Abstract
Genetic tumor syndromes are due to inherited genetic mutations, which have recently come to the attention of clinicians due to the widespread adoption of DNA sequencing, ultimately leading to imaging for surveillance. As a result, radiologists must be familiar with the clinical, genetic, and radiologic features of these syndromes. This article reviews genetic tumor syndromes of the head and neck according to the recently updated WHO’s 5th edition.
Keywords: Genetic tumor syndrome, head and neck cancers, familial cancers
Introduction
Inherited genetic mutations that greatly increase the risk of malignancy are often referred to as genetic tumor syndromes, hereditary cancer syndromes, or familial cancer syndromes. In recent decades, there has been a significant increase in the recognition of identifiable tumor-predisposing syndromes, primarily driven by the widespread adoption of DNA sequencing in diagnostic pathology and clinical genetics. This advancement in technology has illuminated a fascinating shift in our understanding of tumors, once considered sporadic, now revealing novel syndromes and their associated tumors. 1 Imaging plays an important role as it is essential for screening, surveillance, diagnosis, and monitoring.
Radiologists may be the first to alert clinicians about potential inherited syndromes. As screening and surveillance have increased, the radiologist should be familiar with different genetic syndromes and their head and neck manifestations. The imaging identification of familial tumor syndromes holds paramount importance, as it necessitates vigilant monitoring and the provision of genetic counseling to family members. These genetic discoveries remain pivotal in elucidating the underlying mechanisms governing neoplastic development across diverse tissue types.
This article focuses on head and neck imaging findings and clinical features with the evaluation of germline mutations as vital tools in discerning the intricate nuances of tumors according to the newly established WHO’s 5th edition section on genetic tumor syndromes. 2 The following syndromes involving the head and neck region will be discussed: Gorlin Syndrome, Neurofibromatosis 1, Neurofibromatosis 2, Familial Adenomatous Polyposis and Gardner Syndrome, Cowden Syndrome, Brooke-Spiegler Syndrome, Familial Paraganglioma Syndrome, Multiple Endocrine Neoplasia Type 2, Li-Fraumeni Syndrome, Fanconi Anaemia, Dyskeratosis Congenita, Ataxia Telangiectasia, Bloom Syndrome, von Hippel-Lindau Syndrome, and Tuberous Sclerosis. Table 1 summarizes the genetic mutations associated with these syndromes.
Table 1.
Genetic Tumor Syndromes, associated mutations, and inheritance patterns. AD = Autosomal Dominant, AR = Autosomal Recessive.
| Syndrome | Mutations | Inheritance |
|---|---|---|
| Naevoid basal cell carcinoma syndrome/Gorlin Syndrome | Gene PTCH1 on chromosome 9q22-31 | AD |
| Neurofibromatosis 1 | Neurofibromatosis gene 1, on chromosome 17q11.2 | AD |
| Neurofibromatosis 2 | Gene NF2 on chromosome 22q11.2 | AD |
| Familial adenomatous polyposis and Gardner syndrome | Adenomatous polyposis coli (APC) gene on chromosome 5q21 | AD |
| Cowden syndrome | Chromosome 10q23, the phosphate and tensin homolog (PTEN) gene | AD |
| Brooke-Spiegler Syndrome | CYLD gene on chromosome 16q12.1 | AD |
| Familial paraganglioma syndrome | Mutations in succinate dehydrogenase (SDH) | AD |
| Multiple endocrine neoplasia type 2 | RET proto-oncogene located on chromosome 10q11.2 | AD |
| Li-Fraumeni syndrome | Chromosome 17p13.1, in the tumor suppressor gene 53 (TP53) | AD |
| Fanconi Anaemia | 22 genes are identified: Fanconi anemia complementation group A (FANC-A), FANC-C, and FANC-G | AR, X-linked, AD |
| Dyskeratosis congenita | 14 genes have been identified to be associated with DC, including DKC1, TERC, TERT, and TINF2 | X-linked, AD, AR |
| Ataxia telangiectasia | ATM gene located on chromosome 11q22-23 | AR |
| Bloom syndrome | BLM gene on chromosome 15q26 | AR |
| Von Hippel–Lindau syndrome | VHL gene on chromosome 3p25.5 | AD |
| Tuberous sclerosis | Gene TSC1 on chromosome 9q32 or gene TSC2 on chromosome 16p13.3 | AD |
Naevoid basal cell carcinoma syndrome (Gorlin syndrome)
Naevoid basal cell carcinoma syndrome, or Gorlin syndrome (GS) or basal cell naevus syndrome, is a genetic tumor syndrome of autosomal dominant (AD) inheritance occurring in 1 in 57,000 to 164,000 patients. 3 GS shows variable expressivity with high penetrance. GS is caused by mutations in the gene PTCH1 on chromosome 9q22-31, which codes for transmembrane protein acting as a binding site for the Sonic Hedgehog (SHH) ligand and plays a downstream role as a tumor suppressor gene. 3
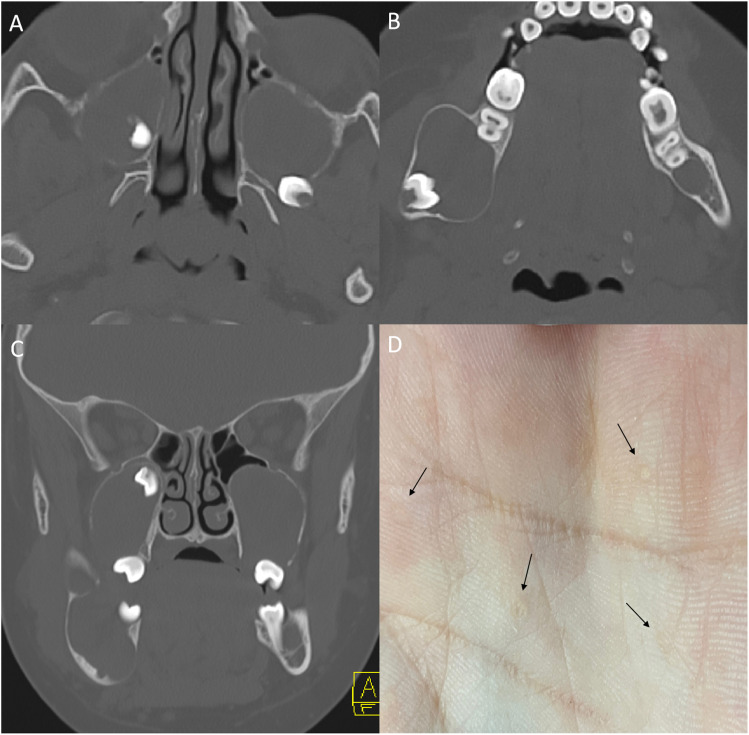
GS is characterized by a triad of multiple basal cell carcinoma (BCC), keratocysts of the jaw, and skeletal abnormalities. Common clinical manifestations of GS encompass palmar and plantar pits (Figure 1); multiple BCCs; fibromas affecting the heart and ovaries; abnormalities in the spine and ribs such as bifid ribs or pectus excavatum/carvinatum, sprengel deformity; odontogenic cysts (Figure 1); tumors involving the CNS; and ophthalmological irregularities.4,5
Figure 1.
Naevoid basal cell carcinoma syndrome (Gorlin syndrome) in a 12-year-old boy. Multiple maxillary and mandibular odontogenic keratocysts on axial (a, b) and coronal (c) CT images seen as unilocular expansile cysts associated with unerupted and impacted teeth. Palmar pits seen as small dermal depressions (D, arrow).
