Abstract
Background
The best strategy to achieve optimal reperfusion outcomes during mechanical thrombectomy remains to be defined. The RapidPulseTM Cyclic Aspiration System is a novel technology, delivering high-frequency pulsed vacuum forces to increase the efficiency of aspiration thrombectomy.
Methods
Prospective, multicenter, open-label, core lab-adjudicated, two-arm study comparing safety and efficacy of a feasibility version of the RapidPulseTM system compared with contemporary controls. Primary endpoint was the rate of mTICI ≥ 2c after first-pass effect (FPE). Additional efficacy endpoints were the rates of mTICI 2b after first pass (modified FPE (mFPE)), last pass with study device defined as frontline technical success, and after all passes including rescue therapy. The primary safety endpoints included symptomatic ICH (sICH) within 24 h post-procedure.
Results
Between February 2022 to December 2022, 80 subjects were consented and enrolled in the study (n = 40 treatment arm, n = 40 control arm). In the intent to treat (ITT) population, mean age was 67.8 ± 11.5 years; 19 (47.5%) were male. Median NIHSS score was 16 (IQR: 13–22). Median ASPECTS score was 9 (IQR: 8–10). The rate of mTICI ≥ 2c after first pass was 53.9% in ITT population (60.0% in per-protocol population) versus 38.5% in the corresponding control population. Functional independence (mRS 0-2) at 90 days was achieved in 61.1% (22/36) in the RapidPulseTM arm and 52.8% (19/36) in the control arm. In the RapidPulseTM arm, no sICH within 24 h and no device-related morbidity or mortality occurred.
Conclusion
Preliminary data suggests RapidPulseTM Aspiration System is highly effective and safe for recanalization of large vessel occlusions.
Keywords: Stroke, mechanical thrombectomy, cyclic aspiration, LVO
Background
The benefit of mechanical thrombectomy over medical treatment for acute ischemic stroke (AIS) patients with large vessel occlusions (LVOs) has been proven in many clinical trials.1–5 Subsequently, the first-pass effect (FPE), defined as obtaining near complete (TICI 2c) or full recanalization (TICI 3) in the first attempt, has emerged as the key factor to maximize the potential treatment effect. 6
Despite intense research, the best technique and device strategy to optimize first pass success remains elusive. None of the currently commercially available reperfusion strategies achieve the goal of excellent recanalization at first attempt in more than half of the cases.3,5,7,8 Similarly, randomized trials comparing aspiration thrombectomy versus stent retriever thrombectomy have failed to show superior recanalization results.9,10
The RapidPulseTM Aspiration System (RapidPulse, Miami, FL) is a novel product with a proprietary algorithm which alternates the pressure at the interface of the catheter opening and the clot between full vacuum and precise positive pressure.
The RapidPulse-FS study, completed with a feasibility version of this new technology, aimed to evaluate the initial safety and FPE performance of the RapidPulseTM Aspiration System in the treatment of the AIS patients with LVOs.
Materials & methods
Study device
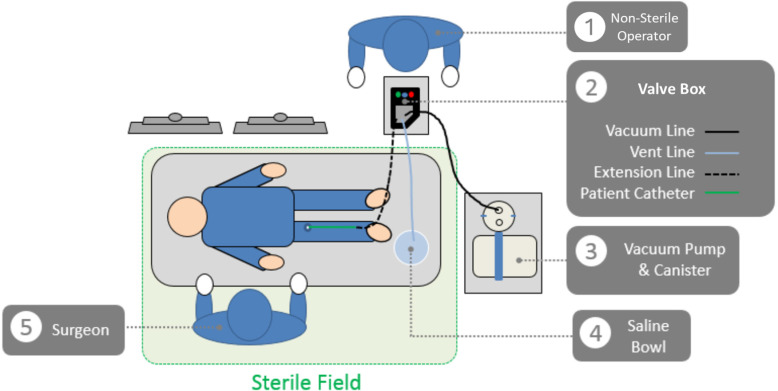
The RapidPulseTM Aspiration System is a novel thrombectomy system that is designed to remove thrombus from the neurovasculature by precisely pulsing aspiration. The feasibility version of the System consists of a Valve Box, which is a controllable valve system that intermittently cycles the vacuum pressure provided from a commercially available thrombectomy pump between fully off and fully on multiple times per second (Figure 1). The Valve Box is powered electrically (standard 120–220 V; AC power is transformed to 12 V DC) to alternately open and close the vacuum line and a saline-filled vent line. For this study, the system was used in conjunction with the commercially available Medtronic React 71 thrombectomy catheter and thrombectomy pump. The system is setup and operated by trained hospital staff; operation requires one operator within the sterile field to perform the thrombectomy while setup and operation of the non-sterile components are completed by an operator outside the sterile field. The diagram in Figure 2 depicts the configuration of the device and its integration into the angiosuite.
