Abstract
Background:
There is emerging interest in the application of foot temperature monitoring as means of diabetic foot ulcer (DFU) prevention. However, the variability in temperature readings of neuropathic feet remains unknown. The aim of this study was to analyze the long-term consistency of foot thermograms of diabetic feet at the risk of DFU.
Methods:
A post-hoc analysis of thermal images of 15 participants who remained ulcer-free during a 12-month follow-up were unblinded at the end of the trial. Skin foot temperatures of 12 plantar, 15 dorsal, 3 lateral, and 3 medial regions of interests (ROIs) were derived on monthly thermograms. The temperature differences (∆Ts) of corresponding ROIs of both feet were calculated.
Results:
Over the 12-month study period, out of the total 2026 plantar data points, 20.3% ROIs were rated as abnormal (absolute ∆T ≥ 2.2°C). There was a significant between-visit variability in the proportion of plantar ROIs with ∆T ≥ 2.2°C (range 7.6%-30.8%, chi-square test, P = .001). The proportion of patients presenting with hotspots (ROIs with ∆T ≥ 2.2°C), abnormal plantar foot temperature (mean ∆T of 12 plantar ROIs ≥ 2.2°C), and abnormal whole foot temperature (mean ∆T of 33 ROIs ≥ 2.2°C) varied between visits and showed no pattern (P > .05 for all comparisons). This variability was not related to the season of assessment.
Conclusions:
Despite the high rate of hotspots on monthly thermograms, all feet remained intact. This study underscores a significant between-visit inconsistency in thermal images of neuropathic feet which should be considered when planning DFU-prevention programs for self-testing and behavior modification.
Keywords: diabetic foot ulcer, neuropathy, infrared thermography, diabetic foot ulcer prevention, spot thermometry, temperature monitoring
Introduction
Diabetic foot ulcer (DFU) is one of the most severe complications of diabetic neuropathy. It is associated with reduced quality of life, significant morbidity, and premature mortality.1,2 Undetected tissue damage in feet that have lost protective sensation can rapidly progress to ulcer, infection, and gangrene, and sometimes this “diabetic foot attack” may lead to a lower-limb amputation. 3 Even when DFUs heal, the risk of recurrence is high—almost 40% of patients develop another DFU in one year, 60% within three years, and 65% within five years. 4 Treatment costs are immense, and the long-term personal and societal outcome of diabetic foot disease is devastating. 5
A variety of strategies and interventions have been tested to alleviate the burden of foot disease and prevent DFU recurrence. 6 One of the techniques which has shown promise is skin foot temperature monitoring as a utility to identify inflamed areas of the foot at risk of DFU. The concept that “the skin heats up before the foot breaks down” originates from Dr Paul W. Brand’s work in the sixties and seventies of the last century. 7 More recently, with the increased availability of low-cost hand-held infrared spot thermometers, there has been a significant rise in observational studies and clinical trials, aimed at investigating the usefulness of early detection of hotspots (defined as areas of the foot with raised temperature which are assumed to indicate preulcerative inflammation of the skin) as a method of DFU prevention. These studies utilize self-testing of temperatures at predefined plantar foot landmarks, and a temperature difference (∆T) of 2.2°C (4°F) or greater between corresponding sites of feet is commonly accepted as a warning sign of inflammation. 6 Detection of hotspots should prompt the individual to reduce ambulatory activity until these inflamed areas have resolved, and the ∆T has decreased below 2.2°C.
Initial research applying this threshold of self-testing of the hallux; first, third, and fifth metatarsal heads (MHs); central midfoot; and heel has demonstrated a significant reduction in ulceration rates.8 -10 However, two recent studies, a single-center study utilizing the same testing sites and a further multicenter clinical trial employing an enhanced assessment with two additional sites (based on participant’s foot ulcer history or presence of preulcerative lesions), have failed to replicate these results.11,12 While attempts have been made to improve adherence to at-home thermometry and to reduction of ambulatory activity once a hotspot is determined, concerns have been raised regarding the implementation of this method beyond clinical trials. 13 Spot thermometry has been perceived by people living with diabetes as a cumbersome and non-user-friendly method. In addition, monitoring limited to predefined plantar foot landmarks can miss preulcerative inflammation of other parts of the foot, as more than half of the DFUs have nonplantar location. 14
We have previously hypothesized that the assessment of feet with thermal imaging would enable the detection of areas with raised temperature, which could be missed with spot thermometry. Detailed analysis of foot thermograms (temperature maps) could elucidate the frequency and distribution of regions with ∆T beyond the 2.2°C threshold (∆T ≥ 2.2°C). This could enable the development of robust and reliable clinical tools for DFU prevention. Thus, the aim of this study was to evaluate prospectively the consistency of ∆Ts over a 12-month follow-up period of 33 foot landmarks derived on plantar, dorsal, medial, and lateral thermal images of feet of people with diabetes who are at risk of DFU.