Multiple odontogenic keratocysts (OKCs) are most consistent with GS in the first two decades of life. 6 They are primarily identified incidentally on plain radiographs and followed with MRI due to radiation concerns with CT. 7 CT/MRI shows uni- or multilocular cysts with scalloped margins around teeth within the mandible and maxilla (Figure 1). OKCs are difficult to differentiate from other types of odontogenic cysts. Therefore, associated findings such as teeth malocclusion, impacted teeth, dental ectopy, and dental agenesis coinciding with multisystem involvement may raise suspicion for GS. 7
In the CNS, early onset medulloblastoma is most common and can be the presenting sign in pediatric patients with age of onset younger than 17 years old. 8 Desmoplastic medulloblastoma (DMB) and medulloblastoma with extensive nodularity (MBEN) variants are the most common subtypes, usually located laterally in the cerebellar hemisphere, in contrast to the classic central location of medulloblastoma. 9 CT demonstrates a hyperattenuating mass. On MRI, the tumor shows a hypointense T1 and isointense T2 mass with heterogeneous enhancement and marked surrounding edema. 3 In contrast to classic medulloblastoma, a central scar-like pattern is present in both variants. MBEN shows multiple grape-like tumor nodules. Due to a high risk of drop metastasis, contrast-enhanced imaging of the entire spine is warranted upon classic imaging findings of a tumor. 3 Other less frequent tumors in GS may include astrocytoma, craniopharyngioma, oligodendroglioma, and meningioma. Benign incidental findings consist of intracranial dural calcifications; cysts of the choroid plexus, brain parenchyma, eye and skull; and osseous bridging of the sella turcica, which is specific to GS. 3
It is important to rule out GS before starting radiation therapy (RT) for head and neck tumors as RT predisposes to spontaneous secondary malignancies. 10 An annual brain MRI is recommended for intracranial tumors until 8 years of age. For patients with a positive family history of GS, surveillance MRI is recommended every 3 months until the age of two, and biannually thereafter. 10
Neurofibromatosis type 1
Neurofibromatosis 1 (NF1), also known as von Recklingson’s disease, is an autosomal dominant inherited genetic tumor syndrome with full penetrance and variable expressivity. 3 NF1 affects the glial cells of the central and peripheral nervous system. It is caused by mutations in the Neurofibromatosis gene 1, located on chromosome 17q11.2. The NF1 gene encodes for the protein neurofibromin, a tumor suppressor gene. The prevalence of NF1 is 1 in 3000 worldwide. 11 Clinical features include multiple cutaneous, subcutaneous, or deep neurofibromas; plexiform neurofibromas; optic and non-optic gliomas; cafe au lait macules; intertriginous freckling; characteristic ocular signs; and osseous abnormalities such as pseudoarthrosis, scoliosis, and sphenoid wing dysplasia. 11
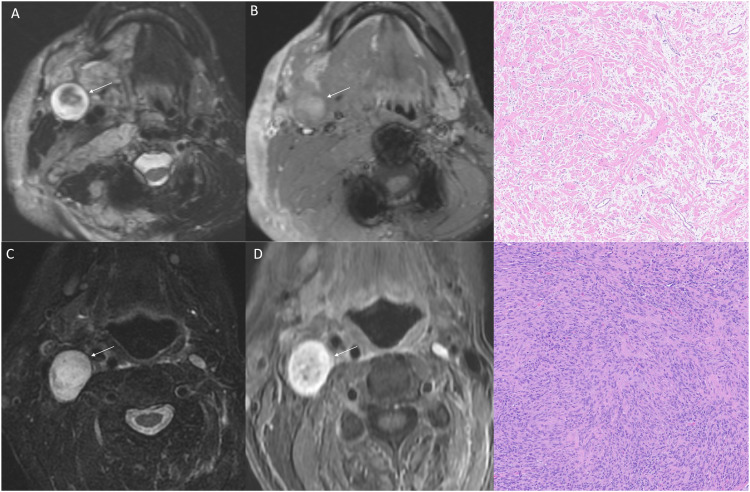
Neurofibromas are benign tumors of Schwann cells. Cutaneous neurofibromas are 1–2 cm soft nodules first present during puberty, increasing in number and size with age. Plexiform neurofibromas (PNF) seen in NF1 are present from birth in 30%–50% of patients and grow during the first decade of life. 12 PNFs differ from other types of neurofibromas in that they arise from multiple nerve fascicles and grow along the fascicle length while carrying a lifetime risk of malignant transformation. 12 On CT, PNFs are seen as hypodense areas due to their fat-rich myxoid content. MRI shows a lobulated hyperintense signal on T2; however, the overall lesion is lower in intensity than muscle tissue. On non-contrast MRI, a target sign is seen, with central hypointensity due to fibrous and myxoid components, while each nerve fascicle appears as the target focus (Figure 2). 13 However, contrast MRI shows a reverse target sign. Growth in tumor size, edema surrounding lesions, cystic changes on T2, and heterogeneous signal on T1 post-contrast sequences suggest malignant transformation to malignant peripheral nerve sheath tumors (MPNSTs) and warrant biopsy. 13
Figure 2.
Neurofibroma versus schwannoma on MRI. Axial fat-saturated T2WI (a) and post-contrast (b) images in a 21-year-old man with NF-1 reveals a T2 hyperintense mass (arrow) with central hypointensity (“target sign”) with heterogenous enhancement. Pathology revealed neurofibroma with plexiform features. Right neck schwannoma in a different patient with NF-2 showing relative homogenous T2 hyperintensity (c, arrow) with homogenous enhancement (d, arrow). Mixed Antoni A and B cells suggestive of schwannoma on histopathology.
Optic pathway glioma is another common feature of NF1 seen in nearly 15%–20% of patients. 14 On MRI, the tumor appears hypointense to isointense on T1 and has a central hyperintense mass with peripheral hypointensity of the dural sheath on T2. 14 These characteristics can help determine the local extent of the tumor. Diffuse tumors are difficult to distinguish exact boundaries. Although commonly associated with NF1, optic nerve tortuosity and nerve sheath thickening are not symptomatic. 14 An MRI of the brain with dedicated high-resolution sequences of the optic nerve and chiasm should be performed in a child with visual disturbance and suspected or proven NF1.11,14 Pilocytic astrocytoma is the most common non-optic glioma observed; it is usually located in the posterior cranial fossa with mass effect. On MRI, it presents as hypointense on T1, isointense on T2, and variable enhancement.11,14
Sphenoid wing dysplasia is a hallmark of NF1. Dysplastic changes include greater and lesser wing hypoplasia, widening of the middle cranial fossa, wide orbital fissure, and a flat posterior orbit. On imaging, the absence or extreme dysplasia of the sphenoid wing is described as a bare orbit sign. 3 Focal abnormal signal intensities (FAIs), also known as unidentified bright objects (UBOs), are also seen, most commonly in the cerebellum, brainstem, and basal ganglia in NF1. On imaging, FAIs appear as foci of various sizes of T2 hyperintensity, while isointense on T1 and DWI. FAIs are hypothesized to be increased intramyelinic edema, indicative of their T2 signal, and decrease with age. 15
Neurofibromatosis type 2
Neurofibromatosis 2 (NF2) is an autosomal dominant disorder characterized by the development of bilateral acoustic neuromas (vestibular schwannomas) and other CNS tumors. Loss of function mutations in the gene NF2 on chromosome 22q11.2 that encodes merlin protein, a tumor suppressor gene, leads to the development of NF2. 3 Multisystemic involvement of NF2 may also include meningiomas, ependymomas, retinal hamartomas, cataracts, mono- or polyneuropathy, and peripheral nerve schwannomas.
The typical onset of schwannomas is 18 to 24 years old, most commonly involving the vestibular division of the vestibulocochlear nerve and, less commonly, the facial nerve and trigeminal nerve. 16 Spinal nerve root and peripheral nerve involvement are also frequently seen. Bilateral vestibular schwannomas are pathognomonic for NF2. CT imaging is less sensitive but may show intense contrast enhancement, along with smooth osseous remodeling. 16 On MRI, it appears as a T1 iso- or hypointense lesion, which intensely enhances with contrast (Figure 2). Heterogenous hypo- and hyperintense signal on T2 reflect the Antoni A and Antoni B areas, respectively. Antoni A is elongated, thickly bundled, well-organized fascicles, and Antoni B is less compact and randomly distributed within a loose myxoid (Figure 2). 16 Hemosiderin deposition and cystic degeneration within Antoni B areas cause heterogeneity of signal.
Many other primary CNS manifestations may also be present. Around 50% of patients with NF2 develop meningioma in their lifetime. 17 Ependymoma is a benign tumor of ependymal cells lining ventricles and the spinal cavity. They are most commonly at the posterior fossa and cervicomedullary canal. On imaging, an ependymoma appears as a weakly enhancing lesion on T1. Meningiomatosis is a small plaque-like leptomeningeal and perivascular proliferation of small blood vessels extending into perivascular spaces. They appear as hypoattenuating lesions on CT with heterogeneous enhancement. A gyriform pattern is visible on MRI. Intense enhancement is observed on T1 post-contrast imaging, while hyperintensity is a distinctive characteristic on FLAIR and T2-weighted sequences. These lesions are considered pre-neoplastic for meningiomas.3,17 Glial micro-hamartomas are stable collections of immature glial cells at the cortical gray-white junction, which is also pathognomonic for NF2. 17 On imaging, they appear as hyperintense well-circumscribed lesions on FLAIR and T2, making it difficult to differentiate from focal cortical dysplasia.