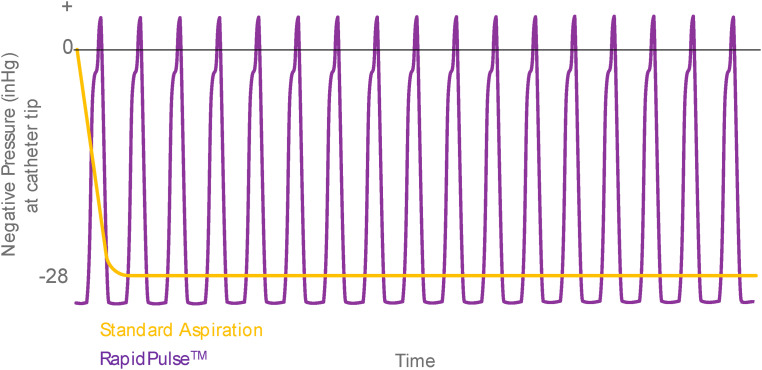
Figure 1.
Comparison of RapidPulseTM aspiration system algorithm to standard aspiration.
Note: Standard aspiration delivers a one-time effect when vacuum goes from “off” to “on.” The RapidPulseTM Aspiration System cycles from full vacuum to slight positive pressure at high frequency which increases the rate of clot ingestion without the distal propagation of clot material.
Figure 2.
The RapidPulse aspiration system.
Study design
The investigation was a prospective, multi-center, open-label trial which compared the safety and performance of 40 subjects treated with the RapidPulseTM Aspiration System and 40 non-randomized prospectively selected retrospective controls meeting identical study selection (inclusion/ exclusion) criteria, over a 7-month period.
The treatment arm used the React 071 (Medtronic, USA) catheter, a commercially available pump, and canister in conjunction with the RapidPulseTM Aspiration System. The control group was selected from the database of the participant centers and was treated with commercially available 0.070–0.072-inch inner diameter aspiration catheters and commercially available pumps within 12 months of the participating center's study initiation.
Independent review committees were established for the data quality assurance of the study. All adverse events through 90 days post-procedure and device failures were reviewed by an independent clinical events committee (CEC). The CEC adjudicated the events and determined seriousness, severity, and nature of the event as well as its relationship to the device or the procedure. An independent imaging core laboratory (ICL) provided blinded assessment of baseline imaging, all angiograms, and 24-h follow-up images. Functional independence at 90 days was evaluated by trained hospital staff personnel using the modified Rankin Scale, as per the site's standard of care procedures. Patients in the Treatment and Control arms, or their legally authorized representatives, provided written Informed Consent (IC) as required by local regulations using a form approved by each participating center's local Ethics Committee. A waiver of consent was obtained for 11 of the 40 patients in the Control arm as it was not required by the center's Ethics Committee for the collection of clinical data prior to study participation. The study was registered on clinicaltrials.gov (NCT05122637).
Patient population and participating centers
Five sites across Turkey, Brazil, Spain, Latvia, and Denmark enrolled 80 subjects. The patient population included patients (males and females) experiencing AIS secondary to intracranial LVO (intracranial internal carotid artery (ICA), M1, M2, basilar or vertebral arteries) identified within 24 h of symptom onset (or last seen normal). Five (5) investigational centers enrolled patients in the Treatment arm and three (3) of the five centers enrolled patients in the Control arm. The Ethics Committee did not approve retrospective enrollment of patients in the Control arm at one center and a second center only enrolled patients in the Treatment arm since it was initiated last into the study after enrollment in the Control arm cohort had concluded.
Adult patients (age 18 + years) who had confirmed LVOs on MR/CT-angiography or digital subtraction angiography involving intracranial internal carotid artery (ICA), the proximal middle cerebral artery (MCA, M1- or M2-segments), or the vertebrobasilar arteries, otherwise independent (pre-morbid mRS score 0-2 or pre-morbid mRS 0-1 for age 80 years), baseline NIHSS score of were eligible for the study. The thrombectomy procedure had to be performed within 24 h from stroke onset defined as time last known well. Baseline imaging inclusion criteria were defined as ASPECTS score 6 on non-contrast CT (or PC-ASPECTS 7 for vertebrobasilar occlusions), ischemic core lesion 70cc based on CT-perfusion (CTP, CBV 30%), or DWI-MRI or MR-perfusion. Patients who were ineligible to receive or had failed intravenous thrombolytics including tissue plasminogen-activator (t-PA) and/or Tenecteplase (TNK) were included. Key exclusion criteria included any evidence of intracranial hemorrhage, significant mass, and/or midline shift, 11 known or suspected ICAD and/or tandem occlusion that could prevent thrombus removal. The full inclusion/exclusion criteria are provided in Supplement material 1 and identically applied to the Treatment and Control groups.
