first visit and the 3rd‐month visit was <0.001, except for CRP (p = 0.097). Although the change in the median value of CRP was not statistically significant, there was a decrease. A statistically significant decrease was observed in the median values of all other parameters. When comparing the 1st visit with the visit in 6th month, a statistically significant decrease was observed in all parameters (p < 0.001). Although the CRP level decreased, it did not reach statistical significance (p = 0.022). When comparing the 3rd month to 6th month, a minimal increase in SDAI (p = 0.684), DAS28‐ ESR (p = 0.290), and CRP (p = 0.458) was noted, although none of these values were statistically significant. There was a decrease in all other parameters, but it was not statistically significant. As a result, it can be said that there were significant decreases in the disease activity indexes in both the 3rd and 6th month visits compared to the first visit, and there was no increase in the 6th month compared to the 3rd month, suggesting a loss of activity (Table S1).
DISCUSSION
In our study, we analyzed 9 years of data from 112 patients with RA, who were treated with tofacitinib in a tertiary care hospital. We found that disease activity indexes significantly decreased in the 3rd and 6th months after initiation of tofacitinib treatment compared with baseline, and there was no loss of effectiveness at the end of the sixth month. In addition, one MI, two PE, three HZ and one BCC were observed during this treatment. Of these adverse events, PE and HZ were more frequent and MACE and non‐melanoma skin cancer (BCC) were less frequent than reported in the literature.
In a review from Spain, the mean age of patients ranged from 43.7 ± 12.2 years to 61.2 ± 13.2 years, the proportion of female patients ranged from 58% to 94.4%, and the mean disease duration ranged from 8.7 ± 6.5 years to a median of 18 (P25, P75; 9–22) years. 3 , 11 The data in our study were consistent with the literature. The most common comorbidities in our study were HT (51.8%), dyslipidemia (27.7%), DM (25.9%), cardiovascular disease (25%), and asthma (25%). In two studies reviewed, comorbidity rates were reported as dyslipidemia (32.5% and 39.4%, respectively), HT (30% and 30.3%, respectively), and DM (15% and 9.1%, respectively). 3
In a prospective observational study from Japan evaluating the efficacy of tofacitinib, ACPA positivity was 87.6%. 12 In another study, ACPA positivity was 87.2% and RF positivity was 79.7%. 11 In our study, ACPA positivity was 66.1% and RF positivity was 60.7%, lower than reported in the literatüre. In a Canadian study evaluating 3 years of experience with tofacitinib in patients with RA, the rate of patients taking one or more bDMARDs before treatment with tofacitinib was 67%, and the mean number of bDMARDs was 2.6 ± 1.6. In the same study, the most frequently used bDMARDs were abatacept (13.1%), etanercept (12.8%), and tocilizumab (12.4%), respectively. 12 In our study, the proportion of patients who had previously taken at least one bDMARD was 45.5%, and the median number of bDMARDs was 0.78 ± 1.18 (median 0). The most commonly non‐anti‐TNF bDMARDs used bDMARDs in our study were tocilizumab (15.2%). Among anti‐TNF agents, the most commonly used bDMARDs were adalimumab (13.4%), and etanercept (11.6%). The usage rate of RTX was 11.6%. In our study, the rate of bDMARD use before tofacitinib and the number of bDMARDs used were lower than those reported in the literature. We can say that anti‐TNF drugs and tocilizumab in particular are used more frequently in the treatment of RA. In a prospective observational study conducted in Israel comparing the actual efficacy of tofacitinib with other bDMARDs, tofacitinib was the first choice in 17% of 139 tofacitinib users. 11 In our study, tofacitinib was the first choice among DMARDs other than csDMARDs in 54.5% of patients.
In a study of 70 patients who started treatment with tofacitinib between 2013 and 2016, the mean DAS28‐ESR score was significantly lower at week 4 compared with baseline (p < 0.0001), and this efficacy of tofacitinib persisted over 24 weeks. The proportion of patients achieving remission increased significantly for each clinical index from baseline to week 24 (DAS28‐ESR: 0% to 21.4%, SDAI: 0% to 26.1%, CDAI: 0% to 20.3%). Health Assessment Questionnaire‐Disability Index (HAQ‐DI) Values decreased from 0.3 (0–1.0) at baseline to 0.1 (0–0.88) at week 24 (median (interquartile range), p < 0.05). 13 In a prospective observational study conducted in Japan evaluating 113 patients treated with tofacitinib, the CDAI50 response was successful in 57.5% of patients initially treated with tofacitinib in combination with methotrexate or as monotherapy, whereas 42.5% showed no CDAI50 response. 14 We can say that our trial data are generally consistent with the literature, the drug is effective from the first month and effectiveness persists at the end of the sixth month.
