Abstract
Background:
Once weekly Glucagon-Like Peptide-1 Receptor Agonists (GLP-1 RA) have been shown to improve glycemic outcomes and cause significant weight loss. However, 9% to 27% of individuals have little or no response to these drugs. In this article, we investigated the efficacy of GLP-1 RA therapy among adults with type 1 diabetes and obesity likely related to genetic mutations compared with obesity likely unrelated to genetic mutations.
Methods:
In this retrospective study, we compared body weight and glycated hemoglobin (HbA1c) change with the use of GLP-1 RA therapy (including a dual agonist, Tirzepatide) over six months among adults with type 1 diabetes and obesity likely (n = 11, median age 39.5 years with a median BMI of 43.0 kg/m2) versus unlikely related to genetic mutation(s) (n = 15, median age 45.8 years with a median BMI of 38.7 kg/m2).
Results:
Six months of GLP-1 RA treatment resulted in a numerically lower reduction of weight (−5.75 ± 9.46 kg vs −8.65 ± 9.36 kg, P = .44) and HbA1c (−0.28 ± 0.96% vs −0.43 ± 0.57%, P = .64) among individuals with obesity likely versus unlikely related to a genetic mutation(s), respectively. Fewer individuals with genetic obesity met goal weight loss ≥5% or HbA1c decrease ≥0.4% than did individuals with obesity unlikely related to a genetic cause (36.4% vs 80.0%, P = .04).
Conclusions:
The weight loss and glycemic lowering effects of GLP-1 RA therapy may be decreased in people with type 1 diabetes and obesity likely related to genetic causes. Further research is needed to understand GLP-1 RA mechanisms via energy regulating genes.
Keywords: glucagon-like peptide-1 receptor agonist (GLP-1 RA), type 1 diabetes mellitus, obesity, genetics, genetic mutations
Introduction
Glucagon-Like Peptide-1 Receptor Agonist (GLP-1 RA) therapy has become a standard of care for management of type 2 diabetes. 1 These drugs have been shown to significantly reduce body weight, improve glycemic control, and decrease the risk of cardiovascular complications and all-cause mortality.2,3 Weekly Semaglutide (Wegovy) and dual agonist, Tirzepatide (Zepbound), are also approved by the United States Food and Drug Administration (FDA) for obesity management.
Over the past few decades, rates of obesity experienced by people living with type 1 diabetes have continued to increase. 4 Subsequently, off-label use of GLP-1 RA is gaining traction within this population.5,6,7 A concurrent proof of concept study at the Barbara Davis Center (BDC) demonstrated significant reduction in body weight and glycated hemoglobin (HbA1c) with the use of Tirzepatide in adults with type 1 diabetes and overweight/obesity. 8
Research into obesity itself has demonstrated the complexity and often polygenic nature of the disease, which is spurred by a heritability ranging from 40 to 70%. 9 Correspondingly, research into correlations between certain genetic mutations and obesity has spiked in recent years and may pave the way for future understanding and mitigation of the disease.
It has been reported that between 9% and 27% percent of those who use Semaglutide or Tirzepatide therapy do not respond to the drug, defined as less than 5% weight loss over an average of 72 weeks of treatment. 10 The STEP 1 study demonstrated a 13.6% nonresponse rate to the GLP-1 RA Semaglutide in individuals with overweight or obesity (where nonresponse was defined as loss of <5% of body weight). 10 We have observed a lack of weight loss and/or glycemic lowering effect with GLP-1 RA therapy in a few individuals with type 1 diabetes in our clinical practice and in the aforementioned feasibility study. 8
Assuming good adherence to medications in the clinical trials, lack of either glycemic or weight loss efficacy may be due to genetic variation at incretin receptors, 10 genetic mutations in leptin-melanocortin signaling pathways, or genes that interact with this pathway peripherally or influence the development of neurons responsible for energy homeostasis.
In this exploratory study, we sought to evaluate response to GLP-1 RA therapy in those with type 1 diabetes and obesity likely related to genetic mutations.
