Abstract
Background
Posterior cerebral arteries with acute ischemic strokes (PCA-AISs) comprise around 2% of all acute ischemic strokes and may result in significant long-term deficits. Current guidance regarding endovascular thrombectomy (EVT) for PCA-AIS is insufficient as no published randomized trials exist.
Methods
An analysis of the National Inpatient Sample database compared medical management versus EVT for PCA-AIS. Propensity score matching was applied to adjust for nonrandomization.
Results
The study included 19,655 patients. Before matching, the EVT cohort had significantly higher National Institutes of Health Stroke Scale (NIHSS) (10.21 vs. 4.67, p < 0.001), had lower rates of favorable functional outcomes, functional independence, and higher rates of intracranial hemorrhage (ICH) and inpatient mortality. After matching, no differences in functional outcomes were identified, but revealed a higher proportion of ICH in the EVT group (17.45% vs. 8.98%, p < 0.001). However, NIHSS subgroup analysis identified improved functional outcomes associated with the EVT group who presented with an NIHSS between 10 and 19 both in terms of rates of favorable functional outcomes (35.56% vs. 12.09%, p < 0.001) and rates of functional independence (26.67% vs. 9.34%, p < 0.01). On further investigation, the clinical benefit, in the NIHSS 10-19 subgroup, was driven by patients receiving EVT in combination with intravenous thrombolysis (IVT).
Conclusions
This analysis shows that current national practices utilize EVT for more severe PCA strokes. Clinical benefit was only detected in patients with moderate stroke severity (NIHSS 10-19) who were treated with combined EVT and IVT. Further work is needed to investigate the features of PCA-AIS that might benefit from EVT the most.
Keywords: Posterior cerebral artery stroke, mechanical thrombectomy, national inpatient sample, acute ischemic stroke
Introduction
Isolated posterior cerebral artery (PCA) occlusion strokes account for ∼ 2% of acute ischemic stroke (AIS) patients and are marked by unique clinical presentations and potential long-term impairments.1,2 In the acute phase, patients often exhibit mild-to-moderate deficits and generally have relatively low admission scores on the National Institutes of Health Stroke Scale (NIHSS). While PCA occlusion patients tend to appear less debilitated on admission than those suffering anterior circulation large vessel occlusion, the condition can still be highly debilitating, with over a third to one-half of affected individuals unable to live independently after 3 months. 3 This underlines the pressing need to refine management strategies.
Current evidence for endovascular thrombectomy (EVT) of PCA occlusions lacks strength. Guidelines from the American Heart Association and American Stroke Association recommend consideration for EVT on a case-by-case basis. 4 Previous observational studies comparing medical management (MM) + EVT and MM alone have reported similar three-month functional outcomes for both treatment groups.3,5,6 In a recent observational study by Sabben et al., 7 EVT + MM showed no significant benefit compared to MM alone, with a non-significant trend towards lower odds of good functional outcomes and higher rates of symptomatic intracranial hemorrhage (sICH) and early neurologic deterioration.
In our study, we utilized the National Inpatient Sample (NIS) database to assess the effectiveness of MM versus EVT for treating PCA strokes. We further analyzed the effects of intravenous thrombolysis (IVT) on these outcomes with subgroup analysis. By analyzing a large representative dataset of inpatient admissions for PCA occlusion we hope to provide additional evidence to inform clinical decision-making in this specific population.
Methods
Data source
The NIS is the largest, national inpatient database in the United States, containing 7 million unweighted hospital admissions annually. It is developed and maintained by the Healthcare Cost and Utilization Project (HCUP) and comprised of de-identified inpatient hospitalization data derived from billing and discharge information of participating hospitals. Utilization of provided discharge weights reported by participating institutions allows for estimates of nationally representative statistics.
Patient selection
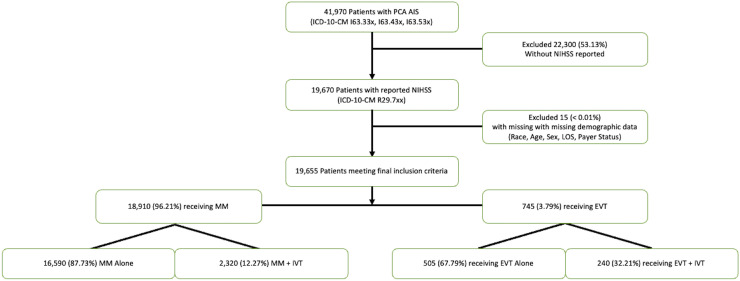
Weighted discharge NIS data from 2016 to 2020 was surveyed for patients admitted with primary International Classification of Disease, Tenth Version, (ICD-10) admission diagnosis of PCA stroke (cerebral infarction due to thrombosis, embolism, or unspecified occlusion or stenosis of the posterior cerebral artery – ICD-10-CM I63.33x, I63.43x, I63.53x) and a reported NIHSS (R29.7xx). Patients were excluded if there was a concomitant diagnosis code identifying an infarct of another vascular territory. Therefore, limiting the patient population to patients with only PCA or bilateral PCA infarcts. There were no patients included with a concomitant basilar artery occlusion. Patients were excluded if an NIHSS was not present. EVT procedural codes were then interrogated to determine treatment exposure. Those who did not receive EVT were assigned to the MM group. Additional diagnostic and procedural codes were utilized to identify whether the patient received IVT, developed intracerebral hemorrhage (ICH), or required other procedures. A flowchart depicting the patient selection process is shown in Figure 1.