Methods
Data Provenance
This is a post-hoc analysis of prospective data from a recent single-blind multicenter clinical trial which investigated the usefulness of monthly thermography to reduce DFU recurrence in high-risk diabetic foot patients (ClinicalTrials.gov website NCT02579070). 15 The study was conducted in accordance to the Declaration of Helsinki with trial approval (Research Ethics Committee reference 15/LO/1940). 15 Participants provided informed written consent.
Study Participants
Trial inclusion and exclusion criteria have been previously reported. 15 In brief, study participants were 18 years or older and had type 1 or type 2 diabetes, intact feet, peripheral neuropathy (defined as vibration perception threshold [VPT] of 25 Volts or greater), and a history of at least one healed DFU. Subjects were recruited and randomized in three clinical hospital trusts in England to either active monthly thermography and standard foot care (intervention group) or blinded monthly thermography and standard foot care (control group). All participants received standard podiatric treatment and offloading with insoles and therapeutic footwear and were followed up in the study until they developed a DFU or for 12 months.
The present study included the subset of participants who were randomized to the control group at King’s College Hospital (blinded monthly thermography and standard foot care) and who remained ulcer free during the 12-month study period. Fifteen out of 34 participants fulfilled these criteria. Their thermal images acquired at baseline and then monthly for one year were included in the analysis.
Thermal Images
Study participants were assessed with thermal imaging, using a battery-powered hand-held device. 16 It included two cameras (an infrared camera and a visible light camera) which captured simultaneously a thermal and a visual image, respectively. The device was characterized and calibrated at the UK’s National Physical Laboratory and its reliability in assessing skin foot temperatures in healthy subjects and people with diabetes has been previously reported.17,18 At each visit, thermal images of the plantar, dorsal, medial, and lateral sites of both feet were acquired in a study room controlled for temperature and humidity after a 10-minute acclimatization period.15,17,19
For blinding purposes, the study device was set up to operate on the visual mode, and only the visual images were available for previewing on the device. During the clinical trial, access to thermal images was granted to the researchers only for participants allocated to the active group. At the end of the clinical trial, the thermal images of those study participants who remained ulcer free over the 12-month study period were unblinded and analyzed as described below.
Image Processing and Data Analysis
The thermal images were downloaded onto a computer and were analyzed using dedicated image analysis software by a trained operator (HL) who was unaware of the patents’ identity or their medical history. The software automatically calculated temperatures of manually selected circular regions of interest (ROIs) with a cross-sectional area of 1 cm2. 17 Temperatures at 33-foot landmarks of both feet were defined by manual annotation of each ROI. 19
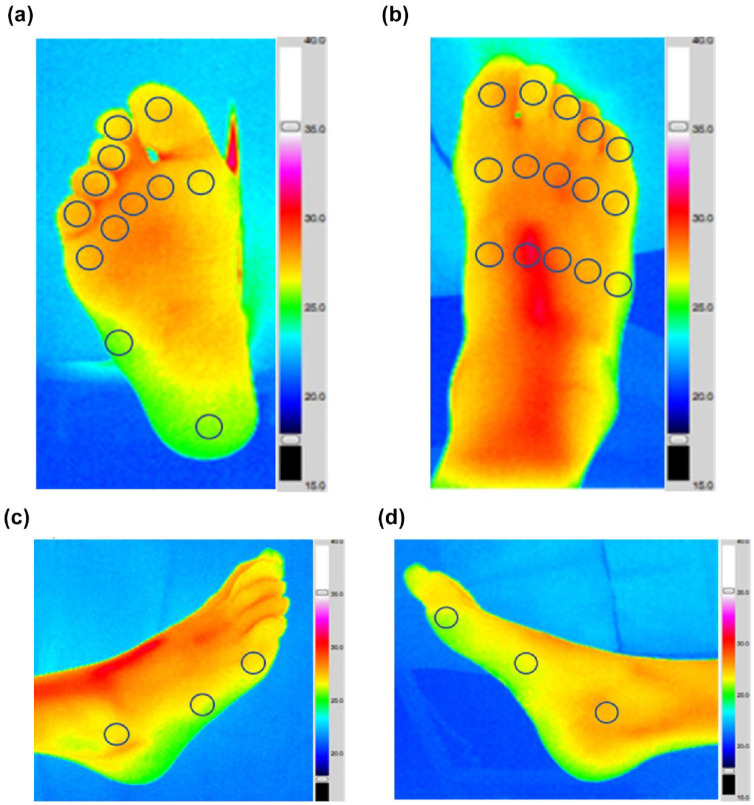
These included (1) 12 plantar sites (first to fifth toes, first to fifth MHs, fifth metatarsal base, and center of the heel); (2) 15 dorsal sites (first to fifth proximal metatarso-phalangeal joints [MPJs], first to fifth MHs, and first to fifth tarso-metatarsal joints); (3) three lateral sites (fifth MPJ, fifth metatarsal base and lateral malleolus); and (4) three medial sites (first MPJ, first metatarsal base, and medial malleolus; Figure 1).
Figure 1.
