Abstract
Background:
Hybrid Closed-Loop Systems (HCLs) may not perform optimally on postprandial glucose control. We evaluated how first-generation and advanced HCLs manage meals varying in carbohydrates, fat, and protein.
Method:
According to a cross-sectional design, seven-day food records and HCLs reports from 120 adults with type 1 diabetes (MiniMed670G: n = 40, MiniMed780G: n = 49, Control-IQ [C-IQ]: n = 31) were analyzed. Breakfasts (n = 570), lunches (n = 658), and dinners (n = 619) were divided according to the median of their carbohydrate (g)/fat (g) plus protein (g) ratio (C/FP). After breakfast (4-hour), lunch (6-hour), and dinner (6-hour), continuous glucose monitoring (CGM) metrics and early and late glucose incremental area under the curves (iAUCs) and delivered insulin doses were evaluated. The association of C/FP and HCLs with postprandial glucose and insulin patterns was analyzed by univariate analysis of variance (ANOVA) with a two-factor design.
Results:
Postprandial glucose time-in-range 70 to 180 mg/dL was optimal after breakfast (78.3 ± 26.9%), lunch (72.7 ± 26.1%), and dinner (70.8 ± 27.3%), with no significant differences between HCLs. Independent of C/FP, late glucose-iAUC after lunch was significantly lower in C-IQ users than 670G and 780G (P < .05), with no significant differences at breakfast and dinner. Postprandial insulin pattern (Ins3-6h minus Ins0-3h) differed by type of HCLs at lunch (P = .026) and dinner (P < .001), being the early insulin dose (Ins0-3h) higher than the late dose (Ins3-6h) in 670G and 780G users with an opposite pattern in C-IQ users.
Conclusions:
Independent of different proportions of dietary carbohydrates, fat, and protein, postprandial glucose response was similar in users of different HCLs, although obtained through different automatic insulin delivery patterns.
Keywords: carbohydrate/fat-protein ratio, HCL algorithms, hybrid closed-loop system, meal composition, postprandial glucose, type 1 diabetes
Introduction
Postprandial glucose excursions are a main determinant of overall blood glucose control in individuals with type 1 diabetes (T1D). 1 Hybrid Closed-Loop Systems (HCLs) improve glycemic control in terms of time-in-range (TIR) and HbA1c in individuals with T1D.2,3 This improvement is especially related to better management of glycemia during fasting periods. Hybrid Closed-Loop Systems still underperform during the postprandial phase due to the requirement of manual input of insulin bolus, the limitations of subcutaneous insulin delivery, and, most relevant, the complexity of postprandial glucose response (PGR). The PGR has a large intra-individual and inter-individual variability, 4 depending on many nutritional factors beyond carbohydrate amount that remains the only factor considered for premeal insulin calculation.5-7 Increasing evidence highlights the role of fats and proteins in shaping PGR. Fat reduces the early glucose response while leading to late (>3 hours) hyperglycemia; proteins determine late hyperglycemia in a dose-dependent manner.1,6 In addition, in a cross-sectional study, the ratio between carbohydrate and fat was a main predictor of PGR in patients with T1D not using HCLs. 8
First-generation HCLs could not counteract the late postprandial glucose increase induced by meals relatively rich in fat and protein consumed in real-life by adults with T1D. 9 The advanced HCLs have been provided with automated correction boluses to counteract the rapid postprandial increase of glycemia. Boluses are delivered every 5 minutes triggered by a meal-detection module (MiniMed780G system [780G], Medtronic, Dublin, Irland), or once per hour, at 40% of the estimated needed correction (Control-IQ system [C-IQ], Tandem Diabetes Care, San Diego, California). A recent post hoc analysis of a randomized cross-over trial comparing MiniMed670G (670G) (Medtronic) with 780G system in adolescents and young adults with T1D did not find significant differences between the two systems in blood glucose control following a meal. 10 The study only included a short postprandial observation period (3 hours) and, therefore, possible differences between algorithms in managing the late increase in postprandial glycemia related to the composition of meals, ie, content of fat, proteins, low-glycemic index (GI) foods, could have been missed.
Therefore, our cross-sectional study aimed to compare the performance in real-life of first-generation (670G) and advanced (780G, C-IQ) HCLs on glycemic control over an extended postprandial period, according to the nutritional composition of meals in adults with T1D.
Methods
Participants
Patients with T1D on HCLs (670G) or advanced HCLs (780G, C-IQ) since at least six months, aged >18 years, trained in carbohydrate counting, not pregnant/breastfeeding, or suffering from acute or chronic diseases significantly affecting their health status, were eligible for the study. All eligible participants consecutively attending the Diabetes Clinic of Federico II University Hospital (Naples, Italy) for the annual evaluation of diabetes complication status from January 2020 to May 2023 were enrolled in the study.
Each participant gave informed consent for using her or his data following the approval of the Ethical Committee of Federico II University.
Study Design
A cross-sectional study was performed. One week before the clinic visit, participants were invited by phone call to complete a seven-day food record, reinforcing instructions on how to identify and estimate portion sizes for various food items. They were asked to report on a food record form sent by mail all foods and beverages consumed, including portions measured by household units (cups, spoons, etc.) or weight, providing as much information as possible (ie, cooking methods, brands names).
During the hospital visit, participants underwent standardized anthropometric measurements, and venous blood was drawn for HbA1c and other biochemical evaluations. A registered dietitian reviewed the food records to check for any potential error or missing information. Food records retrieved 1847 meals (570 breakfasts, 658 lunches, and 619 dinners) available for food composition evaluation. Energy intake, nutrient composition, GI, and glycemic load (GL) were assessed using the MetaDieta Software version 4.6.1 (METEDA S.r.l., Roma, Italy).