Familial adenomatous polyposis and Gardner syndrome
Familial Adenomatous Polyposis (FAP) is an autosomal dominant disease. It is characterized by the development of thousands of adenomatous colorectal polyps along with multisystem manifestations. Inherited or acquired loss of function mutations in the Adenomatous Polyposis Coli (APC) gene on chromosome 5q21 lead to cell proliferation and carcinogenesis through the Wnt signaling pathway. 18 Gardner syndrome is the historical term used to describe a variant of FAP where patients have extraintestinal manifestations. These extraintestinal manifestations can include thyroid cancer, pancreatic tumors, and hepatoblastomas. In the head and neck region, it may manifest as ophthalmic abnormalities, osteomas, desmoid tumors, and dental abnormalities. The most consistent extraintestinal manifestation includes retinal pigment epithelium hypertrophy. 18
Medulloblastoma accounts for approximately 80% of the CNS tumors seen in FAP, other less common tumors involve high-grade astrocytomas and ependymomas. 18 Medulloblastoma is most common in the first decade of life. Imaging findings are similar to other genetic/sporadic forms, as discussed in Gorlin Syndrome. 18
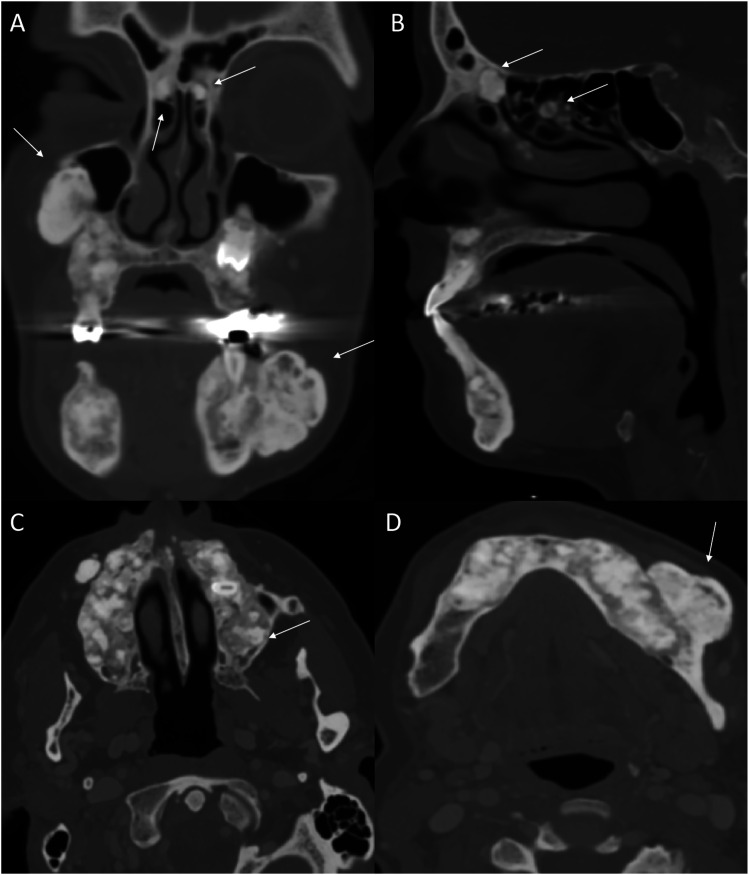
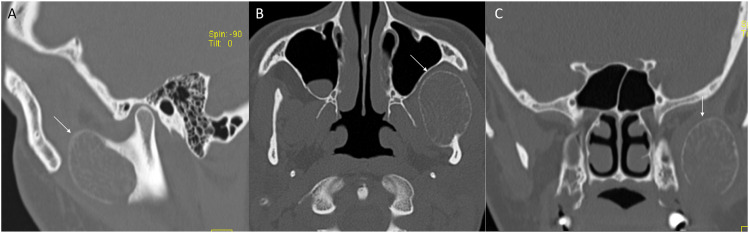
Craniofacial osteomas are also common in FAP, typically located at the mandible and skull base. On CT, they are seen as highly hyperattenuating round lesions, which may be sessile or pedunculated. Mature osteoma might have central radiolucency, suggesting the presence of marrow (Figure 3). 19 MRI presentation is low intensity on T1 and T2. The presence of osteomas may indicate FAP as they precede the clinical and radiologic evidence of colonic manifestations, thus prompting early intervention.18,19 Dentition abnormalities are also common. Patients may have a supranumery tooth, dentigerous cyst, or secondary retention of teeth (Figure 3). FAP patients have an overall mild risk of developing thyroid nodules and/or carcinoma, with a higher incidence among females. 18 The most common subtype is papillary thyroid cancer. Thyroid abnormalities are usually screened and managed with ultrasound examination.
Figure 3.
Numerous craniofacial osteomas in a 35-year-old woman with Gardner syndrome. Multiplanar CT images (a-d) reveal numerous sessile and intraosseous osteomas in/along the maxilla, mandible and within the paranasal sinuses (arrows). These are seen as dense osseous lesion with few showing central lucency suggesting presence of underlying marrow.
Cowden syndrome
Cowden Syndrome (CS), also known as Cowden Disease or Multiple Hamartomas Syndrome, is a genetic disorder characterized by the presence of multiple hamartomas throughout the body. Patients with CS often develop malignancies in the breast, thyroid glands, colon, endometrium, and CNS. 20 Molecular studies have identified germline mutations at chromosome 10q23, the phosphate and tensin homolog (PTEN) gene. 20
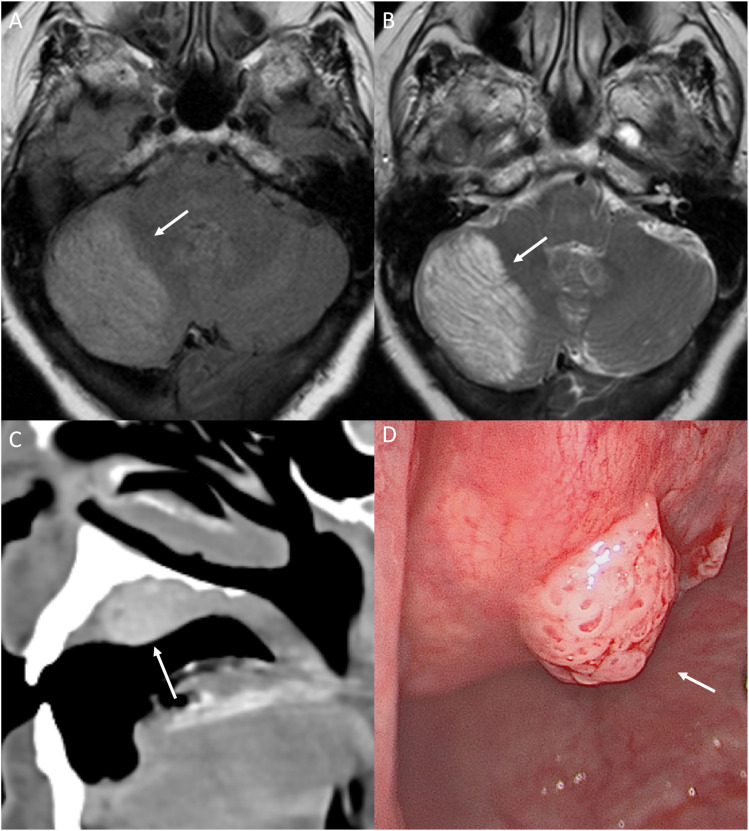
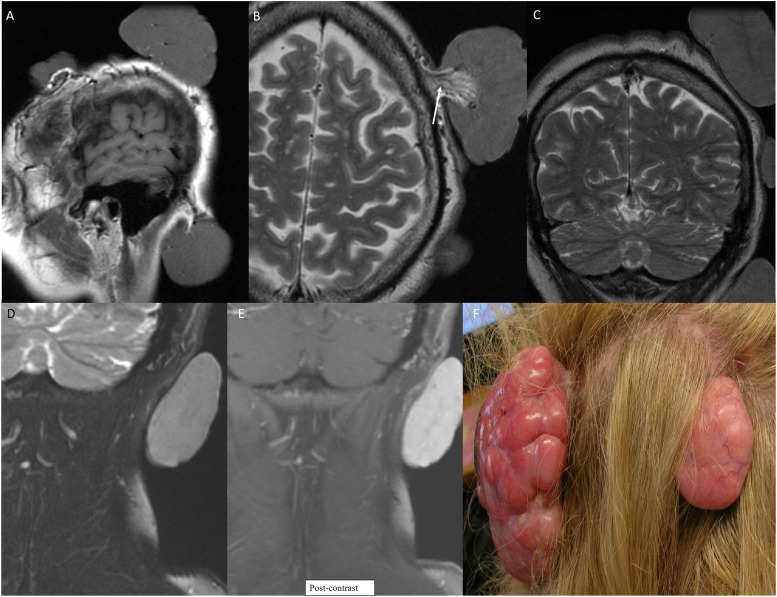
Dysplastic cerebellar gangliocytoma (DCG) in CS is described as Lhermitte-Duclos disease (LDD). LDD is a benign hamartoma usually seen in the 3rd or 4th decade of life. Clinically, LDD presents as symptoms of posterior fossa mass, including ataxia, headache, vision abnormalities, and increased intracranial pressure. 21 On CT, DCG appears as a hypoattenuating, non-enhancing, well-circumscribed lesion limited to one cerebellar hemisphere, sometimes with focal calcifications. MRI demonstrates a hypointense, non-enhancing T1, and hyperintense T2 lesion with enlarged cortical folia (Figure 4). On MRI, thickened dysplastic cerebellar folia are seen as linear striations over the lesion in an alternating pattern of parallel high and low signals, otherwise known as the “Tiger Stripe Sign”—pathognomonic for CS (Figure 4). MRI findings are sufficient to diagnose, and patients do not need a biopsy to confirm. 22 CS patients also have various vascular malformations present in the CNS. Rarely, patients may develop meningiomas.