Representative thermograms of an individual with a history of healed DFU and neuropathy. (a) Plantar ROIs (n = 12). (b) Dorsal ROIs (n = 15). (c) Lateral ROIs (n = 3). (d) Medial ROIs (n = 3).
The ∆Ts between contralateral ROIs for the initial visit and the subsequent 12-monthly follow-up visits were calculated by subtracting the left foot temperature (LF) from the right foot (RF) temperature, as shown in the equation below.
The plantar foot ∆T for each participant at each visit was calculated as the mean of the sum of the ∆Ts of all 12 plantar ROIs.
The whole foot ∆T for each participant at each visit was calculated as the mean of the sum of ∆Ts of all 33 ROIs (12 plantar, 15 dorsal, 3 lateral, and 3 medial).
The ∆Ts for each ROI, for the plantar foot, and for the whole foot at each visit were computed using Microsoft Excel. Each of these derived variables were binary rated as normal (if ∆T < 2.2°C) or abnormal (if ∆T ≥ 2.2°C). Data are presented as mean (±standard deviation), median (interquartile range), or percentages as appropriate. Kruskal-Wallis (k-independent samples) and chi-square tests were used to compare the between-visit variability using Statistical Package for Social Sciences (SPSS). A P <.05 was considered to indicate statistical significance.
Results
Fifteen study participants fulfilled the inclusion criteria: There were 10 men and 5 women, of whom 5 had type 1 and 10 had type 2 diabetes mellitus. All participants had peripheral neuropathy. Almost half had a history of two or more healed DFUs. Most feet exhibited some degree of deformity (Table 1).
Table 1.
Demographic and Clinical Features of the Study Cohort.
| Study participants (n = 15) | |
|---|---|
| Males: females | 10:5 |
| Age (years) | 63 ± 10 |
| Type 1: type 2 diabetes | 5:10 |
| Duration of diabetes (years) | 26 ± 12 |
| Body mass index (kg/cm2) | 29 ± 8.4 |
| Right foot VPT (Volts) | 39 ± 11 |
| Left foot VPT (Volts) | 43 ± 10 |
| Mean VPT of right foot and left foot (Volts) | 39 ± 10 |
| Number of participants with history of | |
| One healed DFU | 8 |
| Two healed DFUs | 6 |
| More than 2 healed DFUs | 1 |
| Right foot deformities | |
| No deformity | 5 |
| Lesser toe deformities | 5 |
| More prominent deformities a | 5 |
| Left foot deformities | |
| No deformity | 6 |
| Lesser toe deformities | 4 |
| More prominent deformities a | 5 |
Data are presented as number or mean ± SD as appropriate.
Abbreviations: VPT, vibration perception threshold; DFU, diabetic foot ulcer.
Prominent deformities (prominent metatarsal heads, rocker bottom Charcot foot deformity, medial convexity Charcot foot deformity, prominent styloid process of the fifth metatarsal base, pes cavus [high arch, clawed toes, prominent metatarsal heads]).
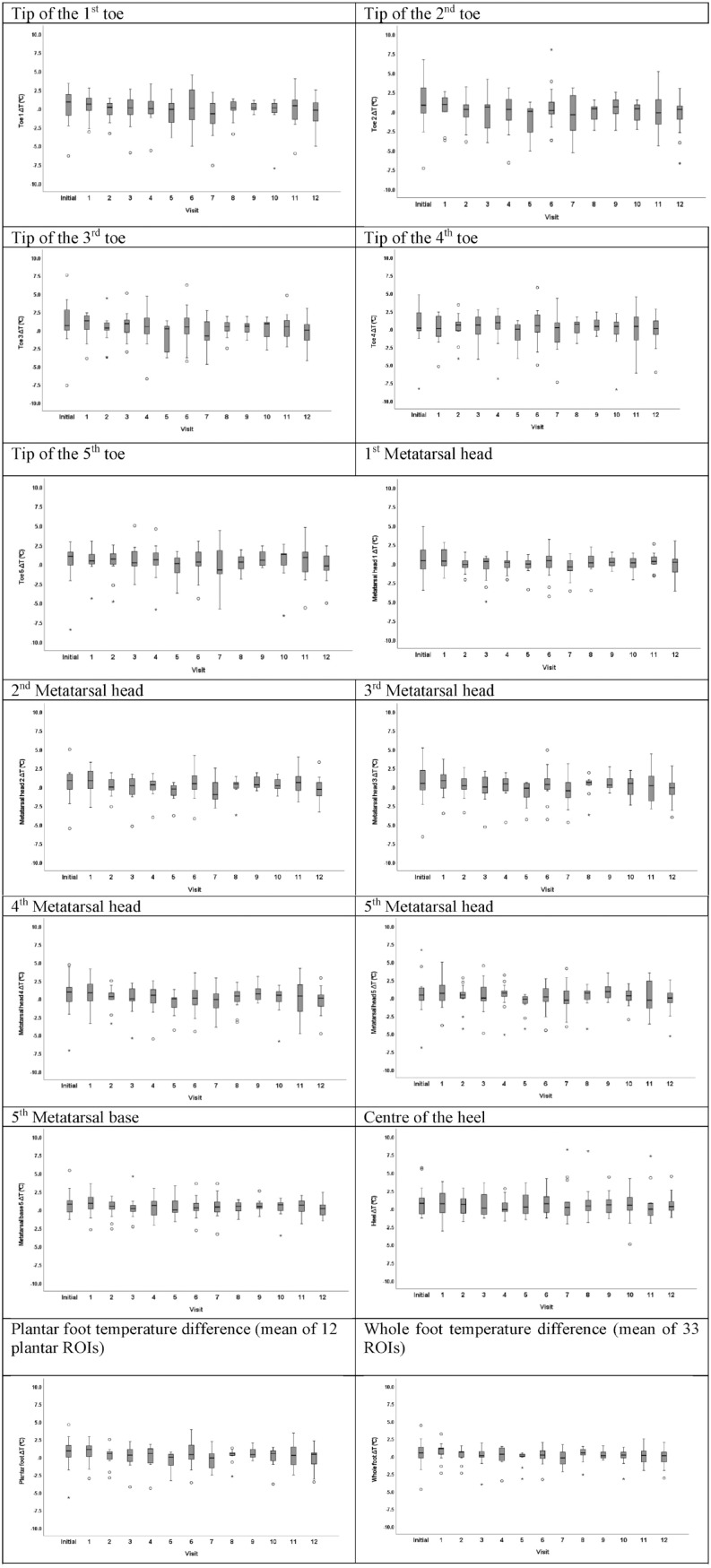