Methods
Study Design, Population, and Data Collection
This was a single-center, retrospective study. As part of clinical care, we (V.N.S. and H.K.A.) offered genetic testing for eligible individuals who were followed for management of their diabetes at the Barbara Davis Center Adult Clinic. This genetic testing was offered at no cost through the Rhythm Pharmaceutical sponsored “Uncovering Rare Obesity” program. 11 Adults with a Body Mass Index (BMI) ≥40 kg/m2 and a history of early-onset, severe obesity were eligible for free testing through this program. 11 All patients signed a written consent form prior to saliva or blood sample collection. Samples were shipped to Prevention Genetics for genetic testing. The 79 genes screened were primarily associated with the leptin-melanocortin pathway, genes affecting energy balance, and Bardet-Biedl syndrome and related ciliopathies. Further details about this genetic testing are available at https://www.preventiongenetics.com/sponsoredTesting/Rhythm. Prevention Genetics reported genetic testing per American College of Medical Genetics and Genomics (ACMG) guidelines. 12
We reviewed Electronic Medical Records (EMR) for individuals meeting the following criteria: adults (age >18 years) with type 1 diabetes who have participated in the “Uncovering Rare Obesity” program and have used GLP-1 RA therapy for management of obesity. As a control, we used three individuals who tested negative for a genetic mutation associated with obesity alongside a cohort of adults with type 1 diabetes who were followed for up to 8 months to evaluate safety and efficacy of Tirzepatide at our center. The details of this cohort are published in the same issue of the JDST. 8 Baseline (defined as the date of GLP-1 RA therapy initiation) and follow-up clinic data were collected for the following variables; age, sex, race, ethnicity, diabetes duration, insulin delivery method, use of Continuous Glucose Monitor (CGM), Total Daily insulin Dose (TDD), insurance type, height, weight, BMI, HbA1c, and presence of comorbidities/diabetes complications. EMR-based data collection methods at our site were previously published.13,14 This study was approved by the Colorado Multiple Institutional Review Board (COMIRB) under the exempt category.
Study Outcomes
The primary outcome of this study was percentage change in body weight and absolute change in HbA1c at six months across two groups: one with obesity likely related to a genetic mutation(s) and a control group with obesity unlikely related to a genetic mutation(s). The secondary outcomes were differences in baseline diabetes duration, TDD, and BMI between the two cohorts.
Statistical Analysis
We analyzed response to GLP-1 RA by comparing the change in weight and HbA1c as continuous variables using unpaired t-tests. We also compared responses to GLP-1 RA therapy across four main parameters. First, response was assessed based on relative weight change, defined as response if greater than or equal to a 5% decrease in body weight between baseline and six months and nonresponse if less than a 5% decrease. Second, response was assessed via absolute change in HbA1c, defined as response if greater than or equal to a 0.4-unit decrease between baseline and six months and nonresponse if less than a 0.4-unit decrease. The final two parameters were the frequency of (1) meeting either goal (≥5% decrease in body weight or ≥0.4 decrease in HbA1c) and (2) meeting both goals (≥5% decrease in body weight and ≥0.4 decrease in HbA1c).
Results
Of 126 individuals with a BMI ≥40 kg/m2 who were seen for clinical care at the BDC, 23 (18.3%) were eligible for and consented to genetic testing. 18 (78.3%) of these individuals tested positive for an obesity-related mutation(s), and five (21.7%) tested negative. GLP-1 RA therapy was used by 14 of the 18 individuals positive for a mutation(s) and four of the five individuals negative for a mutation(s). 11 of the 14 former individuals were analyzed as the “Mutation” cohort as three did not have analyzable data at six months from baseline. Three of the four latter individuals (1 did not have analyzable data at six months from baseline)—alongside 12 individuals who were not eligible for genetic testing (thus low probability of genetic obesity) and were treated with GLP-1 RA therapy for glycemic or obesity management at our center—were analyzed as the “Control Group” cohort. A flowchart detailing the cohort selection process is provided in Supplementary Figure 1. Specific genetic testing results are provided in Supplementary Table 1.