Figure 1.
Flowchart of patient selection from the national inpatient sample.
Demographic and clinical characteristics
Baseline demographic data, provided by the NIS, was collected including age, sex, race, primary payer status, hospital location, and teaching status. Comorbidity burden was assessed by interrogating ICD-10-CM codes with diagnosis of diabetes, hypertension, congestive heart failure, coronary artery disease, atrial fibrillation, chronic kidney disease, and dyslipidemia. Stroke severity was determined strictly by baseline NIHSS.
Clinical end points
The primary clinical end point of this study was functional outcome, determined by discharge disposition. We utilized the administrative stroke outcome variable (ASOV), a dichotomous variable that has been validated and has shown substantial agreement with modified Rankin Score (mRS) > 3 at 90 days. 8 ASOV is categorized as a poor functional outcome if the patient was discharged to hospice care, long-term acute care, a skilled nursing facility, or suffered inpatient mortality. Favorable functional outcomes were defined as a routine discharge or discharge to a short-term rehabilitation hospital. In a sub-analysis, we analyzed rates of functional independence, defined as a routine discharge to home without assistance. This discharge disposition has been demonstrated to have a strong correlation with mRS ≤ 2 at 90 days.9,10 Secondary safety outcomes were the rates of ICH and intubation between the treatment groups.
Subgroups
For our primary analysis, we included all patients, regardless of NIHSS, with PCA stroke. For exploratory purposes, we performed secondary logistic regression subgroup analyses on patients with NIHSS ≥ 0–9, ≥ 10–19, and ≥ 20. Additional logistic regression analyses were performed comparing functional outcomes, ICH, and inpatient mortality between those receiving EVT with or without IVT both across all patients and within each NIHSS subgroup.
Statistical analysis
Statistical analyses were performed accounting for the sampling design of the NIS, with appropriate strata, weights, and clusters according to HCUP guidelines.
We used propensity score matching to test the differences in functional outcomes of those treated with EVT and MM accounting for the nonrandomized nature of the study. We matched patients based on comorbidities, age, gender, mechanical ventilation status, and NIHSS to address for confounding on the indication of EVT. We performed a full weighted matching with probit regression with the covariates. This resulted in adequate balance in all covariates in the matching process. Further, this method maintained the original sample size. To determine the effect of EVT on functional outcomes we performed univariate logistic regression models. We also performed logistic regression models comparing IVT with or without EVT to MM on the outcome variables.
Statistical analyses were conducted with R 4.2.2 (R Core Team, Vienna, Austria) and RStudio (Posit Software, Boston, MA, USA). Propensity matching was performed with the “MatchIt” package 11 and all complex survey samples, including regression analysis and statistical tests, were performed with the “Survey” package. 12 An alpha threshold of 0.05 was selected to define significance, with all p-values being two-sided. The data were organized into tables, including the available demographic, clinical, and prognostic data. Descriptive statistics were performed, including weighted means and frequencies. Survey-weighted t-tests and chi-squared analysis were performed to compare the means and association of categorical variables, respectively.
Results
Primary results of unmatched patients
There was a total of 19,655 patients, 18,910 in the MM group and 745 in the EVT group. NIHSS was significantly higher among the EVT cohort than MM (10.21 ± 7.26 vs. 4.67 ± 5.58, p < 0.001). There were no significant differences in baseline patient comorbidities. EVT patients had higher rates of mechanical ventilation than MM patients (26.85% vs. 2.54%, p < 0.001). IVT was more frequently administered in the EVT cohort than in the MM cohort (32.21% vs. 12.27%, p < 0.001). EVT patients had significantly lower rates of favorable functional outcomes and functional independence compared to the MM cohort, (30.87% vs. 39.79%, p = 0.03) and (26.17% vs. 38.08%, p < 0.001), respectively. The EVT cohort demonstrated significantly greater inpatient mortality (10.74% vs. 1.85%, p < 0.001) and rate of ICH (17.45% vs. 7.91%, p < 0.001) than the MM cohort. Full characteristics and outcomes of unmatched patients can be found in the Supplemental Material.
Primary results of matched patients
After propensity score matching the population sizes remained unchanged. There was no significant difference in NIHSS, demographic, or clinical characteristics such as age, gender, and various comorbidities, or intubation rates between the two matched groups. The EVT cohort had a higher rate of IVT use compared to the MM cohort (32.21% vs. 17.06%, p < 0.001). There were no differences in favorable functional outcomes or functional independence between the treatment groups. Inpatient mortality was not significantly different between the two groups. ICH was significantly more frequent in the EVT treatment group (17.45% vs. 8.98%, p < 0.001). See Table 1.
Table 1.