Overall, 169 thermal imaging sequences (plantar, dorsal, lateral, and medial views) were analyzed. The range of ∆Ts at each visit for the individual plantar ROIs, for the plantar foot, and for the whole foot are presented in Figure 2. During the 12-month study period, for the overall study sample, the median ∆T of the plantar foot ranged from 0°C to 1.1°C, and the median ∆T for the whole foot ranged from 0°C to 1°C. During the 12-month follow-up period, there were no statistically significant differences in the between-visit variability of the plantar foot ∆Ts (P = .573) and of the whole foot ∆Ts (P = .176; Figure 2).
Figure 2.
Thermal imaging data of people living with diabetes at the initial and the subsequent 12-monthly follow-up visits.
Data are presented as box and whiskers plots indicating the median temperature difference for the overall study sample and the 25th and 75th percentile at the initial visit and at the 12-monthly consecutive study visits at each plantar ROI, for the plantar foot, and for the whole foot. A total of 169 imaging sequences including plantar, dorsal, medial, and lateral views were analyzed. The number of participants who were assessed at each visit was as follows: Initial visit = 15 participants; month 1 = 13 participants; month 2 = 13 participants; month 3 = 13 participants; month 4 = 13 participants; month 5 = 12 participants; month 6 = 14 participants; month 7 = 13 participants; month 8 = 11 participants; month 9 = 12 participants; month 10 = 13 participants; month 11 = 12 participants; month 12 = 15 participants.
For the overall sample, out of the total number of 2026 ROIs identified on plantar foot thermograms over the 12-month follow-up (on average 155 data points per visit), the ∆Ts of 1616 (79.7%) plantar ROIs were normal (absolute ∆T < 2.2°C), whereas in the remaining 410 ROIs (20.3%), the ∆Ts were abnormal (absolute ∆T ≥ 2.2°C). There was a significant between-visit variability in the proportion of plantar ROIs with ∆T ≥ 2.2°C (range 7.6% to 30.8%, chi-square test, P = .001; Table 2).
Table 2.
Between-Visit Variability in Thermal Images of 15 Participants With a History of Healed DFU(s) Over a One-Year Follow-Up Period.
| Initial | Month 1 | Month 2 | Month 3 | Month 4 | Month 5 | Month 6 | Month 7 | Month 8 | Month 9 | Month 10 | Month 11 | Month 12 | P value | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proportion of plantar hotspots with ∆T ≥ 2.2°C (12 ROIs in 15 study participants) | 27.9% | 25.2% | 17.9% | 23.1% | 16.7% | 19.4% | 27.4% | 30.8% | 9.8% | 7.6% | 10.3% | 23.6% | 19.4% | .001 |
| Proportion of patients presenting with at least one plantar ROI with ∆T ≥ 2.2°C | 53.3% | 46.2% | 53.8% | 53.8% | 69.2% | 66.7% | 71.4% | 61.5% | 45.5% | 58.3% | 76.9% | 66.7% | 60.0% | >.05 |
| Proportion of patients presenting with plantar foot ∆T ≥ 2.2°C | 26.7% | 23.1% | 15.4% | 23.1% | 7.7% | 8.3% | 21.4% | 23.1% | 9.1% | 0.0% | 7.7% | 16.7% | 20.0% | >.05 |
| Proportion of patients presenting with wholefoot ∆T ≥ 2.2°C | 20.0% | 15.4% | 7.7% | 7.7% | 7.7% | 8.3% | 7.1% | 7.7% | 9.1% | 0.0% | 7.7% | 8.3% | 6.7% | >.05 |
Abbreviations: ∆T, temperature difference; ROI, region of interest.