Outcomes
Analysis population
The intent to treat (ITT) population included all subjects in whom the aspiration catheter was successfully navigated to the clot interface and the RapidPulseTM Aspiration System was used. Per-Protocol (PP) population included all ITT subjects with no major eligibility criteria violations. Study eligibility deviations were reviewed and determined prior to database lock.
Primary efficacy endpoint
The primary effectiveness endpoint was the FPE defined by mTICI 2c after one reperfusion attempt as assessed by the ICL. 12
Primary safety endpoints
The primary safety endpoints included symptomatic intracranial hemorrhage (sICH) within 24 h post-procedure, all device-related and procedure-related adverse events, and all-cause mortality through 90 days as assessed by an independent CEC. In addition, any new infarct on CT or MR, any embolism in previously uninvolved territory, and any intracranial hemorrhage according to the Heidelberg classification at 24 h were blindly assessed by the ICL. 13 sICH was defined, per the modified SITS-MOST definition, as local or remote parenchymal hemorrhage type 2 (PH2), subarachnoid hemorrhage (SAH), and/or intraventricular hemorrhage (IVH) on the post-treatment imaging scan, combined with a neurological deterioration of 4 points or more on the NIHSS from baseline, or from the lowest NIHSS value between baseline and 24 h, or leading to death that the CEC judged as causative of the deterioration. 14
Secondary endpoints
The prespecified secondary endpoints included the rates of (a) frontline technical success defined as mTICI 2b after the last pass with the study device, (b) final mTICI 2b after all passes including rescue therapy, (c) modified first-pass reperfusion effect (mFPE) defined as mTICI 2b after one device pass, and (d) functional independence defined by Modified Rankin Scale (mRS) score 0-2 at 90 days.
Statistical analysis
The primary objective of this study was to assess the initial technical performance, effectiveness, and safety of the RapidPulseTM Aspiration System in comparison to the standard of care control methods as a means of determining a reasonable potential effect size for a pivotal study design. As this is a feasibility study, statistical analysis and sample size justification were based on two-sided 95% confidence intervals (CIs) and no formal statistical hypotheses were tested. Two study populations in the treatment arm consisting of an ITT and PP population were compared to the control arm population which included subjects with successful device navigation to the treatment location. The ITT population included all subjects with an attempted RapidPulseTM Aspiration System procedure (defined as cases where the aspiration catheter could be successfully navigated into the proximal face of the clot and the RapidPulseTM Aspiration System was attempted). The PP population included all subjects with no major protocol deviations; major deviations included deviations in study enrollment such as deviation in IC process and eligibility criteria.
Descriptive statistical summaries and formal comparisons were performed to characterize the RapidPulseTM Aspiration System and to compare the two cohorts. Covariate-corrected percent for the study arms and for the mean differences for all sites as well as for those sites only enrolling patients treated with the RapidPulseTM Aspiration System were constructed. The target sample size selected for this study was forty (40) evaluable subjects in the Treatment Arm and forty (40) evaluable subjects in the Control Arm. The sample size was selected to ensure that enough subjects were included in both populations to adequately describe the potential variability of the populations. All analyses were performed after the clinical database was locked using SAS Version 9.4 or later (SAS Institute, Cary NC).
The endpoints were evaluated as success/fail outcomes binary measures. This included the primary endpoint as well as the technical, clinical, safety, and exploratory endpoints. The success/fail outcomes are presented for the treatment and control arms separately as percent (primary efficacy, technical, clinical, safety, and exploratory) consistent with literature reporting along with corresponding two-sided 95% CIs calculated using an exact binomial distribution. The treatment effect (RapidPulseTM Aspiration System, Control) was analyzed using logistic regression to control for the following baseline covariates in addition to treatment:
Site of occlusion (ICA vs M1 segment of middle cerebral artery vs M2 segment of middle cerebral artery vs basilar artery vs vertebral artery)
Intravenous thrombolytics (alteplase or Tenecteplase) (yes vs no)
Time from stroke to groin puncture (<6 h, > 6 h)
Age (< 80years, ≥ 80 years)
NIHSS Baseline Score (ordinal scale)
Pre-morbid mRS (ordinal scale)
ASPECT score.