Data on blood glucose control, including two-week CGM metrics and insulin doses, were downloaded from the Medtronic cloud (CareLink, https://carelink.medtronic.eu/) for the 670G and 780G and from the Diasend or Glooko clouds (Diasend, https://diasend.com/; Glooko, https://eu.my.glooko.com/) for the C-IQ.
Postprandial Blood Glucose Response and Insulin Doses
Continuous glucose monitoring data were collected at 5-minute intervals from 30 minutes before the premeal insulin bolus until the bolus for the following meal. An observation period of 4 hours after breakfast and 6 hours after lunch and dinner was analyzed. Missing values related to sensor malfunctioning were calculated using the linear trend at point method. 11
Postprandial blood glucose response was evaluated by calculating, using the trapezoidal method, the incremental area under the curve (iAUC) during the early (0-2 hours, iAUC0-2h, for breakfast; 0-3 hours, iAUC0-3h, for lunch and dinner) and late (2-4 hours, iAUC2-4h, for breakfast; 3-6 hours, iAUC3-6h, for lunch and dinner) observation periods. The difference between late and early iAUC was used to define the shape of glucose response for breakfast (iAUC2-4h minus iAUC0-2h) and lunch or dinner (iAUC3-6h minus iAUC0-3h). Glucose peak and nadir were individuated as the highest and lowest blood glucose values measured over the postprandial observation period, respectively. The maximal glucose rise was calculated as the difference between the glucose peak and the average blood glucose level during the 30 minutes preceding the meal. Time to peak was individuated as the minute at which the glucose peak was reached.
Postprandial blood glucose control was also evaluated by CGM metrics: percentage of time spent with blood glucose between 70 and 180 mg/dL (TIR), 180 and 250 mg/dL (time-above-range, TAR 180-250) or above 250 mg/dL (TAR >250), and between 70 and 54 mg/dL (time-below-range, TBR = 54-70) or below 54 mg/dL (TBR <54). 12
Insulin doses, including basal infusion, postmeal micro-boluses automatically given by the system, and adjustment boluses that the participants delivered according to the suggestion of the HCLs, were collected from 30 minutes before the premeal insulin bolus until the bolus corresponding to the following meal. Insulin data are shown as: (1) insulin dose delivered in a 5-minute period, (2) sum of insulin doses injected during the early (Ins0-2h for breakfast; Ins0-3h for lunch and dinner), and late (Ins2-4h for breakfast, Ins3-6h for lunch and dinner) observation period, and (3) differences between the insulin dose injected during the late and early observation period.
Statistical Analyses
Data are expressed as mean ± standard deviation (SD) unless otherwise stated.
The Kolmogorov-Smirnov test was performed to check if variables followed a normal distribution. Variables not normally distributed were analyzed after logarithmic transformation.
To minimize biases in interpreting blood glucose response and insulin delivery patterns between different HCLs, comparisons were made within meals with similar macronutrient composition. The ratio between Carbohydrate and Fat plus Protein (C/FP) of each meal was calculated. Comparisons were made between the groups of meals above (↑CHO) and below (↑FatProt) the median of C/FP for each type of meal (breakfast [2.18], lunch [1.59], and dinner [1.02]).
Univariate two-factor analysis of variance (ANOVA) and post hoc analysis was conducted to examine the separate influence of C/FP and HCLs and their interaction on PGR and insulin delivery pattern. Glucose-iAUCs, insulin doses delivered during the early and late postprandial periods, or differences between the insulin dose delivered during the late and early observation period were entered as dependent variables, C/FP group, and HCLs as fixed factors, and mean and trend of blood glucose concentration during the 30 minutes preceding meals and premeal bolus as covariates. Differences in clinical characteristics of participants and PGR and nutritional composition among breakfast, lunch, and dinner were evaluated by one-way ANOVA and post hoc analysis. Differences in nutritional composition between ↑CHO and ↑FatProt meals were evaluated by unpaired t-test.
The statistical analysis was performed according to standard methods using the SPSS software version 29 (SPSS/PC; SPSS, Chicago, Illinois).
Results
Demographic and Clinical Characteristics
Demographic and clinical characteristics of the participants in the whole cohort and divided according to type of HCLs are shown in Table 1. The HCLs groups were comparable for sex, age, body mass index (BMI), and duration of diabetes. Participants on C-IQ showed lower HbA1c levels (P = .019 vs 780G) and a higher percentage of TAR >250 (P < .05 vs 670G and 780G).
Table 1.
Anthropometrics, General Characteristics, and Two-Week CGM Metrics of the Study Participants in the Whole Cohort and According to the Type of Hybrid Closed-Loop System (Mean ± SD).