Demographic, comorbidities, and outcome measures for all patients (matched).
| Total cohort (n = 19,655) | MM (n = 18,910) | EVT (n = 745) | p value | |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age; mean years (SD) | 69.69 ± 14.40 | 69.69 ± 14.45 | 69.76 ± 13.36 | 0.957 |
| Female; n (%) | 7729 (39.3%) | 7419 (39.2%) | 310 (41.6%) | 0.605 |
| Stroke severity | ||||
| NIHSS; mean (SD) | 10.80 ± 8.90 | 10.82 ± 8.95 | 10.21 ± 7.26 | 0.427 |
| Coma; n (%) | 196 (1.0%) | 181 (1.0%) | 15 (2.0%) | 0.302 |
| Mechanical ventilation; n (%) | 4491 (22.9%) | 4291 (22.7%) | 200 (26.9%) | 0.346 |
| Comorbidities | ||||
| Diabetes mellitus; n (%) | 5535 (28.2%) | 5310 (28.1%) | 225 (30.2%) | 0.617 |
| Hypertension; n (%) | 11,178 (56.9%) | 10,793 (57.1%) | 385 (51.7%) | 0.231 |
| Congestive heart failure; n (%) | 4225 (21.5%) | 4045 (21.4%) | 180 (24.2%) | 0.483 |
| Coronary artery disease; n (%) | 7811 (39.7%) | 7551 (39.9%) | 260 (34.9%) | 0.283 |
| Atrial fibrillation; n (%) | 8788 (44.7%) | 8453(44.7%) | 335 (45.0%) | 0.956 |
| Chronic kidney disease; n (%) | 3039 (15.5%) | 2909 (15.4%) | 130 (17.5%) | 0.541 |
| Dyslipidemia; n (%) | 12,007 (61.1%) | 11,552 (61.1%) | 455 (61.1%) | 0.998 |
| Outcome measurements | ||||
| Favorable functional outcome (mRS ≤ 3); n (%) | 4984 (25.4%) | 4754 (25.1%) | 230 (30.9%) | 0.142 |
| Functional independence (mRS ≤ 2) | 4536 (23.1%) | 4341 (23.0%) | 195 (26.2%) | 0.385 |
| Inpatient mortality | 1663 (8.5%) | 1583 (8.4%) | 80 (10.7%) | 0.395 |
| Intracranial hemorrhage | 1829 (9.3%) | 1699 (9.0%) | 130 (17.5%) | 0.003 |
| Length of stay (mean days) (SD) | 7.61 ± 9.06 | 7.59 ± 8.83 | 8.25 ± 13.82 | 0.601 |
| tPA | 3466 (17.6%) | 3226 (17.1%) | 240 (32.2%) | <0.001 |
mRS: modified Rankin Scale; NIHSS: National Institute of Health Stroke Scale; tPA: tissue plasminogen activator; MM: medical management; EVT: endovascular thrombectomy.
Subgroup analyses
NIHSS ≥ 0-9
Among the matched patients treated with NIHSS ≥ 0-9, there were 16,395 patients in the MM group and 420 patients in the EVT group. The NIHSS was not significantly different between the two groups and baseline patient characteristics were matched. IVT usage was not significantly different between the EVT and MM groups (27.38% vs. 19.27%, p = 0.09). There was no difference in favorable functional outcomes or functional independence between the EVT and MM treatment groups. Neither inpatient mortality nor ICH were significantly different between the two groups. See Table 2.
Table 2.
Demographic, comorbidities, and outcome measures for NIHSS (0-9) patients (matched).
| Total cohort (n = 16,815) | MM (n = 16,395) | EVT (n = 420) | p value | |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age; mean years (SD) | 68.08 ± 14.46 | 68.09 ± 14.50 | 68.01 ± 13.11 | 0.963 |
| Female; n (%) | 6828 (40.6%) | 6653 (40.6%) | 175 (41.7%) | 0.853 |
| Stroke severity | ||||
| NIHSS; mean (SD) | 4.92 ± 2.63 | 4.92 ± 2.64 | 4.93 ± 2.56 | 0.976 |
| Coma; n (%) | 95 (0.6%) | 80 (0.5%) | 15 (3.6%) | 0.019 |
| Mechanical ventilation; n (%) | 2818 (16.8%) | 2748 (16.8%) | 70 (16.7%) | 0.985 |
| Comorbidities | ||||
| Diabetes mellitus; n (%) | 5120 (30.5%) | 4995 (30.5%) | 125 (29.8%) | 0.894 |
| Hypertension; n (%) | 10,160 (60.4%) | 9905 (60.4%) | 255 (60.7%) | 0.956 |
| Congestive heart failure; n (%) | 3507 (20.9%) | 3422 (20.9%) | 85 (20.2%) | 0.894 |
| Coronary artery disease; n (%) | 5289 (31.5%) | 5154 (31.4%) | 135 (32.1%) | 0.895 |
| Atrial fibrillation; n (%) | 6488 (38.6%) | 6333 (38.6%) | 155 (36.9%) | 0.772 |
| Chronic kidney disease; n (%) | 2191 (13.0%) | 2136 (13.0%) | 55 (13.1%) | 0.986 |
| Dyslipidemia; n (%) | 9772 (58.1%) | 9522 (58.1%) | 250 (59.5%) | 0.806 |
| Outcome measurements | ||||
| Favorable functional outcome (mRS ≤ 3); n (%) | 5450 (32.4%) | 5310 (32.4%) | 140 (33.3%) | 0.859 |
| Functional independence (mRS ≤ 2) | 5128 (30.5%) | 4993 (30.5%) | 135 (32.1%) | 0.748 |
| Inpatient mortality | 831 (5.0%) | 801 (4.9%) | 30 (7.1%) | 0.432 |
| Intracranial hemorrhage | 1815 (10.8%) | 1760 (10.7%) | 55 (13.1%) | 0.528 |
| Length of stay (mean days) (SD) | 6.62 ± 7.85 | 6.57 ± 7.49 | 8.50 ± 16.54 | 0.298 |
| tPA | 3274 (19.5%) | 3159 (19.3%) | 115 (27.4%) | 0.091 |
mRS: modified Rankin Scale; NIHSS: National Institute of Health Stroke Scale; tPA: tissue plasminogen activator; MM: medical management; EVT: endovascular thrombectomy.