Figure 4.
Neurological and head and neck manifestation in a 64-year-old with PTEN hamartoma tumor syndrome (Cowden syndrome). FLAIR (a) and T2 (b) axial images through the posterior fossa reveal characteristic imaging findings of dysplastic cerebellar gangliocytoma (Lhermitte-Duclos disease). This is seen as widened cerebellar folia with a striated/tigroid appearance, also described as “corduroy/laminated” appearance with diffusely increased T2 signal (arrow). Oral papilloma along the hard palate on CT (c, arrow), seen as a nodular enhancing well-defined mass. Photograph of the lobulated smooth papilloma along the hard palate (d, arrow). Oral papillomas are a major diagnostic criterion for Cowden syndrome and are present in virtually all patients.
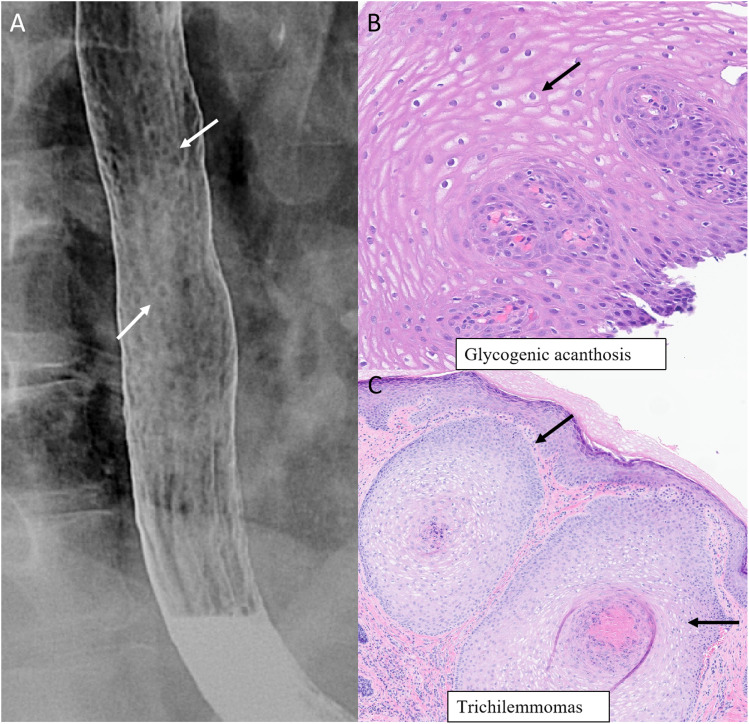
Thyroid abnormalities may also be seen with CS, such as multinodular goiter, thyroid adenoma, Hashimoto thyroiditis, and non-medullary thyroid carcinoma. Thyroid cancer is the second most common cancer seen after breast cancer. 21 Mucocutaneous lesions (papillomas, acral keratosis, etc.) are pathognomonic criteria for diagnosis of Cowden syndrome (Figure 4, Figure 5). 21
Figure 5.
Systemic manifestations in a 62-year-old woman with PTEN hamartoma tumor syndrome (Cowden syndrome). Multiple small nodules and plaques measuring 5–10 mm in the upper to mid thoracic esophagus on double-contrast esophagram (a, arrows). Biopsy with histopathology of the esophageal mucosa reveals squamous mucosa with abundant intracytoplasmic glycogen occupying essentially the entire thickness of the mucosal epithelium (b, arrow) (H&E−200x). Biopsy of dermal nodules along the left cheek region (C, arrows) with histopathology (H&E−100x) revealing nodules composed of clear cells between thickened basement membrane suggestive of trichilemmomas.
Brooke-Spiegler syndrome
Brooke-Spiegler syndrome (BSS) is a rare autosomal dominant disorder caused by mutations in the CYLD gene located on chromosome 16. 23 BSS is also known as familial cylindromatosis or CYLD cutaneous syndrome. It is characterized by the development of multiple adnexal tumors (skin appendage tumors) of the head and neck, such as spiradenomas (sweat gland tumors), cylindromas, spira-cylindroma, and trichoepithelioma (hair follicle tumors).
Basal cell adenoma (BCA) of the salivary gland is also characteristic of BSS. Rarely BCA can transform into basal cell adenocarcinoma (BCAC). 23 Histologically, BCA and BCAC are very rare biphasic tumors of the salivary gland. On MRI, BCA appears as a homogenous hypointense lesion compared to surrounding parotid tissue but isointense to muscle tissue on T1 sequences. On T2 sequences, lesions are homogeneously hyperintense to muscle tissue but slightly hypointense to the normal parotid gland. BCAC may have secondary hemorrhagic and solid-cystic components. 3 Scalp cylindromas appear as nodular, smooth, well-defined lesions (Figure 6).
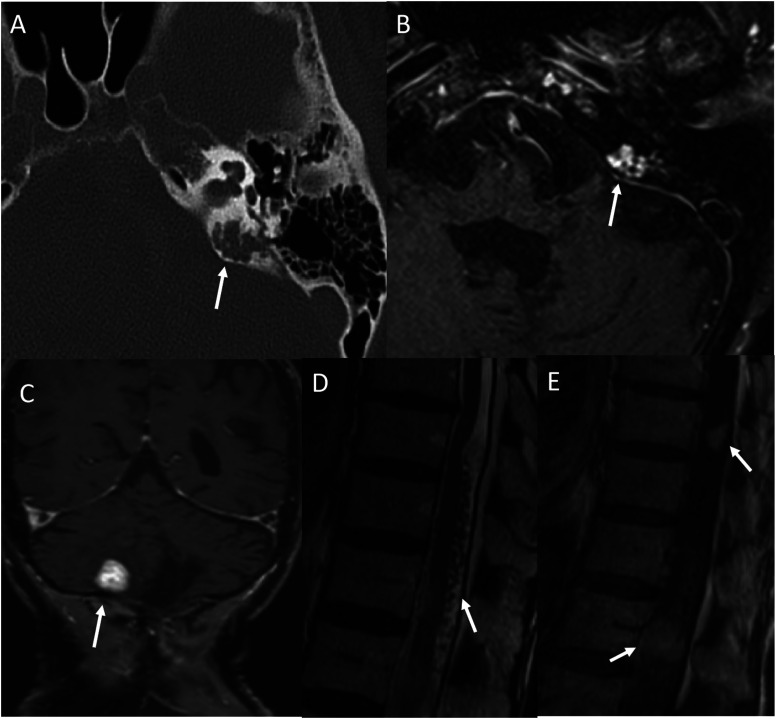
Figure 6.
Multiple variable sized scalp nodules in a 55-year-old with Brooke–Speigler Syndrome. Multiple MR images (a-e) reveal large scalp nodules including few sessile and few pedunculated. Pedunculated nodule along the left scalp shows highly vascular pedicle (b, arrow). Avid contrast enhancement within the lesion on coronal post-contrast image (e). Photograph of the lesions (f) depicting the large multilobulated nodules of the scalp. Biopsy of nodules revealed spiradenomas.