Baseline demographic characteristics of the two cohorts are presented in Table 1. In short, adults with obesity likely related to a genetic mutation(s) were younger (median 39.5 vs 45.8 years) with a shorter duration of diabetes (median 12.8 vs 24.0 years) and a higher BMI (median 43.0 vs 38.7 kg/m2) than controls. Use of technologies for type 1 diabetes management such as CGM and insulin pumps as well as glycemic control (HbA1c) was similar between the two groups. As expected, TDD was higher among adults with obesity likely related to a genetic mutation(s) than among controls (median 0.68 vs 0.46 units/kg/day). The types of GLP-1 RA used between the two groups are provided in Supplementary Table 2.
Table 1.
Baseline Characteristics.
| Variable | Total cohort N = 26 |
With mutation, n = 11 | Control, n = 15 |
|---|---|---|---|
| Age (years) | 39.5 (35.0-46.8) | 39.5 (35.3-44.1) | 45.8 (34.9-48.0) |
| Female, n (%) | 16 (61.5) | 5 (45.5) | 11 (73.3) |
| Non-Hispanic White, n (%) | 23 (88.5) | 9 (81.8) | 14 (93.3) |
| Diabetes Duration (years) | 20.3 (10.3-34.0) | 12.8 (7.5-26.1) | 24.0 (15.3-42.7) |
| Insulin Delivery Method, n (%) | |||
| MDI | 10 (38.5) | 2 (18.2) | 8 (53.3) |
| Pump w/o CGM | 2 (7.7) | 2 (18.2) | 0 (0.0) |
| AID | 14 (53.8) | 7 (63.6) | 7 (46.7) |
| Use of CGM, n (%) | 20 (76.9) | 8 (72.7) | 12 (80.0) |
| Total Daily Insulin Dose a (units/kg/day) | 0.61 (0.43-0.79) | 0.68 (0.56-0.89) | 0.46 (0.42-0.64) |
| Insurance Type, n (%) | |||
| Private | 23 (88.5) | 9 (81.8) | 14 (93.3) |
| Government | 3 (11.5) | 2 (18.2) | 1 (6.7) |
| Weight (kg) BMI (kg/m2) |
114.1 (97.5-129.0) 39.4 (36.2-43.2) |
128.2 (113.0-139.2) 43.0 (38.7-43.9) |
109.7 (92.1-117.3) 38.7 (33.7-41.5) |
| HbA1c (%) | 7.3 (6.9-7.7) | 7.3 (6.9-8.2) | 7.2 (6.9-7.6) |
| Comorbidities, n (%) | |||
| CVD | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Dyslipidemia | 13 (50.0) | 5 (45.5) | 8 (53.3) |
| Neuropathy | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Nephropathy | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Retinopathy | 13 (50.0) | 4 (36.4) | 9 (60.0) |
Data are median (interquartile range) or n (percent).
Abbreviations: MDI, Multiple Daily Injections; CGM, Continuous Glucose Monitor; AID, Automated Insulin Delivery; BMI, Body Mass Index; HbA1c, Glycated Hemoglobin; CVD, Cardiovascular Disease.
No data for one participant.
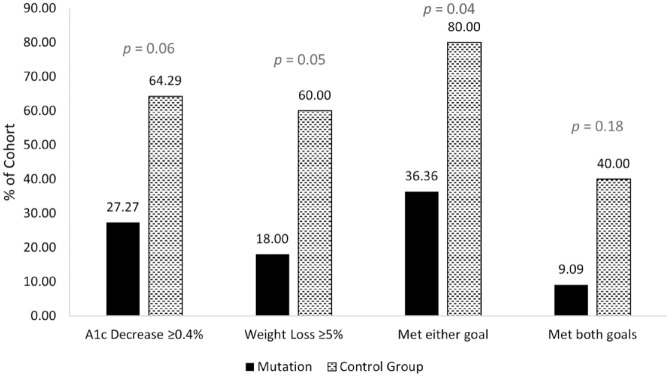
There was an observed lower absolute and relative change in body weight (mean −5.75 ± 9.46 vs −8.65 ± 9.36 kg, P = .44; −4.78 ± 8.83 vs −8.57 ± 9.53 %, P = .31) and absolute change in HbA1c (mean −0.28 ± 0.96 vs −0.43 ± 0.57, P = .64) at six months among adults with obesity likely related to a genetic mutation(s) than among controls; however, these differences remained statistically non-significant (Table 2). There were significantly less people with obesity likely related to a genetic mutation(s) who met either weight loss of ≥5% or HbA1c reduction of ≥0.4% at six months from baseline than the control group (Figure 1). Table 3 provides detailed results of the types of genetic mutations and correlated responses to GLP-1 RA therapy based on weight loss and glycemic lowering.