| Overall (n = 120) |
670G (n = 40) |
780G (n = 49) |
C-IQ (n = 31) |
|
|---|---|---|---|---|
| Gender (M/F) | 61/59 | 21/19 | 24/25 | 16/15 |
| Age (years) | 40.6 ± 12.6 | 42.3 ± 12.2 | 40.7 ± 12.3 | 38.2 ± 13.7 |
| Body weight (kg) | 72.5 ± 14.5 | 72.8 ± 11.6 | 72.9 ± 15.5 | 71.6 ± 15.6 |
| Body mass index (kg/m2) | 25.8 ± 3.8 | 25.6 ± 3.2 | 26.3 ± 4.2 | 25.2 ± 3.6 |
| Duration of diabetes (years) | 22.9 ± 12.2 | 21.5 ± 13.6 | 24.5 ± 11.3 | 22.1 ± 11.9 |
| HbA1c (%) HbA1c (mmol/mol) |
7.2 ± 0.9 54.9 ± 10.6 |
7.3 ± 0.7 54.2 ± 12.7 |
7.4 ± 0.9 57.7 ± 9.7 |
6.9 ± 0.8
a
51.5 ± 9.1 a |
| TIR 70-180 mg/dL (%) | 71.9 ± 11.1 | 72.2 ± 9.6 | 74.0 ± 8.2 | 68.1 ± 15.4 |
| TAR 180-250 mg/dL (%) | 20.0 ± 6.7 | 20.7 ± 6.5 | 19.2 ± 6.1 | 20.6 ± 7.7 |
| TAR >250 mg/dL (%) | 5.9 ± 6.1 | 5.2 ± 4.0 | 4.3 ± 3.5 | 9.4 ± 9.4 b |
| TBR 54-70 mg/dL (%) | 1.7 ± 1.8 | 1.6 ± 1.4 | 1.9 ± 2.3 | 1.4 ± 1.2 |
| TBR <54 mg/dL (%) | 0.4 ± 0.9 | 0.3 ± 0.5 | 0.5 ± 1.3 | 0.4 ± 0.6 |
| GMI (%) | 7.0 ± 0.4 | 6.9 ± 0.3 | 6.9 ± 0.3 | 7.1 ± 0.6 |
| CV (%) | 33.1 ± 4.3 | 32.6 ± 3.5 | 32.8 ± 4.6 | 34.2 ± 4.5 |
Abbreviations: CGM, continuous glucose monitoring; TIR, time-in-range; TAR, time-above-range; TBR, time-below-range; GMI, glucose management indicator; CV, coefficient of variation.
P < .05 vs 780G; bP < .05 vs 670G and 780G, by post hoc ANOVA.
As shown in Table 2, energy intake and meal composition differed between breakfast, lunch, and dinner. Breakfast had lower energy content, macronutrient amount, glycemic index (GI), and glycemic load (GL) than lunch and dinner (P < .05 for all). Lunch had more carbohydrate and fiber and less protein and fat and a lower GI than dinner (P < .05 for all). The C/FP was lower at dinner (1.3 ± 1.0) than breakfast (3.6 ± 6.2) and lunch (1.9 ± 1.9) (P < .001, by ANOVA). Table 2 reports meal composition at breakfast, lunch, and dinner also according to ↑FatProt and ↑CHO groups.
Table 2.
Energy Intake and Dietary Composition of Breakfast, Lunch, and Dinner in the Whole Cohort and in ↑FatProt and ↑CHO Groups (Mean ± SD).
| ↑FatProt | ↑CHO | |||
|---|---|---|---|---|
| BREAKFAST (n = 570) | Overall | C/FP ≤2.18 | C/FP >2.18 | P-value |
| Energy (kcal) | 200 ± 102 a | 202 ± 104 | 197 ± 100 | .539 |
| Carbohydrate (g) | 30.2 ± 15.8 a | 26.3 ± 13.9 | 34.2 ± 16.7 | <.001 |
| Sugar (g) | 14.8 ± 9.1 | 13.1 ± 7.3 | 16.6 ± 10.4 | <.001 |
| Fiber (g) | 1.6 ± 1.8 a | 1.3 ± 1.3 | 2.0 ± 2.2 | <.001 |
| Protein (g) | 7.2 ± 3.9 a | 8.6 ± 3.6 | 5.9 ± 3.6 | <.001 |
| Fat (g) | 6.1 ± 4.7 a | 7.5 ± 5.1 | 4.8 ± 3.8 | <.001 |
| Glycemic index (%) | 50.7 ± 15.4 a | 48.2 ± 15.3 | 53.3 ± 15.0 | <.001 |
| Glycemic load (units) | 14.6 ± 9.8 a | 13.1 ± 8.7 | 16.1 ± 10.6 | <.001 |
| C/FP | 3.6 ± 6.2 a | |||
| LUNCH (n = 658) | C/FP ≤1.59 | C/FP >1.59 | ||
| Energy (kcal) | 618 ± 268 | 680 ± 290 | 557 ± 229 | <.001 |
| Carbohydrate (g) | 75.1 ± 32.8 b | 65.2 ± 30.4 | 85.1 ± 32.2 | <.001 |
| Sugar (g) | 14.9 ± 11.2 | 13.9 ± 10.4 | 15.8 ± 11.9 | .038 |
| Fiber (g) | 7.7 ± 5.4 b | 7.7 ± 5.5 | 7.8 ± 5.3 | .759 |
| Protein (g) | 27.8 ± 17.4 b | 35.4 ± 18.8 | 20.1 ± 11.4 | <.001 |
| Fat (g) | 23.3 ± 15.8 b | 30.7 ± 17.5 | 16.0 ± 9.4 | <.001 |
| Glycemic index (%) | 53.4 ± 13.7 b | 56.0 ± 13.9 | 50.9 ± 13.1 | <.001 |
| Glycemic load (units) | 38.4 ± 20.4 | 34.9 ± 17.4 | 42.0 ± 22.5 | <.001 |
| C/FP | 1.9 ± 1.9 b | |||
| DINNER (n = 619) | C/FP ≤1.02 | C/FP >1.02 | ||
| Energy (kcal) | 640 ± 287 | 646 ± 285 | 634 ± 290 | .611 |
| Carbohydrate (g) | 64.2 ± 36.7 | 46.1 ± 24.8 | 82.3 ± 37.9 | <.001 |
| Sugar (g) | 14.7 ± 12.0 | 11.6 ± 10.1 | 17.7 ± 13.0 | <.001 |
| Fiber (g) | 7.1 ± 4.7 | 6.3 ± 4.5 | 8.0 ± 4.7 | <.001 |
| Protein (g) | 33.7 ± 16.5 | 39.1 ± 16.8 | 28.2 ± 14.3 | <.001 |
| Fat (g) | 27.5 ± 18.5 | 33.5 ± 19.8 | 21.5 ± 14.9 | <.001 |
| Glycemic index (%) | 63.4 ± 17.7 | 61.4 ± 15.1 | 65.4 ± 19.8 | .005 |
| Glycemic load (units) | 40.0 ± 31.1 | 27.5 ± 17.1 | 52.7 ± 36.5 | <.001 |
| C/FP | 1.3 ± 1.0 |
P < .05 vs lunch and dinner; bP < .05 vs dinner, by post hoc ANOVA. Unpaired t-test.
