Abstract
Background:
In-hospital hyperglycemia poses significant risks for patients with diabetes mellitus undergoing coronary artery bypass graft (CABG) surgery. Electronic glycemic management systems (eGMSs) like InsulinAPP offer promise in standardizing and improving glycemic control (GC) in these settings. This study evaluated the efficacy of the InsulinAPP protocol in optimizing GC and reducing adverse outcomes post-CABG.
Methods:
This prospective, randomized, open-label study was conducted with 100 adult type 2 diabetes mellitus (T2DM) patients post-CABG surgery, who were randomized into two groups: conventional care (gCONV) and eGMS protocol (gAPP). The gAPP used InsulinAPP for insulin therapy management, whereas the gCONV received standard clinical care. The primary outcome was a composite of hospital-acquired infections, renal function deterioration, and symptomatic atrial arrhythmia. Secondary outcomes included GC, hypoglycemia incidence, hospital stay length, and costs.
Results:
The gAPP achieved lower mean glucose levels (167.2 ± 42.5 mg/dL vs 188.7 ± 54.4 mg/dL; P = .040) and fewer patients-day with BG above 180 mg/dL (51.3% vs 74.8%, P = .011). The gAPP received an insulin regimen that included more prandial bolus and correction insulin (either bolus-correction or basal-bolus regimens) than the gCONV (90.3% vs 16.7%). The primary composite outcome occurred in 16% of gAPP patients compared with 58% in gCONV (P < .010). Hypoglycemia incidence was lower in the gAPP (4% vs 16%, P = .046). The gAPP protocol also resulted in shorter hospital stays and reduced costs.
Conclusions:
The InsulinAPP protocol effectively optimizes GC and reduces adverse outcomes in T2DM patients’ post-CABG surgery, offering a cost-effective solution for inpatient diabetes management.
Keywords: type 2 diabetes mellitus, digital health protocol, in-hospital hyperglycemia, insulin therapy, medical informatics applications, non-critically ill inpatients
Introduction
Patients with diabetes mellitus (DM) face an elevated risk of hospitalization due to conditions such as coronary, cerebrovascular, peripheral vascular diseases, and infections.1,2 In-hospital hyperglycemia (HH) is prevalent among this population, with estimates ranging from 22% to 46% in general hospitals.3,4 Notably, rates soar to 70% to 80% among those hospitalized for acute coronary syndromes and cardiac surgeries, irrespective of prior diabetes diagnosis. 5
Failure to promptly diagnose and manage HH poses significant risks, including a six-fold increase in nosocomial infections, hindered recovery post-acute myocardial infarction and stroke, heightened thrombotic event risks, and other adverse clinical outcomes.6,7 Despite the critical role of insulin therapy in managing complications, achieving optimal glycemic control (GC) remains challenging. 8 Studies have highlighted shortcomings in guideline adherence globally, with up to 50% of patients in the United States 9 and 75% in Brazil receiving inadequate insulin therapy. 10 Barriers to adherence include limited understanding of complications, insufficient training, and fear of hypoglycemia.11,12
Digital health applications have shown promise in aiding GC, especially for non-critically ill patients. 13 However, few randomized trials have evaluated specifically the impact of in-hospital insulin therapy in adverse nosocomial outcomes, beyond the intensive care unit (ICU) or perioperative settings. 14 In addition, existing tools often lack support for Portuguese language or fail to accommodate insulins provided by the Brazil’s Unified Health System (“Sistema Único de Saúde”).
Addressing this gap, InsulinAPP emerged in 2015 as an innovative digital solution, aimed at standardizing and facilitating insulin prescriptions for managing HH. 15 A prior study by Toyoshima et al 15 demonstrated its efficacy, reporting a 30% reduction in blood glucose levels with minimal hypoglycemia. Jones et al 16 further evaluated this electronic glycemic management system (eGMS), endorsing its quality, usability, and versatility as a user-friendly tool for physicians navigating hospital hyperglycemia complexities.
In this context, our prospective and randomized study aims to assess whether optimizing GC, as per the InsulinAPP protocol, significantly mitigates adverse clinical outcomes associated with HH.
Methods
Participants and Study Design
A prospective, open-label, randomized study was conducted at the Instituto do Coração (InCor) of Hospital das Clinicas of São Paulo University School of Medicine (HCFMUSP). From January 2018 to December 2021, we enrolled 100 adult individuals with type 2 diabetes mellitus (T2DM) aged between 18 and 80 years, discharged from the ICU to stepdown wards for post-operative follow-up of coronary artery bypass graft (CABG) surgery.
The study adhered to the principles of the Declaration of Helsinki and the Guidelines for Good Pharmacoepidemiology Practices. Approval of the trial protocol was obtained from the Research Ethics Committee of HCFMUSP School of Medicine (Certificate of Presentation of Ethical Review: 82183417.3.0000.0068).