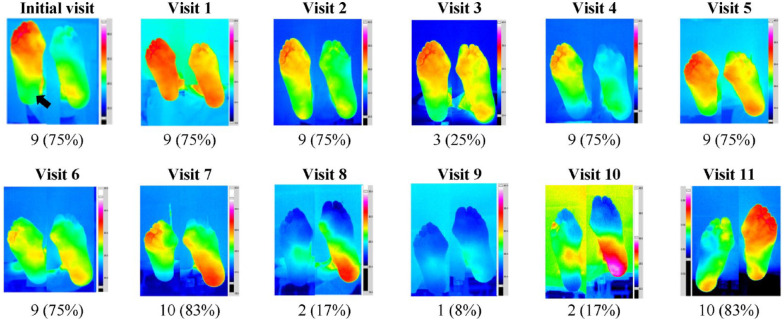
The proportion of study participants presenting with (1) at least one plantar ROI with ∆T ≥ 2.2°C (range 46.2%-77%); (2) with plantar foot ∆T ≥ 2.2°C (range 0%-26.7%); and (3) with whole foot ∆T ≥ 2.2°C (range 0%-20%) varied between visits (Table 2; P > .05 for all comparisons). A representative example of this between-visit inconsistency is demonstrated in Figure 3.
Figure 3.
Representative example of between-visit variability of monthly plantar thermal images.
Consecutive plantar thermal images of the right and left foot in a person with diabetes and a history of a healed DFU of the right heel (black arrow, initial visit). At the end of the study, the thermal images were unblended, and the ∆Ts of 12 plantar ROIs were calculated. At each visit, the number (%) of ROIs with ∆T ≥ 2.2°C is presented. There was a substantial between-visit variability in the appearances of the thermograms of both feet. Overall, 82 of 144 plantar ROIs exceeded the 2.2°C threshold (range 1-10 ROIs with ∆Ts ≥ 2.2°C per visit). The feet remained intact, and none of these hotspots progressed to the development of a DFU.
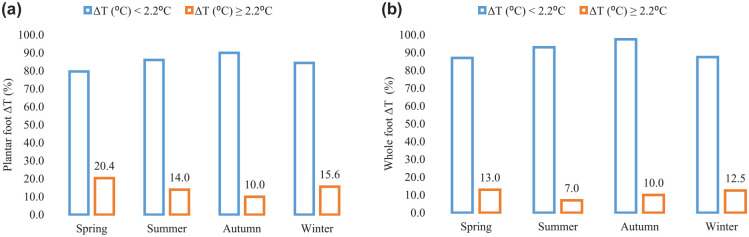
Finally, to assess the impact of seasons on temperature readings, the chronological data set of 169 thermograms was regrouped according to the month of assessment (Figure 4). There was no pattern in the between-season variability of the proportion of patients presenting with plantar foot ∆T ≥ 2.2 (°C) and with whole foot ∆T ≥ 2.2 (°C) (P > .05 for both comparisons).
Figure 4.
Seasonal variability of the plantar and whole foot ∆Ts of neuropathic feet at risk of DFU. (a) Plantar foot. (b) Whole foot.
The consecutive 169-foot thermograms of all study participants were reorganized according to the month (season) of acquisition and were grouped into four categories: Spring (March-May), Summer (June-August), Autumn (September-November), and Winter (December-February). The bars represent the seasonal variability in the proportion of patients with normal (∆T < 2.2°C, blue bars) and abnormal (∆T ≥ 2.2°C, orange bars) temperature differences of the plantar foot (a) and of the whole foot (b).
Discussion
This is the first study to report a comprehensive analysis of thermal imaging data of high-risk diabetic foot patients who remained ulcer free over a 12-month follow-up period. Blinded thermography revealed that on average, 20% of all plantar ROIs had ∆T ≥ 2.2°C, and more than half of the study participants presented with abnormal plantar and/or whole foot ∆Ts ≥ 2.2°C at least once during the 12-month follow-up. The observed between-visit variability showed no specific trend and was not affected by the season of assessment. None of these ROIs with abnormal ∆T progressed to a DFU at the next clinical visit. The reported variability in ∆Ts of commonly assessed plantar, dorsal, lateral, and medial foot ROIs should be considered when planning DFU-prevention studies.
There is an increased interest in the optimization of at-home temperature monitoring as a method of timely detection of skin inflammation for DFU prevention.6,20 Recent meta-analysis of five controlled trials involving 772 participants randomly assigned to daily temperature self-testing and activity reduction in response to identification of a hotspot showed a pooled odds reduction of 50% in the number of new DFU events and a relative risk of 0.51 (0.31-0.84). Nevertheless, due to the risk of bias and inconsistency, this meta-analysis graded the evidence with a low level of certainty. 21 Thus, before this prevention method is adopted in everyday practice, there is a need for improved understanding of the thermal imaging patterns and their consistency in neuropathic feet at risk of DFU.