In our study, drug median survival was 68 (95% CI: 54.8–81.2) months and 82.1% of patients were taking tofacitinib at the end of 12 months and 76.7% were taking tofacitinib at the end of 24 months. There was a statistically significant difference in survival between patients not taking bDMARDs and patients who had previously taken at least one bDMARD (p < 0.001). In the Canadian study, the survival rate of tofacitinib was 62.7% at 1 year (365 days) and 49.6% at 2 years (730 days). Drug survival at 2 years was 55.8% in patients receiving no biologics, while drug survival at 2 years was 45.4% in patients receiving three or more bDMARDs. Median drug survival was approximately 722, > 730, 613, 667, and 592 days in bDMARD‐naive, post‐1 bDMARD, post‐2 bDMARD, and post‐≥3 bDMARD patients, respectively. 11 Including patients who had not previously taken bDMARDs, drug survival was higher in our study. The observed difference in drug survival between bDMARD‐naive patients and those who had previously received bDMARDs can be attributed to several factors. Patients who did not respond adequately to prior bDMARDs may represent a population with more treatment‐resistant disease, leading to shorter drug survival with tofacitinib as well. In contrast, bDMARD‐naive patients may have less severe or less refractory disease, allowing for a longer duration of tofacitinib efficacy. Additionally, patients previously treated with bDMARDs may have experienced side effects or diminishing efficacy over time, which could predispose them to earlier discontinuation of tofacitinib. This difference in drug survival highlights the importance of individualized treatment approaches in RA management, based on the patient's treatment history. In our study, there was no statistically significant difference in drug survival between patients receiving tofacitinib monotherapy and patients receiving methotrexate or leflunomide in combination with tofacitinib (p = 0.835). In a prospective observational study conducted in Israel with real‐world data from RA patients treated with tofacitinib, it was shown that concomitant use of methotrexate did not affect event‐free survival with tofacitinib (HR: 1.18, p = 0.138, 95% CI: 0.95–1.48). 11
When considering tofacitinib‐related adverse events, 13.4% (15/112) of patients experienced at least one adverse event (4.54/100 patient‐years). When analyzing the post‐marketing safety database for tofacitinib from 2012 to 2015, predominantly from the United States (73.9%), 4352 (17.1%) serious adverse events and 102 (0.4%) fatal cases were reported in a total of 34,223 patient‐years with tofacitinib. The distribution of serious adverse events per 100 patient‐years was 2.57 infections, 0.91 GI‐related symptoms, 0.60 respiratory disease, 0.45 neoplasms, 0.43 cardiac disease, and 0.12 hepatobiliary disease. Skin cancer (except melanoma) was the most common neoplasm and was observed in 16 cases and lymphoma in 15 cases during the 3‐year surveillance period. 9 In the randomized controlled ORAL surveillance study assessing malignancy risk, the overall malignancy rate was 4.19% (122/2911), breast cancer 0.74% (7/2293), lymphoma 0.34% (10/2911), lung cancer 1.03% (30/2911), prostate cancer 1.46% (9/618), melanoma 0.07% (2/2911), colorectal cancer 0.27% (8/2911), pancreatic cancer 0.14% (4/2911), non‐melanoma skin cancer 2.2% (64/2911). 15 In our study, BCC was observed in only one patient. In the ORAL surveillance study involving 4362 patients over 50 years of age who were taking tofacitinib and had at least one risk factor for cardiovascular disease and had been treated with methotrexate in the past, it was found that the risk of major adverse cardiovascular events was higher in the tofacitinib group compared with TNF inhibitors. 16 , 17 In the Consortium of Rheumatology Researchers of North America Rheumatoid Arthritis (CORRONA RA) registry in the United States, tofacitinib was not associated with an increased risk of major adverse cardiovascular events (MI, stroke, transient ischemic attack, cardiovascular death) compared with bDMARDs (TNF‐i or non‐TNF‐i). 18 The most common infections observed in our study were HZ (0.9/100 patient‐years) and urinary tract infections (0.9/100 patient‐years). Other infections were pneumonia (0.6/100 patient‐years), cellulitis (0.6/100 patient‐years), and tuberculous meningitis (0.3/100 patient‐years). In a 9.5‐year review of global clinical trials, the most common infectious adverse events were nasopharyngitis, upper respiratory tract infections, and urinary tract infections. Serious infections included pneumonia, HZ, urinary tract infections, and cellulitis. The overall rate of severe and nonsevere HZ was 3.9/100 patient‐years, the rate of multidermatic severe HZ (53/6194) was 0.3/100 patient‐years, and the rate of tuberculosis (36/6194) was 0.2/100 patient‐years. 19 In our study, a slightly higher rate of drug‐associated infections was observed (3.33/100 patient‐years) than in the literature, whereas the HZ infection rate was lower and MI had a lower rate of cardiovascular adverse events at 0.3/100 patient‐years. In one study, PE was 0.1/100 patient‐years (28/7061 patients) in RA patients taking tofacitinib. 20 In our study, PE was observed in 0.6/100 patient‐years (2/112 patients), which was higher than that reported in the literature. There were 0.3/100 patient‐years (1/112 patients) with elevated liver enzymes. In a previous study, the rate of patients with elevated liver enzymes was 1.7% (48/2882). 19 In our study, female sex, presence of hypertension, long duration of tofacitinib use, and absence of steroids at the time of switch to tofacitinib were associated with the occurrence of adverse events.