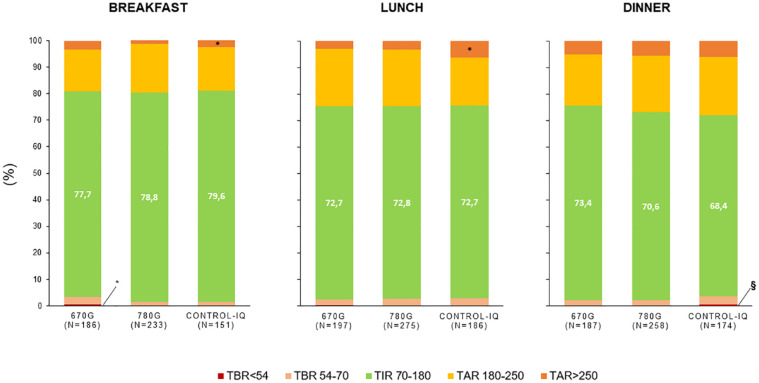
Postprandial CGM Metrics
Blood glucose control after meals was generally adequate, being TIR>70% and TBR<5% in all the HCLs groups (Figure 1). Time-in-range was 78.3 ± 26.9 after breakfast, 72.7 ± 26.1 after lunch, and 70.8 ± 27.3 after dinner (P > .05 for HCLs effect, for each meal). The C-IQ users showed a higher TAR >250 mg/dL than 780G at breakfast (4.4 ± 13.9% vs 1.5 ± 7.4%, P = .015) and lunch (6.2 ± 16.3% vs 3.1 ± 10.0%, P = .012) and a higher TBR<54 mg/dL than 780G and 670G at dinner (0.6 ± 3.2% vs 0.2 ± 1.1%, P = .019 for 780G; 0.2 ± 1.0%, P = .029 for 670G). At breakfast, 670G users had a higher TBR<54 mg/dL than 780G (0.5 ± 2.6% vs 0.0 ± 0.0%, P = .012).
Figure 1.
Postprandial CGM metrics at breakfast (0-4 hours), lunch (0-6 hours), and dinner (0-6 hours) according to the type of hybrid closed-loop system.
*P < .05 vs 780G, §P < .05 vs 670G and 780G, by post hoc ANOVA.
Postprandial Blood Glucose and Insulin Doses
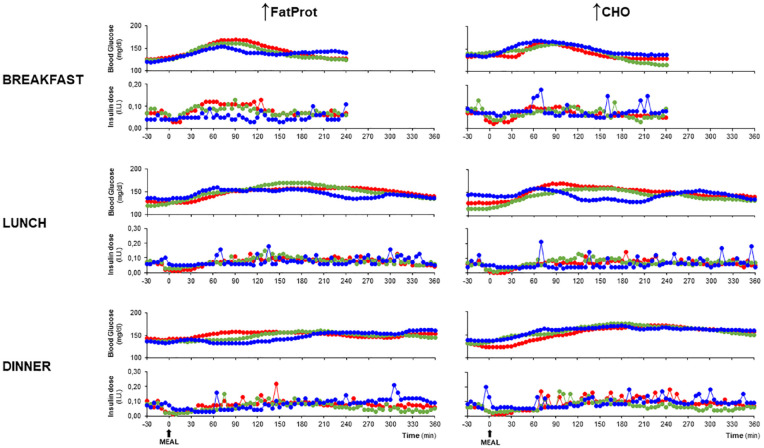
Postprandial blood glucose and insulin profiles (Figure 2) and glucose-iAUC and insulin doses (Figure 3) for the different HCLs types at breakfast, lunch, and dinner are shown according to ↑FatProt and ↑CHO groups.
Figure 2.
Blood glucose and insulin profiles from 30 minutes before to the maximal observational period after meal ingestion at breakfast, lunch, and dinner according to ↑FatProt and ↑CHO groups and type of hybrid closed-loop system.
( 670G,
670G,  780G,
780G,  C-IQ).
C-IQ).
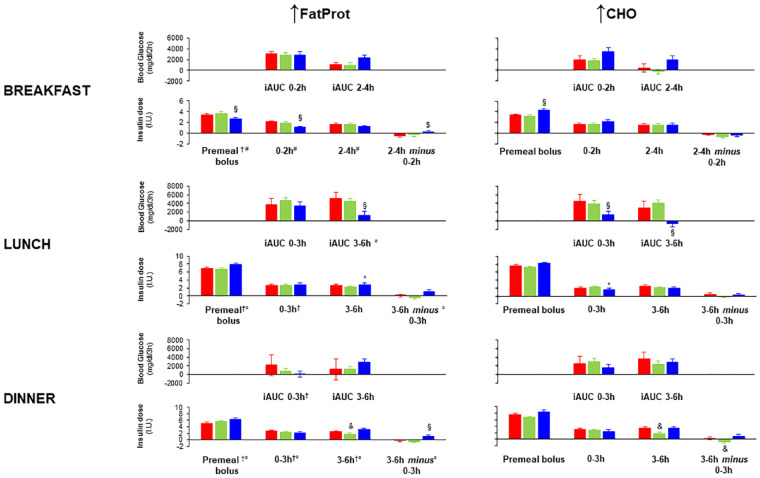
Figure 3.