To our knowledge, no other study has examined the long-term stability of thermal images of neuropathic feet at risk of DFU. The present methodological analysis of 33 ROIs acquired from consecutive monthly thermograms of feet of a typical study cohort at high risk of DFU showed significant variability not previously known. These included inconsistencies related to ∆Ts of individual ROIs, as well as plantar and whole foot ∆Ts.
The analyses of ∆Ts as a function of time showed a trend of a wider range of variability in ∆Ts for the tips of the toes, as compared to ∆Ts of the MHs, fifth metatarsal base, and the center of the heel (Figure 2). In healthy individuals, the plantar temperature is stable and is unrelated to temperatures of foot subregions (with toes having the lowest temperature and the foot arch having the highest temperature). 22 However, in diabetic feet with neuropathy, the plantar temperature is affected by the time taken for stabilization because of sympathetic dysfunction. 23 To standardize the assessment, in our cohort, thermal images were consistently acquired after barefoot rest for 10 minutes on a podiatry chair (10 minutes after socks off). 15
During the 12-month follow-up, the plantar ∆Ts varied between 0°C and 1.1°C. The maximum ∆T of 1.1°C was below 1.35°C, a threshold, recently reported as the most optimal cutoff for determining urgency of treatment for DFU prevention. 24 Indeed, in our study, all participants remained ulcer free, and apart from the routine standard care at 4-weekly intervals, no one required additional treatment. Yet, DFU prevention, based on comparative assessment of temperatures of pragmatically selected ROIs, is not always feasible when both feet have pathology or when one limb has partial or whole foot amputation. In these circumstances, the development of a unilateral temperature-monitoring approach for feet in remission may be facilitated by the application of accessible home thermal imaging devices that can deliver an instantaneous thermal map of the foot. 25
The limited use of ∆Ts of individual ROIs was further reinforced by the significant between-visit variability in the proportion of hotspots, identified in our series during the 12-month follow-up period. Observational data from healthy volunteers using the same device reported that 34 of 103 individuals presented with at least one plantar hotspot, and in 5% of the study participants, the plantar foot ∆T was ≥2.2°C. 19 In contrast, in the present longitudinal study, on average, 63% of the study participants had at least one plantar hotspot at each visit, and overall, 20% out of 2026 data points of all 12 plantar ROIs had a ∆T ≥ 2.2°C. None of these events triggered any action as these images were unblinded only after the end of the study. One can argue that if known, these abnormalities should have prompted a further investigation on the next day as a measure of consistency. Indeed, a sizeable reduction in the proportion of hotspots has been noted with repeated temperature assessments on two consecutive days.26,27
Of further note is the validity of the 2.2°C threshold as a forerunner of DFU—some studies have reported a low specificity of 40% of this threshold,24,27 whereas others have questioned whether the skin indeed heats up before it breaks down. 28 Yet, a further study by Frykberg et al 29 using a wireless thermometric mat demonstrated that the 2.2°C threshold accurately forecasted 97% of the 53 recurrent DFUs, which developed despite the also noted high false-positive rate of 57%. It is yet to be clarified whether the comparative temperature monitoring is the way forward in DFU prevention or whether at-home temperature monitoring with spot thermometers should be superseded with more refined methods of automated image acquisition, pattern recognition, and analyses. Such advances could overcome the need of relying on a “normal” contralateral foot and advance existing methods of temperature assessment and DFU prevention.
Finally, to investigate the possible impact of seasonality on ∆Ts, data were replotted according to the season of taking the thermal images. The highest proportions of hotspots were noted in spring—20.4% (plantar foot) and 13% (whole foot), respectively, (Figure 4). Interestingly, a correlation between the severity of diabetic foot infections and warmer temperatures has been found. 30 Higher incidences of both nontraumatic major lower-limb amputations (P = .0012) and monthly hospital admissions (P < .001) have been reported for countries with warmer climate. 30 Also, feet of people with diabetes and neuropathy show an inverse correlation between temperatures of the toes (r = −0.38, P < .05) and metatarsals (r = −0.43, P < .01) with seasonality. 31 Although we did not find any specific pattern in the seasonal variability of the proportion of ROIs with ∆Ts ≥ 2.2°C, further research is needed to understand the impact of environmental temperature on long-term skin foot temperature assessment of the diabetic neuropathic foot.
The study has some limitations: First, there was a 4-week interval between patients’ attendances for standard care and image acquisition. Such a long period could have had a significant impact on the long-term consistency of thermal patterns in feet with neuropathy. Second, the original study was powered to compare different treatment strategies, not between-visit variability in thermal imaging. Thus, a larger, nonselected study sample is needed to confirm these findings for improved understanding of the clinical significance of these “hot spots” as well as for identifying the optimum frequency of temperature monitoring (daily, every other day, or weekly) for effective DFU prevention.