Multiple imputation was performed for missing data for the following two endpoints in the ITT population:
Modified Rankin Scale Score (mRS) 0 - 2 at 90 days post-index procedure
All-cause mortality.
Results
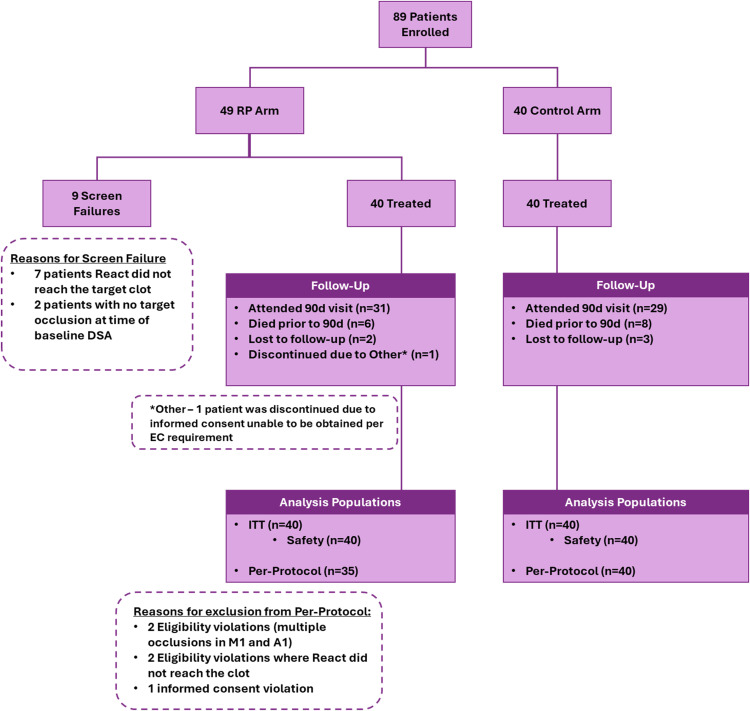
Between February 2022 and December 2022, 80 subjects were consented and enrolled in the study (n = 40 RapidPulseTM or treatment arm, n = 40 contemporary control arm). Inclusion, analysis and treatment algorithm of the RapidPulseTM and control populations are shown in Figure 3. Of the 40 RapidPulseTM subjects in the ITT population, 31 completed the study, two were lost to follow-up, six expired from reasons unrelated to the investigational device, and one subject was withdrawn post-procedure due to IC violations determined by the site's respective Ethics Committee. Of the 35 subjects in the RapidPulseTM arm meeting the PP population criteria, 28 completed the study, two were lost to follow-up and five subjects expired. Of the 40 patients available for comparative analysis in the control arm; 29 completed the 90-day visit, eight expired during follow-up, and three were lost to follow-up.
Figure 3.
Rapidpulsetm FS study subject inclusion and treatment algorithm.
Subject populations in the treatment and control arms had similar baseline stroke severity mean NIHSS (17.2 vs 18.5) and mean ASPECT scores (8.6 vs. 7.9) as assessed by an independent ICL as well as similar mean times from stroke onset to intervention (5.7 vs. 5.8 h.), respectively. The target vessel occlusion locations as assessed by the ICL are presented in Table 1.
Table 1.
Demographic and characteristics.
| RapidPulseTM arm, ITT (N = 40) | RapidPulseTM arm, PP (N = 35) | Control arm (N = 40) | |
|---|---|---|---|
| Mean age — yr ± SD | 67.8 ± 11.5 | 68.3 ± 10.4 | 60.1 ± 14.5 |
| Male sex — no. (%) | 19 (47.5%) | 18 (51.4%) | 22 (55.0%) |
| iv-TPA given — no. (%) | 16 (40.0) | 15 (42.9%) | 16 (40.0%) |
| Medical history — no. (%) | |||
| Diabetes mellitusa | 8/37 (21.6%) | 8 (23.5%) | 8 (20.0%) |
| Hypertensiona | 25/39 (64.1%) | 23 (65.7%) | 22 (56.4%) |
| Smokinga | 8/34 (23.5%) | 6 (19.4%) | 9 (24.3%) |
| Atrial fibrillationa | 7/34 (20.6%) | 7 (22.6%) | 12 (30.0%) |
| ASPECTS (Core Lab) | |||
| Mean | 8.6 | 8.7 | 7.9 |
| Median | 9 | 9 | 8 |
| interquartile range | 8–10 | 8–10 | 6–10 |
| NIHSS total score (Admission) | |||
| Mean | 17.2 | 16.8 | 18.5 |
| Median | 16 | 16 | 19.5 |
| interquartile range | 13 – 22 | 13–22 | 15–22 |
| Site of intracranial occlusion (Core Lab) — no. (%) | |||
| ICA | 4 (10.3%) | 4 (11.4%) | 10 (25.0%) |
| MCA-M1 | 32 (82.1%) | 29 (82.9%) | 23 (57.5%) |
| MCA-M2 | 2 (5.1%) | 1 (2.9%) | 4 (10.0%) |
| BA | 1 (2.6%) | 1 (2.9%) | 3 (7.5%) |
| Onset-to-puncture (min) | |||
| median | 270 | 252 | 318 |
| interquartile range | 174–384 | 174–348 | 216–432 |
| Procedure time (min) | |||
| median | 33 | 33 | 33 |
| interquartile range | 27–66 | 24–52 | 14–67 |
| Onset-to-revascularization (min) | |||
| median | 288 | 282 | 342 |
| interquartile range | 192–402 | 192–372 | 246–450 |
Complete medical history could not be collected in some instances. The denomiantor reflecting missing data is provided where applicable.