Early and late glucose-iAUCs, and premeal bolus, and early and late automatic insulin delivery after meal ingestion at breakfast, lunch, and dinner according to ↑FatProt and ↑CHO groups and type of hybrid closed-loop system.
Breakfast: ( 670G n = 186,
670G n = 186,  780G n = 233,
780G n = 233,  C-IQ n = 151).
C-IQ n = 151).
Lunch: ( 670G n = 197,
670G n = 197,  780G n = 275,
780G n = 275,  C-IQ n = 186).
C-IQ n = 186).
Dinner: ( 670G n = 187,
670G n = 187,  780G n = 258,
780G n = 258,  C-IQ n = 174).
C-IQ n = 174).
†P < .05 for C/FP group effect; #P < .05 for HCLs-C/FP group interaction; °P < .05 for HCLs effect, by two-factor univariate analysis of variance (ANOVA).
*P < .05 vs 780G; §P < .05 vs 670G and 780G; $P < .05 vs 670G, &P < .05 vs 670G and C-IQ; by post hoc ANOVA.
Breakfast
Nadir, maximal glucose rise, and early and late glucose-iAUCs did not differ significantly between ↑FatProt and ↑CHO groups and HCLs types (Figure 3, Table 3).
Table 3.
Glucose Peak, Time to Peak, Nadir, and Maximal Rise at Breakfast, Lunch, and Dinner According to ↑FatProt and ↑CHO Groups and Type of Hybrid Closed-Loop System (Mean ± SD).
| Overall | ↑FatProt | ↑CHO | |||||
|---|---|---|---|---|---|---|---|
| BREAKFAST | 670G | 780G | C-IQ | 670G | 780G | C-IQ | |
| Glucose Peak (mg/dL)
a
Time to Peak (min) b Nadir Glucose (mg/dL) Maximal Glucose Rise (mg/dL) |
196 ± 47.9 c | 205 ± 49.6 | 196 ± 44.1 | 181 ± 40.4 d | 189 ± 39.4 | 195 ± 50.9 | 200 ± 55.6 |
| 109 ± 82.5 c | 121 ± 86.7 | 113 ± 94.1 | 109 ± 81.7 | 123 ± 87.8 | 90.7 ± 71.8 | 109 ± 82.4 | |
| 95.7 ± 31.1 | 95.7 ± 33.6 | 94.9 ± 27.1 | 98.4 ± 27.7 | 89.4 ± 26.8 | 98.5 ± 34.3 | 91.1 ± 21.3 | |
| 64.8 ± 49.3 c | 65.6 ± 52.1 | 66.5 ± 47.5 | 59.7 ± 41.0 | 58.9 ± 45.0 | 59.8 ± 47.0 | 79.2 ± 59.0 | |
| LUNCH | |||||||
| Glucose Peak (mg/dL) Time to Peak (min) Nadir Glucose (mg/dL) e Maximal Glucose Rise (mg/dL) |
205 ± 49.7 | 198 ± 39.2 | 207 ± 44.8 | 212 ± 65.0 | 206 ± 47.9 | 196 ± 45.6 | 209 ± 50.2 |
| 150 ± 98.7 | 157 ± 106 | 153 ± 95.8 | 137 ± 101 | 152 ± 99.1 | 142 ± 90.6 | 160 ± 102 | |
| 93.2 ± 31.7 | 90.4 ± 23.4 | 100 ± 32.0 | 98.9 ± 41.8 | 90.2 ± 28.6 | 85.8 ± 27.6 | 90.7 ± 30.6 | |
| 78.8 ± 50.6 | 75.5 ± 46.2 | 81.8 ± 47.5 | 78.1 ± 58.9 | 81.0 ± 52.7 | 78.2 ± 52.1 | 78.2 ± 50.6 | |
| DINNER | |||||||
| Glucose Peak (mg/dL)
e
Time to Peak (min) e Nadir Glucose (mg/dL) Maximal Glucose Rise (mg/dL) |
210 ± 53.1 | 207 ± 50.3 | 201 ± 46.9 | 204 ± 50.9 | 215 ± 62.9 | 219 ± 56.3 | 212 ± 46.5 |
| 159 ± 104 | 136 ± 100 | 155 ± 106 | 161 ± 111 | 174 ± 97.1 | 164 ± 101 | 168 ± 101 | |
| 98.4 ± 36.9 | 101 ± 31.9 | 100 ± 34.3 | 99.7 ± 43.7 | 92.9 ± 33.2 | 103 ± 36.8 | 95.0 ± 37.3 | |
| 74.2 ± 56.5 | 68.8 ± 61.0 | 62.4 ± 50.2 | 68.2 ± 53.4 | 85.7 ± 66.8 | 84.8 ± 55.1 | 75.5 ± 45.4 | |
P < .05 for HCLs-C/FP group interaction; bP < .05 for HCLs; cP < .05 vs lunch and dinner, by post hoc ANOVA; dP < .05 vs 670G and 780G, by post hoc ANOVA; eP < .05 for C/FP group effect, by two-factor univariate analysis of variance (ANOVA).