Eligibility Criteria
Eligible participants were adult patients with T2DM, in the postoperative period following CABG surgery, discharged from the ICU to stepdown wards. Non-inclusion criteria included hyperglycemia without a known history of diabetes (stress hyperglycemia), planned hospital discharge within 48 hours of randomization, occurrence of any composite of primary outcome event within 24 hours, and laboratory evidence of diabetic ketoacidosis upon admission.
Randomization
Resident and fellow physician teams (clusters) managing post-operative patients were randomly assigned to either conventional care group (gCONV) or the InsulinAPP protocol group (gAPP) using a computer-generated randomization process. The assignments were made at the beginning of the study period, and the intervention allocation was rotated bimonthly among the teams.
Procedures
In the ICU, patients received continuous intravenous insulin infusion, with doses according to the computerized institutional protocol of InsulinAPP-ICU (http://www.insulinapp-uti.com.br). There was a transition from intravenous to subcutaneous insulin therapy before discharge from the ICU to the stepdown unit (Figure 1).
Figure 1.
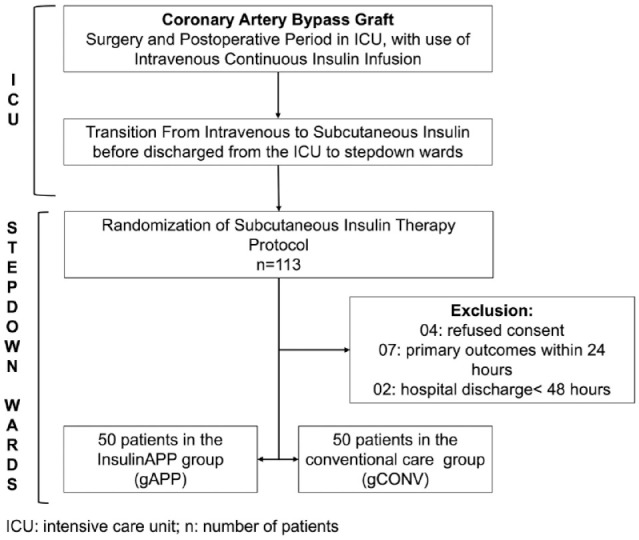
Organizational chart of patient flow in the study.
ICU: intensive care unit; n: number of patients.
In the stepdown unit, all patients received an insulin therapy using human insulins: Neutral Protamine Hagedorn (NPH) insulin as basal insulin and regular insulin as prandial and correctional insulin. The patients’ non-insulin diabetes medications were discontinued during hospitalization. The gCONV group received conventional care based on clinical experience, whereas the gAPP group followed the eGMS protocol (http://www.insulinapp.com.br), which recommended basal-bolus insulin therapy or bolus-correction scheme based on predefined criteria (Figure 1).
The application recommends an initial basal-bolus insulin therapy regimen for individuals with T2DM whose previous total daily insulin dose (TDID) exceeded 0.2 units/kg/day or whose initial blood glucose (BG) levels were above 250 mg/dL. The basal-bolus insulin regime consisted of 50% of TDID allocated to basal insulin using NPH three times daily (before breakfast, before lunch, and at bedtime) and the remaining 50% for prandial insulin (regular insulin three times daily, prior to meals). If a TDID was less than 0.2 units/kg/day and BG levels were below 250 mg/dL, the recommendation of the insulin prescription was with a bolus-correction scheme, which corresponds to a fixed pre-prandial dose (regular insulin three times daily, prior to meals), associated with a correction insulin dose. InsulinAPP also helps in deciding how to manage patients’ GC throughout their hospitalization. If the patient remained hyperglycemic with glycemic averages above 180 mg/dL during reassessments and using the insulin bolus-correction scheme, it suggests switching to the basal-bolus insulin scheme. Our group detailed the protocol in a previously published article. 17
Point-of-care BG levels were assessed four times a day (pre-breakfast, pre-lunch, pre-dinner, and at bedtime). The BG concentrations between 100 and 180 mg/dL were considered within the therapeutic range. Hypoglycemia was defined as a BG less than 70 mg/dL.
The patients underwent surgery according to the institutional standard protocol, performed by the same surgical team. Electronic prescriptions determined the insulin protocol, frequency of bedside glucose testing, and diet orders. Follow-up data were collected from the patient’s electronic medical record.
Outcomes
The primary outcome encompassed hospital-acquired infection, deterioration of renal function, and symptomatic atrial arrhythmia with a high ventricular response. Events were counted from the second day post-randomization until 30 days post-hospital discharge. The first day following randomization was excluded, as the intervention was expected to have a minimal impact on GC and, consequently, a minor effect on clinical outcomes.
The events of the primary composite outcome were defined as follows:
Hospital-acquired infection: the need for a new course of antibiotic therapy (excluding prophylactic use). 18
Deterioration of renal function: an increase of 50% in creatinine or ≥0.3 mg/dL compared to the ICU discharge value. 19
Symptomatic atrial arrhythmia with a high ventricular response: newly diagnosed atrial fibrillation/flutter that required treatment, identified by electrocardiographic parameters along with symptoms of palpitation, embolism, or low flow. 20
The BG measurements were taken starting from the first 24 hours of protocol inclusion until the primary outcome event. If the patient did not experience any of the events considered in the composite outcome during their hospital stay, glucose levels were analyzed until hospital discharge.