Table 2.
Weight and HbA1c Change Between Baseline and 6 Months.
| Variable | With mutation, n = 11 | Control group, n = 15 | P value |
|---|---|---|---|
| Weight, Kg | |||
| Baseline | 125.92 ± 22.52 | 105.90 ± 21.97 | .01 |
| Change at six months | −5.75 ± 9.46 | −8.65 ± 9.36 | .44 |
| % Change at six months | −4.78 ± 8.83 | −8.57 ± 9.53 | .31 |
| HbA1c (%) | |||
| Baseline | 7.58 ± 1.26 | 7.27 ± 0.65 | .47 |
| Change at six months a | −0.28 ± 0.96 | −0.43 ± 0.57 | .64 |
Missing six-month HbA1c data for one individual.
Figure 1.
GLP-1 RA response frequency analysis.
Table 3.
Mutations and GLP-1 RA Response.
| Gene name | Gene transcript | Response—weight a | Response—HbA1c b |
|---|---|---|---|
| IFT172 MAGEL2 |
NM_015662.2 NM_019066.4 |
No (−0.72%) | Yes (−0.6) |
| CREBBP MAGEL2 |
NM_004380.2 NM_019066.4 |
No (−3.93%) | No (0.3) |
| PLXNA2 | NM_025179.3 | No (−0.43%) | Yes (−2.5) |
| ALMS1 ALMS1 BDNF |
NM_015120.4 NM_015120.4 NM_001143810.1 |
No (−1.65%) | No (−0.2) |
| RAI1 | NM_030665.3 | No (−3.85%) | No (0.2) |
| PLXNA4 | NM_020911.1 | No (−4.11%) | No (0.0) |
| SIM1 VPS13B WDPCP |
NM_005068.2 NM_152564.4 NM_015910.5 |
Yes (−30.48%) | Yes (−1.5) |
| IFT172 | NM_015662.2 | Yes (−5.73%) | No (−0.1) |
| NRP2 BBS12 |
NM_201266.1 NM_152618.2 |
No (1.33%) | No (0.5) |
| CEP290 UCP3 |
NM_025114.3 NM_003356.3 |
No (−4.11%) | No (−0.1) |
| NCOA1 (aka SRC1) | NM_003743.4 | No (1.06%) | No (0.9) |
“Yes” if ≥5% relative decrease from baseline.
“Yes” if ≥0.4 absolute decrease from baseline.
Discussion
To our knowledge, this is the first study on the response to GLP-1 RA therapy in patients with type 1 diabetes and obesity likely related to genetic mutations. We found a lower level of body weight and HbA1c reduction with the use of GLP-1 RA therapy among those likely to have a genetic form of obesity than among controls. Although changes in body weight and HbA1c between groups were clinically significant, they were not statistically significant, most likely due to the small sample size. Two individuals, one with a mutation on the NCOA1 (aka SRC1) gene and another with a mutation on the NRP2 and BBS12 genes, did not respond to GLP-1 RA therapy at all. They experienced no reduction in body weight or HbA1c—on the contrary, they experienced an increase in both—over the course of treatment.