ICA: internal carotid artery; ITT; intent to treat.
Baseline characteristics of the ITT and PP populations are shown in Table 2. In the ITT population, mean age was 67.8 ± 11.5 years; 19 (47.5%) were male. Median NIHSS score was 16 (IQR: 13–22). Median ASPECTS score was 9 (IQR: 8–10). IV t-PA was administered in 16 (40.0%) subjects of the treatment arm and in 16 (40.0%) subjects of the control arm.
Table 2.
Technical endpoints and clinical outcomes.
| Reperfusion outcomes (Core Lab) | RapidPulseTM arm, ITT (N = 40) | RapidPulseTM Arm, PP (N = 35) | Control arm (N = 40) |
|---|---|---|---|
| First-pass mTICI scores — no. (%) | |||
| TICI 3 | 13 (33.3%) | 13 (37.1%) | 11 (28.2%) |
| TICI 2c | 8 (20.5%) | 8 (22.9%) | 4 (10.3%) |
| TICI 2b | 7 (17.9%) | 6 (17.1%) | 10 (25.6%) |
| TICI 0-2a | 11 (28.2%) | 8 (22.9%) | 14 (35.9%) |
| Final pass mTICI scores — no. (%) | |||
| TICI 3 | 18 (46.2%) | 18 (51.4%) | 21 (53.8%) |
| TICI 2c | 11 (28.2%) | 8 (22.9%) | 9 (23.1%) |
| TICI 2b | 8 (20.5%) | 8 (22.9%) | 9 (23.1%) |
| TICI 0-2a | 2 (5.1%) | 1 (2.9%) | 0 (0.0%) |
| Clinical outcomes | Treatment arm, ITT (N = 40) | Treatment arm, PP (N = 35) | Control arm (N = 40) |
| NIHSS score at 24h | |||
| median | 9 | 9 | 9 |
| interquartile range | 3–14 | 2–13 | 3–15 |
| Functional ındependence — no. (%) | |||
| mRS 0-2 at 90 day | 22 (61.1%) | 21 (63.6%) | 19 (52.8%) |
ITT: intent to treat.
The procedure was performed using general anesthesia or conscious sedation in both arms with a comparable number of subjects undergoing each procedure. Most occlusions were in the MCA territory (34/40 in the RapidPulseTM ITT arm and 27/40 in the Control arm) assessed by the independent core laboratory. The baseline mTICI was 0 in all cases per independent ICL assessment. The median procedure time was identical in both cohorts (33 min) and the overall mean onset to revascularization was 5.7 h in the RapidPulseTM arm compared to 6.2 h in the Control arm.
Rescue therapy was used in 18 (45.0%) cases in the RapidPulseTM ITT arm, 13 (37.1%) in the RapidPulseTM PP population, and in 16 (40.0%) in the control arm. All patients in the treatment arm (N = 40) were successfully treated with the RapidPulseTM Aspiration System. Procedure-related treatment-emergent adverse events occurred in 18 (45.0%) cases in the treatment arm and in 21 (53.0%) in the control arm. No aspiration pump or RapidPulseTM device-related adverse events were observed in the study. Supplement material 3 provides a table of all procedure-related adverse events.