NIHSS ≥ 10-19
Among the matched patients treated with NIHSS ≥ 10-19, there were 1860 patients in the MM group and 255 in the EVT group. The NIHSS was not significantly different between the two groups and baseline characteristics were matched. The EVT group had greater rates of IVT (47.06% vs. 23.02%, p < 0.01). The rates of favorable functional outcomes were significantly higher in the EVT group compared to the MM group (35.56% vs. 12.09%, p < 0.001). Rates of functional independence were also significantly higher in the EVT group compared to the MM (26.67% vs. 9.34%, p < 0.01). Neither inpatient mortality nor ICH rates were different between the two treatment groups. See Table 3.
Table 3.
Demographic, comorbidities, and outcome measures for NIHSS ≥ 10-19 patients (matched).
| Total cohort (n = 2085) | MM (n = 1860) | EVT (n = 255) | p value | |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age; mean years (SD) | 72.48 ± 13.63 | 72.53 ± 13.73 | 72.07 ± 12.93 | 0.850 |
| Female; n (%) | 892 (42.8%) | 797 (42.8%) | 95 (42.2%) | 0.945 |
| Stroke severity | ||||
| NIHSS; mean (SD) | 14.20 ± 3.05 | 14.23 ± 3.09 | 13.93 ± 2.69 | 0.582 |
| Coma; n (%) | 2 (0.1%) | 2 (0.1%) | 0 (0.00%) | 0.734 |
| Mechanical ventilation; n (%) | 662 (31.8%) | 592 (31.9%) | 70 (31.1%) | 0.933 |
| Comorbidities | ||||
| Diabetes mellitus; n (%) | 580 (27.8%) | 515 (27.7%) | 65 (28.9%) | 0.878 |
| Hypertension; n (%) | 846 (40.6%) | 751 (40.4%) | 95 (42.2%) | 0.832 |
| Congestive heart failure; n (%) | 661 (31.69%) | 586 (31.5%) | 75 (33.3%) | 0.830 |
| Coronary artery disease; n (%) | 809 (38.8%) | 709 (38.1%) | 100 (44.4%) | 0.470 |
| Atrial fibrillation; n (%) | 1149 (55.1%) | 1019 (54.8%) | 130 (57.8%) | 0.744 |
| Chronic kidney disease; n (%) | 389 (18.6%) | 344 (18.5%) | 45 (20.0%) | 0.828 |
| Dyslipidemia; n (%) | 1322 (63.4%) | 1172 (63.0%) | 150 (66.7%) | 0.665 |
| Outcome measurements | ||||
| Favorable functional outcome (mRS ≤ 3); n (%) | 305 (14.6%) | 225 (12.1%) | 80 (35.6%) | <0.001 |
| Functional independence (mRS ≤ 2) | 234 (11.2%) | 174 (9.3%) | 60 (26.7%) | 0.004 |
| Inpatient mortality | 319 (15.3%) | 289 (15.5%) | 30 (13.3%) | 0.758 |
| Intracranial hemorrhage | 279 (13.4%) | 229 (12.3%) | 50 (22.2%) | 0.118 |
| Length of stay (mean days) (SD) | 7.75 ± 8.44 | 7.73 ± 8.25 | 7.89 ± 9.97 | 0.920 |
| tPA | 533 (25.6%) | 428 (23.0%) | 105 (46.7%) | 0.006 |
mRS: modified Rankin Scale; NIHSS: National Institute of Health Stroke Scale; tPA: tissue plasminogen activator; MM: medical management; EVT: endovascular thrombectomy.
NIHSS ≥ 20
Among the matched patients treated with NIHSS ≥ 20, there were 655 patients in the MM group and 100 in the EVT group. NIHSS was not significantly different between the two groups and baseline characteristics were matched. IVT rates were not significantly different between the EVT and MM groups. There was no difference in functional independence or favorable functional outcomes between groups. There were no differences in inpatient mortality or ICH rates between the treatment groups. See Table 4.
Table 4.
Demographic, comorbidities, and outcome measures for NIHSS ≥ 20 patients (matched).