Important to note is the difference in baseline body weight and BMI between the two cohorts. The mutation group had a higher baseline bodyweight (128.2 [113.0-139.2] kg) and BMI (43.0 [38.7−43.9] kg/m2, as compared with the reference group, 109.7 (92.1-117.3) kg and 38.7 (33.7-41.5) kg/m2, respectively. A post hoc analysis of Tirzepatide clinical trials demonstrated that greater baseline BMI was generally associated with greater absolute change in body weight in those with type 2 diabetes. 15 Taking these findings into account when reviewing our results, it would seem that the mutation group—with a greater baseline BMI—would have experienced greater change in body weight. However, the mutation group experienced both a lower absolute and a lower relative reduction in body weight at six months than did the reference group (−5.57 vs −8.65 kg; −4.78 vs −8.57 %). In addition, we hypothesize that the higher baseline weight of those in the mutation cohort may be associated with the mutations themselves. The mutations screened for are associated primarily with hypothalamic regulatory centers which are both essential to the regulation of energy intake and expenditure as well as are sites of endogenous GLP-1 action. If the mutations seen have effect on these regulatory centers, this cohort would be expected to see a higher baseline weight due to atypical endogenous GLP-1 function. Furthermore, our hypothesis follows that these individuals, alongside their atypical endogenous GLP-1 function, respond atypically to exogenous GLP-1 RAs.
A study published in 2018 investigated GLP-1 RA response in 14 patients with obesity caused by melanocortin-4 receptor (MC4-R) mutations. 16 Their results demonstrated statistically similar responses to Liraglutide treatment (assessed through weight loss and glucose lowering) between those with MC4-R mutations and those without. Similarly, a case report published in 2022 demonstrated weight loss with the use of GLP-1 RA therapy (Liraglutide and Semaglutide) in an individual with Bardet-Biedl syndrome. This individual was confirmed to have a homozygous mutation at c.1599_1602del p.(Thr53411efs*21) in the BBS10 gene (12q21.2). 17 Although these publications indicate similar response to GLP-1 RA therapy in individuals with obesity-related genetic mutations as compared with those without, they highlight an important aspect of GLP-1 RA action, that being, the complexity of its pharmacological mechanisms. Endogenous GLP-1, produced primarily in the gut, acts upon a variety of pathways in the body; GLP-1 receptors exist in the liver, pancreas, and other tissues, but primarily within the central nervous system. In the first publication mentioned above, 16 the population studied had genetic mutations associated with MC4-R. These specific receptors lie in the paraventricular nucleus (PVN) of the hypothalamus and play an important role in the regulation of energy intake and expenditure. However, the PVN is far from being the only site in which GLP-1 action takes place. Other areas of the hypothalamus, including the arcuate nucleus (ARC) which contains anorexigenic pro-opiomelanocortin (POMC) neurons and orexigenic agouti-related peptide (AgRP)/neuropeptide Y (NPY) neurons as well as the dorsomedial hypothalamus (DMH), also contain GLP-1 receptors. Furthermore, the hypothalamus is not the only neural center in which endogenous GLP-1 acts. GLP-1 receptors are also found in the area postrema (AP), nucleus tractus solitarii (NTS), ventral tegmental area (VTA), and the nucleus accumbens (NAc). 18 Alongside its direct action on the central nervous system, GLP-1 receptors have also been found in muscle and adipose tissue, 19 indicating peripheral mechanisms that may further influence exogenous GLP-1 RA effectiveness.
To understand how GLP-1 RA can still elicit effect in individuals with mutations on regulatory centers of energy intake and expenditure, it is important to understand the complexity of its action within the human body. In light of both the findings of these two publications as well as the findings of our own study, further research on the specific pharmacological mechanisms of exogenous GLP-1 RAs is needed to understand how mutations blocking/inactivating specific neural pathways may or may not impact pharmacological effectiveness.
The clinical and research implications of this study may help inform indications for GLP-1 RA use. First, given that GLP-1 RA therapy is expensive, nonresponse in an individual may prompt consideration for obesity-related genetic testing. Doing so may indicate the need for alternative treatment options. Second, our data demonstrates the need for investigative research into the physiological factors that affect GLP-1 RA efficacy, specifically regarding obesity-related genetic mutations. This may improve our understanding of GLP-1 RA mechanisms at the central (neurological) and peripheral (muscular and adipose tissue) systems influencing weight and glycemic regulation.