Sample Size and Power Calculation
The power calculation was formulated based on the preceding RABBIT-2 Surgery study, which demonstrated a reduction in the composite outcome, including wound infection, pneumonia, bacteremia, respiratory failure, and acute renal failure in the basal-bolus group (8.6% vs 24.3% in the sliding scale insulin [SSI] group, P < .010). 21 Furthermore, prior studies that specifically evaluated cardiac surgery showed a prevalence of nosocomial complications during the post-operative period in approximately 30% to 50% of individuals with diabetes. 22 Hence, presuming an incidence of adverse outcomes in 50% of individuals in the conservative group and 20% in the gAPP and assuming an alpha-error rate of 5%, we estimated that a sample of 50 subjects per group was necessary to achieve 90% statistical power.
Statistical Analysis
Study results were scrutinized on an intention-to-treat basis. Study population data and clinical outcomes were described using proportions. Variables with a normal distribution are presented as the mean ± standard deviation (SD), whereas variables with non-normal distributions are expressed as the median and interquartile range (p25; p75). For comparison of quantitative variables with a normal distribution, unpaired Student’s t-test was employed. If data normalization was achieved only after logarithmic transformation, the transformed variable was used. Non-parametric data were analyzed using the Mann-Whitney test. Categorical variables were expressed as frequencies and percentages and compared using the chi-square test (χ2).
A multivariate logistic regression analysis was performed to evaluate the factors associated with the primary composite outcome of acute kidney injury, atrial arrhythmias, and nosocomial infection. The analysis included confounding factors based on prior literature and clinical relevance.
For statistical analysis and the construction of graphs, Stata 15.1 software (College Station, Texas) was used. A two-tailed P-value less than .050 was deemed statistically significant throughout the analysis.
Results
Participants Characteristics
The study included 100 individuals, with 64% male and 82% identifying as white people, with a mean age of 64.2 ± 9.1 years. There were no significant demographic differences between the gAPP and gCONV groups at hospital admission (Table 1).
Table 1.
Demographic, Clinical, and Laboratory Characteristics of Participants at Hospital Admission.
| Variable | Total (N = 100) | Conventional | InsulinAPP | P |
|---|---|---|---|---|
| Study population, N | 100 | 50 | 50 | - |
| Age (years) a | 64.2 ± 9.1 | 64.4 ± 9.9 | 63.9 ± 8.3 | .979 |
| Female gender | 36 (36%) | 20 (40%) | 16 (32%) | .405 |
| Reported race (white/black and brown/others), N | 82 /15/2 | 41/7/2 | 42/8/0 | .148 |
| Weight (kg) a | 77.6 ± 14.9 | 79.2 ± 15.6 | 76.1 ± 14.2 | .290 |
| BMI (kg/m2) a | 28.6 ± 4.9 | 29.4 ± 5.2 | 27.8 ± 4.6 | .132 |
| HbA1c (%) b | 7.4 (6.5; 8.6) | 7.2 (6.5; 8.6) | 7.5 (6.4; 8.5) | .570 |
| FBG (mg/dL) a | 166.7 ± 72.2 | 160.2 ± 78.1 | 173.1 ± 66.2 | .184 |
| Serum creatinine (mg/dL) b | 1.04 (0.89; 1.27) | 1.06 (0.90; 1.34) | 1.02 (0.89; 1.19) | .390 |
Data are presented as amean ± SD; bmedian (p25; p75) or n (%); unless otherwise noted.
BMI: body mass index; HbA1c: glycated hemoglobin; FBG: fasting blood glucose; N: number of patients; SD. standard deviation.
Surgical Details
The mean surgical duration was 370 ± 112 minutes, with 80% of procedures using extracorporeal circulation (ECC) (Table 2). Internal mammary artery grafting was universal, with 9% undergoing bilateral grafting. Saphenous vein graft was used in 90% of procedures.
Table 2.
Surgical Details and Clinical and Laboratory Parameters at Discharge From the ICU.