| Total cohort (n = 755) | MM (n = 655) | EVT (n = 100) | p value | |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age; mean years (SD) | 73.37 ± 13.58 | 73.59 ± 13.42 | 71.90 ± 14.90 | 0.633 |
| Female; n (%) | 275 (36.5%) | 235 (36.0%) | 40 (40.0%) | 0.759 |
| Stroke severity | ||||
| NIHSS; mean (SD) | 24.14 ± 3.08 | 24.16 ± 3.13 | 24.05 ± 2.76 | 0.876 |
| Coma; n (%) | 32 (4.2%) | 32 (4.9%) | 0 (0.0%) | 0.499 |
| Mechanical ventilation; n (%) | 443 (58.6%) | 383 (58.4%) | 60 (60.0%) | 0.901 |
| Comorbidities | ||||
| Diabetes mellitus; n (%) | 267 (35.4%) | 232 (35.4%) | 35 (35.0%) | 0.974 |
| Hypertension; n (%) | 166 (22.0%) | 146 (22.3%) | 20 (20.0%) | 0.845 |
| Congestive heart failure; n (%) | 214 (28.3%) | 189 (28.8%) | 25 (25.0%) | 0.739 |
| Coronary artery disease; n (%) | 421 (55.8%) | 371 (56.7%) | 50 (50.0%) | 0.598 |
| Atrial fibrillation; n (%) | 166 (22.0%) | 146 (22.3%) | 20 (20.0%) | 0.845 |
| Chronic kidney disease; n (%) | 227 (30.1%) | 197 (30.1%) | 30 (30.0%) | 0.992 |
| Dyslipidemia; n (%) | 417 (55.3%) | 362 (55.3%) | 55 (55.0%) | 0.979 |
| Outcome measurements | ||||
| Favorable functional Outcome (mRS ≤ 3); n (%) | 74 (9.9%) | 64 (9.8%) | 10 (10.0%) | 0.983 |
| Functional independence (mRS ≤ 2) | 11 (1.4%) | 11 (1.6%) | 0 (0.0%) | 0.454 |
| Inpatient mortality | 198 (26.2%) | 178 (27.2%) | 20 (20.0%) | 0.532 |
| Intracranial hemorrhage | 144 (19.0%) | 119 (18.1%) | 25 (25.0%) | 0.521 |
| Length of stay (mean days) (SD) | 9.55 ± 8.69 | 9.79 ± 8.81 | 8.00 ± 7.89 | 0.413 |
| tPA | 158 (20.9%) | 138 (21.0%) | 20 (20.0%) | 0.927 |
mRS: modified Rankin Scale; NIHSS: National Institute of Health Stroke Scale; tPA: tissue plasminogen activator; MM: medical management; EVT: endovascular thrombectomy.
IVT analyses
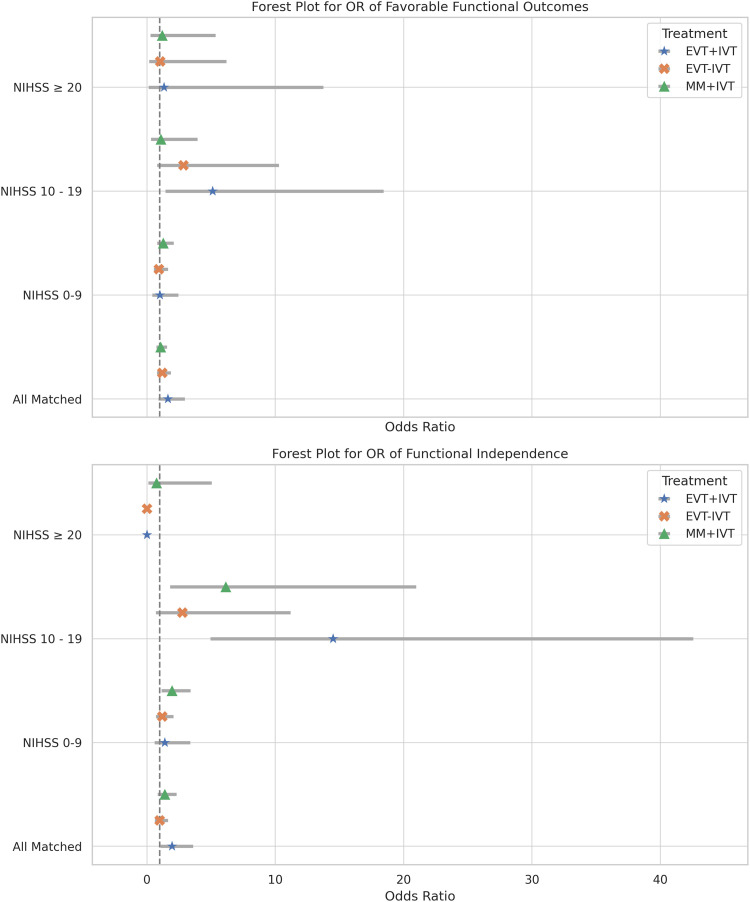
Further subgroup analysis was conducted as a logistic regression with MM without IVT (MM–IVT) as the reference group. Results of logistic regression analysis including all NIHSS and IVT subgroups can be found in Figure 2. The full tables of IVT subgroup comparisons can be found in the Supplemental Material.
Figure 2.
Forest plot of LR. The dashed line represents MM–IVT to which all other sub-groups were compared. The top plot shows the LR of favorable functional outcomes. The bottom plot shows the LR of functional independence. All NIHSS and IVT subgroups are displayed.
LR: logistic regressions; MM: medical management; NIHSS: National Institute of Health Stroke Scale; IVT: intravenous thrombolysis.
IVT subgroup: All matched
Compared to MM–IVT, patients treated with EVT and IVT (EVT + IVT) demonstrated significantly greater odds of achieving functional independence (OR = 1.95, 95% CI [1.06–3.60]; p = 0.03). Patients treated with MM and IVT (MM + IVT) and those treated with EVT without IVT (EVT–IVT) demonstrated significantly greater odds of ICH (OR = 2.25, 95% CI [1.10–5.95]; p = 0.03) and (OR = 1.85, 95% CI [0.07–3.20]; p = 0.03), respectively. No treatment group had higher odds of inpatient mortality compared to MM–IVT.