Primary efficacy endpoints
A summary of the primary and secondary efficacy endpoints is provided in Table 3. In the RapidPulseTM arm ITT population, first pass rates were mTICI-3 in 13 cases (33.3%), mTICI-2c in 8 cases (20.5%), mTICI-2b in 7 cases (17.9%), mTICI 0-2a in 11 cases (28.2%). In the control arm, first pass rates were mTICI-3 in 11 cases (28.2%), mTICI-2c in 4 cases (10.3%), mTICI-2B in 10 cases (25.6%), mTICI 0-2A in 14 cases (35.9%). The rate of the primary efficacy endpoint (FPE) was 53.9% (21/39) in the unimputed ITT treatment population, and 38.5% (15/39) in the corresponding control population. The equal-tailed 95% CI ranges from 37.2% to 69.9% for the RapidPulseTM arm and from 23.4% to 55.4% for the control. In the unimputed PP RapidPulseTM population, the FPE rate was 60.0% (21/35) with CI ranging from 42.1% to 76.1%. Median pass with RapidPulse system was 2 (IQR 1-3) in ITT population, 1 (IQR 1-2) in PP population and 1.5 (1–3) in control arm.
Table 3.
Safety outcomes.
| Safety outcomes (CEC and/or Core Lab adjudicated) | RapidPulseTM, ITT (N = 40) | Control arm (N = 40) |
|---|---|---|
| Symptomatic ICH at 24 h — no. (%) | 0 (0.0%) | 1 (2.5%) |
| Mortality — no. (%) | 6 (15.0%) | 8 (20.0%) |
| Embolism in new territory — no. (%) | 1 (2.6%) | 1 (2.5%) |
| Any intracranial hemorrhage at 24 h — no. (%) | 13 (33.3%) | 18 (45.0%) |
| Hemorrhagic transformation at 24 h — no. (%) | ||
| All subtypes | 13 (32.5%) | 18 (45.0%) |
| HI-1 | 3 (7.5%) | 2 (5%) |
| HI-2 | 0 (0%) | 0 (0%) |
| PH-1 | 4 (10%) | 7 (17.5%) |
| PH-2 | 3 (7.5%) | 8 (20%) |
| SAH | 3 (7.5%) | 1 (2.5%) |
CEC: clinical event committee; ITT: intent to treat.
Secondary efficacy endpoints
The frontline technical success (mTICI ≥ 2b after the last pass) was 84.2% (32/38) in the RapidPulseTM ITT arm, 91.2% (31/34) in the RapidPulseTM PP arm, and 79.5% (31/39) in the control arm. The rate of final mTICI ≥ 2b after all passes (including any rescue therapy) was 94.9% (37/39) the RapidPulseTM ITT arm, 97.1% (34/35) in the RapidPulseTM PP arm, and 100% (39/39) in the Control arm. The rate of mFPE (mTICI ≥ 2b after one device pass) was 71.8% (28/39) in the RapidPulseTM ITT arm, 77.1% (27/35) in the RapidPulseTM PP arm, and 64.1% (25/39) in the Control arm.
Functional independence (mRS 0-2) at 90 days was reported in 61.1% (22/36) of the patients in the RapidPulseTM arm and 52.8% (19/36) of those in the Control arm.
Primary safety endpoints
Safety endpoints for the ITT and Control populations are shown in Table 3. There were no aspiration pump or RapidPulseTM device-related adverse events including device-related vasospasm in the study. Further, there were no neurological adverse events identified by the CEC during follow-up. A total of 13 cases (13/39, 33.3%) of any intracranial hemorrhage were reported in the RapidPulseTM arm and 18 (18/40, 45.0%) cases in the Control arm. There was 1 case of emboli to new territory reported in the RapidPulseTM arm and 1 case in the Control arm.
Discussion
In this multi-center, open-label, feasibility trial with contemporary controls, the RapidPulseTM Aspiration System demonstrated promising results for all predefined performance parameters including FPE, mFPE, and frontline technical success (mTICI ≥ 2b after last pass) as well as safety and functional outcomes. The independently assessed primary effectiveness FPE rate in the ITT treatment population was 53.9% (21/39) versus 38.5% (15/39) in the contemporary aspiration thrombectomy control population. Although the design of the study did not include any hypothetical testing of superiority or non-inferiority against controls, the rates of FPE, mFPE, and good functional outcomes in this study compares favorably with the results of multiple recent trials.8,9,15,16
Some variables, such as site of occlusion and onset-to-puncture time, show differences between the treatment arm and the control arm. These differences may be attributed to the smaller sample sizes and limited time window for enrollment of control group in this feasibility study. Additionally, the RapidPulseTM Aspiration System resulted in equivalent or higher rates of FPE for occlusions in all locations included within the study. However, onset-to-puncture has not been found to be a good predictor of FPE nor has site of occlusion. 17
Several published reports support that cyclic aspiration significantly outperforms static aspiration in speed and overall clearance of synthetic clots across multiple experimental and clinical models.16,18 There is a growing tendency to use mTICI ≥ 2c obtained in first pass as an efficacy benchmark for technical success given its strong correlation with good functional outcome in multiple studies. However, recent reports and meta-analyses consistently show that the FPE of near-complete reperfusion (TICI 2c-3) remains relatively low with existing mechanical thrombectomy technology. A recent 2021 systematic review and meta-analysis by Abbasi et al. of 67 studies comprising 16,870 patients reported overall rates of FPE and mFPE of 28% and 45%, respectively. 19 In the Aspiration versus Stent Retriever (ASTER) RCT clinical trial, the study's core lab reported FPE was 28.9%. 9 Most recently, in the ETIS prospective real-world registry by Di Maria et al., a 22.8% FPE TICI ≥ 2c was reported whereas a higher rate of 38.5% was reported for the ARISE II Embotrap prospective core lab-adjudicated study.15,20 In this study of the RapidPulseTM Aspiration System, core lab-adjudicated results showed favorably higher rate of FPE (mTICI 2c-3) for 53.9% of the ITT group and 60.0% in the PP treatment group.