| Variable | Total (N = 100) | Conventional (N = 50) | InsulinApp (N = 50) | P |
|---|---|---|---|---|
| Emergency admission | 36 (36%) | 19 (38%) | 17 (34%) | .793 |
| Surgical time (minutes) a | 370 ± 112 | 383 ± 84 | 356 ± 134 | .240 |
| ECC use | 80 (80%) | 41 (82%) | 39 (78%) | .617 |
| ECC time (minutes) a | 100 ± 31 | 101 ± 38 | 99 ± 22 | .995 |
| Anoxia time (minutes) a | 81 ± 31 | 83 ± 38 | 80 ± 23 | .835 |
| Number of bridges b | 3 (3; 4) | 3 (3; 4) | 3 (3; 4) | .820 |
| ICU length of stay (days) b | 4 (4; 6) | 5 (4; 6) | 4 (3; 5) | .210 |
| Serum creatinine (mg/dL) c a | 1.32 ± 1.01 | 1.29 ± 0.48 | 1.35 ± 1.35 | .122 |
| Estimated glomerular filtration rate (mL/min per 1.73 m2)a,c | 65.32 ± 27.30 | 60.41 ± 26.93 | 70.44 ± 27.01 | .244 |
| Hematocrit (%)a,c | 28.22 ± 4.02 | 28.66 ± 4.01 | 27.78 ± 4.03 | .280 |
| Leukocytes (×103/mm3)a,c | 10.00 ± 3.48 | 10.34 ± 4.14 | 9.64 ± 2.65 | .580 |
| C-reactive protein (mg/L)a,c | 147.28 ± 76.83 | 147.74 ± 82.29 | 146.82 ± 71.79 | .958 |
| APACHE II scoreb,c | 10 (9; 12) | 11 (9; 13) | 10 (8; 12) | .225 |
| Duration of CIIb,c | 17.50 (11.00; 32.25) | 20.50 (13.00; 33.00) | 15.50 (9.25; 30.25) | .087 |
| Total intravenous insulin therapy (units)b,c | 262.50 (165.00; 483.75) | 291.10 (184.60; 468.60) | 230.95 (137.82; 450.72) | .123 |
Data are presented as amean ± SD; bmedian (p25; p75) or n (%).
Data obtained upon discharge from the ICU.
CII: continuous insulin infusion; ECC: extracorporeal circulation; ICU: intensive care unit; N: number of patients; SD: standard deviation; APACHE: Acute Physiology and Chronic Health Evaluation.
Post-operative Management
The median ICU stay was 4 days (4; 6 days) for all individuals, which intravenous insulin administered to maintain serum glucose levels between 140 and 180 mg/dL. Ventilatory and hemodynamic parameters did not significantly differ between groups (Table 2).
Glycemic Control and Insulin Therapy
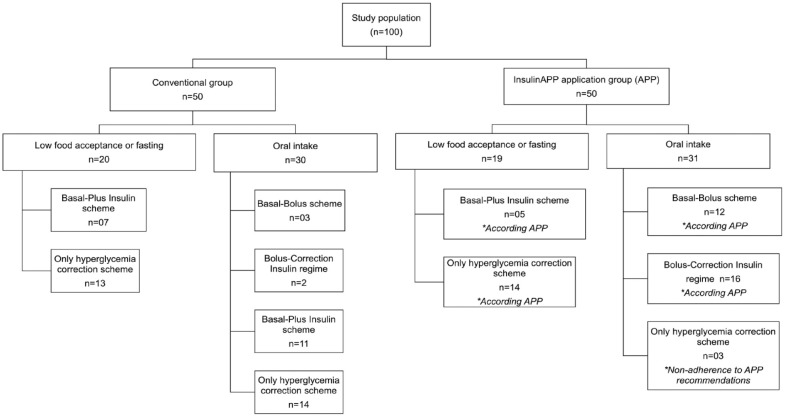
The median preoperative HbA1c was 7.4%, with no statistical difference between groups (Table 1). Initial post-randomization BG levels were comparable between the groups. However, subsequent mean glucose levels were lower in the gAPP compared with the gCONV (167.2 ± 42.5 vs 188.7 ± 54.4 mg/dL; P = .040) (Figure 2), with lower glycemic dispersion (20.2 ± 11.0 vs 26.4 ± 12.2%, P = .020) and fewer percentage of patients-day with BG above 180 mg/dL (51.3% vs 74.8%, respectively, P = .011) (Table 3). In the gAPP group, 90.3% of individuals on oral diets received an insulin regimen that included prandial bolus and correction insulin (either bolus-correction or basal-bolus regimens) compared with 16.7% in the gCONV (P = .001) (Figure 3). Adherence to the app-guided treatment in the gAPP was 94%.
Figure 2.
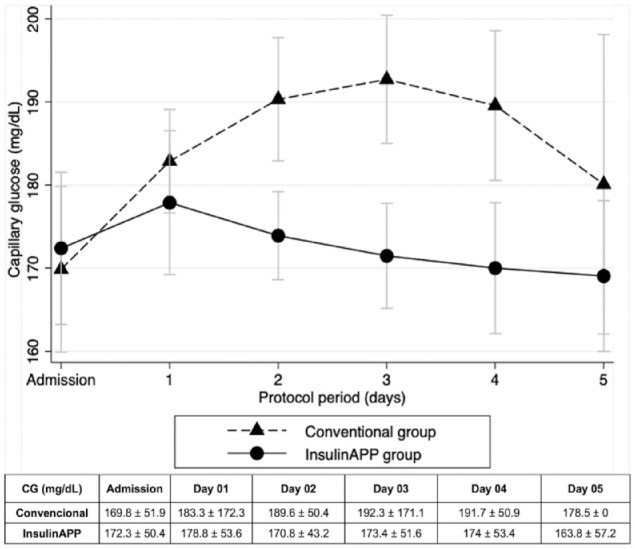
Average daily blood glucose throughout the study.