Familial paraganglioma syndromes
Familial paraganglioma syndromes (FPS), or hereditary paraganglioma-pheochromocytoma syndromes, are autosomal dominant disorders arising from mutations in succinate dehydrogenase (SDH). SDH has different subunits, and most mutations are found in subunit B (SDHB), subunit C (SDHC), and subunit D (SDHD). 24
FPS is characterized by benign development of sympathetic and/or parasympathetic ganglia-derived tumors such as paragangliomas and pheochromocytomas. Paragangliomas (PGLs) are benign neuroendocrine tumors arising from paraganglia from the skull base to the pelvis and are most frequently seen in the head and neck region. 24 They are divided into sympathetic and parasympathetic PGLs. On MRI, PGLs are seen as hypointense on T1 and iso- or hyperintense on T2, with flow voids consistent with high vascularity.25,26 Rarely, secondary hemorrhage can be seen on both sequences.The combination of hemorrhage and flow voids gives a classic salt and pepper imaging appearance.25,26 CT is important to evaluate bony involvement, and if suspected, a dedicated skull base or temporal bone CT should be performed. CTA and MRA show multiple enhancing peritumoral vascular structures feeding the tumors. [18F] Fluorodopa PET CT, initially developed for dopaminergic neurotransmission, has been found highly sensitive and specific for PGLs (Figure 7). 26 ADC values are different for different mutation types and can help aid in the diagnosis of specific FPS. 27
Figure 7.
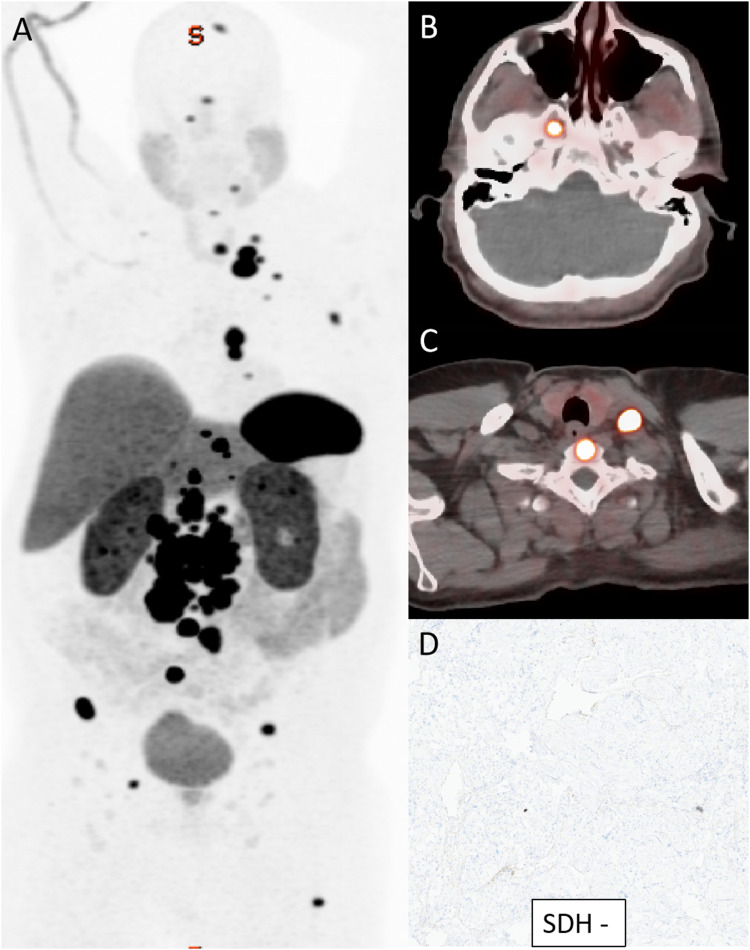
Whole-body Gallium-68 DOTATATE PET/CT DOTATE in a 35-year-old man with SDH-deficient metastatic paraganglioma. Whole body PET (a) shows numerous soft tissue and osseous metastatic paraganglioma with avid Dotatate uptake. Corresponding axial PET/CT (b, c) images with markedly avid soft tissue lesions along the skull base, neck soft tissues and the vertebrae. Loss of normal SDH expression on immunohistochemical staining (d) confirming SDH-deficiency.
Carotid body PGLs occur at the carotid artery bifurcation. Imaging depicts the splaying of both the internal carotid artery and external carotid artery. 25 The presence of flow voids helps differentiate PGLs from neurofibromas and schwannomas. 25 Vagal paraganglioma tumors are benign tumors of the vagus nerve. They can be located anywhere along the course of the vagus nerve but are most often seen at the exit of the jugular foramen. On imaging, due to the posterolateral location of the nerve to the carotid vessels, the tumor displaces vessels anteromedially. 25 Jugular paraganglioma tumors arise from the adventitia of the jugular bulb and are typically located at the jugular foramen. Given its location, it is important to differentiate them from vagal paraganglioma. Jugular paraganglioma tumors are aggressive in nature and expand into surrounding structures, including pneumatized portions of the temporal bone, other foramina, and the eustachian tube. Bony erosion gives a classic moth-eaten appearance on imaging. 25 Tympanic paragangliomas are located in the middle ear, arising from the glomus body that runs with the tympanic branch of the glossopharyngeal nerve. PGLs of larynx, nasopharynx, orbit, tongue, and thyroid are very rare and can be seen incidentally.
Multiple endocrine neoplasia type 2 (MEN 2)
Multiple endocrine neoplasia type 2 (MEN 2) also known as Sipple syndrome, affects multiple endocrine organs. It is inherited in an AD pattern, with germline activating mutations in the RET proto-oncogene located on chromosome 10q11.2. There are more than 200 different mutations identified. 28 MEN 2 has been classified into two subcategories: MEN 2A and MEN 2B.
MEN 2A is very common (∼95% of all MEN 2 cases) in comparison to MEN 2B (∼5%), which reflects a prevalence of 13-24 cases and 1-2 cases per million, respectively. 28 Thyroid gland and adrenal gland involvement are common in both subtypes, while MEN 2A involves primary hyperparathyroidism (HPTH) and MEN 2B is associated with ganglioneuromatosis, musculoskeletal and ophthalmic abnormalities.
MEN 2A has four different clinical subtypes: classic, cutaneous lichen amyloidosis, subtype associated with Hirschsprung disease, and familial medullary thyroid cancer (FMTC). 28 MEN 2A-Classic is characterized by the presence of MTC, pheochromocytoma, and primary HPTH. MEN 2 A with cutaneous lichen amyloidosis typically manifests as multiple amyloid deposits in the upper dermis around the scapular region. MEN 2 A associated with Hirschsprung disease is characterized by the presence of both MEN 2A endocrinopathy with congenital colonic agangliosis. FMTC is the subtype of MEN 2A with early onset MTC in multiple family members and delayed onset or absence of pheochromocytoma or primary HPTH. In some literature, FMTC is sub-categorized as a possible third subtype of MEN syndrome. 29
In MEN syndromes, parathyroid involvement is typically seen as an adenoma. The role of imaging in pre-operative localization of glands has decreased as it is now common practice to surgically evaluate all four glands. In contrast, the role of imaging has become increasingly important in post-surgical persistent hypercalcemia. 30 Ultrasound (US) has been widely utilized for pre-operative evaluation and localization of PTH glands. On US, it appears as a hypoechoic, well-defined round or oval lesion posterior to the thyroid gland. Larger lesions appear lobulated with internal echogenicity. 30 Rarely, non-contrast CT may identify an ectopic gland in the mediastinum. SPECT and 99mTc MIBI scan with early and delayed thin slice imaging has a higher sensitivity than other imaging modalities. 31 MTC associated with MEN syndromes have a younger age of onset and are locally aggressive with early metastasis (Figure 8). Pheochromocytomas associated with MEN 2 syndromes are locally aggressive and have a high chance of recurrence (Figure 8).28,29
Figure 8.