In the early phase (0-2 hours), C-IQ delivered less insulin for ↑FatProt and more insulin for ↑CHO meals than the 670G and 780G (P < .001 for HCLs-C/FP group interaction). Premeal insulin boluses differed between C/FP groups, being significantly lower with ↑FatProt than ↑CHO (3.2 ± 2.2 vs 3.6 ± 2.2 IU, P = .031), and among HCLs groups in each C/FP group (P < .001 for HCLs-C/FP interaction), being lower with ↑FatProt and higher with ↑CHO in C-IQ users than 670G and 780G users (Figure 3).
Lunch
Glucose peak was significantly delayed, and the maximal rise was higher than at breakfast (Table 3). In the ↑FatProt group, glucose-iAUC3-6h was lower with C-IQ than 670G, (P = .007) and 780G (P = .008). In the ↑CHO, both glucose-iAUC0-3h and iAUC3-6h were lower with C-IQ than 670G and 780G (P < .05 for all) (Figure 3).
More insulin was delivered in the ↑FatProt than the ↑CHO group in the early phase (2.7 ± 2.1 vs 2.0 ± 1.7 IU, P < .001). In the ↑FatProt group in the late phase, more insulin was injected by C-IQ than 780G (P = .032) (Figure 3). In the ↑CHO group in the early phase, less insulin was injected by C-IQ than 780G (P = .009) (Figure 3). Less insulin was delivered in the late than early phase with 780G (late minus early insulin dose, −0.4 ± 2.2 IU) and the opposite with C-IQ and 670G (late minus early insulin dose, 0.8 ± 2.1 and 0.3 ± 2.5 IU, respectively) (P = .026 for HCLs effect).
Premeal insulin boluses differed according to the C/FP, being lower in the ↑FatProt group than the ↑CHO group (7.0 ± 3.8 vs 7.7 ± 3.1 IU, P =.040), and according to type of HCLs, being higher with C-IQ than 670G and 780G (8.1 ± 3.8 vs 7.3 ± 2.9 and 6.8 ± 3.5 IU, respectively, P =.001).
Dinner
The glucose peak was significantly delayed, and the maximal rise was higher than at breakfast (Table 3). Glucose-iAUC0-3h was significantly lower in the ↑FatProt than the ↑CHO group (1057 ± 6930 vs 2799 ± 7894 mg/dL/180 min, P =.017).
In the early phase, less insulin was delivered in the ↑FatProt than the ↑CHO group (2.8 ± 2.2 vs 2.4 ± 1.8 IU, P = .016). Early insulin release differed also according to HCLs type, being higher with 670G than C-IQ and 780G (2.8 ± 2.2 vs 2.3 ± 1.8 and 2.5 ± 1.9 IU, respectively, P = .025).
Late insulin doses were significantly lower with 780G than 670G and C-IQ (1.8 ± 2.0 vs 2.9 ± 2.3 and 3.2 ± 3.2 IU, respectively, P < .001). Less insulin was delivered in the late than early phase with 780G (late minus early insulin dose, −0.9 ± 2.4 IU) and the opposite with C-IQ (late minus early insulin dose, 0.8 ± 3.3 IU) (P < . 001 for HCLs effect).
Premeal insulin boluses differed according to C/FP, being lower in the ↑FatProt than the ↑CHO group (5.6 ± 3.9 vs 7.4 ± 4.4 IU, P < .001). Premeal boluses differed also according to HCLs type, being higher with C-IQ than 670G and 780G (7.5 ± 5.3 vs 6.2 ± 3.6 and 6.0 ± 3.7 IU, respectively, P =.002).
Discussion
This is the first study that compares the performances of a first-generation device (670G) and two advanced hybrid closed-loop systems (780G and C-IQ) on postprandial glucose control in individuals with T1D, also accounting for macronutrient meal composition.
First, we show in people with T1D on advanced technologies that the meal content influences the postprandial glucose profile with an evident impact of the relative amount of carbohydrate, fat, and protein. This implied that the shape of glucose response also differed among breakfast, lunch, and dinner, confirming the findings of a larger study in people with T1D. 9 Meal types significantly differed in postprandial glucose peak that was earlier after breakfast, richer in carbohydrates, than lunch and dinner, richer in fat and protein. This relation between macronutrient composition and glucose shape is in line with previous reports in T1D people not on HCLs13-15 showing that fat and protein increased the early insulin requirement when carbohydrates were the main component of a meal and delayed insulin demand when carbohydrates were relatively less. The different nutrients likely influenced gastric emptying and/or the availability of carbohydrates for absorption, delaying, and/or prolonging the postprandial blood glucose increase.16-18
Comparing HCLs performances, the relative meal content of carbohydrate, fat, and protein influenced postprandial glucose profiles similarly in the users of the three HCLs. The similarity among HCLs was still evident after correction for premeal blood glucose mean and trend, which significantly predicted postprandial blood glucose and insulin delivery patterns. This suggests that meal composition is the most challenging factor, among many (eg, insulin on board, time of the day, second meal effect, exercise) possibly contributing to the observed differences among meals. Our data show that first-generation and advanced HCLs algorithms carry similar limitations in dealing with the impact of different nutritional factors on PGR.