The results of our study should be interpreted with caution. This was a retrospective, exploratory analysis with a small sample size. The majority of individuals in the “Mutation” group were heterozygous for mutations screened and the clinical significance of most of these mutations is currently unknown. Not all individuals in the control group were tested for genetic obesity; however, we assume that they had a low pretest probability for a genetic form of obesity given these participants did not meet criteria for this specific genetic testing. Furthermore, there was an imbalance in the types of GLP-1 RAs used between our two cohorts. 86.6% of the control group use the dual-agonist Tirzepatide compared with 36.3% of the mutation cohort (Supplementary Table 2). Studies have demonstrated a greater impact on weight loss with the use of Tirzepatide as compared with Semaglutide. 20 As Semaglutide has been shown to have a greater impact on weight loss than Liraglutide, Dulaglutide, and Exenatide,21-23 Tirzepatide may do so as well. Consequently, the use of different types of GLP-1 RAs between the two groups may have confounded weight loss and HbA1c outcomes. Similarly, weight loss with GLP-1 RA is dose dependent and given the retrospective nature of this study, we did not have data on dosages of GLP-1 RA over time between the two groups. Lastly, the population of individuals analyzed in this study are primarily Non-Hispanic White (88.5%). This limits generalizability of our study results across other racial/ethnic groups.
With an understanding of these limitations, we present our results as primary evidence to spur future research into the associations between obesity-related genetic mutations and response to GLP-1 RA therapy.
Conclusions
Genetic mutations on the leptin-melanocortin pathway, other energy intake and expenditure pathways, and/or genes related to Bardet-Biedl syndrome and similar ciliopathies may influence the glycemic and weight lowering efficacy of GLP-1 RA therapy. Further research is needed to understand the pharmacological mechanisms of exogenous GLP-1 RA via central and peripheral energy regulating pathways.
Supplemental Material
Supplemental material, sj-docx-1-dst-10.1177_19322968241245680 for Reduced Efficacy of Glucagon-Like Peptide-1 Receptor Agonists Therapy in People With Type 1 Diabetes and Genetic Forms of Obesity by Matthew P. Klein, Halis Kaan Akturk, Janet K. Snell-Bergeon and Viral N. Shah in Journal of Diabetes Science and Technology
Supplemental material, sj-jpg-2-dst-10.1177_19322968241245680 for Reduced Efficacy of Glucagon-Like Peptide-1 Receptor Agonists Therapy in People With Type 1 Diabetes and Genetic Forms of Obesity by Matthew P. Klein, Halis Kaan Akturk, Janet K. Snell-Bergeon and Viral N. Shah in Journal of Diabetes Science and Technology
Acknowledgments
The authors thank Rhythm Pharmaceuticals’ genetic testing program “Uncovering Rare Obesity” through which eligible individuals were tested for genetic forms of obesity at no cost (https://uncoveringrareobesity.com). All genetic testing was performed at Prevention Genetics (https://www.preventiongenetics.com).
Footnotes
Correction (May 2024): Article updated; please see https://doi.org/10.1177/19322968241256557 for more details.