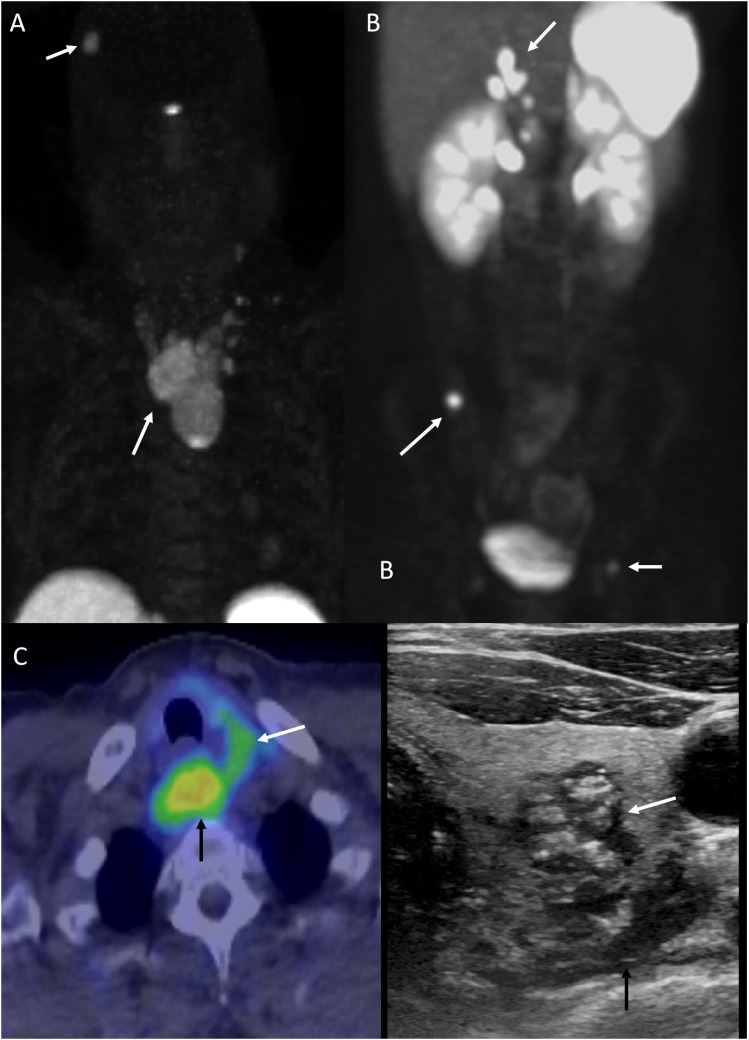
Multiple endocrine neoplasia (MEN) type II in a 47-year-old man with medullary thyroid carcinoma (MTC) and pheochromocytomas. FDG-DOTATATE scan (a-c) reveals medullary thyroid carcinoma arising from the left thyroid lobe with locoregional nodal metastasis. The complex nodule shows central calcification with irregular margins on ultrasound (c, white arrow). Nodal metastasis is most prominent in the retrotracheal region (b, c, black arrows). Metastatic recurrent pheochromocytomas is seen as multiple focal areas of abnormally increased radiotracer activity corresponding to subtle soft tissue nodularity in the right adrenal bed, along the upper abdominal and intrahepatic IVC, portacaval region, and the right parietal calvarium (a, b, arrows).
Hyperparathyroidism-Jaw tumor syndrome (HPT-JT)
Hyperparathyroidism-Jaw tumor syndrome (HPT-JT) is a genetic tumor syndrome inherited in an AD pattern. It is characterized by PTH tumors, ossifying fibromas of craniofacial bones, as well as uterine and renal abnormalities. Mutations involving cell division cycle 73 (CDC73) gene, encoding for parafibromin, is responsible for the development of HPT-JT. 32 Parafibromin is a ubiquitously expressed protein with antiproliferative properties.32,33
HPTH is a primary feature of HPT-JT; PTH gland involvement with HPT-JT syndrome is often more malignant and usually involves a single gland in comparison to other hereditary forms of HPTH, where benign disease is seen in multiple glands. Fibro-osseous tumors of craniofacial bones are seen in 33% of cases with the mandible being most frequently affected, followed by the maxilla.32,33 On CT, they appear as well-circumscribed hyperdense, expansile lesions with rare internal calcification (Figure 9). 34 Differential diagnosis involves fibrous dysplasia of the bone, characterized by indistinct boundaries, and osteolytic brown tumors of HPTH, which present as purely lytic lesions without sclerotic margins. 34 On MRI fibro-osseous tumors are low to intermediate intensity on T1 non-enhanced and T2 sequences, demonstrating contrast enhancement and restricted diffusion. Increased bone uptake is seen on 99mTc scan. 34
Figure 9.
Mandibular ossifying fibroma in a 26-year-old woman. Multiplanar CT images (a-c) reveal an expansile unilocular lesion arising from the left mandibular ramus with calcific and fibrous matrix, discernible sclerotic rim and absence of any aggressive imaging features (arrows).
Li-Fraumeni Syndrome (LFS)
Li-Fraumeni Syndrome is an AD inherited tumor syndrome with early onset (<45 years old) of widespread cancers. Carrier rate of LFS is 1 in 5000 to 20,000 families. 35 Individuals with LFS have mutations on chromosome 17p13.1, in the tumor suppressor gene 53 (TP53). TP53 encodes p53 protein which plays a crucial role in cell cycle regulation, DNA repair, and apoptosis. Patients with LFS are at an increased risk of developing various types of cancers including breast cancer, soft tissue sarcomas, brain tumors, adrenal gland tumors, and leukemia. 35 Carriers of the LFS gene have a 100% risk of developing cancer by their fourth decade. 35
In CNS tumors, malignant gliomas are the most common; followed by choroid plexus carcinomas, medulloblastomas, and meningiomas. 36 Imaging findings of these malignancies resemble their sporadic counterpart. Patients with LFS have early childhood and/or adult-onset malignant gliomas. Imaging characteristics of malignant gliomas vary based on secondary changes such as necrosis, solid and cystic components, hemorrhage, and peritumoral edema. 35 Choroid plexus carcinomas appear as enhancing lobulated lesions in the ventricles. These carcinomas can have dissemination, therefore requiring whole neuroaxis imaging once diagnosed. 35
Most cancers resulting from LFS in the head and neck region are located at the orbit, soft tissue, nasopharynx, and glands. In order of prevalence, thyroid cancer is the most common followed by lymphoma, neural origin tumors, and sarcoma. The National Comprehensive Cancer Network (NCCS) suggests annual brain MR screening in LFS patients.35,36
Fanconi Anemia
Fanconi Anemia (FA) is a rare (1-2 in 100,000 prevalence) genetic tumor syndrome involving any of the 22 genes encoding functional proteins for the DNA cross-link repair pathway. 37 The most common genes with identified mutations are Fanconi Anemia Complementation Group A (FANC-A), FANC-C, and FANC-G. 37 The majority of these germline mutations are inherited in an autosomal recessive pattern (AR), but a few are X-linked or AD.
Patients with FA have a 500 to 800 fold higher risk of developing squamous cell carcinoma (SCC) than the general population. 38 The head and neck are the most frequently affected areas due to DNA damage caused by exposure to ultraviolet light. HNSCC in these populations present with early onset and advanced stage at the time of diagnosis, and higher chances of recurrence. The risk of hematological malignancies also increases, especially myelodysplastic and acute myeloid leukemia. Treating patients with radiation requires caution due to impaired DNA cross-link repair, which reduces the threshold for treatment-induced malignancies.