However, the similar glucose response stemmed from significantly different patterns of automatic insulin delivery. The main limitation of the currently available algorithms is their inability to include the different nutritional factors in the prediction of PGR. 19 This was made evident by the insulin delivery patterns observed in this study, clearly indicating that insulin changes followed blood glucose rises and falls in a reacting modality. A representative example of this feature was the opposite trend between ↑FatProt and ↑CHO groups in early insulin dose between lunch and dinner (higher in the ↑FatProt than in the ↑CHO group at lunch and the opposite at dinner). The differences in insulin delivery patterns observed between the two advanced HCLs are likely related to their timing of intervention. The meal-detection module embedded in the 780G system is probably a trigger of a more pronounced early insulin delivery than the C-IQ. The lower aggressiveness of C-IQ may explain the higher percentage of TAR>250 mg/dL observed with this system. Therefore, proper timing and dose of premeal insulin bolus could assume different relevance according to the type of meal and algorithm. Current possible strategies may include, in the case of a high GI meal, adequate anticipation of the bolus to prevent an early glucose rise while using the C-IQ system, or late hypoglycemia while using the 780G system. In the case of a high-fat meal, using a prolonged bolus may support the reactive automated insulin infusion with the C-IQ, whereas a “reinforced” premeal or a split bolus may compensate for the lack of the effective trigger of early hyperglycemia with the 780G meal-detection module. 20
However, implementing tailored meal insulin strategies still requires filling the gap in the knowledge of the impact on postprandial blood glucose control of different foods and mixed meals. 9 Moreover, for the insulin dose calculation, advanced tools are needed in real-life to allow the evaluation of whole nutritional properties of meals, beyond the carbohydrate content.21,22
Of note, in this real-life evaluation, glucose control over a long postprandial observation period was rather good compared with previous reports. 10 This may be related to the comprehensive educational nutritional therapy to which our patients are subjected that includes a lower use of snacking. The reduced number of daily meals could help overcome the current limitations of HCLs in managing postprandial glycemia with advantages in overall blood glucose control and general health status through positive effects on body weight control.7,23 It should also be considered that the optimal carbohydrate counting and bolusing behavior in the study participants may have reduced the possibility of discovering potential differences between algorithms’ functioning.
This study has some strengths and limitations. As a strength, this is the first study comparing postprandial glucose control between two advanced HCLs, also considering the nutritional composition of meals. The rigorous evaluation of dietary habits provided reliable and precious information that cannot be obtained in large real-world studies analyzing HCLs performance.24,25
A limitation of our study is the cross-sectional design that does not allow to exclude potential biases related to clinical and/or psychosocial differences between the users of the different HCLs. Furthermore, although the gold standard for dietary composition assessment was used (ie, seven-day food records), 26 we cannot exclude biases related to self-reporting. In this respect, the use of standardized meals could have been advantageous.
It must be considered that the comparison between systems may be affected by the inaccurate setting of the correction factors, such as the insulin sensitivity factor, required by the C-IQ system. However, the dependence of the performance on the users’ settings or the algorithm itself may not be clinically relevant in real-world conditions.
Moreover, differences in technical features of Guardian and Dexcom sensors may have affected the comparison in absolute measures of postprandial blood glucose control, as suggested by not optimal concordance between HbA1c and CGM metrics in users of different systems. However, the unmatching between HbA1c and CGM metrics could not have influenced the insulin delivery pattern that reacts to relative changes in blood glucose concentrations.
Conclusions
In conclusion, we showed that currently available HCLs have similar performances on postprandial glucose control that is related to the relative meal content of carbohydrate, fat, and protein. The similar effect on postprandial glucose was obtained by the different HCLs through different patterns of automatic insulin delivery. Therefore, for the optimal management of PGR, predictive algorithm features should consider the nutritional composition of meals. In the meantime, achieving adequate postprandial glucose control is feasible with currently available HCLs, provided that comprehensive nutritional education is delivered.
Footnotes
Abbreviations: C/FP, ratio between carbohydrate and fat plus protein; GI, glycemic index; GL, glycemic load; HCLs, hybrid closed-loop systems; iAUC, incremental area under the curve; PGR, postprandial glucose response; T1D, type 1 diabetes; TAR, time-above-range; TBR, time-below-range; TIR, time-in-range
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the “Finanziamento della Ricerca di Ateneo, Linea B 2021” Federico II University of Naples and CRESCENDO DP supported by the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie program.