Table 3.
Details of Glycemic Control And Insulin Therapy After Randomization.
| Variable | Total (N = 100) | Conventional (N = 50) | InsulinAPP (N = 50) | P |
|---|---|---|---|---|
| BG prior to randomization (mg/dL) | 171 ± 51 | 170 ± 52 | 172 ± 50 | .839 |
| Initial prescription | ||||
| Only correction scheme | 44 (44%) | 27 (54%) | 17 (34%) | .001 |
| Bolus-correction | 18 (18%) | 2 (4%) | 16 (32%) | - |
| Basal-plus | 23 (23%) | 18 (36%) | 5 (10%) | - |
| Basal-bolus | 15 (15%) | 3 (6%) | 12 (24%) | - |
| Total daily insulin dose (unit/day) | 25.8 ± 23.9 | 23.5 ± 25.4 | 28.1 ± 22.3 | .207 |
| Insulin to body weight ratio (unit/kg/day) | 0.34 ± 0.32 | 0.31 ± 0.33 | 0.38 ± 0.31 | .250 |
| NPH insulin dose (unit/day) | 24.8 ± 10.4 | 27.0 ± 12.2 | 22.1 ± 7.1 | .160 |
| Regular insulin dose (unit/day) | 16.4 ± 17.4 | 12.1 ± 16.6 | 20.6 ± 17.3 | .002 |
| Capillary glucose (mg/dL) | 177.9 ± 49.7 | 188.7 ± 54.4 | 167.2 ± 42.5 | .040 |
| Glycemic dispersion (%) | 23.3 ± 12.0 | 26.4 ± 12.2 | 20.2 ± 11.0 | .020 |
| Proportion of days with average GC on target (100-180 mg/dL) | 53 (53%) | 23 (46%) | 30 (60%) | .161 |
| Percentage of patient-days with any glucose >180 mg/dL (%) | 63.1 | 74.8 | 51.3 | .011 |
| Number of patients with hypoglycemia (<70 mg/dL) | 10 (10%) | 8 (16%) | 2 (4%) | .046 |
| Low food acceptance or fasting | 39 (39%) | 20 (40%) | 19 (38%) | .838 |
| Use of prandial insulin in patients with oral intake | 33/61 (54.1%) | 5/30 (16.7%) | 28/31 (90.3%) | .001 |
Data are presented as mean ± SD; or n (%); unless otherwise noted.
BG: blood glucose; GC: glycemic control; NPH: Neutral Protamine Hagedorn.
Figure 3.
Flowchart of the insulin protocol used in study subjects.
Clinical Outcomes
The primary composite outcome was significantly lower in the gAPP group (16%) compared with the gCONV group (58%) (P < .001) (Table 4). Hospital stays and costs were significantly reduced in gAPP (Table 4). The readmission rate was higher in gCONV, but not significantly different (Table 4). Post-pericardiotomy syndrome incidence did not differ between groups (11% vs 13%, P = .640). No deaths occurred until hospital discharge or within 30 days after the procedure.
Table 4.
Composite Clinical Outcomes and Hospital Acquired Events.
| Variable | Total (N = 100) | Conventional (N = 50) | InsulinAPP (N = 50) | P |
|---|---|---|---|---|
| Primary composite outcome | 37 (37%) | 29 (58%) | 8 (16%) | <.001 |
| Hospital stays duration post-randomization (days) a | 14.2 ± 16.0 | 18.6 ± 17.7 | 9.8 ± 12.8 | <.001 |
| Average cost per hospital stays (US$) a | 2947.31 ± 1536.64 | 3231.00 ± 1937.93 | 2663.63 ± 922.46 | .012 |
| Re-hospitalization rate | 14 (14%) | 9 (18%) | 5 (10%) | .249 |
Data are presented as amean ± SD or n (%).
Primary composite outcome: acute kidney injury, atrial arrhythmias, and nosocomial infection.
N: number of patients; SD: standard deviation.
Risk Factors for Primary Outcomes
The first outcome occurred in the gCONV after a median of 5 (4-10) days, whereas in the gAPP, it was 7 (4; 18) days, with no significant difference (P = .740). However, the Kaplan-Meier curve for the cumulative risk of hospital complications showed a greater chance of events in the gCONV than in the gAPP, with a hazard ratio of 2.17 (95% confidence interval [CI] = 1.28-3.66, P = .003) (Figure 4).
Figure 4.