The strengths of this study are as follows: First, it reported robust thermal imaging data of feet at high risk of DFU, acquired consistently with a reliable thermal imaging device; second, the 12-month imaging data were unblinded at the end of the study and analyzed by a blinded researcher, resulting in a low risk of bias; third, this rigorous assessment of 33-foot landmarks has indicated that in neuropathic feet at risk of DFU, ∆Ts of plantar ROIs, plantar foot, and the whole foot commonly exceed the accepted threshold of 2.2°C as a forerunner of DFU and yet the feet remained ulcer free.
Conclusions
A ∆T of ≥2.2°C between corresponding sites of neuropathic feet at risk of DFU on foot thermograms does not always lead to a foot ulcer. Thermal images should be interpreted in the context of clinical presentation. This study underscored that thermal images of the at-risk neuropathic foot are subject to significant between-visit variability. The complexity of this inconsistency should be considered when devising DFU-prevention programs for self-testing and behavior modification.
Acknowledgments
The authors would like to thank the participants of this study, all study collaborators of the multi-disciplinary multi-center study, and the project advisory board for their support and contribution.
Footnotes
Abbreviations: ∆T, temperature difference; DFU, diabetic foot ulcer; LF, left foot; MH, metatarsal head; MPJ, metatarso-phalangeal joint; RF, right foot; ROI, region of interest; VPT, vibration perception threshold.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: P.P. is currently working for Thermetrix Ltd, Abercynon, UK, a company that produces a thermal foot scanner. No potential conflicts of interest relevant to this article were reported by the remaining authors.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The research was funded by the National Institute for Health Research (NIHR) II-LA-0813-20007 program. Dr H.L. was on a fellowship at King’s College Hospital supported by Ministry of Health, Singapore, for Healthcare Manpower Development Award FY2018 and Tan Tock Seng Hospital Scholarship.
ORCID iD: Nina L. Petrova  https://orcid.org/0000-0001-6937-6565
https://orcid.org/0000-0001-6937-6565
References
- 1. Boulton AJM, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet. 2005;366(9498):1719-1724. [DOI] [PubMed] [Google Scholar]
- 2. Edmonds M, Manu C, Vas P. The current burden of diabetic foot disease. J Clin Orthop Trauma. 2021;17:88-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vas PRJ, Edmonds M, Kavarthapu V, et al. The diabetic foot attack: “tis too late to retreat!”. Int J Low Extrem Wounds. 2018;17(1):7-13. [DOI] [PubMed] [Google Scholar]
- 4. Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med. 2017;376(24):2367-2375. [DOI] [PubMed] [Google Scholar]
- 5. Jodheea-Jutton A, Hindocha S, Bhaw-Luximon A. Health economics of diabetic foot ulcer and recent trends to accelerate treatment. Foot (Edinb). 2022;52:101909. [DOI] [PubMed] [Google Scholar]
- 6. Bus SA, Sacco ICN, Monteiro-Soares M, et al. Guidelines on the prevention of foot ulcers in persons with diabetes (IWGDF 2023 update). Diabetes Metab Res Rev. 2023;40(3):e3651. [DOI] [PubMed] [Google Scholar]
- 7. Boulton AJ. Diabetic foot: what can we learn from leprosy? Legacy of Dr Paul W. Diabetes Metab Res Rev. 2012;28(suppl 1):3-7. [DOI] [PubMed] [Google Scholar]
- 8. Lavery LA, Higgins KR, Lanctot DR, et al. Home monitoring of foot skin temperatures to prevent ulceration. Diabetes Care. 2004;27(11):2642-2647. [DOI] [PubMed] [Google Scholar]
- 9. Armstrong DG, Holtz-Neiderer K, Wendel C, Mohler MJ, Kimbriel HR, Lavery LA. Skin temperature monitoring reduces the risk for diabetic foot ulceration in high-risk patients. Am J Med. 2007;120(12):1042-1046. [DOI] [PubMed] [Google Scholar]
- 10. Lavery LA, Higgins KR, Lanctot DR, et al. Preventing diabetic foot ulcer recurrence in high-risk patients: use of temperature monitoring as a self-assessment tool. Diabetes Care. 2007;30(1):14-20. [DOI] [PubMed] [Google Scholar]
- 11. Skafjeld A, Iversen MM, Holme I, Ribu L, Hvaal K, Kilhovd BK. A pilot study testing the feasibility of skin temperature monitoring to reduce recurrent foot ulcers in patients with diabetes: a randomized controlled trial. BMC Endocr Disord. 2015;15:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bus SA, Aan de Stegge WB, van Baal JG, Busch-Westbroek TE, Nollet F, van Netten JJ. Effectiveness of at-home skin temperature monitoring in reducing the incidence of foot ulcer recurrence in people with diabetes: a multicenter randomized controlled trial (DIATEMP). BMJ Open Diabetes Res Care. 2021;9(1):e002392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rovers FJ, Van Netten JJ, Busch- Westbroek TE, Aan de Stegge WB, Bus SA. Adherence to at-home monitoring of foot temperatures in people with diabetes at high risk of ulceration [published online ahead of print July 15, 2022]. Int J Low Extrem Wounds. doi: 10.1177/15347346221114565. [DOI] [PubMed] [Google Scholar]