IVT subgroup: NIHSS ≥0-9
No treatment group was associated with higher odds of favorable functional outcomes compared to MM–IVT. Neither EVT–IVT nor EVT + IVT was associated with higher odds of functional independence. MM + IVT had significantly higher odds of functional independence compared to MM–IVT (OR = 1.96, 95% CI [1.13–3.40]; p = 0.017). Neither EVT–IVT nor EVT + IVT was associated with higher odds of functional independence compared to MM–IVT (OR = 1.190, 95% CI [0.69–2.05]; p = 0.53) and (OR = 1.40, 95% CI [0.59–3.37]; p = 0.65), respectively. No treatment group was associated with increased odds of inpatient mortality or ICH, however, MM + IVT was associated with lower odds of inpatient mortality (OR = 0.04, 95% CI [0.01–0.15]; p < 0.001).
IVT subgroup: NIHSS ≥ 10-19
EVT + IVT was associated with significantly higher odds of favorable functional outcome (OR = 5.13, 95% CI [1.42–18.44]; p = 0.01) compared to MM–IVT, whereas EVT–IVT did not meet significance (OR = 2.85, 95% CI [0.79–10.28]; p = 0.11). Similarly, EVT + IVT was significantly associated with higher odds of functional independence compared to MM–IVT (OR = 14.50, 95% CI [4.94–42.55]; p < 0.001) as was MM + IVT (OR = 6.15, 95% CI [1.80–20.98]; p < 0.01). EVT–IVT was not associated with higher odds of functional independence compared to MM–IVT (OR = 2.76, 95% CI [0.68–11.18]; p = 0.15). No treatment group was associated with increased odds of inpatient mortality or ICH.
IVT subgroup: NIHSS ≥ 20
No treatment group was associated with higher odds of favorable functional outcomes compared to MM–IVT. Both EVT + IVT and EVT–IVT were associated with lower odds of functional independence (OR = 6.77 × 10−8, 95% CI [2.08 × 10−8–2.20 × 10−7]; p < 0.001) and (OR = 6.77 × 10−8, 95% CI [1.67 × 10−8–2.74 × 10−7]; p < 0.001) relative to MM–IVT. MM + IVT was not associated with higher or lower odds of functional independence compared to MM–IVT (0.753 [0.113–5.030]; p = 0.767). EVT–IVT was associated with increased odds of inpatient mortality (OR = 3.12, 95% CI [1.14–8.55]; p = 0.028). No treatment group was associated with increased odds of ICH.
Discussion
Our analysis focused on comparing real-world outcomes of mechanical thrombectomy to medical management for patients with PCA stroke using a national cohort. There were a few key findings of note. Based on the unmatched data, EVT-treated patients had more severe strokes, were more likely to receive IVT, and had worse clinical outcomes, higher mortality, and higher rates of ICH than patients treated with MM. After statistically matching for comorbidities, ventilation status, and NIHSS, EVT failed to demonstrate a positive association with improved functional outcomes across all patients. However, on our subgroup analysis, the NIHSS 10-19 subgroup did demonstrate a clinical benefit in terms of favorable functional outcomes associated with EVT. Subsequent analysis indicated that the benefit was driven primarily by patients who received both IVT and EVT, with those undergoing EVT alone demonstrating a benefit that did not quite meet the threshold for significance.
There are currently no completed randomized controlled studies on EVT for PCA AIS with existing literature consisting of retrospective studies, often with limited case numbers.3,13 Our findings echo those from previous studies including the three most robust retrospective studies, the TOPMOST trial, 14 the study by Sabben et al., 7 and the recent PLATO study by Nguyen et al. 15 In the retrospective TOPMOST trial comparing endovascular therapy (EVT) versus MM for P2 and P3 occlusions, no association between EVT and enhanced clinical outcomes was observed across the entire patient cohort. Nonetheless, a subgroup analysis identified a correlation between EVT and improved discharge NIHSS scores in patients presenting with NIHSS ≥ 10. 14 Sabben et al. 7 studied EVT versus MM for P1 and P2 occlusions in a larger cohort and found no association between EVT and favorable outcomes in any subgroup. Moreover, EVT was linked to a nearly two-fold risk of sICH and an increased risk of early neurological deterioration. On subgroup analysis, the lower rates of favorable outcomes (mRS 0-2) at 90-day and elevated risk of sICH were isolated to patients with NIHSS < 10, and patients with NIHSS ≥ 10 were trending towards greater odds of excellent clinical outcomes (mRS 0-1) and demonstrated no increased risk of sICH. The international PLATO cohort study reported increased odds of ICH and inpatient mortality associated with EVT. However, they also reported that EVT was associated with higher odds of excellent clinical outcomes and complete vision recovery. 15
Both the TOPMOST trial and Sabben et al. suggested that EVT in PCA occlusion is not beneficial for patients with NIHSS <10. Our work corroborates these findings. The reasons for this are not immediately clear and are likely multi-factorial. For example, patient selection for treatment and contemporary study designs are dependent on the NIHSS which under-performs in the evaluation of posterior circulation stroke. 16 Furthermore, navigating thrombectomy instruments predominantly designed for large anterior circulation vessels, through the tortuous posterior circulation with its sharp branching angles, finer caliber PCA, with its complex relationship to fine perforating and choroidal vessels, and frequent atherosclerosis 17 and anatomic variations elevates procedural risk and often leads to higher rates of futile recanalization.17–19 This is reflected by the high rates of ICH in our study, TOPMOST and Sabben et al. These collective results suggest that the elevated risk of PCA EVT may prove overall harmful when NIHSS is low.