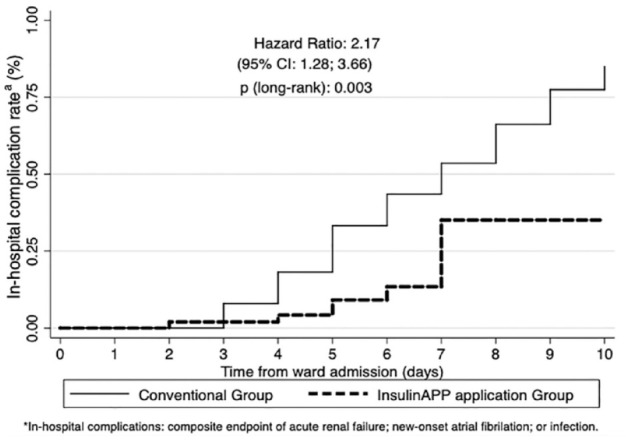
Kaplan-Meier curve for cumulative risk of hospital complications.
aIn-hospital complications: composite endpoint of acute renal failure, new-onset atrial fibrillation, or infection.
In the logistic regression analysis, four confounding factors were defined based on clinical relevance and prior literature: intervention group (eGMS vs conventional), HbA1c >8.5%, 23 extracorporeal circulation, 24 and female gender. 25 These parameters were significant predictors in the univariate analysis. The multivariate analysis confirmed that the intervention group, extracorporeal circulation, and gender remained significant predictors, with adjusted odds ratio (OR) indicating the strength and direction of these associations. The detailed ORs, confidence intervals, coefficients, and P-values for each variable are presented in Table 5.
Table 5.
Univariate and Multivariate Logistic Regression Analysis of Factors Associated With the Primary Composite Outcome.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Variable | Odds ratio (95% CI) | Coefficient | P | Odds ratio (95% CI) | Coefficient | P |
| InsulinAPP group | 0.14 (0.05-0.35) | -1.981 | <.001 | 0.10 (0.03-0.32) | -2.276 | <.001 |
| HbA1c >8.5% at admission | 2.30 (0.92-5.79) | 0.834 | .076 | 2.06 (0.63-6.73) | 0.722 | .232 |
| Extracorporeal circulation | 4.19 (1.14-15.45) | 1.432 | .031 | 5.61 (1.19-26.55) | 1.724 | .030 |
| Female gender | 5.13 (2.12-12.44) | 1.636 | <.001 | 6.63 (2.19-20.09) | 1.891 | .001 |
Hypoglycemia and Safety
Hypoglycemia was less prevalent in the gAPP (4%) compared with the gCONV group (16%) (P = .046) (Table 3). In the gCONV group, 75% of hypoglycemia cases occurred in individuals on an insulin correction regimen, and 25% occurred in those on a basal-plus regimen. In the gAPP group, half of hypoglycemic events occurred in individuals on a bolus-correction regimen, and the other half occurred in those on a basal-bolus insulin regimen.
Discussion
Our study presents a paradigm shift in in-hospital insulin therapy for non-critically ill patients, showcasing the efficacy and safety of the digital protocol over conventional care. Notably, eGMS not only improved GC but also mitigated adverse clinical outcomes, including infection, acute kidney injury, and new atrial arrhythmias. Remarkably, our study fills a crucial gap in the literature by providing prospective evidence of a digital tool’s efficacy in enhancing glycemic parameters and reducing hospital complications.
Consensus and guidelines have recommended using an alternative insulin regimen to the basal-bolus regimen for patients with less severe hyperglycemia.26,27 The app’s emphasis on bolus-correction regimen as an alternative insulin therapy regimen is based on the fact that post-prandial hyperglycemia generally occurs earlier in the pathophysiology of diabetes. 28 This may explain why the contribution of post-prandial glucose predominates in patients with fairly good control, whereas the contribution of fasting hyperglycemia increases as GC worsens. 29 A retrospective study with the use of InsulinAPP in patients in a hospitalist ward showed that 93% of blood glucose measurements were within the target range of 70 to 180 mg/dL with the use of the insulin bolus-correction scheme and without episodes of hypoglycemia. 15 In addition, this scheme allows the use of only one type of insulin, making it easier to prescribe insulin. Our findings highlight the superiority of a protocol incorporating prandial insulin over correction insulin alone, aligning with previous literature demonstrating the therapeutic success of basal-bolus and basal-plus regimens over SSI regimen.21,30 This strategic balance not only resulted in a lower average glycemia but also exhibited a reduction in the glucose variation coefficient, few instances of significant hyperglycemia, and decreased prevalence of hypoglycemia.
Furthermore, InsulinAPP’s adaptive nature challenges conventional guidelines, offering alternative regimens tailored to individual patient needs. The safety profile of our digital tool is noteworthy, with a significantly lower incidence of hypoglycemia compared with conventional care. Moreover, our intervention exhibits tangible benefits, translating into reduced post-operative complications and costs. The financial implications are compelling, with better GC correlating with reduced costs and hospital stays.