Abbreviations: ACMG, American College of Medical Genetics and Genomics; AgRP/NPY, Agouti-Related Peptide/NeuroPeptide Y; AID, Automated Insulin Delivery; AP, Area Postrema; BDC, Barbara Davis Center; BMI, Body Mass Index; CGM, Continuous Glucose Monitoring; COMIRB, Colorado Multiple Institutional Review Board; CVD, Cardiovascular Disease; DMH, DorsoMedial Hypothalamus; EMR, Electronic Medical Records; FDA, Food and Drug Administration; GLP-1 RA, Glucagon-Like Peptide-1 Receptor Agonist; HbA1c, Glycated Hemoglobin; IQR, Interquartile Range; MC4-R, Melanocortin-4 Receptor; MDI, Multiple Daily Injections; NAc, Nucleus Accumbens; NTS, Nucleus Tractus Solitarii; POMC, Pro-OpioMelanoCortin; PVN, Paraventricular Nucleus; TDD, Total Daily [insulin] Dose; VTA, Ventral Tegmental Area.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: HKA received research support through Medtronic, Tandem Diabetes, Mannkind, Eli Lilly, and Dexcom. He received honorarium for consulting from Medtronic, Tandem Diabetes, and Dexcom through the University of Colorado. VNS received research support from NovoNordisk, Insulet, Tandem Diabetes Care, and received honoraria from Dexcom, Embecta, Tandem Diabetes Care, Insulet, and NovoNordisk for speaking or consulting arrangements. MPK and JKS report no conflict of interest.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Matthew P. Klein  https://orcid.org/0009-0002-5279-3447
https://orcid.org/0009-0002-5279-3447
Halis Kaan Akturk  https://orcid.org/0000-0003-4518-5179
https://orcid.org/0000-0003-4518-5179
Janet K. Snell-Bergeon  https://orcid.org/0000-0002-9337-3740
https://orcid.org/0000-0002-9337-3740
Viral N. Shah  https://orcid.org/0000-0002-3827-7107
https://orcid.org/0000-0002-3827-7107
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Committee ADAPP. 9. Pharmacologic approaches to glycemic treatment: standards of care in diabetes—2024. Diabetes Care. 2024;47:S158-S178. doi: 10.2337/dc24-S009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nauck MA, Quast DR, Wefers J, Meier JJ. GLP-1 receptor agonists in the treatment of type 2 diabetes–state-of-the-art. Mol Metab. 2021;46:101102. doi: 10.1016/j.molmet.2020.101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kristensen SL, Rørth R, Jhund PS, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7(10):776-785. [DOI] [PubMed] [Google Scholar]
- 4. Fang M, Echouffo-Tcheugui JB, Selvin E. Prevalence and management of obesity in U.S. adults with type 1 diabetes. Ann Intern Med. 2023;176(3):427-429. doi: 10.7326/M22-3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Curtis L, Holt H, Richardson T, Knott J, Partridge H. GLP-1 analogue use in patients with sub-optimally controlled type 1 diabetes or obesity improves weight and HbA1c. Pract Diabetes. 2016;33(1):13-17. [Google Scholar]
- 6. Mohandas D, Calma J, Gao C, Basina M. Evaluating the efficacy and safety of long-acting GLP-1 receptor agonists in T1DM patients. Endocrines. 2023;4:93-101. doi: 10.3390/endocrines4010008. [Google Scholar]
- 7. Edwards K, Li X, Lingvay I. Clinical and safety outcomes with GLP-1 receptor agonists and SGLT2 inhibitors in type 1 diabetes: a real-world study. J Clin Endocrinol Metab. 2023;108(4):920-930. doi: 10.1210/clinem/dgac618. [DOI] [PubMed] [Google Scholar]
- 8. Akturk HK, Dong F, Snell-Bergeon JK, Karakus KE, Shah VN. Efficacy and safety of tirzepatide in adults with type 1 diabetes: a proof of concept observational study. J Diabetes Sci Technol. 2024: doi: 10.1177/19322968231223991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Feng B, Xu P, He Y. Novel targets in glucose homeostasis and obesity—lesson from rare mutations. Curr Diab Rep. 2020;20. doi: 10.1007/s11892-020-01351-7. [DOI] [PubMed] [Google Scholar]
- 10. Austin GO, Tomas A. Variation in responses to incretin therapy: modifiable and non-modifiable factors. Front Mol Biosci. 2023;10:1170181. doi:10.3389/fmolb.2023.1170181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Uncovering Rare Obesity: Rhythm Pharmaceuticals, Inc., 2023, https://uncoveringrareobesity.com/.