Conversely, at increased stroke severity, the benefit of recanalization appears to outweigh the technical risks. Both TOPMOST and Sabben et al. found promising results in patients with NIHSS >10. Our analysis finds similar results but suggests the benefit may be confined to an even tighter subset of patients, those with NIHSS ≥ 10-19. Further, our analysis finds that even within this subset, the significant benefit was confined to those receiving both EVT and IVT. 4 Whether this benefit is due to chemical thrombolysis, earlier time to thrombectomy, or both, cannot be determined from our analysis due to limitations of the NIS database.
In our analysis, we did not find a clinical benefit from EVT for patients with very severe strokes, NIHSS ≥ 20. This group likely represents cases with longer time to treatment, advanced injury with larger core infarct, and predisposition to swelling and related complications. This is supported by lower rates of tPA administered in the NIHSS ≥ 20 EVT-treated patients compared to those with NIHSS 10-19 treated with EVT. Our findings suggest that in the setting of advanced presentation, EVT does not improve outcomes.
The role of thrombolysis
Sabben et al.'s 7 subgroup analysis revealed an interaction between EVT and IVT-use in terms of END such that EVT increased the risk of END and that this risk was more significant among patients who did not receive IVT. This led us to include a sub-group analysis comparing IVT vs non-IVT outcomes among EVT. Our subgroup analysis revealed that the functional benefits seen among the NIHSS ≥ 10-19 patients were only maintained among those who received IVT in addition to EVT. The role of IVT in patient outcomes is heavily confounded by the fact that IVT has a specific time window for administration. It is likely the case that IVT reflects a shorter time of onset which itself is a positive prognostic factor for improved functional outcomes of stroke care and EVT. This likely also explains why, unlike EVT alone, combined IVT and EVT were not associated with statistically greater odds of ICH compared to MM as would be expected. 20 In the unmatched data we see that EVT was employed for more severe strokes based on NIHSS and that this population was also more likely to receive IVT. This combination of high NIHSS and IVT usage might reflect a more severe patient population across the board, which is similar to a meta-analysis that reported similar findings of higher NIHSS among EVT cohorts of PCA-AIS patients. 3
Limitations
The inherent nature of sampling from a large national database and retrospective study design limits this study. The data contained within the NIS is strictly from participating hospitals in the United States. This limits the interpretation of the results as they may not be generalizable to other populations. The NIS contains limited information at the patient level, providing only a snapshot of their inpatient hospital stay and their disposition status. Individual laboratory values, exam findings, and imaging are not available, all of which are of immense value in the assessment of stroke and the decision-making for thrombectomy. This means that there is also no long-term clinical data available for follow-up. We attempt to address this lack of longitudinal outcome data by relying on the discharge disposition which has been previously validated to predict mRS at 90 days post-EVT.8–10 Another limitation of our study, as with many other studies of posterior circulation strokes, was the use of NIHSS for patient stratification as the neurologic functions tested are not necessarily correlated with posterior stroke severity which is what prompted the development of the posterior-NIH stroke scale. 16 The use of NIHSS is the only validated marker for stroke symptomology available within the NIS data. Additionally, while we were able to standardize our patient cohort by eliminating non-PCA ischemic territories, the inherent limitations of the database prevent us from discerning the PCA segment affected by ischemic infarct. The location could have very significant potential confounding effects on our analysis which we are unable to account for. Regarding the rates of ICH, we utilized an ICD-10-CM for ICH that was broad in nature, however it is exclusive to ICH and excludes subarachnoid hemorrhage. This code does not specify the size or whether the patient experiences symptoms of ICH. Further, this code does not delineate a temporal relationship with the utilization of EVT or IVT and does not distinguish whether ICH developed as a sequela of the procedure or was a hemorrhagic conversion from the core infarct. We used this broad ICD coding to cast a wide net and capture the majority of cases of ICH in the NIS as we anticipate heterogeneity in real-world coding practices. Regarding the effect of thrombolysis, we emphasize that the utilization and effect of IVT in our cohort must be interpreted with caution due to the confounding of time on recanalization effects. Lastly, while our pairwise matching is a statistical means to control for confounding variables available within the data set, it cannot account for variables such as imaging features, time since last well-known, stroke progression, and other factors that go into patient selection. It seems likely that this is a significant limitation to our and any other study utilizing NIS to compare outcomes, and more work is needed to develop predictive markers that can help further categorize cases for more homogenous comparison.
Conclusion
Overall, the number of patients being treated with EVT is low, and patient selection likely plays a large role in outcomes, and this is largely determined by imaging-based selection which we are unable to investigate in this data set. Our findings suggest that EVT may not be beneficial for all cases of PCA stroke but may have clinical benefits in select PCA patients. The optimal patient population based on our analysis is those presenting with an NIHSS between 10-19 and those who have received IVT. Further research is needed to determine the optimal patient selection criteria for EVT as it appears not all patients will necessarily benefit.
Supplemental Material
Supplemental material, sj-docx-1-ine-10.1177_15910199231223535 for Endovascular thrombectomy for posterior cerebral artery strokes in the national inpatient sample (EaT PeCANpIeS) study by Aaron Brake, Lane Fry, Cody Heskett, Abdul-Rahman Alkiswani, Gabriel LeBeau, Frank De Stefano, Catherine Lei, Kevin Le, Adam G Rouse, Jeremy Peterson and Koji Ebersole in Interventional Neuroradiology
Acknowledgments
None.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Lane Fry https://orcid.org/0000-0003-0728-2242
Adam G Rouse https://orcid.org/0000-0003-3785-1442
Jeremy Peterson https://orcid.org/0000-0001-9421-5632
Supplemental material: Supplemental material for this article is available online.