Regarding safety outcomes, there were no sICH within 24 h, device-related morbidity or mortality reported in the RapidPulseTM Treatment group. Further, there were no neurological adverse events identified by the CEC during follow-up. Only one case, reported as symptomatic hemorrhage (2.5%) was identified in the Control group. The rate of any kind of hemorrhage (33.3% vs 45.0%, treatment vs control, respectively) was in line with other thrombectomy studies.
Limitations
This was an early feasibility study, although the patients included in the Control group were contemporary, consecutive, and from the same treating centers, they were not randomized. However, core lab adjudication for angiographic and imaging outcome were used in both arms as an effort to overcome some of these limitations.
Conclusions
In this prospective multi-center trial with contemporary aspiration thrombectomy controls and independent outcome adjudication, the RapidPulseTM Aspiration System showed favorable rates of FPE and correlated good functional outcomes compared with the control group and the results of recent thrombectomy device studies. A larger pivotal trial is planned to further characterize and substantiate the safety and performance of the RapidPulseTM Aspiration System in the treatment of AIS due to LVOs. The larger pivotal trial will be conducted using a second generation of the RapidPulse Aspiration System, based on the clinical experiences with the device in this feasibility study.
Supplemental Material
Supplemental material, sj-docx-1-ine-10.1177_15910199241239094 for RapidpulseTM cyclic aspiration system for acute ischemic stroke due to large vessel occlusions by Arsida Bajrami, Serdar Geyik, Ozgur Ertugrul, Eren Erdem, Jose I Gallego Leon, Giorgio Barbieri, Carlos Dominguez Rodriguez, Jose Carlos Rayón-Aledo, Antonio I Sagredo Barra, Fernando S Sanchez Blanco, Carmen Serna Candel, Francisco Jose Montalverne, Lidemarcks I Andrade, Diego Bandeira, Jose Bezerra, Hellen Carm, Henrique Coelho Silva, Alessandra Braga Cruz Guedes de Morais, Adson Freitas de Lucena, Fabricio O Lima, George Mendes, Felipe A Rocha, Karlis Kupcs, Helmuts Kidikas, Janis Vetra, Gyula Gal, Anabel Diaz and Raul G Nogueira in Interventional Neuroradiology
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Rapidpulse,
ORCID iDs: Serdar Geyik https://orcid.org/0000-0003-0767-5628
Jose Carlos Rayón-Aledo https://orcid.org/0000-0001-7797-9497
Alessandra Braga Cruz Guedes de Morais https://orcid.org/0000-0002-6829-3239
Gyula Gal https://orcid.org/0000-0002-9575-4109
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Eng J Med 2015; 372: 11–20. [DOI] [PubMed] [Google Scholar]
- 2.Goyal M, Demchuk A, Menon B, et al. Randomized assesment of rapid endovascular treatment of stroke. N Eng J Med 2015; 372: 1019–1030. [DOI] [PubMed] [Google Scholar]
- 3.Saver J, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs t-PA alone in stroke. N Eng J Med 2015; 372: 2285–2299. [DOI] [PubMed] [Google Scholar]
- 4.Campbell BC, Mitchell BJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Eng J Med 2015; 372: 1009–1018. [DOI] [PubMed] [Google Scholar]
- 5.Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 h after symptom onset in ischemic stroke. N Eng J Med 2015; 372: 2296–2306. [DOI] [PubMed] [Google Scholar]
- 6.Zaidat OO, Castonguay AC, Linfante I, et al. First pass effect: a new measure for thrombectomy devices. Stroke 2018; 49: 660–666. [DOI] [PubMed] [Google Scholar]