- 14. Prompers L, Huijberts M, Apelqvist J, et al. High prevalence of ischaemia, infection and serious comorbidity in patients with diabetic foot disease in Europe. Diabetologia. 2007;50(1):18-25. [DOI] [PubMed] [Google Scholar]
- 15. Petrova NL, Donaldson NK, Tang W, et al. Infrared thermography and ulcer prevention in the high-risk diabetic foot: data from a single-blind multicentre controlled clinical trial. Diabet Med. 2020;37(1):95-104. [DOI] [PubMed] [Google Scholar]
- 16. Machin G, Whittam A, Ainarkar S, et al. A medical thermal imaging device for the prevention of diabetic foot ulceration. Physiol Meas. 2017;38(3):420-430. [DOI] [PubMed] [Google Scholar]
- 17. Petrova NL, Whittam A, MacDonald A, et al. Reliability of a novel thermal imaging system for temperature assessment of healthy feet. J Foot Ankle Res. 2018;11:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Macdonald A, Petrova N, Ainarkar S, et al. Between visit variability of thermal imaging of feet in people attending podiatric clinics with diabetic neuropathy at high risk of developing foot ulcers. Physiol Meas. 2019;40(8):084004. [DOI] [PubMed] [Google Scholar]
- 19. Macdonald A, Petrova N, Ainarkar S, et al. Thermal symmetry of healthy feet: a precursor to a thermal study of diabetic feet prior to skin breakdown. Physiol Meas. 2017;38(1):33-44. [DOI] [PubMed] [Google Scholar]
- 20. Frykberg RG, Vileikyte L, Boulton AJM, Armstrong DG. The at-risk diabetic foot: time to focus on prevention. Diabetes Care. 2022;45(10):e144-e145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Golledge J, Fernando ME, Alahakoon C, et al. Efficacy of at home monitoring of foot temperature for risk reduction of diabetes-related foot ulcer: a meta-analysis. Diabetes Metab Res Rev. 2022;38(6):e3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sun PC, Jao SH, Cheng CK. Assessing foot temperature using infrared thermography. Foot Ankle Int. 2005;26(10):847-853. [DOI] [PubMed] [Google Scholar]
- 23. Sun PC, Lin HD, Jao SH, Ku YC, Chan RC, Cheng CK. Relationship of skin temperature to sympathetic dysfunction in diabetic at-risk feet. Diabetes Res Clin Pract. 2006;73(1):41-46. [DOI] [PubMed] [Google Scholar]
- 24. van Netten JJ, Prijs M, van Baal JG, Liu C, van der Heijden F, Bus SA. Diagnostic values for skin temperature assessment to detect diabetes-related foot complications. Diabetes Technol Ther. 2014;16(11):714-721. [DOI] [PubMed] [Google Scholar]
- 25. Lavery LA, Petersen BJ, Linders DR, Bloom JD, Rothenberg GM, Armstrong DG. Unilateral remote temperature monitoring to predict future ulceration for the diabetic foot in remission. BMJ Open Diabetes Res Care. 2019;7(1):e000696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wijlens AM, Holloway S, Bus SA, van Netten JJ. An explorative study on the validity of various definitions of a 2·2°C temperature threshold as warning signal for impending diabetic foot ulceration. Int Wound J. 2017;14(6):1346-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Featherston J, Wijlens AM, van Netten JJ. Is a left-to-right >2.2 degrees C difference a valid measurement to predict diabetic foot ulceration in people with diabetes and a history of diabetic foot ulceration? [published online ahead of print December 20, 2021]. Int J Low Extrem Wounds. doi: 10.1177/15347346211062719. [DOI] [PubMed] [Google Scholar]
- 28. Aan de Stegge WB, Van Netten JJ, Bus SA. Does the skin heat up before it breaks down in diabetic foot ulceration. Diabetes Metab Res Rev. 2023;39(5):e3621. [DOI] [PubMed] [Google Scholar]
- 29. Frykberg RG, Gordon IL, Reyzelman AM, et al. Feasibility and efficacy of a smart mat technology to predict development of diabetic plantar ulcers. Diabetes Care. 2017;40(7):973-980. [DOI] [PubMed] [Google Scholar]
- 30. Leung HB, Ho YC, Wong WC, Guerin J. Seasonal variations in non-traumatic major lower limb amputation in Hong Kong Chinese diabetic patients. Hong Kong Med J. 2007;13(5):379-381. [PubMed] [Google Scholar]
- 31. Schmidt BM, Allison S, Wrobel JS. Describing normative foot temperatures in patients with diabetes-related peripheral neuropathy. J Diabetes Sci Technol. 2020;14(1):22-27. [DOI] [PMC free article] [PubMed] [Google Scholar]