References
- 1.Duloquin G, Graber M, Garnier L, et al. Incidence of acute ischemic stroke with visible arterial occlusion: a population-based study (Dijon Stroke Registry). Stroke 2020; 51: 2122–2130. [DOI] [PubMed] [Google Scholar]
- 2.Saver JL, Chapot R, Agid R, et al. Thrombectomy for distal, medium vessel occlusions: a consensus statement on present knowledge and promising directions. Stroke 2020; 51: 2872–2884. [DOI] [PubMed] [Google Scholar]
- 3.Berberich A, Finitsis S, Strambo D, et al. Endovascular therapy versus no endovascular therapy in patients receiving best medical management for acute isolated occlusion of the posterior cerebral artery: a systematic review and meta-analysis. Eur J Neurol 2022; 29: 2664–2673. [DOI] [PubMed] [Google Scholar]
- 4.Powers WJ, Rabinstein AA, Ackerson T, , et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 Update to the 2018 guidelines for the early management of acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke [Internet]. 2019[cited 2023 May 22]; 50: e373. [DOI] [PubMed] [Google Scholar]
- 5.Cunha B, Baptista M, Pamplona J, et al. Acute treatment of isolated posterior cerebral artery occlusion: single center experience. J Stroke Cerebrovasc Dis 2022; 31: 106239. [DOI] [PubMed] [Google Scholar]
- 6.Herweh C, Abdalkader M, Nguyen TN, et al. Mechanical thrombectomy in isolated occlusion of the proximal posterior cerebral artery. Front Neurol 2021; 12: 697348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sabben C, Charbonneau F, Delvoye F, et al. Endovascular therapy or medical management alone for isolated posterior cerebral artery occlusion: a multicenter study. Stroke 2023; 54: 928–937. [DOI] [PubMed] [Google Scholar]
- 8.Patel PD, Salwi S, Liles C, et al. Creation and validation of a stroke scale to increase utility of national inpatient sample administrative data for clinical stroke research. J Stroke Cerebrovasc Dis Off J Natl Stroke Assoc 2021; 30: 105658. [DOI] [PubMed] [Google Scholar]
- 9.Qureshi AI, Chaudhry SA, Sapkota BL, et al. Discharge destination as a surrogate for Modified Rankin Scale defined outcomes at 3- and 12-months poststroke among stroke survivors. Arch Phys Med Rehabil 2012 Aug; 93: 1408–1413. .e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ElHabr AK, Katz JM, Wang J, et al. Predicting 90-day modified Rankin Scale score with discharge information in acute ischaemic stroke patients following treatment. BMJ Neurol Open 2021; 3: e000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ho D, Imai K, King Get al. et al. Matchit: nonparametric preprocessing for parametric causal inference. J Stat Softw 2011; 42: 1–28. [Google Scholar]
- 12.Lumley T. Analysis of complex survey samples. J Stat Softw 2004; 9: 1–19. [Google Scholar]
- 13.Abdelnaby R, Mohamed KA, ELgenidy A, et al. Endovascular therapy in acute isolated posterior cerebral artery occlusion: systematic review and meta-analysis. Clin Neuroradiol 2023; 33: 405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meyer L, Stracke CP, Jungi N, et al. Thrombectomy for primary distal posterior cerebral artery occlusion stroke: the TOPMOST study. JAMA Neurol 2021; 78: 434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen TN, Qureshi MM, Strambo D, et al. Endovascular versus medical management of posterior cerebral artery occlusion stroke: the PLATO study. Stroke 2023; 54: 1708–1717. [DOI] [PubMed] [Google Scholar]
- 16.Alemseged F, Rocco A, Arba F, et al. Posterior National Institutes of Health stroke scale improves prognostic accuracy in posterior circulation stroke. Stroke 2022; 53: 1247–1255. [DOI] [PubMed] [Google Scholar]
- 17.Lee SJ, Hong JM, Kim JSet al. et al. Endovascular treatment for posterior circulation stroke: ways to maximize therapeutic efficacy. J Stroke 2022; 24: 207–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang F, Wang J, He Q, et al. Mechanical thrombectomy for posterior circulation occlusion: a comparison of outcomes with the anterior circulation occlusion–a meta-analysis. J Atheroscler Thromb 2020; 27: 1325–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nouh A, Remke J, Ruland S. Ischemic posterior circulation stroke: a review of anatomy, clinical presentations, diagnosis, and current management. Front Neurol 2014; 5: 30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kandregula S, Savardekar AR, Sharma P, et al. Direct thrombectomy versus bridging thrombolysis with mechanical thrombectomy in middle cerebral artery stroke: a real-world analysis through National Inpatient Sample data. Neurosurg Focus 2021; 51: E4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-ine-10.1177_15910199231223535 for Endovascular thrombectomy for posterior cerebral artery strokes in the national inpatient sample (EaT PeCANpIeS) study by Aaron Brake, Lane Fry, Cody Heskett, Abdul-Rahman Alkiswani, Gabriel LeBeau, Frank De Stefano, Catherine Lei, Kevin Le, Adam G Rouse, Jeremy Peterson and Koji Ebersole in Interventional Neuroradiology