- 7.Bajrami A, Ertugrul O, Senadim S, et al. First pass results of mechanical thrombectomy with two-drop zone NeVaTM device. Interv Neuroradiol 2022: 15910199221135309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta R, Saver JL, Levy E, et al. New class of radially adjustable stentrievers for acute ischemic stroke: primary results of the multicenter tiger trial. Stroke 2021; 52: 1534–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lapergue B, Blanc R, Gory B, et al. Effect of endovascular contact aspiration vs stent retriever on revascularization in patients with acute ischemic stroke and large vessel occlusion: the Aster randomized clinical trial. JAMA 2017; 318: 443–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lapergue B, Blanc R, Costalat V, et al. Effect of thrombectomy with combined contact aspiration and stent retriever vs stent retriever alone on revascularization in patients with acute ischemic stroke and large vessel occlusion: the ASTER2 randomized clinical trial. JAMA 2021; 326: 1158–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liao CC, Chen YF, Xiao F. Brain midline shift measurement and its automation: a review of techniques and algorithms. Int J Biomed Imaging 2018; 2018: 4303161. PMID: 29849536; PMCID: PMC5925103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jadhav AP, Desai SM, Zaidat OO, et al. First pass effect with neurothrombectomy for acute ischemic stroke: analysis of the systematic evaluation of patients treated with stroke devices for acute ischemic stroke registry. Stroke 2022; 53: e30–e32. Epub 2021 Nov 17. PMID: 34784741. [DOI] [PubMed] [Google Scholar]
- 13.Von Kummer R, Broderick J, Campbell B, et al. The Heidelberg bleeding classification. Stroke 2015; 46: 2981–2986. [DOI] [PubMed] [Google Scholar]
- 14.Mazya M, Egido JA, Ford GA, et al. Predicting the risk of symptomatic intracerebral hemorrhage in ischemic stroke treated with intravenous alteplase: safe implementation of treatments in stroke (SITS) symptomatic intracerebral hemorrhage risk score. Stroke 2012; 43: 1524–1531. [DOI] [PubMed] [Google Scholar]
- 15.Zaidat OO, Bozorgchami H, Ribo M, et al. Primary results of the multicenter ARISE II study (analysis of revascularization in ischemic stroke with embotrap). Stroke 2018; 49: 1107–1115. [DOI] [PubMed] [Google Scholar]
- 16.Simon S, Grey CP, Massenzo T, et al. Exploring the efficacy of cyclic vs static aspiration in a cerebral thrombectomy model: an initial proof of concept study. J Neurointerv Surg 2014; 6: 677–683. [DOI] [PubMed] [Google Scholar]
- 17.Lee IH, Choi JI, Ha SKet al. et al. Predictive factors of first-pass effect in patients who underwent successful endovascular thrombectomy for emergent large vessel occlusion. J Korean Neurosurg Soc 2024; 67: 14–21. Epub 2023 Jul 10. PMID: 37424093; PMCID: PMC10788560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arslanian RA, Marosfoi M, Caroff J, et al. Complete clot ingestion with cyclical ADAPT increases first-pass recanalization and reduces distal embolization. J Neurointerv Surg 2019; 11: 931–936. [DOI] [PubMed] [Google Scholar]
- 19.Abbasi M, Liu Y, Fitzgerald s, et al. Systematic review and meta-analysis of current rates of first pass effect by thrombectomy technique and associations with clinical outcomes. J Neurointerv Surg 2021; 13: 212–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Maria F, Kyheng M, Consoli A, et al. Identifying the predictors of first-pass effect and its influence on clinical outcome in the setting of endovascular thrombectomy for acute ischemic stroke: results from a multicentric prospective registry. Int J Stroke 2021; 16: 20–28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-ine-10.1177_15910199241239094 for RapidpulseTM cyclic aspiration system for acute ischemic stroke due to large vessel occlusions by Arsida Bajrami, Serdar Geyik, Ozgur Ertugrul, Eren Erdem, Jose I Gallego Leon, Giorgio Barbieri, Carlos Dominguez Rodriguez, Jose Carlos Rayón-Aledo, Antonio I Sagredo Barra, Fernando S Sanchez Blanco, Carmen Serna Candel, Francisco Jose Montalverne, Lidemarcks I Andrade, Diego Bandeira, Jose Bezerra, Hellen Carm, Henrique Coelho Silva, Alessandra Braga Cruz Guedes de Morais, Adson Freitas de Lucena, Fabricio O Lima, George Mendes, Felipe A Rocha, Karlis Kupcs, Helmuts Kidikas, Janis Vetra, Gyula Gal, Anabel Diaz and Raul G Nogueira in Interventional Neuroradiology