Dyskeratosis congenita
Dyskeratosis congenita (DC), also known as Zinsser-Engman-Cole syndrome, is a very rare inherited tumor syndrome with a yearly prevalence of 1 in 10 million. 39 At the molecular level, mutations in genes maintaining telomere length lead to the development of DC syndrome. In total, 14 genes have been identified to be associated with DC, including DKC1, TERC, TERT, and TINF2. 39
Clinically, a triad of dysplastic nails; lacy, reticular pigmentation of the upper chest and/or neck; and oral leukoplakia are common. Patients also have hematological abnormalities, increased risk of solid malignancies (HNSCC), pulmonary fibrosis, ophthalmological abnormalities, and gastrointestinal telangiectasias. 23
Hoyeraal Hreidarsson syndrome (HHS) is cerebellar hypoplasia associated with DC. Cerebellar hypoplasia in HHS involves hypoplasia of the cerebellar hemisphere and vermis. MRI of the cerebellum shows areas of hypointense signal on T2-sequences, which correspond to parenchymal calcifications, better seen on CT. 39 A small cerebellum, enlarged foramen magnum and fourth ventricle can also be seen, best visualized on sagittal sequences. 39
Ataxia-telangiectasia
Ataxia telangiectasia (AT) is a primary immunodeficiency disease caused by AR inherited mutations in the ATM gene located on chromosome 11q22-23. 40 Mutations in the ATM gene lead to impaired DNA double-strand break repairs causing accumulation of spontaneous DNA mutations and tumorigenesis. 40
Clinical features of AT involve immunodeficiency and neurodegeneration, including progressive cerebellar ataxia, postural instability, oculomotor apraxia, and dysarthria. 40 On imaging, cerebellar degeneration with thinning of the cerebellar cortex and vermal atrophy is present, often best seen on T2-sequences. 40 Other features are similar to cerebellar hypoplasia as described in DC. 41
Bloom syndrome
Bloom syndrome is a rare genetic instability syndrome most prevalent in the Ashkenazi Jewish population of Europe and Israel. 42 Patients inherit mutations in the BLM gene in an AR pattern, which encodes RecQL3 helicase-a DNA repair enzyme. 23
Clinical features of Bloom syndrome include long limbs; large ears, hands, and feet; and a long narrow face with a small, prominent jaw giving a distinct bird-like appearance. 42 Bloom syndrome patients have a high predisposition to develop skin cancer, most commonly multiple BCC of the head and neck. Hematological malignancies are also common and aggressive. 42
Von Hippel–Lindau syndrome
Von Hipple–Lindau (VHL) syndrome is an AD inherited disorder that results from germline mutations in the VHL gene on chromosome 3p25.5. The prevalence of VHL is estimated to be 1 in 31,000-53,000. 43 VHL syndrome is characterized by the presence of hemangioblastomas, endolymphatic sac tumors, renal cysts and tumors, pheochromocytoma, and epididymal cystadenomas (Figure 10). 43
Figure 10.
Von Hippel-Lindau (vHL) disease in a 48-year-old man with left endolymphatic sac tumor and multiple hemangioblastomas. Erosion of the left petrous bone (a, arrow) in the region of vestibular aqueduct in a “moth-eaten” pattern with avid enhancement seen on post-contrast MR image (b, arrow). Multiple cerebellar and spinal (intramedullary and peri-medullary) enhancing tumors (c, f, arrows), consistent with hemangioblastomas. Marked vascularity of the hemangioblastoma seen as multiple dilated and tortuous vessels along the posterior cord surface (d, arrow).
Hemangioblastomas in the CNS are often located in the cerebellum and spinal canal. Intramedullary spinal hemangioblastomas are more frequent than extramedullary hemangioblastomas. 40 Hemangioblastomas can be cystic, hemorrhagic, or mixed. The most common presentation on imaging is a cystic mass with an enhancing mural nodule. 40 On MRI, hemangioblastomas present as a hypo-to isointense mass on T1 and hyperintense on T2 with serpiginous flow voids; intense contrast enhancement is present due to high vascularity (Figure 10). 40 Patients diagnosed with VHL are recommended to undergo brain and spine MR screening once baseline at 20 years old and annually after that. 40
Tuberous sclerosis
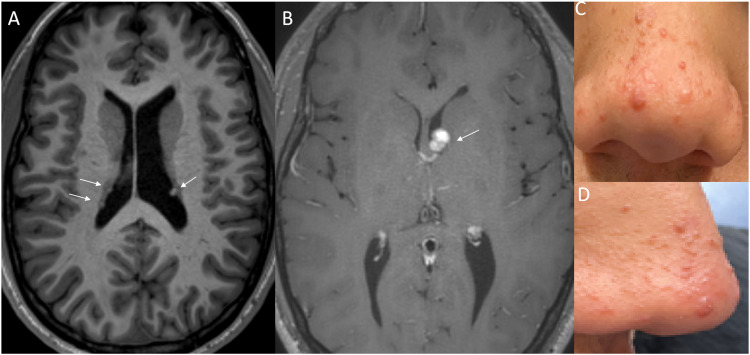
Tuberous Sclerosis (TS), also known as Bourneville disease, is characterized by the presence of multisystemic hamartomas and a clinical triad (Vogt triad) of seizures, intellectual disability, and facial angiofibroma. TS is an AD genetic disorder caused by mutations in gene TSC1 on chromosome 9q32 or gene TSC2 on chromosome 16p13.3, coding for hamartin and tuberin, respectively. 44 The clinical features include subependymal nodules, subependymal giant cell astrocytomas (SEGAs), white matter heterotopia, cardiac rhabdomyosarcoma, lymphangiomyomatosis, and angiomyolipoma (Figure 11).
Figure 11.
Stigmata of Tuberous Sclerosis in a 16-year-old boy. Axial T1WI reveals few subependymal nodules/hamartomas (a, arrows). Contrast-enhanced image (b) reveals an enhancing mass adjacent to the foramen of Monroe on the left (arrow) compatible with subependymal giant cell astrocytoma (SGCA). Facial angiofibromas along the nose (c, d), which represent the most conspicuous cutaneous manifestations of tuberous sclerosis.
Due to disorganized cortical lamination, tubers can be present anywhere from the cortex to deep white matter, causing locoregional symptoms, including seizures. 45 On CT, tubers appear as hypodense lesions with rare calcifications. On MRI, tubers are hypointense on T1 and hyperintense on FLAIR and T2 sequences. Classically, this pattern is reversed in children due to a lack of myelination. 46 Pathophysiologically and symptomatically like tubers, white matter heterotopia are nodules, cysts, or hypo-myelinated lesions that may or may not result in tubers. On MRI, white matter heterotopia is seen as iso- or hypointense lesions on T1 and hyperintense lesions on T2. 46
Subependymal nodules are one of the significant features in the diagnosis of TS. They are most common in the fourth ventricle and tend to calcify. On non-contrast CT, subependymal nodules appear as multiple small foci with dense calcification. MRI shows iso-to hyperintense lesions on T1, T2, and FLAIR with susceptibility artifact due to calcification. 47 SEGAs are the most common CNS tumors in TS, presumed to arise from subependymal nodules. Their clinical features depend on location, but are most common near the foramen of Monro, causing obstructive hydrocephalus and area postrema symptoms. On MRI, SEGAs show variable enhancement, iso-to hypointensity compared to cortex on T1-sequences and variable intensity on T2-sequences (Figure 11). In comparison to subependymal nodules, SEGAs are larger and more avidly enhancing.
Surveillance with brain MRI is recommended at intervals of 1–3 years until 25 years of age. Patients without SEGAs do not require prolonged surveillance; however, those with SEGAs should undergo more frequent monitoring.