- 12. Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405-424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shah VN, Kanapka LG, Akturk HK, et al. Time in range is associated with incident diabetic retinopathy in adults with type 1 diabetes: a longitudinal study. Diabetes Technol Ther. 2024;26(4):246-251. doi: 10.1089/dia.2023.0486. [DOI] [PubMed] [Google Scholar]
- 14. Karakus KE, Akturk HK, Alonso GT, Snell-Bergeon JK, Shah VN. Association between diabetes technology use and glycemic outcomes in adults with type 1 diabetes over a decade. Diabetes Care. 2023;46(9):1-6. doi: 10.2337/dc23-0495. [DOI] [PubMed] [Google Scholar]
- 15. Kwan A, Maldonado JM, Wang H, Rasouli N, Wilding J. 719-P: tirzepatide induces weight loss in patients with type 2 diabetes regardless of baseline BMI: a post hoc analysis of SURPASS-1 through -5 studies. Diabetes. 2022;71:719. doi: 10.2337/db22-719-P. [DOI] [Google Scholar]
- 16. Iepsen EW, Zhang J, Thomsen HS, et al. Patients with obesity caused by melanocortin-4 receptor mutations can be treated with a glucagon-like peptide-1 receptor agonist. Cell Metab. 2018;28(1):23-32. [DOI] [PubMed] [Google Scholar]
- 17. Ganawa S, Santhosh SH, Parry L, Syed AA. Weight loss with glucagon-like peptide-1 receptor agonists in Bardet-Biedl syndrome. Clin Obes. 2022;12(5):e12546. doi: 10.1111/cob.12546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van Bloemendaal L, Ten Kulve JS, la Fleur SE, Ijzerman RG, Diamant M. Effects of glucagon-like peptide 1 on appetite and body weight: focus on the CNS. J Endocrinol. 2014;221(1):T1-16. doi: 10.1530/JOE-13-0414. [DOI] [PubMed] [Google Scholar]
- 19. Hammoud R, Drucker DJ. Beyond the pancreas: contrasting cardiometabolic actions of GIP and GLP1. Nat Rev Endocrinol 2023;19:201-216. [DOI] [PubMed] [Google Scholar]
- 20. Frías JP, Davies MJ, Rosenstock J, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385(6):503-515. doi: 10.1056/NEJMoa2107519. [DOI] [PubMed] [Google Scholar]
- 21. Rubino DM, Greenway FL, Khalid U, et al. Effect of weekly subcutaneous semaglutide vs daily liraglutide on body weight in adults with overweight or obesity without diabetes: the STEP 8 randomized clinical trial. JAMA. 2022;327(2):138-150. doi: 10.1001/jama.2021.23619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lingvay I, Bauer R, Baker-Knight J, Lawson J, Pratley R. An indirect treatment comparison of semaglutide 2.0 mg vs dulaglutide 3.0 mg and 4.5 mg using multilevel network meta-regression. J Clin Endocrinol Metab. 2022;107(5):1461-1469. doi: 10.1210/clinem/dgab905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ahmann AJ, Capehorn M, Charpentier G, et al. Efficacy and safety of once weekly semaglutide versus exenatide ER in subjects with type 2 diabetes (SUSTAIN3): a 56-week, open-label, randomized clinical trial. Diabetes Care. 2018;41(2):258-266. doi: 10.2337/dc17-0417. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-dst-10.1177_19322968241245680 for Reduced Efficacy of Glucagon-Like Peptide-1 Receptor Agonists Therapy in People With Type 1 Diabetes and Genetic Forms of Obesity by Matthew P. Klein, Halis Kaan Akturk, Janet K. Snell-Bergeon and Viral N. Shah in Journal of Diabetes Science and Technology
Supplemental material, sj-jpg-2-dst-10.1177_19322968241245680 for Reduced Efficacy of Glucagon-Like Peptide-1 Receptor Agonists Therapy in People With Type 1 Diabetes and Genetic Forms of Obesity by Matthew P. Klein, Halis Kaan Akturk, Janet K. Snell-Bergeon and Viral N. Shah in Journal of Diabetes Science and Technology