In a Canadian study evaluating experience with tofacitinib, tofacitinib was discontinued in 1226/3678 patients at the end of a 3‐year follow‐up period. Inefficacy (35.7%; 438/1226 patients) and side effects (26.9%; 330/1226 patients) were the most common reasons for treatment discontinuation. 11 In our study, the most common reasons for drug discontinuation were ineffectiveness (25%; 28/112 patients) and side effects (9.8%; 11/112 patients) which were consistent with the literature.
Our limitations were that our study was a single centered, retrospective study and the number of patients was small. One of the notable limitations of our study is the absence of a control group. Without a direct comparison to a placebo or another treatment group, it is difficult to fully assess the relative efficacy and safety of tofacitinib in comparison to other available therapies. This limitation reduces the ability to generalize the findings across broader patient populations or different treatment settings. The lack of a control group also restricts the capacity to attribute observed changes in disease activity solely to the effects of tofacitinib, as other uncontrolled factors may have influenced the outcomes. However, we believe that the real‐world nature of our data still provides important insights into the long‐term safety and efficacy of tofacitinib in rheumatoid arthritis (RA) patients, which is valuable for clinicians making treatment decisions in similar populations.
In our study, approximately 9 years of single‐center real‐life experience of tofacitinib used in RA patients was evaluated. Although its effectiveness has been shown in most studies, we think that tofacitinib, which has safety concerns such as HZ, MACE, embolism‐thrombosis and cancer development, will have a more predictable preference process in terms of safety, especially as real‐life data increase in different populations. The higher frequency of PE and HZ in our cohort could be related to the underlying comorbidities of the patients, such as cardiovascular disease and immunosuppression, both of which can increase the risk of these adverse events. Additionally, prolonged tofacitinib exposure in a real‐world setting, compared to shorter clinical trial durations, may explain the higher incidence of these adverse events. Differences in preventive measures, such as HZ vaccination, may also play a role. On the contrary, the lower frequency of MACE and BCC may be due to the characteristics of our patient population, including potentially lower baseline cardiovascular risk profiles or lower cumulative exposure to risk factors for skin cancer. These discrepancies highlight the importance of individualized patient monitoring and the need for further studies to validate our findings in larger, more diverse cohorts.
AUTHOR CONTRIBUTIONS
A.E., S.M., and B.N.C. wrote the manuscript; A.E., Y.P., and E.D. designed the research; A.E., S.İ., and B.Y. performed the research; A.E. and S.M. analyzed the data; A.E., S.M., and B.N.C. contributed new reagents/analytical tools.
FUNDING INFORMATION
No funding was received for this work.
CONFLICT OF INTEREST STATEMENT
The authors declared no competing interests for this work.
Supporting information
Table S1
Ekin A, Misirci S, İldemir S, et al. Efficacy and safety of tofacitinib in rheumatoid arthritis: Nine years of real‐world data. Clin Transl Sci. 2024;17:e70084. doi: 10.1111/cts.70084
DATA AVAILABILITY STATEMENT
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. O'Shea JJ, Laurence A, IB MI. Back to the future: oral targeted therapy for RA and other autoimmune diseases. Nat Rev Rheumatol. 2013;9(3):173‐182. doi: 10.1038/nrrheum.2013.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Smolen JS, Landewé RBM, Bergstra SA, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease‐modifying antirheumatic drugs: 2022 update. Ann Rheum Dis. 2023;82(1):3‐18. doi: 10.1136/ard-2022-223356 [DOI] [PubMed] [Google Scholar]
- 3. Román Ivorra JA, Llevat N, Montoro M. Real‐world evidence of tofacitinib in rheumatoid arthritis patients in Spain. Drug Discov Ther. 2022;16(2):63‐71. doi: 10.5582/ddt.2022.01028 [DOI] [PubMed] [Google Scholar]