Conclusion
Clearly, the radiologist plays an integral role in both surveillance and early detection of tumors in the setting of genetic tumor syndromes involving the head and neck. Knowledge of these key elements of genetic tumor syndromes can often lead to accurate first-time diagnosis by the radiologist, thus enhancing value in patient care.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs
Sneh Brahmbhatt https://orcid.org/0009-0008-8147-3219
Alok A. Bhatt https://orcid.org/0000-0003-3400-7123
References
- 1.Jiang H, He K. Statistics in the genomic era. Genes 2020; 11: 443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Classification of Tumours Editorial Board . Head and neck tumours. Lyon (France): international agency for research on cancer. In: WHO classification of tumours series. 5th edn. 2022, https://publications.iarc.fr/ [Google Scholar]
- 3.Vijapura C, Saad Aldin E, Capizzano AA, et al. Genetic syndromes associated with central nervous system tumors. Radiographics 2017; 37: 258–280. [DOI] [PubMed] [Google Scholar]
- 4.Kimonis VE, Mehta SG, Digiovanna JJ, et al. Radiological features in 82 patients with nevoid basal cell carcinoma (NBCC or Gorlin) syndrome. Genet Med 2004; 6: 495–502. [DOI] [PubMed] [Google Scholar]
- 5.Lo ML, Nocini PF, Savoia A, et al. Nevoid basal cell carcinoma syndrome. Clinical findings in 37 Italian affected individuals. Clin Genet 1999; 55: 34–40. [DOI] [PubMed] [Google Scholar]
- 6.Evans DG, Ladusans EJ, Rimmer S, et al. Complications of the naevoid basal cell carcinoma syndrome: results of a population based study. J Med Genet 1993; 30: 460–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sartip K, Kaplan A, Obeid G, et al. Neuroimaging of nevoid basal cell carcinoma syndrome (NBCCS) in children. Pediatr Radiol 2013; 43: 620–627. [DOI] [PubMed] [Google Scholar]
- 8.Levy RA, Blaivas M, Muraszko K, Robertson PL. Desmoplastic medulloblastoma: MR findings. AJNR Am J Neuroradiol. 1997; 18(7): 1364–1366. [PMC free article] [PubMed] [Google Scholar]
- 9.Maria G, Armando C, Francesca B, et al. Medulloblastoma Variants: Age-Dependent Occurrence and Relation to Gorlin Syndrome—A New Clinical Perspective. Clin Cancer Res. 2009; 1157: 2463–2471. doi: 10.1158/1078-0432.CCR-08-2023 [DOI] [PubMed] [Google Scholar]
- 10.Choudry Q, Patel HC, Gurusinghe NT, et al. Radiation-induced brain tumours in nevoid basal cell carcinoma syndrome: implications for treatment and surveillance. Childs Nerv Syst 2007; 23: 133–136. [DOI] [PubMed] [Google Scholar]
- 11.FriedmanNeurofibromatosis JM.1. 1998. Oct 2 [Updated 2022 Apr 21]. In: Adam MP, Feldman J, Mirzaa GM, et al. , editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2024. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1109/ [Google Scholar]
- 12.Grover DSB, Kundra DR, Grover DH, Gupta DV, Gupta DR. Imaging diagnosis of plexiform neurofibroma- unravelling the confounding features: A report of two cases. Radiol Case Rep. 2021; 1716(9): 2824-2833. doi: 10.1016/j.radcr.2021.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DrSB G, Kundra DR, Grover DH, et al. Imaging diagnosis of plexiform neurofibroma- unravelling the confounding features: a report of two cases. Radiol Case Rep 2021; 16: 2824–2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Blank PMK, Fisher MJ, Liu GT, et al. Optic pathway gliomas in neurofibromatosis type 1: an update: surveillance, treatment indications, and biomarkers of vision. J Neuro-Ophthalmol Off J North Am Neuro-Ophthalmol Soc 2017; 37: S23–S32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Russo C, Russo C, Cascone D, et al. Non-oncological neuroradiological manifestations in NF1 and their clinical implications. Cancers 2021; 13: 1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skolnik AD, Loevner LA, Sampathu DM, et al. Cranial nerve schwannomas: diagnostic imaging approach. Radiographics 2016; 36: 1463–1477. [DOI] [PubMed] [Google Scholar]
- 17.Coy S, Rashid R, Stemmer-Rachamimov A, et al. An update on the CNS manifestations of neurofibromatosis type 2. Acta Neuropathol 2020; 139: 643–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Groen EJ, Roos A, Muntinghe FL, et al. Extra-Intestinal manifestations of familial adenomatous polyposis. Ann Surg Oncol 2008; 15: 2439–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koh K-J, Park H-N, Kim K-A. Gardner syndrome associated with multiple osteomas, intestinal polyposis, and epidermoid cysts. Imaging Sci Dent 2016; 46: 267–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pilarski R. PTEN hamartoma tumor syndrome: a clinical overview. Cancers 2019; 11: 844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lok C, Viseux V, Avril MF, et al. Brain magnetic resonance imaging in patients with cowden syndrome. Medicine (Baltim) 2005; 84: 129. [DOI] [PubMed] [Google Scholar]
- 22.Joo G, Doumanian J. Radiographic findings of dysplastic cerebellar gangliocytoma (Lhermitte-Duclos disease) in a woman with cowden syndrome: a case study and literature review. J Radiol Case Rep 2020; 14: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nosé V, Lazar AJ. Update from the 5th edition of the world health organization classification of head and neck tumors: familial tumor syndromes. Head Neck Pathol 2022; 16: 143–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benn DE, Robinson BG, Clifton-Bligh RJ. 15 years of paraganglioma: clinical manifestations of paraganglioma syndromes types 1–5. Endocr Relat Cancer 2015; 22: T91–T103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thelen J, Bhatt AA. Multimodality imaging of paragangliomas of the head and neck. Insights Imaging 2019; 10: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin EP, Chin BB, Fishbein L, et al. Head and neck paragangliomas: an update on the molecular classification, state-of-the-art imaging, and management recommendations. Radiol Imaging Cancer 2022; 4: e210088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ota Y, Naganawa S, Kurokawa R, et al. Assessment of MR imaging and CT in differentiating hereditary and nonhereditary paragangliomas. AJNR Am J Neuroradiol 2021; 42: 1320–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mathiesen JS, Effraimidis G, Rossing M, et al. Multiple endocrine neoplasia type 2: a review. Semin Cancer Biol 2022; 79: 163–179. [DOI] [PubMed] [Google Scholar]
- 29.Wells SA, Asa SL, Dralle H, et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid Off J Am Thyroid Assoc 2015; 25: 567–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scarsbrook AF, Thakker RV, Wass JAH, et al. Multiple endocrine neoplasia: spectrum of radiologic appearances and discussion of a multitechnique imaging approach. Radiographics 2006; 26: 433–451. [DOI] [PubMed] [Google Scholar]
- 31.Kushchayev SV, Kushchayeva YS, Tella SH, et al. Medullary thyroid carcinoma: an update on imaging. J Thyroid Res 2019; 2019: 1893047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jha S, Simonds WF. Molecular and clinical spectrum of primary hyperparathyroidism. Endocr Rev 2023; 44: 779–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamashita Y, Akiyama T, Mizusawa N, et al. A case of hyperparathyroidism-jaw tumour syndrome found in the treatment of an ossifying fibroma in the maxillary bone. Int J Oral Maxillofac Surg 2007; 36: 365–369. [DOI] [PubMed] [Google Scholar]
- 34.Khan MM, Fazli H, Khan TB, et al. Hyperparathyroidism jaw-tumor syndrome: a case report from a radiological view. Cureus 2022; 14. DOI: 10.7759/cureus.28329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naganawa S, Donohue T, Capizzano A, et al. Neuroradiology manifestations of Li-Fraumeni syndrome: epidemiology, genetics, imaging findings, and management. Neurographics 2020; 10: 228–235. [Google Scholar]
- 36.McBride KA, Ballinger ML, Killick E, et al. Li-Fraumeni syndrome: cancer risk assessment and clinical management. Nat Rev Clin Oncol 2014; 11: 260–271. [DOI] [PubMed] [Google Scholar]
- 37.Beckham TH, Leeman J, Jillian Tsai C, et al. Treatment modalities and outcomes of Fanconi anemia patients with head and neck squamous cell carcinoma: series of 9 cases and review of the literature. Head Neck 2019; 41: 1418–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scheckenbach K, Wagenmann M, Freund M, et al. Squamous cell carcinomas of the head and neck in Fanconi anemia: risk, prevention, therapy, and the need for guidelines. Klin Pädiatr 2012; 224: 132–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang M-J, Cao Y-X, Wu H-Y, et al. Brain imaging features of children with Hoyeraal-Hreidarsson syndrome. Brain Behav 2021; 11: e02079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Farina L, Uggetti C, Ottolini A, et al. Ataxia-telangiectasia: MR and CT findings. J Comput Assist Tomogr 1994; 18: 724–727. [PubMed] [Google Scholar]
- 41.Arora H, Chacon AH, Choudhary S, et al. Bloom syndrome. Int J Dermatol 2014; 53: 798–802. [DOI] [PubMed] [Google Scholar]
- 42.Karsdorp N, Elderson A, Wittebol-Post D, et al. Von Hippel-Lindau disease: new strategies in early detection and treatment. Am J Med 1994; 97: 158–168. [DOI] [PubMed] [Google Scholar]
- 43.Cheadle JP, Reeve MP, Sampson JR, et al. Molecular genetic advances in tuberous sclerosis. Hum Genet 2000; 107: 97–114. [DOI] [PubMed] [Google Scholar]
- 44.Lu DS, Karas PJ, Krueger DA, Weiner HL. Central nervous system manifestations of tuberous sclerosis complex . Am J Med Genet Part C . 2018; 178C: 291–298. 10.1002/ajmg.c.31647 [DOI] [PubMed] [Google Scholar]
- 45.Wang MX, Segaran N, Bhalla S, et al. Tuberous sclerosis: current update. Radiographics 2021; 41: 1992–2010. [DOI] [PubMed] [Google Scholar]
- 46.Alshoabi SA, Hamid AM, Alhazmi FH, et al. Diagnostic features of tuberous sclerosis complex: case report and literature review. Quant Imag Med Surg 2022; 12: 84661–84861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gaillard A-L, Crombé A, Jecko V, et al. Magnetic resonance imaging diagnosis of subependymal giant cell astrocytomas in follow-up of children with tuberous sclerosis complex: should we always use contrast enhancement? Pediatr Radiol 2020; 50: 1397–1408. [DOI] [PubMed] [Google Scholar]