Our study does have various limitations, including reflecting the activities of a single academic cardiology center and limited statistical power for severe hypoglycemia detection. Future multicenter studies across diverse medical settings and ethnic groups are necessary to confirm the generalizability of our findings. Despite limitations, our study provides valuable insights into the utility of InsulinAPP. The broader applicability of our findings reinforces the safety and efficacy of human insulin use, particularly in resource-constrained settings like public hospitals in Brazil and other developing countries.
Conclusions
In conclusion, our study demonstrates that GC guided by the InsulinAPP digital protocol significantly improves glycemic profiles and reduces unfavorable clinical outcomes in noncritical post-operative cardiac surgery patients discharged from the ICU. The protocol’s effectiveness in reducing hyperglycemia rates translates into shorter hospital stays and reduced resource utilization compared with conventional treatment. These findings underscore the potential of eGMS as a cost-effective and accessible solution for optimizing in-hospital insulin therapy in similar patient populations.
Footnotes
Abbreviations: AKI, acute kidney injury; BG: blood glucose; BMI, body mass index; CABG, coronary artery bypass grafting; CI, confidence interval; DM, diabetes mellitus; ECC, extracorporeal circulation; eGMS, electronic glycemic management system; gAPP, InsulinAPP application group; GC, glycemic control; gCONV, conventional protocol group; HbA1c, glycated hemoglobin; HH, in-hospital hyperglycemia; HR, hazard ratio; ICU, intensive care unit; InCor, Instituto do Coração; n, number of individuals; NPH, Neutral Protamine Hagedorn; p25; p75, interquartile range; SD, standard deviation; SSI, sliding scale insulin; T2DM, type 2 diabetes mellitus; TDID, total daily insulin dose.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Alexandre Barbosa Câmara de Souza  https://orcid.org/0000-0002-4029-3667
https://orcid.org/0000-0002-4029-3667
Marcos Tadashi Kakitani Toyoshima  https://orcid.org/0000-0002-9146-4606
https://orcid.org/0000-0002-9146-4606
Marcia Nery  https://orcid.org/0000-0003-2415-9668
https://orcid.org/0000-0003-2415-9668
References
- 1. Trence DL, Kelly JL, Hirsch IB. The rationale and management of hyperglycemia for in-patients with cardiovascular disease: time for change. J Clin Endocrinol Metab. 2003;88(6):2430-2437. doi: 10.1210/JC.2003-030347. [DOI] [PubMed] [Google Scholar]
- 2. Sawin G, Shaughnessy AF. Glucose control in hospitalized patients. Am Fam Physician. 2010;81:1121-1124. [PubMed] [Google Scholar]
- 3. Swanson CM, Potter DJ, Kongable GL, Cook CB. Update on inpatient glycemic control in hospitals in the United States. Endocr Pract. 2011;17(6):853-861. doi: 10.4158/EP11042.OR. [DOI] [PubMed] [Google Scholar]
- 4. Dhatariya K, Corsino L, Umpierrez GE. Management of diabetes and hyperglycemia in hospitalized patients (Updated 30 December 2020). In: Feingold KR, Anawalt B, Blackman MR, et al., eds. Endotext [Internet]. South Dartmouth, MA: MDText.com, Inc.; 2000. https://www.ncbi.nlm.nih.gov/books/NBK279093/ [Google Scholar]
- 5. Tsai LL, Jensen HA, Thourani VH. Intensive glycemic control in cardiac surgery. Curr Diab Rep. 2016;16:1-9. doi: 10.1007/s11892-016-0719-5. [DOI] [PubMed] [Google Scholar]
- 6. Reyes-Umpierrez D, Davis G, Cardona S, et al. Inflammation and oxidative stress in cardiac surgery patients treated to intensive versus conservative glucose targets. J Clin Endocrinol Metab. 2017;102:309-315. doi: 10.1210/jc.2016-3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kafaki SB, Alaedini K, Qorbani A, Asadian L, Haddadi K. Hyperglycemia: a predictor of death in severe head injury patients. Clin Med Insights Endocrinol Diabetes. 2016;9:43-46. doi: 10.4137/CMED.S40330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Umpierrez G, Cardona S, Pasquel F, et al. Randomized controlled trial of intensive versus conservative glucose control in patients undergoing coronary artery bypass graft surgery: GLUCOCABG trial. Diabetes Care. 2015;38(9):1665-1672. doi: 10.2337/dc15-0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wexler DJ, Meigs JB, Cagliero E, Nathan DM, Grant RW. Prevalence of hyper- and hypoglycemia among inpatients with diabetes: a national survey of 44 U.S. Diabetes Care. 2007;30(2):367-369. doi: 10.2337/DC06-1715. [DOI] [PubMed] [Google Scholar]
- 10. Moreira ED, Silveira PCB, Neves RCS, Souza C, Nunes ZO, Almeida MDCC. Glycemic control and diabetes management in hospitalized patients in Brazil. Diabetol Metab Syndr. 2013;5. doi: 10.1186/1758-5996-5-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aloi JA, Mulla C, Ullal J, Lieb DC. Improvement in inpatient glycemic care: pathways to quality. Curr Diab Rep. 2015;15(4):18. doi: 10.1007/S11892-015-0587-4. [DOI] [PubMed] [Google Scholar]