- 4. Meyer DM, Jesson MI, Li X, et al. Anti‐inflammatory activity and neutrophil reductions mediated by the JAK1/JAK3 inhibitor, CP‐690,550, in rat adjuvant‐induced arthritis. J Inflamm. 2010;7(1):41. doi: 10.1186/1476-9255-7-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kawalec P, Sladowska K, Malinowska‐Lipien I, Brzostek T, Kózka M. European perspective on the management of rheumatoid arthritis: clinical utility of tofacitinib. Ther Clin Risk Manag. 2017;14:15‐29. doi: 10.2147/TCRM.S138677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee EB, Fleischmann R, Hall S, et al. Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med. 2014;370(25):2377‐2386. doi: 10.1056/NEJMoa1310476 [DOI] [PubMed] [Google Scholar]
- 7. Dhillon S. Tofacitinib: a review in rheumatoid arthritis. Drugs. 2017;77(18):1987‐2001. doi: 10.1007/s40265-017-0835-9 [DOI] [PubMed] [Google Scholar]
- 8. Bertoldi I, Caporali R. Tofacitinib: real‐world data and treatment persistence in rheumatoid arthritis. Open Access Rheumatol Res Rev. 2021;13:221‐237. doi: 10.2147/OARRR.S322086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Caporali R, Zvaglia D. Real‐world experience with tofacitinib for the treatment of rheumatoid arthritis. Clin Exp Rheumatol. 2019;37(3):485‐495. [PubMed] [Google Scholar]
- 10. Alzahrani Z, Alhazmi A, Almalki H, Aljehani N, Dumyati M, Alabdali H. Efficacy and safety of tofacitinib in rheumatoid arthritis (RA): a retrospective study from two centers in Jeddah, Saudi Arabia. Cureus. 2022;14(12):e32240. doi: 10.7759/cureus.32240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shouval A, Lidar M, Reitblat T, et al. Real‐world effectiveness of tofacitinib in patients with rheumatoid arthritis: a prospective observational study. Clin Exp Rheumatol. 2022;39(6):1378‐1384. doi: 10.55563/clinexprheumatol/do2uxu [DOI] [PubMed] [Google Scholar]
- 12. Hirose W, Harigai M, Amano K, et al. Real‐world effectiveness and safety of tofacitinib and abatacept in patients with rheumatoid arthritis. Rheumatol Adv Pract. 2022;6(3):rkac090. doi: 10.1093/rap/rkac090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Iwamoto N, Tsuji S, Takatani A, et al. Efficacy and safety at 24 weeks of daily clinical use of tofacitinib in patients with rheumatoid arthritis. PLoS One. 2017;12(5):e0177057‐e0177057. doi: 10.1371/journal.pone.0177057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mori S, Yoshitama T, Ueki Y. Tofacitinib therapy for rheumatoid arthritis: a direct comparison study between biologic‐naïve and experienced patients. Intern Med. 2018;57(5):663‐670. doi: 10.2169/internalmedicine.9341-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Curtis JR, Yamaoka K, Chen YH, et al. Malignancy risk with tofacitinib versus TNF inhibitors in rheumatoid arthritis: results from the open‐label, randomised controlled ORAL Surveillance trial. Ann Rheum Dis. 2022;82(3):331‐343. doi: 10.1136/ard-2022-222543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mease P, Charles‐Schoeman C, Cohen S, et al. Incidence of venous and arterial thromboembolic events reported in the tofacitinib rheumatoid arthritis, psoriasis and psoriatic arthritis development programmes and from real‐world data. Ann Rheum Dis. 2020;79(11):1400‐1413. doi: 10.1136/annrheumdis-2019-216761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Charles‐Schoeman C, Buch MH, Dougados M, et al. Risk of major adverse cardiovascular events with tofacitinib versus tumour necrosis factor inhibitors in patients with rheumatoid arthritis with or without a history of atherosclerotic cardiovascular disease: a post hoc analysis from ORAL Surveillance. Ann Rheum Dis. 2023;82(1):119‐129. doi: 10.1136/ard-2022-222259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kremer JM, Bingham CO 3rd, Cappelli LC, et al. Postapproval comparative safety study of tofacitinib and biological disease‐modifying Antirheumatic drugs: 5‐year results from a United States‐based rheumatoid arthritis registry. ACR Open Rheumatol. 2021;3(3):173‐184. doi: 10.1002/acr2.11232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mimori T, Harigai M, Atsumi T, et al. Post‐marketing surveillance of tofacitinib in Japanese patients with rheumatoid arthritis: an interim report of safety data. Arthritis Rheumatol. 2017;69(Suppl 10). https://acrabstracts.org/abstract/post‐marketing‐surveillance‐of‐tofacitinib‐in‐japanese‐patients‐with‐rheumatoid‐arthritis‐an‐interim‐report‐of‐safety‐data/. Accessed October 21, 2024. [Google Scholar]
- 20. Cohen SB, Tanaka Y, Mariette X, et al. Long‐term safety of tofacitinib up to 9.5 years: a comprehensive integrated analysis of the rheumatoid arthritis clinical development programme. RMD Open. 2020;6(3):e001395. doi: 10.1136/rmdopen-2020-001395 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.