ORCID iDs: Giuseppe Scidà  https://orcid.org/0000-0002-8095-0799
https://orcid.org/0000-0002-8095-0799
Giuseppe Della Pepa  https://orcid.org/0000-0001-5862-3794
https://orcid.org/0000-0001-5862-3794
Giovanni Annuzzi  https://orcid.org/0000-0002-9324-6047
https://orcid.org/0000-0002-9324-6047
Lutgarda Bozzetto  https://orcid.org/0000-0001-6549-4476
https://orcid.org/0000-0001-6549-4476
References
- 1. Gingras V, Taleb N, Roy-Fleming A, Legault L, Rabasa-Lhoret R. The challenges of achieving postprandial glucose control using closed-loop systems in patients with type 1 diabetes. Diabetes Obes Metab. 2018;20(2):245-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brown SA, Kovatchev BP, Raghinaru D, et al. Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med. 2019;381(18):1707-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bergenstal RM, Garg S, Weinzimer SA, et al. Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA. 2016;316(13):1407-1408. [DOI] [PubMed] [Google Scholar]
- 4. Bozzetto L, Pacella D, Cavagnuolo L, et al. Postprandial glucose variability in type 1 diabetes: the individual matters beyond the meal. Diabetes Res Clin Pract. 2022;192:110089. [DOI] [PubMed] [Google Scholar]
- 5. Bell KJ, Barclay AW, Petocz P, Colagiuri S, Brand-Miller JC. Efficacy of carbohydrate counting in type 1 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2014;2(2):133-140. [DOI] [PubMed] [Google Scholar]
- 6. Bell KJ, Smart CE, Steil GM, Brand-Miller JC, King B, Wolpert HA. Impact of fat, protein, and glycemic index on postprandial glucose control in type 1 diabetes: implications for intensive diabetes management in the continuous glucose monitoring era. Diabetes Care. 2015;38(6):1008-1015. [DOI] [PubMed] [Google Scholar]
- 7. Bozzetto L, Corrado A, Scidà G. Dietary treatment of type 1 diabetes: beyond carbohydrate counting to fight cardiovascular risk. Nutr Metab Cardiovasc Dis. 2023;33(2):299-306. [DOI] [PubMed] [Google Scholar]
- 8. Shilo S, Godneva A, Rachmiel M, et al. Prediction of personal glycemic responses to food for individuals with type 1 diabetes through integration of clinical and microbial data. Diabetes Care. 2022;45(3):502-511. [DOI] [PubMed] [Google Scholar]
- 9. Vetrani C, Calabrese I, Cavagnuolo L, et al. Dietary determinants of postprandial blood glucose control in adults with type 1 diabetes on a hybrid closed-loop system. Diabetologia. 2022;65(1):79-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weinzimer SA, Bailey RJ, Bergenstal RM, et al. A comparison of postprandial glucose control in the medtronic advanced hybrid closed-loop system versus 670G. Diabetes Technol Ther. 2022;24(8):573-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kenneth SB, Anthony JB, Data analysis with IBM SPSS statistics. Dealing with missing data and outliers. Packt Publishing; 2017. [Google Scholar]
- 12. Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40(12):1631-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Campbell MD, Walker M, Ajjan RA, Birch KM, Gonzalez JT, West DJ. An additional bolus of rapid-acting insulin to normalise postprandial cardiovascular risk factors following a high-carbohydrate high-fat meal in patients with type 1 diabetes: a randomised controlled trial. Diab Vasc Dis Res. 2017;14(4):336-344. [DOI] [PubMed] [Google Scholar]
- 14. Kaya N, Kurtoğlu S, Gökmen Özel H. Does meal-time insulin dosing based on fat-protein counting give positive results in postprandial glycaemic profile after a high protein-fat meal in adolescents with type 1 diabetes: a randomised controlled trial. J Hum Nutr Diet. 2020;33(3):396-403. [DOI] [PubMed] [Google Scholar]
- 15. Al Balwi R, Al Madani W, Al Ghamdi A. Efficacy of insulin dosing algorithms for high-fat high-protein mixed meals to control postprandial glycemic excursions in people living with type 1 diabetes: a systematic review and meta-analysis. Pediatr Diabetes. 2022;23(8):1635-1646. [DOI] [PubMed] [Google Scholar]
- 16. Bozzetto L, Alderisio A, Clemente G, et al. Gastrointestinal effects of extra-virgin olive oil associated with lower postprandial glycemia in type 1 diabetes. Clin Nutr. 2019;38(6):2645-2651. [DOI] [PubMed] [Google Scholar]
- 17. Thomsen C, Rasmussen O, Lousen T, et al. Differential effects of saturated and monounsaturated fatty acids on postprandial lipemia and incretin responses in healthy subjects. Am J Clin Nutr. 1999;69(6):1135-1143. [DOI] [PubMed] [Google Scholar]
- 18. Lodefalk M, Aman J, Bang P. Effects of fat supplementation on glycaemic response and gastric emptying in adolescents with type 1 diabetes. Diabet Med. 2008;25(9):1030-1035. [DOI] [PubMed] [Google Scholar]
- 19. Annuzzi G, Bozzetto L, Cataldo A, Criscuolo S, Pesola M. Predicting and monitoring blood glucose through nutritional factors in type 1 diabetes by artificial neural networks. ACTA IMEKO. 2023;12:1-7. [Google Scholar]
- 20. Smith TA, Marlow AA, King BR, Smart CE. Insulin strategies for dietary fat and protein in type 1 diabetes: a systematic review. Diabet Med. 2021;38(11):e14641. [DOI] [PubMed] [Google Scholar]
- 21. Lithgow K, Edwards A, Rabi D. Smartphone app use for diabetes management: evaluating patient perspectives. JMIR Diabetes. 2017;2(1):e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alfonsi JE, Choi EEY, Arshad T, et al. Carbohydrate counting app using image recognition for youth with type 1 diabetes: pilot randomized control trial. JMIR Mhealth Uhealth. 2020;8(10):e22074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Alderisio A, Bozzetto L, Franco L, Riccardi G, Rivellese AA, Annuzzi G. Long-term body weight trajectories and metabolic control in type 1 diabetes patients on insulin pump or multiple daily injections: a 10-year retrospective controlled study. Nutr Metab Cardiovasc Dis. 2019;29(10):1110-1117. [DOI] [PubMed] [Google Scholar]
- 24. Castañeda J, Mathieu C, Aanstoot HJ, et al. Predictors of time in target glucose range in real-world users of the MiniMed 780G system. Diabetes Obes Metab. 2022;24(11):2212-2221. [DOI] [PubMed] [Google Scholar]
- 25. Messer LH, Breton MD. Therapy settings associated with optimal outcomes for t:slim X2 with control-IQ technology in real-world clinical care. Diabetes Technol Ther. 2023;25(12):877-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Biró G, Hulshof KF, Ovesen L, Amorim Cruz JA, EFCOSUM Group. Selection of methodology to assess food intake. Eur J Clin Nutr. 2002;56(suppl 2):S25-S32. [DOI] [PubMed] [Google Scholar]