- 12. Ham P. Glycemic control in the hospital: what to do when experts disagree. Am Fam Physician. 2010;81:1078-1080. [PubMed] [Google Scholar]
- 13. Morris AH, Stagg B, Lanspa M, et al. Enabling a learning healthcare system with automated computer protocols that produce replicable and personalized clinician actions. J Am Med Inform Assoc. 2021;28:1330-1344. doi: 10.1093/jamia/ocaa294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gianchandani R, Umpierrez GE. Inpatient use of computer-guided insulin devices moving into the non-intensive care unit setting. Diabetes Technol Ther. 2015;17(10):673-675. doi: 10.1089/dia.2015.0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Toyoshima MTK, Brandes PHR, Lauterbach G, et al. InsulinAPP application protocol for the inpatient management of type 2 diabetes on a hospitalist-managed ward: a retrospective study. Arch Endocrinol Metab. 2022;66(4):498-505. doi: 10.20945/2359-3997000000496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jones JML, Feitosa ACR. Estudo de validação de aplicativo para insulinização de pacientes com diabetes hospitalizados: InsulinAPP [Dissertação de Mestrado]. Escola Bahiana de Medicina e Saúde Pública, 2020. [Google Scholar]
- 17. Toyoshima MT, de Souza AB, Admoni SN, et al. New digital tool to facilitate subcutaneous insulin therapy orders: an inpatient insulin dose calculator. Diabetol Metab Syndr. 2015;7:114. doi: 10.1186/s13098-015-0111-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pomposelli JJ, Baxter JK, 3rd, Babineau TJ, et al. Early postoperative glucose control predicts nosocomial infection rate in diabetic patients. JPEN J Parenter Enteral Nutr. 1998;22(2):77-81. doi: 10.1177/014860719802200277. [DOI] [PubMed] [Google Scholar]
- 19. Mehta RL, Kellum JA, Shah SV, et al. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/CC5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Estrada CA, Young JA, Nifong LW, Chitwood WR. Outcomes and perioperative hyperglycemia in patients with or without diabetes mellitus undergoing coronary artery bypass grafting. Ann Thorac Surg. 2003;75:1392-1399. doi: 10.1016/S0003-4975(02)04997-4. [DOI] [PubMed] [Google Scholar]
- 21. Umpierrez GE, Smiley D, Jacobs S, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care. 2011;34(2):256-261. doi: 10.2337/dc10-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Whang W, Bigger JT., Jr. Diabetes and outcomes of coronary artery bypass graft surgery in patients with severe left ventricular dysfunction: results from the CABG Patch Trial database. J Am Coll Cardiol. 2000;36(4):1166-1172. doi: 10.1016/S0735-1097(00)00823-8. [DOI] [PubMed] [Google Scholar]
- 23. Halkos ME, Puskas JD, Lattouf OM, et al. Elevated preoperative hemoglobin A1c level is predictive of adverse events after coronary artery bypass surgery. J Thorac Cardiovasc Surg. 2008;136(3):631-640. doi: 10.1016/J.JTCVS.2008.02.091. [DOI] [PubMed] [Google Scholar]
- 24. Martin ET, Kaye KS, Knott C, et al. Diabetes and risk of surgical site infection: a systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2016;37(1):88-99. doi: 10.1017/ICE.2015.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Antunes PE, de Oliveira JF, Antunes MJ. Coronary surgery in patients with diabetes mellitus: a risk-adjusted study on early outcome. Eur J Cardiothorac Surg. 2008;34(2):370-375. doi: 10.1016/j.ejcts.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 26. Korytkowski MT, Muniyappa R, Antinori-Lent K, et al. Management of hyperglycemia in hospitalized adult patients in non-critical care settings: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2022;107:2101-2128. doi: 10.1210/CLINEM/DGAC278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pasquel FJ, Lansang MC, Dhatariya K, Umpierrez GE. Management of diabetes and hyperglycaemia in the hospital. Lancet Diabetes Endocrinol. 2021;9(3):174-188. doi: 10.1016/S2213-8587(20)30381-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pratley RE, Weyer C. The role of impaired early insulin secretion in the pathogenesis of type II diabetes mellitus. Diabetologia. 2001;44(8):929-945. doi: 10.1007/S001250100580. [DOI] [PubMed] [Google Scholar]
- 29. Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA(1c). Diabetes Care. 2003;26(3):881-885. doi: 10.2337/DIACARE.26.3.881. [DOI] [PubMed] [Google Scholar]
- 30. Nau KC, Lorenzetti RC, Cucuzzella M, Devine T, Kline J. Glycemic control in hospitalized patients not in intensive care: beyond sliding-scale insulin. Am Fam Physician. 2010;81:1130-1135. [PubMed] [Google Scholar]